Abstract
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy worldwide, with oral squamous cell carcinoma (OSCC) accounting for a significant portion of cases. Despite advancements in treatment, only modest gains have been made in HNSCC/OSCC control. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have emerged as targeted therapies for OSCC in clinical trials. However, their clinical efficacy remains a challenge. Cannabidiol (CBD), a non-psychoactive phytochemical from cannabis, has demonstrated anticancer and immunomodulatory properties. CBD induces apoptosis and autophagy and modulates signaling pathways often dysregulated in HNSCC. This review summarizes the molecular mechanisms of EGFR-TKIs and CBD and their clinical insights and further discusses potential implications of combination targeted therapies.
1. Introduction
Head and neck squamous cell carcinoma (HNSCC), ranked as the sixth most common cancer globally, is predominantly composed of OSCC [1,2,3]. The disease is responsible for approximately 450,000 deaths worldwide each year, highlighting its significant global health burden. According to GLOBOCAN 2022 estimates from the International Agency for Research on Cancer (IARC), Papua New Guinea, Southeast Asian countries, and Australia have some of the highest age-standardized incidence rates (ASIRs) of HNSCC globally, with ASIRs of 17.5, 9–10, and 8.3 per 100,000, respectively [1]. While the rising occurrence of HPV-related oropharyngeal infections has driven the increased prevalence of HNSCC in the United States and Western Europe, the elevated incidence of HNSCC in Southeast Asia and Australia/New Zealand is linked to the use of particular products containing carcinogens [3,4]. In the Asia-Pacific region, lip, oral cavity, oropharyngeal, and nasopharyngeal cancers continue to pose significant health challenges due to their high absolute incidence rates (Figure 1).
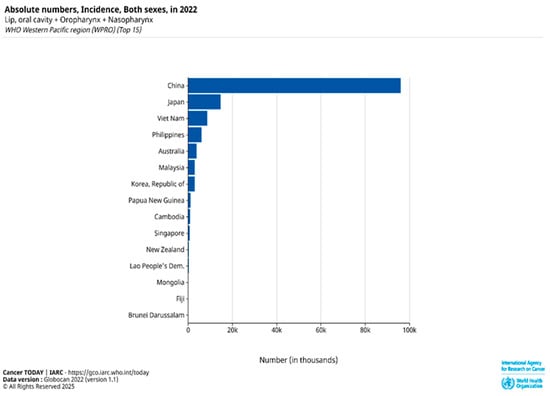
Figure 1.
Absolute number incidence of lip, oral cavity, oropharyngeal, and nasopharyngeal cancers in both sexes across the top 15 countries in the WHO Western Pacific Region (WPRO) based on GLOBOCAN 2022 data [1].
Despite advances in standard treatments and progress in research, only modest gains have been made in HNSCC/OSCC control [5]. Advanced-stage OSCC has a poor prognosis, with a 5-year survival rate of less than 30% in unresectable or metastatic cases due to the limited effectiveness of current therapies. Emerging clinical evidence suggests epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (TKIs), either alone or in combination with other therapies, such as chemotherapy, radiotherapy, or immunotherapy (e.g., nivolumab or pembrolizumab), have shown promise as targeted regimens for head and neck cancers, including oral cancers [4,5]. However, patients with mutations in the phosphatidylinositol 3-kinase (PI3K) pathway have shown poor response to irreversible EGFR-TKIs because these mutations activate signals that continue to promote cancer cell growth, even when EGFR is blocked [6,7].
Cannabidiol (CBD) has been shown to inhibit PI3K/Akt signaling, induce apoptosis and autophagy, and exhibit anti-inflammatory and anti-angiogenesis properties [8]. CBD’s ability to modulate signaling pathways, reduce inflammation, and induce cancer cell death could complement the targeted action of TKIs [9]. This synergy could potentially enhance the effectiveness of TKIs in eradicating cancer cells and thus prevent multidrug resistance [9,10,11,12]. Coordinated targeting of EGFR via CBD and TKIs could represent a logical approach for cancer treatment, particularly in HNSCC, where CBD receptors and EGFR signaling pathways interact [13]. Although this review focuses on the potential synergy between CBD and EGFR-TKIs, it is important to note that CBD’s anticancer effects are not limited to EGFR inhibition.
Preclinical studies suggest that CBD can also influence other receptor tyrosine kinases (RTKs), such as HER2, VEGFR, and PDGFR, which are involved in tumor growth, angiogenesis, and metastasis. CBD has been reported to inhibit HER2-driven tumor cell proliferation by binding to the HER2 kinase domain, thereby reducing activation of the downstream PI3K/AKT and MAPK/ERK pathways, which are critical for cancer cell survival [14]. Furthermore, CBD also suppresses VEGF signaling by reducing hypoxia-inducible factor 1-alpha (HIF-1α) expression and preventing VEGFR activation on endothelial cells, ultimately inhibiting angiogenesis [15]. Moreover, CBD interferes with PDGFR activity and downstream signaling pathways, like PI3K/AKT and MAPK/ERK, both of which contribute to cancer cell migration and resistance mechanisms. Since PDGFR is active not only in tumor cells but also in the tumor microenvironment, its inhibition by CBD may disrupt stromal support and reduce tumor progression [16].
Combinatorial therapies are increasingly being explored in cancer treatment to overcome drug resistance, boost therapeutic efficacy, and minimize toxicity. The incorporation of CBD into oral cancer therapy holds promise for improving patient outcomes by alleviating treatment-induced side effects and symptoms. CBD exhibits multiple pharmacological properties, including anticonvulsant, neuroprotective, antioxidant, analgesic, and anti-nausea effects. CBD also significantly lowers muscular tension, restlessness, fatigue, and cognitive impairments, such as difficulty concentrating. The U.S. Food and Drug Administration (FDA) has approved two cannabinoid-based oral medications, dronabinol (Marinol) and nabilone (Cesamet), specifically to relieve nausea and vomiting caused by cancer chemotherapy, underscoring their medical usefulness in oncology [17]. Furthermore, the novel combination strategy may enhance treatment outcomes in oral cancer by mitigating resistance to chemotherapeutic agents. Drug resistance remains a major clinical challenge contributing to therapeutic failure in cancer patients. Notably, CBD has demonstrated the ability to inhibit the growth and metastasis of cisplatin-resistant non-small cell lung cancer (NSCLC) [18], highlighting its potential to overcome drug resistance in other cancer types. Collectively, these contributions have the potential to substantially enhance patient outcomes, positioning this research as a pivotal advancement in the field of oncology.
This review summarizes the molecular mechanisms of EGFR-TKIs and CBD and their clinical insights and further discusses potential implications of combination targeted therapies.
2. Overview of Oral Cancer
The major constituent of head and neck cancers is OSCC, which is derived from the mucosal epithelium in the oral cavity, pharynx, and larynx and is known collectively as HNSCC [3,19]. OSCC accounts for more than 90% of head and neck cancers, which include malignancies of the nasal cavity, oral cavity, paranasal sinuses, nasopharynx, hypopharynx, and larynx [20,21,22]. The most common intraoral location of the OSCC is the tongue, followed by the floor of the mouth (which is the second common site), while the gingival, buccal mucosa, labial mucosa, and hard palate are less common sites [23].
The main risk factors for oral cancer include smoking, alcohol consumption, betel quid use, and overexposure to sunlight, which have been particularly associated with cancers of the skin and lip [24]. Accumulating evidence suggests that alcohol consumption is a predominant risk factor, followed by tobacco [3,21]. Further investigations are needed to determine the prevalence of oral malignancies attributed to oncogenic human papillomavirus (HPV) infection [19]. The interaction between these factors and viral infection in the situation of oral malignancy is a subject of ongoing investigations.
Certain high-risk HPVs are etiological factors for oral cancer; about 20% of OSCC are positive for HPVs, especially HPV-16 and -18 [25]. HPV types such as HPV-16 and -18 can integrate their genomes into the host DNA, leading to the expression of E6 and E7 oncogenes, which deregulate key molecules involved in the cell cycle [26,27]. Furthermore, the oncogenic proteins E6 and E7 inactivate tumour suppressors p53 and pRb, respectively [28]. Many clinical studies have shown that HPV status is an independent prognostic factor, with better survival rates observed in HPV-positive individuals compared to those who are HPV-negative [29]. Subsequently, HNSCC with positive HPV is more responsive to radiotherapy, and OPC with HPV has a better prognosis than conventional squamous cell carcinoma (SCC). This is because HPV-positive tumors usually have fewer genetic changes and are more sensitive to radiation. They also tend to trigger a stronger immune response, which helps improve treatment outcomes [30,31]; however, their association with the prognosis of OSCC has not been well-documented [32].
The WHO predicts a global increase of approximately 20% in OSCC incidence over the coming decades [33,34]. It is important to mention that the incidence of oropharyngeal malignancies has increased in many countries, including those in Europe [35], as well as Japan [36], South Korea [37], Brazil [38], Canada [39], the United States [40], England [41], Denmark [42], Australia [43], Scotland [44], and New Zealand [45].
Current scientific evidence indicates that the transition from normal epithelium to oral carcinoma results from the accumulation of genetic and epigenetic changes over time. These changes include the inactivation of tumor suppressor genes (TSG) (e.g., TP53, CDKN2A/p16, ING1, and ING3) [46], the activation of oncogenes (e.g., EGFR/erbB1, ERBB2/HER2), and mutations in DNA mismatch repair genes (e.g., MLH1, MSH2, MSH6, PMS2) [47,48]. As illustrated in Figure 2 and summarized in Table 1, this development follows a sequence of histopathological stages starting with normal squamous epithelium and advancing through hyperplasia, mild to severe dysplasia, carcinoma in situ, and ultimately SCC. Specific molecular changes accompany each stage. Early in the process, p16 deletion and EGFR overexpression are commonly observed. As dysplasia progresses to a more severe stage, TP53 mutations and p21 deletions contribute to cell cycle dysregulation. In carcinoma in situ, there is often amplification of cyclin D1 and chromosomal deletions at q24 and q31, promoting cellular proliferation. As the lesion progresses to invasion, PTEN inactivation and deletions on 18q and 4q26–28 further disrupt tumor suppressor functions. Finally, the loss of E-cadherin impairs cell–cell adhesion [23,46,47].
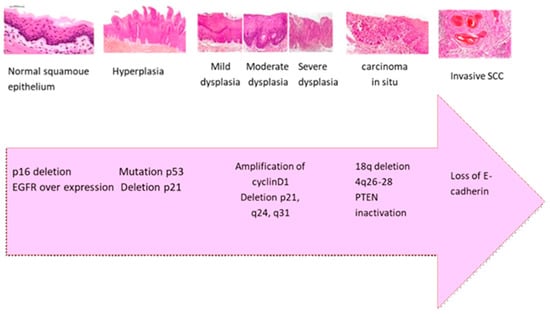
Figure 2.
The transition from normal squamous epithelium to invasive SCC involves sequential morphological changes (including dysplasia, carcinoma in situ, and invasive carcinoma, magnification 400×) accompanied by key molecular events such as p16 deletion, EGFR overexpression, TP53 mutation, cyclin D1 amplification, and PTEN inactivation. These alterations cause malignant transformation, enhanced proliferation, and loss of cellular adhesion, particularly E-cadherin, contributing to tumor invasiveness and metastasis [23,46,47].

Table 1.
Histopathological and molecular progression of OSCC [23,46,47].
HNSCC is typically addressed through initial surgery if the tumor is resectable, followed by adjuvant therapy that may involve radiation and, if necessary, chemotherapy [49,50]. For early-stage tumors (stages I and II) in the oral cavity, the primary approaches include either surgery or definitive radiation therapy. On the other hand, locoregionally advanced cancers (stages III and IVa,b) in the oral cavity are managed with surgery, followed by adjuvant radiation therapy, with or without concurrent chemotherapy [50]. About two-thirds of HNSCCs manifest in advanced stages, encompassing both local stages (III/IVa/IVb) and the metastatic stage (IVc) [51].
The effectiveness of surgery is hindered by limitations related to gender, age, and health condition. To address this limitation, standard chemotherapy agents, like cisplatin and 5-fluorouracil (5-FU), are employed alongside radiotherapy. However, this approach is restricted by prevalent resistance, lack of specificity, and undesirable side effects, including nephrotoxicity, mucositis, and fatigue, particularly in older patients [52,53].
In recent years, increasing rates of local recurrence and distant metastasis have created a bottleneck in improving survival outcomes for patients with HNSCC. To overcome this challenge, there is a pressing need for the development and integration of molecularly targeted antibodies and immunotherapies that offer high efficacy, strong selectivity, low toxicity, and the ability to counteract resistance to conventional treatments, such as radiotherapy and chemotherapy [52,53,54].
As summarized in Table 2, EGFR-targeted therapies for HNSCC include both monoclonal antibodies and TKIs. Monoclonal antibodies such as cetuximab, panitumumab, zalutumumab, and nimotuzumab bind to the extracellular domain of EGFR, blocking ligand binding and receptor activation while also promoting antibody-dependent cellular cytotoxicity (ADCC). In contrast, EGFR-TKIs like gefitinib, erlotinib, lapatinib, afatinib, and dacomitinib inhibit the intracellular tyrosine kinase domain of EGFR, thereby preventing downstream signaling pathways, such as PI3K/AKT and MAPK/ERK, which drive tumor proliferation and survival.

Table 2.
A summary of target therapies for EGFR in the treatment of HNSCCs [53].
Immunotherapy combinations are being actively investigated in Phase III trials to enhance efficacy and reduce toxicity in HNSCC. These combinations primarily involve immune checkpoint inhibitors, such as PD-1 inhibitors, like pembrolizumab and nivolumab, or PD-L1 inhibitors, such as avelumab and durvalumab, used either alone or in combination with other chemotherapy, radiotherapy, EGFR-targeting antibodies or other immunotherapeutic agents [54]. Notably, a recent Phase III trial (KEYNOTE-689 trial (NCT03765918)) exploring the addition of an innovative neoadjuvant and adjuvant pembrolizumab in resectable HNSCC has demonstrated significantly improved event-free survival [55].
EGFR antibody–immunotherapy approaches, which combine EGFR-targeting antibodies, like cetuximab, with immune checkpoint inhibitors, are also under investigation to enhance immune activation and overcome resistance [56]. Cetuximab, an anti-EGFR IgG1 monoclonal antibody, not only inhibits EGFR-mediated signaling but also engages immune effector functions, such as antibody-dependent cellular cytotoxicity (ADCC), thereby providing a rationale for combination with immunotherapy [56]. For instance, the GORTEC 2017-01 (REACH) Phase III trial (NCT02999087) is evaluating the efficacy and safety of combining cetuximab and avelumab (anti-PD-L1) with radiotherapy versus standard radiotherapy combinations in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN) [57].
Furthermore, novel targeted agents, such as antibody drug conjugates (ADCs), are designed to deliver cytotoxic payloads directly to tumor cells with high specificity. Emerging ADC formats involving bispecific ADCs, dual-drug ADCs, and conditionally activated probody drug conjugates are being developed to improve tumor targeting while reducing toxicity [58]. For example, MRG003, an anti-EGFR antibody–drug conjugate, is currently being evaluated in an ongoing Phase III clinical trial (NCT05751512) to assess its efficacy and safety compared to cetuximab or methotrexate in patients with recurrent/metastatic (R/M) HNSCC who have progressed on prior treatments [59]. The novel targeted agent PDS0101, a therapeutic vaccine that targets HPV16 E6/E7 oncoproteins, is currently being evaluated in the Phase III VERSATILE-003 trial (NCT04260126). Administered in combination with pembrolizumab, this vaccine has demonstrated promising results in earlier trials and represents a targeted immunotherapeutic strategy for HPV+HNSCC [60].
3. Epidermal Growth Factor Receptor (EGFR)
EGFR is defined as a family of receptor tyrosine kinases (RTKs) that form a subset of tyrosine kinases crucial for cell communication and governing various intricate biological processes such as cellular proliferation, movement, specialization, and metabolic activities. Human biology encompasses 58 identified RTKs, all exhibiting a uniform protein architecture consisting of an extracellular ligand-binding domain, a solitary transmembrane helix, and an intracellular segment housing a regulatory region adjacent to the membrane, a tyrosine kinase domain (TKD), and a C-terminal tail [61].
The EGFR family consists of four distinct receptors, including the epidermal growth factor receptor (also known as ErbB-1/HER1), ErbB-2 (neu, HER2), ErbB-3 (HER3), and ErbB-4 (HER4) [62]. EGFR is triggered by binding to specific ligands such as epidermal growth factor (EGF), transforming growth factor-alpha (TGF-alpha), amphiregulin (AREG), epigen, β-cellulin, and heparin-binding EGF(HB-EGF). Ligand binding to domains I and III of the RTK induces the EGFR for a conformational alteration that causes the exposure of the dimerization loop in domain II of the receptor. As a result of this exposure of the dimerization loop, the receptor at the plasma membrane undergoes homodimerization and heterodimerization [62,63]. This interaction stimulates the RTK, leading to phosphorylation of the intracellular domain, and mediates the interaction between the receptor and downstream effectors, such as Shc1 and Grb2 [64]. This further activates downstream signaling pathways, such as PI3/Akt (survival and apoptosis evasion) and Ras-Raf-MEK-MAPK (proliferation) [65]. HER3 is the only family member without intrinsic kinase activity [63].
EGFR is also present in healthy tissues but is upregulated in malignancies such as breast cancer, glioblastoma, and SCC [66]. In HNSCCs, high EGFR expressions are positively associated with earlier relapse, shorter disease-free survival, and reduced overall survival compared to patients with lower EGFR expression levels [67,68]. EGFR overexpression is associated with poor outcomes, the inhibition of apoptosis, and increased metastatic potential [53,59]. Notably, EGFR overexpression is observed in approximately 90% of the HNSCC cases, making it a negative prognostic marker [62,68].
Given these associations, current therapeutic strategies for HNSCC focus on EGFR inhibition. EGFR inhibition agents function by either blocking the ligand binding at the extracellular domain of EGFR (e.g., cetuximab, panitumumab, zalutumumab, and nimotuzumab) or hindering EGFR autophosphorylation in the cytoplasmic domain by competing with ATP (e.g., gefitinib, erlotinib, lapatinib, afatinib, and dacomitinib). Such interferences consequently disrupt the downstream cell signaling cascade [67]. In Section 4, a brief explanation of TKIs and their mechanism of action will be provided. Molecular targeted therapies, such as gefitinib and afatinib, are being used in tongue cancer; therefore, further details about gefitinib will be briefly discussed.
4. EGFR–Tyrosine Kinase Inhibitors
4.1. Gefitinib
Gefitinib (ZD1839 or Iressa) is a widely used EGFR-TKI. According to preclinical in vitro research, EGFR targeting with gefitinib leads to decreased cell survival, proliferation, and migration with sensitivity to the drug. Depending on the cancer cell type and the presence or absence of a sensitizing EGFR mutation, gefitinib exhibited half-maximal inhibitory concentrations (IC50) ranging from submicromolar levels to 13 µM across various non-small cell lung cancer (NSCLC) cell lines [69].
In early clinical trials, patients with a wide range of solid tumor types, including head and neck, lung, colon, breast, and prostate cancers, have revealed that gefitinib is generally well tolerated [70]. Moreover, it is well established that gefitinib inhibits solid tumors in vivo and induces autophagy [71,72]. This drug has been used to treat many types of cancer with EGFR mutations effectively [73]. In 2003, the FDA approved gefitinib as the first-line treatment for patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC) [74].
Several different EGFR mutations have been discovered, although they are rare in HNSCC patients. These mutations tend to concentrate around the active site crevice of the tyrosine kinase domain. In exon 18, point mutations within the nucleotide binding loop have been identified, including substitutions at glycine 719 (Gly719), which may be replaced by serine, cysteine, or alanine [75]. In exon 19, minor deletions are observed, most notably the deletion of residues 746–750, which is common in lung cancer but rarely reported in HNSCC [75,76]. Exon 20 mutations include insertions that can enhance kinase activity, though they are infrequent in HNSCC [75]. In exon 21, the L858R point mutation, which substitutes leucine 858 with arginine, is the most prevalent EGFR mutation in lung cancer, accounting for around 40% of all cases, but it is rarely seen in HNSCC [75,76]. These mutations enhance receptor activation by stabilizing the kinase domain in its active conformation, which facilitates dimerization [61,77]. Many studies have demonstrated that lung cancer patients with EGFR kinase domain mutations benefit from EGFR-TKI treatments, while those with wild-type EGFR do not show similar advantages [61]. However, in a small-scale HNSCC clinical study, an EGFR mutation involving a deletion in exon 19 was associated with a poor response to cetuximab treatment and worse prognosis [78], as shown in Table 3.

Table 3.
A summary of EGFR mutations across NSCLC vs. HNSCC, with prevalence and TKI sensitivity.
Gefitinib has been investigated in HNSCC cell lines, where it has been frequently shown to delay tumor growth and enhance apoptosis [79,80]. This has been attributed to its inactivation of functional ERK and AKT signaling pathways in either unstimulated or EGF-stimulated conditions [80]. However, a meta-analysis suggested that gefitinib did not significantly improve the progression-free survival (PFS), overall survival rate (OSR), or objective response rate (ORR) in patients with advanced HNSCC [51]. Similarly, unfavorable results were observed in a randomized study comparing gefitinib to methotrexate in recurrent and/or metastatic HNSCC, where the EGFR gene copy number did not predict a survival advantage in individuals treated with the EGFR-TKI. Intriguingly, the response to TKIs in a specific subset of HNSCC might be attributed to mutations in ErbB2 rather than EGFR, although this initial observation awaits further validation [81].
4.2. Afatinib
Afatinib (BIBW 2992, Gilotrif™) is a second-generation, orally administered TKI that irreversibly targets members of the ErbB receptor family, including EGFR, HER2, and HER4 [82]. It is FDA-approved as a first-line treatment for metastatic NSCLC with common EGFR mutations [83].
Mechanistically, afatinib is an irreversible pan-ErbB TKI that covalently binds to the kinase domains of EGFR, HER2, and HER4, leading to sustained inhibition of their enzymatic activity. This covalent binding blocks both ligand-dependent and ligand-independent receptor activation, preventing autophosphorylation and transphosphorylation events within ErbB dimers. As a result, downstream signaling pathways such as PI3K/AKT, RAS/RAF/MEK/ERK, and JAK/STAT are suppressed, reducing cell proliferation, survival, and angiogenesis [84]. Afatinib also inhibits receptor heterodimerization and crosstalk, which are key mechanisms of resistance in tumors. Importantly, afatinib retains activity against resistant EGFR mutation T790M in NSCLC, activating truncation variants, like EGFRvIII, occasionally identified in HNSCC [82,84,85,86].
Afatinib has demonstrated lower IC50 values compared to gefitinib across EGFR-amplified cell lines, including those resistant to gefitinib [84,85]. It also shows activity in HER2-amplified breast cancer cell lines resistant to trastuzumab and lapatinib [82]. In HNSCC, where erlotinib and gefitinib have shown limited efficacy as monotherapies, afatinib has emerged as a promising drug due to its ability to inhibit the ErbB family and its encouraging preclinical and early clinical studies [85,87]. Recent clinical trials support the effectiveness of afatinib. For example, the UPSTREAM trial reported improved progression-free survival in biomarker-selected HNSCC patients, particularly those with EGFR amplification, HER2 mutations, or PTEN-high tumors [88]. Additionally, the ALPHA study demonstrated an improved ORR when afatinib was combined with pembrolizumab in platinum-refractory HNSCC [89].
5. Clinical Trials of Targeted Therapies in HNSCC
EGFR is overexpressed in 90% of HNSCC patients, despite the absence of active EGFR mutations [85,90]. Several clinical trials have investigated the safety and efficacy of TKIs and immunotherapies in HNSCC. Table 4 summarizes FDA-approved monoclonal antibodies targeting EGFR and PD-1 receptors, including cetuximab, pembrolizumab, and nivolumab, along with their mechanisms of action, indications, trial phases, and common adverse effects. Table 5 presents EGFR-targeting TKIs, such as erlotinib, gefitinib, lapatinib, afatinib, and dacomitinib, which have been evaluated across multiple clinical trial phases [85,91]. For instance, in a multi-center Phase II trial (NCT00387127), lapatinib was compared to placebo in combination with cisplatin and radiotherapy in patients with unresected HNSCC [92]. The study evaluated complete response rates at 6 months post-chemoradiation and found that lapatinib was generally well tolerated, with numerical improvements in progression-free survival (PFS), particularly in p16-negative patients.
Similarly, a Phase II trial demonstrated that afatinib monotherapy was effective in recurrent or metastatic HNSCC resistant to platinum-based treatments, suggesting that sequential ErbB inhibition may benefit certain patient subgroups [85,92]. Despite afatinib’s clinical activity in HNSCC, its approval remains limited to EGFR mutation-positive NSCLC due to the lack of activating mutations in HNSCC [90,93]. Nevertheless, afatinib has shown promise in R/M HNSCC following platinum failure [85,90,94]. However, adjuvant afatinib after chemoradiotherapy (CRT) did not improve disease-free survival (DFS) and was associated with increased adverse events, making it unsuitable for routine use in primary unresected HNSCC [82,85,95,96].
The suboptimal response to EGFR inhibitors remains a major challenge in HNSCC [97]. To address this, emerging strategies are exploring combinations of TKIs with immunotherapy and radiotherapy. For example, the NIVOPOSTOP trial (NCT03576417) is a pivotal Phase III randomized clinical trial that evaluated the addition of nivolumab, an anti-PD-1 immune checkpoint inhibitor, to standard postoperative cisplatin-based chemoradiotherapy in patients with locally advanced HNSCC showing high-risk pathological features after surgery. The trial recently demonstrated that the addition of adjuvant nivolumab significantly improved the 3-year disease-free survival in high-risk, resected locally advanced HNSCC (63.1% vs. 52.5%; HR 0.76; p = 0.034), providing important clinical evidence supporting the integration of immunotherapy in the treatment of HNSCC [97].
Globally, 1266 clinical studies related to HNSCC were registered on ClinicalTrials.gov with start dates on or before 31 January 2022. Of these, 393 have been completed, while 590 are in various stages of recruitment. Despite the volume of research, a significant number of completed trials remain unpublished [98].

Table 4.
Monoclonal antibodies target EGFR and PD-1 receptors, approved for treating HNSCC.
Table 4.
Monoclonal antibodies target EGFR and PD-1 receptors, approved for treating HNSCC.
| Medicine | Mechanism of Action | Year | Indication | Clinical Trial | Side Effect | Ref. |
|---|---|---|---|---|---|---|
| Cetuximab | Monoclonal antibody targeting EGFR | 2006 | HNSCC after platinum-based therapy (in combination with radiotherapy) | NCT00004227 | Acneiform skin rash Fatigue Diarrhea Hypomagnesemia | [67] |
| 2009 | R/M HNSCC (in combination with platinum–fluorouracil chemotherapy) | NCT00122460 | Acneiform skin rash Fatigue Diarrhea Hypomagnesemia | [68] | ||
| Pembrolizumab | Monoclonal antibody targeting PD-1 receptor | 2016 | HNSCC after platinum-based chemotherapy | NCT01848834 | Autoimmune toxicities Colitis Pneumonitis Thyroiditis | [99] |
| 2019 | Metastatic or unresectable recurrent HNSCC (in combination with platinum and fluorouracil (FU) for all patients and as a single agent for patients whose tumors express PD-L1). | NCT02358031 | Hepatitis Dermatitis Hypophysitis | [100] | ||
| 2025 | Resectable locally advanced HNSCC (CPS ≥ 1); neoadjuvant + adjuvant ± cisplatin + RT | KEYNOTE-689 | Pneumonitis, colitis, hepatitis, endocrinopathies | [55] | ||
| Nivolumab | Monoclonal antibody targeting PD-1 receptor | 2014 | R/M HNSCC with disease progression on or after a platinum-based therapy | NCT02105636 | Autoimmune toxicities Colitis Pneumonitis Thyroiditis Hepatitis Dermatitis Hypophysitis | [101] |

Table 5.
EGFR-TKIs are approved for the treatment of HNSCC in clinical trials.
Table 5.
EGFR-TKIs are approved for the treatment of HNSCC in clinical trials.
| Medicine | MOA | Phase | Indication | Stage of Disease | Result | Ref. |
|---|---|---|---|---|---|---|
| Erlotinib | EGFR-TKI | I | Cissplatin+radiotherapy | Locally advanced | Safe combination | [102] |
| II | Drug alone | Recurrent or metastatic | Stabilized disease | [87] | ||
| I/II | Cissplatin + radiotherapy | Locally advanced | Feasible and well tolerated | [103] | ||
| Geftinib | EGFR-TKI | I | Cisplatin+radiotherapy | Locally advanced | Well tolerated with concomitant radiotherapy/chemoradiotherapy | [104] |
| II | Drug alone | Recurrent/metastatic | Feasible and active | [105] | ||
| III | Cisplatin + radiotherapy | Untreated/unresected/stage III/IV/nonmetastatic | Well tolerated | [106] | ||
| II | Carbo/paclitaxel+RT | Locally advanced | Overall survival and complete response improvement | [107] | ||
| Lapatinib | EGFR-TKI | II | Cisplatin + radiotherapy | Locally advanced | Positive clinical activity was well tolerated | [92] |
| II | Druga alone | Before chemoradiotherapy | Positive clinical activity, reduction, and cell proliferation index | [108] | ||
| Afatinib | EGFR-TKI | II | Compared with cetuximab | Metastatic and recurrent HNSCC | Sequential EGFR/ErbB treatment with afatinib and cetuximab provided sustained clinical benefits in patients after crossover, suggesting a lack of cross-resistance | [109] |
| Dacomitinib | EGFR-TKI | II | Drug alone | Metastatic and recurrent HNSCC | Well tolerated | [110] |
MOA means mechanism of action; RT refers to radiotherapy.
6. Cannabidiol (CBD)
CBD (Figure 3) is a constituent of the Cannabis sativa L. (marijuana) plant family, and research on the chemical structure of CBD compounds has been ongoing since the 1960s. Cannabis plants contain two primary components, CBD and tetrahydrocannabinol (THC), each exerting distinct bioactive and medicinal effects on the human body [111].
In addition to its well-known antioxidant and immunomodulatory effects, CBD interacts with pathways in the body that are also involved in cancer progression. Accumulating evidence suggests that CBD demonstrates anti-tumor efficacy across various malignancies such as lung, breast, prostate, and colorectal cancer [112]. Studies using xenograft mouse models have shown that CBD can significantly reduce the migration, invasion, and viability of HNSCC cells in a dose- and time-dependent manner [113,114]. Additionally, CBD has demonstrated anti-tumor effects in HNSCC human cell lines and can enhance the efficacy of chemotherapy drugs [5]. CBD’s anticancer effects have been attributed to its interaction with the endocannabinoid system (ECS), apoptosis induction, and the suppression of oncogenic signaling [115].
The specific mechanism through which CBD reduces EGFR-mediated intracellular signaling is still not fully understood. The molecular structure of CBD is rich with aromatic rings and hydroxyl groups, which play crucial roles in establishing hydrophobic interactions and hydrogen bonds with numerous amino acids within the EGFR active site. Notably, CBD contains two hydroxyl groups. The significance of the phenolic hydroxyl groups in cannabinoids for both binding to and inhibiting EGFR-TK has been confirmed. CBD can be securely docked into the ATP-binding site of EGFR-TK through various weak interactions, as shown in Figure 3. These interactions include hydrophobic interactions among several amino acid residues and strong hydrogen bonds formed between a hydroxyl group of cannabinoids and Thr766, Gln767, and Met769 of EGFR-TK [116,117].
Figure 3.
The chemical structure of cannabidiol (CBD) [118].
The combination of CBD with chemotherapeutic agents vinorelbine and 7-ethyl-10-hydroxycamptothecin has enhanced their efficacy in MCF7 breast cancer cells. A key molecular mechanism contributing to these synergistic effects is the enhancement of the pro-apoptotic activity of chemotherapeutic agents by CBD [119]. CBD’s induction of programmed cell death (apoptosis) was linked to the reduction in the levels of mTOR, AKT, 4EBP1, and cyclin D and the increased expression of PPAR-γ [9]. Moreover, CBD impeded the invasive and metastatic characteristics of aggressive triple-negative breast cancer (TNBC) by inhibiting the activation of the EGF/EGFR pathway and its downstream components (AKT and NF-κB). This was coupled with the downregulation of Id-1 protein by ERK and reactive oxygen species (ROS) [11]. Recent studies have attributed the anticancer activities of CBD to its binding to endocannabinoid receptors CB1 and CB2 [112], which subsequently render anti-inflammatory, pro-apoptotic, and antiproliferative effects [120].
6.1. Endocannabinoid Receptors (CB1 and CB2)
It is important to note that the effects of Δ9-THC (Delta-9-tetrahydrocannabinol) and cannabidiol depend on their ability to target specific endocannabinoid receptors, which belong to the G protein-coupled receptor family. CB1 receptors are primarily located in the central nervous system, particularly in the brain, while CB2 receptors are predominantly expressed in the immune system [118,121]. CBD has low affinity for CB1 and CB2 receptors compared to Δ9-THC, which is consistent with its lack of noticeable psychoactivity. Despite its low affinity towards CB1 and CB2 receptors, CBD can behave as a CB2 receptor inverse agonist [122], which binds as an agonist but inhibits CB2 receptors. Recent studies have linked cannabinoid receptors to the onset and advancement of tumors. The upregulation of CB1 or CB2 expression has been observed in hepatocellular carcinoma, renal cancer, and breast cancer. Moreover, this upregulation is correlated with the severity of the disease and a less favorable prognosis [123,124].
Nevertheless, conflicting functions have been documented for cannabinoid receptor pathways. While some studies suggest that cannabinoid receptors exhibit anti-tumoral effects, others demonstrate a tumor-promoting role for cannabinoids [8,125]. Changes in the endocannabinoid system (ECS) have been observed in breast cancer, notably with an increase in the expression of CB2 receptors. Specifically, research has found that over 90% of HER-2-positive tumors exhibit elevated expression of the cannabinoid receptor [126,127]. This overexpression of CB2 receptors is associated with a negative prognosis, likely due to the activation of HER2 pro-oncogenic pathways [126,128].
6.2. CBD as a Promising Anticancer Agent
It has been demonstrated that CBD has a major impact on cancer hallmarks through multiple pathways. The first pathway is the modulation of endoplasmic reticulum (ER) stress. ER homeostasis refers to the balance between the protein load within the ER and its capacity to properly fold proteins. This balance can be disrupted by various physiological conditions, leading to protein folding demand and folding capacity. As a result, unfolded proteins accumulate, inducing ER stress [129]. Subsequently, unfolded protein response (UPR) is activated in response to ER stress, leading to the activation of three parallel pathways, which are protein kinase RNA-like ER kinases (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE-1) [130]. In a cancer environment, ER stress and UPR have a distinguishable function in malignancy development. This results in malignant survival, oncogenic transformation, and cancer progression. CBD is a potent ER stress inducer, which causes prolonged toxic signals to induce apoptosis in cancer cells [129,131]. For example, the controlled exacerbation of pre-existing ER stress in the cancer cell can overload the ER stress scheme, which leads to the stimulation of the pro-apoptotic component of the UPR [132].
Another possible mechanism of CBD in malignancy is the modulation of major cell cycle pathways. In a notable study on gastric cancer cells, CBD was reported to induce cell cycle arrest at the G0–G1 phase, which was associated with a reduction in CDK2/cyclin E protein levels [133]. Addressing local recurrence and the metastasis of cancer remains a contemporary challenge in the treatment of HNSCC. To combat this issue, the application of combination therapy containing drugs with multiple anticancer mechanisms has been proposed as a potential therapeutic strategy to overcome multidrug resistance and to improve therapeutic efficacy [134].
In addition, the role of classical cannabinoid receptors in the progression of HPV-positive HNSCC has been investigated. The study revealed that the knockdown of CB1 and CB2 receptors suppressed the growth of HPV-positive HNSCC cells, whereas activation with CB1/CB2 agonists increased proliferation and migration and reduced apoptosis. These effects occurred via the specific activation of the p38 MAPK pathway. Moreover, increased p38 MAPK activation has been observed in HPV-positive HNSCC patients exposed to cannabinoids [135]. However, the role of cannabinoids in the HPV-positive HNSCC microenvironment remains largely unexplored. A recent preclinical study provided direct evidence that CBD suppresses HPV-positive HNSCC by promoting apoptosis, activating MAPK signaling (ERK1/2, JNK/SAPK, and MK2), and enhancing anti-tumor immunity through CD4+/CD8+ T-cell infiltration and activation, particularly in immune-competent models [136].
Hijiya et al. (2017) have demonstrated an independent association between CB1 overexpression and an unfavorable prognosis in esophageal SCC [137]. Additionally, in another study, the high expression of CB2 in HNSCC has been correlated with reduced disease-specific survival [138]. On the contrary, some researchers found elevated levels of CB1 and CB2 in mobile tongue SCC, and this heightened expression was associated with a more favorable prognosis [139]. Further mechanistic studies are warranted to elucidate the mechanisms underlying such discrepancy between clinical association studies.
Research has explored the anti-angiogenic properties of CBD using human umbilical vein endothelial cells. The findings indicate that CBD causes endothelial cells to cease proliferation without triggering cell death and effectively reduces their ability to migrate, invade, and form new blood vessel structures both in vitro and in vivo. These actions are associated with reduced levels of several key angiogenesis-related molecules, such as matrix metalloproteinases MMP2 and MMP9, urokinase-type plasminogen activator (uPA), endothelin-1 (ET-1), platelet-derived growth factor-AA (PDGF-AA), and the chemokine CXCL16 [15], as seen in Table 6, which shows the mechanism of action of CBD, molecular targets, and biological effects.

Table 6.
CBD’s mechanism of action, molecular targets, and biological effects.
CBD has been shown to enhance chemosensitivity and reverse drug resistance in HNSCC models. Go et al. (2020) reported that CBD significantly reduced cell viability, migration, and invasion in various HNSCC cell lines and xenograft models. When combined with chemotherapeutics, like fluoropyrimidines, platinum analogs, or taxanes, CBD exhibited synergistic cytotoxic effects. Mechanistically, CBD induced apoptosis and autophagy while downregulating key genes involved in DNA repair, cell proliferation, and cell cycle progression, which are related to therapeutic resistance in HNSCC. Furthermore, CBD modulates signaling pathways, such as PI3K/AKT/mTOR and MAPK/ERK, which are often dysregulated in HNSCC, thereby overcoming survival mechanisms and enhancing drug efficacy [5].
6.3. Synergism Between CBD and TKIs on EGFR
As mentioned previously, EGFR is overexpressed in many types of cancer, including HNSCC. Experimental data have shown that CBD can inhibit EGF-induced EGFR-TK activity, leading to the suppression of tumor cell growth and the induction of apoptosis in vitro and in vivo [11]. CBD has demonstrated a high binding affinity for purified EGFR-TK and a potent inhibitory effect against EGFR-TK, with IC50 values of 32 nM (compared with an IC50 value of 3 nM for afatinib), which is consistent with the in silico characterization of molecular interactions between CBD and EGFR-TK [14]. Gefitinib exhibited enhanced therapeutic efficacy and reduced cytotoxicity when combined with CBD [14]. These results provide strong support for the development of CBD as a potential adjunct to EGFR TKI-based treatment options in patients with EGFR-positive cancers.
Importantly, EGFR activation can happen via transactivation by other receptors and mediators. For example, human corneal epithelial cell (HCEC) proliferation and migration are enhanced in response to CB1 and transient receptor potential cation channel vanilloid member 1 (TRPV1) activation—targets of CBD—which transactivate EGFR and subsequently trigger the MAPK and Akt/PI3K pathways [12,13]. CBD has been shown to directly stimulate the TRPV1 receptor, which triggers intracellular signaling through the influx of calcium. Capsaicin, a model TRPV1 agonist, leads to apoptosis induction through mitochondrial membrane depolarization, the generation of ROS, and the activation of caspases 9 and 3 [140]. Similarly, CBD increased ROS production, mediated by elevated intracellular Ca2+ [141], which results in altering the mitochondrial membrane potential and causes the production of ROS in MDA-MB-231 cells [141]. TRPV1-mediated release of cytokine mediators, such as interleukin (IL)-6 and the chemoattractant (IL-8), is achieved via both the EGFR-dependent and EGFR-independent signaling pathways [142]. These examples highlight the complexity of CBD-EGFR interactions.
7. Conclusions
This review explores the molecular rationale for combining CBD with EGFR TKIs in the treatment of HNSCC. Despite promising preclinical evidence demonstrating CBD’s anticancer and immunomodulatory effects, no clinical data currently support its use as an adjunct to EGFR-TKIs in HNSCC; thus, this remains a hypothesis requiring further investigation. Significant knowledge gaps exist regarding how CBD interacts with dysregulated signaling pathways in HNSCC in the presence and absence of an EGFR-TKI. Future research should focus on elucidating these mechanisms through rigorous in vitro and in vivo studies. Testing this hypothesis is critical, as combining CBD with EGFR-TKIs could lay a transformative foundation for significantly enhancing treatment efficacy and patient outcomes in HNSCC, potentially converting a suboptimal targeted therapy into a highly effective therapeutic strategy. Further research is warranted to establish greater confidence in supporting experimental and clinical correlative data and address key gaps in current knowledge.
Author Contributions
Conceptualization, Y.L. and Z.S.G.A.; methodology, Y.L. and Z.S.G.A.; software, Z.S.G.A.; validation, Y.L. and Z.S.G.A.; writing—original draft preparation, Z.S.G.A.; writing—review and editing, Y.L., T.T.L., and A.S.; visualization, Z.S.G.A.; supervision, Y.L., T.T.L., and A.S.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study did not involve human participants or animals.
Informed Consent Statement
Not applicable.
Acknowledgments
Zainab is a recipient of the PhD scholarship from Al-Qadisiyah University, College of Dentistry, Iraq.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- International Agency for Research on Cancer. GLOBOCAN 2020: Cancer Incidence and Mortality Worldwide. Available online: https://gco.iarc.fr/today/ (accessed on 16 May 2024).[Green Version]
- Kunhabdulla, H.; Manas, R.; Shettihalli, A.K.; Reddy, C.R.M.; Mustak, M.S.; Jetti, R.; Abdulla, R.; Sirigiri, D.R.; Ramdan, D.; Ammarullah, M.I. Identifying Biomarkers and Therapeutic Targets by Multiomic Analysis for HNSCC: Precision Medicine and Healthcare Management. ACS Omega 2024, 9, 12602–12610. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Zhao, B.; Kim, J.; Sui, G.; Shi, J. Small molecule compounds of natural origin target cellular receptors to inhibit cancer development and progression. Int. J. Mol. Sci. 2022, 23, 2672. [Google Scholar] [CrossRef]
- Go, Y.Y.; Kim, S.R.; Kim, D.Y.; Chae, S.-W.; Song, J.-J. Cannabidiol enhances cytotoxicity of anti-cancer drugs in human head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 20622. [Google Scholar] [CrossRef]
- Michmerhuizen, N.L.; Leonard, E.; Matovina, C.; Harris, M.; Herbst, G.; Kulkarni, A.; Zhai, J.; Jiang, H.; Carey, T.E.; Brenner, J.C. Rationale for using irreversible epidermal growth factor receptor inhibitors in combination with phosphatidylinositol 3-kinase inhibitors for advanced head and neck squamous cell carcinoma. Mol. Pharmacol. 2019, 95, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Paragliola, F.; Sparano, F.; Iacovino, M.L.; Castrichino, A.; Doria, F.; Sica, A. Head and neck cancer: The role of anti-EGFR agents in the era of immunotherapy. Ther. Adv. Med. Oncol. 2021, 13, 1758835920949418. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Antitumorigenic targets of cannabinoids–current status and implications. Expert Opin. Ther. Targets 2016, 20, 1219–1235. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Block, E.R.; Klarlund, J.K. Wounding sheets of epithelial cells activates the epidermal growth factor receptor through distinct short-and long-range mechanisms. Mol. Biol. Cell 2008, 19, 4909–4917. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson III, W.E. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- Spix, J.K.; Chay, E.Y.; Block, E.R.; Klarlund, J.K. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp. Cell Res. 2007, 313, 3319–3325. [Google Scholar] [CrossRef]
- Lamtha, T.; Tabtimmai, L.; Songtawee, N.; Tansakul, N.; Choowongkomon, K. Structural analysis of cannabinoids against EGFR-TK leads a novel target against EGFR-driven cell lines. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100132. [Google Scholar] [CrossRef]
- Lamtha, T.; Jongkon, N.; Lertvanithphol, T.; Horprathum, M.; Seetaha, S.; Choowongkomon, K. Cannabinoids as Promising Inhibitors of HER2-Tyrosine Kinase: A Novel Strategy for Targeting HER2-Positive Ovarian Cancer. ACS Omega 2025, 10, 6191–6200. [Google Scholar] [CrossRef]
- Solinas, M.; Massi, P.; Cantelmo, A.; Cattaneo, M.; Cammarota, R.; Bartolini, D.; Cinquina, V.; Valenti, M.; Vicentini, L.; Noonan, D. Cannabidiol inhibits angiogenesis by multiple mechanisms. Br. J. Pharmacol. 2012, 167, 1218–1231. [Google Scholar] [CrossRef] [PubMed]
- Massi, P.; Solinas, M.; Cinquina, V.; Parolaro, D. Cannabidiol as potential anticancer drug. Br. J. Clin. Pharmacol. 2013, 75, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Misri, S.; Kaul, K.; Mishra, S.; Charan, M.; Verma, A.K.; Barr, M.P.; Ahirwar, D.K.; Ganju, R.K. Cannabidiol inhibits tumorigenesis in cisplatin-resistant non-small cell lung cancer via TRPV2. Cancers 2022, 14, 1181. [Google Scholar] [CrossRef]
- Yakin, M.; Gavidi, R.O.; Cox, B.; Rich, A. Oral cancer risk factors in New Zealand. N. Z. Med. J. 2017, 130, 30. [Google Scholar]
- Koch, W.M.; Nance, M. Classification, clinical features, and molecular genetic models. In Epidemiology, Pathogenesis, and Prevention of Head and Neck Cancer; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–21. [Google Scholar]
- Lee, J.; Moon, C. Current status of experimental therapeutics for head and neck cancer. Exp. Biol. Med. 2011, 236, 375–389. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Gan, C.; Wang, K.; Sun, B.; Wang, M.; Zhu, F. Temporal and spatial patterns of recurrence in oral squamous cell carcinoma, a single-center retrospective cohort study in China. BMC Oral Health 2023, 23, 679. [Google Scholar] [CrossRef]
- Tanaka, T.; Ishigamori, R. Understanding carcinogenesis for fighting oral cancer. J. Oncol. 2011, 2011, 603740. [Google Scholar] [CrossRef]
- Taberna, M.; Mena, M.; Pavón, M.; Alemany, L.; Gillison, M.; Mesía, R. Human papillomavirus-related oropharyngeal cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef]
- Syrjanen, S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 2005, 32 (Suppl. 1), S59–S66. [Google Scholar] [CrossRef]
- Munger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J. Virol. 2004, 78, 11451–11460. [Google Scholar] [CrossRef]
- de Abreu, P.M.; Co, A.C.G.; Azevedo, P.L.; do Valle, I.B.; de Oliveira, K.G.; Gouvea, S.A.; Cordeiro-Silva, M.F.; Louro, I.D.; de Podesta, J.R.V.; Lenzi, J.; et al. Frequency of HPV in oral cavity squamous cell carcinoma. BMC Cancer 2018, 18, 324. [Google Scholar] [CrossRef] [PubMed]
- Ringstrom, E.; Peters, E.; Hasegawa, M.; Posner, M.; Liu, M.; Kelsey, K.T. Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin. Cancer Res. 2002, 8, 3187–3192. [Google Scholar] [PubMed]
- Ou, P.; Gear, K.; Rahnama, F.; Thomas, S.; Nagappan, R.; Kee, D.; Waldvogel-Thurlow, S.; Jain, R.; McIvor, N.; Izzard, M. Human papillomavirus and oropharyngeal squamous cell carcinoma: A New Zealand cohort study. ANZ J. Surg. 2018, 88, E278–E283. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Sloan, P.; Shaw, R. Refining the diagnosis of oropharyngeal squamous cell carcinoma using human papillomavirus testing. Oral Oncol. 2010, 46, 492–496. [Google Scholar] [CrossRef]
- Benson, E.; Li, R.; Eisele, D.; Fakhry, C. The clinical impact of HPV tumor status upon head and neck squamous cell carcinomas. Oral Oncol. 2014, 50, 565–574. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein–Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Massano, J.; Regateiro, F.S.; Januário, G.; Ferreira, A. Oral squamous cell carcinoma: Review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 67–76. [Google Scholar] [CrossRef]
- Nayanar, S.K.; Tripathy, J.P.; Duraisamy, K.; Babu, S. Prognostic efficiency of clinicopathologic scoring to predict cervical lymph node metastasis in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 36–42. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Kabir, Z.; Harding, M. Lip, Oral Cavity and Pharyngeal Cancer Burden in the European Union from 1990–2019 Using the 2019 Global Burden of Disease Study. Int. J. Environ. Res. Public Health 2022, 19, 6532. [Google Scholar] [CrossRef]
- Kawakita, D.; Oze, I.; Iwasaki, S.; Matsuda, T.; Matsuo, K.; Ito, H. Trends in the Incidence of Head and Neck Cancer by Subsite between 1993 and 2015 in Japan. Cancer Med. 2022, 11, 1553–1560. [Google Scholar] [CrossRef]
- Jung, Y.S.; Seok, J.; Hong, S.; Ryu, C.H.; Ryu, J.; Jung, K.W. The emergence of oral cavity cancer and the stabilization of oropharyngeal cancer: Recent contrasting epidemics in the South Korean population. Cancer 2021, 127, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.d.S.; Latorre, M.d.R.D.d.O.; Conceição, G.M.d.S.; Curado, M.P.; Antunes, J.L.F.; Toporcov, T.N. The emerging risk of oropharyngeal and oral cavity cancer in HPV-related subsites in young people in Brazil. PLoS ONE 2020, 15, e0232871. [Google Scholar] [CrossRef] [PubMed]
- Ghazawi, F.M.; Lu, J.; Savin, E.; Zubarev, A.; Chauvin, P.; Sasseville, D.; Zeitouni, A.; Litvinov, I.V. Epidemiology and patient distribution of oral cavity and oropharyngeal SCC in Canada. J. Cutan. Med. Surg. 2020, 24, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Ellington, T.D.; Henley, S.J.; Senkomago, V.; O’Neil, M.E.; Wilson, R.J.; Singh, S.; Thomas, C.C.; Wu, M.; Richardson, L.C. Trends in incidence of cancers of the oral cavity and pharynx—United States 2007–2016. Morb. Mortal. Wkly. Rep. 2020, 69, 433. [Google Scholar] [CrossRef]
- Owosho, A.A.; Velez, M.; Tyburski, A.; Hofheins, J.; Wiley, R.; Stansbury, T.; Gbadamosi, S.O.; Ryder, J.S. Epidemiological trends of oropharyngeal and oral cavity squamous cell carcinomas in Northern New England, 2000–2013. Cancer Causes Control 2019, 30, 291–299. [Google Scholar] [CrossRef]
- Johansen, J.; Grau Eriksen, J.; Research, A.o.G.C. Trends in cancer of the head and neck in the elderly in Denmark, 1980–2012. Acta Oncol. 2016, 55 (Suppl. 1), 13–18. [Google Scholar] [CrossRef]
- Farah, C.; Simanovic, B.; Dost, F. Oral cancer in Australia 1982–2008: A growing need for opportunistic screening and prevention. Aust. Dent. J. 2014, 59, 349–359. [Google Scholar] [CrossRef]
- Purkayastha, M.; McMahon, A.D.; Gibson, J.; Conway, D.I. Trends of oral cavity, oropharyngeal and laryngeal cancer incidence in Scotland (1975–2012)—A socioeconomic perspective. Oral Oncol. 2016, 61, 70–75. [Google Scholar] [CrossRef]
- Chelimo, C.; Elwood, J.M. Sociodemographic differences in the incidence of oropharyngeal and oral cavity squamous cell cancers in New Zealand. Aust. N. Z. J. Public Health 2015, 39, 162–167. [Google Scholar] [CrossRef]
- Beder, L.B.; Gunduz, M.; Ouchida, M.; Fukushima, K.; Gunduz, E.; Ito, S.; Sakai, A.; Nagai, N.; Nishizaki, K.; Shimizu, K. Genome-wide analyses on loss of heterozygosity in head and neck squamous cell carcinomas. Lab. Investig. 2003, 83, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Luthra, R.; Saraiya, D.S.; Urbauer, D.L.; Broaddus, R.R. Identification of cancer patients with Lynch syndrome: Clinically significant discordances and problems in tissue-based mismatch repair testing. Cancer Prev. Res. 2012, 5, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Wiesenfeld, D. Oral Cancer. Aust. Dent. J. 2018, 63 (Suppl. 1), S91–S99. [Google Scholar] [CrossRef]
- Anderson, G.; Ebadi, M.; Vo, K.; Novak, J.; Govindarajan, A.; Amini, A. An updated review on head and neck cancer treatment with radiation therapy. Cancers 2021, 13, 4912. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, J.; Jiang, H.; Ma, H.; Yang, B. Anti-epidermal growth factor receptor therapy for advanced head and neck squamous cell carcinoma: A meta-analysis. Eur. J. Clin. Pharmacol. 2012, 68, 561–569. [Google Scholar] [CrossRef]
- Tang, X.; He, J.; Li, B.; Zheng, Y.; Li, K.; Zou, S.; Chen, L. Efficacy and Safety of Gefitinib in Patients with Advanced Head and Neck Squamous Cell Carcinoma: A Meta-Analysis of Randomized Controlled Trials. J. Oncol. 2019, 2019, 6273438. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Grzybowska-Szatkowska, L. Application of molecular targeted therapies in the treatment of head and neck squamous cell carcinoma. Oncol. Lett. 2018, 15, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, A.; Yang, F.; Du, W. Immunotherapeutic strategies for head and neck squamous cell carcinoma (HNSCC): Current perspectives and future prospects. Vaccines 2022, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Uppaluri, R.; Haddad, R.I.; Tao, Y.; Le Tourneau, C.; Lee, N.Y.; Westra, W.; Chernock, R.; Tahara, M.; Harrington, K.J.; Klochikhin, A.L. Neoadjuvant and Adjuvant Pembrolizumab in Locally Advanced Head and Neck Cancer. N. Engl. J. Med. 2025, 393, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune checkpoint inhibitors combined with targeted therapy: The recent advances and future potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef]
- Tao, Y.; Aupérin, A.; Sun, X.; Sire, C.; Martin, L.; Coutte, A.; Lafond, C.; Miroir, J.; Liem, X.; Rolland, F. Avelumab–cetuximab–radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: The safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur. J. Cancer 2020, 141, 21–29. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Anami, Y.; Ha, S.Y.; Yamazaki, C.M. Exploring the next generation of antibody–drug conjugates. Nat. Rev. Clin. Oncol. 2024, 21, 203–223. [Google Scholar] [CrossRef]
- Jiménez-Labaig, P.; Rullan, A.; Hernando-Calvo, A.; Llop, S.; Bhide, S.; O’Leary, B.; Braña, I.; Harrington, K.J. A systematic review of antibody-drug conjugates and bispecific antibodies in head and neck squamous cell carcinoma and nasopharyngeal carcinoma: Charting the course of future therapies. Cancer Treat. Rev. 2024, 128, 102772. [Google Scholar] [CrossRef]
- Price, K.A.R.; Harrington, K.J.; Worden, F.P.; Mesia, R.; Even, C.; Schaaf, D.; Jones, S.; Saba, N.F.; Haddad, R.I. VERSATILE-003: A phase 3, randomized, open-label trial of PDS0101 and pembrolizumab compared with pembrolizumab for first-line treatment of patients with HPV16-positive recurrent/metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2025, 43, TPS6111. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Ribeiro, F.A.; Noguti, J.; Oshima, C.T.; Ribeiro, D.A. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: A promising approach. Anticancer Res. 2014, 34, 1547–1552. [Google Scholar]
- Guy, P.M.; Platko, J.V.; Cantley, L.C.; Cerione, R.A.; Carraway, K.L., 3rd. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 1994, 91, 8132–8136. [Google Scholar] [CrossRef]
- Purba, E.R.; Saita, E.I.; Maruyama, I.N. Activation of the EGF Receptor by Ligand Binding and Oncogenic Mutations: The “Rotation Model”. Cells 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Cohen, R.B.; Burtness, B.A. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin. Adv. Hematol. Oncol. 2008, 6, 742–750. [Google Scholar] [PubMed]
- Psyrri, A.; Seiwert, T.Y.; Jimeno, A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Ono, M.; Hirata, A.; Kometani, T.; Miyagawa, M.; Ueda, S.-i.; Kinoshita, H.; Fujii, T.; Kuwano, M. Sensitivity to gefitinib (Iressa, ZD1839) in non-small cell lung cancer cell lines correlates with dependence on the epidermal growth factor (EGF) receptor/extracellular signal-regulated kinase 1/2 and EGF receptor/Akt pathway for proliferation. Mol. Cancer Ther. 2004, 3, 465–472. [Google Scholar] [CrossRef]
- Von Pawel, J. Gefitinib (Iressa, ZD1839): A novel targeted approach for the treatment of solid tumors. Bull. Cancer 2004, 91, 10070–10076. [Google Scholar]
- Baselga, J.; Rischin, D.; Ranson, M.; Calvert, H.; Raymond, E.; Kieback, D.; Kaye, S.B.; Gianni, L.; Harris, A.; Bjork, T. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J. Clin. Oncol. 2002, 20, 4292–4302. [Google Scholar] [CrossRef]
- Chang, C.Y.; Kuan, Y.H.; Ou, Y.C.; Li, J.R.; Wu, C.C.; Pan, P.H.; Chen, W.Y.; Huang, H.Y.; Chen, C.J. Autophagy contributes to gefitinib-induced glioma cell growth inhibition. Exp. Cell Res. 2014, 327, 102–112. [Google Scholar] [CrossRef]
- Guo, M.; Liu, S.; Lu, F. Gefitinib-sensitizing mutations in esophageal carcinoma. N. Engl. J. Med. 2006, 354, 2193–2194. [Google Scholar] [CrossRef]
- Fukuoka, M.; Yano, S.; Giaccone, G.; Tamura, T.; Nakagawa, K.; Douillard, J.Y.; Nishiwaki, Y.; Vansteenkiste, J.; Kudoh, S.; Rischin, D.; et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J. Clin. Oncol. 2003, 21, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Bonner, J.A.; Bredel, M. EGFR mutations in head and neck squamous cell carcinoma. Int. J. Mol. Sci. 2022, 23, 3818. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-H.; Boggon, T.J.; Li, Y.; Woo, M.S.; Greulich, H.; Meyerson, M.; Eck, M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007, 11, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Eastwood, M.P.; Zhang, X.; Kim, E.T.; Arkhipov, A.; Dror, R.O.; Jumper, J.; Kuriyan, J.; Shaw, D.E. Oncogenic mutations counteract intrinsic disorder in the EGFR kinase and promote receptor dimerization. Cell 2012, 149, 860–870. [Google Scholar] [CrossRef]
- Smilek, P.; Neuwirthová, J.; Jarkovsky, J.; Dusek, L.; Rottenberg, J.; Kostrica, R.; Srovnal, J.; Hajduch, M.; Drabek, J.; Klozar, J. Epidermal growth factor receptor (EGFR) expression and mutations in the EGFR signaling pathway in correlation with anti-EGFR therapy in head and neck squamous cell carcinomas. Neoplasma 2012, 59, 508. [Google Scholar] [CrossRef]
- Citro, S.; Bellini, A.; Miccolo, C.; Ghiani, L.; Carey, T.E.; Chiocca, S. Synergistic antitumour activity of HDAC inhibitor SAHA and EGFR inhibitor gefitinib in head and neck cancer: A key role for DeltaNp63alpha. Br. J. Cancer 2019, 120, 658–667. [Google Scholar] [CrossRef]
- Khalil, A.; Jameson, M.J. The EGFR Inhibitor Gefitinib Enhanced the Response of Human Oral Squamous Cell Carcinoma to Cisplatin In Vitro. Drugs R&D 2017, 17, 545–555. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: Understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur. J. Cancer 2003, 39, 1348–1354. [Google Scholar] [CrossRef]
- Li, D.; Ambrogio, L.; Shimamura, T.; Kubo, S.; Takahashi, M.; Chirieac, L.; Padera, R.; Shapiro, G.; Baum, A.; Himmelsbach, F. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008, 27, 4702–4711. [Google Scholar] [CrossRef]
- Park, K.; Tan, E.-H.; O’Byrne, K.; Zhang, L.; Boyer, M.; Mok, T.; Hirsh, V.; Yang, J.C.-H.; Lee, K.H.; Lu, S. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016, 17, 577–589. [Google Scholar] [CrossRef]
- Solca, F.; Dahl, G.; Zoephel, A.; Bader, G.; Sanderson, M.; Klein, C.; Kraemer, O.; Himmelsbach, F.; Haaksma, E.; Adolf, G.R. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J. Pharmacol. Exp. Ther. 2012, 343, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ferrarotto, R.; Gold, K.A. Afatinib in the treatment of head and neck squamous cell carcinoma. Expert Opin. Investig. Drugs 2014, 23, 135–143. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Dunn, E.F.; Harari, P.M. Understanding resistance to EGFR inhibitors—Impact on future treatment strategies. Nat. Rev. Clin. Oncol. 2010, 7, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Soulieres, D.; Senzer, N.N.; Vokes, E.E.; Hidalgo, M.; Agarwala, S.S.; Siu, L.L. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J. Clin. Oncol. 2004, 22, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Galot, R.; Le Tourneau, C.; Guigay, J.; Licitra, L.; Tinhofer, I.; Kong, A.; Caballero, C.; Fortpied, C.; Bogaerts, J.; Govaerts, A.-S. Personalized biomarker-based treatment strategy for patients with squamous cell carcinoma of the head and neck: EORTC position and approach. Ann. Oncol. 2018, 29, 2313–2327. [Google Scholar] [CrossRef]
- Kao, H.-F.; Liao, B.-C.; Huang, Y.-L.; Huang, H.-C.; Chen, C.-N.; Chen, T.-C.; Hong, Y.-J.; Chan, C.-Y.; Chia, J.-S.; Hong, R.-L. Afatinib and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma (ALPHA Study): A phase II study with biomarker analysis. Clin. Cancer Res. 2022, 28, 1560–1571. [Google Scholar] [CrossRef]
- Maarof, N.N.; Alsalahi, A.; Abdulmalek, E.; Fakurazi, S.; Tejo, B.A.; Abdul Rahman, M.B. Efficacy of afatinib in the treatment of patients with non-small cell lung cancer and head and neck squamous cell carcinoma: A systematic review and meta-analysis. Cancers 2021, 13, 688. [Google Scholar] [CrossRef]
- Vermorken, J.; Rottey, S.; Ehrnrooth, E.; Pelling, K.; Lahogue, A.; Wind, S.; Machiels, J.-P. A phase Ib, open-label study to assess the safety of continuous oral treatment with afatinib in combination with two chemotherapy regimens: Cisplatin plus paclitaxel and cisplatin plus 5-fluorouracil, in patients with advanced solid tumors. Ann. Oncol. 2013, 24, 1392–1400. [Google Scholar] [CrossRef]
- Harrington, K.; Berrier, A.; Robinson, M.; Remenar, E.; Housset, M.; de Mendoza, F.H.; Fayette, J.; Mehanna, H.; El-Hariry, I.; Compton, N. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: Rationale for future randomised trials in human papilloma virus-negative disease. Eur. J. Cancer 2013, 49, 1609–1618. [Google Scholar]
- Thongprasert, S.; Geater, S.L.; Clement, D.; Abdelaziz, A.; Reyes-Igama, J.; Jovanovic, D.; Alexandru, A.; Schenker, M.; Sriuranpong, V.; Serwatowski, P. Afatinib in locally advanced/metastatic NSCLC harboring common EGFR mutations, after chemotherapy: A Phase IV study. Lung Cancer Manag. 2019, 8, LMT15. [Google Scholar] [CrossRef]
- Burtness, B.; Haddad, R.; Dinis, J.; Trigo, J.; Yokota, T.; de Souza Viana, L.; Romanov, I.; Vermorken, J.; Bourhis, J.; Tahara, M. Afatinib vs placebo as adjuvant therapy after chemoradiotherapy in squamous cell carcinoma of the head and neck: A randomized clinical trial. JAMA Oncol. 2019, 5, 1170–1180. [Google Scholar] [CrossRef]
- Macha, M.A.; Rachagani, S.; Qazi, A.K.; Jahan, R.; Gupta, S.; Patel, A.; Seshacharyulu, P.; Lin, C.; Li, S.; Wang, S. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget 2017, 8, 20961. [Google Scholar] [CrossRef]
- Sok, J.C.; Coppelli, F.M.; Thomas, S.M.; Lango, M.N.; Xi, S.; Hunt, J.L.; Freilino, M.L.; Graner, M.W.; Wikstrand, C.J.; Bigner, D.D. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin. Cancer Res. 2006, 12, 5064–5073. [Google Scholar] [CrossRef]
- Chen, L.F.; Cohen, E.E.; Grandis, J.R. New strategies in head and neck cancer: Understanding resistance to epidermal growth factor receptor inhibitors. Clin. Cancer Res 2010, 16, 2489–2495. [Google Scholar] [CrossRef]
- Goel, B.; Tiwari, A.K.; Pandey, R.K.; Singh, A.P.; Kumar, S.; Sinha, A.; Jain, S.K.; Khattri, A. Therapeutic approaches for the treatment of head and neck squamous cell carcinoma–An update on clinical trials. Transl. Oncol. 2022, 21, 101426. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein Jr, G.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Rusthoven, K.E.; Feigenberg, S.J.; Raben, D.; Kane, M.; Song, J.I.; Nicolaou, N.; Mehra, R.; Burtness, B.; Ridge, J.; Swing, R. Initial results of a Phase I dose-escalation trial of concurrent and maintenance erlotinib and reirradiation for recurrent and new primary head-and-neck cancer. Int. J. Radiat. Oncol. * Biol. * Phys. 2010, 78, 1020–1025. [Google Scholar] [CrossRef]
- Herchenhorn, D.; Dias, F.L.; Viegas, C.M.; Federico, M.H.; Araújo, C.M.M.; Small, I.; Bezerra, M.; Fontão, K.; Knust, R.E.; Ferreira, C.G. Phase I/II study of erlotinib combined with cisplatin and radiotherapy in patients with locally advanced squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. * Biol. * Phys. 2010, 78, 696–702. [Google Scholar] [CrossRef]
- Chen, C.; Kane, M.; Song, J.; Campana, J.; Raben, A.; Hu, K.; Harrison, L.; Quon, H.; Dancey, J.; Baron, A. Phase I trial of gefitinib in combination with radiation or chemoradiation for patients with locally advanced squamous cell head and neck cancer. J. Clin. Oncol. 2007, 25, 4880–4886. [Google Scholar] [CrossRef]
- Perez, C.A.; Song, H.; Raez, L.E.; Agulnik, M.; Grushko, T.A.; Dekker, A.; Stenson, K.; Blair, E.A.; Olopade, O.I.; Seiwert, T.Y. Phase II study of gefitinib adaptive dose escalation to skin toxicity in recurrent or metastatic squamous cell carcinoma of the head and neck. Oral Oncol. 2012, 48, 887–892. [Google Scholar] [CrossRef]
- Gregoire, V.; Hamoir, M.; Chen, C.; Kane, M.; Kawecki, A.; Julka, P.K.; Wang, H.-M.; Prasad, S.; D’Cruz, A.K.; Radosevic-Jelic, L. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: A phase II, randomized, double-blind, placebo-controlled study. Radiother. Oncol. 2011, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; Haraf, D.J.; Kunnavakkam, R.; Stenson, K.M.; Blair, E.A.; Brockstein, B.; Lester, E.P.; Salama, J.K.; Dekker, A.; Williams, R. Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. J. Clin. Oncol. 2010, 28, 3336. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; Hitt, R.; Sebastian, P.; Carracedo, C.; Lokanatha, D.; Bourhis, J.; Temam, S.; Cupissol, D.; De Raucourt, D.; Maroudias, N. Effects of lapatinib monotherapy: Results of a randomised phase II study in therapy-naive patients with locally advanced squamous cell carcinoma of the head and neck. Br. J. Cancer 2011, 105, 618–627. [Google Scholar] [CrossRef]
- Seiwert, T.; Fayette, J.; Cupissol, D.; Del Campo, J.; Clement, P.; Hitt, R.; Degardin, M.; Zhang, W.; Blackman, A.; Ehrnrooth, E. A randomized, phase II study of afatinib versus cetuximab in metastatic or recurrent squamous cell carcinoma of the head and neck. Ann. Oncol. 2014, 25, 1813–1820. [Google Scholar] [CrossRef]
- Razak, A.A.; Soulieres, D.; Laurie, S.; Hotte, S.; Singh, S.; Winquist, E.; Chia, S.; Le Tourneau, C.; Nguyen-Tan, P.-F.; Chen, E. A phase II trial of dacomitinib, an oral pan-human EGF receptor (HER) inhibitor, as first-line treatment in recurrent and/or metastatic squamous-cell carcinoma of the head and neck. Ann. Oncol. 2013, 24, 761–769. [Google Scholar] [CrossRef]
- Shannon, S.; Opila-Lehman, J. Cannabidiol oil for decreasing addictive use of marijuana: A case report. Integr. Med. A Clin. J. 2015, 14, 31. [Google Scholar]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of cannabidiol (CBD) in cancer treatment: A review. Biology 2022, 11, 817. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Chiu, S.-C.; Chao, S.-C.; Liao, H.-Y.; Lee, S.-P.; Huang, C.-C.; Cho, D.-Y. Real-Time Monitoring of the Cytotoxic and Antimetastatic Properties of Cannabidiol in Human Oral Squamous Cell Carcinoma Cells Using Electric Cell-Substrate Impedance Sensing. Int. J. Mol. Sci. 2022, 23, 15842. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Crippa, J.A.d.S.; Hallak, J.E.C.; Moreira, F.; Guimarães, F.S. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med. Biol. Res. 2006, 39, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The current state and future perspectives of cannabinoids in cancer biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Sadek, M.M.; Serrya, R.A.; Kafafy, A.-H.N.; Ahmed, M.; Wang, F.; Abouzid, K.A. Discovery of new HER2/EGFR dual kinase inhibitors based on the anilinoquinazoline scaffold as potential anti-cancer agents. J. Enzym. Inhib. Med. Chem. 2014, 29, 215–222. [Google Scholar] [CrossRef]
- Hashibe, M.; Straif, K.; Tashkin, D.P.; Morgenstern, H.; Greenland, S.; Zhang, Z.-F. Epidemiologic review of marijuana use and cancer risk. Alcohol 2005, 35, 265–275. [Google Scholar] [CrossRef]
- Alsherbiny, M.A.; Bhuyan, D.J.; Low, M.N.; Chang, D.; Li, C.G. Synergistic interactions of cannabidiol with chemotherapeutic drugs in mcf7 cells: Mode of interaction and proteomics analysis of mechanisms. Int. J. Mol. Sci. 2021, 22, 10103. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacological Actions of Cannabinoids; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Pertwee, R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Thomas, A.; Baillie, G.; Phillips, A.; Razdan, R.; Ross, R.A.; Pertwee, R. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef]
- Mukhopadhyay, B.; Schuebel, K.; Mukhopadhyay, P.; Cinar, R.; Godlewski, G.; Xiong, K.; Mackie, K.; Lizak, M.; Yuan, Q.; Goldman, D. Cannabinoid receptor 1 promotes hepatocellular carcinoma initiation and progression through multiple mechanisms. Hepatology 2015, 61, 1615–1626. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhu, L.; Zou, Y.; Kong, W.; Dong, B.; Huang, J.; Chen, Y.; Xue, W.; Huang, Y. Cannabinoid receptor 2 as a novel target for promotion of renal cell carcinoma prognosis and progression. J. Cancer Res. Clin. Oncol. 2018, 144, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Bifulco, M. The endocannabinoid signaling system in cancer. Trends Pharmacol. Sci. 2013, 34, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- Pérez-Gómez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martín-Villar, E.; Flores, J.M. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. J. Natl. Cancer Inst. 2015, 107, djv077. [Google Scholar] [CrossRef]
- de la Harpe, A.; Beukes, N.; Frost, C.L. CBD activation of TRPV1 induces oxidative signaling and subsequent ER stress in breast cancer cell lines. Biotechnol. Appl. Biochem. 2022, 69, 420–430. [Google Scholar] [CrossRef]
- Schröder, M. Endoplasmic reticulum stress responses. Cell. Mol. Life Sci. 2008, 65, 862–894. [Google Scholar] [CrossRef]
- Verfaillie, T.; Garg, A.D.; Agostinis, P. Targeting ER stress induced apoptosis and inflammation in cancer. Cancer Lett. 2013, 332, 249–264. [Google Scholar] [CrossRef]
- Schonthal, A.H. Targeting endoplasmic reticulum stress for cancer therapy. Front. Biosci. Sch. 2012, 4, 412–431. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Pan, Z.; Li, M.; Liu, X.; Chen, X.; Qu, G.; Zhou, L.; Xu, M.; Zheng, Q. Cannabidiol induces cell cycle arrest and cell apoptosis in human gastric cancer SGC-7901 cells. Biomolecules 2019, 9, 302. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Lu, T.-R.; Yarla, N.S.; Wu, H.-Y.; Xu, B.; Ding, J.; Zhu, H. Drug combination in clinical cancer treatments. Rev. Recent Clin. Trials 2017, 12, 202–211. [Google Scholar] [CrossRef]
- Liu, C.; Sadat, S.H.; Ebisumoto, K.; Sakai, A.; Panuganti, B.A.; Ren, S.; Goto, Y.; Haft, S.; Fukusumi, T.; Ando, M. Cannabinoids promote progression of HPV-positive head and neck squamous cell carcinoma via p38 MAPK activation. Clin. Cancer Res. 2020, 26, 2693–2703. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Sadat, S.; Ebisumoto, K.; Al-Msari, R.; Miyauchi, S.; Roy, S.; Mohammadzadeh, P.; Lips, K.; Nakagawa, T.; Saddawi-Konefka, R. CBD promotes antitumor activity by modulating tumor immune microenvironment in HPV associated head and neck squamous cell carcinoma. Front. Immunol. 2025, 16, 1528520. [Google Scholar] [CrossRef] [PubMed]
- Hijiya, N.; Shibata, T.; Daa, T.; Hamanaka, R.; Uchida, T.; Matsuura, K.; Tsukamoto, Y.; Nakada, C.; Iha, H.; Inomata, M. Overexpression of cannabinoid receptor 1 in esophageal squamous cell carcinoma is correlated with metastasis to lymph nodes and distant organs, and poor prognosis. Pathol. Int. 2017, 67, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nulent, T.J.K.; Van Diest, P.J.; Van Der Groep, P.; Leusink, F.K.; Kruitwagen, C.L.; Koole, R.; Van Cann, E.M. Cannabinoid receptor-2 immunoreactivity is associated with survival in squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2013, 51, 604–609. [Google Scholar] [CrossRef]
- Theocharis, S.; Giaginis, C.; Alexandrou, P.; Rodriguez, J.; Tasoulas, J.; Danas, E.; Patsouris, E.; Klijanienko, J. Evaluation of cannabinoid CB1 and CB2 receptors expression in mobile tongue squamous cell carcinoma: Associations with clinicopathological parameters and patients’ survival. Tumor Biol. 2016, 37, 3647–3656. [Google Scholar] [CrossRef]
- Maccarrone, M.; Lorenzon, T.; Bari, M.; Melino, G.; Finazzi-Agro, A. Anandamide induces apoptosis in human cells via vanilloid receptors: Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000, 275, 31938–31945. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Z.; Capó-Aponte, J.E.; Zhang, F.; Pan, Z.; Reinach, P.S. Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells. Exp. Eye Res. 2010, 91, 462–471. [Google Scholar] [CrossRef] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).