Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation of Rhizospheric and Endophytic Fungi
2.3. Phenotypic Identification
2.4. Screening of PGP Trails
2.4.1. Production of Indole-Acetic Acid
2.4.2. Phosphate Solubilization
2.4.3. Antagonistic Activity against Alternaria alternata In Vitro
2.4.4. Production of Siderophore
2.4.5. Extracellular Enzymes
2.5. Application of Fungal Isolates as PGP agents for Wheat Plants
2.5.1. Vigor of Wheat Plant Seedlings
2.5.2. Pot Experiment
2.6. Molecular Confirmation and Phylogenetic Analysis of Most Potent PGPF Isolates
2.7. Statistical Analysis
3. Results
3.1. Isolation and Identification of Rhizospheric and Endophytic Fungi
3.2. Screening the PGP Traits of Fungal Isolates
3.2.1. IAA Production
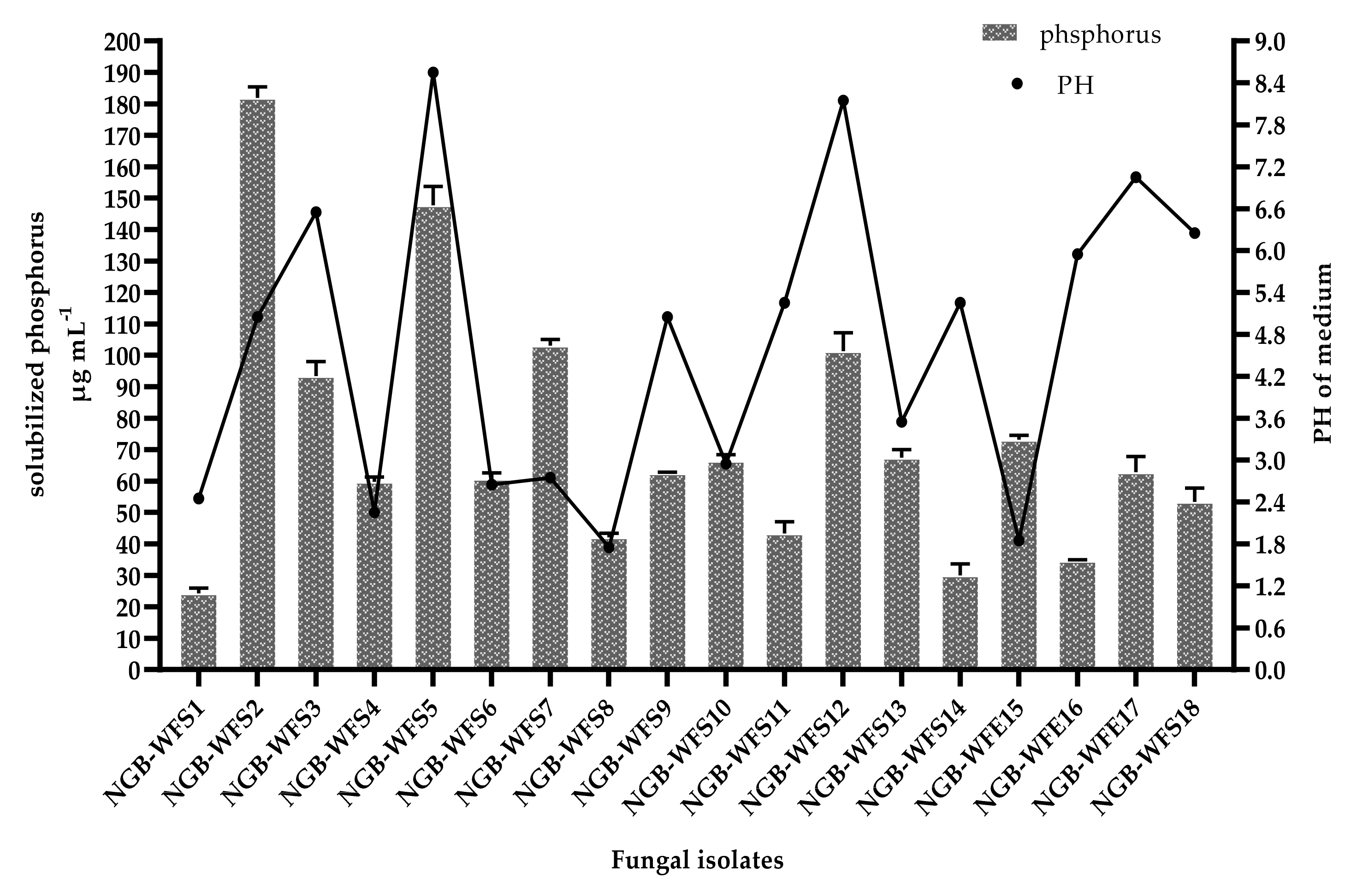
3.2.2. Phosphate Solubilization Efficiency
3.2.3. Antagonistic Activity and Siderophore Production
3.2.4. Extracellular Enzymes
3.3. Application of Fungal Isolates as PGP Agents for Wheat Plant
3.3.1. Grain Germination and Seedling Vigor Test
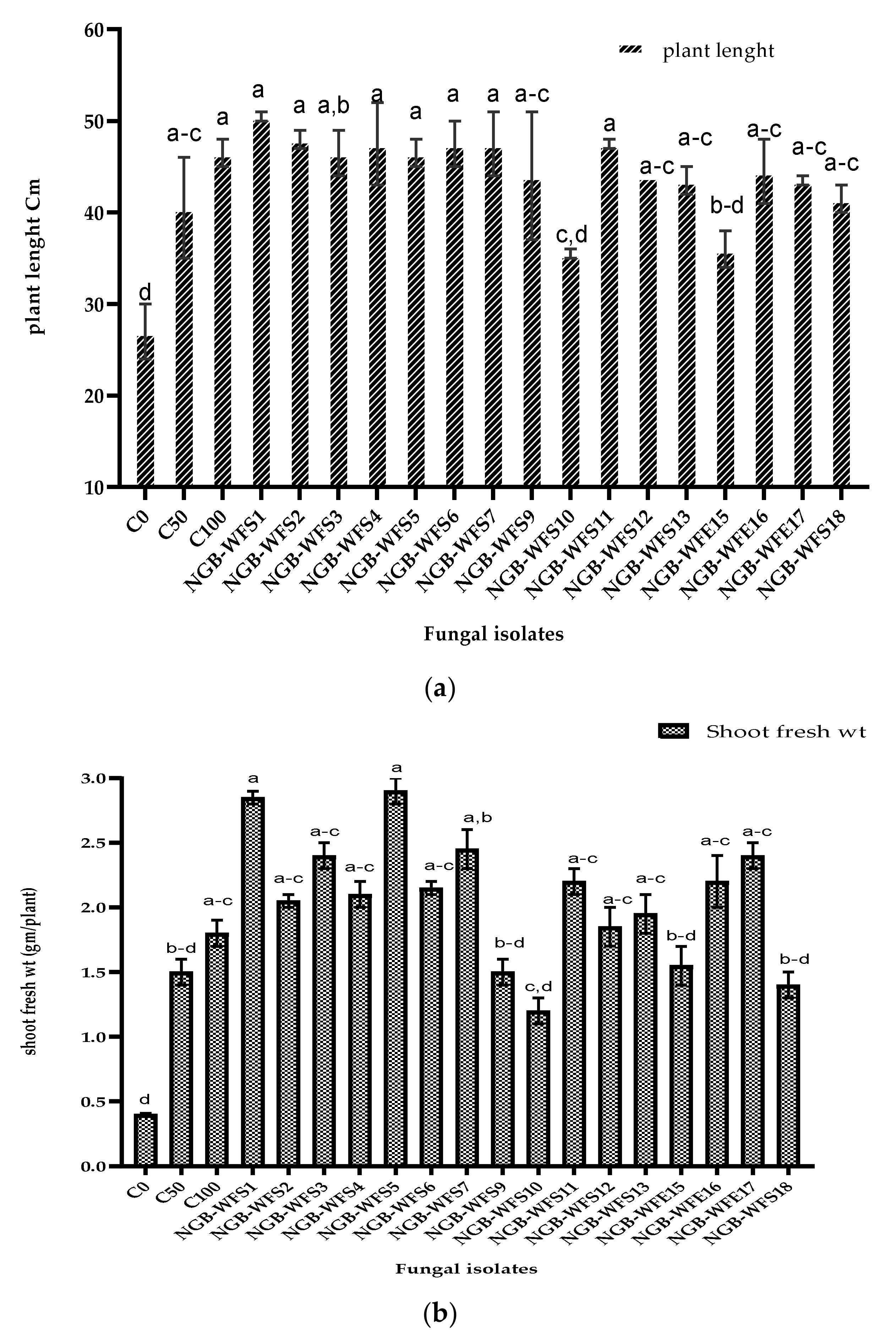
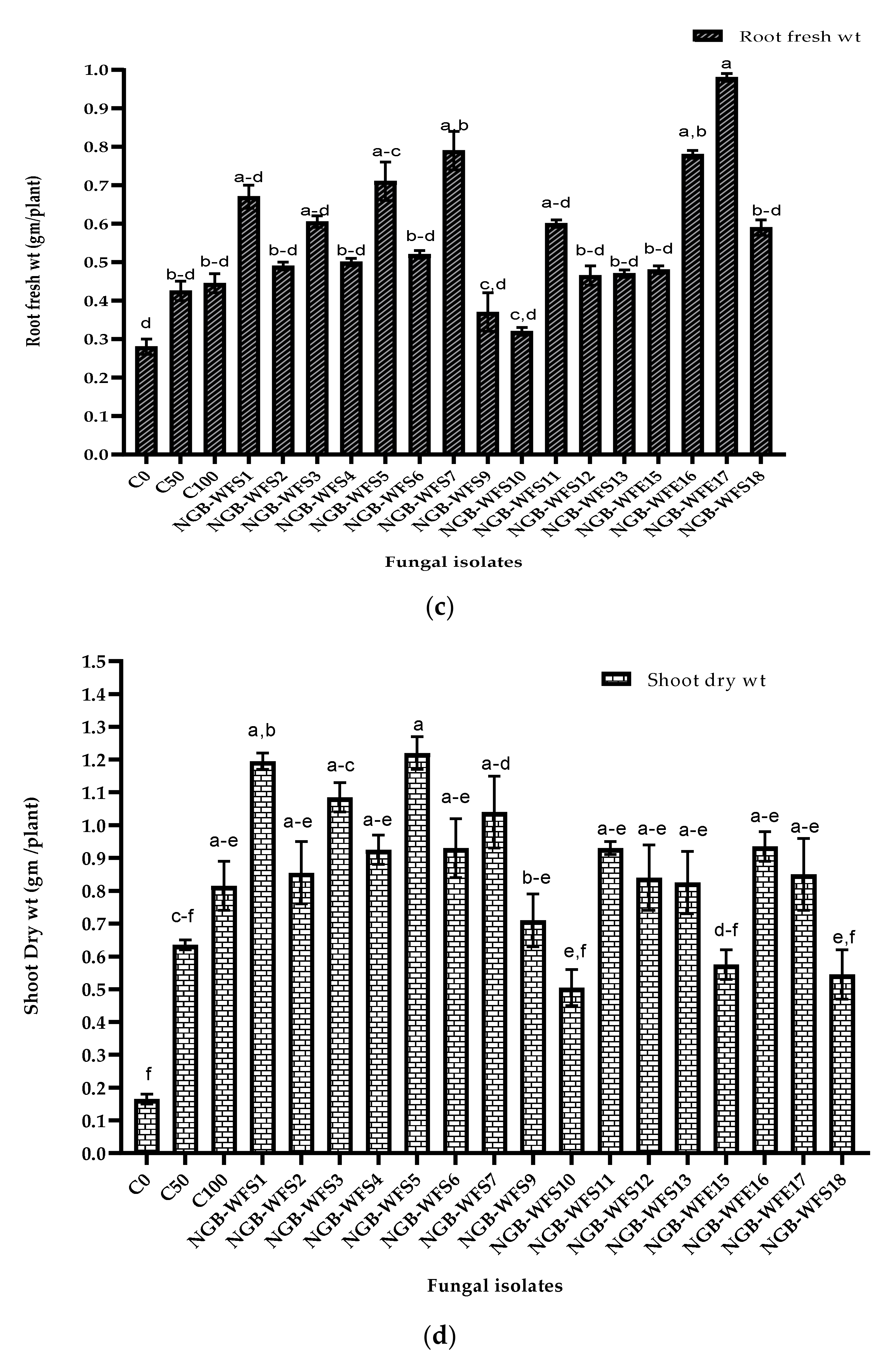
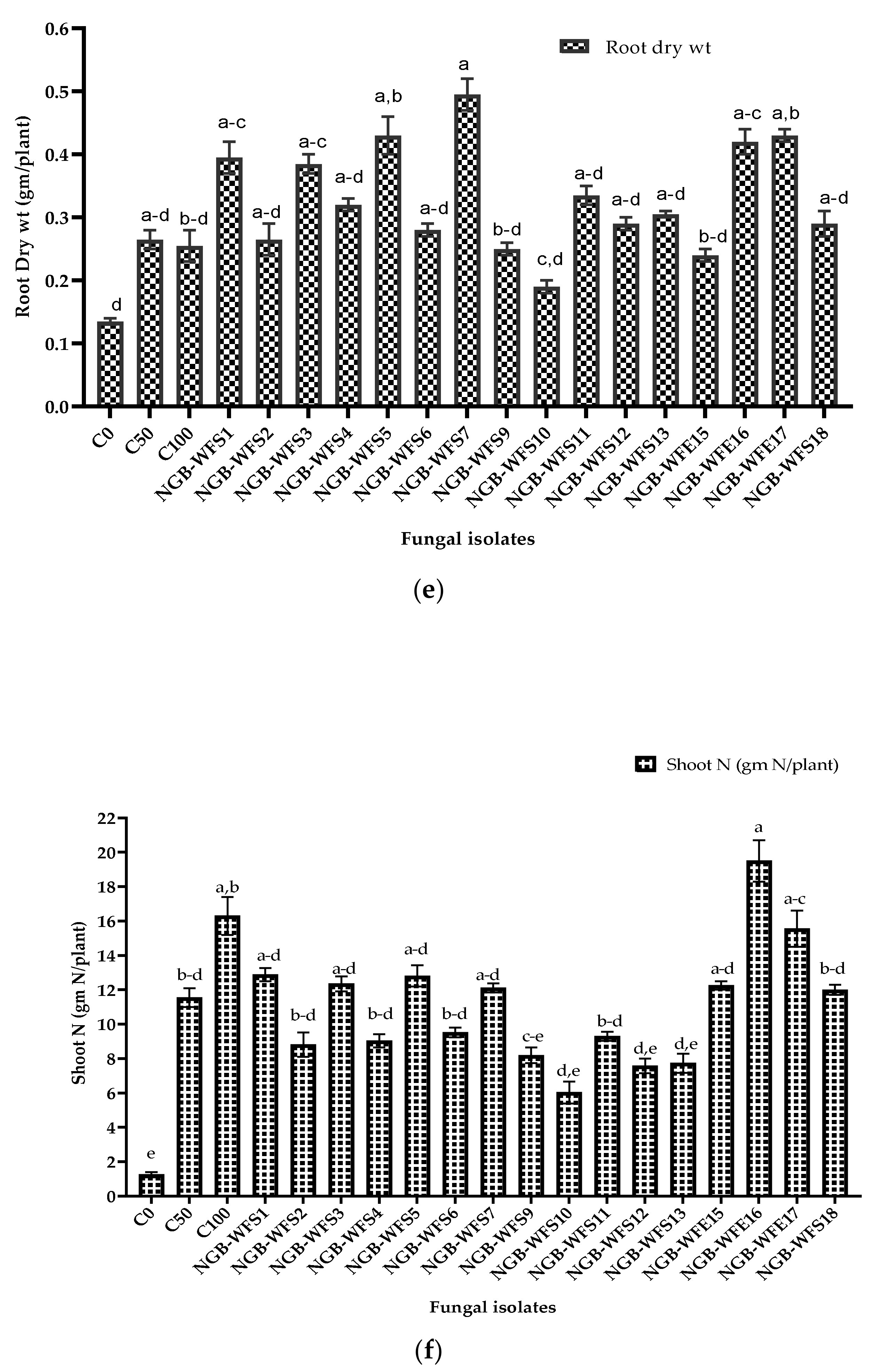
3.3.2. Pot Experiment
3.4. Molecular Confirmation and Phylogenetic Analysis of Most Potent PGPF Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nema, R.; Khare, S.; Jain, P.; Pradhan, A.; Gupta, A.; Singh, D. Natural Products Potential and Scope for Modern Cancer Research. Am. J. Plant Sci. 2013, 4, 1270–1277. [Google Scholar] [CrossRef] [Green Version]
- Jorquera, M.A.; Shaharoona, B.; Nadeem, S.M.; de la Luz Mora, M.; Crowley, D.E. Plant Growth-Promoting Rhizobacteria Associated with Ancient Clones of Creosote Bush (Larrea tridentata). Microb. Ecol. 2012, 64, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Kocsubé, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Pattnaik, S.S.; Busi, S. Rhizospheric fungi: Diversity and potential biotechnological applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 63–84. [Google Scholar]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef] [Green Version]
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Schaechter, M. Eukaryotic Microbes; Academic Press: Cambridge, MA, USA, 2011; ISBN 0123838770. [Google Scholar]
- Muslim, A.; Hyakumachi, M.; Kageyama, K.; Suwandi, S. Induction of systemic resistance in cucumber by hypovirulent binucleate Rhizoctonia against anthracnose caused by Colletotrichum orbiculare. Trop. Life Sci. Res. 2019, 30, 109. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F.-S.; Wu, X.-Q.; Luan, F.-G.; Zhang, L.-P.; Fang, X.-M.; Wan, S.-Z.; Hu, X.-F.; Ye, J.-R. Isolation and characterization of two phosphate-solubilizing fungi from rhizosphere soil of moso bamboo and their functional capacities when exposed to different phosphorus sources and pH environments. PLoS ONE 2018, 13, e0199625. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.A.; Papavizas, G.C. Biocontrol of plant diseases: The approach for tomorrow. Crop Prot. 1991, 10, 95–105. [Google Scholar] [CrossRef]
- Cherif, H.; Marasco, R.; Rolli, E.; Ferjani, R.; Fusi, M.; Soussi, A.; Mapelli, F.; Blilou, I.; Borin, S.; Boudabous, A. Oasis desert farming selects environment-specific date palm root endophytic communities and cultivable bacteria that promote resistance to drought. Environ. Microbiol. Rep. 2015, 7, 668–678. [Google Scholar] [CrossRef] [Green Version]
- Amrani, S.; Djouadi, S.; Bouheraama, A.; Mohamed, T.A.; Abo Nouh, F.; Mansour, S.; Gezaf, S.; El-Azeem, A.; Ahmed, M.; Abo Nahas, H. Checklist of Algerian fungi–Part 5: Dothideomycetes (Ascomycota). Microb. Biosyst. 2021, 5, 83–121. [Google Scholar] [CrossRef]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel Papaverine Metal Complexes with Potential Anticancer Activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). World Food and Agriculture; FAO Statistical Pocketbook; FAO: Rome, Italy, 2015. [Google Scholar]
- Bin-Jumah, M.; Abdel-Fattah, A.-F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-Induced Peripheral Neuropathy: Manifestations, Mechanisms, and Potential Treatment Modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Asseng, S.; Kheir, A.M.S.; Kassie, B.T.; Hoogenboom, G.; Abdelaal, A.I.N.; Haman, D.Z.; Ruane, A.C. Can Egypt become self-sufficient in wheat? Environ. Res. Lett. 2018, 13, 94012. [Google Scholar] [CrossRef] [Green Version]
- Laurent, E.-A.; Ahmed, N.; Durieu, C.; Grieu, P.; Lamaze, T. Marine and fungal biostimulants improve grain yield, nitrogen absorption and allocation in durum wheat plants. J. Agric. Sci. 2020, 158, 279–287. [Google Scholar] [CrossRef]
- Ruisi, P.; Frangipane, B.; Amato, G.; Frenda, A.S.; Plaia, A.; Giambalvo, D.; Saia, S. Nitrogen uptake and nitrogen fertilizer recovery in old and modern wheat genotypes grown in the presence or absence of interspecific competition. Front. Plant Sci. 2015, 6, 185. [Google Scholar] [CrossRef] [Green Version]
- Ruisi, P.; Saia, S.; Badagliacca, G.; Amato, G.; Frenda, A.S.; Giambalvo, D.; Di Miceli, G. Long-term effects of no tillage treatment on soil N availability, N uptake, and 15N-fertilizer recovery of durum wheat differ in relation to crop sequence. Field Crop. Res. 2016, 189, 51–58. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant. Cell Environ. 2016, 39, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- El Hadidi, M.N.; Hosni, H.A. Biodiversity in the Flora of Egypt. In The Biodiversity of African Plants: Proceedings XIVth AETFAT Congress; Wageningen, The Netherlands, 22–27 August 1994, van der Maesen, L.J.G., van der Burgt, X.M., van Medenbach de Rooy, J.M., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 785–787. ISBN 978-94-009-0285-5. [Google Scholar]
- Garrett, S.D. Colonization of unsterilized filter paper by cereal foot-rot fungi. Trans. Br. Mycol. Soc. 1980, 74, 259–263. [Google Scholar] [CrossRef]
- Smith, N.R.; Dawson, V.T. The bacteriostatic action of rose bengal in media used for plate counts of soil fungi. Soil Sci. 1944, 58, 467–472. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Salem, F.M. Biodiversity of laccase producing fungi in Egypt. Mycosphere 2012, 3, 900–920. [Google Scholar] [CrossRef]
- Samson, R.A. Review of The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. Mycologia 1981, 73, 582–584. [Google Scholar] [CrossRef]
- Christensen, M.; Tuthill, D.E. Aspergillus: An Overview. In Advances in Penicillium and Aspergillus Systematics; Samson, R.A., Pitt, J.I., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1986; pp. 195–209. ISBN 978-1-4757-1856-0. [Google Scholar]
- Klich, M.A. Biogeography of Aspergillus species in soil and litter. Mycologia 2002, 94, 21–27. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Abu-Elsaoud, A.; Darwish, A.M.G.; Balbool, B.A.; Abo Nouh, F.; Abo Nahas, H.H.; El-Azeem, A.; Ahmed, M.; Ali, N.H.; Kirk, P. The Egyptian Ascomycota 1: Genus Aspergillus. Microb. Biosyst. 2020, 5, 61–99. [Google Scholar] [CrossRef]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Ellis, M.B. More Dematiaceous Hyphomicetes; Commonwealth Mycological Institute: Kew, UK, 1976. [Google Scholar]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971; ISBN 978-0-85198-046-1. [Google Scholar]
- Seifert, K.A. Compendium of Soil Fungi—By K.H. Domsch, W. Gams & T.-H. Anderson. Eur. J. Soil Sci. 2008, 59, 1007. [Google Scholar]
- Guarro, J. Atlas of Soil Ascomycetes; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2012; ISBN 9070351889. [Google Scholar]
- Kirk, P.M. Authors of Fungal Names: A List of Authors of Scientific Names of Fungi, with Recommended Standard Forms of Their Names, Including Abbreviations; C.A.B. International: Wallingford, UK, 1992; ISBN 978-0-85198-833-7. [Google Scholar]
- Kirk, P.; Cannon, P.; Minter, D.; Stalpers, J. Ainsworth & Bisby’s Dictionary of the Fungi; CABI: Wallingford, UK, 2008; ISBN 9780851998268. [Google Scholar]
- Bose, A.; Shah, D.; Keharia, H. Production of indole-3-acetic-acid (IAA) by the white rot fungus Pleurotus ostreatus under submerged condition of Jatropha seedcake. Mycology 2013, 4, 103–111. [Google Scholar] [CrossRef]
- Sharar, M.; Saied, E.M.; Rodriguez, M.C.; Arenz, C.; Montes-Bayón, M.; Linscheid, M.W. Elemental Labelling and Mass Spectrometry for the Specific Detection of Sulfenic Acid Groups in Model Peptides: A Proof of Concept. Anal. Bioanal. Chem. 2017, 409, 2015–2027. [Google Scholar] [CrossRef]
- Pikovskaya, R.I. Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologiya 1948, 17, 362–370. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Pentice Hall of India Pvt. Ltd.: New Delhi, India, 1973. [Google Scholar]
- Jomduang, J.; Sariah, M. Antagonistic effect of Malaysian isolates of Trichoderma harzianum and Gliocladium virens on Sclerotium rolfsii. Pertanika J. Trop. Agric. Sci. 1997, 20, 35–42. [Google Scholar]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, M.E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef] [PubMed]
- Mandels, M.; Reese, E.T. Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J. Bacteriol. 1957, 73, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gohel, H.R.; Contractor, C.N.; Ghosh, S.K.; Braganza, V.J. A comparative study of various staining techniques for determination of extra cellular cellulase activity on Carboxy Methyl Cellulose (CMC) agar plates. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 261–266. [Google Scholar]
- Ren, J.; Saied, E.M.; Zhong, A.; Snider, J.; Ruiz, C.; Arenz, C.; Obeid, L.M.; Girnun, G.D.; Hannun, Y.A. Tsc3 Regulates SPT Amino Acid Choice in Saccharomyces Cerevisiae by Promoting Alanine in the Sphingolipid Pathway. J. Lipid Res. 2018, 59, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Eid, A.M.; Hassan, S.E.-D.; Salem, S.S.; Radwan, A.A.; Halawa, M.; Saleh, F.M.; Saad, H.A.; Saied, E.M.; Fouda, A. The Catalytic Activity of Biosynthesized Magnesium Oxide Nanoparticles (MgO-NPs) for Inhibiting the Growth of Pathogenic Microbes, Tanning Effluent Treatment, and Chromium Ion Removal. Catalysts 2021, 11, 821. [Google Scholar] [CrossRef]
- Saied, E.M.; Diederich, S.; Arenz, C. Facile Synthesis of the CERT Inhibitor HPA-12 and Some Novel Derivatives. Chem. Asian J. 2014, 9, 2092–2094. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria 1. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Khokhar, I.; Haider, M.S.; Mukhtar, I.; Ali, A.; Mushtaq, S.; Ashfaq, M. Effect of Penicillium species culture filtrate on seedling growth of wheat. Int. Res. J. Agric. Sci. Soil Sci. 2013, 3, 24–29. [Google Scholar]
- Patil, D.P.; Muley, S.M.; Pawar, P. V Impact of fungal culture filtrate (mycotoxins) on seed germination of some pulses. World J. Sci. Technol. 2012, 2, 1–2. [Google Scholar]
- Abd El-Megeed, F.H.; Youseif, S.H. Molecular identification and plant growth pro-moting activities of endophytic Pantoea sp. isolat-ed from Zygophyllum album medicinal plant. Egypt. J. Genet. Cytol. 2018, 47, 69–86. [Google Scholar]
- Ortiz, J.; Soto, J.; Almonacid, L.; Fuentes, A.; Campos-Vargas, R.; Arriagada, C. Alleviation of metal stress by Pseudomonas orientalis and Chaetomium cupreum strains and their effects on Eucalyptus globulus growth promotion. Plant Soil 2019, 436, 449–461. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Osman, M.E.; El- Beih, A.; Khatab, O.-K.; Moghannem, S.A.M.; Abdullah, N.H. Optimization of Bioactive Compounds Production by Endophytic Chaetosphaeronema sp. (KY321184) Using Experimental Design Method. Egypt. J. Bot. 2018, 58, 343. [Google Scholar] [CrossRef] [Green Version]
- Shivanna, M.B.; Meera, M.S.; Kubota, M.; Hyakumachi, M. Promotion of growth and yield in cucumber by zoysiagrass rhizosphere fungi. Microbes Environ. 2005, 20, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Chowdappa, P.; Kumar, S.P.M.; Lakshmi, M.J.; Upreti, K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Haudecoeur, E.; Faure, D.; Kerr, K.F.; Nester, E.W. Comparative transcriptome analysis of Agrobacterium tumefaciens in response to plant signal salicylic acid, indole-3-acetic acid and γ-amino butyric acid reveals signalling cross-talk and Agrobacterium–plant co-evolution. Cell. Microbiol. 2008, 10, 2339–2354. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3 acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of Azotobacter and fluorescent Pseudomonas in the presence and absence of tryptophan. Turkish J. Biol. 2005, 29, 29–34. [Google Scholar]
- Turbat, A.; Rakk, D.; Vigneshwari, A.; Kocsubé, S.; Thu, H.; Szepesi, Á.; Bakacsy, L.; D Škrbić, B.; Jigjiddorj, E.-A.; Vágvölgyi, C. Characterization of the Plant Growth-Promoting Activities of Endophytic Fungi Isolated from Sophora flavescens. Microorganisms 2020, 8, 683. [Google Scholar] [CrossRef]
- Daniels, C.; Michán, C.; Ramos, J.L. New molecular tools for enhancing methane production, explaining thermodynamically limited lifestyles and other important biotechnological issues. Microb. Biotechnol. 2009, 2, 533. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Pandey, A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 2019, 9, 2–9. [Google Scholar] [CrossRef]
- Pandey, A.; Das, N.; Kumar, B.; Rinu, K.; Trivedi, P. Phosphate solubilization by Penicillium spp. isolated from soil samples of Indian Himalayan region. World J. Microbiol. Biotechnol. 2008, 24, 97–102. [Google Scholar] [CrossRef]
- Kumar, C.M.S.; Jacob, T.K.; Devasahayam, S.; Thomas, S.; Geethu, C. Multifarious plant growth promotion by an entomopathogenic fungus Lecanicillium psalliotae. Microbiol. Res. 2018, 207, 153–160. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Bioassay, characterization and estimation of siderophores from some important antagonistic Fungi. J. Biopestic. 2017, 10, 105–112. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.W.; Hodgkiss, I.J.; Hyde, K.D. Enzyme production by endophytes of Brucea javanica. J. Agric. Technol. 2005, 1, 55–66. [Google Scholar]
- de Almeida Lopes, K.B.; Carpentieri-Pipolo, V.; Oro, T.H.; Stefani Pagliosa, E.; Degrassi, G. Culturable endophytic bacterial communities associated with field-grown soybean. J. Appl. Microbiol. 2016, 120, 740–755. [Google Scholar] [CrossRef]
- Rajini, S.B.; Nandhini, M.; Udayashankar, A.C.; Niranjana, S.R.; Lund, O.S.; Prakash, H.S. Diversity, plant growth-promoting traits, and biocontrol potential of fungal endophytes of Sorghum bicolor. Plant Pathol. 2020, 69, 642–654. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Elhaissoufi, W.; Khourchi, S.; Ibnyasser, A.; Ghoulam, C.; Rchiad, Z.; Zeroual, Y.; Lyamlouli, K.; Bargaz, A. Phosphate Solubilizing Rhizobacteria Could Have a Stronger Influence on Wheat Root Traits and Aboveground Physiology Than Rhizosphere P Solubilization. Front. Plant Sci. 2020, 11, 979. [Google Scholar] [CrossRef]
- Qiang, X.; Ding, J.; Lin, W.; Li, Q.; Xu, C.; Zheng, Q.; Li, Y. Alleviation of the detrimental effect of water deficit on wheat (Triticum aestivum L.) growth by an indole acetic acid-producing endophytic fungus. Plant Soil 2019, 439, 373–391. [Google Scholar] [CrossRef]
- Emami, S.; Alikhani, H.A.; Pourbabaee, A.A.; Etesami, H.; Motasharezadeh, B.; Sarmadian, F. Consortium of endophyte and rhizosphere phosphate solubilizing bacteria improves phosphorous use efficiency in wheat cultivars in phosphorus deficient soils. Rhizosphere 2020, 14, 100196. [Google Scholar] [CrossRef]
- Qarni, A.; Billah, M.; Hussain, K.; Shah, S.H.; Ahmed, W.; Alam, S.; Sheikh, A.A.; Jafri, L.; Munir, A.; Malik, K.M. Isolation and Characterization of Phosphate Solubilizing Microbes from Rock Phosphate Mines and their Potential Effect for Sustainable Agriculture. Sustainability 2021, 13, 2151. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Cetner, M.D.; Samborska, I.A.; Lukasik, I.; Oukarroum, A.; Rusinowki, S.; Pietkiewicz, S.; Swiatek, M.; Dabrowski, P. Effective microorganisms impact on photosynthetic activity of Arabidopsis plant grown under salinity stress conditions. Ann. Warsaw Univ. Life Sci. Life Reclam. 2016, 48, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Shi, J.; He, C.; He, X. Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China. Agronomy 2021, 11, 648. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.-J. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Haden, V.R.; Peng, S.; Bouman, B.A.M.; Visperas, R.M.; Nie, L.; Huang, J.; Cui, K. Improvement in nitrogen availability, nitrogen uptake and growth of aerobic rice following soil acidification. Soil Sci. Plant Nutr. 2009, 55, 705–714. [Google Scholar] [CrossRef]
- van Asten, P.J.A.; Van Bodegom, P.M.; Mulder, L.M.; Kropff, M.J. Effect of straw application on rice yields and nutrient availability on an alkaline and a pH-neutral soil in a Sahelian irrigation scheme. Nutr. Cycl. Agroecosyst. 2005, 72, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Sultana, F. Application and mechanisms of plant growth promoting fungi (PGPF) for phytostimulation. In Organic Agriculture; IntechOpen: London, UK, 2020. [Google Scholar]






| Longitude | Latitude | Site of Collection | Plant Family | Host Plant |
|---|---|---|---|---|
| 28.309 N | 33.984 E | Wadi Tarafa | Labiatae | Cleome droserifolia (Forssk.) Delile |
| 28.539 N | 33.979 E | Shaq Elgragnia | Labiatae | Thymus bovei Benth. |
| 28.542 N | 33.964 E | WadiElArba’een | Labiatae | Marrubium alysson L. |
| 33.934 E | 28.553 N | Shaq Itlah | Compositae | Sonchus oleraceus L. |
| 28.568 N | 33.929 E | Wadi Eltalaa | Zygophyllaceae | Peganum harmala L. |
| 33.934 E | 28.553 N | Shaq Itlah | Cruciferae | Diplotaxis harra (Forssk.) Boiss. |
| 34.0148 E | 28.550 N | Wadi Sdod | Adianteacea | Cheilanthes vellea (Aiton) F. Muell. |
| 28.539 N | 33.977 E | Gebel Musa | Compositae | Conyza stricta Willd. |
| 33.940 N | 28.538 E | Elfaraa | Caryophyllaceae | Silene schimperiana Boiss. |
| Isolates Code | Host Plant | Phenotypic Identification | Molecular Confirmation | Accession No. |
|---|---|---|---|---|
| NGB-WFS1 * | Cleome droserifolia (Forssk.) Delile | Botryotrichum atrogriseum J.F.H. Beyma | Botryotrichum atrogriseum | LC642736 |
| NGB-WFS2 | Thymus bovei Benth. | Penicillium chrysogenum Thom | N/A | N/A |
| NGB-WFS3 * | Thymus bovei Benth. | Penicillium chrysogenum Thom | Penicillium | LC642737 |
| NGB-WFS4 | Marrubium alysson L. | Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde | N/A | N/A |
| NGB-WFS5 | Sonchus oleraceus L. | Aspergillus fumigatiaffinis S.B. Hong, Frisvad & Samson | Aspergillus fumigatiaffinis | LC642738 |
| NGB-WFS6 | Peganum harmala L. | Alternaria alternata (Preuss) Woudenb. & Crous | N/A | N/A |
| NGB-WFS7 * | Peganum harmala L. | Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde | Chaetosphaeronema sp. | LC642739 |
| NGB-WFS8 | Peganum harmala L. | Acrophialophora levis Samson & T. Mahmood | N/A | N/A |
| NGB-WFS9 | Peganum harmala L. | Aspergillus versicolor (Vuill.) Tirab. | N/A | N/A |
| NGB-WFS10 | Diplotaxis harra (Forssk.) Boiss. | Sterile mycelium | N/A | N/A |
| NGB-WFS11 | Sonchus oleraceus L. | Geotrichum sp. | N/A | N/A |
| NGB-WFS12 | Sonchus oleraceus L. | Penicillium chrysogenum Thom | N/A | N/A |
| NGB-WFS13 | Diplotaxis harra (Forssk.) Boiss. | Aspergillus versicolor (Vuill.) Tirab. | N/A | N/A |
| NGB-WFS14 | Diplotaxis harra (Forssk.) Boiss. | Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde | N/A | N/A |
| NGB-WFE15 | Cheilanthes vellea (Aiton) F.Muell | Penicillium chrysogenum Thom | N/A | N/A |
| NGB-WFE16 * | Conyza stricta Willd. | Fusarium oxysporum Schltdl. | Fusarium petersiae | LC642740 |
| NGB-WFE17 | Silene schimperiana Boiss. | Alternaria botrytis (Preuss) Woudenb. & Crous | N/A | N/A |
| NGB-WFS18 | Diplotaxis harra (Forssk.) Boiss. | Trichoderma atroviride P. Karst. | N/A | N/A |
| Isolates | Pathogen Growth Inhibition % | Siderophore Production (% SU) | ||||
|---|---|---|---|---|---|---|
| NGB-WFS1 | 65.9 | ± | 0.8 b–e | 25.9 | ± | 0.10 h |
| NGB-WFS2 | 59.5 | ± | 2.4 e,f | 3.1 | ± | 0.05 n |
| NGB-WFS3 | 63.5 | ± | 7.9 c–f | 19.3 | ± | 0.05 i |
| NGB-WFS4 | 69.1 | ± | 0.8 b–e | 33.7 | ± | 0.05 g |
| NGB-WFS5 | 73.8 | ± | 4.0 b–d | 1.5 | ± | 0.10 o |
| NGB-WFS6 | 64.3 | ± | 0.8 b–f | 33.6 | ± | 0.55 g |
| NGB-WFS7 | 62.7 | ± | 0.8 d–f | 23.0 | ± | 0.25 i |
| NGB-WFS8 | 77.0 | ± | 0.8 a,b | 0.0 | ± | 0.00 p |
| NGB-WFS9 | 56.4 | ± | 0.8 e,f | 44.7 | ± | 0.15 e |
| NGB-WFS10 | 59.6 | ± | 5.6 e,f | 61.7 | ± | 0.05 c |
| NGB-WFS11 | 59.5 | ± | 0.8 e,f | 57.1 | ± | 1.85 d |
| NGB-WFS12 | 58.0 | ± | 4.0 e,f | 16.8 | ± | 0.10 l |
| NGB-WFS13 | 55.6 | ± | 4.8 e,f | 36.2 | ± | 0.30 f |
| NGB-WFS14 | 52.4 | ± | 4.8 f | 17.3 | ± | 0.30 k |
| NGB-WFE15 | 57.9 | ± | 0.8 e,f | 75.4 | ± | 0.14 b |
| NGB-WFE16 | 76.2 | ± | 9.5 a–c | 96.5 | ± | 0.43 a |
| NGB-WFE17 | 63.5 | ± | 1.6 c–f | 14.6 | ± | 0.32 m |
| NGB-WFS18 | 87.3 | ± | 1.6 a | 44.4 | ± | 0.09 e |
| Isolate Code | Enzymatic Index | |||
|---|---|---|---|---|
| Xylanase | Chitinase | Xylanase | Chitinase | |
| NGB-WFS1 | 1.4 b,c | 1.8 a | 1.1 b–d | 1.3 d,e |
| NGB-WFS2 | 2.0 a | 1.5 a,b | 2.0 a,b | 1.2 d,e |
| NGB-WFS3 | 1.6 b | 1.6 a,b | 1.4 a–d | 1.2 d,e |
| NGB-WFS4 | 1.0 d | 1.0 a–c | 0.0 e | 1.0 e |
| NGB-WFS5 | 1.0 d | 1.0 a–c | 1.0 c,d | 1.1 d,e |
| NGB-WFS6 | 1.1 d | 0.0 d | 0.0 e | 1.0 e |
| NGB-WFS7 | 1.4 b,c | 1.3 a–c | 1.3 a–d | 1.4 d |
| NGB-WFS8 | 1.1 d | 1.0 a–c | 0.0 e | 1.0 e |
| NGB-WFS9 | 1.6 b | 1.3 a–c | 1.4 a–d | 1.8 c |
| NGB-WFS10 | 1.0 d | 1.1 a–c | 0.5 d,e | 3.9 a |
| NGB-WFS11 | 1.1 d | 1.8 a | 1.3 a–d | 3.0 b |
| NGB-WFS12 | 1.6 b | 1.4 a–c | 1.5 a–c | 1.3 d,e |
| NGB-WFS13 | 1.3 c,d | 1.0 b,c | 1.3 a–d | 1.4 d |
| NGB-WFS14 | 1.5 b,c | 1.3 a–c | 1.4 a–d | 1.0 e |
| NGB-WFE15 | 1.1 d | 1.7 a,b | 1.1 b–d | 1.1 d,e |
| NGB-WFE16 | 1.3 c,d | 0.7 c,d | 2.1 a | 1.0 e |
| NGB-WFE17 | 1.0 d | 1.5 a,b | 1.1 b–d | 1.2 d,e |
| NGB-WFS18 | 1.0 d | 1.0 a–c | 1.9 a–c | 1.0 e |
| Isolate Code | Percent of Grain Germination (%) | Seedling Vigor | ||
|---|---|---|---|---|
| Fungi Filterate | Fungi Spore Suspension | Fungi Filterate | Fungi Spore Suspension | |
| NGB-WFS1 | 90.5 a | 79.5 a–c | 1236 a–e | 1304 a,b |
| NGB-WFS2 | 81.5 a,b | 50.5 d | 1362 a–d | 871 b,c |
| NGB-WFS3 | 78 a,b | 75 a–d | 1238 a–e | 1270 a,b |
| NGB-WFS4 | 77 a,b | 80 a–c | 872 d–f | 1043 a–c |
| NGB-WFS5 | 87 a | 70 a–d | 1500 a,b | 1147 a–c |
| NGB-WFS6 | 94 a | 81 a–c | 1651 a,b | 1308 a,b |
| NGB-WFS7 | 53.5 a,b | 61.5 b–d | 603 f | 727 c |
| NGB-WFS8 | 86 a | 0.00 e | 1482 a–c | 0 d |
| NGB-WFS9 | 72 a,b | 81.5 a,b | 1327 a–d | 1362 a,b |
| NGB-WFS10 | 69.5 a,b | 70.5 a–d | 1168 b–e | 1276 a,b |
| NGB-WFS11 | 76.5 a,b | 90.5 a | 1385 a–d | 1533 a |
| NGB-WFS12 | 73.5 a,b | 68.5 a–d | 1287 a–e | 1178 a–c |
| NGB-WFS13 | 67 a,b | 71 a–d | 1200 a–e | 1199 a–c |
| NGB-WFS14 | 76 a,b | 0.00 e | 729 e,f | 0 d |
| NGB-WFE15 | 66.5 a,b | 61.5 b–d | 1131 b–f | 1081 a–c |
| NGB-WFE16 | 96.5 a | 53 c,d | 1767 a | 883 b,c |
| NGB-WFE17 | 78.5 a,b | 74 a–d | 1339 a–d | 1200 a–c |
| NGB-WFS18 | 77 a,b | 68.5 a–d | 1402 a–d | 887 b,c |
| Control | 73.5 a,b | 70.5 a–d | 910 c–f | 888 b,c |
| Property | Value |
|---|---|
| Particle size distribution (%) | |
| Sand | 90.1 |
| Silt | 3.9 |
| Clay | 6.0 |
| Texture grade | Sandy |
| CaCo3 (%) | 1.61 |
| Saturation percent S.P (%) | 21.50 |
| pH | 7.82 |
| E.C. (dS m−1 at 25 °C) | 0.32 |
| Soluble cations (meq/L) | |
| Ca2+ | 0.54 |
| Mg2+ | 0.33 |
| Na+ | 1.62 |
| K+ | 0.65 |
| Soluble anions (meq/L) | |
| CO3−2 | 0.00 |
| HCO3− | 0.88 |
| Cl− | 0.59 |
| SO4−2 | 1.67 |
| Total N (%) | 0.021 |
| Total Soluble-N (mg kg−1) | 16.30 |
| Available-P (mg kg−1) | 6.71 |
| Available-K (mg kg−1) | 52.10 |
| Organic matter (%) | 0.23 |
| DTPA extractable (ppm) | |
| Fe | 1.62 |
| Mn | 0.31 |
| Zn | 0.42 |
| Cu | 0.18 |
| Isolate | Chlorophyll a (mg g−1) | Chlorophyll b (mg g−1) | Total Chlorophyll (mg g−1) | Carotenoids (mg g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NGB-WFS1 | 2.0 | ± | 0.07 a | 0.56 | ± | 0.06 a,b | 2.6 | ± | 0.13 a–d | 0.77 | ± | 0.06 a,b |
| NGB-WFS2 | 1.8 | ± | 0.12 a–d | 0.43 | ± | 0.06 b–d | 2.2 | ± | 0.18 c–g | 0.63 | ± | 0.07 b–e |
| NGB-WFS3 | 1.7 | ± | 0.00 a–e | 0.40 | ± | 0.01 c,d | 2.1 | ± | 0.01 d–g | 0.60 | ± | 0.01 c–e |
| NGB-WFS4 | 1.6 | ± | 0.03 a–e | 0.38 | ± | 0.00 c,d | 2.1 | ± | 0.04 d–g | 0.58 | ± | 0.02 c–e |
| NGB-WFS5 | 1.7 | ± | 0.02 a–e | 0.40 | ± | 0.01 c,d | 2.1 | ± | 0.03 d–g | 0.58 | ± | 0.03 c–e |
| NGB-WFS6 | 1.5 | ± | 0.41 d,e | 0.34 | ± | 0.12 d,e | 1.9 | ± | 0.53 f,g | 0.54 | ± | 0.14 d,e |
| NGB-WFS7 | 1.5 | ± | 0.08 c–e | 0.37 | ± | 0.05 c,e | 1.9 | ± | 0.14 f,g | 0.56 | ± | 0.07 d,e |
| NGB-WFS9 | 1.6 | ± | 0.26 b–e | 0.38 | ± | 0.07 c,d | 2.0 | ± | 0.34 e–g | 0.59 | ± | 0.04 c–e |
| NGB-WFS10 | 1.7 | ± | 0.21 a–e | 0.40 | ± | 0.09 c,d | 2.1 | ± | 0.31 d–g | 0.58 | ± | 0.10 c–e |
| NGB-WFS11 | 1.8 | ± | 0.02 a–d | 0.45 | ± | 0.00 b–d | 2.3 | ± | 0.02 c–f | 0.66 | ± | 0.02 b–d |
| NGB-WFS12 | 1.6 | ± | 0.21 a–e | 0.37 | ± | 0.07 c–e | 2.0 | ± | 0.30 e–g | 0.59 | ± | 0.05 c–e |
| NGB-WFS13 | 1.7 | ± | 0.00 a–e | 0.40 | ± | 0.00 c,d | 2.1 | ± | 0.01 d–g | 0.62 | ± | 0.04 c–e |
| NGB-WFE15 | 1.9 | ± | 0.02 a–c | 0.43 | ± | 0.04 b–d | 3.1 | ± | 0.00 a | 0.65 | ± | 0.03 b–d |
| NGB-WFE16 | 1.8 | ± | 0.05 a–d | 0.34 | ± | 0.04 d,e | 3.0 | ± | 0.11 a,b | 0.59 | ± | 0.02 c–e |
| NGB-WFE17 | 1.9 | ± | 0.01 a–c | 0.45 | ± | 0.02 b–d | 2.7 | ± | 0.13 a–c | 0.66 | ± | 0.01 b–d |
| NGB-WFS18 | 2.0 | ± | 0.00 a,b | 0.66 | ± | 0.02 a | 3.0 | ± | 0.05 a,b | 0.81 | ± | 0.01 a |
| Control (0 N) | 1.1 | ± | 0.28 f | 0.23 | ± | 0.08 e | 1.3 | ± | 0.37 h | 0.49 | ± | 0.00 e |
| Control (50 N) | 1.8 | ± | 0.27 a–e | 0.42 | ± | 0.06 a,b | 2.2 | ± | 0.35 c–g | 0.63 | ± | 0.12 b–e |
| Control (100 N) | 2.0 | ± | 0.21 a,b | 0.52 | ± | 0.06 b–d | 2.5 | ± | 0.39 b–e | 0.72 | ± | 0.05 a–c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, A.H.; Abd El-Megeed, F.H.; Hassanein, N.M.; Youseif, S.H.; Farag, P.F.; Saleh, S.A.; Abdel-Wahab, B.A.; Alsuhaibani, A.M.; Helmy, Y.A.; Abdel-Azeem, A.M. Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input. J. Fungi 2022, 8, 94. https://doi.org/10.3390/jof8020094
Mohamed AH, Abd El-Megeed FH, Hassanein NM, Youseif SH, Farag PF, Saleh SA, Abdel-Wahab BA, Alsuhaibani AM, Helmy YA, Abdel-Azeem AM. Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input. Journal of Fungi. 2022; 8(2):94. https://doi.org/10.3390/jof8020094
Chicago/Turabian StyleMohamed, Akram H., Fayrouz H. Abd El-Megeed, Naziha M. Hassanein, Sameh H. Youseif, Peter F. Farag, Saleh A. Saleh, Basel A. Abdel-Wahab, Amnah Mohammed Alsuhaibani, Yosra A. Helmy, and Ahmed M. Abdel-Azeem. 2022. "Native Rhizospheric and Endophytic Fungi as Sustainable Sources of Plant Growth Promoting Traits to Improve Wheat Growth under Low Nitrogen Input" Journal of Fungi 8, no. 2: 94. https://doi.org/10.3390/jof8020094









