The Fungal Defensin Family Enlarged
Abstract
:1. Introduction
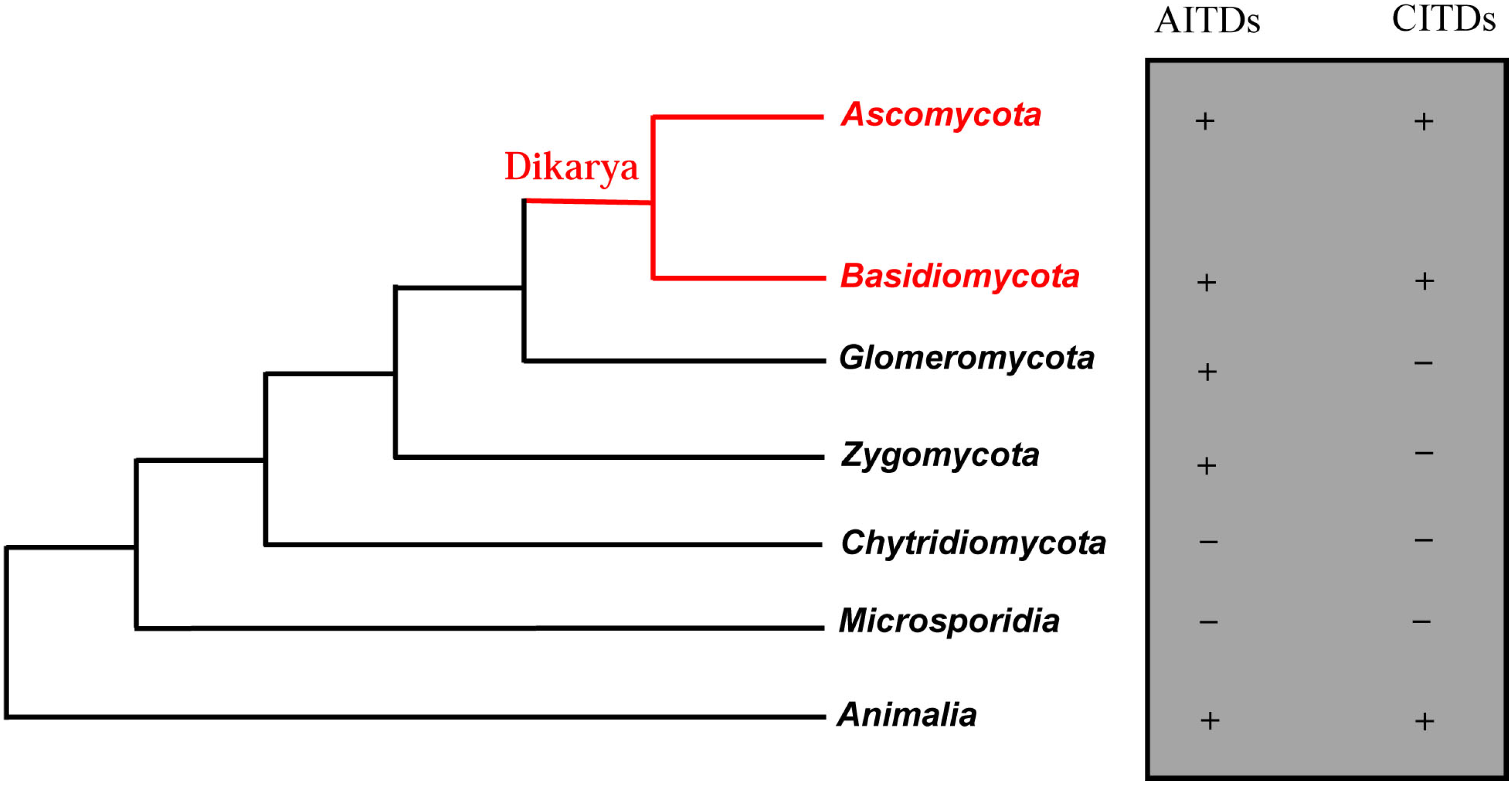
2. Discovery of New fDLPs
| Name|Accession No. | Class | Species (phylum: subphylum: class) | Size | MW | NC |
|---|---|---|---|---|---|
| Pyronesin1|CATG01000243 (G) | fDEF1 | Pyronema omphalodes (Ascomycota: Pezizomycotina: Pezizomycetes) | 40 | 4317 | +1.2 |
| Pyronesin2|CATG01000243 (G) | P. omphalodes | 40 | 4402 | +0.2 | |
| Pyronesin3|CATG01000243 (G) | P. omphalodes | 40 | 4389 | +1.2 | |
| Pyronesin4|CATG01000243 (G) | P. omphalodes | 40 | 4416 | +2.2 | |
| Pyronesin5|CATG01000243 (G) | P. omphalodes | 40 | 4375 | +1.2 | |
| Pyronesin6|CATG01000243 (G) | P. omphalodes | 40 | 4291 | +2.4 | |
| Abisin1|AEOK01000166 (G) | Agaricus bisporus (Basidiomycota: Agaricomycotina: Agaricomycetes) | 40 | 4097 | ‒3.8 | |
| Abisin2|AEOK01000166 (G) | A. bisporus | 40 | 4097 | ‒3.8 | |
| Abisin3|AEOK01000166 | A. bisporus | 39 | 3926 | ‒2.8 | |
| Beauvesin1|ADAH01000714 (G) | Beauveria bassiana (Ascomycota: Pezizomycotina: Sordariomycetes) | 52 | 5475 | +2.9 | |
| Pyrelysin|GAJI01023341 (T) | Pyrenochaeta lycopersici (Ascomycota: Pezizomycotina: Dothideomycetes) | 55 | 5858 | +5.4 | |
| Risin|JAQX01005622 | Rhizophagus irregularis (Glomeromycota: Glomeromycetes) | 55 | 5972 | +6.1 | |
| Trimensin|FG132536 (E) | Trichophyton mentagrophytes (Ascomycota: Pezizomycotina: Eurotiomycetes) | 38 | 4156 | +2.2 | |
| Lecasin|AWYC01000479 | fDEF2 | Lecanosticta acicola (Ascomycota: Pezizomycotina: Dothideomycetes) | 42 | 4314 | ‒4.8 |
| Pochlasin1|AOSW01002431 | Pochonia chlamydosporia (Ascomycota: Pezizomycotina: Sordariomycetes) | 43 | 4339 | ‒3.5 | |
| Perisin|AFRD01000258 | Periglandula ipomoeae (Ascomycota: Pezizomycotina: Sordariomycetes) | 43 | 4080 | ‒1.5 | |
| Masysin|CANK01000016 | Malassezia sympodialis (Basidiomycota: Ustilaginomycotina: Exobasidiomycetes) | 35 | 3432 | +2.2 | |
| Maglosin1N|AAYY01000039 (G) | Malassezia globosa (Basidiomycota: Ustilaginomycotina: Exobasidiomycetes) | 40 | 3980 | +1.2 | |
| Maglosin2N|AAYY01000024 (G) | M. globosa | 40 | 4022 | +0.2 | |
| Maglosin1C|AAYY01000039 (G) | M. globosa | 41 | 3910 | +2.7 | |
| Maglosin2C|AAYY01000024 (G) | M. globosa | 40 | 3835 | +2.7 | |
| Beauvesin2C|ADAH01000123 (G) | fDEF3 | B. bassiana | 41 | 4243 | +0.9 |
| ManisinC|ADNJ01000735 | Metarhizium anisopliae (Ascomycota: Pezizomycotina: Sordariomycetes) | 41 | 4211 | ‒0.1 | |
| Pochlasin2C|AOSW01005877 | P. chlamydosporia | 41 | 4381 | +0.2 | |
| AsosinC|BACA01000303 | Aspergillus sojae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 38 | 4002 | ‒1.0 | |
| Beauvesin2N|ADAH01000123 (G) | fDEF4 | B. bassiana | 48 | 5067 | +2.9 |
| ManisinN|ADNJ01000735 | M. anisopliae | 46 | 4921 | +0.2 | |
| Pochlasin2N|AOSW01005877 | P. chlamydosporia | 49 | 5185 | +2.9 | |
| AsosinN|BACA01000303 | A. sojae | 49 | 5140 | ‒1.1 | |
| Rhimisin1|ANKS01000620 | fDEF6 | Rhizopus microsporus (Zygomycota: Mucoromycotina: Mucorales) | 45 | 4867 | +10.0 |
| Rhimisin2|ANKS01000620 | R. microsporus | 44 | 4638 | +3.4 | |
| Rhimisin3|ANKS01001486 | R. microsporus | 44 | 4768 | +1.5 | |
| Rhimisin4|ANKS01001486 | R. microsporus | 45 | 4811 | +8.0 | |
| Rhidesin1|AACW02000043 | Rhizopus delemar (Zygomycota: Mucoromycotina: Mucorales) | 55 | 5885 | +10.4 | |
| Rhidesin2|AACW02000259 | R. delemar | 48 | 5270 | +0.5 | |
| Mirresin|AZYI01000143 | Mucor irregularis (Zygomycota: Mucoromycotina: Mucorales) | 60 | 6424 | +13.4 | |
| Mucisin|AOCY01001156 (G) | Mucor circinelloides (Zygomycota: Mucoromycotina: Mucorales) | 53 | 5548 | +2.2 | |
| Phycomysin|EX863311 (E) | Phycomyces blakesleeanus (Zygomycota: Mucoromycotina: Mucorales) | 50 | 5342 | +9.4 | |
| TritoDLP|ACPI01000196 (G) | fDEF8 | Trichophyton tonsurans (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4323 | +3.7 |
| TrequiDLP|ABWI01000729 (G) | Trichophyton equinum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4323 | +3.7 | |
| TriveDLP|ACYE01000402 | Trichophyton verrucosum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4403 | +4.7 | |
| ArgyDLP|ABQE01000293 | Arthroderma gypseum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 41 | 4247 | +3.7 | |
| ArbeDLP|ABSU01000004 | Arthroderma benhamiae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4493 | +4.7 | |
| TriruDLP|ACPH01000567 (G) | Trichophyton rubrum (Ascomycota: Pezizomycotina: Eurotiomycetes) | 42 | 4479 | +4.7 | |
| MicaDLP|ABVF01000093 | Arthroderma otae (Ascomycota: Pezizomycotina: Eurotiomycetes) | 43 | 4745 | +3.2 |
| Name|Accession No. | Organism | Scaffold (Contig) | Range | Size | MW | NC |
|---|---|---|---|---|---|---|
| Malpisin1-1|AZCI01001104 | Mortierella alpina B6842 | jtg7180000084593f_7180000084594f | 55070–55405 | 41 | 4048 | ‒0.0 |
| Malpisin1-2|AZCI01001104 | 55870–56127 | 48 | 5166 | ‒3.3 | ||
| Malpisin1-3|AZCI01001104 | 56393–56635 | 45 | 5047 | +0.7 | ||
| Malpisin1-4|AZCI01001104 | 63869–64117 | 39 | 4117 | ‒1.0 | ||
| Malpisin1-5|AZCI01000882 | Contig 7180000084767 | 22045–22248 | 37 | 4259 | ‒2.8 | |
| Malpisin1-6|AZCI01000882 | 25851–26051 | 39 | 4543 | ‒3.5 | ||
| Malpisin1-7|AZCI01000882 | 42456–42677 | 33 | 3624 | ‒1.0 | ||
| Malpisin1-8|AZCI01000882 | 43573–43800 | 47 | 5078 | +1.7 | ||
| Malpisin1-9|AZCI01000882 | 45037–45261 | 48 | 5203 | +3.0 | ||
| Malpisin1-10|AZCI01000882 | 45559–45738 | 35 | 3914 | ‒0.0 | ||
| Malpisin1-11|AZCI01000882 | 46707–46913 | 43 | 4941 | +5.4 | ||
| Malpisin1-12|AZCI01001135 | jtg7180000084204f_7180000084205f_7180000084206f | 135437–135676 | 44 | 4722 | ‒0.0 | |
| Malpisin1-13|AZCI01001084 | jtg7180000084699f_7180000084700f | 362415–362627 | 47 | 4919 | ‒1.8 | |
| Malpisin1-14|AZCI01001006 | jtg7180000084769f_7180000084770f_7180000084771f_7180000084772f | 179488–179673 | 38 | 4188 | +0.2 | |
| Malpisin2-1|ADAG01001070 | Mortierella alpina ATCC 32222 | Contig 1070 | 9785–10114 | 39 | 4105 | +1.0 |
| Malpisin2-2|ADAG01001070 | 10532–10792 | 48 | 5187 | ‒2.3 | ||
| Malpisin2-3|ADAG01001070 | 11052–11297 | 44 | 4783 | ‒0.0 | ||
| Malpisin2-4|ADAG01001070 | 11773–12021 | 39 | 4052 | ‒1.8 | ||
| Malpisin2-5|ADAG01000791 | Contig 791 | 4894–5097 | 37 | 4259 | ‒2.8 | |
| Malpisin2-7|ADAG01000903 | Contig 903 | 13145–13357 | 33 | 3899 | +1.2 | |
| Malpisin2-8|ADAG01000903 | 14223–14450 | 47 | 5065 | +1.7 | ||
| Malpisin2-9|ADAG01000903 | 15634–15852 | 45 | 4917 | +4.0 | ||
| Malpisin2-10|ADAG01000903 | 16158–16337 | 35 | 3937 | +0.2 | ||
| Malpisin2-11|ADAG01000903 | 17264–17446 | 39 | 4531 | +5.0 |
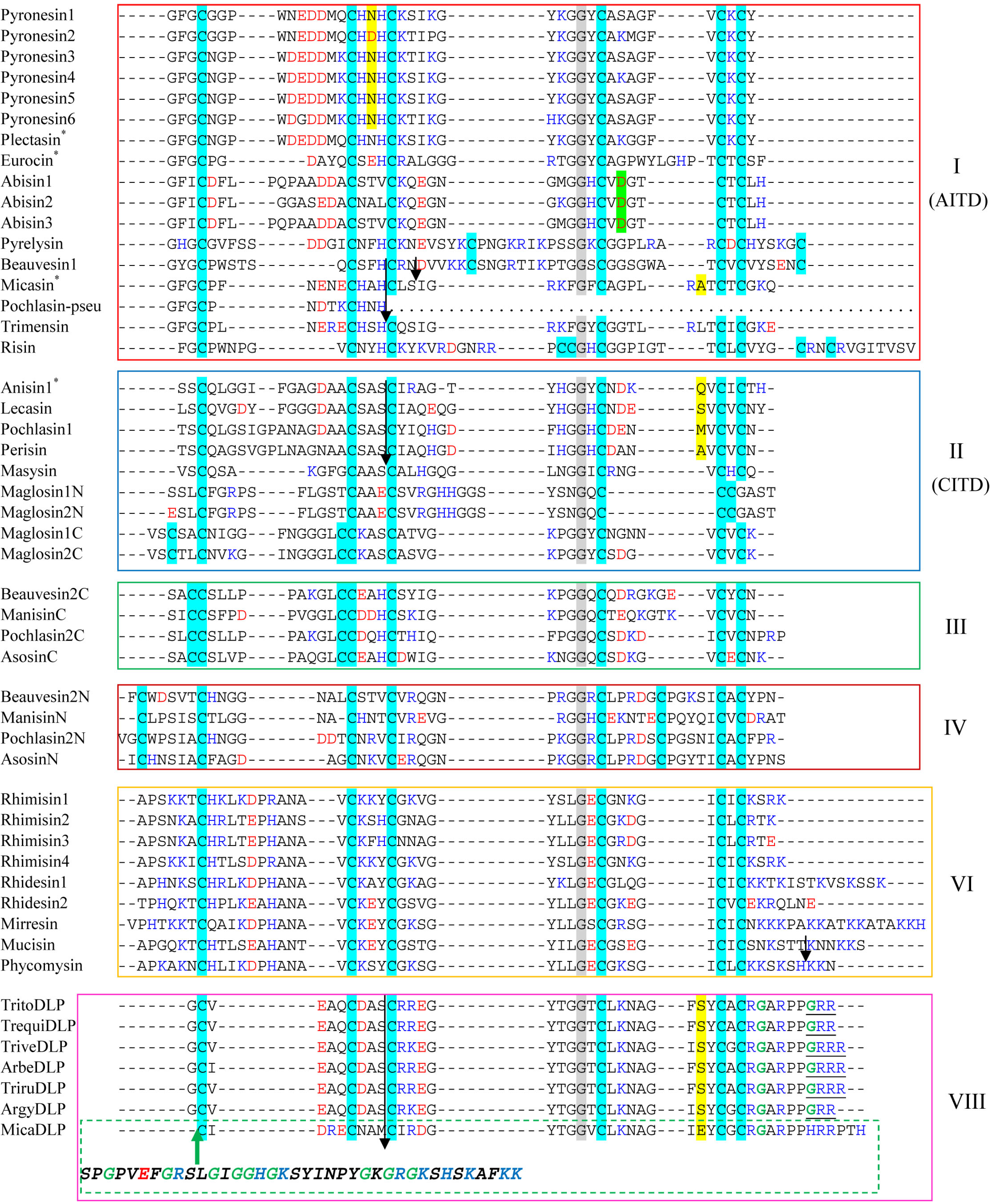

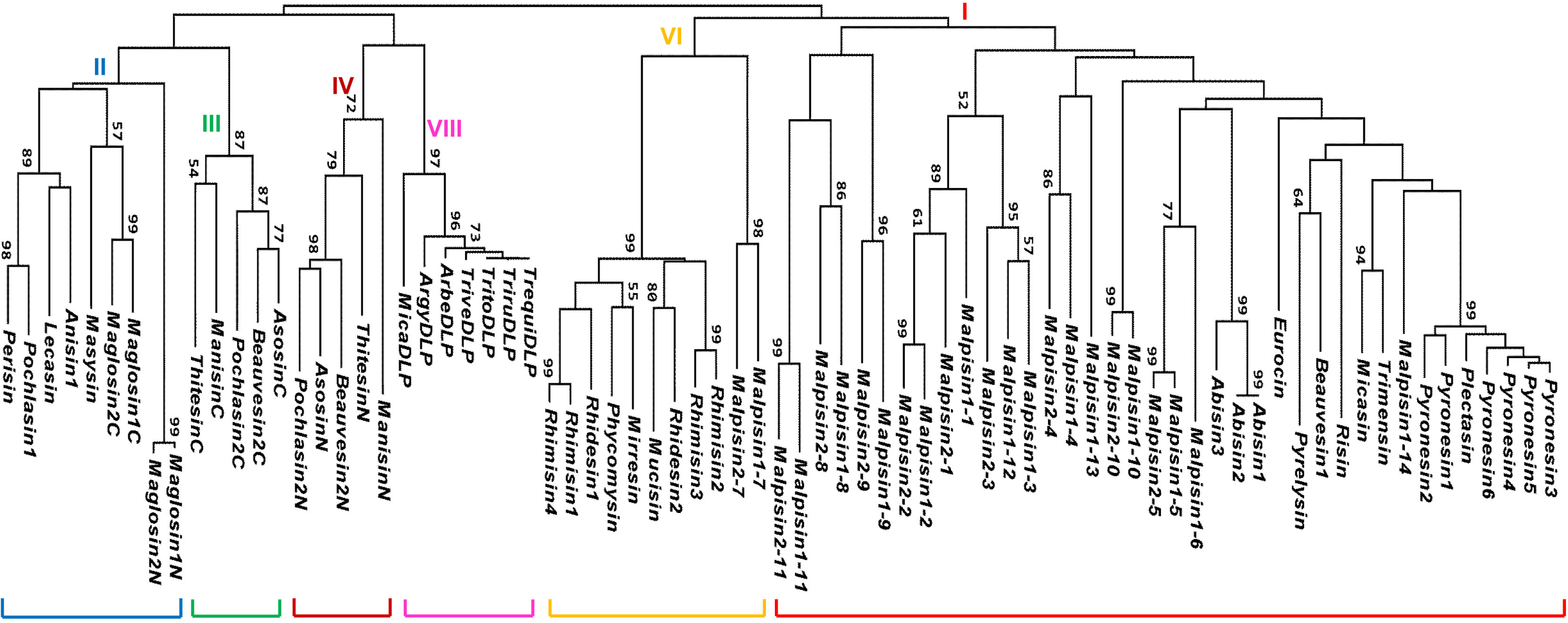
3. Gene Duplication of fDLPs
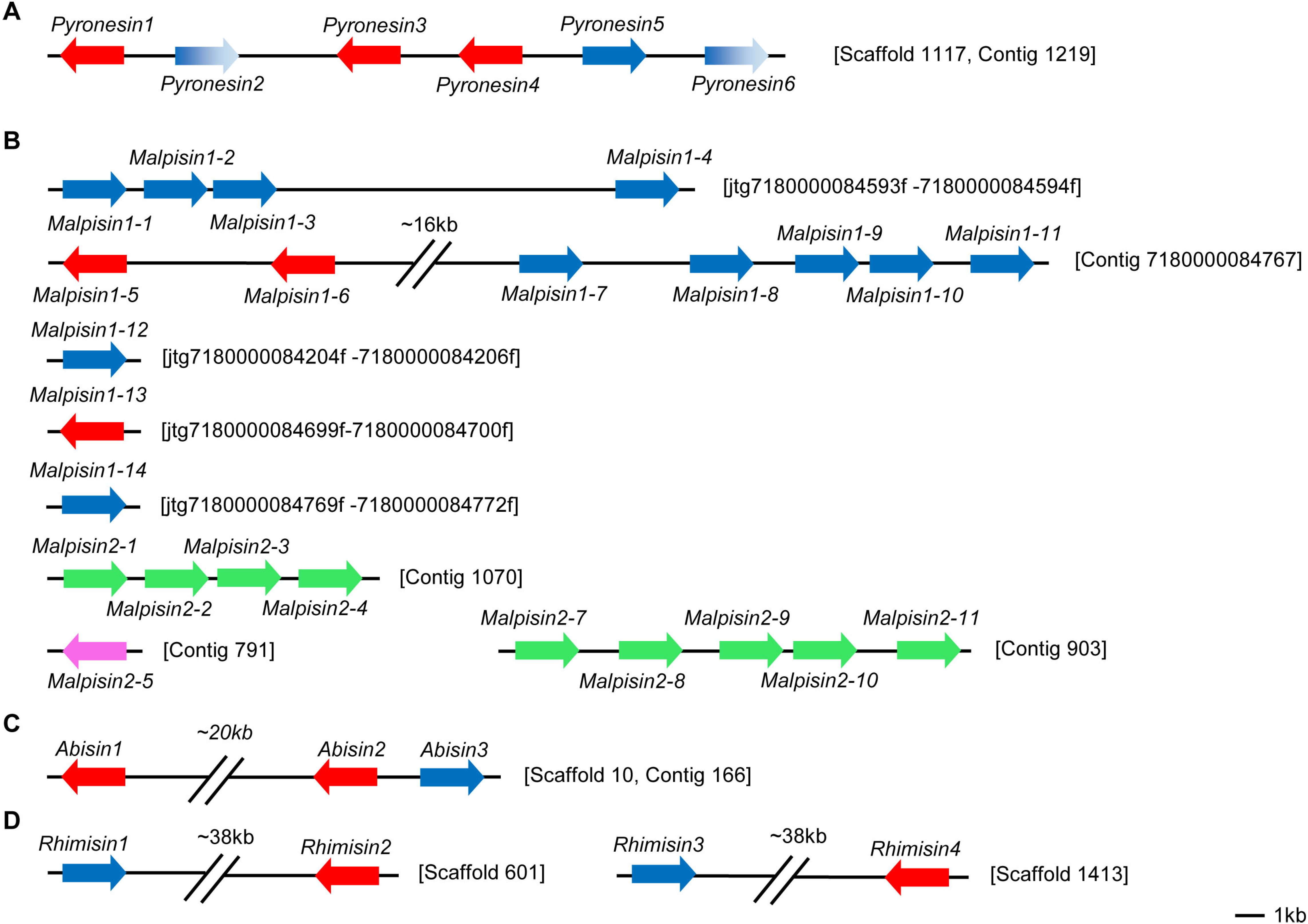
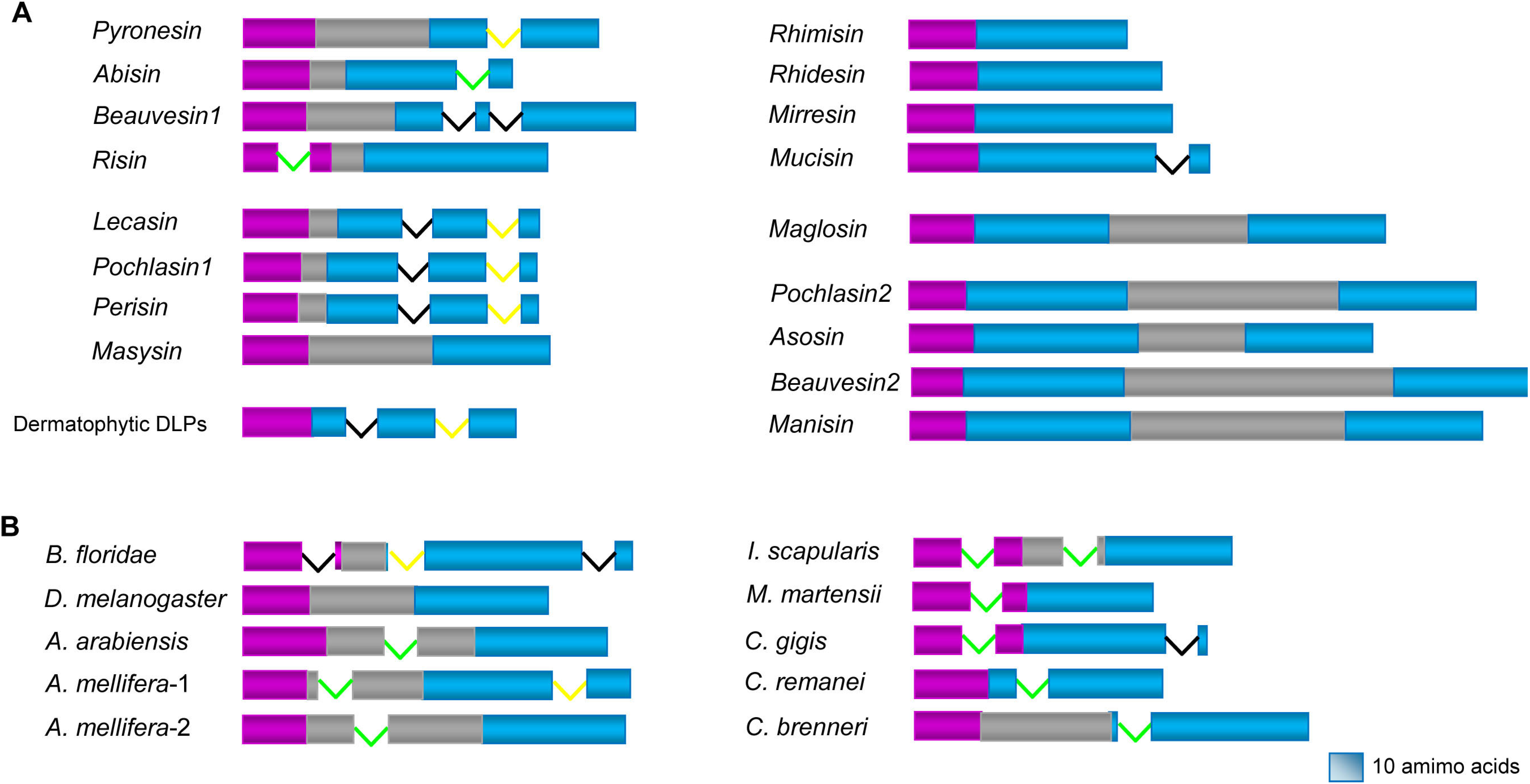
4. Variable Gene Structures of fDLPs
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgements
Author Contributions
Conflicts of Interest
References
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sonksen, C.P.; Ludvigsen, S.; Raventos, D.; Buskov, S.; Christensen, B.; De Maria, L.; et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, B.; Harvey, P.J.; Craik, D.J. Dermatophytic defensin with antiinfective potential. Proc. Natl. Acad. Sci. USA 2012, 109, 8495–8500. [Google Scholar] [CrossRef]
- Zhu, S. Discovery of six families of fungal defensin-like peptides provides insights into origin and evolution of the CSαβ defensins. Mol. Immunol. 2008, 45, 828–838. [Google Scholar] [CrossRef]
- Schneider, T.; Kruse, T.; Wimmer, R.; Wiedemann, I.; Sass, V.; Pag, U.; Jansen, A.; Nielsen, A.K.; Mygind, P.H.; Raventos, D.S.; et al. Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 2010, 328, 1168–1172. [Google Scholar] [CrossRef]
- Oeemig, J.S.; Lynggaard, C.; Knudsen, D.H.; Hansen, F.T.; Norgaard, K.D.; Schneider, T.; Vad, B.S.; Sandvang, D.H.; Nielsen, L.A.; Neve, S.; et al. Eurocin, a new fungal defensin: Structure, lipid binding, and its mode of action. J. Biol. Chem. 2012, 287, 42361–42372. [Google Scholar] [CrossRef]
- Hara, S.; Mukae, H.; Sakamoto, N.; Ishimoto, H.; Amenomori, M.; Fujita, H.; Ishimatsu, Y.; Yanagihara, K.; Kohno, S. Plectasin has antibacterial activity and no affect on cell viability or IL-8 production. Biochem. Biophys. Res. Commun. 2008, 374, 709–713. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Hady, W.A.; Deslandes, A.; Rey, A.; Fraisse, L.; Kristensen, H.H.; Yeaman, M.R.; Bayer, A.S. Efficacy of NZ2114, a novel plectasin-derived cationic antimicrobial peptide antibiotic, in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2011, 55, 5325–5330. [Google Scholar] [CrossRef]
- Ostergaard, C.; Sandvang, D.; Frimodt-Moller, N.; Kristensen, H.H. High cerebrospinal fluid (CSF) penetration and potent bactericidal activity in CSF of NZ2114, a novel plectasin variant, during experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 2009, 53, 1581–1585. [Google Scholar] [CrossRef]
- Andes, D.; Craig, W.; Nielsen, L.A.; Kristensen, H.H. In vivo pharmacodynamic characterization of a novel plectasin antibiotic, NZ2114, in a murine infection model. Antimicrob. Agents Chemother. 2009, 53, 3003–3009. [Google Scholar] [CrossRef]
- Lutzoni, F.; Kauff, F.; Cox, C.J.; McLaughlin, D.; Celio, G.; Dentinger, B.; Padamsee, M.; Hibbett, D.; James, T.Y.; Baloch, E.; et al. Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. Am. J. Bot. 2004, 91, 1446–1480. [Google Scholar] [CrossRef]
- Seidah, N.G.; Chrétien, M. Proprotein and prohormone convertases: A family of subtilases generating diverse bioactive polypeptides. Brain Res. 1999, 848, 45–62. [Google Scholar]
- Benson, D.A.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Wheeler, D.L. GenBank. Nucleic Acids Res. 2008, 36, D25–30. [Google Scholar] [CrossRef]
- Traeger, S.; Altegoer, F.; Freitag, M.; Gabaldon, T.; Kempken, F.; Kumar, A.; Marcet-Houben, M.; Poggeler, S.; Stajich, J.E.; Nowrousian, M. The genome and development-dependent transcriptomes of Pyronema confluens: A window into fungal evolution. PLoS Genet. 2013, 9, e1003820. [Google Scholar] [CrossRef]
- Tian, C.; Gao, B.; Fang, Q.; Ye, G.; Zhu, S. Antimicrobial peptide-like genes in Nasonia vitripennis: A genomic perspective. BMC Genomics 2010, 11, 187. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, S. Comparative genomics analysis of five families of antimicrobial peptide-like genes in seven ant species. Dev. Comp. Immunol. 2012, 38, 262–274. [Google Scholar] [CrossRef]
- Neves, F.; Abrantes, J.; Pinheiro, A.; Almeida, T.; Costa, P.P.; Esteves, P.J. Convergent evolution of IL-6 in two leporids (Oryctolagus and Pentalagus) originated an extended protein. Immunogenetics 2014. [Google Scholar] [CrossRef]
- Zhu, S.; Peigneur, S.; Gao, B.; Lu, X.; Cao, C.; Tytgat, J. Evolutionary diversification of Mesobuthus α-scorpion toxins affecting sodium channels. Mol. Cell. Proteomics 2012. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W.; Feng, Y.; Ren, Y.; Gu, Z.; Chen, H.; Wang, H.; Thomas, M.J.; Zhang, B.; Berquin, I.M.; et al. Genome characterization of the oleaginous fungus Mortierella alpina. PLoS ONE 2011, 6, e28319. [Google Scholar] [CrossRef]
- Etienne, K.A.; Chibucos, M.C.; Su, Q.; Orvis, J.; Daugherty, S.; Ott, S.; Sengamalay, N.A.; Fraser, C.M.; Lockhart, S.R.; Bruno, V.M. Draft genome sequence of Mortierella alpina isolate CDC-B6842. Genome Announc. 2014, 2, e01180-13. [Google Scholar]
- Schutte, B.C.; Mitros, J.P.; Bartlett, J.A.; Walters, J.D.; Jia, H.P.; Welsh, M.J.; Casavant, T.L.; McCray, P.B., Jr. Discovery of five conserved β-defensin gene clusters using a computational search strategy. Proc. Natl. Acad. Sci. USA 2002, 99, 2129–2133. [Google Scholar] [CrossRef]
- Semple, C.A.; Rolfe, M.; Dorin, J.R. Duplication and selection in the evolution of primate β-defensin genes. Genome Biol. 2003, 4, R31. [Google Scholar] [CrossRef]
- Froy, O.; Gurevitz, M. Arthropod and mollusk defensins - evolution by exon-shuffling. Trends Genet. 2003, 19, 684–687. [Google Scholar] [CrossRef]
- Rodriguez de la Vega, R.C.; Possani, L.D. On the evolution of invertebrate defensins. Trends Genet. 2005, 21, 330–332. [Google Scholar] [CrossRef]
- Gao, B.; Rodriguez Mdel, C.; Lanz-Mendoza, H.; Zhu, S. AdDLP, a bacterial defensin-like peptide, exhibits anti-Plasmodium activity. Biochem. Biophys. Res. Commun. 2009, 387, 393–398. [Google Scholar] [CrossRef]
- Yu, J.K.; Wang, M.C.; Shin-I, T.; Kohara, Y.; Holland, L.; Satoh, N.; Satou, Y. A cDNA resource for the cephalochordate amphioxus Branchiostoma floridae. Dev. Genes Evol. 2008, 218, 723–727. [Google Scholar] [CrossRef]
- Zhu, S.; Peigneur, S.; Gao, B.; Umetsu, Y.; Ohki, S.; Tytgat, J. Experimental conversion of a defensin into a neurotoxin: implications for origin of toxic function. Mol. Biol. Evol. 2014, 31, 546–559. [Google Scholar] [CrossRef]
- Sverdlov, A.V.; Rogozin, I.B.; Babenko, V.N.; Koonin, E.V. Reconstruction of ancestral protosplice sites. Curr. Biol. 2004, 14, 1505–1508. [Google Scholar] [CrossRef]
- Rogozin, I.B.; Sverdlov, A.V.; Babenko, V.N.; Koonin, E.V. Analysis of evolution of exon-intron structure of eukaryotic genes. Brief. Bioinformatics 2005, 6, 118–134. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, B.; Zhu, S. Fungal defensins, an emerging source of anti-infective drugs. Chin. Sci. Bull. 2014, 59, 931–935. [Google Scholar] [CrossRef]
- Eigentler, A.; Pocsi, I.; Marx, F. The anisin1 gene encodes a defensin-like protein and supports the fitness of Aspergillus nidulans. Arch. Microbiol. 2012, 194, 427–437. [Google Scholar] [CrossRef]
- Hegedus, N.; Marx, F. Antifungal proteins: more than antimicrobials? Fungal Biol. Rev. 2013, 26, 132–145. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, J.; Gao, B.; Zhu, S. The Fungal Defensin Family Enlarged. Pharmaceuticals 2014, 7, 866-880. https://doi.org/10.3390/ph7080866
Wu J, Gao B, Zhu S. The Fungal Defensin Family Enlarged. Pharmaceuticals. 2014; 7(8):866-880. https://doi.org/10.3390/ph7080866
Chicago/Turabian StyleWu, Jiajia, Bin Gao, and Shunyi Zhu. 2014. "The Fungal Defensin Family Enlarged" Pharmaceuticals 7, no. 8: 866-880. https://doi.org/10.3390/ph7080866
APA StyleWu, J., Gao, B., & Zhu, S. (2014). The Fungal Defensin Family Enlarged. Pharmaceuticals, 7(8), 866-880. https://doi.org/10.3390/ph7080866





