The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review
Abstract
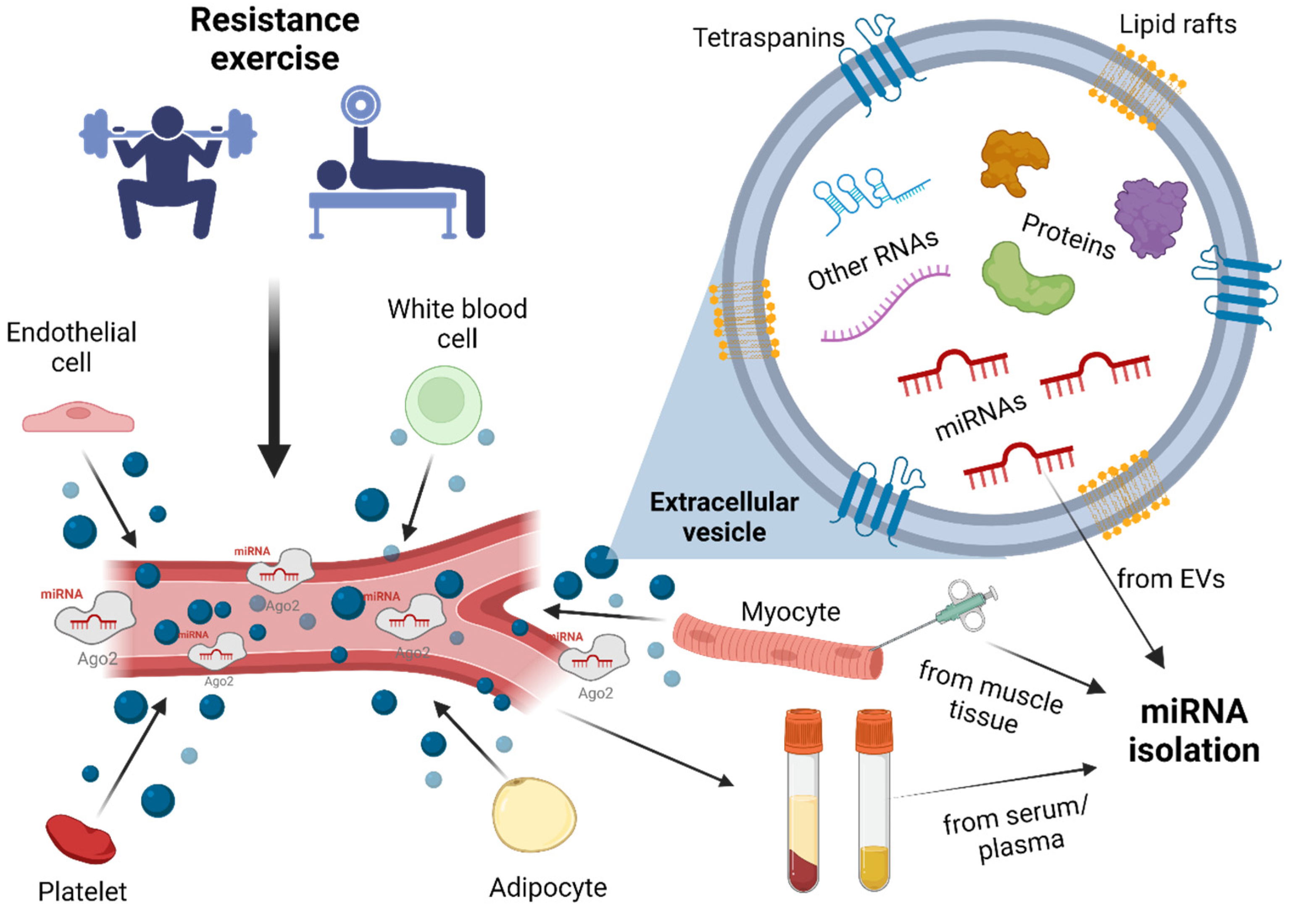
1. Introduction
2. Materials and Methods
3. Extracellular Vesicles
4. miRNAs
4.1. Muscle-Specific miRNAs
4.2. Circulating miRNAs
5. Ci-miRNAs After Acute RE
6. The Relationship Between Acute RE and the miRNA Profile from Muscle Biopsy
7. The miRNA Profile After RE with Protein Consumption
8. Studies on miRNA Profile in Response to Chronic RE
9. The Effect of RE on Extracellular Vesicle Profile Changes
10. MiRNA Detection Methods
11. Discussion
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AER | Aerobic exercise |
| AGO | Argonaute protein |
| BFR | Blood flow restriction |
| ci-miRNA | Circulating miRNA |
| DUC | Differential ultracentrifugation |
| EV | Extracellular vesicle |
| HI | High-intensity |
| LI | Low-intensity |
| MH | Muscle hypertrophy |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| NGS | New Generation Sequencing |
| NTA | Nanoparticle tracking analysis |
| PA | Physical activity |
| PFP | Platelet-free plasma |
| RE | Resistance exercise |
| RM | Repetition maximum |
| RT-PCR | Real-time polymerase chain reaction |
| SEC | Size exclusion chromatography |
| T2DM | Type 2 diabetes mellitus |
| TEM | Transmission electron microscopy |
| UC | Ultracentrifugation |
| WB | Western blotting |
| yr | Year |
References
- Fuller, O.K.; Whitham, M.; Mathivanan, S.; Febbraio, M.A. The Protective Effect of Exercise in Neurodegenerative Diseases: The Potential Role of Extracellular Vesicles. Cells 2020, 9, 2182. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Grgic, J.; Van Every, D.W.; Plotkin, D.L. Loading Recommendations for Muscle Strength, Hypertrophy, and Local Endurance: A Re-Examination of the Repetition Continuum. Sports 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal Muscle Wasting in Cachexia and Sarcopenia: Molecular Pathophysiology and Impact of Exercise Training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Ishihara, K.; Tekus, E.; Varga, C.; Posa, A.; Balogh, L.; Boldogh, I.; Koltai, E. Exercise, Oxidants, and Antioxidants Change the Shape of the Bell-Shaped Hormesis Curve. Redox Biol. 2017, 12, 285–290. [Google Scholar] [CrossRef]
- Kirby, T.J.; McCarthy, J.J. MicroRNAs in Skeletal Muscle Biology and Exercise Adaptation. Free Radic. Biol. Med. 2013, 64, 95–105. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Leuchtmann, A.B.; Adak, V.; Dilbaz, S.; Handschin, C. The Role of the Skeletal Muscle Secretome in Mediating Endurance and Resistance Training Adaptations. Front. Physiol. 2021, 12, 709807. [Google Scholar] [CrossRef]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E. Physical Exercise Induces Rapid Release of Small Extracellular Vesicles into the Circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef]
- Wackerhage, H.; Schoenfeld, B.J.; Hamilton, D.L.; Lehti, M.; Hulmi, J.J. Stimuli and Sensors That Initiate Skeletal Muscle Hypertrophy Following Resistance Exercise. J. Appl. Physiol. 2019, 126, 30–43. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Nindl, B.C. Recovery Responses of Testosterone, Growth Hormone, and IGF-1 after Resistance Exercise. J. Appl. Physiol. 2017, 122, 549–558. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Wang, C. A Review of the Regulatory Mechanisms of Extracellular Vesicles-Mediated Intercellular Communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Sapp, R.M.; Shill, D.D.; Roth, S.M.; Hagberg, J.M. Circulating MicroRNAs in Acute and Chronic Exercise: More than Mere Biomarkers. J. Appl. Physiol. 2017, 122, 702–717. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; Spencer, S.J. Emerging Roles of Extracellular Vesicles in the Intercellular Communication for Exercise-Induced Adaptations. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E320–E329. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2021, 11, 604274. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and Biogenesis of Shed Microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Doncheva, A.I.; Romero, S.; Ramirez-Garrastacho, M.; Lee, S.; Kolnes, K.J.; Tangen, D.S.; Olsen, T.; Drevon, C.A.; Llorente, A.; Dalen, K.T.; et al. Extracellular Vesicles and microRNAs Are Altered in Response to Exercise, Insulin Sensitivity and Overweight. Acta Physiol. 2022, 236, e13862. [Google Scholar] [CrossRef]
- Ferguson, S.W.; Nguyen, J. Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. J. Control. Release 2016, 228, 179–190. [Google Scholar] [CrossRef]
- Just, J.; Yan, Y.; Farup, J.; Sieljacks, P.; Sloth, M.; Venø, M.; Gu, T.; de Paoli, F.V.; Nyengaard, J.R.; Bæk, R.; et al. Blood Flow-Restricted Resistance Exercise Alters the Surface Profile, MiRNA Cargo and Functional Impact of Circulating Extracellular Vesicles. Sci. Rep. 2020, 10, 5835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-Induced PTEN Loss by Exosomal MicroRNA Primes Brain Metastasis Outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Chitti, S.V.; Fonseka, P.; Mathivanan, S. Emerging Role of Extracellular Vesicles in Mediating Cancer Cachexia. Biochem. Soc. Trans. 2018, 46, 1129–1136. [Google Scholar] [CrossRef]
- Sanwlani, R.; Fonseka, P.; Chitti, S.V.; Mathivanan, S. Milk-Derived Extracellular Vesicles in Inter-Organism, Cross-Species Communication and Drug Delivery. Proteomes 2020, 8, 11. [Google Scholar] [CrossRef]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of Recipient Cell-Dependent Differences in Exosome Uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering Exosomes as Refined Biological Nanoplatforms for Drug Delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice after Intravenous Injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Ghahremani, M.H.; Soleimani, M. Delivery of Exogenous MiR-124 to Glioblastoma Multiform Cells by Wharton’s Jelly Mesenchymal Stem Cells Decreases Cell Proliferation and Migration, and Confers Chemosensitivity. Stem Cell Rev. Rep. 2018, 14, 236–246. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 3. [Google Scholar] [CrossRef]
- Laulagnier, K.; Javalet, C.; Hemming, F.J.; Chivet, M.; Lachenal, G.; Blot, B.; Chatellard, C.; Sadoul, R. Amyloid Precursor Protein Products Concentrate in a Subset of Exosomes Specifically Endocytosed by Neurons. Cell. Mol. Life Sci. 2018, 75, 757–773. [Google Scholar] [CrossRef]
- Lagos-Quintana, M.; Rauhut, R.; Lendeckel, W.; Tuschl, T. Identification of Novel Genes Coding for Small Expressed RNAs. Science 2001, 294, 853–858. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific MicroRNAs in Skeletal Muscle Development. Dev. Biol. 2016, 410, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.-H.; Burge, C.B.; Bartel, D.P. Most Mammalian MRNAs Are Conserved Targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.-H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA Genes Are Transcribed by RNA Polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human MicroRNAs Are Processed from Capped, Polyadenylated Transcripts That Can Also Function as MRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Lee, Y. MicroRNA Maturation: Stepwise Processing and Subcellular Localization. EMBO J. 2002, 21, 4663–4670. [Google Scholar] [CrossRef]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 Mediates the Nuclear Export of Pre-MicroRNAs and Short Hairpin RNAs. Genes. Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a Bidentate Ribonuclease in the Initiation Step of RNA Interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Hutvágner, G.; Haley, B.; Zamore, P.D. Evidence That SiRNAs Function as Guides, Not Primers, in the Drosophila and Human RNAi Pathways. Mol. Cell 2002, 10, 537–548. [Google Scholar] [CrossRef]
- Höck, J.; Meister, G. The Argonaute Protein Family. Genome Biol. 2008, 9, 210. [Google Scholar] [CrossRef]
- Meister, G. Argonaute Proteins: Functional Insights and Emerging Roles. Nat. Rev. Genet. 2013, 14, 447–459. [Google Scholar] [CrossRef]
- Gibbings, D.J.; Ciaudo, C.; Erhardt, M.; Voinnet, O. Multivesicular Bodies Associate with Components of MiRNA Effector Complexes and Modulate MiRNA Activity. Nat. Cell Biol. 2009, 11, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA Packaging into Extracellular Vesicles: An Orchestra of RNA-binding Proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, J.R.; Georges, S.A.; Seay, H.R.; Tapscott, S.J.; McManus, M.T.; Goldhamer, D.J.; Swanson, M.S.; Harfe, B.D. Essential Role for Dicer during Skeletal Muscle Development. Dev. Biol. 2007, 311, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Mytidou, C.; Koutsoulidou, A.; Katsioloudi, A.; Prokopi, M.; Kapnisis, K.; Michailidou, K.; Anayiotos, A.; Phylactou, L.A. Muscle-derived Exosomes Encapsulate MyomiRs and Are Involved in Local Skeletal Muscle Tissue Communication. FASEB J. 2021, 35, e21279. [Google Scholar] [CrossRef]
- Lee, R.C.; Ambros, V. An Extensive Class of Small RNAs in Caenorhabditis elegans. Science 2001, 294, 862–864. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206: The Skeletal Muscle-Specific MyomiR. Biochim. Et. Biophys. Acta (BBA)-Gene Regul. Mech. 2008, 1779, 682–691. [Google Scholar] [CrossRef]
- Sempere, L.F.; Freemantle, S.; Pitha-Rowe, I.; Moss, E.; Dmitrovsky, E.; Ambros, V. Expression Profiling of Mammalian MicroRNAs Uncovers a Subset of Brain-Expressed MicroRNAs with Possible Roles in Murine and Human Neuronal Differentiation. Genome Biol. 2004, 5, R13. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-Kinase/Akt Signaling by Muscle-Enriched MicroRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of Stress-Dependent Cardiac Growth and Gene Expression by a MicroRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef]
- Walden, T.B.; Timmons, J.A.; Keller, P.; Nedergaard, J.; Cannon, B. Distinct Expression of Muscle-specific MicroRNAs (Myomirs) in Brown Adipocytes. J. Cell Physiol. 2009, 218, 444–449. [Google Scholar] [CrossRef]
- Chen, X.; Liang, H.; Zhang, J.; Zen, K.; Zhang, C.-Y. Secreted MicroRNAs: A New Form of Intercellular Communication. Trends Cell Biol. 2012, 22, 125–132. [Google Scholar] [CrossRef]
- Boon, R.A.; Vickers, K.C. Intercellular Transport of MicroRNAs. Arter. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef]
- Castaño, C.; Mirasierra, M.; Vallejo, M.; Novials, A.; Párrizas, M. Delivery of Muscle-Derived Exosomal MiRNAs Induced by HIIT Improves Insulin Sensitivity through down-Regulation of Hepatic FoxO1 in Mice. Proc. Natl. Acad. Sci. USA 2020, 117, 30335–30343. [Google Scholar] [CrossRef]
- Guescini, M.; Maggio, S.; Ceccaroli, P.; Battistelli, M.; Annibalini, G.; Piccoli, G.; Sestili, P.; Stocchi, V. Extracellular Vesicles Released by Oxidatively Injured or Intact C2C12 Myotubes Promote Distinct Responses Converging toward Myogenesis. Int. J. Mol. Sci. 2017, 18, 2488. [Google Scholar] [CrossRef] [PubMed]
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and Systemic Molecular Responses to a Single Flywheel Based Iso-Inertial Training Session in Resistance-Trained Men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Ge, Y.; Li, S.; Pincas, H.; Jain, N.; Seenarine, N.; Amper, M.A.S.; Goodpaster, B.H.; Walsh, M.J.; Coen, P.M.; et al. Sedentary and Trained Older Men Have Distinct Circulating Exosomal MicroRNA Profiles at Baseline and in Response to Acute Exercise. Front. Physiol. 2020, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Sun, B.; Yin, X.; Guo, X.; Chao, D.; Zhang, C.; Zhang, C.-Y.; Chen, X.; Ma, J. Time-Course Responses of Circulating MicroRNAs to Three Resistance Training Protocols in Healthy Young Men. Sci. Rep. 2017, 7, 2203. [Google Scholar] [CrossRef]
- Benavente, C.; León, J.; Feriche, B.; Schoenfeld, B.J.; Bonitch-Góngora, J.; Almeida, F.; Pérez-Regalado, S.; Padial, P. Hormonal and Inflammatory Responses to Hypertrophy-Oriented Resistance Training at Acute Moderate Altitude. Int. J. Environ. Res. Public Health 2021, 18, 4233. [Google Scholar] [CrossRef]
- Khalid, K.; Szewczyk, A.; Kiszałkiewicz, J.; Migdalska- Sęk, M.; Domańska-Senderowska, D.; Brzeziański, M.; Lulińska, E.; Jegier, A.; Brzeziańska-Lasota, E. Type of Training Has a Significant Influence on the GH/IGF-1 Axis but Not on Regulating MiRNAs. Biol. Sport. 2020, 37, 217–228. [Google Scholar] [CrossRef]
- Schober-Halper, B.; Hofmann, M.; Oesen, S.; Franzke, B.; Wolf, T.; Strasser, E.-M.; Bachl, N.; Quittan, M.; Wagner, K.-H.; Wessner, B. Elastic Band Resistance Training Influences Transforming Growth Factor-ß Receptor I MRNA Expression in Peripheral Mononuclear Cells of Institutionalised Older Adults: The Vienna Active Ageing Study (VAAS). Immun. Ageing 2016, 13, 22. [Google Scholar] [CrossRef]
- Uhlemann, M.; Möbius-Winkler, S.; Fikenzer, S.; Adam, J.; Redlich, M.; Möhlenkamp, S.; Hilberg, T.; Schuler, G.C.; Adams, V. Circulating MicroRNA-126 Increases after Different Forms of Endurance Exercise in Healthy Adults. Eur. J. Prev. Cardiol. 2014, 21, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.; Kon, M.; Wada, S.; Ushida, T.; Suzuki, K.; Akimoto, T. Profiling of Circulating MicroRNAs after a Bout of Acute Resistance Exercise in Humans. PLoS ONE 2013, 8, e70823. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; Lessard, S.J.; Ezzyat, Y.; Fielding, R.A.; Rivas, D.A. Circulating MicroRNA Are Predictive of Aging and Acute Adaptive Response to Resistance Exercise in Men. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Fujita, H.; Numao, K.; Nakamura, Y.; Shimizu, H.; Sekiguchi, M.; Hohjoh, H. MiR-199-3p Enhances Muscle Regeneration and Ameliorates Aged Muscle and Muscular Dystrophy. Commun. Biol. 2021, 4, 427. [Google Scholar] [CrossRef]
- Vogel, J.; Niederer, D.; Engeroff, T.; Vogt, L.; Troidl, C.; Schmitz-Rixen, T.; Banzer, W.; Troidl, K. Effects on the Profile of Circulating MiRNAs after Single Bouts of Resistance Training with and without Blood Flow Restriction—A Three-Arm, Randomized Crossover Trial. Int. J. Mol. Sci. 2019, 20, 3249. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Markworth, J.F.; Aasen, K.M.M.; Zeng, N.; Cameron-Smith, D.; Mitchell, C.J. Acute Resistance Exercise Modulates MicroRNA Expression Profiles: Combined Tissue and Circulatory Targeted Analyses. PLoS ONE 2017, 12, e0181594. [Google Scholar] [CrossRef]
- Hashida, R.; Matsuse, H.; Kawaguchi, T.; Yoshio, S.; Bekki, M.; Iwanaga, S.; Sugimoto, T.; Hara, K.; Koya, S.; Hirota, K.; et al. Effects of a Low-intensity Resistance Exercise Program on Serum MiR-630, MiR-5703, and Fractalkine/CX3CL1 Expressions in Subjects with No Exercise Habits: A Preliminary Study. Hepatol. Res. 2021, 51, 823–833. [Google Scholar] [CrossRef]
- Drummond, M.J.; McCarthy, J.J.; Fry, C.S.; Esser, K.A.; Rasmussen, B.B. Aging Differentially Affects Human Skeletal Muscle MicroRNA Expression at Rest and after an Anabolic Stimulus of Resistance Exercise and Essential Amino Acids. Am. J. Physiol.-Endocrinol. Metab. 2008, 295, E1333–E1340. [Google Scholar] [CrossRef]
- Rivas, D.A.; Lessard, S.J.; Rice, N.P.; Lustgarten, M.S.; So, K.; Goodyear, L.J.; Parnell, L.D.; Fielding, R.A. Diminished Skeletal Muscle MicroRNA Expression with Aging Is Associated with Attenuated Muscle Plasticity and Inhibition of IGF-1 Signaling. FASEB J. 2014, 28, 4133–4147. [Google Scholar] [CrossRef]
- Zacharewicz, E.; Della Gatta, P.; Reynolds, J.; Garnham, A.; Crowley, T.; Russell, A.P.; Lamon, S. Identification of MicroRNAs Linked to Regulators of Muscle Protein Synthesis and Regeneration in Young and Old Skeletal Muscle. PLoS ONE 2014, 9, e114009. [Google Scholar] [CrossRef]
- Ogasawara, R.; Akimoto, T.; Umeno, T.; Sawada, S.; Hamaoka, T.; Fujita, S. MicroRNA Expression Profiling in Skeletal Muscle Reveals Different Regulatory Patterns in High and Low Responders to Resistance Training. Physiol. Genom. 2016, 48, 320–324. [Google Scholar] [CrossRef]
- Santos Morais Junior, G.; Carolino Souza, V.; Machado-Silva, W.; Dallanora Henriques, A.; Melo Alves, A.; Barbosa Morais, D.; Nóbrega, O.; Brito, C.J.; dos Santos Silva, R.J. Acute Strength Training Promotes Responses in Whole Blood Circulating Levels of MiR-146a among Older Adults with Type 2 Diabetes Mellitus. Clin. Interv. Aging 2017, 12, 1443–1450. [Google Scholar] [CrossRef]
- Russell, A.P.; Wallace, M.A.; Kalanon, M.; Zacharewicz, E.; Della Gatta, P.A.; Garnham, A.; Lamon, S. Striated Muscle Activator of Rho Signalling (STARS) Is Reduced in Ageing Human Skeletal Muscle and Targeted by MiR-628-5p. Acta Physiol. 2017, 220, 263–274. [Google Scholar] [CrossRef]
- Bjørnsen, T.; Wernbom, M.; Løvstad, A.; Paulsen, G.; D’Souza, R.F.; Cameron-Smith, D.; Flesche, A.; Hisdal, J.; Berntsen, S.; Raastad, T. Delayed Myonuclear Addition, Myofiber Hypertrophy, and Increases in Strength with High-Frequency Low-Load Blood Flow Restricted Training to Volitional Failure. J. Appl. Physiol. 2019, 126, 578–592. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Zeng, N.; Markworth, J.F.; Figueiredo, V.C.; Hedges, C.P.; Petersen, A.C.; Della Gatta, P.A.; Cameron-Smith, D.; Mitchell, C.J. Whey Protein Supplementation Post Resistance Exercise in Elderly Men Induces Changes in Muscle MiRNA’s Compared to Resistance Exercise Alone. Front. Nutr. 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Telles, G.D.; Libardi, C.A.; Conceição, M.S.; Vechin, F.C.; Lixandrão, M.E.; De Andrade, A.L.L.; Guedes, D.N.; Ugrinowitsch, C.; Camera, D.M. Time Course of Skeletal Muscle MiRNA Expression after Resistance, High-Intensity Interval, and Concurrent Exercise. Med. Sci. Sports Exerc. 2021, 53, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Gombos, Z.; Fridvalszki, M.; Langmar, G.; Tarcza, Z.; Merkely, B.; Naito, H.; Ichinoseki-Sekine, N.; Takeda, M.; Murlasits, Z.; et al. Blood Flow Restriction in Human Skeletal Muscle during Rest Periods after High-Load Resistance Training down-Regulates MiR-206 and Induces Pax7. J. Sport. Health Sci. 2021, 10, 470–477. [Google Scholar] [CrossRef]
- Buchanan, S.R.; Miller, R.M.; Nguyen, M.; Black, C.D.; Kellawan, J.M.; Bemben, M.G.; Bemben, D.A. Circulating MicroRNA Responses to Acute Whole-Body Vibration and Resistance Exercise in Postmenopausal Women. Front Endocrinol 2022, 13, 1038371. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Figueiredo, V.C.; Markworth, J.F.; Zeng, N.; Hedges, C.P.; Roberts, L.A.; Raastad, T.; Coombes, J.S.; Peake, J.M.; Mitchell, C.J.; et al. Cold Water Immersion in Recovery Following a Single Bout Resistance Exercise Suppresses Mechanisms of miRNA Nuclear Export and Maturation. Physiol. Rep. 2023, 11, e15784. [Google Scholar] [CrossRef]
- Benavente, C.; Padial, P.; Scott, B.R.; Almeida, F.; Olcina, G.; Pérez-Regalado, S.; Feriche, B. Strength and Muscle Mass Development after a Resistance-Training Period at Terrestrial and Normobaric Intermittent Hypoxia. Pflug. Arch. 2024, 476, 1221–1233. [Google Scholar] [CrossRef]
- Takamura, D.; Iwata, K.; Inoue, S.; Hatakeyama, J.; Moriyama, H. Circulating MiR-29c, MiR-195, and MiR-486 Are Objective Indicators to Determine the Moderate Intensity of Resistance Exercise. Cureus 2024, 16, e76212. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Bjørnsen, T.; Zeng, N.; Aasen, K.M.M.; Raastad, T.; Cameron-Smith, D.; Mitchell, C.J. MicroRNAs in Muscle: Characterizing the Powerlifter Phenotype. Front. Physiol. 2017, 8, 383. [Google Scholar] [CrossRef]
- Burke, B.I.; Ismaeel, A.; Long, D.E.; Depa, L.A.; Coburn, P.T.; Goh, J.; Saliu, T.P.; Walton, B.J.; Vechetti, I.J.; Peck, B.D.; et al. Extracellular Vesicle Transfer of MiR-1 to Adipose Tissue Modifies Lipolytic Pathways Following Resistance Exercise. JCI Insight 2024, 9, e182589. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.B.; Brown, K.A.; DeRuisseau, K.; Kanaley, J.A.; Ploutz-Snyder, L.L. Skeletal Muscle Adaptations Following Blood Flow-Restricted Training during 30 Days of Muscular Unloading. J. Appl. Physiol. 2010, 109, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Koltai, E.; Bori, Z.; Chabert, C.; Dubouchaud, H.; Naito, H.; Machida, S.; Davies, K.J.; Murlasits, Z.; Fry, A.C.; Boldogh, I.; et al. SIRT1 May Play a Crucial Role in Overload-induced Hypertrophy of Skeletal Muscle. J. Physiol. 2017, 595, 3361–3376. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.P.; Weiss, J.A.; Nie, Y.; Garner, R.T.; Drohan, C.J.; Kuang, S.; Stout, J.; Gavin, T.P. Skeletal Muscle IGF-1 Is Lower at Rest and after Resistance Exercise in Humans with Obesity. Eur. J. Appl. Physiol. 2020, 120, 2835–2846. [Google Scholar] [CrossRef]
- Elia, L.; Contu, R.; Quintavalle, M.; Varrone, F.; Chimenti, C.; Russo, M.A.; Cimino, V.; De Marinis, L.; Frustaci, A.; Catalucci, D.; et al. Reciprocal Regulation of MicroRNA-1 and Insulin-Like Growth Factor-1 Signal Transduction Cascade in Cardiac and Skeletal Muscle in Physiological and Pathological Conditions. Circulation 2009, 120, 2377–2385. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Zeng, N.; Markworth, J.F.; Figueiredo, V.C.; Roberts, L.A.; Raastad, T.; Coombes, J.S.; Peake, J.M.; Cameron-Smith, D.; Mitchell, C.J. Divergent Effects of Cold Water Immersion versus Active Recovery on Skeletal Muscle Fiber Type and Angiogenesis in Young Men. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 314, R824–R833. [Google Scholar] [CrossRef]
- Rivas, D.A.; Peng, F.; Benard, T.; Ramos da Silva, A.S.; Fielding, R.A.; Margolis, L.M. MiR-19b-3p Is Associated with a Diametric Response to Resistance Exercise in Older Adults and Regulates Skeletal Muscle Anabolism via PTEN Inhibition. Am. J. Physiol.-Cell Physiol. 2021, 321, C977–C991. [Google Scholar] [CrossRef] [PubMed]
- Davidsen, P.K.; Gallagher, I.J.; Hartman, J.W.; Tarnopolsky, M.A.; Dela, F.; Helge, J.W.; Timmons, J.A.; Phillips, S.M. High Responders to Resistance Exercise Training Demonstrate Differential Regulation of Skeletal Muscle MicroRNA Expression. J. Appl. Physiol. 2011, 110, 309–317. [Google Scholar] [CrossRef]
- Mueller, M.; Breil, F.A.; Lurman, G.; Klossner, S.; Flück, M.; Billeter, R.; Däpp, C.; Hoppeler, H. Different Molecular and Structural Adaptations with Eccentric and Conventional Strength Training in Elderly Men and Women. Gerontology 2011, 57, 528–538. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Page, R.A.; Sukala, W.R.; Giri, M.; Ghimbovschi, S.D.; Hayat, I.; Cheema, B.S.; Lys, I.; Leikis, M.; Sheard, P.W.; et al. Multi-Omic Integrated Networks Connect DNA Methylation and MiRNA with Skeletal Muscle Plasticity to Chronic Exercise in Type 2 Diabetic Obesity. Physiol. Genom. 2014, 46, 747–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Birbrair, A.; Wang, Z.-M.; Messi, M.L.; Marsh, A.P.; Leng, I.; Nicklas, B.J.; Delbono, O. Improved Knee Extensor Strength with Resistance Training Associates with Muscle Specific MiRNAs in Older Adults. Exp. Gerontol. 2015, 62, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hagstrom, A.; Denham, J. MicroRNAs in High and Low Responders to Resistance Training in Breast Cancer Survivors. Int. J. Sports Med. 2018, 39, 482–489. [Google Scholar] [CrossRef]
- Horak, M.; Zlamal, F.; Iliev, R.; Kucera, J.; Cacek, J.; Svobodova, L.; Hlavonova, Z.; Kalina, T.; Slaby, O.; Bienertova-Vasku, J. Exercise-Induced Circulating MicroRNA Changes in Athletes in Various Training Scenarios. PLoS ONE 2018, 13, e0191060. [Google Scholar] [CrossRef]
- Gazova, A.; Samakova, A.; Laczo, E.; Hamar, D.; Polakovicova, M.; Jurikova, M.; Kyselovic, J. Clinical Utility of MiRNA-1, MiRNA-29g and MiRNA-133s Plasma Levels in Prostate Cancer Patients With High-Intensity Training After Androgen-Deprivation Therapy. Physiol. Res. 2019, 68, S139–S147. [Google Scholar] [CrossRef]
- Olioso, D.; Dauriz, M.; Bacchi, E.; Negri, C.; Santi, L.; Bonora, E.; Moghetti, P. Effects of Aerobic and Resistance Training on Circulating Micro-RNA Expression Profile in Subjects With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 1119–1130. [Google Scholar] [CrossRef]
- Schwarz, N.A.; McKinley-Barnard, S.K.; Blahnik, Z.J. Effect of Bang® Pre-Workout Master Blaster® Combined with Four Weeks of Resistance Training on Lean Body Mass, Maximal Strength, MircoRNA Expression, and Serum IGF-1 in Men: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2019, 16, 54. [Google Scholar] [CrossRef]
- Liu, H.-W.; Cheng, H.-C.; Tsai, S.-H.; Sun, W.-H. Effect of Progressive Resistance Training on Circulating Adipogenesis-, Myogenesis-, and Inflammation-Related MicroRNAs in Healthy Older Adults: An Exploratory Study. Gerontology 2020, 66, 562–570. [Google Scholar] [CrossRef]
- Banitalebi, E.; Ghahfarrokhi, M.M.; Dehghan, M. Effect of 12-Weeks Elastic Band Resistance Training on MyomiRs and Osteoporosis Markers in Elderly Women with Osteosarcopenic Obesity: A Randomized Controlled Trial. BMC Geriatr. 2021, 21, 433. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, B.; Visavadiya, N.; de Paz, J.; Whitehurst, M.; Cuevas, M.; González-Gallego, J.; Huang, C.-J. Resistance Training Diminishes the Expression of Exosome CD63 Protein without Modification of Plasma MiR-146a-5p and CfDNA in the Elderly. Nutrients 2021, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Torma, F.; Bakonyi, P.; Regdon, Z.; Gombos, Z.; Jokai, M.; Babszki, G.; Fridvalszki, M.; Virág, L.; Naito, H.; Iftikhar Bukhari, S.R.; et al. Blood Flow Restriction during the Resting Periods of High-Intensity Resistance Training Does Not Alter Performance but Decreases MIR-1 and MIR-133A Levels in Human Skeletal Muscle. Sports Med. Health Sci. 2021, 3, 40–45. [Google Scholar] [CrossRef]
- de Luca Corrêa, H.; Neves, R.V.P.; Deus, L.A.; Reis, A.L.; Raab, A.T.O.; Rodrigues-Silva, P.L.; Barbosa, J.M.S.; de Araújo, T.B.; da Silva, M.G.S.; Ferreira, C.E.S.; et al. MicroRNA Levels in Hemodialysis Patients Following Resistance Training: Associations with Functional Performance, Inflammatory Profile, Sestrins-2, and Nitric Oxide. Exp. Gerontol. 2022, 162, 111761. [Google Scholar] [CrossRef]
- Agostini, S.; Mancuso, R.; Citterio, L.A.; Mihali, G.A.; Arosio, B.; Clerici, M. Evaluation of Serum MiRNAs Expression in Frail and Robust Subjects Undergoing Multicomponent Exercise Protocol (VIVIFRAIL). J. Transl. Med. 2023, 21, 67. [Google Scholar] [CrossRef]
- Fei, S.; Rule, B.D.; Godwin, J.S.; Mobley, C.B.; Roberts, M.D.; von Walden, F.; Vechetti, I.J. miRNA-1 Regulation Is Necessary for Mechanical Overload-induced Muscle Hypertrophy in Male Mice. Physiol. Rep. 2025, 13, e70166. [Google Scholar] [CrossRef]
- Vechetti, I.J.; Peck, B.D.; Wen, Y.; Walton, R.G.; Valentino, T.R.; Alimov, A.P.; Dungan, C.M.; Van Pelt, D.W.; von Walden, F.; Alkner, B.; et al. Mechanical Overload-induced Muscle-derived Extracellular Vesicles Promote Adipose Tissue Lipolysis. FASEB J. 2021, 35, e21644. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A. Adaptations Of Skeletal Muscle To Prolonged, Intense Endurance Training. Clin. Exp. Pharmacol. Physiol. 2002, 29, 218–222. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Bøtker, H.E.; Lassen, T.R.; Jespersen, N.R. Clinical Translation of Myocardial Conditioning. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H1225–H1252. [Google Scholar] [CrossRef]
- Polakovičová, M.; Musil, P.; Laczo, E.; Hamar, D.; Kyselovič, J. Circulating MicroRNAs as Potential Biomarkers of Exercise Response. Int. J. Mol. Sci. 2016, 17, 1553. [Google Scholar] [CrossRef]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying MiRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef]
- Lovett, J.A.C.; Durcan, P.J.; Myburgh, K.H. Investigation of Circulating Extracellular Vesicle MicroRNA Following Two Consecutive Bouts of Muscle-Damaging Exercise. Front. Physiol. 2018, 9, 1149. [Google Scholar] [CrossRef]
- Xhuti, D.; Nilsson, M.I.; Manta, K.; Tarnopolsky, M.A.; Nederveen, J.P. Circulating Exosome-like Vesicle and Skeletal Muscle MicroRNAs Are Altered with Age and Resistance Training. J. Physiol. 2023, 601, 5051–5073. [Google Scholar] [CrossRef]
- Kawanishi, N.; Tominaga, T.; Suzuki, K. Electrical Pulse Stimulation-Induced Muscle Contraction Alters the MicroRNA and MRNA Profiles of Circulating Extracellular Vesicles in Mice. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2023, 324, R761–R771. [Google Scholar] [CrossRef]
- Conkright, W.R.; Kargl, C.K.; Hubal, M.J.; Tiede, D.R.; Beckner, M.E.; Sterczala, A.J.; Krajewski, K.T.; Martin, B.J.; Flanagan, S.D.; Greeves, J.P.; et al. Acute Resistance Exercise Modifies Extracellular Vesicle MiRNAs Targeting Anabolic Gene Pathways: A Prospective Cohort Study. Med. Sci. Sports Exerc. 2024, 56, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Hale, A.; Weiner, R.B.; Lewis, G.D.; Systrom, D.; Wang, F.; Wang, T.J.; Chan, S.Y. Dynamic Regulation of Circulating MicroRNA during Acute Exhaustive Exercise and Sustained Aerobic Exercise Training. J. Physiol. 2011, 589, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; Perry, M.M.; Moschos, S.A.; Larner-Svensson, H.M.; Lindsay, M.A. Role of MiRNA-146a in the Regulation of the Innate Immune Response and Cancer. Biochem. Soc. Trans. 2008, 36, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Conkright, W.R.; Beckner, M.E.; Sterczala, A.J.; Mi, Q.; Lovalekar, M.; Sahu, A.; Krajewski, K.T.; Martin, B.J.; Flanagan, S.D.; Greeves, J.P.; et al. Resistance Exercise Differentially Alters Extracellular Vesicle Size and Subpopulation Characteristics in Healthy Men and Women: An Observational Cohort Study. Physiol. Genom. 2022, 54, 350–359. [Google Scholar] [CrossRef]
- Panteleev, M.A.; Abaeva, A.A.; Balandina, A.N.; Belyaev, A.V.; Nechipurenko, D.Y.; Obydennyi, S.I.; Sveshnikova, A.N.; Shibeko, A.M.; Ataullakhanov, F.I. Extracellular Vesicles of Blood Plasma: Content, Origin, and Properties. Biochem. Suppl. Ser. A Membr. Cell Biol. 2017, 11, 187–192. [Google Scholar] [CrossRef]
- Strauss, R.G. Mechanisms of Adverse Effects during Hemapheresis. J. Clin. Apher. 1996, 11, 160–164. [Google Scholar] [CrossRef]
- Lacroix, R.; Judicone, C.; Mooberry, M.; Boucekine, M.; Key, N.S.; Dignat-George, F. Standardization of Pre-Analytical Variables in Plasma Microparticle Determination: Results of the International Society on Thrombosis and Haemostasis SSC Collaborative Workshop. J. Thromb. Haemost. 2013, 11, 1190–1193, Erratum in J. Thromb. Haemost. 2017, 15, 1236. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Zhang, A.; Cai, H.; Price, S.R.; Wang, X.H. MicroRNA-23a and MicroRNA-27a Mimic Exercise by Ameliorating CKD-Induced Muscle Atrophy. J. Am. Soc. Nephrol. 2017, 28, 2631–2640. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Wang, B.; Klein, J.D.; Price, S.R.; Wang, X.H. MiRNA-23a/27a Attenuates Muscle Atrophy and Renal Fibrosis through Muscle-kidney Crosstalk. J. Cachexia Sarcopenia Muscle 2018, 9, 755–770. [Google Scholar] [CrossRef]
- Borja-Gonzalez, M.; Casas-Martinez, J.C.; McDonagh, B.; Goljanek-Whysall, K. Aging Science Talks: The Role of MiR-181a in Age-Related Loss of Muscle Mass and Function. Transl. Med. Aging 2020, 4, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Lee, J.; Donaldson, A.V.; Connolly, M.; Sharif, M.; Natanek, S.A.; Rosendahl, U.; Polkey, M.I.; Griffiths, M.; Kemp, P.R. MiR-422a Suppresses SMAD4 Protein Expression and Promotes Resistance to Muscle Loss. J. Cachexia Sarcopenia Muscle 2018, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.T.; Weiss, J.A.; Nie, Y.; Sullivan, B.P.; Kargl, C.K.; Drohan, C.J.; Kuang, S.; Stout, J.; Gavin, T.P. Effects of Obesity and Acute Resistance Exercise on Skeletal Muscle Angiogenic Communication Pathways. Exp. Physiol. 2022, 107, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Garner, R.T.; Solfest, J.S.; Nie, Y.; Kuang, S.; Stout, J.; Gavin, T.P. Multivesicular Body and Exosome Pathway Responses to Acute Exercise. Exp. Physiol. 2020, 105, 511–521. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The Potential of Endurance Exercise-Derived Exosomes to Treat Metabolic Diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Kyriakidou, Y.; Cooper, I.; Kraev, I.; Lange, S.; Elliott, B.T. Preliminary Investigations Into the Effect of Exercise-Induced Muscle Damage on Systemic Extracellular Vesicle Release in Trained Younger and Older Men. Front. Physiol. 2021, 12, 723931. [Google Scholar] [CrossRef]
- Fernandez-Sanjurjo, M.; Pinto-Hernandez, P.; Dávalos, A.; Díaz-Martínez, Á.E.; Martín-Hernández, R.; Castilla-Silgado, J.; Toyos-Rodríguez, C.; Whitham, M.; Amado-Rodríguez, L.; Muñiz-Albaiceta, G.; et al. Next-generation Sequencing Reveals That MiR-16-5p, MiR-19a-3p, MiR-451a, and MiR-25-3p Cargo in Plasma Extracellular Vesicles Differentiates Sedentary Young Males from Athletes. Eur. J. Sport. Sci. 2024, 24, 766–776. [Google Scholar] [CrossRef]
- O’Bryan, S.M.; Lavin, K.M.; Graham, Z.A.; Drummer, D.J.; Tuggle, S.C.; Van Keuren-Jensen, K.; Reiman, R.; Alsop, E.; Kadakia, M.P.; Craig, M.P.; et al. Muscle-Derived MicroRNAs Correlated with Thigh Lean Mass Gains during Progressive Resistance Training in Older Adults. J. Appl. Physiol. 2024, 137, 262–273. [Google Scholar] [CrossRef]
- György, B.; Pálóczi, K.; Balbisi, M.; Turiák, L.; Drahos, L.; Visnovitz, T.; Koltai, E.; Radák, Z. Effect of the 35 Nm and 70 Nm Size Exclusion Chromatography (SEC) Column and Plasma Storage Time on Separated Extracellular Vesicles. Curr. Issues Mol. Biol. 2024, 46, 4337–4357. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA Profiling: Approaches and Considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Le Carré, J.; Lamon, S.; Léger, B. Validation of a Multiplex Reverse Transcription and Pre-Amplification Method Using TaqMan® MicroRNA Assays. Front. Genet. 2014, 5, 413. [Google Scholar] [CrossRef]
- Chen, C. Real-Time Quantification of MicroRNAs by Stem-Loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.W.I.; Simon, P.; Krämer-Albers, E.-M. Considerations for the Analysis of Small Extracellular Vesicles in Physical Exercise. Front. Physiol. 2020, 11, 576150. [Google Scholar] [CrossRef]
| Reference | Subjects | Exercise Stimulus | Additional Intervention | Samples | MiRNA Detection Method | MiRNA Responses |
|---|---|---|---|---|---|---|
| Drummond et al., 2008 [78] | Healthy, untrained young (n = 6, age 29 ± 2 yr) and elderly men (n = 6, age 70 ± 2 yr) | Acute RE: leg extension 10 reps of 8 sets 70% at 1RM | 20 g of EAA ingestion 1 h after the training session | Muscle biopsies (Vastus lateralis): pre, 1 h, 3 h, 6 h | Pri-miRNA: SYBR Green RT-PCR miRNA: mirVana SYBR Green RT-PCR | Young: 3 h: 1↓ 6 h: Pri-206↑ Pri-1-2, 1, Pri-133a-1, Pri-133a-2↓ Elderly: 3 h: Pri-206↑ Pri-133a-2↓ |
| Sawada et al., 2013 [72] | Healthy, recreationally active men (n = 12), age 29 ± 1.2 yr | Acute RE: Bench press and bilateral leg press, 5 sets of 10 reps at 70% 1RM | Blood (serum), pre, immed, 1 h, 1 day, and 3 days after exercise | miRCURY™ LNA miRNA Array, TaqMan MicroRNA assay RT-PCR | 3 days after: 149↑ 146a, 221↓ | |
| Rivas et al., 2014 [79] | Healthy young (n = 8, age 22 ± 1 yr) and elderly men (n = 8, age 74 ± 2 yr) | Acute RE: bilateral knee extension, bilateral leg press of 3 sets 80% at 1RM | Young and elderly men comparison | Muscle biopsies (Vastus lateralis): pre, 6 h | miScript SYBR Green PCR Array | Young: 423-5p↑ 16-5p, 23b-3p, 24-3p, 26a-5p, 26b-5p, 27a-3p, 27b-3p, 29a-3p, 29c-3p, 30a-5p, 30d-5p, 95-3p,107, 126-3p, 133a, 133b, 140-3p, 181a-5p, 324-3p, 378a-5p↓ Elderly: 423-5p↑ |
| Uhlemann et al., 2014 [71] | Healthy, trained subjects (n = 11) | Acute RE: Lat pulldown, leg press and butterfly, 3 sets of 15 reps, additional eccentric load | Blood (plasma) pre, immed, 1 h | TaqMan miRNA Assay RT-PCR | Immed. after RE: 133↑ | |
| Zacharewicz et al., 2014 [80] | Healthy, untrained young (n = 10, age 24.2 ± 0.9 yr) and elderly men (n = 10, age 66.6 ± 1.1 yr) | Acute RE: leg extension, 14 reps of 3 sets 60% at 1RM | Young and elderly men comparison | Muscle biopsies (Vastus lateralis): pre, 2 h | TaqMan Array Human MicroRNA A + B Cards v3.0 | 26 miRNAs were regulated with age and/or exercise, 7 of these were differentially and the other 7 were regulated in either young or old subjects. Young: 486-3p↑ 149-3p, 99b-5p, 520g-5p↓ Old: 99a-5p↑ 196b-5p, 489-5p, 628-5p, 186-5p, 335-5p↓ |
| Ogasawara et al., 2016 [81] | Healthy, untrained young men (n = 10) | Acute RE: bilateral knee extension and flexion, 10 reps of 3 sets at 70% of 1RM | High and low responders comparison | Muscle biopsies (Vastus lateralis): pre, 3 h after | NanoString nCounter human miRNA expression assay | 84 miRNAs differentially expressed |
| Cui et al., 2017 [67] | Healthy, active, young men with no gym experience (n = 15), age 19.36 ± 0.14 yr | Acute RE: Strength endurance: bench press, squat, pulldown, overhead press, standing dumbbell curl, 3 sets of 16–20 reps at 40% 1RM | Blood (plasma), pre, immed, 1 h, 24 h | TaqMan Low-Density Array, TaqMan RT-PCR | 1 h after: 532↑ Immed after exercise: 208b↓ | |
| Cui et al., 2017 [67] | Healthy, active, young men with no gym experience (n = 15), age 19.72 ± 0.20 yr | Acute RE: Muscular hypertrophy: bench press, squat, pulldown, overhead press, standing dumbbell curl, 3 sets of 12 reps at 70% 1RM | Blood (plasma), pre, immed, 1 h, 24 h | TaqMan Low-Density Array, TaqMan RT-PCR | Immed after exercise: 21, 133a↓ 1 h after: 181a, 206↑ 221↓ 24 h after: 133b↑ | |
| Cui et al., 2017 [67] | Healthy, active, young men with no gym experience (n = 15), age 18.87 ± 0.12 yr | Acute RE: Maximum strength: bench press, squat, pulldown, overhead press, standing dumbbell curl, 4 sets of 6 reps at 90% 1RM | Blood (plasma): pre, immed, 1 h, 24 h | TaqMan Low-Density Array, TaqMan RT-PCR | Immed after exercise: 133a↓ 1 h after: 133b↑ | |
| D’Souza et al., 2017 [76] | Healthy, resistance-trained, young men (n = 9), age 24.6 ± 4.9 yr | Acute RE: Leg press, 2 sets of 10 reps 50–70% at 1 RM, 6 sets of 8–10 reps 80% at 1 RM, leg extension, 8 sets of 8–10 reps 80% at 1RM | Blood (plasma), muscle biopsies (Vastus lateralis): pre, 2 h, 4 h | TaqMan Advanced miRNA RT-PCR | Muscle: 2 h after: 133a, 206↑ 23a, 378b↓ 4 h after: 486, 146a↑ 23a↓ Blood: 4 h after: 133a, 149↑ | |
| Margolis et al., 2016 [73] | Healthy, inactive, young (n = 9, age 22 ± 1) and old (n = 9, age 74 ± 2 yr) | Acute RE: Bilateral leg extension and leg press, 3 sets of 10 reps at 80% 1RM | Blood (serum), pre, immed, and 6 h after | miScript miRNA PCR Array SYBR Green, TaqMan MicroRNA Assays | 6 h after exercise in young: 17-5p, 19a-3p, 19b-3p, 20a-5p, 26b-5p, 93-5p, 106-5p, 143-3p, 195-5p↑ | |
| Morais Junior et al., 2017 [82] | T2DM and healthy age 68.2 ± 5.3 yr men and women (n = 23) | Acute strength exercise: circuit fashion (8 exercises) 3 sets 40 s and 20 s | Strength and cardiovascular training circuit comparisons | Blood (serum): pre, post | TaqMan RT-PCR | 146a-5p↑ |
| Russell et al., 2017 [83] | Healthy young (n = 10, age 18–30) and elderly (n = 10, 60–75) men | Acute RE: leg extension, 3 sets of 14 reps | Young/elderly comparisons | Muscle biopsies (vastus lateralis): pre, 2 h | TaqMan Array Human MicroRNA A + B Cards version Set v3.0 | 26 miRNAs that were significant (previously reported Zacharewicz et al., 2014 [80]) young: 520g-3p, 628-5p↓ |
| Bjørnsen et al., 2019 [84] | Recreationally active age 24 ± 2 yr men and women (n = 13) | Acute RE: two 5-day blocks of 7 BFRRE sessions, separated by a 10-day rest period. 4 sets of unilateral knee extensions to voluntary failure at 20% of 1RM | Partial BFR | Muscle biopsies (vastus lateralis): pre, during: Acute 1, Day 4, Rest week, Acute 2, 3 days, and 10 days after | TaqMan Advanced miRNA RT-PCR | Rest week: 208b↓ Acute2: 208b, 486↓ Post 10: 16, 486↑ |
| D’Souza et al., 2019 [85] | Healthy, recreationally active elderly men (n = 23) age 67.9 ± 0.9 yr | Acute RE: bilateral barbell smith racks squat, 45° leg press, seated knee extensions, 8–10 reps of 3 sets 80% at 1RM (circuit manner) | Protein ingestion: placebo, 20 or 40 g whey protein | Muscle biopsies (Vastus lateralis): pre, 2 h and 4 h | TaqMan Advanced miRNA RT-PCR | 16-5p was altered in all groups placebo: 4 h: 15a, 499a↑ 40g: 2 h, 4 h: 451a↑ |
| Vogel et al., 2019 [75] | Healthy men and females (n = 18) age 25 ± 2 yr | Acute low intensity (LI) RE: leg flexion/extension, total 75 reps in 4 sets 30% at 1RM | BFR | Blood (plasma): pre, immed | RT-PCR: miRCURY LNA miRNA PCR assays | 143-3p↓ |
| Vogel et al., 2019 [75] | Healthy men and females (n = 18) age 25 ± 2 yr | Acute RE: leg flexion/extension, total 75 reps in 4 sets 30% at 1RM | Blood (plasma): pre, immed | RT-PCR: miRCURY LNA miRNA PCR assays | ||
| Vogel et al., 2019 [75] | Healthy men and females (n = 18) age 25 ± 2 yr | Acute RE: leg flexion/extension, total 30 reps in 3 sets 70% at 1RM | Blood (plasma): pre, immed | RT-PCR: miRCURY LNA miRNA PCR assays | 10b-5p, 30a-5p, 139-5p, 143-3p, 195-5p↑ | |
| Hashida et al., 2021 [77] | Physically inactive men (n = 7) | LI RE | Blood (serum): pre, post | miRNA microarray | 7 miRNAs significantly changed, of these: 630, 5703↑ | |
| Telles et al., 2021 [86] | Healthy, untrained young men (n = 9) age 23.9 ± 2.8 yr | Acute RE: leg press, leg extension, 8–12 reps of 2 sets until muscle failure, high intensity (HI) interval exercise: 12 × 1-min sprints, RE and HI interval exercise combined | Muscle biopsies (Vastus lateralis): pre, immed, 4 h, 8 h | TaqMan Advanced miRNA RT-PCR | RE: 1-3p, 23a-3p, 133-a-3p, 133-b. 181a-3p, 206, 486↑ | |
| Torma et al., 2021 [87] | Healthy men (n = 7) age 24.5 ± 4.7 yr | Acute RE: squats, 10 reps of 7 sets 70% at 1RM | BFR during rest periods | Muscle biopsies (Vastus lateralis): 2 h | TaqMan miRNA RT-PCR | BFR leg: 206↓ |
| Buchanan et al., 2022 [88] | Postmenopausal women (n = 10) age 65–76 yr | Acute RE: 3 sets of 10 repetitions per exercise at 70–75% of 1RM: leg press, shoulder press, lat pulldown, leg extension, and hip adduction | RE and whole body vibration comparison | Blood (serum): pre, post, 1 h, 24 h, 48 h | SYBR Green RT-PCR | RE: no changes |
| D’Souza et al., 2023 [89] | Physically active young 22 ± 2 yr men (n = 9) | Acute RE: unilateral knee extension, unilateral 45° leg press, 6 sets (8, 8, 10, 12, 10, and 10 reps), single-leg squats and walking lunges, 3 sets (12 reps) | Cold water immersion, or active recovery after RE | Muscle biopsies (Vastus lateralis): pre, 2 h, 24 h, 48 h | TaqMan Advanced miRNA RT-PCR | Cold water immersion: 24 h: 133a, 126↑ 48 h: 126↑ active recovery: 48 h: 1↑ |
| Benavente et al., 2024 [90] | Strength-trained males aged (Normoxia 22.7 ± 3.4 yr, Hipobaric hypoxia 22.8 ± 4.2 yr, Normobaric hypoxia 21.9 ± 2.2 yr) (n = 33) | Acute and Chronic RE: full body routine, 6 exercises, 3 sets of 6–12 reps at 65–80% of 1RM | Normoxia, Hypobaric hypoxia, Normobaric hypoxia | Blood (serum): 72 h pre, Post first RE, Post last RE | SYBR Green RT-PCR | Post first RE: Hypobaric hypoxia: 206↓ Normobaric hypoxia: 206↑ Post last RE: Normoxia: 206↑ Hypobaric hypoxia: 206↑ Normobaric hypoxia: 206↑ |
| Takamura et al., 2024 [91] | Healthy, untrained males, age LI 26.17 ± 4.40 yr, HI 25 ± 3.2 yr (n = 12) | Acute RE: leg extension and leg curl, 5 sets of 10 reps at 10% of 1RM (LI), or 80% of 1RM (HI) | LI and HI exercise | Blood (plasma): pre, post | TaqMan RT-PCR | HI: 195↑ LI: 29c, 486↑ |
| Reference | Subjects | Exercise Stimulus | Additional Intervention | Samples | MiRNA Detection Method | MiRNA Responses |
|---|---|---|---|---|---|---|
| Davidsen et al., 2011 [100] | Healthy young, physically active men (n = 56), age 18–30 yr | 12-week training, 5 sessions/week, pushing, pulling, and leg exercises | High and low responders comparison | Muscle biopsies (Vastus lateralis): 48 h pre and post after first/last training session | TaqMan RT-PCR | Low responders: 26a, 29a, 378↓ 451↑ |
| Mueller et al., 2011 [101] | Elderly (age 80.1 ± 3.7 yr) men and women RE (n = 13), eccentric ergometer (n = 14) | 12-week, 2 sessions/week, leg press, knee extension, leg curl, hip extension, 3 sets of 8–10 reps | RE or eccentric ergometer sessions | Muscle biopsies (Vastus lateralis): pre, post | miScript primer assay SYBR Green | Both groups: 1↓ |
| Rowlands et al., 2014 [102] | Inactive, Polynesian T2DM males and females age 49 ± 5 yr (n = 17) | 16-week training, 3 sessions/week: two or three sets of eight exercises using machine weights, six to eight repetitions to fatigue | RE and endurance exercise comparison | Muscle biopsies (Vastus lateralis): pre, post | Affymetrix GeneChip microarray, TaqMan miRNA assay RT-PCR | 23a, 195, 3178, 483-5p, 487↑ 193b, 1207-5p↓ |
| Zhang et al., 2015 [103] | Sedentary older men (n = 3) and women (n = 4), age 65–80 yr | 5 months, 3 sessions/week: leg press, knee extension, leg curl, calf press, 70% at 1RM | Muscle biopsies (Vastus lateralis): pre, 5 months after; Blood (plasma): pre, 5 months after | TaqMan miRNA assay RT-PCR | Muscle: 133b↓ | |
| Ogasawara et al., 2016 [81] | Healthy, untrained young men (n = 18), age 21.4 ± 1.1 yr | 12-week training, 3 sessions/week: knee extension, flexion, 10 reps of 3 sets, 70% at 1RM | High and low responders comparison | Muscle biopsies (Vastus lateralis): pre, 12 w after | NanoString nCounter human miRNA expression assay | 102 miRNAs differentially expressed |
| D’Souza et al., 2018 [98] | Physically active young, resistance exercise-trained men, age 21.5 ± 0.6 yr (n = 21) | 12-week training, 2 sessions/week: Bilateral 45° leg press, knee extension, walking, lunges, plyometrics exercises | Cold water immersion, or active recovery after RE; Whey protein isolate consumed 1 h before and following the completion of therapy, plus recovery bar with 18 g of protein and 30.7 g of carbohydrate was also consumed 2 h post-training | Muscle biopsies (Vastus lateralis): pre, post | TaqMan Advanced miRNA RT-PCR | Active recovery: 15a, 16, 208b, 499a↑ |
| Hagstrom and Denham 2018 [104] | Breast cancer survivors (women, n = 24) | 16-week, 3 sessions/week | RE or usual care intervention | Blood (serum): pre, 16 w after | RT-PCR | No change, only between high and low responders: miR-133a-3p, miR-370-3p↑ |
| Horak et al., 2018 [105] | Healthy young men (n = 30), age 22.5 ± 4.06 yr | Explosive strength training, MH and HI interval exercise | Blood (plasma): pre, 5 week after, post | TaqMan miRNA assay RT-PCR | Explosive strength training: 222, 16↓ MH: 93, 16, 222↓ | |
| Gazova et al., 2019 [106] | Sedentary prostate cancer patients (men, age 69.21 ± 5.8 yr, n = 15) | Strength training: 16-week, 3 times/week | Exercise and control group comparisons | Blood (plasma): pre, post | TaqMan miRNA assay RT-PCR | 1, 29, 133↑ |
| Olioso et al., 2019 [107] | Elderly, sedentary individuals with type 2 diabetes mellitus (n = 6: AER = 3; RE = 3), age 40–70 yr | AER or RE: 60 min, 3 times per week, for a period of 4 months, RE: lower, upper body, core exercises, 70–80% at 1RM | Blood (plasma): pre, after exercise training | c-miRNA PCR panel (Exiqon), TaqMan RT-PCR | Irrespective of AER/RE: 423-3p, 451a, 766-3p↑ | |
| Schwarz et al., 2019 [108] | Healthy, recreationally active resistance-trained young men (n = 16), age 22.5 ± 3.1 yr | 4-week periodized training, consisting of 2 lower-body and 2 upper-body sessions. 7 exercises/session. | Pre-Workout drink ingestion: 26.1 g Bang Master Blaster; 26.1 g placebo | Muscle biopsies (vastus lateralis): pre, post 4 weeks | SYBR Green RT-PCR | Both groups: 23a, 23b↑ |
| Liu et al., 2020 [109] | Healthy older men and females (n = 10), age 67.6 ± 2.2 yr | 12-week training | Blood: pre, after 12 weeks | Illumina NextSeq NGS | Adipogenesis-related: 103a-3p, 103b, 143-5p, 146b-3p, 146b-5p, -17-5p, 181a-2-3p, 181b-5p, 199a-5p, 204-3p, and -378c anti-adipogenesis-related: 155-3p, 448, 363-3p myogenesis-related: 125b-1-3p, 128-3p, 133a-3p, 155-3p, 181a-2-3p, 181b-5p, 199a-5p, 223-3p, 499a-5p inflammation-related: 146b-3p, 146b-5p, 155-3p, 181a-2-3p, 181b-5p | |
| Banitalebi et al., 2021 [110] | Untrained females with Osteosarcopenic Obesity (n = 63), age 65–80 yr | 12-week training, 3 sessions/week using elastic bands | Blood (serum): pre, after 12 weeks | SYBR Green RT-PCR | 133, 206 not changed | |
| Estébanez et al., 2021 [111] | Healthy elderly men and females (n = 38), age 70–85 yr | 8-week training, 2 sessions/week, leg press, ankle extension, bench press, leg extension, biceps curl, pec deck, high pulley traction, dumbbell lateral lift, 3 sets of 12-8-12 repetitions | Blood (plasma): pre, after 12 weeks | TaqMan RT-PCR | 146a-5p not changed | |
| Rivas et al., 2021 [99] | Inactive, elderly males and females, age 70–85 yr (n = 73), Losers (78 ± 6), Gainers (77 ± 6) | 6 months training, 3 sessions/week: leg press, seated row, leg extension, chest press, and leg curl at 80% of 1RM, 2 sets of 10 then 3 sets of 12 reps | Whey protein supplement or isocaloric control beverage consumption, twice a day. Leg lean mass losers (Losers) or gainers (Gainers) comparison | Blood (serum): pre, post | TaqMan RT PCR, miRCURY LNA SYBR, Green RT-PCR | No pre, post comparison, only between groups: 19b-3p, 92a, 126, 133a-3p, -133b↑, -1-3p↓ was in Gainers, compared to Losers |
| Torma et al., 2021 [112] | Healthy young men (n = 22), age control: 23.9 ± 1.7, BFR: 24.1 ± 6.1 | 4-week training, 3 sessions/week, 10 reps of 5 sets, 70% at 1RM | BFR during rest periods | Muscle biopsies (vastus lateralis): 72 h pre, 24 h post after first/last training session | TaqMan RT-PCR | BFR: 1, 133a↓ |
| Corrêa et al., 2022 [113] | Older hemodialysis patients (n = 25), age 68 ± 1, men and women | 24-week, 3 sessions/week | RE (n = 13) or control (n = 12) | Blood | TaqMan RT PCR | RE: 1↑, 31↓ |
| Agostini et al., 2023 [114] | Elderly (age > 60 yr) frail (n = 15) and robust (n = 30) males and females | Multicomponent exercise: 12-week, RE, gait training, balance training | Frail and robust subjects, comparisons | Blood (serum): pre, post | Digital Droplet PCR | Both groups: 93-5p, 495-3p↓, 155-5p↑ |
| Reference | Subjects | Exercise Stimulus | Samples | EV Isolation Method | EV Detection Method | miRNA Detection Method | MiRNA Responses |
|---|---|---|---|---|---|---|---|
| Lovett et al., 2018 [122] | Healthy, untrained men (n = 9), age 18–30 yr | Acute plyometric jumps, 10 sets, 10 reps, followed by 5 sets of 4 min downhill running | Blood (plasma): pre, 2 h, 24 h | SEC | TEM, NTA | TaqMan Advanced RT-PCR | 24 h after: 31↓ |
| Annibalini et al., 2019 [65] | Healthy, resistance exercise-trained young men (n = 8), age 23.7 ± 2.8 yr | Acute flywheel RE: squats, 5 sets, 10 maximal reps | Blood (plasma): pre, 2 h, 24 h, 48 h, Muscle biopsies: pre, 2 h | DUC | NTA | SYBR Green RT PCR | 2 h after: 206, 146a↑ |
| Just et al., 2020 [24] | Healthy, recreationally active young men (n = 9), age 21 ± 0.6 yr | Acute blood flow restricted RE: knee extensions, 5 sets, volitional failure 30% at 1RM | Blood (plasma): pre, 1 h | precipitation, SEC | NTA, WB, TEM, EV Array | Illumina NextSeq NGS | 1 h after: 7b-5p, 16-5p, 182-5p, 363-3p, 451a-5p, 1294↑ 17-5p, 19b-3p, 21-5p, 150-5p, 221-3p, 340-5p↓ |
| Vechetti et al., 2021 [116] | Healthy, recreationally active males and females (n = 10), age 26–50 | Acute RE: leg press, knee extension, 4 sets, 7 reps | Blood (plasma): pre, 30 min | magnetic beads | NTA, TEM, WB, EV labeling and tracking | TaqMan RT PCR | 30 min after: 1↑ |
| Xhuti et al., 2023 [123] | Older adults (n = 19), age 74.9 ± 5.7 yr | Chronic home-based RE (12 weeks): 3 sets, 10–15 reps | Blood (plasma): pre, post 12 weeks, Muscle biopsies: pre, post | SEC-UC | IB, NTA, TEM | TaqMan RT PCR | 23a, 27a, 146a, 92a↑ |
| Kawanishi et al., 2023 [124] | C57BL/6 male mice (n = 18), age 10 wk | Acute EPS-induced RE: 6 sets, 10 contractions for 3 s | Blood (serum): pre, post, 1.5 h | precipitation | NTA, WB, EV Quantification Assay | Ion Torrent NGS, TaqMan RT PCR | 1,5 h after: 1a-3p, 133a-3p, 206-3p↑ |
| Conkright et al., 2024 [125] | Participants (n = 10), age 26.9 ± 5.5 yr | Acute RE: back squat, 6 sets, 10 reps, 75% at 1RM | Blood (plasma): pre, post | SEC | Illumina NextSeq NGS | 34 miRNAs were altered | |
| Burke et al., 2024 [93] | Healthy, untrained males and females (n = 32), age 29.2 ± 6.2 yr | Acute RE: back squat, leg press, knee extension, and latissimus pulldown, 3 sets, 8 reps at 80% 1RM, 4th set continued until failure | Blood (serum): pre, post, 30 min, 1 h, 90 min Muscle biopsies (vastus lateralis): pre, 45 min Adipose tissue biopsies: 70 min | SEC | ExoView | TaqMan RT PCR | Serum: post, 90 min: 1↑ Muscle: pri-miR-1a↑ Adipose: 1↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csala, D.; Ádám, Z.; Wilhelm, M. The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review. Curr. Issues Mol. Biol. 2025, 47, 583. https://doi.org/10.3390/cimb47080583
Csala D, Ádám Z, Wilhelm M. The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review. Current Issues in Molecular Biology. 2025; 47(8):583. https://doi.org/10.3390/cimb47080583
Chicago/Turabian StyleCsala, Dávid, Zoltán Ádám, and Márta Wilhelm. 2025. "The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review" Current Issues in Molecular Biology 47, no. 8: 583. https://doi.org/10.3390/cimb47080583
APA StyleCsala, D., Ádám, Z., & Wilhelm, M. (2025). The Role of miRNAs and Extracellular Vesicles in Adaptation After Resistance Exercise: A Review. Current Issues in Molecular Biology, 47(8), 583. https://doi.org/10.3390/cimb47080583






