A Comprehensive Review of Azelaic Acid Pharmacological Properties, Clinical Applications, and Innovative Topical Formulations
Abstract
1. Introduction
1.1. Structure, Physicochemical Properties, and Natural Occurrence of Azelaic Acid
1.2. Synthesis and Industrial Production: Sustainable and Conventional Routes for Azelaic Acid Production
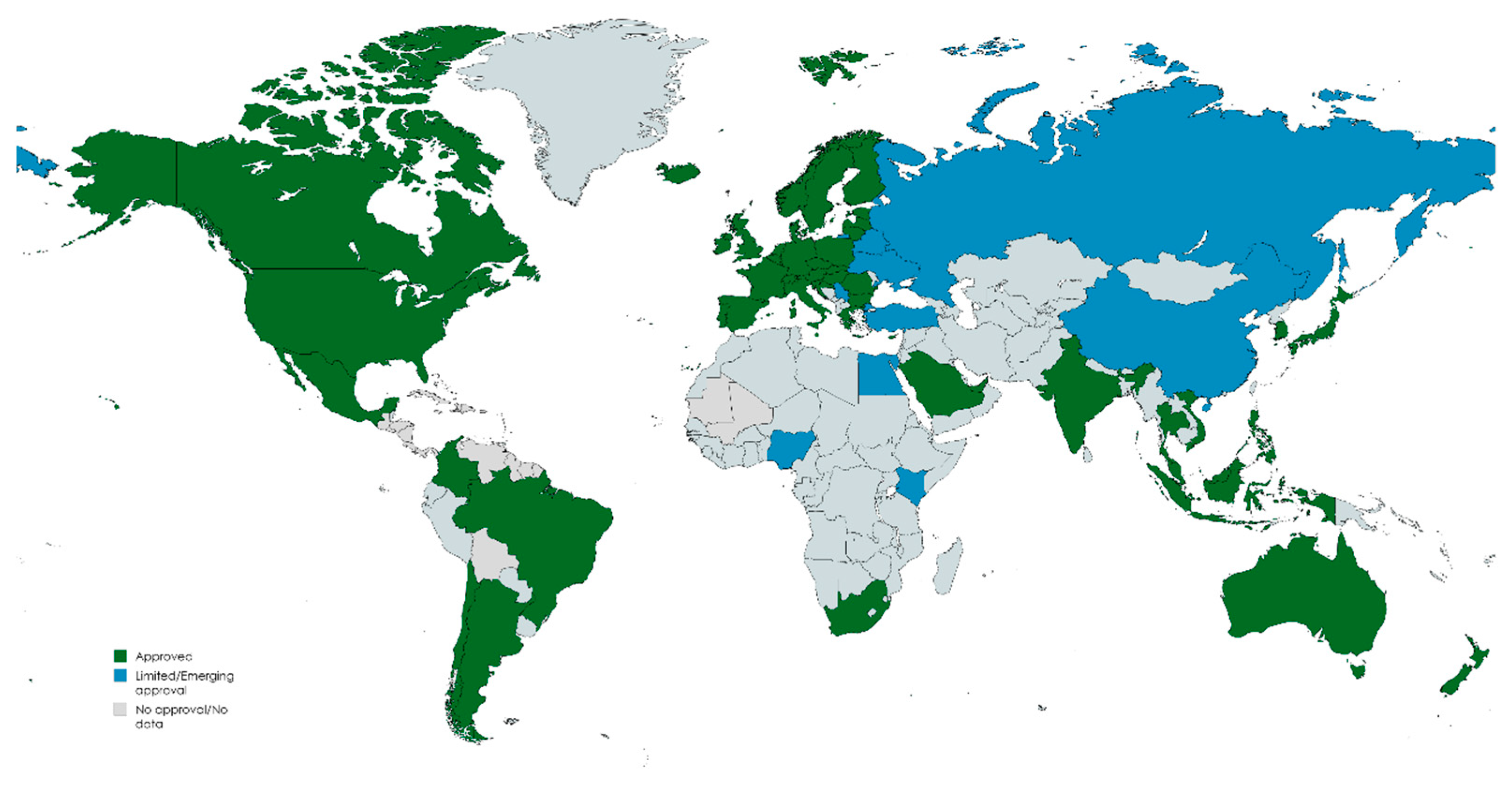
1.3. Global Approvals, Usage Patterns, and Market Trends for Azelaic Acid Products
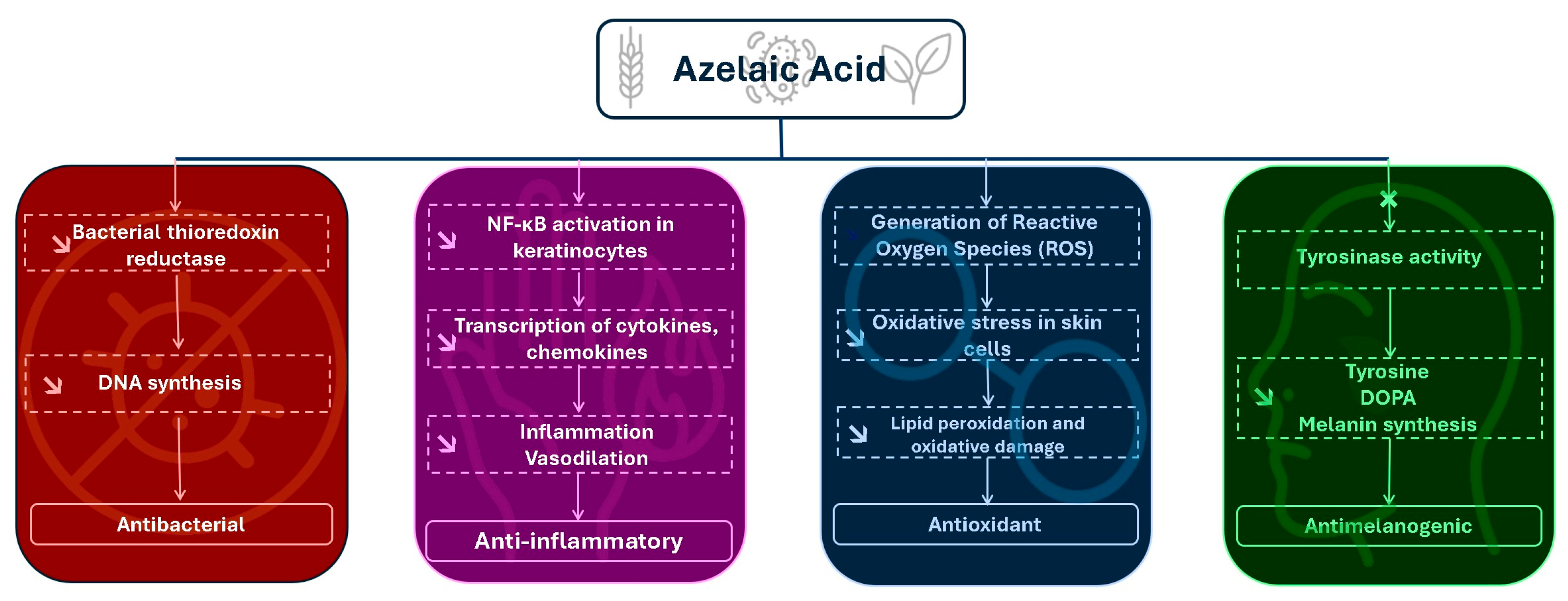
2. Pharmacodynamics and Mechanisms of Action: Biological Activities of Azelaic Acid and Their Therapeutic Relevance
Antibacterial Mechanism and Other Medical Indications
3. Clinical Studies
3.1. Clinical Trials and Outcomes in Acne Vulgaris
3.2. Rosacea: Evidence-Based Efficacy
3.3. Hyperpigmentation Disorders: Monotherapy and Combination Regimens Clinical Trials in Hyperpigmentation Disorders
3.4. Safety and Tolerability Profile
4. Stability and Analytical Methods
4.1. Introduction and Analytical Challenges
4.2. Stability Testing of Azelaic Acid Formulations
4.3. Stability-Indicating Analytical Methods
4.4. Chromatographic Techniques for Azelaic Acid Analysis
4.5. Spectrophotometric Assays for Azelaic Acid
4.6. Method Validation and Performance Parameters
- Specificity: ability to differentiate AzA from excipients and degradants.
- Linearity: typically validated over 2–3 orders of magnitude (e.g., 5–400 μg/mL).
- Accuracy and Precision: recovery of 97–100% and %RSD ≤ 2% in replicate tests.
- LOD/LOQ: for the UV-HPLC method at 206 nm, LOD was ~1.08 μg/mL and LOQ 3.28 μg/mL [85]; LC-MS techniques may offer sub-ng/mL limits.
- Robustness: demonstrated stability under ±10% variations in pH, flow rate, and detection wavelength.
5. Formulation Strategies and Delivery Systems: From Conventional Vehicles to Advanced Nanocarriers
5.1. Conventional Formulations (Gels, Creams, Foams)
5.2. Nanocarrier-Based Formulations
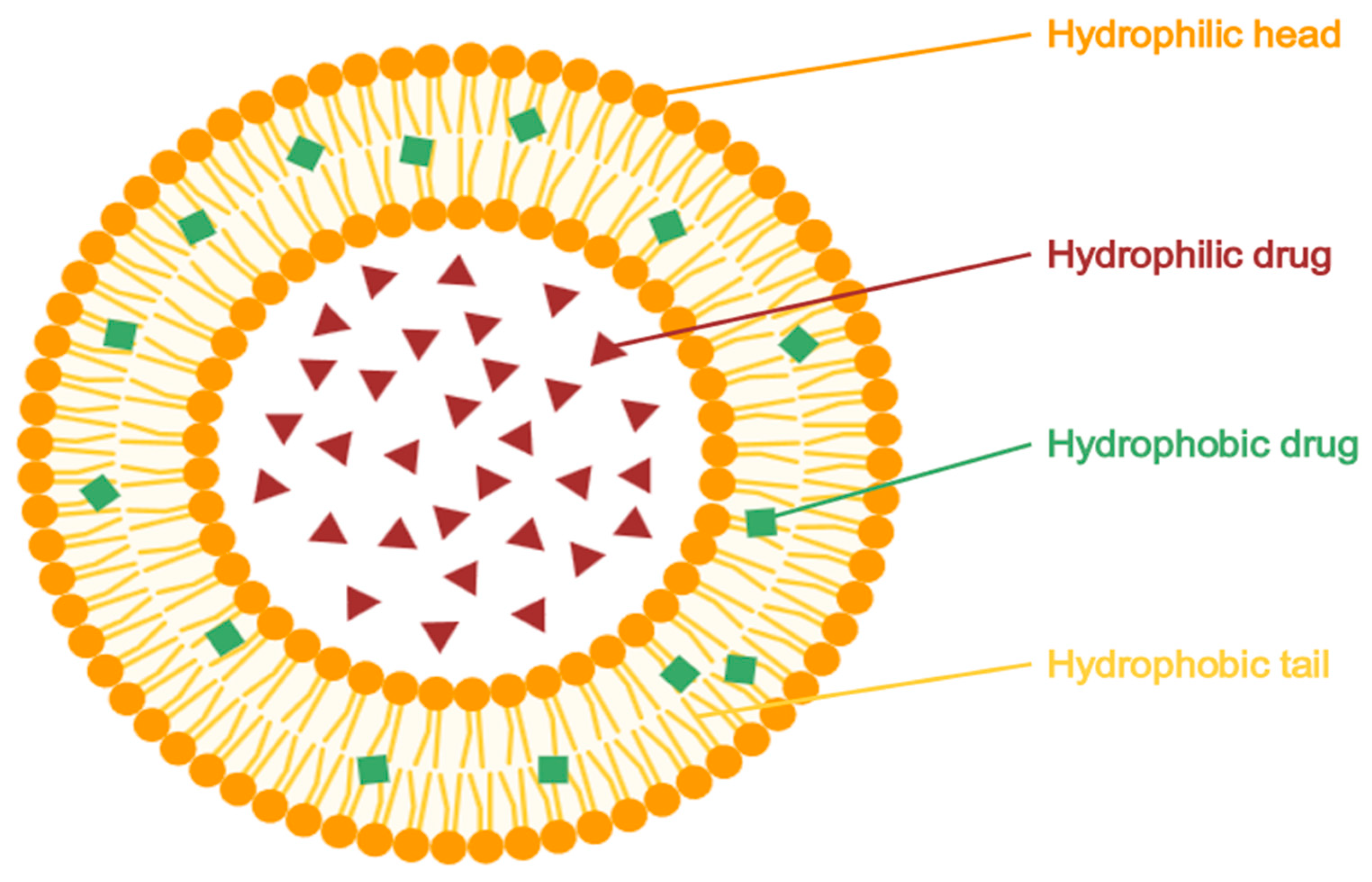
5.2.1. Liposomes
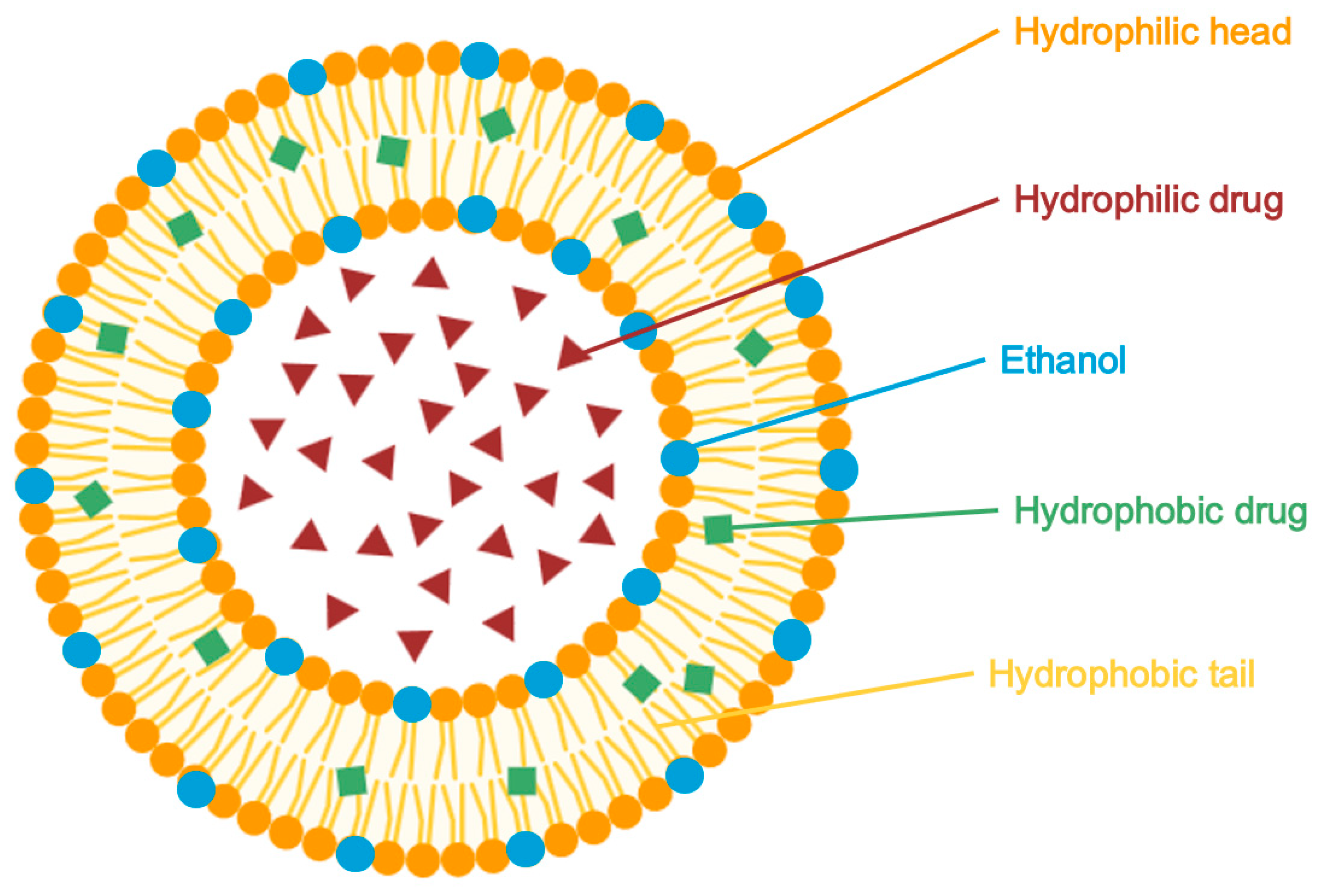
5.2.2. Ethosomes
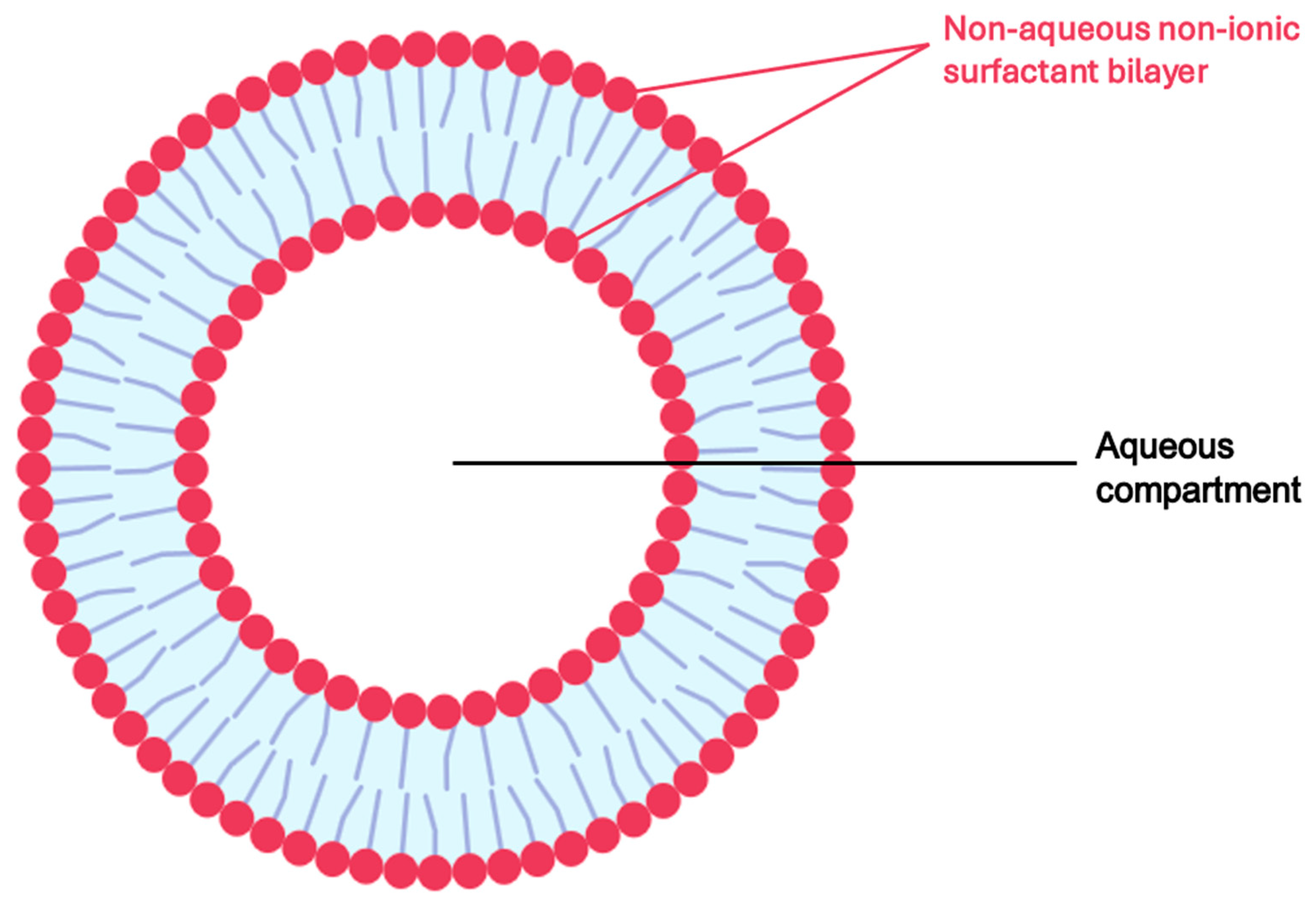
5.2.3. Niosomes
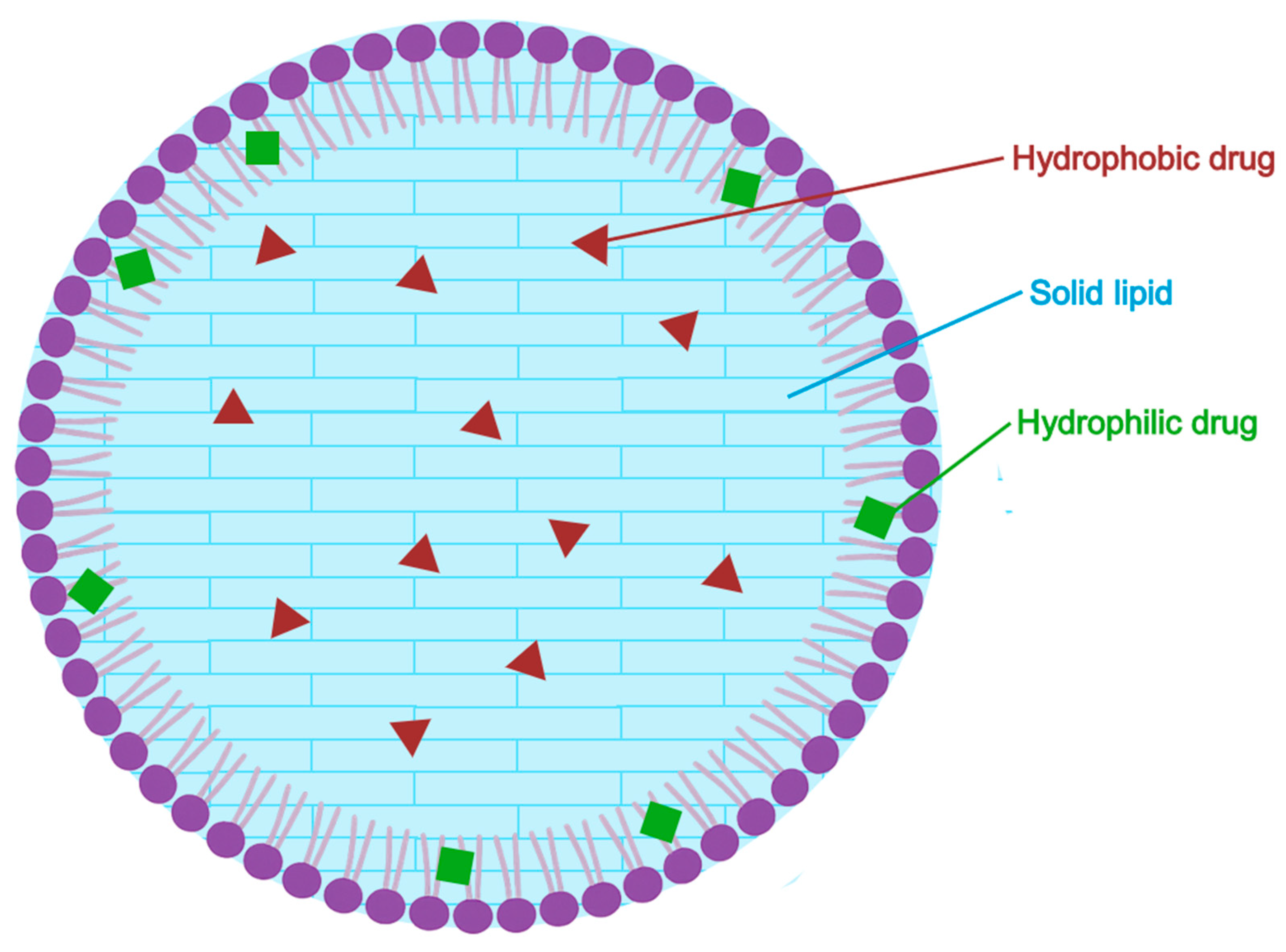
5.2.4. Solid Lipid Nanoparticles (SLN)
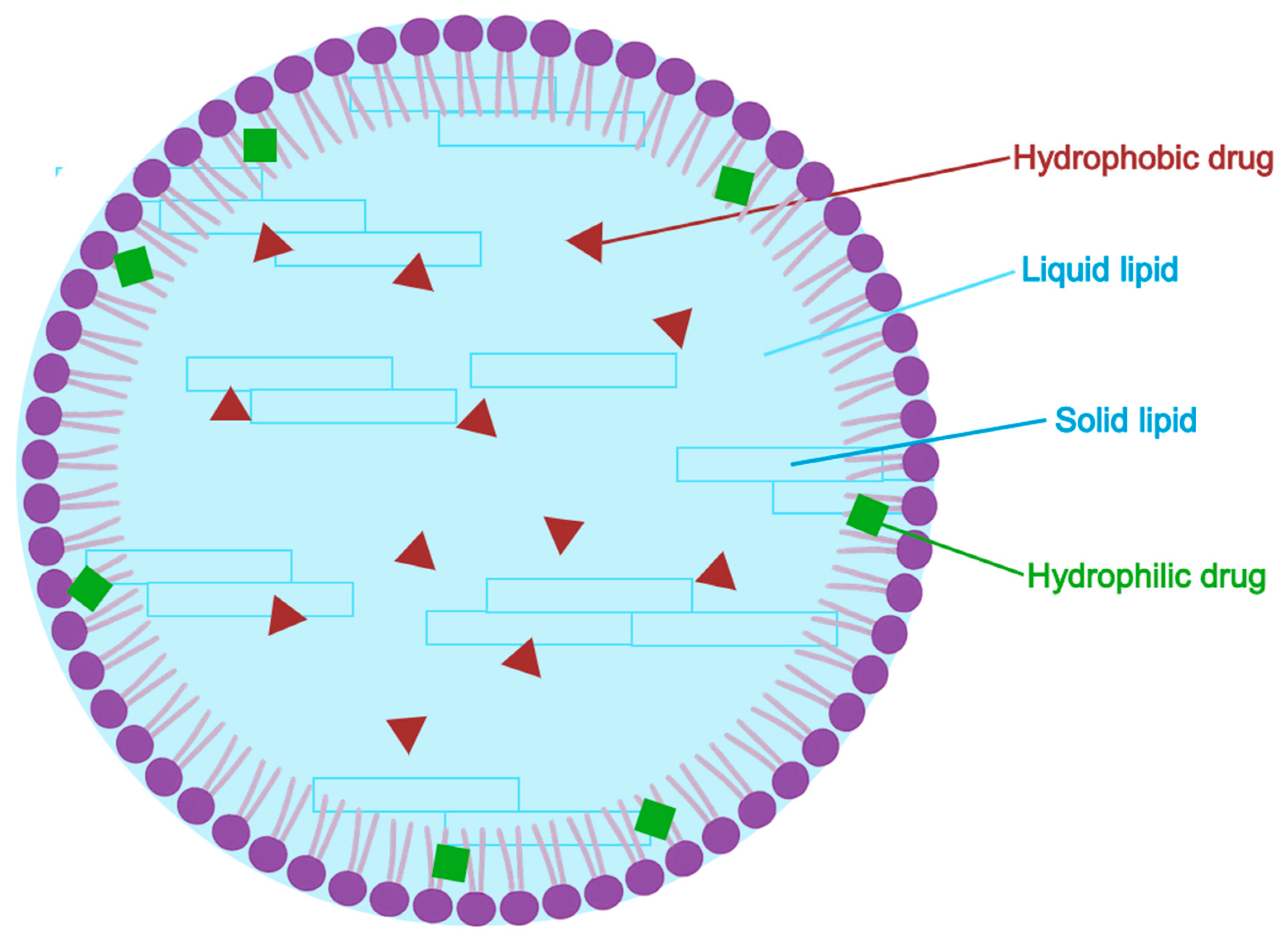
5.2.5. Nanostructured Lipid Carriers (NLC)
5.2.6. Nanosponges
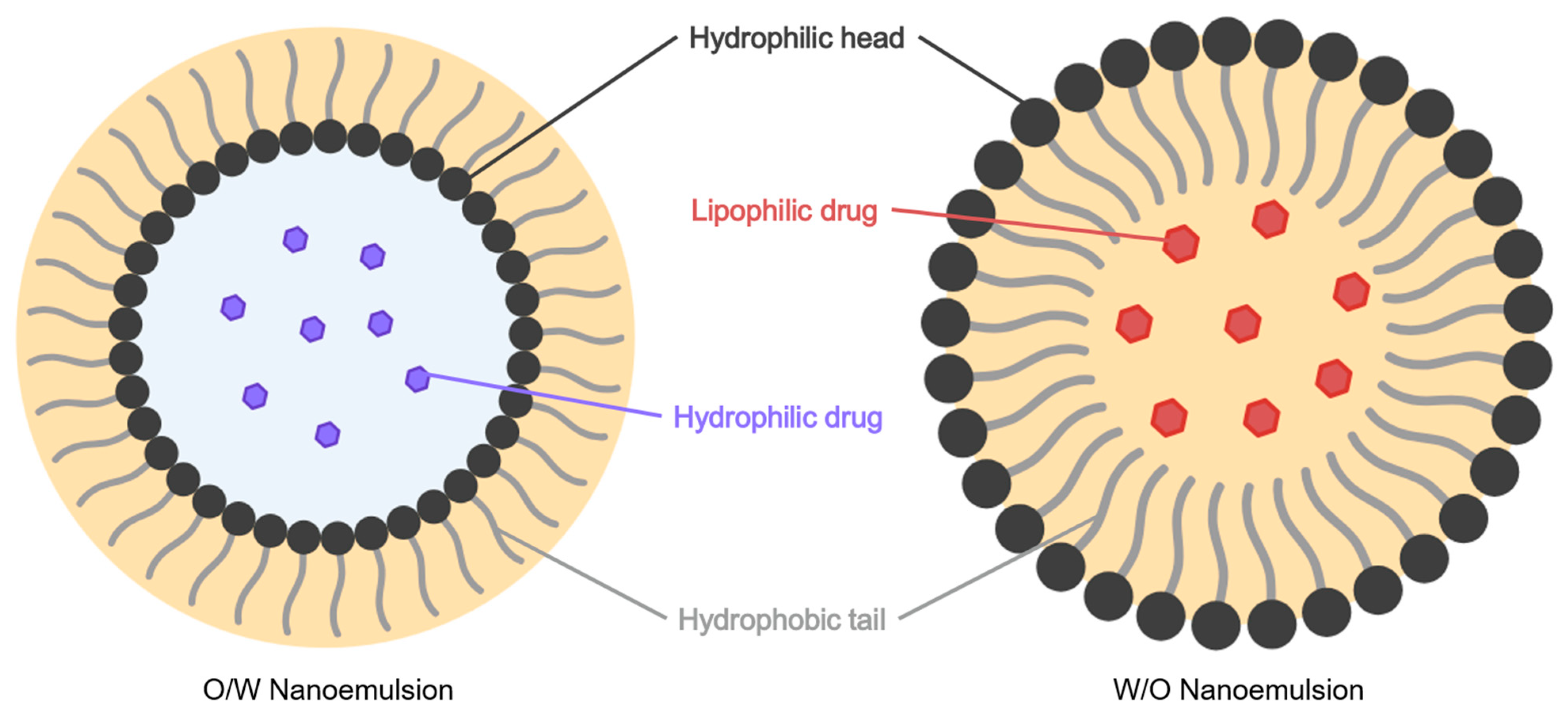
5.2.7. Nanoemulsions
5.3. Emerging Nanocarrier Systems for Potential Application in Azelaic Acid Delivery
5.3.1. Cubosomes (CBs)
5.3.2. Dendrimers
5.3.3. Polymeric Nanoparticles
5.3.4. Transethosomal Gel Systems
5.3.5. Mesoporous Silica Nanoparticles (MSNs)
5.3.6. Lyotropic Liquid Crystals (LLCs)
5.4. Pharmaceutical Cocrystals: Crystal Engineering for Solubility and Delivery
5.5. Deep Eutectic Solvents
5.5.1. Fundamentals and Pharmaceutical Classification
- -
- Type I: Metal salt + metal salt (e.g., AlCl3 + NaCl);
- -
- Type II: Metal salt + hydrated metal salt;
- -
- Type III: Quaternary ammonium salt (e.g., choline chloride ChCl) + HBD (e.g., glycerol);
- -
- Type IV: Metal salt + hydrogen bond donor;
5.5.2. Physicochemical Properties of DESs
5.5.3. DES in Pharmaceutical Drug Delivery: Solubility and Skin Penetration Enhancement
5.5.4. Solubility and Dissolution Enhancement by DES/THEDES
5.5.5. Enhancement of Skin Penetration and Drug Delivery
5.5.6. DES-Based Topical Formulations of Azelaic Acid and Related Systems
5.6. Quality-by-Design (QbD) Optimization
5.7. Challenges and Considerations on Integration of Artificial Intelligence in Azelaic Acid Therapy
6. Combination Therapies
6.1. Synergistic Combinations (Azelaic Acid with Retinoids, Antibiotics, Antioxidants)
6.2. Enhanced Dermatologic Outcomes with Azelaic Acid-Retinoid Combinations
6.3. Azelaic Acid and Antibiotics: A Dual Approach to Inflammatory Skin Disorders
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- PubChem. Azelaic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2266 (accessed on 7 May 2025).
- Feng, X.; Shang, J.; Gu, Z.; Gong, J.; Chen, Y.; Liu, Y. Azelaic acid: Mechanisms of action and clinical applications. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2359–2371. [Google Scholar] [CrossRef]
- Sauer, N.; Oślizło, M.; Brzostek, M.; Wolska, J.; Lubaszka, K.; Karłowicz-Bodalska, K. The multiple uses of azelaic acid in der-matology: Mechanism of action, preparations, and potential therapeutic applications. Postepy Dermatol. Alergol. 2023, 40, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Breathnach, A.S. Pharmacological properties of azelaic acid: A rationale for clinical use. Clin. Drug Investig. 1995, 10 (Suppl. S2), 27–33. [Google Scholar] [CrossRef]
- Ma, Y.; Chun, J.; Wang, S.; Chen, F. Allelopathic Potential of Jatropha curcas. Afr. J. Biotechnol. 2011, 10, 11932–11942. [Google Scholar]
- Spaggiari, C.; Annunziato, G.; Spadini, C.; Montanaro, S.L.; Iannarelli, M.; Cabassi, C.S.; Costantino, G. Extraction and quantification of azelaic acid from different wheat samples (Triticum durum Desf.) and evaluation of their antimicrobial and antioxidant activities. Molecules 2023, 28, 2134. [Google Scholar] [CrossRef]
- Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/search_product.cfm (accessed on 24 July 2025).
- AZELAIC ACID, 95%|Marketing Authorisations|MA|Europe. Available online: https://www.pharmacompass.com/eu-ctd-dossier-marketing-authorisation/azelaic-acid-95 (accessed on 24 July 2025).
- De Leon Izeppi, G.A.; Dubois, J.-L.; Balle, A.; Soutelo-Maria, A. Economic risk assessment using Monte Carlo simulation for the production of azelaic acid and pelargonic acid from vegetable oils. Ind. Crops Prod. 2020, 150, 112411. [Google Scholar] [CrossRef]
- Köckritz, A.; Martin, A. Synthesis of azelaic acid from vegetable oil-based feedstocks. Eur. J. Lipid Sci. Technol. 2011, 113, 83–91. [Google Scholar] [CrossRef]
- Todea, A.; Deganutti, C.; Spennato, M.; Asaro, F.; Zingone, G.; Milizia, T.; Gardossi, L. Azelaic Acid: A Bio-Based Building Block for Biodegradable Polymers. Polymers 2021, 13, 4091. [Google Scholar] [CrossRef]
- Cotarca, L.; Delogu, P.; Nardelli, A.; Maggioni, P.; Bianchini, R.; Sguassero, S.; Alini, S.; Dario, R.; Clauti, G.; Pitta, G.; et al. Efficient and scaleable methods for ω-functionalized nonanoic acids: Development of a novel process for azelaic and 9-aminononanoic acids (Nylon-6,9 and Nylon-9 precursors). Org. Process Res. Dev. 2001, 5, 69–76. [Google Scholar] [CrossRef]
- Cotarca, L.; Delogu, P.; Maggioni, P.; Nardelli, A.; Bianchini, R.; Sguassero, S. Efficient synthesis of ω-functionalized nonanoic acids. Synthesis 1997, 1997, 328–332. [Google Scholar] [CrossRef]
- Elewski, B.E. Azelaic acid 15% gel for treatment of rosacea. Expert. Rev. Dermatol. 2006, 1, 535–545. [Google Scholar] [CrossRef]
- Bjerke, R.; Fyrand, O.; Graupe, K. Double-blind comparison of azelaic acid 20% cream and its vehicle in treatment of papulo-pustular rosacea. Acta Derm. Venereol. 1999, 79, 456–459. [Google Scholar]
- Thielitz, A.; Lux, A.; Wiede, A.; Kropf, S.; Papakonstantinou, E.; Gollnick, H. A randomized investigator-blind parallel-group study to assess efficacy and safety of azelaic acid 15% gel vs. adapalene 0.1% gel in the treatment and maintenance treatment of female adult acne. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Elewski, B.E.; Fleischer, A.B.; Pariser, D.M. A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: Results of a randomized trial. Arch. Dermatol. 2003, 139, 1444. [Google Scholar] [CrossRef]
- King, S.; Campbell, J.; Rowe, R.; Daly, M.; Moncrieff, G.; Maybury, C. A systematic review to evaluate the efficacy of azelaic acid in the management of acne, rosacea, melasma and skin aging. J. Cosmet. Dermatol. 2023, 22, 2650–2662. [Google Scholar] [CrossRef]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid–willow bark extract–panthenol-loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crops Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, G.; Popescu, M.; Bordei, N.; Istrati, D.; Moldovan, L.; Seciu, A.M.; Panteli, M.I.; Rasit, I.; Badea, N. Marigold extract, azelaic acid and black caraway oil into lipid nanocarriers provides a strong anti-inflammatory effect in vivo. Ind. Crops Prod. 2017, 109, 141–150. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Sultan, M.H.; Almoshari, Y.; Sivadasan, D.; Alqahtani, S.S.; Madkhali, O.A.; Ahsan, W. Pharmaceutical applications of therapeutic deep eutectic systems (THEDES) in maximising drug delivery. Heliyon 2024, 10, e29783. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wu, X.; Jia, W.; Zhang, M.C.; Tan, F.; Zhang, J. Effect of ionization and vehicle on skin absorption and penetration of azelaic acid. Drug Dev. Ind. Pharm. 2012, 38, 985–994. [Google Scholar] [CrossRef]
- Burchacka, E.; Potaczek, P.; Paduszyński, P.; Karłowicz-Bodalska, K.; Han, T.; Han, S. New effective azelaic acid liposomal gel formulation of enhanced pharmaceutical bioavailability. Biomed. Pharmacother. 2016, 83, 771–775. [Google Scholar] [CrossRef]
- U.S. FDA. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021470s005lbl.pdf (accessed on 7 May 2025).
- Sunveniz XL 150 mg Prolonged Release Tablets—Summary of Product Characteristics (SmPC)—(emc)|5349. Available online: https://www.medicines.org.uk/emc/product/5349/smpc (accessed on 12 May 2025).
- Azzalure|SPC|Medicines.ie. Available online: https://www.medicines.ie/medicines/skinoren-cream-20-w-w-31387/spc (accessed on 12 May 2025).
- Medicinal Product Activity Control Directorate. Guideline for the Preparation of the Summary of Product Characteristics (SmPC). Available online: https://amdm.gov.md/old_files/files/Autorizarea%20medicamentelor/Ordine/Ghid%20RCP.pdf (accessed on 12 May 2025).
- Azelaic Acid Market. Market.us. Available online: https://market.us/report/azelaic-acid-market/ (accessed on 7 May 2025).
- Allied Market Research. Azelaic Acid Market Size and Share Growth Report 2033. Available online: https://www.alliedmarketresearch.com/azelaic-acid-market-A15507 (accessed on 7 May 2025).
- Verified Market Research. Azelaic Acid Topical Market Size, Trends, Scope and Forecast. Available online: https://www.verifiedmarketresearch.com/product/azelaic-acid-topical-market/ (accessed on 7 May 2025).
- Gasco, M.R.; Gallarate, M.; Pattarino, F. In vitro permeation of azelaic acid from viscosized microemulsions. Int. J. Pharm. 1991, 69, 193–196. [Google Scholar] [CrossRef]
- Hossain, A.; Bu, L.; Hartley, R.S.; Allanson, J.T. Waterborne Topical Compositions Comprising Azelaic Acid. US Patent US20100004296A1, 7 January 2010. [Google Scholar]
- Apriani, E.; Rosana, Y.; Iskandarsyah, I. Formulation, characterization, and in vitro testing of azelaic acid ethosome-based cream against Propionibacterium acnes for the treatment of acne. J. Adv. Pharm. Technol. Res. 2019, 10, 75. [Google Scholar] [CrossRef]
- Nautiyal, A.; Wairkar, S. A reduced dose of azelaic acid-loaded solid lipid nanoparticles for treatment of hyperpigmentation: In vitro characterization and cell line studies. J. Drug Deliv. Sci. Technol. 2023, 80, 104158. [Google Scholar] [CrossRef]
- Arpa, M.D.; Seçen, İ.M.; Erim, Ü.C.; Hoş, A.; Üstündağ Okur, N. Azelaic acid loaded chitosan and HPMC based hydrogels for treatment of acne: Formulation, characterization, in vitro—Ex vivo evaluation. Pharm. Dev. Technol. 2022, 27, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, R. Enhancing efficacy and safety of azelaic acid via encapsulation in cyclodextrin nanosponges: Development, characterization and evaluation. Polym. Bull. 2021, 78, 5275–5302. [Google Scholar] [CrossRef]
- Yeow, A.T.H.; Hayyan, A.; Hayyan, M.; Junaidi, M.U.M.; Saleh, J.; Basirun, W.J.; Nor, M.R.M.; Al Abdulmonem, W.; Salleh, M.Z.M.; Zuki, F.M.; et al. A comprehensive review on the physicochemical properties of deep eutectic solvents. Results Chem. 2024, 7, 101378. [Google Scholar] [CrossRef]
- Ko, J.; Mandal, A.; Dhawan, S.; Shevachman, M.; Mitragotri, S.; Joshi, N. Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 2021, 6, e10191. [Google Scholar] [CrossRef]
- Pedro, S.N.; Mendes, M.S.M.; Neves, B.M.; Almeida, I.F.; Costa, P.; Correia-Sá, I.; Vilela, C.; Freire, M.G.; Silvestre, A.J.D.; Freire, C.S.R. Deep eutectic solvent formulations and alginate-based hydrogels as a new partnership for the transdermal administration of anti-inflammatory drugs. Pharmaceutics 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Shen, H.; Cai, B.; Xie, Y.; Wang, Y.; Wang, J. 15% Azelaic acid gel modify the skin microbiota of acne vulgaris. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100041. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, R.; Yadav, P. Azelaic acid: A promising agent for dermatological applications. Curr. Drug Ther. 2020, 15, 181–193. [Google Scholar]
- Mastrofrancesco, A.; Ottaviani, M.; Aspite, N.; Cardinali, G.; Izzo, E.; Graupe, K.; Zouboulis, C.C.; Camera, E.; Picardo, M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPARγ activation. Exp. Dermatol. 2010, 19, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Sial, N.T.; Malik, A.; Iqbal, U.; Mehmood, M.H.; Rehman, M.F.U. Novel antiarthritic mechanisms of azelaic acid against CFA-induced arthritis in rats by modulating pro- and anti-inflammatory cytokines network. Inflammopharmacology 2024, 32, 2445–2462. [Google Scholar] [CrossRef]
- Li, L.; Lu, H.; Zhang, Y.; Li, Q.; Shi, S.; Liu, Y. Effect of azelaic acid on psoriasis progression investigated based on phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2523–2534. [Google Scholar] [CrossRef]
- Mazurek, K.; Pierzchała, E. Comparison of efficacy of products containing azelaic acid in melasma treatment. J. Cosmet. Dermatol. 2016, 15, 269–282. [Google Scholar] [CrossRef]
- Shucheng, H.; Zhou, X.; Du, D.; Li, J.; Yu, C.; Jiang, X. Effects of 15% azelaic acid gel in the management of post-inflammatory erythema and post-inflammatory hyperpigmentation in acne vulgaris. Dermatol. Ther. 2024, 14, 1293–1314. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Z.; Jin, Y.; Chen, Y.; Yang, T.; Yang, Q.; Wu, B.; Shang, Y.; Liu, X.; Wei, Y.; et al. Azelaic acid exerts antileukemia effects against acute myeloid leukemia by regulating the Prdxs/ROS signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1295984. [Google Scholar] [CrossRef]
- Mayer-da-Silva, A. Azelaic acid: Pharmacology, toxicology and mechanism of action in acne. J. Dermatol. Treat. 1989, 1 (Suppl. S1), 11–15. [Google Scholar] [CrossRef]
- Hung, W.-H.; Chen, P.-K.; Fang, C.-W.; Lin, Y.-C.; Wu, P.-C. Preparation and evaluation of azelaic acid topical microemulsion formulation: In vitro and in vivo study. Pharmaceutics 2021, 13, 410. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm activities of fatty acids including myristoleic acid against Cutibacterium acnes via reduced cell hydrophobicity. Phytomedicine 2021, 91, 153710. [Google Scholar] [CrossRef]
- Cristani, M.; Micale, N. Bioactive compounds from medicinal plants as potential adjuvants in the treatment of mild acne vulgaris. Molecules 2024, 29, 2394. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential role of the microbiome in acne: A comprehensive review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M. Azelaic acid as a competitive inhibitor of thioredoxin reductase in human melanoma cells. Cancer Lett. 1987, 36, 297–305. [Google Scholar] [CrossRef]
- Woo, Y.; Lim, J.; Cho, D.; Park, H. Rosacea: Molecular mechanisms and management of a chronic cutaneous inflammatory condition. Int. J. Mol. Sci. 2016, 17, 1562. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.; Goa, K.L. Azelaic acid: A review of its pharmacological properties and therapeutic efficacy in acne and hyperpigmentary skin disorders. Drugs 1991, 41, 780–798. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment. Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of melanogenesis: An updated review. J. Med. Chem. 2018, 61, 7395–7418. [Google Scholar] [CrossRef]
- Kumari, S.; Thng, S.; Verma, N.; Gautam, H. Melanogenesis inhibitors. Acta Derm. Venereol. 2018, 98, 924–931. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, H.; Dong, P.; Zhang, T.; Lu, C.; Jin, T.; Chai, K.Y. Pegylated azelaic acid: Synthesis, tyrosinase inhibitory activity, antibacterial activity and cytotoxic studies. J. Mol. Struct. 2021, 1224, 129234. [Google Scholar] [CrossRef]
- Sardana, K.; Ghunawat, S. Rationale of using hypopigmenting drugs and their clinical application in melasma. Expert. Rev. Clin. Pharmacol. 2015, 8, 123–134. [Google Scholar] [CrossRef]
- Sieber, M.A.; Hegel, J.K.E. Azelaic acid: Properties and mode of action. Skin. Pharmacol. Physiol. 2014, 27 (Suppl. S1), 9–17. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Gallo, R.L. The critical and multifunctional roles of antimicrobial peptides in dermatology. Dermatol. Clin. 2017, 35, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ollengo, M.A. The Photochemistry and Photostabilization Potential of Plant Extracts on Sunscreen Absorbers. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2014. [Google Scholar]
- Katsambas, A.; Graupe, K.; Stratigos, E. Clinical studies of 20% azelaic acid cream in the treatment of acne vulgaris. Comparison with vehicle and topical tretinoin. Acta Derm. Venereol. 1989, 69, 260–263. [Google Scholar]
- Iraji, F.; Sadeghinia, A.; Shahmoradi, Z.; Siadat, A.; Jooya, A. Efficacy of topical azelaic acid gel in the treatment of mild-moderate acne vulgaris. Indian J. Dermatol. Venereol. Leprol. 2007, 73, 94. [Google Scholar] [CrossRef] [PubMed]
- Maddin, S. A comparison of topical azelaic acid 20% cream and topical metronidazole 0.75% cream in the treatment of patients with papulopustular rosacea. J. Am. Acad. Dermatol. 1999, 40, 961–965. [Google Scholar] [CrossRef]
- Thiboutot, D.; Thieroff-Ekerdt, R.; Graupe, K. Efficacy and safety of azelaic acid (15%) gel as a new treatment for papulopustular rosacea: Results from two vehicle-controlled, randomized phase III studies. J. Am. Acad. Dermatol. 2003, 48, 836–845. [Google Scholar] [CrossRef]
- Frampton, J.E.; Wagstaff, A.J. Azelaic acid 15% gel: In the treatment of papulopustular rosacea. Am. J. Clin. Dermatol. 2004, 5, 57–64. [Google Scholar] [CrossRef]
- Lowe, N.J.; Rizk, D.; Grimes, P.; Billips, M.; Pincus, S. Azelaic acid 20% cream in the treatment of facial hyperpigmentation in darker-skinned patients. Clin. Ther. 1998, 20, 945–959. [Google Scholar] [CrossRef]
- Ahmad, I.; Wahid, Z.; Ansari, M. Melasma: A comparative trial of azelaic acid (20%) cream alone and in combination with tretinoin (0.1%) cream. J. Pak. Assoc. Dermatol. 2004, 14, 10–15. [Google Scholar]
- Gupta, A.K.; Gover, M.D.; Nouri, K.; Taylor, S. The treatment of melasma: A review of clinical trials. J. Am. Acad. Dermatol. 2006, 55, 1048–1065. [Google Scholar] [CrossRef]
- Kakita, L.S.; Lowe, N.J. Azelaic acid and glycolic acid combination therapy for facial hyperpigmentation in darker-skinned patients: A clinical comparison with hydroquinone. Clin. Ther. 1998, 20, 960–970. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Thiboutot, D.; Gallo, R.; Webster, G.; Tanghetti, E.; Eichenfield, L.; Stein-Gold, L.; Berson, D.; Zaenglein, A. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 2: A status report on topical agents. Cutis 2013, 92, 277–284. [Google Scholar] [PubMed]
- Yaghi, M. Acne and pregnancy: A clinical review and practice pearls. Cutis 2024, 113, E26–E32. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Esmaili-Azad, M.; Vaezi, M.; Saljoughi, N. Efficacy and safety of azelaic acid 20% plus hydroquinone 5% in the management of melasma. Iran. J. Dermatol. 2012, 15, 1–6. [Google Scholar]
- Verallo-Rowell, V.M.; Verallo, V.; Graupe, K.; Lopez-Villafuerte, L.; Garcia-Lopez, M. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm. Venereol. Suppl. 1989, 143, 19–23. [Google Scholar]
- Albzea, W.; AlRashidi, R.; Alkandari, D.; Sadan, M.; Alkandari, A.; Alkanderi, J.J.; AlHajri, M.T.; Almutairi, S.N.; Alenzi, A.; Alanazi, S.; et al. Azelaic acid versus hydroquinone for managing patients with melasma: Systematic review and meta-analysis of randomized controlled trials. Cureus 2023, 15, e41796. [Google Scholar] [CrossRef]
- Chilicka, K.; Rogowska, A.M.; Szyguła, R.; Dzieńdziora-Urbińska, I.; Taradaj, J. A comparison of the effectiveness of azelaic and pyruvic acid peels in the treatment of female adult acne: A randomized controlled trial. Sci. Rep. 2020, 10, 12612. [Google Scholar] [CrossRef]
- Hasanbeyzade, S. Comparison of topical 20% azelaic acid and 7.5% dapsone in the treatment of mild-to-moderate papulopustular rosacea. J. Cosmet. Dermatol. 2025, 24, e70212. [Google Scholar] [CrossRef]
- Gao, X.; Xiang, W. Efficacy of widely used topical drugs for rosacea: A systematic review and meta-analysis. Actas Dermo-Sifiliogr. 2025; in press. [Google Scholar] [CrossRef]
- Tirgar Tabari, S.; Moghadam Nia, A.A.; Hajian, K.; Moeinzadeh, A. Comparison of the effect of azelaic acid 20% and clindamycin 1% in the treatment of mild and moderate acne. Iran. J. Dermatol. 2009, 12, 106–110. [Google Scholar]
- Malik, D.S.; Kaur, G. A validated stability-indicating RP-HPLC method for analysis of azelaic acid in pharmaceuticals. Indian J. Pharm. Sci. 2018, 80, 503. [Google Scholar] [CrossRef]
- Han, S.; Karłowicz-Bodalska, K.; Potaczek, P.; Wójcik, A.; Ozimek, Ł.; Szura, D.; Musiał, W. Identification of unknown impurity of azelaic acid in liposomal formulation assessed by HPLC-ELSD, GC-FID, and GC-MS. AAPS PharmSciTech 2014, 15, 111–120. [Google Scholar] [CrossRef]
- Kadam, T.V.; Darekar, A.B. Spectrophotometric method for determination of azelaic acid: Development and validation approach. In Advanced Concepts in Pharmaceutical Research; B P International: London, UK, 2023; Volume 3, pp. 207–214. [Google Scholar]
- Parveen, N.; Sheikh, A.; Molugulu, N.; Annadurai, S.; Wahab, S.; Kesharwani, P. Drug permeation enhancement, efficacy, and safety assessment of azelaic acid loaded SNEDDS hydrogel to overcome the treatment barriers of atopic dermatitis. Environ. Res. 2023, 236, 116850. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Label Information for Azelaic Acid Gel 15%. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2002/021470lbl.pdf (accessed on 20 May 2025).
- Nazzaro-Porro, M. Azelaic acid. J. Am. Acad. Dermatol. 1987, 17, 1033–1041. [Google Scholar] [CrossRef]
- Bayer HealthCare Pharmaceuticals Inc. FINACEA® (Azelaic Acid) Foam, for Topical Use. Initial U.S. Approval: 1995; (Unpublished regulatory document); Bayer HealthCare Pharmaceuticals Inc.: Whippany, NJ, USA, 2015. [Google Scholar]
- U.S. Food and Drug Administration. Label Information for FINACEA® Foam. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207071s000lbl.pdf (accessed on 20 May 2025).
- Del Rosso, J.Q. Azelaic acid topical formulations: Differentiation of 15% gel and 15% foam. J. Clin. Aesthet. Dermatol. 2017, 10, 37–40. [Google Scholar] [PubMed]
- Health Products Regulatory Authority. Azelaic Acid Cream Product Licence. Available online: https://assets.hpra.ie/products/Human/27892/Licence_PA1025-009-001_07042021143645.pdf (accessed on 20 May 2025).
- Indrayanto, G.; Ahmad, F.; Mustafa, A. Benzoic acid. In Analytical Profiles of Drug Substances and Excipients; Elsevier: Amsterdam, The Netherlands, 1999; Volume 26, pp. 1–46. [Google Scholar]
- Fukushima, S.; Takahashi, M.; Yamaguchi, M. Effect of cetostearyl alcohol on stabilization of oil-in-water emulsion. J. Colloid. Interface Sci. 1976, 57, 201–206. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Bhatia, N. Azelaic acid gel 15% in the management of papulopustular rosacea: A status report on available efficacy data and clinical application. Cutis 2011, 88, 67–72. [Google Scholar]
- Patel, V.; Gaurav, V. Role of polyethylene glycol in dermatology. Indian. Dermatol. Online J. 2025, 16, 227–234. [Google Scholar] [CrossRef]
- Ma, H.; Yu, M.; Tan, F.; Li, N. Improved percutaneous delivery of azelaic acid employing microemulsion as nanocarrier: Formulation optimization, in vitro and in vivo evaluation. RSC Adv. 2015, 5, 28985–28995. [Google Scholar] [CrossRef]
- Pasca, P.M.; Antonescu, A.; Fritea, L.; Banica, F.; Vicas, S.I.; Laslo, V.; Zaha, D.C.; Cavalu, S. Novel liposomal formulation with azelaic acid: Preparation, characterization, and evaluation of biological properties. Appl. Sci. 2022, 12, 13039. [Google Scholar] [CrossRef]
- Esposito, E.; Menegatti, E.; Cortesi, R. Ethosomes and liposomes as topical vehicles for azelaic acid: A preformulation study. Int. J. Cosmet. Sci. 2004, 26, 270–271. [Google Scholar] [CrossRef]
- Kashyap, V.; Rani, A. Formulation and evaluation of niosomal gel of azelaic acid for antiacne activity. Int. J. Appl. Pharm. 2023, 15, 237–244. [Google Scholar] [CrossRef]
- Malik, D.S.; Kaur, G. Exploring therapeutic potential of azelaic acid loaded NLCs for the treatment of acne vulgaris. J. Drug Deliv. Sci. Technol. 2020, 55, 101418. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Mistry, A.; Ravikumar, P. Development and evaluation of azelaic acid based ethosomes for topical delivery for the treatment of acne. Indian. J. Pharm. Educ. Res. 2016, 50, S232–S243. [Google Scholar] [CrossRef]
- Yeo, P.L.; Lim, C.L.; Chye, S.M.; Ling, A.P.K.; Koh, R.Y. Niosomes: A review of their structure, properties, methods of preparation, and medical applications. Asian Biomed. 2018, 11, 301–314. [Google Scholar] [CrossRef]
- Jejurkar, A.S.; Newre, A.B.; Ravate, A.B.; Wakle, A.S.; Kawade, R.M. Niosomes: Pharmaceutical novel drug delivery system. Int. J. Pharm. Sci. 2023, 1, 18–24. [Google Scholar]
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Kheirkhah, A.H.; Taghizadeh, M.; Yazdani, Y.; et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int. J. Pharm. 2022, 624, 121878. [Google Scholar] [CrossRef]
- Nguyen, T.-T.-L.; Duong, V.-A. Solid lipid nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure, preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Muller, R.; Petersen, R.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-based nanosponges: Overview and opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Singh, A.; Chauhan, C.S. Nanosponges-A versatile approach of drug delivery system. Int. J. Pharm. Qual. Assur. 2023, 14, 1246–1255. [Google Scholar] [CrossRef]
- Pandey, P.; Purohit, D.; Dureja, H. Nanosponges—A promising novel drug delivery system. Recent. Pat. Nanotechnol. 2018, 12, 180–191. [Google Scholar] [CrossRef]
- Surushe, C.; Thake, J.; Karpe, M.; Kadam, V. Nanosponges: A brief review. Indian J. Pharm. Sci. 2023, 85, 1586–1593. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and nanoemulsions in skin drug delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, B.G.; Parihar, A.; Macwan, M.; Pal, S. A comprehensive review on applications, preparation & characterization of nanoemulsion. Int. J. Clin. Anal. Appl. Pharm. 2023, 8, 104–111. [Google Scholar]
- Berlitz, S.J.; De Villa, D.; Inácio, L.A.M.; Davies, S.; Zatta, K.C.; Guterres, S.S.; Külkamp-Guerreiro, I.C. Azelaic acid-loaded nanoemulsion with hyaluronic acid—A new strategy to treat hyperpigmentary skin disorders. Drug Dev. Ind. Pharm. 2019, 45, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Rahisuddin; Farooqui, N.A.; Sharma, D.K. Enhanced topical delivery of azelaic acid through nanoemulsion formulation: Optimization, characterization, and potential therapeutic application for skin disorders. J. Adv. Zool. 2024, 45, 88. [Google Scholar]
- Susa, F.; Arpicco, S.; Pirri, C.F.; Limongi, T. An overview on the physiopathology of the blood–brain barrier and the lipid-based nanocarriers for central nervous system delivery. Pharmaceutics 2024, 16, 849. [Google Scholar] [CrossRef]
- Alkawak, R.S.Y.; Rajab, N.A. Lornoxicam-loaded cubosomes: Preparation and in vitro characterization. Iraqi J. Pharm. Sci. 2022, 31, 144–153. [Google Scholar] [CrossRef]
- Vardhan, H.; Jain, A.; Singhai, A.K. Potential of dendrimers in drug delivery: An updated review. Asian J. Pharm. Res. 2024, 14, 242–254. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.-P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gorain, B.; Choudhury, H.; Srivastava, A.; Kesharwani, P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon 2019, 5, e01343. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric nanoparticles for drug delivery: Recent developments and future prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic development of transethosomal gel system of piroxicam: Formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech 2017, 18, 58–71. [Google Scholar] [CrossRef]
- Zaki, R.M.; Seshadri, V.D.; Mutayran, A.S.; Elsawaf, L.A.; Hamad, A.M.; Almurshedi, A.S.; Yusif, R.M.; Said, M. Wound healing efficacy of rosuvastatin transethosomal gel, I optimal optimization, histological and in vivo evaluation. Pharmaceutics 2022, 14, 2521. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Imran, M.; Jahan, S.; Akhtar, A.; Hasan, Z. Formulation and characterization of hesperidin-loaded transethosomal gel for dermal delivery to enhance antibacterial activity: Comprehension of in vitro, ex vivo, and dermatokinetic analysis. Gels 2023, 9, 791. [Google Scholar] [CrossRef]
- Nasr, A.M.; Badawi, N.M.; Tartor, Y.H.; Sobhy, N.M.; Swidan, S.A. Development, optimization, and in vitro/in vivo evaluation of azelaic acid transethosomal gel for antidermatophyte activity. Antibiotics 2023, 12, 707. [Google Scholar] [CrossRef] [PubMed]
- Kothawade, S.N.; Pande, V.V. (Eds.) Mesoporous Silica Nanoparticles: Drug Delivery, Catalysis and Sensing Applications; De Gruyter: Berlin, Germany, 2024. [Google Scholar]
- Heidari, R.; Assadollahi, V.; Shakib Manesh, M.H.; Mirzaei, S.A.; Elahian, F. Recent advances in mesoporous silica nanoparticles formulations and drug delivery for wound healing. Int. J. Pharm. 2024, 665, 124654. [Google Scholar] [CrossRef] [PubMed]
- Arshad, T.; Shoaib Khan, H.M.; Akhtar, N.; Hanan, H.; Hussain, M.D.; Kazi, M. Structural elucidation and development of azelaic acid loaded mesoporous silica nanoparticles infused gel: Revolutionizing nanodrug delivery for cosmetics and pharmaceuticals. Heliyon 2024, 10, e29460. [Google Scholar] [CrossRef]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Devel. Ther. 2017, 11, 393–406. [Google Scholar] [CrossRef]
- Adwan, S.; Qasmieh, M.; Al-Akayleh, F.; Ali Agha, A.S.A. Recent advances in ocular drug delivery: Insights into lyotropic liquid crystals. Pharmaceuticals 2024, 17, 1315. [Google Scholar] [CrossRef] [PubMed]
- Gowda, C.M.; Wairkar, S. Azelaic acid-based lyotropic liquid crystals gel for acne vulgaris: Formulation optimization, antimicrobial activity and dermatopharmacokinetic study. Int. J. Pharm. 2024, 667, 124879. [Google Scholar] [CrossRef]
- Witika, B.A.; Choonara, Y.E.; Demana, P.H. A SWOT analysis of nano co-crystals in drug delivery: Present outlook and future perspectives. RSC Adv. 2023, 13, 7339–7351. [Google Scholar] [CrossRef]
- Zotova, J.; Wojnarowska, Z.; Twamley, B.; Tajber, L. Formation of stoichiometric and non-stoichiometric ionic liquid and cocrystal multicomponent phases of lidocaine with azelaic acid by changing counterion ratios. J. Mol. Liq. 2021, 344, 117737. [Google Scholar] [CrossRef]
- Yarava, J.R.; Potnuru, L.R.; Pahari, B.; Tothadi, S.; Ramanathan, K.V. Supramolecular synthon identification in azelaic acid—Isonicotinamide. J. Magn. Reson. Open 2022, 10–11, 100056. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Liu, Y.; Wu, K.; Zhu, Y.; Lu, H.; Liang, B. Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents. Environ. Sci. Pollut. Res. 2021, 28, 35537–35563. [Google Scholar] [CrossRef]
- Abdul Rahman, A.M.; Abu Bakar, A.R.; Yee, A.Q.; Zainudin, M.A.M.; Nik Daud, N.M.A.; Nagoor Gunny, A.A.; Md Sarip, M.S.; Perona, R.V.; Khairuddin, N.H. A review on the role of deep eutectic solvents in mango (Mangifera indica) extraction. RSC Adv. 2025, 15, 4296–4321. [Google Scholar] [CrossRef] [PubMed]
- Zaib, Q.; Masoumi, Z.; Aich, N.; Kyung, D. Review of the synthesis and applications of deep eutectic solvent-functionalized adsorbents for water treatment. J. Environ. Chem. Eng. 2023, 11, 110214. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Nica, M.-A.; Anuța, V.; Nicolae, C.A.; Popa, L.; Ghica, M.V.; Cocoș, F.-I.; Dinu-Pîrvu, C.-E. Exploring deep eutectic solvents as pharmaceutical excipients: Enhancing the solubility of ibuprofen and mefenamic acid. Pharmaceuticals 2024, 17, 1316. [Google Scholar] [CrossRef]
- Cichowska-Kopczyńska, I.; Nowosielski, B.; Warmińska, D. Deep eutectic solvents: Properties and applications in CO2 separation. Molecules 2023, 28, 5293. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Skin. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, M.G.; Freire, C.S.R.; Silvestre, A.J.D. Deep eutectic solvents comprising active pharmaceutical ingredients in the development of drug delivery systems. Expert. Opin. Drug Deliv. 2019, 16, 497–506. [Google Scholar] [CrossRef]
- Boscariol, R.; Caetano, É.A.; Silva, E.C.; Oliveira, T.J.; Rosa-Castro, R.M.; Vila, M.M.D.C.; Balcão, V.M. Performance of choline geranate deep eutectic solvent as transdermal permeation enhancer: An in vitro skin histological study. Pharmaceutics 2021, 13, 540. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; McCabe, C.; Bunge, A.; Gooris, G.S. The skin barrier: An extraordinary interface with an exceptional lipid organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfar, M.; Idkaidek, N.; Al-Remawi, M. A novel eutectic-based transdermal delivery system for risperidone. AAPS PharmSciTech 2021, 22, 4. [Google Scholar] [CrossRef]
- Tomić, I.; Juretić, M.; Jug, M.; Pepić, I.; Cetina Čižmek, B.; Filipović-Grčić, J. Preparation of in situ hydrogels loaded with azelaic acid nanocrystals and their dermal application performance study. Int. J. Pharm. 2019, 563, 249–258. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Alkhawaja, B.; Al-Zoubi, N.; Abdelmalek, S.M.A.; Daadoue, S.; AlAbbasi, D.; Al-Masri, S.; Ali Agha, A.S.; Olaimat, A.R.; Woodman, T. Novel therapeutic deep eutectic system for the enhancement of ketoconazole antifungal activity and transdermal permeability. J. Mol. Liq. 2024, 413, 125975. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.I.; Al-Muhtaseb, N.; Jaber, N.; Al-Remawi, M.; Collier, P.J. Antimicrobial potential of natural deep eutectic solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, Z.; Yin, T.; Wang, X.; Yuan, J. Eutectogel based on multi-functional deep eutectic solvent for acne infection treatment. J. Drug Deliv. Sci. Technol. 2024, 101, 106260. [Google Scholar] [CrossRef]
- Salimi, A.; Sharif Makhmal Zadeh, B.; Godazgari, S.; Rahdar, A. Development and evaluation of azelaic acid-loaded microemulsion for transfollicular drug delivery through guinea pig skin: A mechanistic study. Adv. Pharm. Bull. 2020, 10, 239–246. [Google Scholar] [CrossRef]
- Grzybowski, A.; Jin, K.; Wu, H. Challenges of artificial intelligence in medicine and dermatology. Clin. Dermatol. 2024, 42, 210–215. [Google Scholar] [CrossRef]
- Behara, K.; Bhero, E.; Agee, J.T. AI in dermatology: A comprehensive review into skin cancer detection. PeerJ Comput. Sci. 2024, 10, e2530. [Google Scholar] [CrossRef]
- Fliorent, R.; Fardman, B.; Podwojniak, A.; Javaid, K.; Tan, I.J.; Ghani, H.; Truong, T.M.; Rao, B.; Heath, C. Artificial intelligence in dermatology: Advancements and challenges in skin of color. Int. J. Dermatol. 2024, 63, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Labkoff, S.; Oladimeji, B.; Kannry, J.; Solomonides, A.; Leftwich, R.; Koski, E.; Joseph, A.L.; Lopez-Gonzalez, M.; Fleisher, L.A.; Nolen, K.; et al. Toward a responsible future: Recommendations for AI-enabled clinical decision support. J. Am. Med. Inform. Assoc. 2024, 31, 2730–2739. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Heydari, K.; Powell, D. Wearable AI to enhance patient safety and clinical decision-making. npj Digit. Med. 2025, 8, 176. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine scale-up technologies: Feasibilities and challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Struzek, A.-M.; Scherließ, R. Quality by design as a tool in the optimisation of nanoparticle preparation—A case study of PLGA nanoparticles. Pharmaceutics 2023, 15, 617. [Google Scholar] [CrossRef]
- Waghule, T.; Dabholkar, N.; Gorantla, S.; Rapalli, V.K.; Saha, R.N.; Singhvi, G. Quality by design (QbD) in the formulation and optimization of liquid crystalline nanoparticles (LCNPs): A risk-based industrial approach. Biomed. Pharmacother. 2021, 141, 111940. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Artificial Intelligence for Drug Development. FDA. Available online: https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/artificial-intelligence-drug-development (accessed on 19 May 2025).
- Kania, B.; Montecinos, K.; Goldberg, D.J. Artificial intelligence in cosmetic dermatology. J. Cosmet. Dermatol. 2024, 23, 3305–3311. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, M.S.; Lim, W.; Park, G.H.; Park, I.; Chang, S.E. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J. Investig. Dermatol. 2018, 138, 1529–1538. [Google Scholar] [CrossRef]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Pazoki-Toroudi, H.; Nilforoushzadeh, M.A.; Ajami, M.; Jaffary, F.; Aboutaleb, N.; Nassiri-Kashani, M.; Firooz, A. Combination of azelaic acid 5% and clindamycin 2% for the treatment of acne vulgaris. Cutan. Ocul. Toxicol. 2011, 30, 286–291. [Google Scholar] [CrossRef]
- Thiboutot, D.M.; Fleischer, A.B.; Del Rosso, J.Q.; Rich, P. A multicenter study of topical azelaic acid 15% gel in combination with oral doxycycline as initial therapy and azelaic acid 15% gel as maintenance monotherapy. J. Drugs Dermatol. 2009, 8, 639–648. [Google Scholar]
- Graupe, K.; Verallo-Rowell, V.; Verallo, V.; Zaumseil, R.-P. Combined use of 20% azelaic acid cream and 0.05% tretinoin cream in the topical treatment of melasma. J. Dermatol. Treat. 1996, 7, 235–237. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Breathnach, A.S. Melanin hyperpigmentation of skin: Melasma, topical treatment with azelaic acid, and other therapies. Cutis 1996, 57, 36–45. [Google Scholar]
- Sen, K.G.; Sarkar, S.K.; Islam, M.S.; Mostofa, M.K.; Das, A.R.; Khan, M.U.; Wadud, M.A.; Alam, J.; Saha, S.K.; Akter, S. The role of combination of 20% azelaic acid with 0.05% tretinoin cream in the treatment of melasma—A study in FMCH. Faridpur Med. Coll. J. 2017, 11, 50–53. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Finkel, L.J.; Ditre, C.M.; Hamilton, T.A.; Ellis, C.N.; Voorhees, J.J. Topical tretinoin (retinoic acid) improves melasma. A vehicle-controlled, clinical trial. Br. J. Dermatol. 1993, 129, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Jutley, G.S.; Rajaratnam, R.; Halpern, J.; Salim, A.; Emmett, C. Systematic review of randomized controlled trials on interventions for melasma: An abridged Cochrane review. J. Am. Acad. Dermatol. 2014, 70, 369–373. [Google Scholar] [CrossRef]
- St. Surin-Lord, S.; Miller, J. Topical treatment of truncal acne with tretinoin lotion 0.05% and azelaic acid foam. Case Rep. Dermatol. Med. 2020, 2020, 5217567. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, H.P.; Graupe, K.; Zaumseil, R.P. Comparison of combined azelaic acid cream plus oral minocycline with oral isotretinoin in severe acne. Eur. J. Dermatol. 2001, 11, 538–544. [Google Scholar]
- Barbieri, J.S.; Hoffstad, O.; Margolis, D.J. Duration of oral tetracycline-class antibiotic therapy and use of topical retinoids for the treatment of acne among general practitioners (GP): A retrospective cohort study. J. Am. Acad. Dermatol. 2016, 75, 1142–1150.e1. [Google Scholar] [CrossRef] [PubMed]

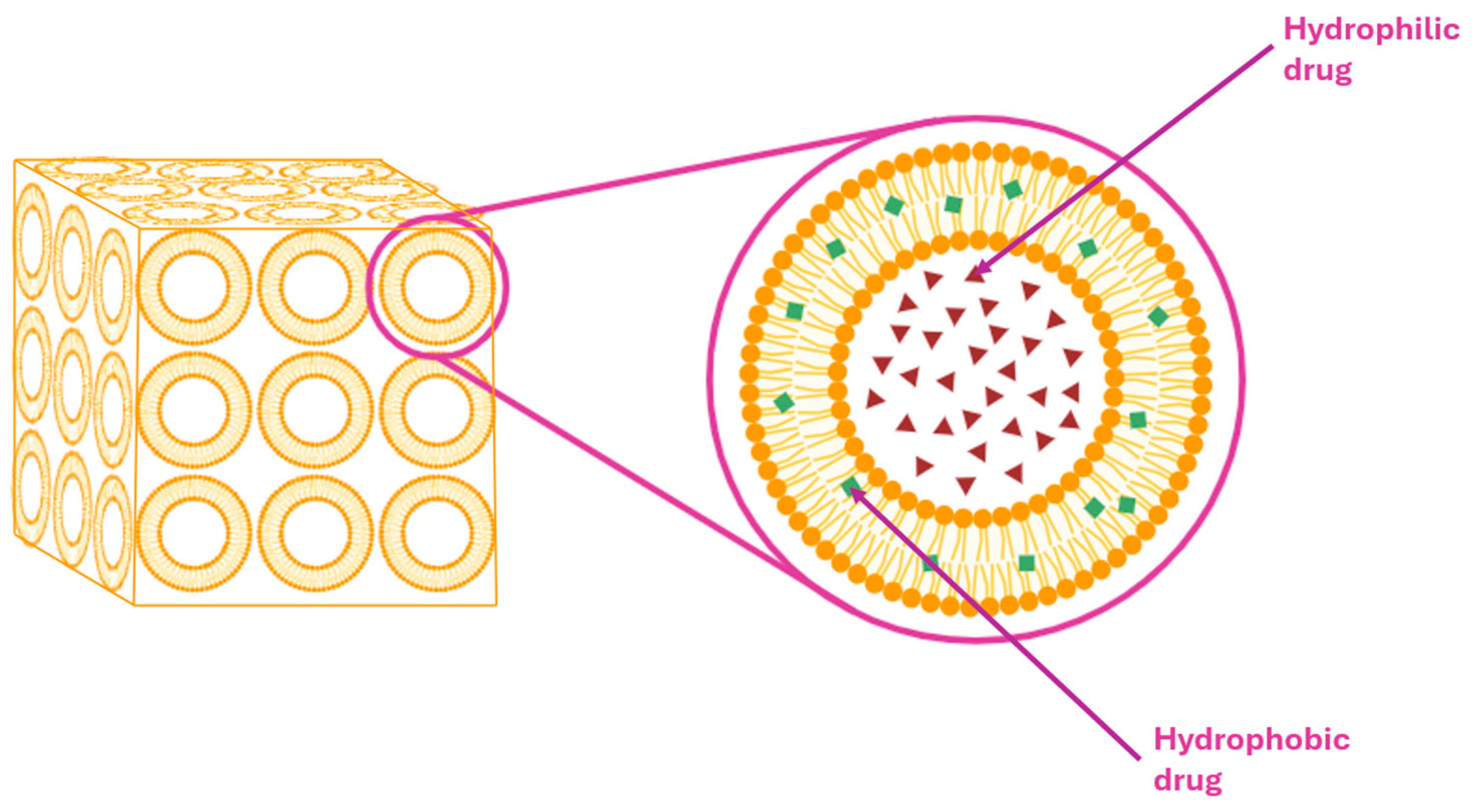
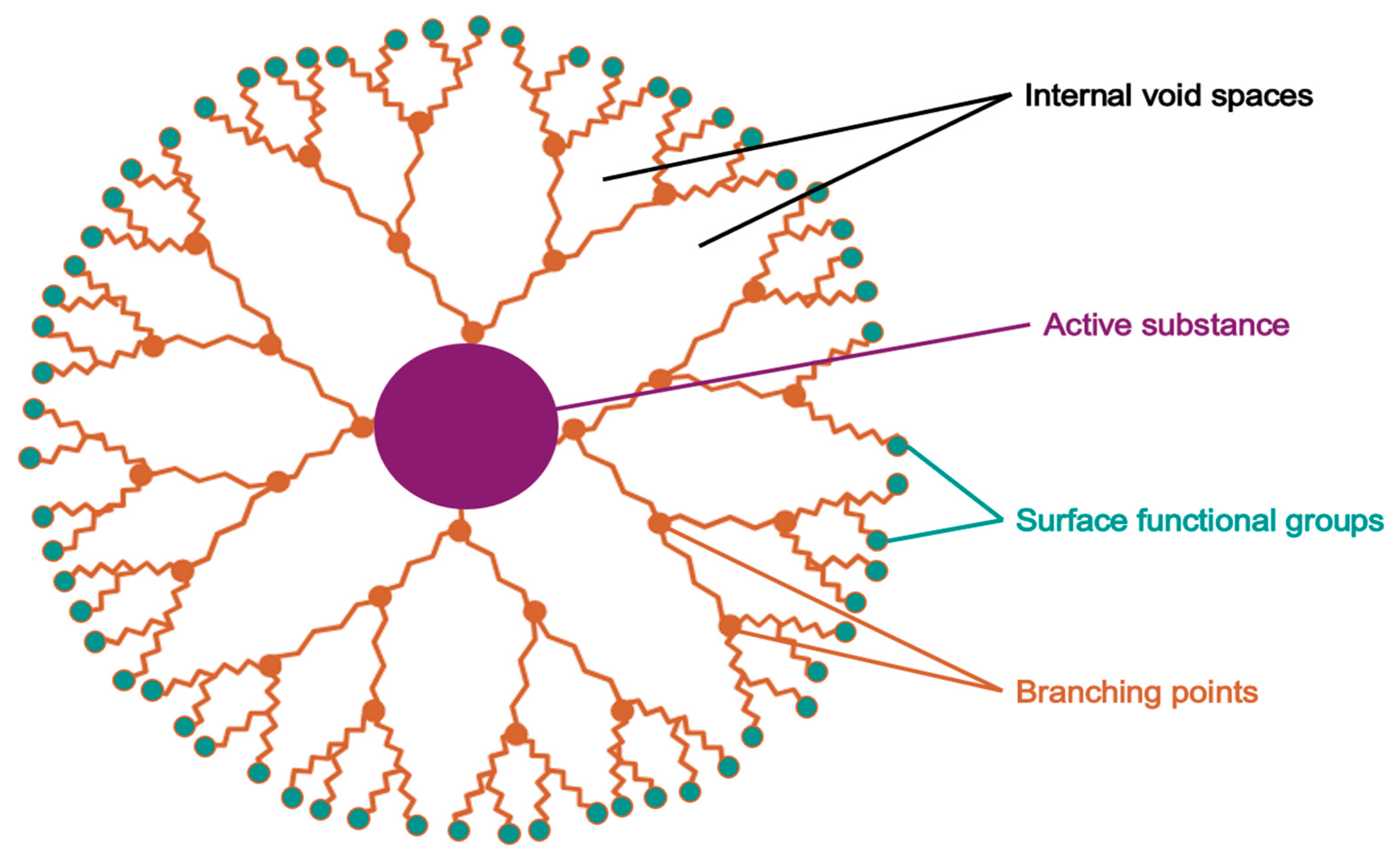
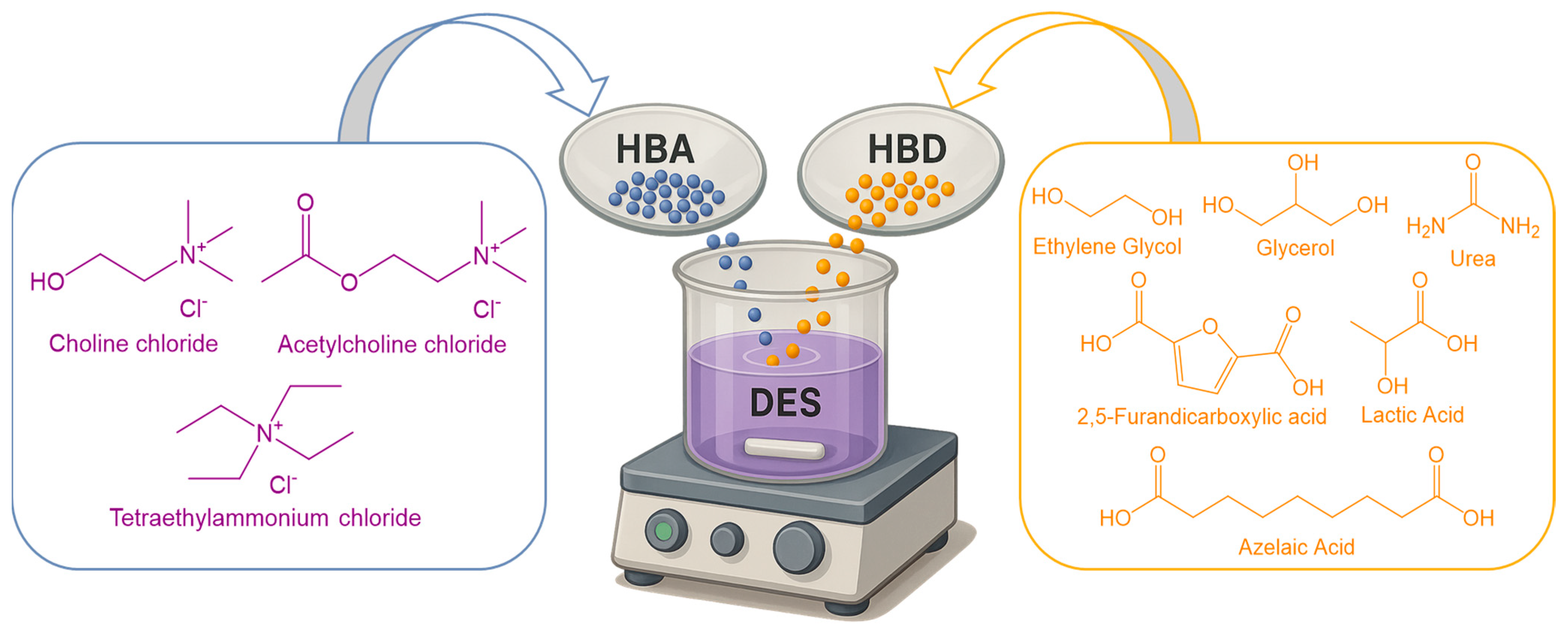
| Route | Starting Material | Key Reagent/Catalyst | Yield (%) | Notes | Ref. |
|---|---|---|---|---|---|
| Ozonolysis (classical) | Oleic acid | O3 + oxidant | 70–95 | Industrial route, concerns regarding ozone safety | [10] |
| H2O2-based oxidation | Vegetable oils | H2O2, catalysts | ≤80 | Industrialized by Matrica S.p.A. (Italy) | [10] |
| Performic acid + O3 | Oleic acid | HCOOH + H2O2 | up to 95 | In situ peracid formation enhances selectivity | [11] |
| Biocatalytic | Oleic acid | Multi-enzyme cascade | 5–10 mM | Low yield, multistep enzymatic route | [12] |
| Chemo-enzymatic | Oleic acid | Lipase + Fe(NO3)3 + TEMPO | 44 | No chromatography required | [12] |
| Fermentative | Oleic acid | C. tropicalis + ozonolysis | 67 | Long fermentation, no pelargonic acid | [12] |
| Chemical | Cyclohexanone + methyl acrylate | Percarboxylic acid + Pd catalyst | >90 | Fully continuous processing | [13,14] |
| Mechanism | Target | Therapeutic Effect | Ref. |
|---|---|---|---|
| Antimicrobial | Cutibacterium acnes, oxidoreductase | Inhibits bacterial growth and colonization | [41,42] |
| Anti-inflammatory | IL-1β, TNF-α, NF-κB, MMPs, LL-37 | Suppresses inflammatory cytokines and oxidative stress | [43,44,45] |
| Antimelanogenic | Tyrosinase, hyperactive melanocytes | Inhibits melanin synthesis and melanocyte proliferation | [3,15,19,46,47] |
| Antioxidant | ROS, lipid peroxidation | Protects skin barrier and reduces oxidative tissue damage | [1,42,48] |
| Activity | Target/Mechanism | Effect/Result | Ref. |
|---|---|---|---|
| Antibacterial | Mitochondrial oxidoreductase inhibition | Impaired bacterial respiration and energy production leading to bacterial growth inhibition. | [5] |
| Antibacterial | KLK5 cleaves hCAP18 into LL-37 | Increased LL-37 promotes inflammation in rosacea | [65] |
| Anti-inflammatory | IL-1β, TNF-α downregulation (via NF-κB) | Reduction in pro-inflammatory cytokine levels | [56] |
| Anti-inflammatory | MMP-9 inhibition | Inhibition of MMP-9 activity in inflamed skin | [56] |
| Antimelanogenic | Tyrosinase inhibition | Significant reduction in melanin index | [59] |
| Antimelanogenic | Reduced melanocyte proliferation | Decrease in melanocyte density on histological analysis | [58] |
| Antioxidant | Reactive oxygen species scavenging | Reduction in reactive oxygen species markers | [64] |
| Immunomodulatory | Suppression of pro-inflammatory cytokine milieu via PPARγ pathway | Modulation of cytokine balance in keratinocyte culture | [43] |
| Anti-keratinization | Inhibition of keratinocyte hyperproliferation via mitochondrial swelling and ER dilation | Reduction in hyperkeratosis and normalization of intra- and interfollicular keratinization | [3] |
| Microbiome modulation | Inhibition of quorum sensing signals in C. acnes | Reduction in pathogenic bacterial communication | [56] |
| Antiangiogenic | Inhibition of VEGF expression via PI3K/AKT pathway | Reduction in angiogenic factors in psoriatic skin | [45] |
| Photoprotective | ROS scavenging by plant polyphenols; enhancement of UV absorber stability | Reduction in UV-induced oxidative stress and potential mitigation of photodamage in sunscreen formulations | [66] |
| Condition | Formulation | Comparator | Duration | Efficacy Result | Ref. |
|---|---|---|---|---|---|
| Hyperpigmentation | Azelaic acid 20% cream | Hydroquinone 2% | 24 weeks | Comparable efficacy; mild local irritation with azelaic acid | [79] |
| Hyperpigmentation | 20% cream | Hydroquinone 4% | 8–24 weeks | Greater MASI reduction with azelaic acid; adverse events similar between groups | [80] |
| Acne vulgaris | Azelaic acid chemical peel | Pyruvic acid peel | 12 weeks | Both effective; azelaic acid better tolerated; pyruvic acid reduced sebum more | [81] |
| Rosacea | Azelaic acid 15% gel | Metronidazole 0.75% gel | 15 weeks | Greater reduction in lesions and erythema with azelaic acid | [18] |
| Rosacea | Azelaic acid 20% cream | Dapsone 7.5% gel | 12 weeks | Similar efficacy; azelaic acid had more local side effects; dapsone better tolerated | [82] |
| Rosacea | Azelaic acid 15% gel | Ivermectin, metronidazole, minocycline | 12–16 weeks | Ivermectin is most effective; azelaic acid better than metronidazole; all well tolerated, with more irritation from azelaic acid | [83] |
| Acne | Azelaic acid 20% cream | Clindamycin 1% lotion | 8 weeks | Similar efficacy; azelaic acid well tolerated; no direct tolerability comparison reported | [84] |
| Acne | Azelaic acid 15% gel | Adapalene 0.1% gel | 12 weeks | Comparable efficacy; adapalene slightly better tolerated | [17] |
| Classical Nanocarriers Tested with Azelaic Acid | ||||||
|---|---|---|---|---|---|---|
| Nanocarrier System | Status with AzA | Key Composition/Type | Main Findings/Notes | Advantages | Limitations | Ref. |
| Liposomes | Tested | Phospholipids (e.g., Phospholipon 50 IP) | Increased SC retention (187.5 mg/cm2), good stability, enhanced antimicrobial effects | Biocompatible, non-irritant | Risk of leakage, low long-term stability | [24,100] |
| Ethosomes | Tested | Phospholipids + high ethanol (20–45%) | Enhanced permeation, improved antimicrobial activity, sustained release (93.4% in 12 h) | High penetration, stable, effective | Potential irritation from ethanol | [34,105] |
| Niosomes | Tested | Non-ionic surfactants (Span 40), cholesterol | High encapsulation efficiency (72.3%), sustained release (82.7%), reduced irritation (with aloe vera) | Stable, biocompatible | Less studied than liposomes | [102] |
| SLNs | Tested | Solid lipids (stearic acid) + surfactants | Enhanced release (93.9%), reduced irritation (HET-CAM), improved depigmentation | Good stability, GRAS lipids | Size growth at room temp | [35] |
| NLCs | Proposed | Solid + liquid lipids | Literature-based evidence, ↑ skin penetration | Literature-based evidence, improved skin penetration | No specific AzA data reported | [110] |
| Nanosponges | Tested | β-Cyclodextrin crosslinked networks | Increased solubility (5-fold), better MIC/MBC than free AzA | Stable, non-irritant, good control | ↓ Tyrosinase inhibition vs. free AzA | [37] |
| Nanoemulsions | Tested | O/W emulsions w/surfactants and HA or vitamins | Enhanced retention, greater tyrosinase inhibition, improved sensorial profile | Stable, well tolerated | Thermodynamically unstable | [121,122] |
| Non-Classical/Emerging Nanocarriers Tested or Proposed with Azelaic Acid | ||||||
| Nanocarrier System | Status with AzA | Key Composition/Type | Main Findings/Notes | Advantages | Limitations | Ref. |
| Cubosomes | Not yet tested | GMO/phytantriol + Pluronic F127 | Proposed: stable, SC-mimicking, dual drug encapsulation | Excellent skin compatibility | Theoretical only | [123,124] |
| Dendrimers | Early-stage | PAMAM G4 + microsponges (with dithranol) | ↑ Stability, ↑ AUC, ↓ irritation (model drug) | Controlled release, high loading | No direct AzA formulation yet | [128] |
| Polymeric NPs | Suggested | Chitosan, PLGA, ethyl cellulose | Based on vit. C, arbutin, glabridin-potential for AzA | Mucoadhesive, biocompatible | Lacking direct AzA evidence | [129,130] |
| Transethosomes | Tested | Lecithin + ethanol + edge activator | ↑ Permeation (3056 µg/cm2), ↑ antifungal activity, 83% cure rate | Deformable, high EE | Needs ethanol tuning | [135] |
| MSNs | Tested | Silica (CTAB/TEOS), high surface area | ↑ Tyrosinase inhibition, ↑ permeation (85.5%), ↑ stability | Porous, functionalizable | Inorganic, limited biodegradability | [136] |
| LLCs | Tested | GMO + poloxamer + EtOH | ↑ Release (91%), ↑ skin retention (146 µg/cm2), antimicrobial | Biphasic release, safe | Complex formulation, rheology control | [139] |
| Type | Formula | Terms | Example |
|---|---|---|---|
| I | M+X− + z MClX | M = Zn, Sn, Al, Ga, Fe, In | ZnCl2 + ChCl |
| II | M+X− + z MClX·Yh2O | M = Co, Cu, Ni, Fe, Cr | CoCl2·6H2O + ChCl |
| III | Cat+X− + zRZ | RZ = OH, COOH, CONH2 (hydrogen bond donor) | Urea + ChCl |
| IV | MClX + RZ → MClX−1−·RZ + MClx+1− | M = Zn, Al and Z = OH, CONH2 | ZnCl2 + urea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovici, A.-G.; Spennato, M.; Bîtcan, I.; Péter, F.; Cotarcă, L.; Todea, A.; Ordodi, V.L. A Comprehensive Review of Azelaic Acid Pharmacological Properties, Clinical Applications, and Innovative Topical Formulations. Pharmaceuticals 2025, 18, 1273. https://doi.org/10.3390/ph18091273
Petrovici A-G, Spennato M, Bîtcan I, Péter F, Cotarcă L, Todea A, Ordodi VL. A Comprehensive Review of Azelaic Acid Pharmacological Properties, Clinical Applications, and Innovative Topical Formulations. Pharmaceuticals. 2025; 18(9):1273. https://doi.org/10.3390/ph18091273
Chicago/Turabian StylePetrovici, Andreea-Georgiana, Mariachiara Spennato, Ioan Bîtcan, Francisc Péter, Livius Cotarcă, Anamaria Todea, and Valentin Laurențiu Ordodi. 2025. "A Comprehensive Review of Azelaic Acid Pharmacological Properties, Clinical Applications, and Innovative Topical Formulations" Pharmaceuticals 18, no. 9: 1273. https://doi.org/10.3390/ph18091273
APA StylePetrovici, A.-G., Spennato, M., Bîtcan, I., Péter, F., Cotarcă, L., Todea, A., & Ordodi, V. L. (2025). A Comprehensive Review of Azelaic Acid Pharmacological Properties, Clinical Applications, and Innovative Topical Formulations. Pharmaceuticals, 18(9), 1273. https://doi.org/10.3390/ph18091273








