Amaranthus graecizans L. Mitigates Hyperlipidemia-Induced Nonalcoholic Fatty Liver Disease in Experimental Rats: Future Pharmaceuticals
Abstract
1. Introduction
2. Results
2.1. Proximate Composition of Amaranthus graecizans L.
2.2. Vitamin and Mineral Content of Amaranthus graecizans L.
2.3. Phenolic Compounds of Amaranthus graecizans L.
2.4. Evaluation of the Body Weight Gain
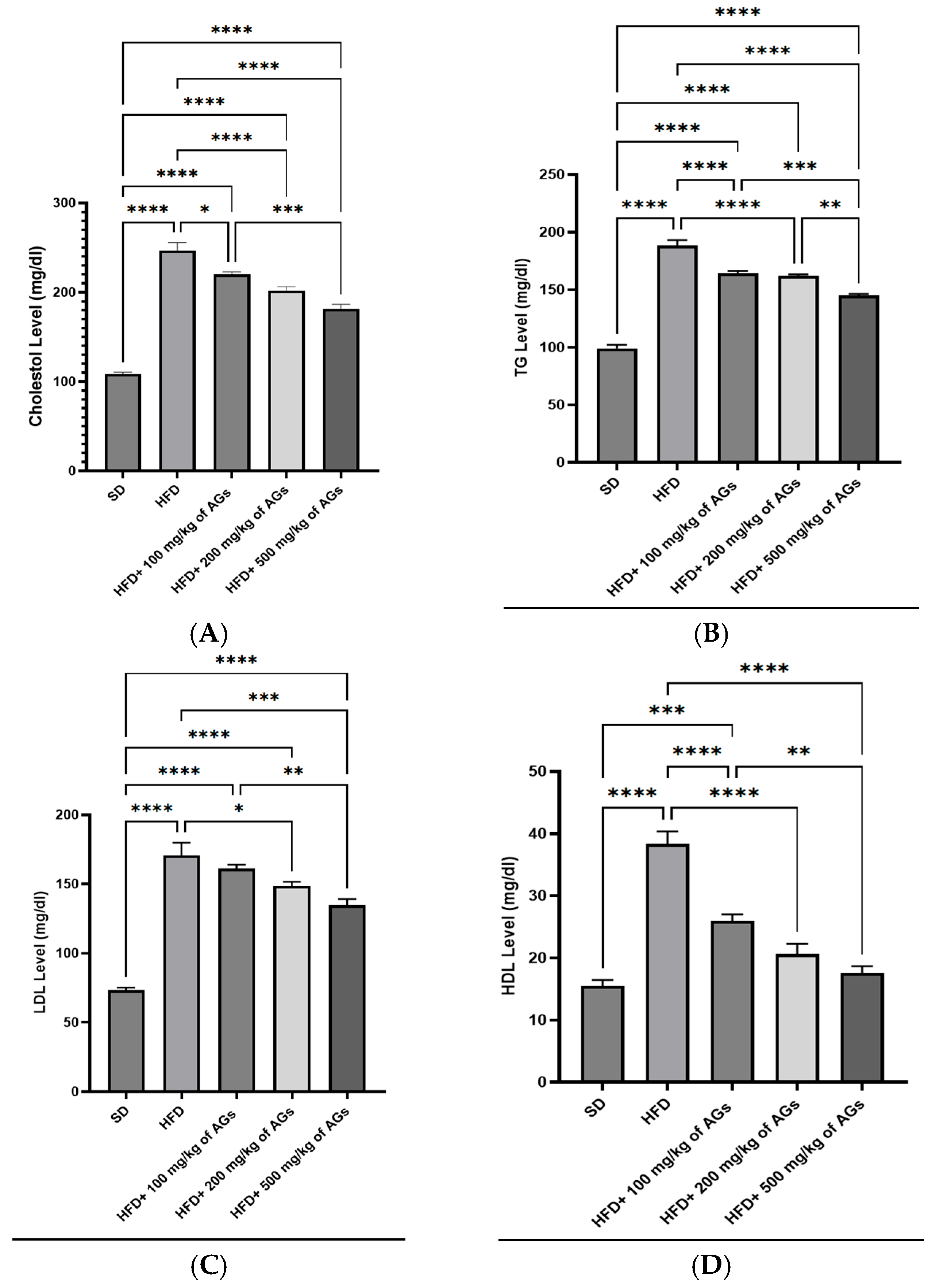
2.5. Effect of AGs on Serum Lipid Profiles
2.6. Effect of AGs on Liver Function
2.7. Histological Examination
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Preparation of AG Powder
4.3. Experimental Animals
4.4. Diet Preparation
4.5. Experimental Design
4.6. Proximate Analysis
4.6.1. Carbohydrates
4.6.2. Crude Protein
4.6.3. Total Fat Content
4.6.4. Moisture
4.6.5. Ash
4.7. Micronutrient Analysis
4.7.1. Vitamins
4.7.2. Minerals
4.8. Phenolic Compounds
4.9. Biochemical Analysis
4.10. Histopathology
4.11. Ethical Approval
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Machado, M.V.; Diehl, A.M. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology 2016, 150, 1769–1777. [Google Scholar] [CrossRef]
- Powell, E.E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Kalligeros, M.; Henry, L. Epidemiology of metabolic dysfunction associated steatotic liver disease. Clin. Mol. Hepatol. 2024, 31, S32–S50. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Wong, R.J.; Harrison, S.A. Nonalcoholic fatty liver disease review: Diagnosis, treatment, and outcomes. Clin. Gastroenterol. Hepatol. 2015, 13, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Salem, V.; AlHusseini, N.; Abdul Razack, H.I.; Naoum, A.; Sims, O.T.; Alqahtani, S.A. Prevalence, risk factors, and interventions for obesity in Saudi Arabia: A systematic review. Obes. Rev. 2022, 23, e13448. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Prasad, H.; Ryan, D.A.; Celzo, M.F.; Stapleton, D. Metabolic syndrome: Definition and therapeutic implications. Postgrad. Med. 2012, 124, 21–30. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovascular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxidative Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Lin, Y.P.; Oba, S.; Yoshioka, Y.; Hoshikawa, K. Prospects and potentials of underutilized leafy amaranths as vegetable use for health-promotion. Plant Physiol. Biochem. 2022, 182, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Peña, N.; Minguez, S.; Escobar, J.D. Current production scenario and functional potential of the whole amaranth plant: A review. In Agricultural Sciences; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2024; Volume 5, ISBN 978-1-83768-606-3. [Google Scholar]
- Jimoh, M.O.; Okaiyeto, K.; Oguntibeju, O.O.; Laubscher, C.P. A systematic review on Amaranthus-related research. Horticulturae 2022, 8, 239. [Google Scholar] [CrossRef]
- Peter, K.; Gandhi, P. Rediscovering the therapeutic potential of Amaranthus species: A review. Egypt. J. Basic Appl. Sci. 2017, 4, 196–205. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, P.C.; Rao, C.V. Nutrient content, mineral content and antioxidant activity of Amaranthus viridis and Moringa oleifera leaves. Res. J. Med. Plant 2012, 6, 253–259. [Google Scholar] [CrossRef]
- Bagepalli, S.A.K.; Kuruba, L.; Jayaveera, K. Comparative antipyretic activity of methanolic extracts of some species of Amaranthus. Asian Pac. J. Trop. Biomed. 2011, 1, S47–S50. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Dutta, A.; Singh, M. Comparative analysis of aqueous extracts of amaranth and coriander in scavenging free radical activity and protection of DNA against oxidative damage. Chiang Mai J. Sci. 2011, 38, 560–571. [Google Scholar]
- Semmler, G.; Datz, C.; Trauner, M. Eating, diet, and nutrition for the treatment of non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S244–S260. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005, 19, 136–138. [Google Scholar] [CrossRef]
- de Moura e Dias, M.; dos Reis, S.A.; da Conceição, L.L.; Sediyama, C.M.N.d.O.; Pereira, S.S.; de Oliveira, L.L.; Gouveia Peluzio, M.d.C.; Martinez, J.A.; Milagro, F.I. Diet-induced obesity in animal models: Points to consider and influence on metabolic markers. Diabetol. Metab. Syndr. 2021, 13, 32. [Google Scholar] [CrossRef]
- Dincheva, I.; Badjakov, I.; Galunska, B. New insights into the research of bioactive compounds from plant origins with nutraceutical and pharmaceutical potential. Plants 2023, 12, 258. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity rat model: A comparison between Wistar and Sprague-Dawley rat. Adipocyte 2015, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, O.C.; Babalola, O.O. Amaranth production and consumption in South Africa: The challenges of sustainability for food and nutrition security. Int. J. Agric. Sustain. 2022, 20, 449–460. [Google Scholar] [CrossRef]
- Koutsoukis, C.; Roukos, C.; Demertzis, P.G.; Kandrelis, S.; Akrida-Demertzi, K. The variation of the chemical composition of the main plant species in a subalpine grassland in northwestern Greece. Legume Sci. 2019, 1, e23. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Maurya, N.K.; Arya, P. Amaranthus grain nutritional benefits: A review. J. Pharmacogn. Phytochem. 2018, 7, 2258–2262. [Google Scholar]
- Schmidt, D.; Verruma-Bernardi, M.R.; Forti, V.A.; Borges, M.T.M.R. Quinoa and amaranth as functional foods: A review. Food Rev. Int. 2023, 39, 2277–2296. [Google Scholar] [CrossRef]
- Ishtiaq, S.; Ahmad, M.; Hanif, U.; Akbar, S.; Mehjabeen Kamran, S.H. Phytochemical and in vitro antioxidant evaluation of different fractions of Amaranthus graecizans subsp. silvestris (Vill.) Brenan. Asian Pac. J. Trop. Med. 2014, 7, S342–S347. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Shehab, N.G.; Abu-Gharbieh, E.; Bayoumi, F.A. Impact of phenolic composition on hepatoprotective and antioxidant effects of four desert medicinal plants. BMC Complement. Altern. Med. 2015, 15, 401. [Google Scholar] [CrossRef]
- Németh, K.; Tóth, B.; Sarnyai, F.; Koncz, A.; Lenzinger, D.; Kereszturi, É.; Visnovitz, T.; Kestecher, B.M.; Osteikoetxea, X.; Csala, M.; et al. High fat diet and PCSK9 knockout modulates lipid profile of the liver and changes the expression of lipid homeostasis related genes. Nutr. Metab. 2023, 20, 19. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and lipids metabolism: A review. Evid. Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, R.; Zi, Z.; Liu, B. A new clinical age of aging research. Trends Endocrinol. Metab. 2024, 36, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.A.M.S. Avaliação de Biodisponibilidade e Mecanismos de Ação Hipocolesterolemizante de Peptídeos do Amaranto (Amaranthus cruentus L. BRS-Alegria). Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2018. [Google Scholar]
- Jeong, Y.H.; Kim, T.H. Evaluation of radical scavenging and α-glucosidase inhibitory effects of gallic acid reactants using polyphenol oxidase. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1385–1390. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The potential and action mechanism of polyphenols in the treatment of liver diseases. Oxidative Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Jin, L.; Qian, C.; Zuo, W.; Lin, J.; Xie, L.; Jin, B.; Zhao, Y.; Huang, L.; et al. Alantolactone attenuates high-fat diet-induced inflammation and oxidative stress in non-alcoholic fatty liver disease. Nutr. Diabetes 2024, 14, 41. [Google Scholar] [CrossRef]
- Saha, P.; Talukdar, A.D.; Nath, R.; Sarker, S.D.; Nahar, L.; Sahu, J.; Choudhury, M.D. Role of natural phenolics in hepatoprotection: A mechanistic review and analysis of regulatory network of associated genes. Front. Pharmacol. 2019, 10, 509. [Google Scholar] [CrossRef]
- Jadhav, V.; Biradar, S. Evaluation of antioxidant activity of Amaranthus viridis L. methanolic extract. Int. J. Pharm. Bio Sci. 2016, 6, 150–153. [Google Scholar] [CrossRef]
- Sarker, U.; Ercisli, S. Salt eustress induction in red amaranth (Amaranthus gangeticus) augments nutritional, phenolic acids and antiradical potential of leaves. Antioxidants 2022, 11, 2434. [Google Scholar] [CrossRef]
- Adegbola, P.I.; Adetutu, A.; Olaniyi, T.D. Antioxidant activity of Amaranthus species from the Amaranthaceae family—A review. S. Afr. J. Bot. 2020, 133, 111–117. [Google Scholar] [CrossRef]
- Ko, H.J.; Chen, J.H.; Ng, L.T. Hepatoprotection of Gentiana scabra extract and polyphenols in liver of carbon tetrachloride-intoxicated mice. J. Environ. Pathol. Toxicol. Oncol. 2011, 30, 179–187. [Google Scholar] [CrossRef]
- Žiberna, L.; Jenko-Pražnikar, Z.; Petelin, A. Serum bilirubin levels in overweight and obese individuals: The importance of anti-inflammatory and antioxidant responses. Antioxidants 2021, 10, 1352. [Google Scholar] [CrossRef]
- Kipp, Z.A.; Xu, M.; Bates, E.A.; Lee, W.H.; Kern, P.A.; Hinds, T.D. Bilirubin levels are negatively correlated with adiposity in obese men and women, and its catabolized product, urobilin, is positively associated with insulin resistance. Antioxidants 2023, 12, 170. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Wang, C.; Ma, C.; Zhou, H.; Li, Y. The potential application of Chinese medicine in liver diseases: A new opportunity. Front. Pharmacol. 2021, 12, 771459. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Nag, N.; Chatterjee, S.; Adhikari, S.; Mazumder, S. Bilirubin clearance and antioxidant activities of ethanol extract of Phyllanthus amarus root in phenylhydrazine-induced neonatal jaundice in mice. J. Physiol. Biochem. 2013, 69, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, J.; Chua, S.S.; Qatanani, M.; Han, Y.; Granata, R.; Moore, D.D. Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc. Natl. Acad. Sci. USA 2003, 100, 4156–4161. [Google Scholar] [CrossRef]
- Kim, S.D.; Morgan, L.; Hargreaves, E.; Zhang, X.; Jiang, Z.; Antenos, M.; Li, B.; Kirby, G.M. Regulation of cytochrome P450 2a5 by Artemisia capillaris and 6,7-dimethylesculetin in mouse hepatocytes. Front. Pharmacol. 2021, 12, 730416. [Google Scholar] [CrossRef]
- Sinal, C.J.; Bend, J.R. Aryl hydrocarbon receptor-dependent induction of Cyp1a1 by bilirubin in mouse hepatoma Hepa 1c1c7 cells. Mol. Pharmacol. 1997, 52, 590–599. [Google Scholar] [CrossRef]
- Gabuza, K.B.; Sibuyi, N.R.S.; Mobo, M.P.; Madiehe, A.M. Differentially expressed serum proteins from obese Wistar rats as a risk factor for obesity-induced diseases. Sci. Rep. 2020, 10, 12415. [Google Scholar] [CrossRef]
- Diniz, A.; Escuder-Gilabert, L.; Lopes, N.P.; Villanueva-Camañas, R.M.; Sagrado, S.; Medina-Hernández, M.J. Characterization of interactions between polyphenolic compounds and human serum proteins by capillary electrophoresis. Anal. Bioanal. Chem. 2008, 391, 625–632. [Google Scholar] [CrossRef]
- Westerbacka, J.; Lammi, K.; Häkkinen, A.M.; Rissanen, A.; Salminen, I.; Aro, A.; Yki-Järvinen, H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 2804–2809. [Google Scholar] [CrossRef]
- Abenavoli, L.; Larussa, T.; Corea, A.; Procopio, A.C.; Boccuto, L.; Dallio, M.; Federico, A.; Luzza, F. Dietary polyphenols and non-alcoholic fatty liver disease. Nutrients 2021, 13, 494. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kitts, D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Pravin, B.; Nanaware, V.; Ashwini, B.; Wondmie, G.F.; Jardan, Y.A.B.; Bourhia, M. Assessing the antioxidant properties of naringin and rutin and investigating their oxidative DNA damage effects in breast cancer. Sci. Rep. 2024, 14, 15314. [Google Scholar] [CrossRef] [PubMed]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant activity of rosmarinic acid extracted and purified from Mentha piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Kumar, U.; Kumar, I.; Singh, P.K.; Yadav, J.S.; Dwivedi, A.; Singh, P.; Mishra, S.; Sharma, R.K. Total phenolic content and antioxidant activities in methanol extracts of medicinal herbs from Indo-Gangetic plains of India. J. Appl. Biol. Biotechnol. 2024, 12, 89–99. [Google Scholar] [CrossRef]
- Olubukola Sinbad, O.; Folorunsho, A.A.; Olabisi, O.L.; Abimbola Ayoola, O.; Johnson Temitope, E. Vitamins as antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Duchnik, E.; Marchlewicz, M. Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J. Physiol. Sci. 2022, 72, 19. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska-Markiewicz, D.; Stachowska, E.; Hawryłkowicz, V.; Stachowska, L.; Prowans, P. The role of resolvins, protectins and marensins in non-alcoholic fatty liver disease (NAFLD). Biomolecules 2021, 11, 937. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Fan, J.G.; Ding, X.D.; Qiao, L.; Wang, G.L. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef]
- Al-Dosari, M.S. The effectiveness of ethanolic extract of Amaranthus tricolor L.: A natural hepatoprotective agent. Am. J. Chin. Med. 2010, 38, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Ashok Kumar, B.S.; Lakshman, K.; Jayaveera, K.N.; Sheshadri Shekar, D.; Nandeesh, R.; Velmurugan, C. Chemoprotective and antioxidant activities of methanolic extract of Amaranthus spinosus leaves on paracetamol induced liver damage in rats. Acta Medica Salin. 2010, 39, 68–74. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Hosseini, R.; Kazemi, A.; Ofori-Asenso, R.; Mazidi, M.; Mazloomi, S.M. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 1587–1598. [Google Scholar] [CrossRef]
- Alzahrani, N.S.; Alshammari, G.M.; El-Ansary, A.; Yagoub, A.E.A.; Amina, M.; Saleh, A.; Yahya, M.A. Anti-Hyperlipidemia, Hypoglycemic, and Hepatoprotective Impacts of Pearl Millet (Pennisetum glaucum L.) Grains and Their Ethanol Extract on Rats Fed a High-Fat Diet. Nutrients 2022, 14, 1791. [Google Scholar] [CrossRef]
- Bose, M.; Lambert, J.D.; Ju, J.; Reuhl, K.R.; Shapses, S.A.; Yang, C.S. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008, 138, 1677–1683. [Google Scholar] [CrossRef]
- Alibas, I. Microwave, vacuum, and air-drying characteristics of collard leaves. Dry. Technol. 2009, 27, 1266–1273. [Google Scholar] [CrossRef]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef]
- Kim, H.C.; Song, J.M.; Kim, C.J.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Shin, S.H. Combined effect of bisphosphonate and recombinant human bone morphogenetic protein 2 on bone healing of rat calvarial defects. Maxillofac. Plast. Reconstr. Surg. 2015, 37, 16. [Google Scholar] [CrossRef]
- Hedge, J.; Hofreiter, B.; Whistler, R. Carbohydrate Chemistry; Academic Press: New York, NY, USA, 1962; pp. 371–380. [Google Scholar]
- Kjeldahl, J. New method for the determination of nitrogen. Sci. Am. 1883, 16, 6470. [Google Scholar] [CrossRef]
- Agroindustriais, P. Official methods of analysis of the Association of Official Analytical Chemists. In Caracterização Propagação e Melhor Genético Pitaya Comer e Nativa Cerrado; AOAC: Rockville, MD, USA, 2013; Volume 26, p. 62. [Google Scholar]
- ICC-Standard No. 110/1; Determination of the Moisture Content of Cereals and Cereal Products (Practical Method). International Association for Cereal Chemistry (ICC): Vienna, Austria, 1976.
- ICC 104/1; Determination of Ash in Cereals and Cereal Products. International Association for Cereal Chemistry (ICC): Vienna, Austria, 1990.




| Nutrients | % |
|---|---|
| Carbohydrates | 31.5 ± 0.5 |
| Protein | 21.3 ± 0.3 |
| Fat | 1.1 ± 0.1 |
| Total fiber | 15 ± 0.1 |
| Ash | 25.6 ± 0.5 |
| Moisture | 5.15 ± 0.5 |
| Vitamins | µg/mL |
|---|---|
| Vitamin C (ascorbic acid) | 8.09 |
| Vitamin B1 (Thiamine) | 7.16 |
| Vitamin B3(Niacin) | 1.39 |
| Vitamin B9 (Folic acid) | 6.37 |
| Vitamin B12 (Cobalamin) | 1.03 |
| Vitamin A (Retinol) | 4.69 |
| Vitamin E (Tocopherol) | 69.78 |
| Vitamin D3(Cholecalciferol) | 0.085 |
| Minerals | mg/g |
| Magnesium (Mg) | 6.55 |
| Potassium (K) | 35 |
| Phosphorus (P) | 2.5 |
| Sodium (Na) | 2.5 |
| Calcium (Ca) | 20 |
| Compound Name | Formula | * RT (mine) | * MW (g/mol) | Peak Area (%) |
|---|---|---|---|---|
| Gallic acid | C7H6O5 | 3.557 | 170.12 | 16.96 |
| Chlorogenic acid | C16H18O9 | 4.190 | 354.31 | 21.20 |
| Catechin | C15H14O6 | 4.627 | 290.26 | 0.59 |
| Methyl gallate | C8H8O5 | 5.471 | 184.147 | 2.47 |
| Caffeic acid | C9H8O4 | 5.850 | 180.16 | 3.21 |
| Syringic acid | C9H10O5 | 6.342 | 198.17 | 1.22 |
| Rutin | C27H30O16 | 6.931 | 610.517 | 8.71 |
| p-Coumaric acid | C9H8O3 | 8.522 | 164.04 | 2.69 |
| Vanillin | C8H8O3 | 9.070 | 152.15 | 4.81 |
| Ferulic acid | C10H10O4 | 9.642 | 194.18 | 2.03 |
| Rosmarinic acid | C18H16O8 | 11.660 | 360.318 | 6.52 |
| Daidzein | C15H10O4 | 15.658 | 254.23 | 0.54 |
| Cinnamic acid | C9H8O2 | 19.162 | 148.1586 | 3.01 |
| Group | Body Weight Gain (g) |
|---|---|
| SD | 9.83 ± 02.78 |
| HFD | 75.67 ± 11.79 **** |
| HFD + 100 mg/kg of AGs | 49.00 ± 04.04 ++++ |
| HFD + 200 mg/kg of AGs | 37.00 ± 09.03 ++++ |
| HFD + 500 mg/kg of AGs | 39.00 ± 07.04 ++++ |
| SD (D12450H) | HFD (D12451) | |||
|---|---|---|---|---|
| gm% | Kcal% | gm% | Kcal% | |
| Carbohydrate | 67.3 | 70% | 41 | 35% |
| Proteins | 19.2 | 20% | 24 | 20% |
| Fat | 4.3 | 10% | 24 | 45% |
| Others | 9.7 | - | 11 | - |
| Total (Kcal/gm) | 100 (3.85 Kcal/g) | 100 (4.73 Kcal/g) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, N.S.; Aljahdali, B.; Alhosain, A.; Alasmari, A.A.; Amna, T.; Yousef, S.M. Amaranthus graecizans L. Mitigates Hyperlipidemia-Induced Nonalcoholic Fatty Liver Disease in Experimental Rats: Future Pharmaceuticals. Pharmaceuticals 2025, 18, 1196. https://doi.org/10.3390/ph18081196
Alzahrani NS, Aljahdali B, Alhosain A, Alasmari AA, Amna T, Yousef SM. Amaranthus graecizans L. Mitigates Hyperlipidemia-Induced Nonalcoholic Fatty Liver Disease in Experimental Rats: Future Pharmaceuticals. Pharmaceuticals. 2025; 18(8):1196. https://doi.org/10.3390/ph18081196
Chicago/Turabian StyleAlzahrani, Nadiah S., Bayan Aljahdali, Aeshah Alhosain, Abeer Abdullah Alasmari, Touseef Amna, and Soha Mohamed Yousef. 2025. "Amaranthus graecizans L. Mitigates Hyperlipidemia-Induced Nonalcoholic Fatty Liver Disease in Experimental Rats: Future Pharmaceuticals" Pharmaceuticals 18, no. 8: 1196. https://doi.org/10.3390/ph18081196
APA StyleAlzahrani, N. S., Aljahdali, B., Alhosain, A., Alasmari, A. A., Amna, T., & Yousef, S. M. (2025). Amaranthus graecizans L. Mitigates Hyperlipidemia-Induced Nonalcoholic Fatty Liver Disease in Experimental Rats: Future Pharmaceuticals. Pharmaceuticals, 18(8), 1196. https://doi.org/10.3390/ph18081196








