Phosphodiesterase Inhibition and Immunotropic Activity of Dipyridamole Dynamic Derivatives
Abstract
1. Introduction
2. Materials and Methods
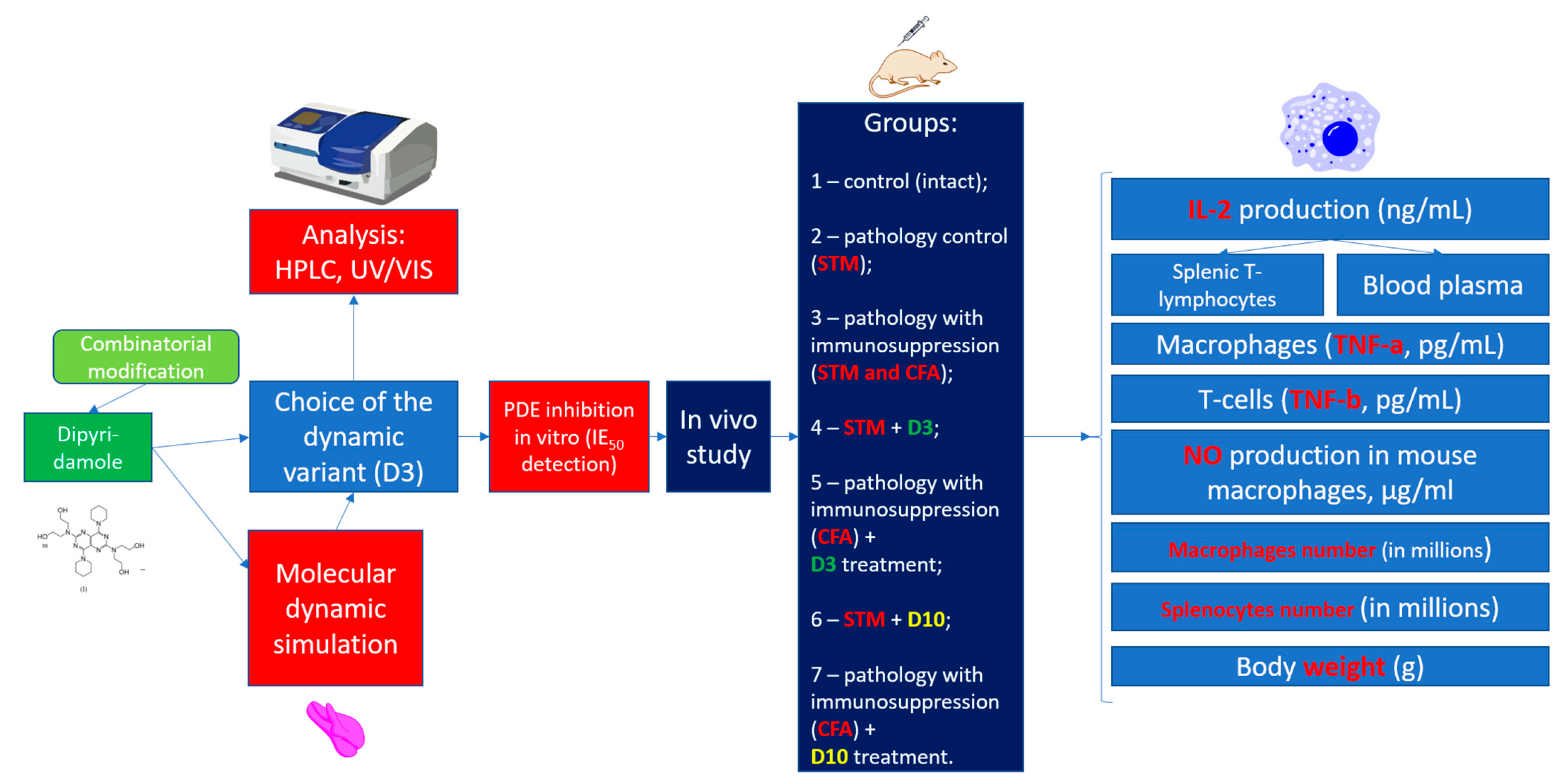
2.1. Planning (Methodology) of Research
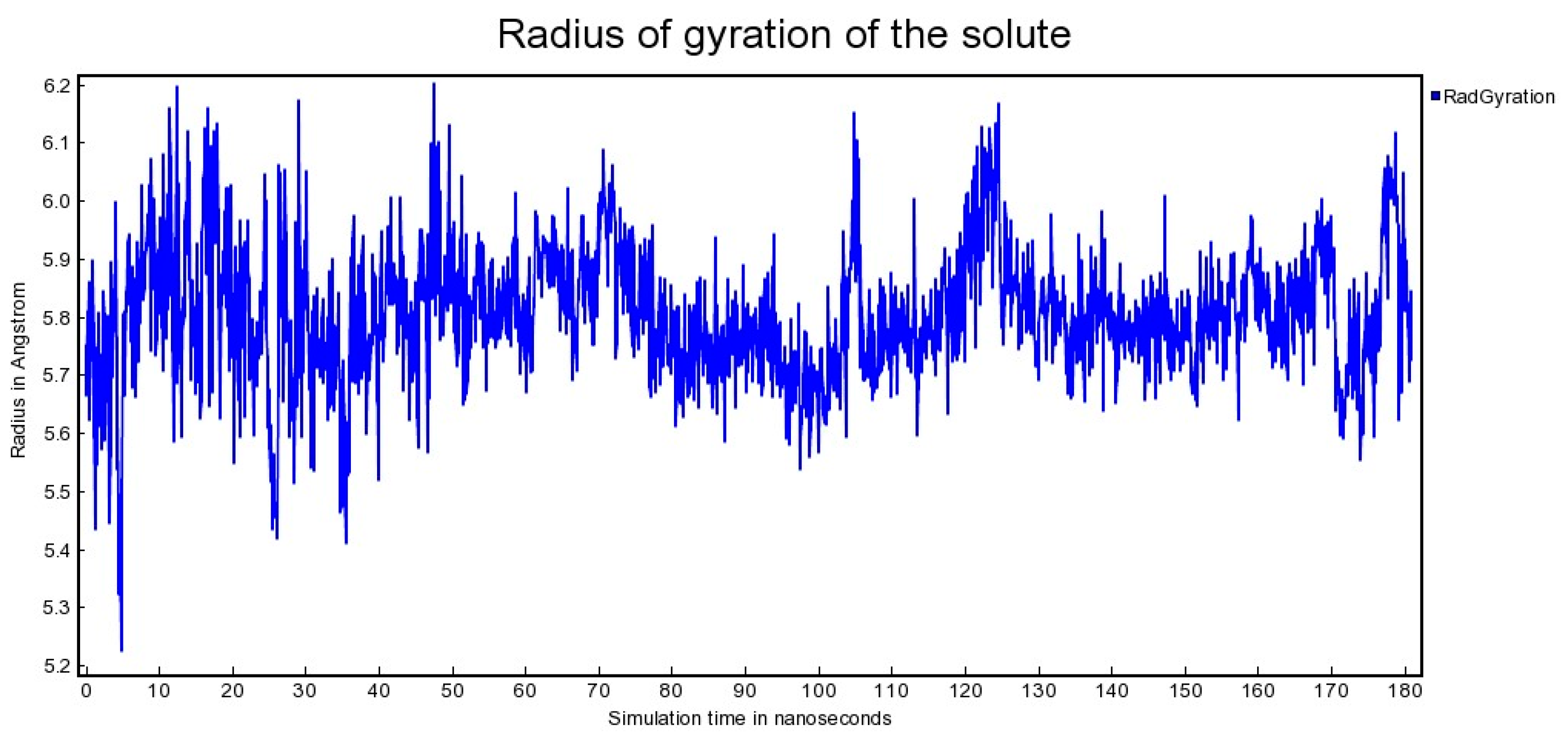
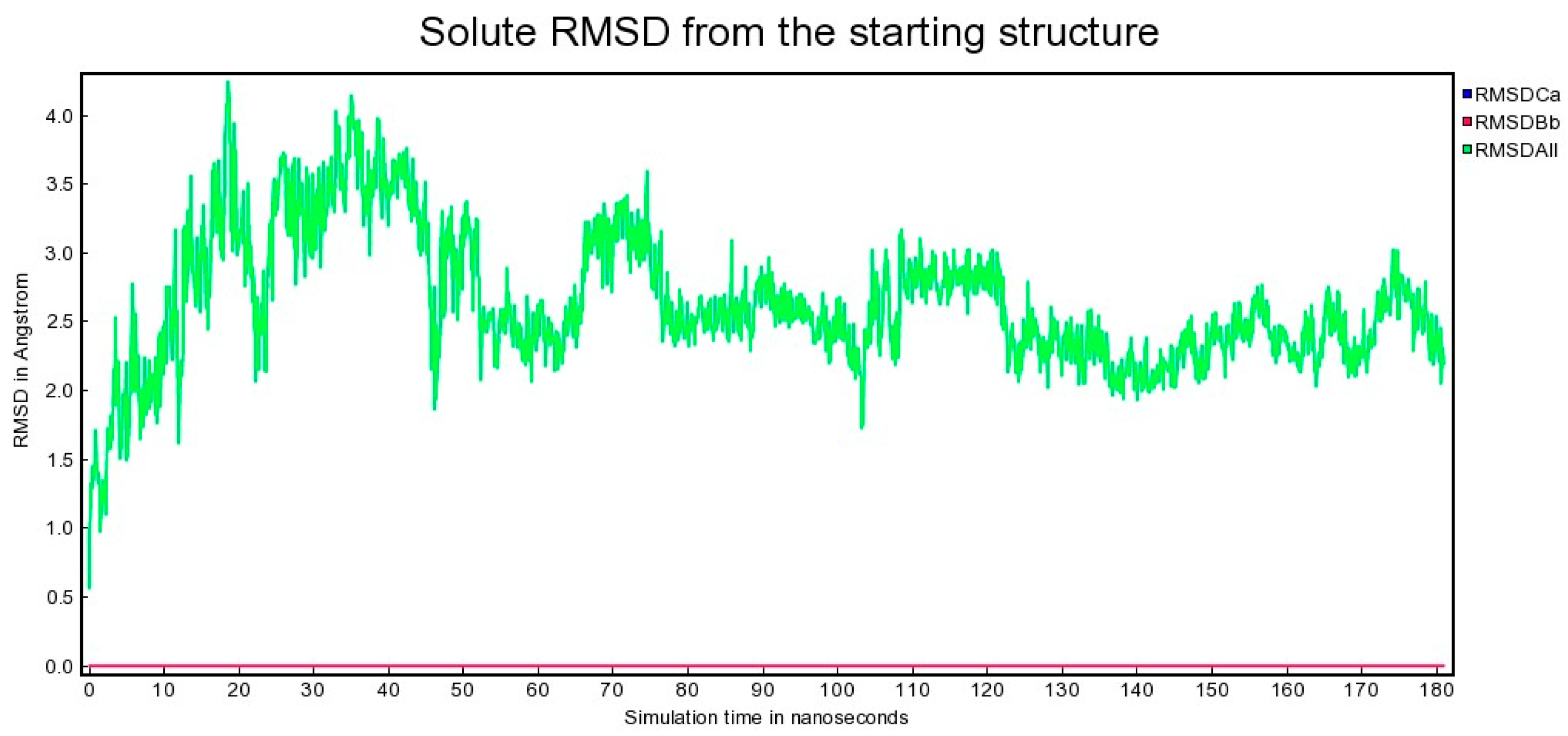
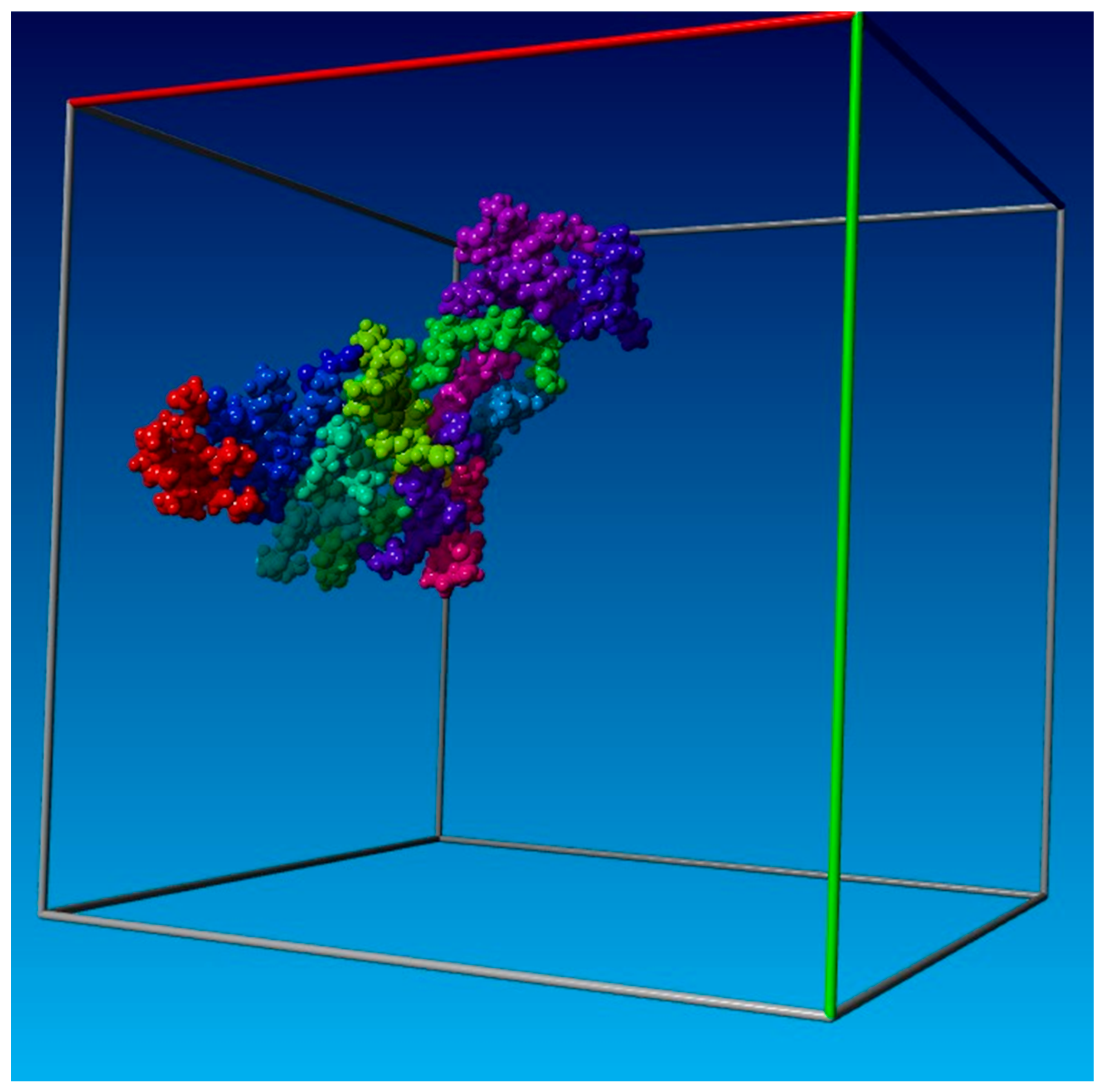
2.2. Molecular Dynamics
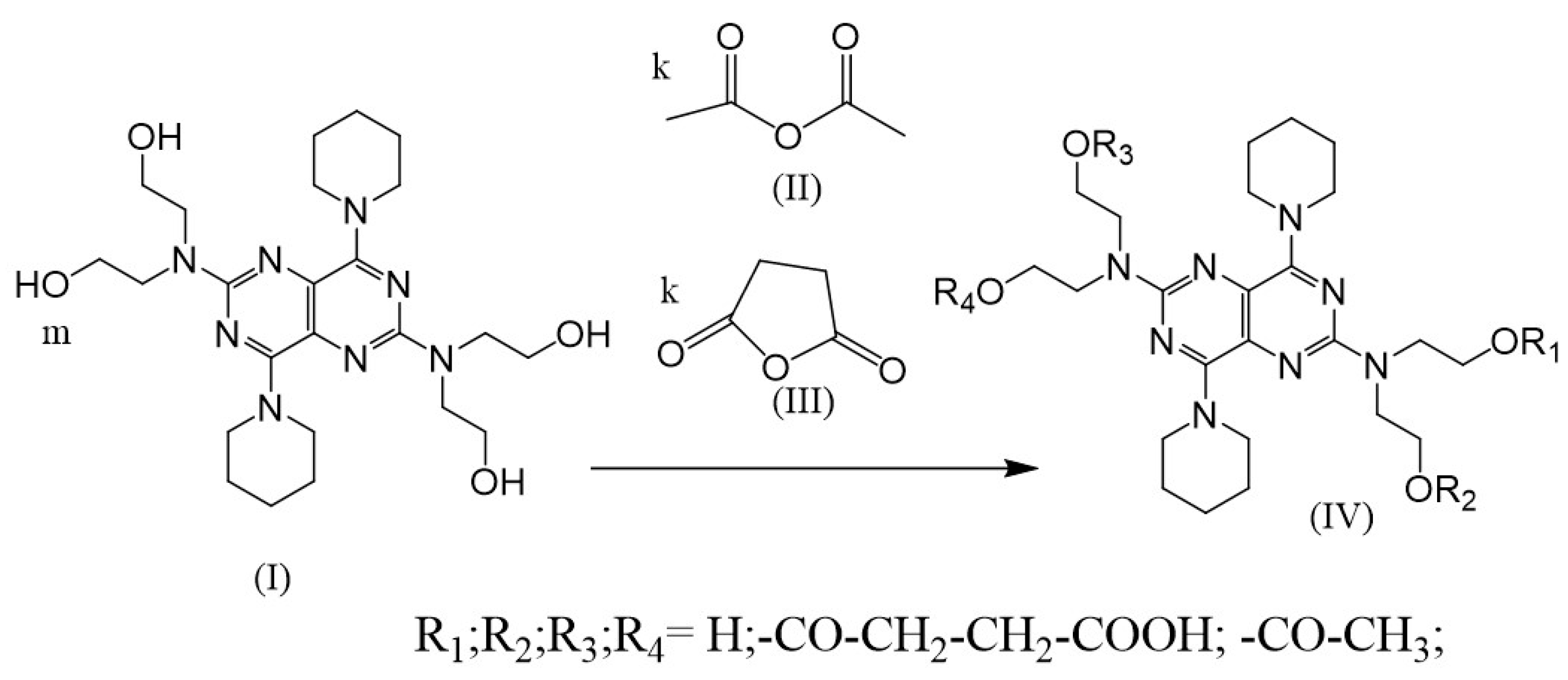
2.3. Synthesis of (IV)
2.4. Detection of Derivative (IV) Effect on Activity of PDE
2.5. Animals, Diarrhea, and Immunodeficiency
- Control (intact);
- Mice with diarrhea caused by STM;
- Mice with diarrhea caused by STM and immunosuppression caused by CFA;
- Mice with diarrhea caused by STM and treated by D3;
- Mice with diarrhea caused by STM and immunosuppression caused by CFA and treated by D3;
- Mice with diarrhea caused by STM and treated by D10;
- Mice with diarrhea caused by STM and immunosuppression caused by CFA and treated by D10.
Immunological Methods
2.6. Preparation of Peritoneal Macrophages
2.7. Preparation of Splenic T Lymphocytes
2.8. IL-2 Assay
2.9. Statistical Analysis
3. Results
3.1. Molecular Dynamics
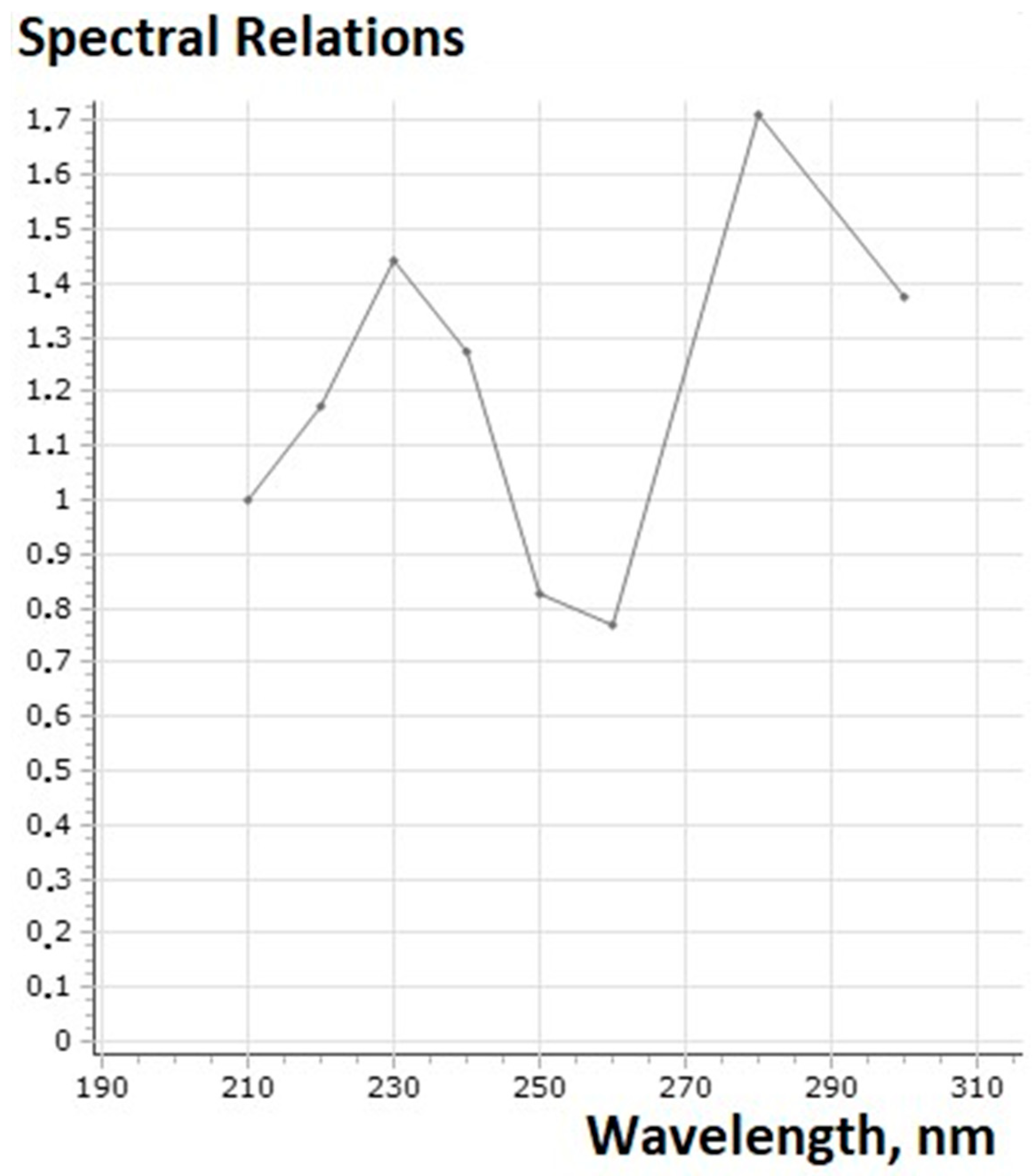
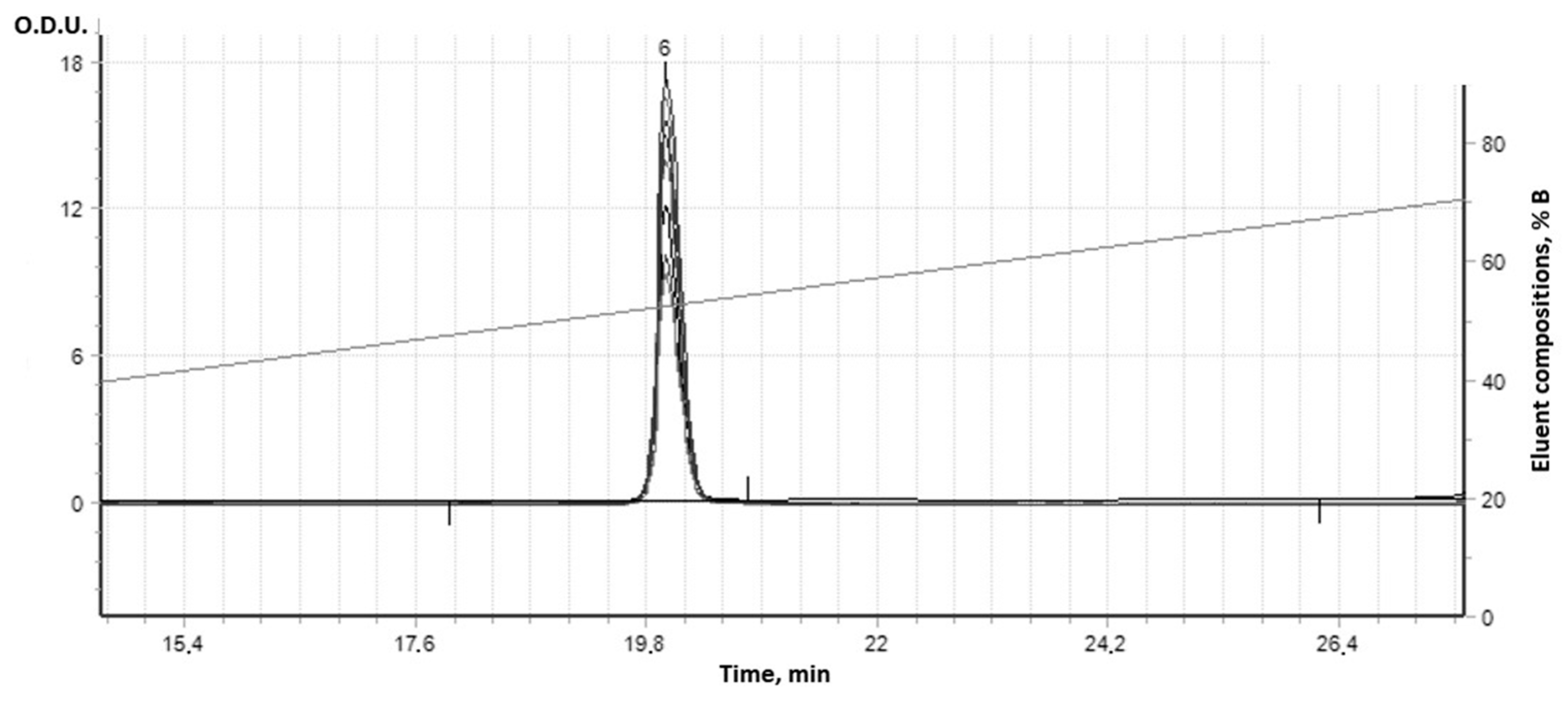
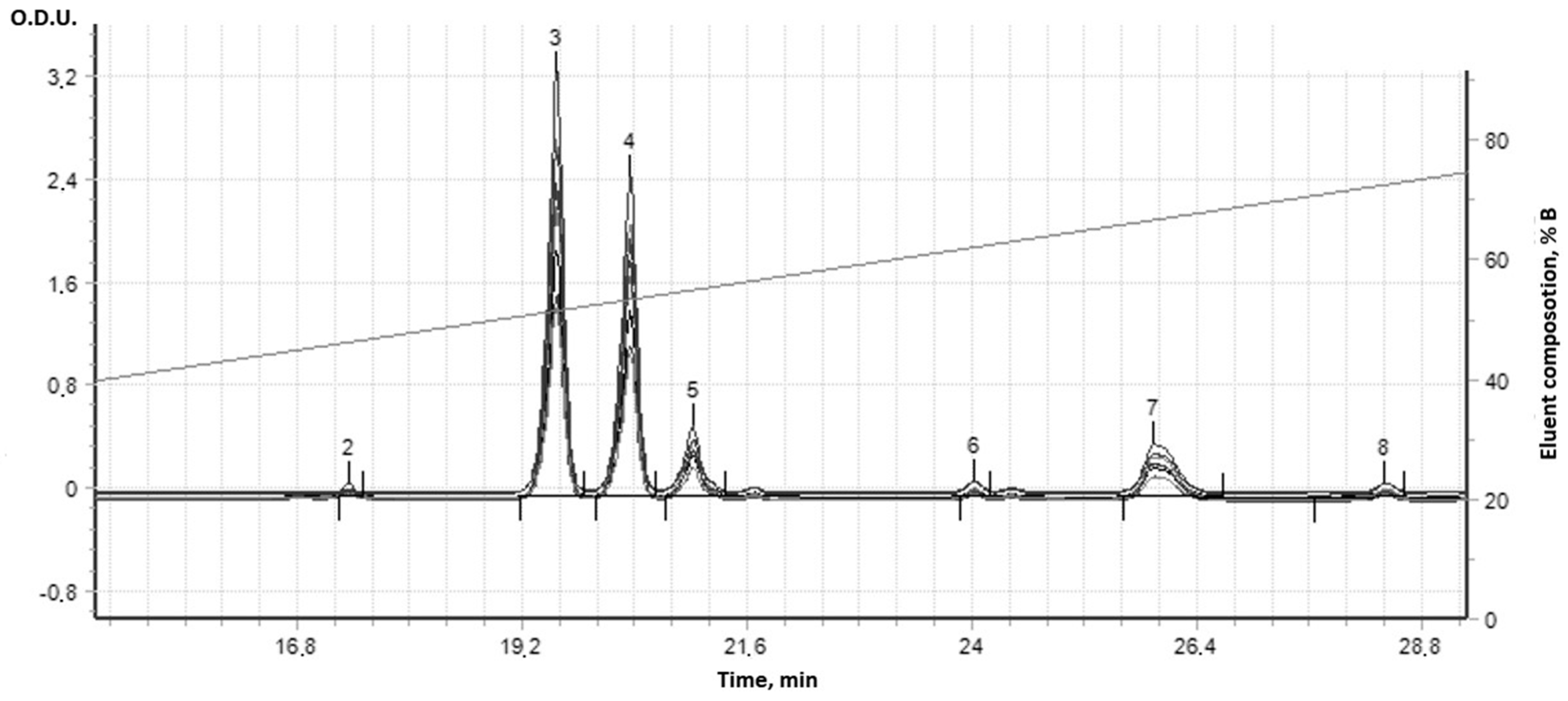
3.2. Synthesis and Analysis of Dynamic Dipyridamole
3.3. Study of the Derivative’s Phosphodiesterase Inhibition Ability
| Item No. | Reagent Molecular Ratio * | IC50 **1, µM | ||
|---|---|---|---|---|
| m | k1 | k2 | ||
| 1 | 18 | 100 *** | 100 *** | >800 |
| 2 | -//- | 50 | 50 | 160 |
| 3 (D3) | -//- | 25 | 25 | 0.05 |
| 4 | -//- | 15 | 15 | 40 |
| 5 | -//- | 7 | 7 | 40 |
| 6 | -//- | 3 | 3 | 20 |
| 7 | -//- | 1 | 1 | 20 |
| 8 | -//- | 0.5 | 0.5 | 10 |
| 9 | -//- | 0.25 | 0.25 | 10 |
| 10 (D10) | - | 0 | 0 | 5 |
| 11 | Control from Kit 3-isobutyl-1-methylxanthine (IBMX) | 5 | ||
3.4. In Vivo Results
3.5. The Effect of D3 and D10 (See Table 2) on Mice Immunity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horcajada, J.P.; Aldonza, R.; Real, M.; Castañeda-Espinosa, S.; Sendra, E.; Gomez-Junyent, J.; López-Montesinos, I.; Gómez-Zorrilla, S.; Briansó, S.; Duran-Taberna, M. Safety and Efficacy of Favipiravir in COVID-19 Patients with Pneumonia. A Randomized, Double-Blind, Placebo-Controlled Study (FAVID). Pneumonia 2024, 16, 3. [Google Scholar] [CrossRef]
- Buraczynska, M.; Zukowski, P.; Buraczynska, K.; Mozul, S.; Ksiazek, A. Renalase Gene Polymorphisms in Patients with Type 2 Diabetes, Hypertension and Stroke. Neuromolecular Med. 2011, 13, 321–327. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X.; Wan, L.; Fu, J.; Huo, Y.; Zhao, Y.; Guo, C. Gene Polymorphisms Affecting the Pharmacokinetics and Pharmacodynamics of Donepezil Efficacy. Front. Pharmacol. 2020, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ballesteros, A.I.; Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Parra-Rojas, I.; de la Cruz-Mosso, U. Association of Vitamin D Metabolism Gene Polymorphisms with Autoimmunity: Evidence in Population Genetic Studies. Int. J. Mol. Sci. 2020, 21, 9626. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kong, X.; Jiang, Y.; Zhao, M.; Gao, P.; Cong, X.; Cao, Y.; Ma, L. Association of AGTR1 A1166C and CYP2C9∗ 3 Gene Polymorphisms with the Antihypertensive Effect of Valsartan. Int. J. Hypertens. 2022, 2022, 7677252. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Pharmacogenomics of Hypertension Treatment. Int. J. Mol. Sci. 2020, 21, 4709. [Google Scholar] [CrossRef] [PubMed]
- Martynov, A.V.; Bomko, T.V.; Nosalskaya, T.N.; Farber, B.S.; Farber, S.B. Oral Long-Acting Pharmaceutical Form of Insulin on the Basis of Self-Organizing Kvasi-Living System of Combinatorial Peptides. Ann. Mechnikov Inst. 2012, 2, 64–70. [Google Scholar]
- Martynov, A.V.; Smelyanskaya, M.V. Antiproliferative Properties of Chemically Modified Recombinant IFN-A2b. J. Interferon Cytokine Res. 2005, 25, 414–417. [Google Scholar] [CrossRef]
- Martynov, A.V.; Farber, B.S.; Farber, S.B.; Kabluchko, T.V. Synthesis of the Ensembles from Succinylated Interleukin-2 Derivatives and Their Biological Activity in Vitro. ScienceRise 2015, 11, 25–30. [Google Scholar] [CrossRef]
- Martynov, A.; Farber, B.; Farber, S. Quasi-Life Self-Organizing Systems: Based on Ensembles of Succinylated Derivatives of Interferon-Gamma. Curr. Med. Chem. 2011, 18, 3431–3436. [Google Scholar] [CrossRef]
- Livotov, P.; Chandra Sekaran, A.P.; Law, R.; udah, M.; Reay, D. Systematic Innovation in Process Engineering: Linking TRIZ and Process Intensification. In Advances in Systematic Creativity: Creating and Managing Innovations; Palgrave Macmillan: Cham, Switzerland, 2019; pp. 27–44. [Google Scholar] [CrossRef]
- Ghosh, R.; Sawant, O.; Ganpathy, P.; Pitre, S.; Kadam, V.J. Phosphodiesterase Inhibitors: Their Role and Implications. Int. J. PharmTech Res. 2009, 1, 1148–1160. [Google Scholar]
- Sharma, A.; Singh Shergill, G.; Gupta, M. A Review on the Therapeutic Potential of Dipyridamole in the Treatment of Covid-19. Plant Arch. 2021, 21, 2072–2077. [Google Scholar] [CrossRef]
- Allahham, M.; Lerman, A.; Atar, D.; Birnbaum, Y. Why Not Dipyridamole: A Review of Current Guidelines and Re-Evaluation of Utility in the Modern Era. Cardiovasc. Drugs Ther. 2021, 36, 525–532. [Google Scholar] [CrossRef]
- Mastikova, M.; Galabov, A.S.; Karparov, A.A.; Doseva-Runevska, P. Antiviral Activity of Dipyridamole in Experimental Viral Infections in Mice. Acta Microbiol. Bulg. 2019, 35, 46–52. [Google Scholar]
- Tenser, R.B.; Gaydos, A.; Hay, K.A. Inhibition of Herpes Simplex Virus Reactivation by Dipyridamole. Antimicrob Agents Chemother 2001, 45, 3657–3659. [Google Scholar] [CrossRef]
- Rodriguez-Pallares, J.; Caruncho, H.J.; Guerra, M.J.; Labandeira-Garcia, J.L. Dipyridamole-Induced Increase in Production of Rat Dopaminergic Neurons from Mesencephalic Precursors. Neurosci. Lett. 2002, 320, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, M.; Hofer, M.; Netíková, J.; Viklická, S.; Pipalová, I.; Bartoníčková, A. Effect of Dipyridamole and Adenosine Monophosphate on Cell Proliferation in the Hemopoietic Tissue of Normal and Gamma-Irradiated Mice. Experientia 1992, 48, 253–257. [Google Scholar] [CrossRef]
- Galabov, A.S. Dipyridamole as an Antiviral Agent. Recent Prog. Microbiol. Biotechnol. 2021, 5, 67–77. [Google Scholar]
- Martynov, A.; Bomko, T.; Nosalskaya, T.; Farber, B.; Brek, O. Non-Classical Effects of the cAMP Accumulation Activators In Vivo. Adv. Pharm. Bull. 2020, 10, 477–481. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, Y.; Yan, C.; Xiang, Y.K. Phosphodiesterase in Heart and Vessels: From Physiology to Diseases. Physiol. Rev. 2024, 104, 765–834. [Google Scholar] [CrossRef]
- Mallavarapu, R.; Katari, N.K.; Dongala, T.; Rekulapally, V.K.; Marisetti, V.M.; Vyas, G. A Validated Stability-Indicating Reversed-Phase-HPLC Method for Dipyridamole in the Presence of Degradation Products and Its Process-Related Impurities in Pharmaceutical Dosage Forms. Biomed. Chromatogr. 2022, 36, e5247. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.; Al-Gareeb, A.; Al-Niemi, M.; Al-Buhadily, A.; Al-Harchan, N.; Lugnier, C. COVID-19 and Phosphodiesterase Enzyme Type 5 Inhibitors. J. Microsc. Ultrastruct. 2020, 8, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Sibille, G.; Luganini, A.; Sainas, S.; Boschi, D.; Lolli, M.L.; Gribaudo, G. The Novel h DHODH Inhibitor MEDS433 Prevents Influenza Virus Replication by Blocking Pyrimidine Biosynthesis. Viruses 2022, 14, 2281. [Google Scholar] [CrossRef]
- Ofori, S.K.; Hung, Y.W.; Schwind, J.S.; Diallo, K.; Babatunde, D.; Nwaobi, S.O.; Hua, X.; Sullivan, K.L.; Cowling, B.J.; Chowell, G. Economic Evaluations of Interventions against Influenza at Workplaces: Systematic Review. Occup. Med. 2022, 72, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Thomé, M.P.; Borde, C.; Larsen, A.K.; Henriques, J.A.P.; Lenz, G.; Escargueil, A.E.; Maréchal, V. Dipyridamole as a New Drug to Prevent Epstein-Barr Virus Reactivation. Antiviral Res. 2019, 172, 104615. [Google Scholar] [CrossRef]
- de Mariz e Miranda, L.S. The Synergy between Nucleotide Biosynthesis Inhibitors and Antiviral Nucleosides: New Opportunities against Viral Infections? Arch. Pharm. 2023, 356, 2200217. [Google Scholar] [CrossRef]
- Lee, P.Y.; Aksentijevich, I.; Zhou, Q. Mechanisms of Vascular Inflammation in Deficiency of Adenosine Deaminase 2 (DADA2). In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2022; Volume 44, pp. 269–280. [Google Scholar]
- Aliter, K.F.; Al-Horani, R.A. Potential Therapeutic Benefits of Dipyridamole in COVID-19 Patients. Curr. Pharm. Des. 2021, 27, 866–875. [Google Scholar] [CrossRef]
- Luganini, A.; Sibille, G.; Mognetti, B.; Sainas, S.; Pippione, A.C.; Giorgis, M.; Boschi, D.; Lolli, M.L.; Gribaudo, G. Effective Deploying of a Novel DHODH Inhibitor against Herpes Simplex Type 1 and Type 2 Replication. Antiviral Res. 2021, 189, 105057. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.; Engelman, D.M.; Schulten, K. Molecular Dynamics Simulations of Micelle Formation around Dimeric Glycophorin A Transmembrane Helices. Biophys. J. 2004, 87, 754–763. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. New Ways to Boost Molecular Dynamics Simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.Y.; Deshmukh, S.A. A Review of Advancements in Coarse-Grained Molecular Dynamics Simulations. Mol. Simul. 2021, 47, 786–803. [Google Scholar] [CrossRef]
- Kadaoluwa Pathirannahalage, S.P.; Meftahi, N.; Elbourne, A.; Weiss, A.C.; McConville, C.F.; Padua, A.; Winkler, D.A.; Costa Gomes, M.; Greaves, T.L.; Le, T.C. Systematic Comparison of the Structural and Dynamic Properties of Commonly Used Water Models for Molecular Dynamics Simulations. J. Chem. Inf. Model. 2021, 61, 4521–4536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, L.; Hou, W.-T.; Zha, Z.; Xu, K.; Zhou, C.-Z.; Li, Q.; Chen, Y. Structural Insights into Human ABCC4-Mediated Transport of Platelet Agonist and Antagonist. Nat. Cardiovasc. Res. 2023, 2, 693–701. [Google Scholar] [CrossRef]
- Lewis-Atwell, T.; Townsend, P.A.; Grayson, M.N. Comparisons of Different Force Fields in Conformational Analysis and Searching of Organic Molecules: A Review. Tetrahedron 2021, 79, 131865. [Google Scholar] [CrossRef]
- Tieleman, D.; Van der Spoel, D.; Berendsen, H. Molecular Dynamics Simulations of Dodecylphosphocholine Micelles at Three Different Aggregate Sizes: Micellar Structure and Chain Relaxation. J. Phys. Chem. B 2000, 104, 6380–6388. [Google Scholar] [CrossRef]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An Overview of the Amber Biomolecular Simulation Package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, C.; Chowdhury, S.; Lee, M.C.; Xiong, G.; Zhang, W.; Yang, R.; Cieplak, P.; Luo, R.; Lee, T. A Point-charge Force Field for Molecular Mechanics Simulations of Proteins Based on Condensed-phase Quantum Mechanical Calculations. J. Comput. Chem. 2003, 24, 1999–2012. [Google Scholar] [CrossRef]
- Martynov, A.; Bomko, T.; Nosalskaya, T.; Farber, B.; Kleyn, I. Synthesis of Dynamic Riboflavin Derivatives and the Study of Their Ability to Urease Photoinactivation. Ann. Mechnikovs Inst. 2019, 3, 44–49. [Google Scholar] [CrossRef]
- Ab139460 PDE Activity Assay Kit (Colorimetric) Instructions for Use; Abcam: Cambridge, UK, 2019.
- Park, K.H.; Choi, Y.; Yoon, D.S.; Lee, K.-M.; Kim, D.; Lee, J.W. Zinc Promotes Osteoblast Differentiation in Human Mesenchymal Stem Cells via Activation of the cAMP-PKA-CREB Signaling Pathway. Stem Cells Dev. 2018, 27, 1125–1135. [Google Scholar] [CrossRef]
- Caporale, V.; Alessandrini, B.; Villa, P.D.; Del Papa, S. Global Perspectives on Animal Welfare: Europe. Rev. Sci. Tech.-Off. Int. Epizoot. 2005, 24, 567. [Google Scholar] [CrossRef]
- Sanders, A. The Universal Declaration of Animal Welfare Should Be Taken up on an International Level [Eлeктpoнний Pecypc]. Available online: https://www.libdemvoice.org/adrian-sanders-mp-writesthe-universal-declaration-of-animal-welfare-should-be-taken-up-on-an-international-level-36897.html (accessed on 4 February 2025).
- Directive, C. 86/609/EEС of 24 November 1986 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Off. J. Eur. Communities 1986, L358, 1–29. [Google Scholar]
- Institute of Laboratory Animal Resources (US); Committee on Care, and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals; US Department of Health and Human Services, Public Health Service, National Institutes of Health: Washington, DC, USA, 2010. [Google Scholar]
- Keeney, K.M.; Yurist-Doutsch, S.; Arrieta, M.-C.; Finlay, B.B. Effects of Antibiotics on Human Microbiota and Subsequent Disease. Annu. Rev. Microbiol. 2014, 68, 217–235. [Google Scholar] [CrossRef]
- Spees, A.M.; Wangdi, T.; Lopez, C.A.; Kingsbury, D.D.; Xavier, M.N.; Winter, S.E.; Tsolis, R.M.; Bäumler, A.J. Streptomycin-Induced Inflammation Enhances Escherichia Coli Gut Colonization through Nitrate Respiration. MBio 2013, 4, e00430-13. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.U.; Naz, S.; Zulfiqar, T.; Khan, J.Z.; Ghazanfar, S.; Tipu, M.K. Immunostimulant, Hepatoprotective, and Nephroprotective Potential of Bacillus subtilis (NMCC-Path-14) in Comparison to Dexamethasone in Alleviating CFA-Induced Arthritis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 3275–3299. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Sun, Y.; Yi, X.; Wei, X.; Liu, W.; Zhang, Q.; Yi, H.; Chen, G. Mesenchymal Stromal Cells Ameliorate Acute Lung Injury Induced by LPS Mainly through Stanniocalcin-2 Mediating Macrophage Polarization. Ann. Transl. Med. 2020, 8, 334. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Snyder, T.M.; Gittelman, R.M.; Klinger, M.; May, D.H.; Osborne, E.J.; Taniguchi, R.; Zahid, H.J.; Kaplan, I.M.; Dines, J.N.; Noakes, M.T. Magnitude and Dynamics of the T-Cell Response to SARS-CoV-2 Infection at Both Individual and Population Levels. medRxiv 2020. [Google Scholar] [CrossRef]
- Austin, D.; Melhem, M.; Gandhi, Y.; Lu, S.; Visser, S. Comparative Analysis of PD-1 Target Engagement of Dostarlimab and Pembrolizumab in Advanced Solid Tumors Using Ex Vivo IL-2 Stimulation Data. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 87–94. [Google Scholar] [CrossRef]
- Lee, S.W. Methods for Testing Statistical Differences between Groups in Medical Research: Statistical Standard and Guideline of Life Cycle Committee. Life Cycle 2022, 2, e1. [Google Scholar] [CrossRef]



| Used Substances | Day of Experiment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Streptomycin | + | + | + | + | + | + * | + * | + * | + * | * | * | * |
| Cyclophosphamide | + ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** | ** |
| D3 and D10 | + | + | + | + | + | + | + | |||||
| Mice Group (n = 12) | Body Weight (g) | |
|---|---|---|
| Before Experiment | On the 12th Day | |
| 1 | 20.80 ± 0.52 | 23.12 ± 0.58 * |
| 2 | 22.00 ± 0.28 | 18.60 ±0.45 * |
| 3 | 20.88 ± 0.40 | 18.34 ± 0.66 * |
| 4 | 20.82 ± 0.56 | 22.88 ± 0.82 * |
| 5 | 20.70 ± 0.56 | 21.10 ± 0.50 |
| 6 | 20.65 ± 0.40 | 21.85 ± 0.40 * |
| 7 | 20.78 ± 0.88 | 22.65 ± 0.28 |
| Mice Group ** (n = 12) | Cells, Tissue, Lymphokines | ||||||
|---|---|---|---|---|---|---|---|
| Il-2 Production (ng/mL) | Macrophages (TNF-a) (pg/mL) | T Cells (TNF-b) (pg/mL) | NO Production in Mouse Macrophages, µg/mL | Macrophages Number (in Millions) | Splenocytes Number (in Millions) | ||
| Splenic T-Lymphocytes | Blood Plasma | ||||||
| 1 control | 73.92 ± 2.39 | 161.75 ± 5.15 | 15.33 ± 3.03 | 12.75 ± 2.01 | 9.92 ± 2.39 | 1.92 ± 0.67 | 127.00 ± 10.01 |
| 2 control | 53.75 ± 4.73 | 107.42 ± 5.74 | 8.58 ± 1.98 | 10.83 ± 3.56 | 17.17 ± 3.74 | 1.33 ± 0.49 | 107.92 ± 10.67 |
| 3 control | 11.75 ± 4.39 | 31.33 ± 4.31 | 2.58 ± 1.0 | 8.25 ± 1.14 | 6.33 ± 1.97 | 1.08 ± 0.29 | 89.00 ± 8.30 |
| 4 | 65.25 ± 5.29 | 155.50 ± 4.64 | 10.92 ± 1.98 | 10.17 ± 4.00 * | 12.25 ± 2.01 | 1.83 ± 0.94 * | 116.33 ± 5.25 |
| 5 | 86.33 ±5.18 | 160.00 ± 12.45 * | 18.17 ± 1.34 | 14.25 ± 3.22 * | 13.08 ± 2.23 | 1.42 ± 0.51 * | 137.50 ± 10.88 |
| 6 | 51.33 ± 4.79 * | 100.92 ± 8.13 | 7.25 ± 2.26 * | 7.92 ± 2.11 | 10.08 ± 3.12 | 1.50 ± 0.52 * | 105.50 ± 5.89 * |
| 7 | 36.50 ± 4.34 | 79.25 ± 5.64 | 2.92 ± 1.44 * | 4.17 ± 1.47 | 6.00 ± 1.41 * | 1.25 ± 0.45 * | 79.67 ± 8.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynov, A.; Farber, B.; Katz, A. Phosphodiesterase Inhibition and Immunotropic Activity of Dipyridamole Dynamic Derivatives. Curr. Issues Mol. Biol. 2025, 47, 214. https://doi.org/10.3390/cimb47040214
Martynov A, Farber B, Katz A. Phosphodiesterase Inhibition and Immunotropic Activity of Dipyridamole Dynamic Derivatives. Current Issues in Molecular Biology. 2025; 47(4):214. https://doi.org/10.3390/cimb47040214
Chicago/Turabian StyleMartynov, Artur, Boris Farber, and Alexander Katz. 2025. "Phosphodiesterase Inhibition and Immunotropic Activity of Dipyridamole Dynamic Derivatives" Current Issues in Molecular Biology 47, no. 4: 214. https://doi.org/10.3390/cimb47040214
APA StyleMartynov, A., Farber, B., & Katz, A. (2025). Phosphodiesterase Inhibition and Immunotropic Activity of Dipyridamole Dynamic Derivatives. Current Issues in Molecular Biology, 47(4), 214. https://doi.org/10.3390/cimb47040214


_Kim.png)



