Anticancer Activity of Ethyl Acetate/Water Fraction from Tanacetum vulgare L. Leaf and Flower Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Extraction
2.3. LC-HRMS Analyses
2.4. Cell Culturing
2.5. Cell Viability Assessment
2.6. Cell Cycle FACS-Analysis
2.7. Pro-Apoptotic Activity
2.8. Genotoxicity Assay
2.9. In Vivo Experiments
2.10. Statistics
3. Results and Discussion
3.1. LC-HRMS Analyses
3.2. In Vitro Anticancer Activity
3.3. Effect on the Cell Cycle
3.4. Pro-Apoptotic Activity
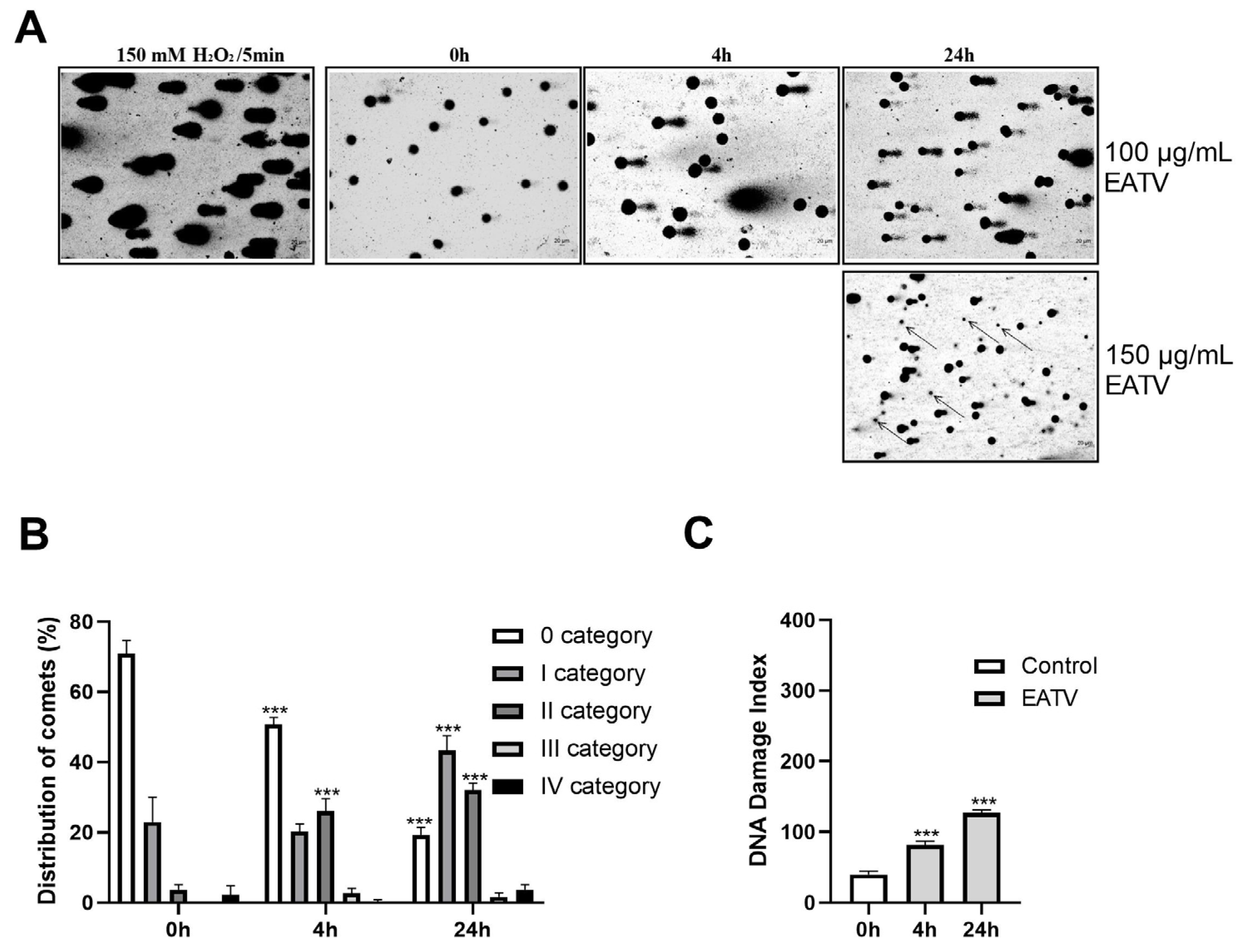
3.5. Comet Assay
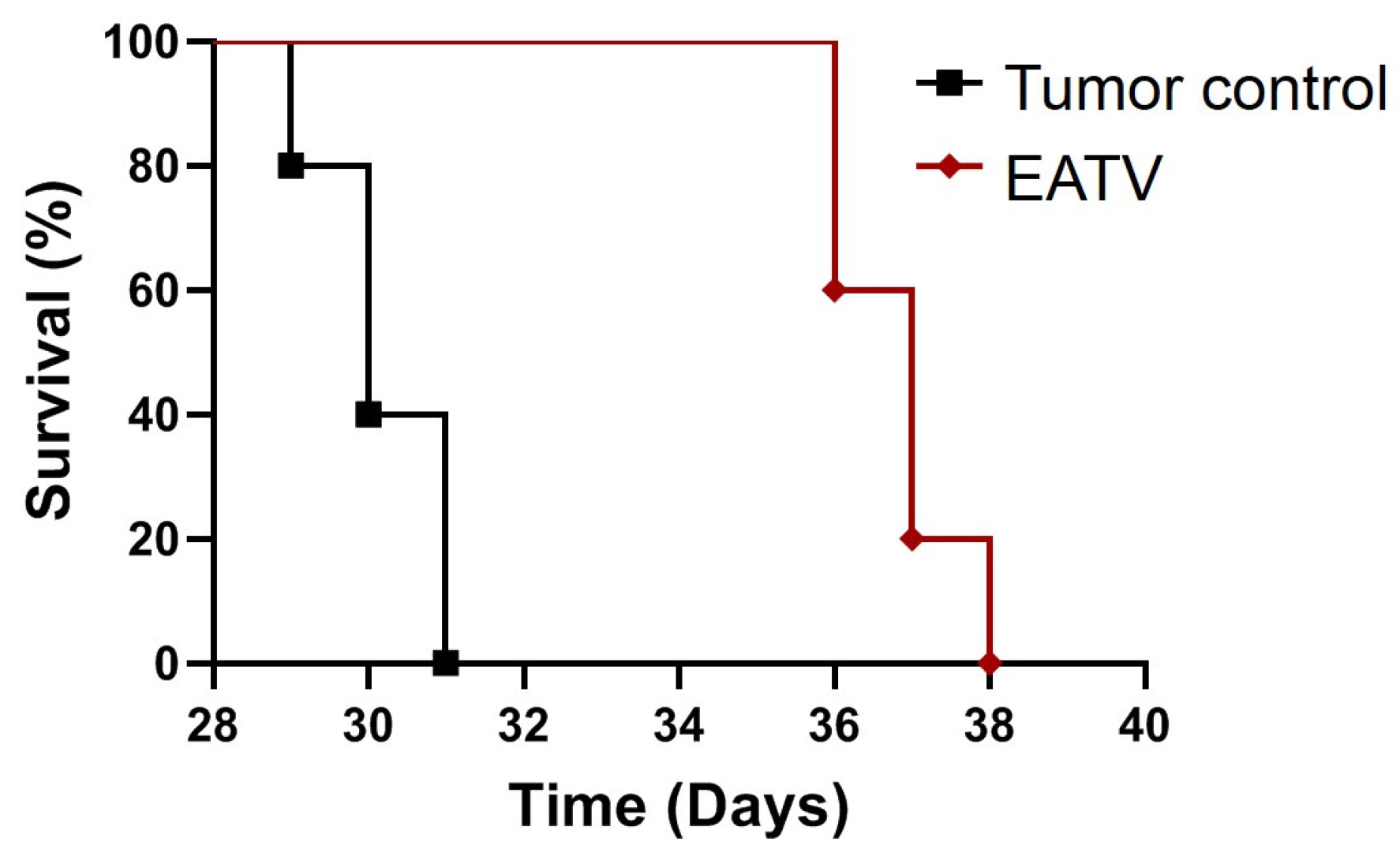
3.6. In Vivo Anticancer Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radulović, N.S.; Genčić, M.S.; Stojanović, N.M.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Stojiljković, N.I. Toxic essential oils. Part V: Behaviour modulating and toxic properties of thujones and thujone-containing essential oils of Salvia officinalis L., Artemisia absinthium L., Thuja occidentalis L. and Tanacetum vulgare L. Food Chem. Toxicol. 2017, 105, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, S.; Israili, Z.H.; Lyoussi, B. Acute and chronic toxicity of a lyophilised aqueous extract of Tanacetum vulgare leaves in rodents. J. Ethnopharmacol. 2008, 117, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Khatib, S.; Sobeh, M.; Faraloni, C.; Bouissane, L. Tanacetum species: Bridging empirical knowledge, phytochemistry, nutritional value, health benefits and clinical evidence. Front. Pharmacol. 2023, 14, 1169629. [Google Scholar]
- Vasileva, A.M.; Iliev, I.A.; Lozanov, V.S.; Dimitrova, M.B.; Mitev, V.I.; Ivanov, I.P. In vitro study on the antitumor activity of Tanacetum vulgare L. extracts. Bulg. Chem. Commun. 2019, 51, 249–255. [Google Scholar]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative studies on the antimicrobial and cytotoxic activities of Tanacetum vulgare L. essential oil and methanol extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar]
- Ivanescu, B.; Tuchilus, C.; Corciova, A.; Lungu, C.; Cosmin, T.M.; Gheldiu, A.-M.; Laurian, V. Antionidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Babich, O.; Larina, V.; Krol, O.; Ulrikh, E.; Sukhikh, S.; Gureev, M.A.; Prosekov, A.; Ivanova, S. In vitro study of biological activity of Tanacetum vulgare extracts. Pharmaceutics 2023, 15, 616. [Google Scholar] [CrossRef]
- Karimian, H.; Mohan, S.; Moghadamtousi, S.Z.; Fadaeinasab, M.; Razavi, M.; Arya, A.; Kamalidehghan, B.; Ali, H.M.; Noordin, M.I. Tanacetum polycephalum (L.) Schultz-Bip. induces mitochondrial-mediated apoptosis and inhibits migration and invasion in MCF7 cells. Molecules 2014, 19, 9478–9501. [Google Scholar] [CrossRef]
- Sinha, S.; Amin, H.; Nayak, D.; Bhatnagar, M.; Kacker, P.; Chakraborty, S.; Kitchlu, S.; Vishwakarma, R.; Goswami, A.; Ghosal, S. Assessment of microtubule depolymerization property of flavonoids isolated from Tanacetum gracile in breast cancer cells by biochemical and molecular docking approach. Chem.-Biol. Interact. 2015, 239, 1–11. [Google Scholar]
- Sulikovska, I.; Djeliova, V.; Kirazov, L.; Ivanov, I.; Dimitrova, M. Evaluation of different staining methods and image analyses of the results from a comet assay in human colorectal cancer cells treated with hydrogen peroxide. Acta Morphol. Anthropol. 2023, 30, 5–13. [Google Scholar] [CrossRef]
- Noroozi, M.; Angerson, W.J.; Lean, M.E.J. Effects of flavonoids and Vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutr. 1998, 67, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Azqueta, A.; Langie, S.A.S.; Boutet-Robinet, E.; Duthie, S.; Ladeira, C.; Møller, P.; Collins, A.R.; Godschalk, R.W.L. DNA repair as a human biomonitoring tool: Comet assay approaches. Mutat. Res. Rev. Mutat. Res. 2019, 781, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Niehans, G.A.; Kratzke, R.A.; Froberg, M.K.; Aeppli, D.M.; Nguyen, P.L.; Geradts, J. G1 checkpoint protein and p53 abnormalities occur in most invasive transitional cell carcinomas of the urinary bladder. Br. J. Cancer 1999, 80, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating in vitro DNA damage using comet assay. J. Vis. Exp. 2017, 128, e56450. [Google Scholar]
- Singh, N.P.; Mccoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Aldubayan, M.A.; Elgharabawy, R.M.; Ahmed, A.S.; Tousson, E. Antineoplastic activity and curative role of avenanthramides against the growth of Ehrlich solid tumors in mice. Oxidative Med. Cell Longev. 2019, 13, 5162687. [Google Scholar] [CrossRef]
- Ali, A.D.; Badr El-Din, K.N.; Abou-El-Magd, R.F. Antioxidant and hepatoprotective activities of grape seeds and skin against Ehrlich solid tumor induced oxidative stress in mice. Egypt. J. Basic Appl. Sci. 2015, 2, 98–109. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Jarrar, B.M. Gold nanoparticles induced cloudy swelling to hydropic degeneration, cytoplasmic hyaline vacuolation, polymorphism, binucleation, karyopyknosis, karyolysis, karyorrhexis and necrosis in the liver. Lipids Health Dis. 2011, 10, 166. [Google Scholar] [CrossRef]
- Yee, P.P.; Li, W. Tumor necrosis: A synergistic consequence of metabolic stress and inflammation. Bioessays 2021, 43, 2100029. [Google Scholar] [CrossRef]
- Kitamura, H.; Kodama, F.; Odagiri, S.; Nagahara, N.; Inoue, T.; Kanisawa, M. Granulocytosis associated with malignant neoplasms: A clinicopathologic study and demonstration of colony-stimulating activity in tumor extracts. Hum. Pathol. 1989, 20, 878–885. [Google Scholar] [CrossRef]
- Hocking, W.; Goodman, J.; Golde, D. Granulocytosis associated with tumor cell production of colony-stimulating activity. Blood 1983, 61, 600–603. [Google Scholar]
- Kolb, R.; Zhang, W. Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue. Cancers 2020, 12, 1686. [Google Scholar] [CrossRef]





| № | RT (min) | [M-H]− m/z Error (ppm) | Molecular Formula | MS2 Fragments m/z, (R.I., %) | Proposed Compound |
|---|---|---|---|---|---|
| Benzoic acid derivative | |||||
| 1 | 24.40 | 419.0975 839.2029 a (−1.59) | C20H20O10 | 153.0177 (10), 152.0099 (61), 121.0278 (5), 109.0277 (19), 108.0199 (100) | Dihydroxybenzoic acid (O-Benzoyl)glucoside (I) |
| 2 | 26.13 | 419.0975 839.2029 a (−1.59) | C20H20O10 | 153.0177 (70), 152.0099 (23), 121.0278 (15), 108.0199 (15), 109.0278 (100), 108.0199 (15) | Dihydroxybenzoic acid (O-Benzoyl)glucoside (II) |
| Hydroxycinnamoyl quinic acids | |||||
| 3 | 7.60 | 353.0870 (−2.16) | C16H18O9 | 191.0548 (67), 179.0336 (15), 135.0435 (100) | 3-caffeoylquinic acid |
| 4 | 11.24 | 353.0870 707.1820 a (−2.20) | C16H18O9 | 191.0548 (100) | 5-caffeoylquinic acid |
| 5 | 13.70 | 337.0925 (−1.23) | C16H18O8 | 191.0549 (100), 163.0386 (11), 119.0486 (34) | p-Coumaroylquinic acid |
| 6 | 14.96 | 367.1024 (−2.90) | C17H20O9 | 191.0548 (100), 173.0442 (6), 134.0357 (31), 11.0435 (6), 93.0329 (49) | 5-Feruloylquinic acid |
| 7 | 20.16 | 515.1185 1031.2456 a (−2.13) | C25H24O12 | 353.0873 (16), 335.0770 (5), 191.0548 (85), 179.0336 (100), 173.0442 (82), 161.0229 (26), 155.0334 (10), 135.0435 (40) | 3,4-dicaffeoylquinic acid |
| 8 | 20.38 | 515.1186 1031.2447 a (−2.64) | C25H24O12 | 353.0872 (62), 191.0548 (100), 179.0336 (56), 161.0229 (5), 135.0435 (21) | 3,5-dicaffeoylquinic acid |
| 9 | 20.62 | 515.1183 1031.2448 a (−2.34) | C25H24O12 | 353.0872 (23), 191.0548 (100), 179.0336 (22), 161.0229 (5), 135.0435 (8) | 1,5-dicaffeoylquinic acid |
| 10 | 22.29 | 515.1182 1031.2447 a (−2.23) | C25H24O12 | 353.0874 (62), 191.0549 (36), 179.0337 (73), 173.0442 (100), 161.0230 (7), 155.0335 (6), 135.0436 (24) | 4,5-dicaffeoylquinic acid |
| 11 | 23.04 | 549.1970 (−1.38) | C27H34O12 | 387.1651 (52), 207.1015 (18), 161.0229 (100) | O-(O-Caffeoyl)glucosyl [(hydroxypentenyl)-3-oxo-cyclopentyl]acetic acid |
| 12 | 27.93 | 677.1503 (−1.27) | C34H30O15 | 515.1187 (27), 353.0873 (87), 335.0768 (18), 191.0548 (66), 179.0336 (80), 173.0441 (80), 161.0229 (100), 135.0435 (44), 111.0435 (5.0), 93.0329 (11), 85.0278 (13) | 3,4,5-tricaffeoylquinic acid |
| Flavonoids | |||||
| 13 | 25.75 | 285.0397 (−2.10) | C15H10O6 | 151.0021 (10), 133.0279 (100), 107.0122 (12) | Luteolin |
| 14 | 25.77 | 345.0610 (−1.67) | C17H14O8 | 330.0379 (34), 315.0144 (68), 287.0194 (100), 149.0229 (52) | Quercetagetin 3,6-dimethyl ether |
| 15 | 26.84 | 315.0507 (−1.19) | C16H12O7 | 300.0268 (16), 272.0276 (13), 271.0243 (83), 255.0292 (32), 243.0291 (24), 227.0341 (6), 173.0441 (100), 148.0150 (6) | Nepetin |
| 16 | 28.60 | 301.0712 (−1.66) | C16H14O6 | 286.0473 (8), 285.0399 (15), 214.0628 (5), 213.0547 (6), 201.0183 (12), 199.0388 (15), 173.0441 (16), 164.0099 (54), 161.0228 (81), 151.0020 (55), 136.0149 (79), 135.0435 (39), 134.0356 (51), 125.0227 (10), 108.0199 (100), 107.0121 (47) | Hesperetin |
| 17 | 28.84 | 269.0452 (−1.40) | C15H10O5 | 151.0021 (9), 149.0228 (8), 117.0329 (100), 107.0121 (14), 83.0121 (7) | Apigenin |
| 18 | 29.59 | 299.0555 (−1.81) | C16H12O6 | 285.0358 (11), 284.0321 (66), 256.0370 (100), 255.0292 (16), 227.0341 (23), 211.0389 (7), 199.0388 (7), 183.0438 (5), 151.0020 (36), 125.0227 (5) | Chrysoeriol |
| 19 | 29.69 | 315.0506 (−1.35) | C16H12O7 | 301.0262 (8), 300.0268 (54), 283.0241 (25), 271.0242 (40), 255.0262 (34), 227.0340 (35), 199.0388 (11), 172.0516 (5), 151.0021 (100), 149.9942 (10), 148.0150 (37), 137.0228 (48), 135.0070 (12), 124.0148 (11), 120.0200 (6), 108.2000 (28), 107.0121 (77) | Isorhamnetin |
| 20 | 29.82 | 299.0555 (−1.81) | C16H12O6 | 285.0358 (17), 284.0321 (100), 256.0370 (26), 255.0292 (17), 227.0341 (26), 211.0389 (11), 199.0388 (10), 187.0387 (6), 183.0438 (7), 151.0021 (41), 135.0435 (5), 134.0356 (11), 133.0278 (13), 107.0121 (32), 83.0121 (17) | Diosmetin |
| 21 | 29.98 | 329.0662 (−1.38) | C17H14O7 | 314.0425 (6), 299.01917 (27), 271.0243 (100), 243.0291 (8), 227.0341 (7), 199.0388 (11), 161.0228 (11), 136.9857 (6), 133.0273 (7) | Cirsiliol |
| 22 | 31.38 | 359.0766 (−1.87) | C18H16O8 | 344.0532 (7), 329.0298 (24), 314.0063 (18), 301.0348 (34), 287.0147 (15), 286.0114 (100), 258.0163 (58), 230.0212 (20), 214.0261 (5), 202.0259 (15), 165.9894 (12), 164.9814 (5), 163.0386 (5) | Quercetagetin-3,6,3′(4′)- trimethyl ether |
| Flavonoid-O-glucuronides | |||||
| 23 | 17.94 | 463.0875 927.1862 a (−1.47) | C21H20O12 | 287.0554 (14), 151.0020 (100), 135.0434 (43), 113.0227 (10) | Eriodictyol-O-glucoronide |
| 24 | 18.37 | 477.0668 (−2.15) | C21H18O13 | 301.0347 (100), 178.9972 (5), 151.0020 (18) | Quercetin-3-O-glucuronide (Miquelianin) |
| 25 | 19.31 | 461.0717 (−1.86) | C21H18O12 | 285.0397 (100), 284.0321(32) | Luteolin-7-O-glucuronide |
| 26 | 21.66 | 445.0768 (−1.81) | C21H18O11 | 269.0449 (100), 175.0234 (7), 113.0227 (22) | Apigenin-7-O-glucuronide |
| 27 | 22.23 | 477.1028 (−2.17) | C22H22O12 | 301.0711 (100), 286.0476 (25), 242.0575 (15), 199.0547 (10), 151.0020 (11), 113.0227 (60) | Homoeriodictyol-O-glucuronide |
| 28 | 22.80 | 475.0873 951.1835 a (−1.81) | C22H20O12 | 299.0553 (88), 284.0319 (100), 175.0230 (27), 113.0227 (60) | Diosmetin-7-O-glucuronide |
| 29 | 24.02 | 505.0982 1011.2038 a (−1.04) | C23H22O13 | 329.0661 (76), 314.0428 (58), 299.0191 (100), 271.0243 (5), 175.0234 (18), 168.0049 (25), 161.0228 (25), 151.0022 (7), 137.0227 (21), 125.0227 (10), 113.0229 (52) | Tricin-7-O-glucuronide |
| 30 | 24.62 | 519.1135 (−1.70) | C24H24O13 | 301.0710 (100), 286.0476 (16), 242.05756 (9), 152.0100 (8), 113.0227 (42), 99.0070 (8), 95.0121 (12) | Hesperetin-O-(O-acetyl)glucuronide or Homoeriodictyol-O-(O-acetyl)glucuronide (Isomer I) |
| 31 | 25.28 | 519.1133 (−2.18) | C24H24O13 | 301.0710 (100), 286.0476 (12), 242.05756 (9), 217.0343 (5), 151.0021 (7), 113.0227 (6), 99.0070 (12) | Hesperetin-O-(O-acetyl)glucuronide or Homoeriodictyol-O-(O-acetyl)glucuronide (Isomer II) |
| Flavonoid-O-glucosides | |||||
| 32 | 18.36 | 463.0878 927.1828 a (−0.86) | C21H20O12 | 301.0328 (31), 300.0269 (100), 285.0398 (13), 284.0322 (5) | Quercetin-3-O-glucoside |
| 33 | 19.32 | 447.0925 (−1.73) | C21H20O11 | 285.0397 (100), 284.0321(32) | Luteolin-7-O-glucoside |
| 34 | 21.48 | 505.0982 (−1.04) | C23H22O13 | 317.1651 (9), 287.0556 (24), 268.0371 (16), 161.0229 (18), 151.0021 (100), 135.0435 (40) | Myricetin-3-O-(O-acetyl)rhamnoside |
| 35 | 25.14 | 609.1242 (−1.26) | C30H26O14 | 447.0926 (5), 323.0767 (7), 285.0398 (100), 284.022(39), 179.0336 (26), 161.0229 (59), 151.0020 (6), 135.0435 (14), 133.0278, 109.0277 (8) | Kaempferol-O-(O-caffeoyl)glucoside or Luteolin-O-(O-caffeoyl)glucoside |
| Organic acid | |||||
| 36 | 32.76 | 329.2328 (−1.51) | C18H34O5 | 229.1436 (23), 211.1332 (100), 209.1171 (14), 183.1377 (40), 171.1012 (89), 165.1270 (11), 139.1112 (43), 137.0956 (10), 127.0762 (37) | 9,12,13-trihydroxyoctadec-10-enoic acid |
| Unknown compounds | |||||
| 37 | 12.45 | 303.1080 (−1.66) | C13H20O8 | 201.0393 (27), 101.0591 (100), 87.0070 (71) | Unknown |
| 38 | 12.57 | 303.1080 (−1.66) | C13H20O8 | 201.0393 (43), 101.0591 (100), 87.0070 (89) | Unknown |
| 39 | 14.42 | 323.0766 647.1610 a (−1.89) | C15H16O8 | 201.0393 (100), 121.0277 (83), 87.0070 (78) | Unknown |
| 40 | 18.81 | 281.1391 (−1.59) | C15H22O5 | 219.1381 (7), 209.0792 (10), 201.1272 (100), 199.1116 (19), 185.0959 (60), 165.0905 (30), 157.0643 (11) | Unknown |
| 41 | 19.52 | 281.1391 (−1.59) | C15H22O5 | 263.1285 (15), 219.1091 (20), 201.1272 (100) | Unidentified Eudesmanolide |
| 42 | 23.29 | 399.1289 (−1.98) | C18H24O10 | 367.1025 (11), 315.0501 (13), 314.0427 (16), 300.0263 (7), 299,0192 (13), 193.0493 (55), 152.0099 (66), 109.0277 (31), 108.0199 (100) | Unknown |
| 43 | 28.04 | 503.2491 (−1.44) | C24H40O11 | 457.2438 (67), 415.2330 (25), 397.2222 (100), 379.2120 (5), 327.1809 (8), 291.1271 (5) | Unknown |
| 44 | 30.68 | 531.2503 (−1.40) | C26H44O11 | 329.2330 (53), 315.1809 (14), 229.1436 (22), 211.1330 (30), 201.0393 (100), 101.0590 (5), 87.0070 (53) | Unknown |
| 45 | 33.05 | 329.2328 (−1.51) | C18H34O5 | 221.1174 (18), 145.0279 (100), 119.0486 (55) | Unknown |
| Group | Necrosis | Mitoses | Tumor Macrophages | Lymphocytes and Neutrofils Infiltration | Neovascularisation | Fibrosis |
|---|---|---|---|---|---|---|
| Tumor control | 10% | 10% | + | ++ | + | − |
| EATV | 30–50% | 5% | + | +++ | + | − |
| 5-FU | 65–75% | 0% | + | ++ | − | − |
| Treatment | Parameters | ||||||
|---|---|---|---|---|---|---|---|
| WBC (109/L) | RBC (1012/L) | HGB (g/L) | PLT (109/L) | Differential Count (%) | |||
| Lym. | Mon. | Gran. | |||||
| Referent values | 0.8–6.8 | 6.36–9.42 | 110–143 | 45–1590 | 55.8–90.6 | 1.8–6.0 | 8.6–38.9 |
| Control | 4.5 ± 0.53 | 8.09 ± 0.37 | 122.3 ± 6.4 | 1167 ± 35.5 | 65.9 ± 4.8 | 5 ± 0.3 | 29.1 ± 4.6 |
| Tumor control | 19.4 ± 0.1 | 8.64 ± 0.7 | 117 ±1.03 | 1060 ± 6.7 | 50.1 ± 0.7 | 5.8 ± 0.7 | 44.1 ± 0.2 |
| EATV | 18.2 ±0.3 | 7.51 ± 0.5 | 98 ± 0.9 | 1467 ± 2 | 16.7 ± 0.4 | 5 ± 0.2 | 78.3 ± 0.8 |
| 5-FU | 27.8 ± 2.29 | 7.97 ± 1.63 | 110 ± 9 | 1548 ± 28.7 | 18.9 ± 6.2 | 3 ± 1.5 | 78.1 ± 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulikovska, I.; Georgieva, A.; Djeliova, V.; Todorova, K.; Vasileva, A.; Ivanov, I.; Dimitrova, M. Anticancer Activity of Ethyl Acetate/Water Fraction from Tanacetum vulgare L. Leaf and Flower Extract. Curr. Issues Mol. Biol. 2025, 47, 215. https://doi.org/10.3390/cimb47040215
Sulikovska I, Georgieva A, Djeliova V, Todorova K, Vasileva A, Ivanov I, Dimitrova M. Anticancer Activity of Ethyl Acetate/Water Fraction from Tanacetum vulgare L. Leaf and Flower Extract. Current Issues in Molecular Biology. 2025; 47(4):215. https://doi.org/10.3390/cimb47040215
Chicago/Turabian StyleSulikovska, Inna, Ani Georgieva, Vera Djeliova, Katerina Todorova, Anelia Vasileva, Ivaylo Ivanov, and Mashenka Dimitrova. 2025. "Anticancer Activity of Ethyl Acetate/Water Fraction from Tanacetum vulgare L. Leaf and Flower Extract" Current Issues in Molecular Biology 47, no. 4: 215. https://doi.org/10.3390/cimb47040215
APA StyleSulikovska, I., Georgieva, A., Djeliova, V., Todorova, K., Vasileva, A., Ivanov, I., & Dimitrova, M. (2025). Anticancer Activity of Ethyl Acetate/Water Fraction from Tanacetum vulgare L. Leaf and Flower Extract. Current Issues in Molecular Biology, 47(4), 215. https://doi.org/10.3390/cimb47040215






