Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers
Abstract
1. Introduction
2. Materials and Methods
3. Classes and Characteristics of Antimicrobial NPs
3.1. Metal and Metal Oxide NPs
3.2. Polymeric NPs
3.3. Lipid-Based NPs
3.4. Hybrid NPs (MOF-Based Nanozymes)
4. Mechanisms of Antimicrobial Action of NPs
5. Biomedical Applications of Antimicrobial NPs
5.1. Wound Healing and Skin Infections
5.2. Implant-Associated and Nosocomial Infections
5.3. Targeted Antibiotic Delivery Systems
5.4. Inhalation-Based Therapies for Respiratory Infections
5.5. Intracellular Infections and NP-Mediated Immunotherapy
5.6. Clinical Trials and Regulatory Perspectives
5.7. Price and Affordability Constraints
6. Translational Challenges and Safety Considerations of Antimicrobial NPs
6.1. Toxicological Concerns and Dose-Dependent Toxicity
6.2. Immune Activation and Inflammatory Risks
6.3. Protein Corona Formation and Biodistribution
6.4. Manufacturing, Scalability, and Standardization
6.5. Green Synthesis and Biocompatibility
6.6. PEGylation and Reduced Toxicity
6.7. Regulatory Frameworks and Clinical Translation
6.8. Microbiota–NP Interactions
7. Future Directions and Research Gaps
| Research Gap | Description | Proposed Future Directions | Key References |
|---|---|---|---|
| Lack of stimuli-responsive drug delivery systems | Current NPs often release drugs passively, leading to off-target effects and reduced therapeutic precision. | Design infection-triggered or pH-/enzyme-responsive systems to enable site-specific activation. | [197] |
| Poor efficacy against biofilms | Biofilm matrices hinder NP penetration and antimicrobial activity, reducing treatment effectiveness. | Develop enzyme-functionalized NPs (e.g., DNase, protease) or ROS-generating NPs to disrupt biofilms. | [199,203] |
| Limited long-term safety and biodistribution data | Most studies assess short-term efficacy, lacking insight into chronic toxicity and organ accumulation. | Utilize radiolabeling or imaging-guided platforms to evaluate NP clearance, persistence, and tissue-specific effects over time. | [204,205] |
| Environmental impact and microbiome disruption | Persistent NPs, especially metal-based ones, may harm beneficial microbes in the environment or gut microbiota. | Prioritize green synthesis methods and biodegradable nanomaterials that limit ecological toxicity. | [206] |
| Fragmented regulatory frameworks | Lack of harmonized guidelines delays clinical translation and hinders global acceptance of NP therapeutics. | Develop unified regulatory protocols addressing NP characterization, manufacturing, and pharmacokinetics. | [29,207] |
| Insufficient integration with diagnostics (theranostics) | Few platforms allow simultaneous infection detection and treatment, limiting real-time precision therapy. | Create dual-function NPs combining antimicrobial delivery with imaging or biosensing elements. | [208] |
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AMR | Antimicrobial Resistance |
| NPs | Nanoparticles |
| MDR | Multidrug-resistant |
| AgNPs | Silver Nanoparticles |
| AI | Artificial Intelligence |
| AuNPs | Gold Nanoparticles |
| CFU | Colony-Forming Units |
| CuO | Copper Oxide |
| CuO NPs | Copper Oxide Nanoparticles |
| DNA | Deoxyribonucleic Acid |
| EMA | European Medicines Agency |
| FDA | U.S. Food and Drug Administration |
| GNS | Gold Nanostar |
| IFN-γ | Interferon Gamma |
| IL-1β | Interleukin-1 Beta |
| MIC | Minimum Inhibitory Concentration |
| Mo@ZIF-8 | Molybdenum-Doped Zeolitic Imidazolate Framework-8 |
| MOF | Metal–Organic Framework |
| MSNs | Mesoporous Silica Nanoparticles |
| NLCs | Nanostructured Lipid Carriers |
| NP(s) | Nanoparticle(s) |
| PEG | Polyethylene Glycol |
| PLGA | Poly(lactic-co-glycolic acid) |
| QS | Quorum Sensing |
| rGO | Reduced Graphene Oxide |
| RNA | Ribonucleic Acid |
| ROS | Reactive Oxygen Species |
| SCFAs | Short-Chain Fatty Acids |
| S. aureus | Staphylococcus aureus |
| E. coli | Escherichia coli |
| P. aeruginosa | Pseudomonas aeruginosa |
| K. pneumoniae | Klebsiella pneumoniae |
| S. typhimurium | Salmonella typhimurium |
| TiO2 | Titanium Dioxide |
| TiO2 NPs | Titanium Dioxide Nanoparticles |
| TiN–Ag | Titanium Nitride–Silver |
| ZnO | Zinc Oxide |
| ZnO NPs | Zinc Oxide Nanoparticles |
| ZnO–Ag | Zinc Oxide–Silver |
| ZIF-8 | Zeolitic Imidazolate Framework-8 |
References
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 8 July 2025).
- WHO. Global Action Plan on Antimicrobial Resistance; 2015. Available online: https://www.emro.who.int/health-topics/drug-resistance/global-action-plan.html (accessed on 8 July 2025).
- Theuretzbacher, U.; Piddock, L.J. Non-traditional antibacterial therapeutic options and challenges. Cell Host Microbe 2019, 26, 61–72. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.; Forde, B.M.; Kidd, T.J.; Harris, P.N.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; de Aberasturi, D.J.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Nazam, N.; Rizvi, S.M.D.; Ahmad, K.; Baig, M.H.; Lee, E.J.; Choi, I. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. Int. J. Mol. Sci. 2019, 20, 2468. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.j.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Tängdén, T.; Giske, C. Global dissemination of extensively drug-resistant carbapenemase-producing E nterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J. Intern. Med. 2015, 277, 501–512. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Yathavan, B.; Chhibber, T.; Steinhauff, D.; Pulsipher, A.; Alt, J.A.; Ghandehari, H.; Jafari, P. Matrix-mediated delivery of silver nanoparticles for prevention of Staphylococcus aureus and Pseudomonas aeruginosa biofilm formation in chronic rhinosinusitis. Pharmaceutics 2023, 15, 2426. [Google Scholar] [CrossRef]
- Alhosani, F.; Islayem, D.; Almansoori, S.; Zaka, A.; Nayfeh, L.; Rezk, A.; Yousef, A.F.; Pappa, A.M.; Nayfeh, A. Antibiofilm activity of ZnO–Ag nanoparticles against Pseudomonas aeruginosa. Sci. Rep. 2025, 15, 17321. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.M.A.; El Maghraby, G.M.; Shafik, M.M.; Al-Madboly, L.A. Silver nanoparticle with potential antimicrobial and antibiofilm efficiency against multiple drug resistant, extensive drug resistant Pseudomonas aeruginosa clinical isolates. BMC Microbiol. 2024, 24, 277. [Google Scholar] [CrossRef]
- Szymczak, M.; Pankowski, J.A.; Kwiatek, A.; Grygorcewicz, B.; Karczewska-Golec, J.; Sadowska, K.; Golec, P. An effective antibiofilm strategy based on bacteriophages armed with silver nanoparticles. Sci. Rep. 2024, 14, 9088. [Google Scholar] [CrossRef]
- Ahmad, I.; Khan, S.A.; Naseer, A.; Nazir, A. Green synthesized silver nanoparticles from Phoenix dactylifera synergistically interact with bioactive extract of Punica granatum against bacterial virulence and biofilm development. Microb. Pathog. 2024, 192, 106708. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The regulation of nanomaterials and nanomedicines for clinical application: Current and future perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, properties, and regulatory issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef]
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 500–525. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Li, Z.; Chu, X. Metal-based nanoparticles in antibacterial application in biomedical field: Current development and potential mechanisms. Biomed. Microdevices 2024, 26, 12. [Google Scholar] [CrossRef]
- Moradialvand, M.; Asri, N.; Jahdkaran, M.; Beladi, M.; Houri, H. Advancements in nanoparticle-based strategies for enhanced antibacterial interventions. Cell Biochem. Biophys. 2024, 82, 3071–3090. [Google Scholar] [CrossRef]
- Okka, E.Z.; Tongur, T.; Aytas, T.T.; Yılmaz, M.; Topel, Ö.; Sahin, R. Green Synthesis and the formation kinetics of silver nanoparticles in aqueous Inula viscosa extract. Optik 2023, 294, 171487. [Google Scholar] [CrossRef]
- Gur, T. Green synthesis, characterizations of silver nanoparticles using sumac (Rhus coriaria L.) plant extract and their antimicrobial and DNA damage protective effects. Front. Chem. 2022, 10, 968280. [Google Scholar] [CrossRef]
- Ershov, V.A.; Ershov, B.G. Effect of silver nanoparticle size on antibacterial activity. Toxics 2024, 12, 801. [Google Scholar] [CrossRef]
- Shahzadi, S.; Fatima, S.; Shafiq, Z.; Janjua, M.R.S.A. A review on green synthesis of silver nanoparticles (SNPs) using plant extracts: A multifaceted approach in photocatalysis, environmental remediation, and biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef]
- Aguilar-Garay, R.; Lara-Ortiz, L.F.; Campos-López, M.; Gonzalez-Rodriguez, D.E.; Gamboa-Lugo, M.M.; Mendoza-Pérez, J.A.; Anzueto-Ríos, Á.; Nicolás-Álvarez, D.E. A comprehensive review of silver and gold nanoparticles as effective antibacterial agents. Pharmaceuticals 2024, 17, 1134. [Google Scholar] [CrossRef]
- El-Fallal, A.A.; Elfayoumy, R.A.; El-Zahed, M.M. Antibacterial activity of biosynthesized zinc oxide nanoparticles using Kombucha extract. SN Appl. Sci. 2023, 5, 332. [Google Scholar] [CrossRef]
- Tazeen, S.K.; Niazi, M.B.K.; Binobead, M.A.; Ahmed, T.; Shahid, M. Characterization and toxicity evaluation of chitosan/ZnO nanocompoite as promising nano-biopolymer for treatment of synthetic wastewater. J. King Saud Univ.-Sci. 2024, 36, 103432. [Google Scholar]
- Azad, A.; Hussain, S.; Akram, H.; Fida, H.; Iqbal, M.A.; Butt, T.E. Environmentally-friendly synthesis of platinum nanoparticles: Phytochemical, antioxidant, and antimicrobial properties of Cichorium intybus in different solvents. Discov. Plants 2024, 1, 24. [Google Scholar] [CrossRef]
- Prashanth, G.; Lalithamba, H.; Rao, S.; Rashmi, K.; Bhagya, N.; Dileep, M.; Gadewar, M.; Ghosh, M.K. Green synthesized Pt-based nanoparticles redefining biomedical frontiers: A brief review. Next Mater. 2025, 8, 100613. [Google Scholar] [CrossRef]
- Su, L.; Gong, X.; Fan, R.; Ni, T.; Yang, F.; Zhang, X.; Li, X. Mechanism of action of platinum nanoparticles implying from antioxidant to metabolic programming in light-induced retinal degeneration model. Redox Biol. 2023, 65, 102836. [Google Scholar] [CrossRef]
- Belloso Daza, M.V.; Scarsi, A.; Gatto, F.; Rocchetti, G.; Pompa, P.P.; Cocconcelli, P.S. Role of Platinum Nanozymes in the Oxidative Stress Response of Salmonella typhimurium. Antioxidants 2023, 12, 1029. [Google Scholar] [CrossRef]
- Gatto, F.; Moglianetti, M.; Pompa, P.P.; Bardi, G. Platinum nanoparticles decrease reactive oxygen species and modulate gene expression without alteration of immune responses in THP-1 monocytes. Nanomaterials 2018, 8, 392. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Awadelkareem, A.M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Hadi, S.; Snoussi, M.; Badraoui, R.; Bardakci, F.; Ashraf, M.S.; Danciu, C. Biosynthesized silver nanoparticles derived from probiotic Lactobacillus rhamnosus (AgNPs-LR) targeting biofilm formation and quorum sensing-mediated virulence factors. Antibiotics 2023, 12, 986. [Google Scholar] [CrossRef]
- Chen, S.; Yao, J.; Huo, S.; Xu, C.; Yang, R.; Tao, D.; Fang, B.; Ma, G.; Zhu, Z.; Zhang, Y. Designing injectable dermal matrix hydrogel combined with silver nanoparticles for methicillin-resistant Staphylococcus aureus infected wounds healing. Nano Converg. 2024, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Hu, F.; Chai, Z.; Zheng, C.; Zhang, W.; Pu, K.; Yang, Z.; Zhang, Y.; Ramrkrishna, S.; Wu, X. Multifunctional hydrogel with mild photothermal properties enhances diabetic wound repair by targeting MRSA energy metabolism. J. Nanobiotechnol. 2025, 23, 380. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Zeiringer, S.; Šarić, A.; Stanković, A.; Učakar, A.; Vidmar, J.; Abram, A.; Njegić Džakula, B.; Kontrec, J.; Zore, A. ZnO Nanoparticle-Infused Vaterite Coatings: A Novel Approach for Antimicrobial Titanium Implant Surfaces. J. Funct. Biomater. 2025, 16, 108. [Google Scholar] [CrossRef]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of antimicrobial properties of titanium dioxide nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef]
- Najm, M.A.; Shakir, H.A.; Hasen, S.T.; Jawad, K.H.; Hasoon, B.A.; Jabir, M.S.; Issa, A.A.; Albukhaty, S.; Gatasheh, M.K.; Molla, M.H. Titanium dioxide nanoparticles augment Ciprofloxacin activity via Inhibition of biofilm formation for multidrug resistance bacteria in-vitro and insilco prediction study. Sci. Rep. 2025, 15, 18014. [Google Scholar] [CrossRef]
- Shariati, A.; Chegini, Z.; Ghaznavi-Rad, E.; Zare, E.N.; Hosseini, S.M. PLGA-based nanoplatforms in drug delivery for inhibition and destruction of microbial biofilm. Front. Cell. Infect. Microbiol. 2022, 12, 926363. [Google Scholar] [CrossRef]
- Türeli, N.G.; Torge, A.; Juntke, J.; Schwarz, B.C.; Schneider-Daum, N.; Türeli, A.E.; Lehr, C.-M.; Schneider, M. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. Eur. J. Pharm. Biopharm. 2017, 117, 363–371. [Google Scholar] [CrossRef]
- Tchatchiashvili, T.; Duering, H.; Mueller-Boetticher, L.; Grune, C.; Fischer, D.; Pletz, M.W.; Makarewicz, O. PEG-PLGA nanoparticles deposited in Pseudomonas aeruginosa and Burkholderia cenocepacia. J. Pharm. Anal. 2024, 14, 100939. [Google Scholar] [CrossRef]
- Coksu, I.; Dokuz, S.; Akgul, B.; Ozbek, T.; Abamor, E.S.; Duranoglu, D.; Acar, S. Enhancing the treatment of Staphylococcus aureus infections: A nanosystem with including dual antimicrobial peptide. J. Drug Deliv. Sci. Technol. 2024, 97, 105830. [Google Scholar] [CrossRef]
- Miranda Calderon, L.G.; Alejo, T.; Santos, S.; Mendoza, G.; Irusta, S.; Arruebo, M. Antibody-functionalized polymer nanoparticles for targeted antibiotic delivery in models of pathogenic bacteria infecting human macrophages. ACS Appl. Mater. Interfaces 2023, 15, 40213–40227. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, W.; Song, L.-J.; Luan, S.-F. Stimuli-responsive nanocarriers for bacterial biofilm treatment. Rare Met. 2022, 41, 482–498. [Google Scholar] [CrossRef]
- Afrasiabi, S.; Partoazar, A.; Chiniforush, N. In vitro study of nanoliposomes containing curcumin and doxycycline for enhanced antimicrobial photodynamic therapy against Aggregatibacter actinomycetemcomitans. Sci. Rep. 2023, 13, 11552. [Google Scholar] [CrossRef]
- Arabestani, M.R.; Bigham, A.; Kamarehei, F.; Dini, M.; Gorjikhah, F.; Shariati, A.; Hosseini, S.M. Solid lipid nanoparticles and their application in the treatment of bacterial infectious diseases. Biomed. Pharmacother. 2024, 174, 116433. [Google Scholar] [CrossRef]
- Motsoene, F.; Abrahamse, H.; Kumar, S.S.D. Multifunctional lipid-based nanoparticles for wound healing and antibacterial applications: A review. Adv. Colloid Interface Sci. 2023, 321, 103002. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, T.; Pan, X. Metal–organic-framework-based materials for antimicrobial applications. ACS Nano 2021, 15, 3808–3848. [Google Scholar] [CrossRef]
- Hu, W.; Ouyang, Q.; Jiang, C.; Huang, S.; Alireza, N.-E.; Guo, D.; Liu, J.; Peng, Y. Biomedical Metal–Organic framework materials on antimicrobial therapy: Perspectives and challenges. Mater. Today Chem. 2024, 41, 102300. [Google Scholar] [CrossRef]
- Omer, M.E.; Halwani, M.; Alenazi, R.M.; Alharbi, O.; Aljihani, S.; Massadeh, S.; Al Ghoribi, M.; Al Aamery, M.; Yassin, A.E. Novel self-assembled polycaprolactone–lipid hybrid nanoparticles enhance the antibacterial activity of ciprofloxacin. SLAS Technol. Transl. Life Sci. Innov. 2020, 25, 598–607. [Google Scholar] [CrossRef]
- Rajak, K.K.; Pahilani, P.; Patel, H.; Kikani, B.; Desai, R.; Kumar, H. Green synthesis of silver nanoparticles using Curcuma longa flower extract and antibacterial activity. arXiv 2023, arXiv:2304.04777. [Google Scholar] [CrossRef]
- Lian, Z.; Lu, C.; Zhu, J.; Zhang, X.; Wu, T.; Xiong, Y.; Sun, Z.; Yang, R. Mo@ ZIF-8 nanozyme preparation and its antibacterial property evaluation. Front. Chem. 2022, 10, 1093073. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Sadiq, S.; Xu, T.; Wu, P.; Khan, I.; Jiao, X.; Khan, A.; Wang, L.; Lin, S. Inhibitory effect of organometallic framework composite nanomaterial ZIF8@ ZIF67 on different pathogenic microorganisms of silkworms. Pestic. Biochem. Physiol. 2025, 208, 106307. [Google Scholar] [CrossRef]
- Cai, W.; Zhang, W.; Chen, Z. Magnetic Fe3O4@ ZIF-8 nanoparticles as a drug release vehicle: pH-sensitive release of norfloxacin and its antibacterial activity. Colloids Surf. B Biointerfaces 2023, 223, 113170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Chen, L.; Yu, J.; Jin, X.; Zeng, R.; Luo, X.; Cong, Y.; Xu, G.; Zhang, J. NIR-triggered and Glucose-Powered Hollow mesoporous Mo-based single-atom nanozymes for cascade chemodynamic diabetic infection therapy. Mater. Today Bio 2025, 31, 101557. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Choudhary, M. Recent Updates on Green Synthesized Silver Nanoparticles: Preparation Technologies, Properties, and Applications In Biomedical Sector. Int. J. Environ. Sci. 2025, 11, 968–982. [Google Scholar] [CrossRef]
- Sharma, R.; Basist, P.; Alhalmi, A.; Khan, R.; Noman, O.M.; Alahdab, A. Synthesis of quercetin-loaded silver nanoparticles and assessing their anti-bacterial potential. Micromachines 2023, 14, 2154. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- El-Habib, I.; Maatouk, H.; Lemarchand, A.; Dine, S.; Roynette, A.; Mielcarek, C.; Traoré, M.; Azouani, R. Antibacterial size effect of ZnO nanoparticles and their role as additives in emulsion waterborne paint. J. Funct. Biomater. 2024, 15, 195. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Rageb, M.; El-Saber, M.M.; El-Masry, R.A.; Ramadan, K.M.; Kandeel, M.; Alhajri, A.S.; Osman, A. Green synthesis, characterization, and hepatoprotective effect of zinc oxide nanoparticles from Moringa oleifera leaves in CCl4-treated albino rats. Heliyon 2024, 10, e30627. [Google Scholar] [CrossRef]
- Khamis, M.; Gouda, G.A.; Nagiub, A.M. Biosynthesis approach of zinc oxide nanoparticles for aqueous phosphorous removal: Physicochemical properties and antibacterial activities. BMC Chem. 2023, 17, 99. [Google Scholar] [CrossRef]
- Ali, S.A.; Ali, E.; Hamdy, G.; Badawy, M.S.E.; Ismail, A.R.; El-Sabbagh, I.A.; El-Fass, M.M.; Elsawy, M.A. Enhancing physical characteristics and antibacterial efficacy of chitosan through investigation of microwave-assisted chemically formulated chitosan-coated ZnO and chitosan/ZnO physical composite. Sci. Rep. 2024, 14, 9348. [Google Scholar] [CrossRef]
- Al-Mohaimeed, A.M.; Al-Onazi, W.A.; El-Tohamy, M.F. Multifunctional eco-friendly synthesis of ZnO nanoparticles in biomedical applications. Molecules 2022, 27, 579. [Google Scholar] [CrossRef]
- Neiva, J.; Benzarti, Z.; Carvalho, S.; Devesa, S. Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization. Materials 2024, 17, 5709. [Google Scholar] [CrossRef]
- Chen, N.-F.; Liao, Y.-H.; Lin, P.-Y.; Chen, W.-F.; Wen, Z.-H.; Hsieh, S. Investigation of the characteristics and antibacterial activity of polymer-modified copper oxide nanoparticles. Int. J. Mol. Sci. 2021, 22, 12913. [Google Scholar] [CrossRef]
- Palani, M.; Kalaiselvan, S.; Mark, J.A.M.; Chandran, K.; Ekhambaram, V. Green synthesis of CuO nanoparticles: A promising role of antioxidant and antimicrobial activity by using Tribulus terrestris L. Asp. Mol. Med. 2024, 4, 100049. [Google Scholar] [CrossRef]
- Lone, A.L.; Rehman, S.U.; Haq, S.; Shahzad, N.; Al-Sadoon, M.K.; Shahzad, M.I.; Razzokov, J.; Shujaat, S.; Samad, A. Unveiling the physicochemical, photocatalytic, antibacterial and antioxidant properties of MWCNT-modified Ag2O/CuO/ZnO nanocomposites. RSC Adv. 2025, 15, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Jeevarathinam, M.; Asharani, I. Synthesis of CuO, ZnO nanoparticles, and CuO-ZnO nanocomposite for enhanced photocatalytic degradation of Rhodamine B: A comparative study. Sci. Rep. 2024, 14, 9718. [Google Scholar] [CrossRef] [PubMed]
- Jabber, A.A.; Abdulridha, A.R. Preparation and characterization of CuO/ZnO nanostructures thin films using thermal evaporation for advanced gas sensing applications. Trends Sci. 2025, 22, 9002. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium dioxide nanoparticles: Recent progress in antimicrobial applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1860. [Google Scholar] [CrossRef]
- Aravind, M.; Amalanathan, M.; Mary, M. Synthesis of TiO2 nanoparticles by chemical and green synthesis methods and their multifaceted properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Amiri, M.R.; Alavi, M.; Taran, M.; Kahrizi, D. Antibacterial, antifungal, antiviral, and photocatalytic activities of TiO2 nanoparticles, nanocomposites, and bio-nanocomposites: Recent advances and challenges. J. Public Health Res. 2022, 11, 22799036221104151. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, Y. The latest research progress on the antibacterial properties of TiO2 nanocomposites. J. Text. Inst. 2025, 116, 634–660. [Google Scholar] [CrossRef]
- Mohammadi, H.; Moradpoor, H.; Beddu, S.; Mozaffari, H.R.; Sharifi, R.; Rezaei, R.; Fallahnia, N.; Ebadi, M.; Mazlan, S.A.; Safaei, M. Current trends and research advances on the application of TiO2 nanoparticles in dentistry: How far are we from clinical translation? Heliyon 2025, 11, e42169. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a novel approach in combating microbes providing an alternative to antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- AlQurashi, D.M.; AlQurashi, T.F.; Alam, R.I.; Shaikh, S.; Tarkistani, M.A.M. Advanced nanoparticles in combating antibiotic resistance: Current innovations and future directions. J. Nanotheranostics 2025, 6, 9. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Processes 2020, 8, 1173. [Google Scholar] [CrossRef]
- Heidegger, S.; Gößl, D.; Schmidt, A.; Niedermayer, S.; Argyo, C.; Endres, S.; Bein, T.; Bourquin, C. Immune response to functionalized mesoporous silica nanoparticles for targeted drug delivery. Nanoscale 2016, 8, 938–948. [Google Scholar] [CrossRef]
- Yang, C.; Luo, Y.; Shen, H.; Ge, M.; Tang, J.; Wang, Q.; Lin, H.; Shi, J.; Zhang, X. Inorganic nanosheets facilitate humoral immunity against medical implant infections by modulating immune co-stimulatory pathways. Nat. Commun. 2022, 13, 4866. [Google Scholar] [CrossRef]
- Wypij, M.; Jędrzejewski, T.; Trzcińska-Wencel, J.; Ostrowski, M.; Rai, M.; Golińska, P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol. 2021, 12, 632505. [Google Scholar] [CrossRef]
- Srećković, N.Z.; Nedić, Z.P.; Monti, D.M.; D’Elia, L.; Dimitrijević, S.B.; Mihailović, N.R.; Katanić Stanković, J.S.; Mihailović, V.B. Biosynthesis of silver nanoparticles using Salvia pratensis L. aerial part and root extracts: Bioactivity, biocompatibility, and catalytic potential. Molecules 2023, 28, 1387. [Google Scholar] [CrossRef]
- Chahardoli, A.; Qalekhani, F.; Hajmomeni, P.; Shokoohinia, Y.; Fattahi, A. Enhanced hemocompatibility, antimicrobial and anti-inflammatory properties of biomolecules stabilized AgNPs with cytotoxic effects on cancer cells. Sci. Rep. 2025, 15, 1186. [Google Scholar] [CrossRef]
- Vijaya, P.; Rekha, B.; Mathew, A.T.; Syed Ali, M.; Yogananth, N.; Anuradha, V.; Kalitha Parveen, P. Antigenotoxic effect of green-synthesised silver nanoparticles from Ocimum sanctum leaf extract against cyclophosphamide induced genotoxicity in human lymphocytes—In vitro. Appl. Nanosci. 2014, 4, 415–420. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.K.; Renouard, S.; Drouet, S.; Blondeau, J.-P.; Anjum, I.; Hano, C.; Abbasi, B.H.; Anjum, S. Effect of UV irradiation (A and C) on Casuarina equisetifolia-mediated biosynthesis and characterization of antimicrobial and anticancer activity of biocompatible zinc oxide nanoparticles. Pharmaceutics 2021, 13, 1977. [Google Scholar] [CrossRef] [PubMed]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2018, 20, 5. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Hu, Y. Zinc oxide nanoparticle-reinforced sodium alginate/hydroxyapatite scaffolds for osteoporosis treatment in fragility fracture patients: Development and characterization using artificial neural networks (ANNs) modeling. Iran. J. Basic Med. Sci. 2024, 27, 1592. [Google Scholar]
- Al-Hamad, K.A.; Asiri, A.; Alqahtani, A.M.; Alotaibi, S.; Almalki, A. Monitoring the Antibacterial Activity of the Green Synthesized ZnO Nanoparticles on the Negative and Positive Gram Bacteria Mimicking Oral Environment by Using a Quartz Tuning Fork (QTF) Micromechanical Sensor. Int. J. Nanomed. 2025, 20, 7975–7985. [Google Scholar] [CrossRef]
- Sarfraz, M.H.; Zubair, M.; Aslam, B.; Ashraf, A.; Siddique, M.H.; Hayat, S.; Cruz, J.N.; Muzammil, S.; Khurshid, M.; Sarfraz, M.F. Comparative analysis of phyto-fabricated chitosan, copper oxide, and chitosan-based CuO nanoparticles: Antibacterial potential against Acinetobacter baumannii isolates and anticancer activity against HepG2 cell lines. Front. Microbiol. 2023, 14, 1188743. [Google Scholar] [CrossRef]
- Badawy, A.A.; Abdelfattah, N.A.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.). Plant Biol. 2021, 10, 233. [Google Scholar] [CrossRef]
- Nassar, A.-R.A.; Atta, H.M.; Abdel-Rahman, M.A.; El Naghy, W.S.; Fouda, A. Myco-synthesized copper oxide nanoparticles using harnessing metabolites of endophytic fungal strain Aspergillus terreus: An insight into antibacterial, anti-Candida, biocompatibility, anticancer, and antioxidant activities. BMC Complement. Med. Ther. 2023, 23, 261. [Google Scholar] [CrossRef]
- El-Sherbiny, G.M.; Kalaba, M.H.; Sharaf, M.H.; Moghannem, S.A.; Radwan, A.A.; Askar, A.A.; Ismail, M.K.; El-Hawary, A.S.; Abushiba, M.A. Biogenic synthesis of CuO-NPs as nanotherapeutics approaches to overcome multidrug-resistant Staphylococcus aureus (MDRSA). Artif. Cells Nanomed. Biotechnol. 2022, 50, 260–274. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Badr, B.M.; Elkady, F.M.; Watanabe, T.; Abdel-Maksoud, M.A.; Alamri, A.M.; Alrokayan, S.; Abdelaziz, A.M. Anti-Virulence Properties of Curcumin/CuO-NPs and Their Role in Accelerating Wound Healing In Vivo. Medicina 2025, 61, 515. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Elhenawy, Y.; Abdel-Hamid, S.M.; Fouad, Y.; Monica, T.; Al-Qabandi, O.; El Fray, M.; Bassyouni, M. New eco-friendly, biocompatible, bactericidal, fungicidal and anticancer-activity-exhibiting nanocomposites based on bimetallic TiO2@ Cr2O3 nanoparticle core and biopolymer shells. J. Compos. Sci. 2023, 7, 426. [Google Scholar] [CrossRef]
- Al-Shabib, N.A.; Husain, F.M.; Qais, F.A.; Ahmad, N.; Khan, A.; Alyousef, A.A.; Arshad, M.; Noor, S.; Khan, J.M.; Alam, P. Phyto-mediated synthesis of porous titanium dioxide nanoparticles from Withania somnifera root extract: Broad-spectrum attenuation of biofilm and cytotoxic properties against HepG2 cell lines. Front. Microbiol. 2020, 11, 1680. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Shahzad, K.; Mushtaq, S.; Ali, I.; Rafe, M.H.; Fazal-ul-Karim, S.M. Antibacterial and antiviral potential of colloidal Titanium dioxide (TiO2) nanoparticles suitable for biological applications. Mater. Res. Express 2019, 6, 105409. [Google Scholar] [CrossRef]
- Ghareeb, A.; Fouda, A.; Kishk, R.M.; El Kazzaz, W.M. Multifaceted biomedical applications of biogenic titanium dioxide nanoparticles fabricated by marine actinobacterium Streptomyces vinaceusdrappus AMG31. Sci. Rep. 2025, 15, 20244. [Google Scholar] [CrossRef]
- Rafe Hatshan, M.; Perianaika Matharasi Antonyraj, A.; Marunganathan, V.; Rafi Shaik, M.; Deepak, P.; Thiyagarajulu, N.; Manivannan, C.; Jain, D.; Melo Coutinho, H.D.; Guru, A. Synergistic Action of Vanillic Acid-Coated Titanium Oxide Nanoparticles: Targeting Biofilm Formation Receptors of Dental Pathogens and Modulating Apoptosis Genes for Enhanced Oral Anticancer Activity. Chem. Biodivers. 2025, 22, e202402080. [Google Scholar] [CrossRef]
- Bharti, S. Harnessing the potential of bimetallic nanoparticles: Exploring a novel approach to address antimicrobial resistance. World J. Microbiol. Biotechnol. 2024, 40, 89. [Google Scholar] [CrossRef]
- Solanki, R.; Makwana, N.; Kumar, R.; Joshi, M.; Patel, A.; Bhatia, D.; Sahoo, D.K. Nanomedicines as a cutting-edge solution to combat antimicrobial resistance. RSC Adv. 2024, 14, 33568–33586. [Google Scholar] [CrossRef]
- Singh, R. Revolutionizing Antimicrobial Therapies Through Biofilm-Targeted Nanomedicine. Curr. Pharm. Res. 2025, 1, 78–97. [Google Scholar] [CrossRef]
- Boersema, G.C.; Smart, H.; Giaquinto-Cilliers, M.; Mulder, M.; Weir, G.R.; Bruwer, F.A.; Idensohn, P.J.; Sander, J.E.; Stavast, A.; Swart, M. Management of non-healable and maintenance wounds: A systematic integrative review and referral pathway. Wound Heal. S. Afr. 2021, 14, 8–17. [Google Scholar]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial contribution in chronicity of wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, J.; Karthikeyan, E.; Rani, E.E.; Karthikha, V.; Sanjana, D.S.; Jeevitha, H.; Rajeshkumar, S.; Venugopal, V.; Priyadharshan, A. Advancing engineered approaches for sustainable wound regeneration and repair: Harnessing the potential of green synthesized silver nanoparticles. Eng. Regen. 2024, 5, 306–325. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.; Vindigni, V.; Azzena, B.; Barbante, C. Active silver nanoparticles for wound healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef]
- Salama, A.; Elsherbiny, N.; Hetta, H.F.; Safwat, M.A.; Atif, H.M.; Fathalla, D.; Almanzalawi, W.S.; Almowallad, S.; Soliman, G.M. Curcumin-loaded gold nanoparticles with enhanced antibacterial efficacy and wound healing properties in diabetic rats. Int. J. Pharm. 2024, 666, 124761. [Google Scholar] [CrossRef]
- Poomrattanangoon, S.; Pissuwan, D. Gold nanoparticles coated with collagen-I and their wound healing activity in human skin fibroblast cells. Heliyon 2024, 10, e33302. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Gao, J.; Zhang, Z.; Wang, L.; Chen, X.; Mi, J.; Yao, Y.; Guan, D.; Chen, B. Transdermal vascular endothelial growth factor delivery with surface engineered gold nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 5173–5180. [Google Scholar] [CrossRef]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The current knowledge on the pathogenesis of tissue and medical device-related biofilm infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Liu, L.; Zhang, P.; Zhang, Y.; Zhou, Z.; Gao, X.; Sun, S. Photothermal Antibacterial Effect of Gold Nanostars Coating on Titanium Implant and Its Osteogenic Performance. Int. J. Nanomed. 2025, 5983–5999. [Google Scholar] [CrossRef] [PubMed]
- Aly, Y.M.; Zhang, Z.; Ali, N.; Milward, M.R.; Poologasundarampillai, G.; Dong, H.; Kuehne, S.A.; Camilleri, J. Ceramic conversion treated titanium implant abutments with gold for enhanced antimicrobial activity. Dent. Mater. 2024, 40, 1199–1207. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Luo, Z.; Zhu, C.; Zhang, Y.; Shu, Z.; Shen, C.; Yao, X.; Wang, Y.; Wang, X. ZnO-CuS/F127 hydrogels with multienzyme properties for implant-related infection therapy by inhibiting bacterial arginine biosynthesis and promoting tissue repair. Adv. Funct. Mater. 2025, 35, 2415778. [Google Scholar] [CrossRef]
- San, H.; Paresoglou, M.; Minneboo, M.; van Hengel, I.A.; Yilmaz, A.; Gonzalez-Garcia, Y.; Fluit, A.C.; Hagedoorn, P.-L.; Fratila-Apachitei, L.E.; Apachitei, I. Fighting antibiotic-resistant bacterial infections by surface biofunctionalization of 3D-printed porous titanium implants with reduced graphene oxide and silver nanoparticles. Int. J. Mol. Sci. 2022, 23, 9204. [Google Scholar] [CrossRef]
- Hojda, S.; Biegun-Żurowska, M.; Skórkowska, A.; Klesiewicz, K.; Ziąbka, M. A Weapon Against Implant-Associated Infections: Antibacterial and Antibiofilm Potential of Biomaterials with Titanium Nitride and Titanium Nitride-Silver Nanoparticle Electrophoretic Deposition Coatings. Int. J. Mol. Sci. 2025, 26, 1646. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-based strategies to combat antibiotic resistance: Mechanisms and applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef]
- Ladva, D.N.; Selvadoss, P.P.; Chitroda, G.K.; Dhanasekaran, S.; Nellore, J.; Tippabathani, J.; Solomon, S.M. Maleimide conjugated PEGylated liposomal antibiotic to combat multi-drug resistant Escherichia coli and Klebsiella pneumoniae with enhanced wound healing potential. Sci. Rep. 2024, 14, 18361. [Google Scholar] [CrossRef]
- Shakya, A.K.; Al-Sulaibi, M.; Naik, R.R.; Nsairat, H.; Suboh, S.; Abulaila, A. Review on PLGA polymer based nanoparticles with antimicrobial properties and their application in various medical conditions or infections. Polymers 2023, 15, 3597. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric nanoparticles for antimicrobial therapies: An up-to-date overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Maurya, P.; Saklani, R.; Singh, S.; Nisha, R.; Mishra, N.; Singh, P.; Pal, R.R.; Kumar, A.; Chourasia, M.K.; Saraf, S.A. Effective uptake of folate-functionalized ethionamide-loaded hybrid system: Targeting alveolar macrophages. Nanomedicine 2022, 17, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Cao, Y.; Liu, K.; Shi, H.; Guo, X.; Liu, W.; Hao, R.; Song, H.; Zhao, R. Nanocarriers for the delivery of antibiotics into cells against intracellular bacterial infection. Biomater. Sci. 2023, 11, 432–444. [Google Scholar] [CrossRef]
- Raj, K.; Attavar, P.C. Multidrug resistance in microorganisms causing ventilator-associated pneumonia: A literature review. Int. J. Res. Publ. Rev. Internet 2024, 5, 5965–5974. [Google Scholar] [CrossRef]
- Reynolds, D.; Burnham, J.P.; Guillamet, C.V.; McCabe, M.; Yuenger, V.; Betthauser, K.; Micek, S.T.; Kollef, M.H. The threat of multidrug-resistant/extensively drug-resistant Gram-negative respiratory infections: Another pandemic. Eur. Respir. Rev. 2022, 31, 220068. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-based drug delivery system: The magic bullet for the treatment of chronic pulmonary diseases. Mol. Pharm. 2021, 18, 3671–3718. [Google Scholar] [CrossRef]
- Pei, J.; Yan, Y.; Palanisamy, C.P.; Jayaraman, S.; Natarajan, P.M.; Umapathy, V.R.; Gopathy, S.; Roy, J.R.; Sadagopan, J.C.; Thalamati, D. Materials-based drug delivery approaches: Recent advances and future perspectives. Green Process. Synth. 2024, 13, 20230094. [Google Scholar] [CrossRef]
- Madkour, L.H. Eco-friendly green biosynthesized metallic nanoparticles and biotechnological applications in pharmaceuticals sciences. J. Mater. Sci. Eng. B 2023, 13, 1–69. [Google Scholar] [CrossRef]
- Jalal, R.R.; Ways, T.M.M.; Elella, M.H.A.; Hassan, D.A.; Khutoryanskiy, V.V. Preparation of mucoadhesive methacrylated chitosan nanoparticles for delivery of ciprofloxacin. Int. J. Biol. Macromol. 2023, 242, 124980. [Google Scholar] [CrossRef]
- Fernández-García, R.; Fraguas-Sánchez, A.I. Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics 2024, 16, 1584. [Google Scholar] [CrossRef]
- Zacaron, T.M.; Silva, M.L.S.e.; Costa, M.P.; Silva, D.M.e.; Silva, A.C.; Apolônio, A.C.M.; Fabri, R.L.; Pittella, F.; Rocha, H.V.A.; Tavares, G.D. Advancements in chitosan-based nanoparticles for pulmonary drug delivery. Polymers 2023, 15, 3849. [Google Scholar] [CrossRef]
- Van Giau, V.; An, S.S.A.; Hulme, J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des. Dev. Ther. 2019, 13, 327–343. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Zhao, C.; Tian, X.; Cun, D.; Yang, M. Mannose-decorated solid-lipid nanoparticles for alveolar macrophage targeted delivery of rifampicin. Pharmaceutics 2024, 16, 429. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Li, Y.; Liu, X.; Zhang, Y.; Gao, F. Intracellular infection-responsive macrophage-targeted nanoparticles for synergistic antibiotic immunotherapy of bacterial infection. J. Mater. Chem. B 2024, 12, 5248–5260. [Google Scholar] [CrossRef] [PubMed]
- Haworth, C.S.; Bilton, D.; Chalmers, J.D.; Davis, A.M.; Froehlich, J.; Gonda, I.; Thompson, B.; Wanner, A.; O’Donnell, A.E. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): Two phase 3, randomised controlled trials. Lancet Respir. Med. 2019, 7, 213–226. [Google Scholar] [CrossRef]
- Singh, A.V.; Varma, M.; Laux, P.; Choudhary, S.; Datusalia, A.K.; Gupta, N.; Luch, A.; Gandhi, A.; Kulkarni, P.; Nath, B. Artificial intelligence and machine learning disciplines with the potential to improve the nanotoxicology and nanomedicine fields: A comprehensive review. Arch. Toxicol. 2023, 97, 963–979. [Google Scholar] [CrossRef]
- Pathi, B.K.; Mishra, S.; Moharana, N.; Kanungo, A.; Mishra, A.; Sahu, S.; Dash, R.K.; Dubey, R.; Das, M.K.; Pathi, B.K. Effect of topical silver nanoparticle formulation on wound bacteria clearance and healing in patients with infected wounds compared to standard topical antibiotic application: A randomized open-label parallel clinical trial. Cureus 2024, 16, e60569. [Google Scholar] [CrossRef]
- Vass, P.; Akdag, D.S.; Broholm, G.E.; Kjaer, J.; Humphreys, A.J.; Ehmann, F. Enabling technologies driving drug research and development. Front. Med. 2023, 10, 1122405. [Google Scholar] [CrossRef]
- Emily, M.; Ioanna, N.; Scott, B.; Beat, F. Reflections on FDA draft guidance for products containing nanomaterials: Is the abbreviated new drug application (ANDA) a suitable pathway for nanomedicines? AAPS J. 2018, 20, 92. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Sati, A.; Ranade, T.N.; Mali, S.N.; Ahmad Yasin, H.K.; Pratap, A. Silver nanoparticles (AgNPs): Comprehensive insights into bio/synthesis, key influencing factors, multifaceted applications, and toxicity─ A 2024 update. ACS Omega 2025, 10, 7549–7582. [Google Scholar] [CrossRef]
- Jebril, S.; Fdhila, A.; Dridi, C. Nanoengineering of eco-friendly silver nanoparticles using five different plant extracts and development of cost-effective phenol nanosensor. Sci. Rep. 2021, 11, 22060. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.; Engstrom, A.M.; Harper, B.J.; Harper, S.L.; Mackiewicz, M.R. Silver nanoparticles stable to oxidation and silver ion release show size-dependent toxicity in vivo. Nanomaterials 2021, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Huo, G.; Qin, C.; Wu, H.; Wang, D.; Dan, M.; Geng, X.; Liu, S. Safety evaluation of PEGylated MNPs and p-PEGylated MNPs in SD rats. Sci. Rep. 2023, 13, 21501. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, J.; Boudreau, M.; Meng, J.; Yin, J.-J.; Liu, J.; Xu, H. Intravenous administration of silver nanoparticles causes organ toxicity through intracellular ROS-related loss of inter-endothelial junction. Part. Fibre Toxicol. 2015, 13, 21. [Google Scholar] [CrossRef]
- Connors, J.; Joyner, D.; Mege, N.J.; Cusimano, G.M.; Bell, M.R.; Marcy, J.; Taramangalam, B.; Kim, K.M.; Lin, P.J.; Tam, Y.K. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 2023, 6, 188. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Dao, H.M.; Xu, H.; Koleng, J.J.; Sakran, W.; Cui, Z. Effect of the amount of cationic lipid used to complex siRNA on the cytotoxicity and proinflammatory activity of siRNA-solid lipid nanoparticles. Int. J. Pharm. X 2023, 6, 100197. [Google Scholar] [CrossRef]
- Lonez, C.; Bessodes, M.; Scherman, D.; Vandenbranden, M.; Escriou, V.; Ruysschaert, J.-M. Cationic lipid nanocarriers activate Toll-like receptor 2 and NLRP3 inflammasome pathways. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 775–782. [Google Scholar] [CrossRef]
- Bai, X.; Wang, J.; Mu, Q.; Su, G. In vivo protein corona formation: Characterizations, effects on engineered nanoparticles’ biobehaviors, and applications. Front. Bioeng. Biotechnol. 2021, 9, 646708. [Google Scholar] [CrossRef]
- González-Vega, J.G.; García-Ramos, J.C.; Chavez-Santoscoy, R.A.; Castillo-Quiñones, J.E.; Arellano-Garcia, M.E.; Toledano-Magaña, Y. Lung models to evaluate silver nanoparticles’ toxicity and their impact on human health. Nanomaterials 2022, 12, 2316. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef] [PubMed]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up polymeric-based nanoparticles drug delivery systems: Development and challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Naveen, K.V.; Saravanakumar, K.; Sathiyaseelan, A.; Wang, M.-H. Eco-friendly synthesis and characterization of Aloe vera/Gum Arabic/silver nanocomposites and their antibacterial, antibiofilm, and wound healing properties. Colloid Interface Sci. Commun. 2022, 46, 100566. [Google Scholar] [CrossRef]
- Dudhagara, P.; Alagiya, J.; Bhagat, C.; Dudhagara, D.; Ghelani, A.; Desai, J.; Patel, R.; Vansia, A.; Nhiem, D.N.; Chen, Y.-Y. Biogenic synthesis of antibacterial, hemocompatible, and antiplatelets lysozyme functionalized silver nanoparticles through the one-step process for therapeutic applications. Processes 2022, 10, 623. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Kumari, S.A.; Tchounwou, P.B. A comparison of poly-ethylene-glycol-coated and uncoated gold nanoparticle-mediated hepatotoxicity and oxidative stress in Sprague Dawley rats. Int. J. Nanomed. 2019, 14, 639–647. [Google Scholar] [CrossRef]
- Cho, W.-S.; Cho, M.; Jeong, J.; Choi, M.; Han, B.S.; Shin, H.-S.; Hong, J.; Chung, B.H.; Jeong, J.; Cho, M.-H. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol. Appl. Pharmacol. 2010, 245, 116–123. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- FDA. Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considering-whether-fda-regulated-product-involves-application-nanotechnology?utm_source = chatgpt.com (accessed on 3 August 2025).
- Law, H. The Role of FDA in the Regulation of Manufactured Nanomaterials. Available online: https://www.steptoe.com/a/web/2497/4145.pdf?utm_source = chatgpt.com (accessed on 3 August 2025).
- Kumari, R.; Suman, K.; Karmakar, S.; Mishra, V.; Lakra, S.G.; Saurav, G.K.; Mahto, B.K. Regulation and safety measures for nanotechnology-based agri-products. Front. Genome Ed. 2023, 5, 1200987. [Google Scholar] [CrossRef]
- Nadar, S.; Safwat, A.; Qin, N.; Czyż, D.M. An uphill path to commercialization of silver nanoparticle antimicrobials: From bench to market. Nanomedicine 2025, 1–4. [Google Scholar] [CrossRef]
- Kumarasamy, R.V.; Natarajan, P.M.; Umapathy, V.R.; Roy, J.R.; Mironescu, M.; Palanisamy, C.P. Clinical applications and therapeutic potentials of advanced nanoparticles: A comprehensive review on completed human clinical trials. Front. Nanotechnol. 2024, 6, 1479993. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Rana, D.; Patel, M.; Bajwa, N.; Prasad, R.; Vora, L.K. Nanoparticle Therapeutics in Clinical Perspective: Classification, Marketed Products, and Regulatory Landscape. Small 2025, 21, 2502315. [Google Scholar] [CrossRef] [PubMed]
- van Den Brule, S.; Ambroise, J.; Lecloux, H.; Levard, C.; Soulas, R.; De Temmerman, P.-J.; Palmai-Pallag, M.; Marbaix, E.; Lison, D. Dietary silver nanoparticles can disturb the gut microbiota in mice. Part. Fibre Toxicol. 2015, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Ghoshdastidar, S.; Rekha, K.R.; Suresh, D.; Mao, J.; Bivens, N.; Kannan, R.; Joshi, T.; Rosenfeld, C.S.; Upendran, A. Developmental exposure to silver nanoparticles leads to long term gut dysbiosis and neurobehavioral alterations. Sci. Rep. 2021, 11, 6558. [Google Scholar] [CrossRef]
- Ren, Q.; Ma, J.; Li, X.; Meng, Q.; Wu, S.; Xie, Y.; Qi, Y.; Liu, S.; Chen, R. Intestinal toxicity of metal nanoparticles: Silver nanoparticles disorder the intestinal immune microenvironment. ACS Appl. Mater. Interfaces 2023, 15, 27774–27788. [Google Scholar] [CrossRef]
- Wang, X.; Cui, X.; Wu, J.; Bao, L.; Chen, C. Oral administration of silver nanomaterials affects the gut microbiota and metabolic profile altering the secretion of 5-HT in mice. J. Mater. Chem. B 2023, 11, 1904–1915. [Google Scholar] [CrossRef]
- Lamas, B.; Martins Breyner, N.; Houdeau, E. Impacts of foodborne inorganic nanoparticles on the gut microbiota-immune axis: Potential consequences for host health. Part. Fibre Toxicol. 2020, 17, 19. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, X.; Du, H.; Guo, X.; Han, Y.; McClements, D.J.; Decker, E.; Xing, B.; Xiao, H. Adverse effects of titanium dioxide nanoparticles on beneficial gut bacteria and host health based on untargeted metabolomics analysis. Environ. Res. 2023, 228, 115921. [Google Scholar] [CrossRef]
- Kumar, A.; Pramanik, J.; Batta, K.; Bamal, P.; Gaur, M.; Rustagi, S.; Prajapati, B.G.; Bhattacharya, S. Impact of metallic nanoparticles on gut microbiota modulation in colorectal cancer: A review. Cancer Innov. 2024, 3, e150. [Google Scholar] [CrossRef]
- Luo, D.; Luo, G.; Xu, H.; Li, K.; Li, Z.; Zhang, C. Inorganic dietary nanoparticles in intestinal barrier function of inflammatory bowel disease: Allies or adversaries? Front. Immunol. 2025, 16, 1563504. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lv, K.; Cheng, Q.; Xing, H.; Xue, W.; Zhang, W.; Lin, Q.; Ma, D. Enhanced bacterial-infected wound healing by nitric oxide-releasing topological supramolecular nanocarriers with self-optimized cooperative multi-point anchoring. Adv. Sci. 2023, 10, 2206959. [Google Scholar] [CrossRef] [PubMed]
- Kalhapure, R.S.; Sikwal, D.R.; Rambharose, S.; Mocktar, C.; Singh, S.; Bester, L.; Oh, J.K.; Renukuntla, J.; Govender, T. Enhancing targeted antibiotic therapy via pH responsive solid lipid nanoparticles from an acid cleavable lipid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Tasia, W.; Lei, C.; Cao, Y.; Ye, Q.; He, Y.; Xu, C. Enhanced eradication of bacterial biofilms with DNase I-loaded silver-doped mesoporous silica nanoparticles. Nanoscale 2020, 12, 2328–2332. [Google Scholar] [CrossRef]
- Hajfathalian, M.; de Vries, C.R.; Hsu, J.C.; Amirshaghaghi, A.; Dong, Y.C.; Ren, Z.; Liu, Y.; Huang, Y.; Li, Y.; Knight, S.A. Theranostic gold-in-gold cage nanoparticles enable photothermal ablation and photoacoustic imaging in biofilm-associated infection models. J. Clin. Investig. 2023, 133, e168485. [Google Scholar] [CrossRef]
- Athauda, I.; Shetty, M.; Pai, P.; Hegde, M.; Gurumurthy, S.; Babitha, K. Enhanced bactericidal effects and drug delivery with gentamicin-conjugated nanoparticles. J. Clust. Sci. 2024, 35, 371–390. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 22: Guidance on Nanomaterials. ISO/TR 10993-22:2017. 2017; International Organization for Standardization: Geneva, Switzerland. [Google Scholar]
- Li, M.; Liu, Y.; Gong, Y.; Yan, X.; Wang, L.; Zheng, W.; Ai, H.; Zhao, Y. Recent advances in nanoantibiotics against multidrug-resistant bacteria. Nanoscale Adv. 2023, 5, 6278–6317. [Google Scholar] [CrossRef]
- Luo, L.; Huang, W.; Zhang, J.; Yu, Y.; Sun, T. Metal-based nanoparticles as antimicrobial agents: A review. ACS Appl. Nano Mater. 2024, 7, 2529–2545. [Google Scholar] [CrossRef]
- Gatto, M.S.; Najahi-Missaoui, W. Lyophilization of nanoparticles, does it really work? Overview of the current status and challenges. Int. J. Mol. Sci. 2023, 24, 14041. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, T.; Wang, Y.; Li, T.; Chi, Q. Multifaceted impacts of nanoparticles on plant nutrient absorption and soil microbial communities. Front. Plant Sci. 2024, 15, 1497006. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Sahlgren, C.; Lindén, M. Towards multifunctional, targeted drug delivery systems using mesoporous silica nanoparticles–opportunities & challenges. Nanoscale 2010, 2, 1870–1883. [Google Scholar]
- Jiang, L.; Ding, L.; Liu, G. Nanoparticle formulations for therapeutic delivery, pathogen imaging and theranostic applications in bacterial infections. Theranostics 2023, 13, 1545. [Google Scholar] [CrossRef]

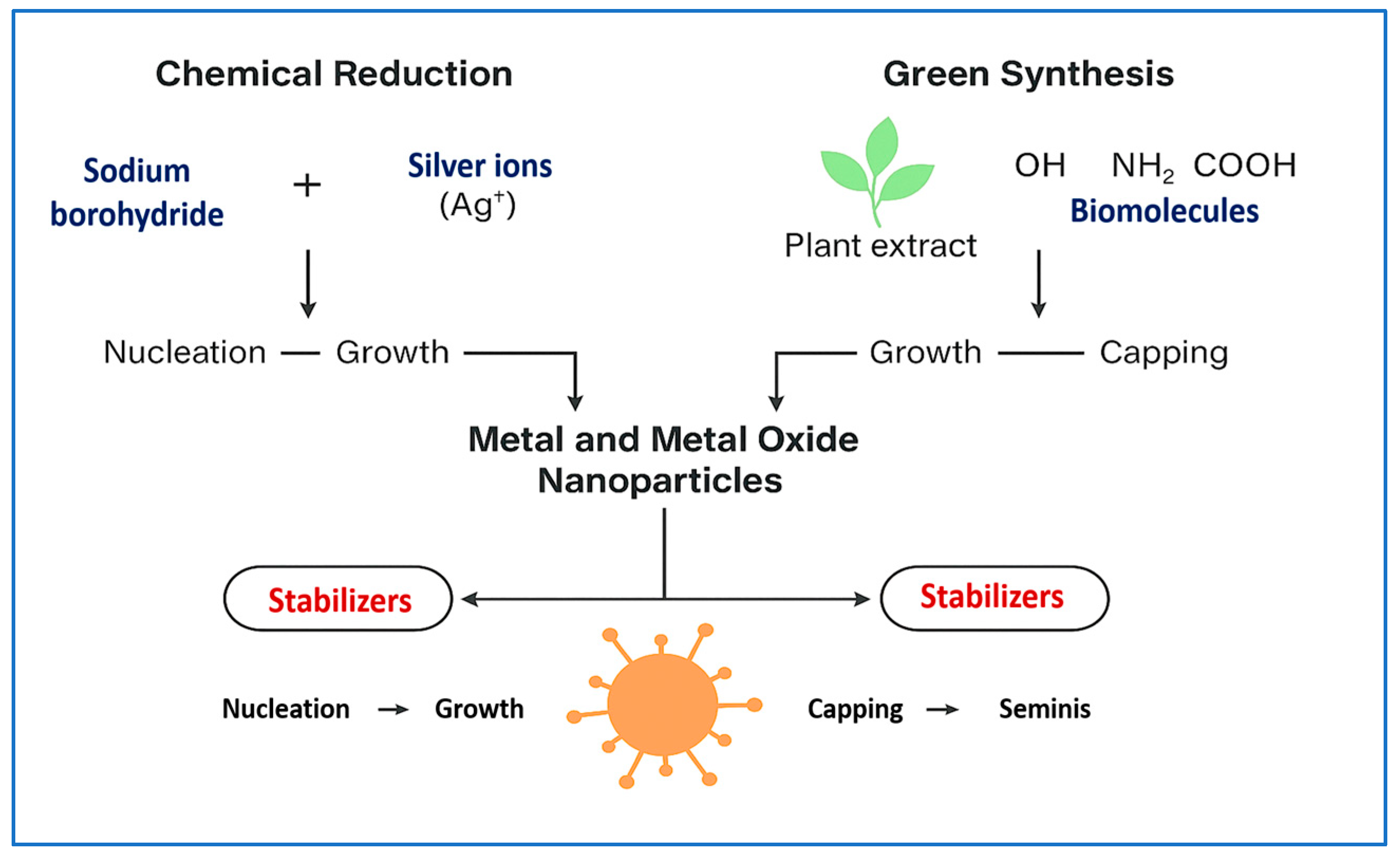
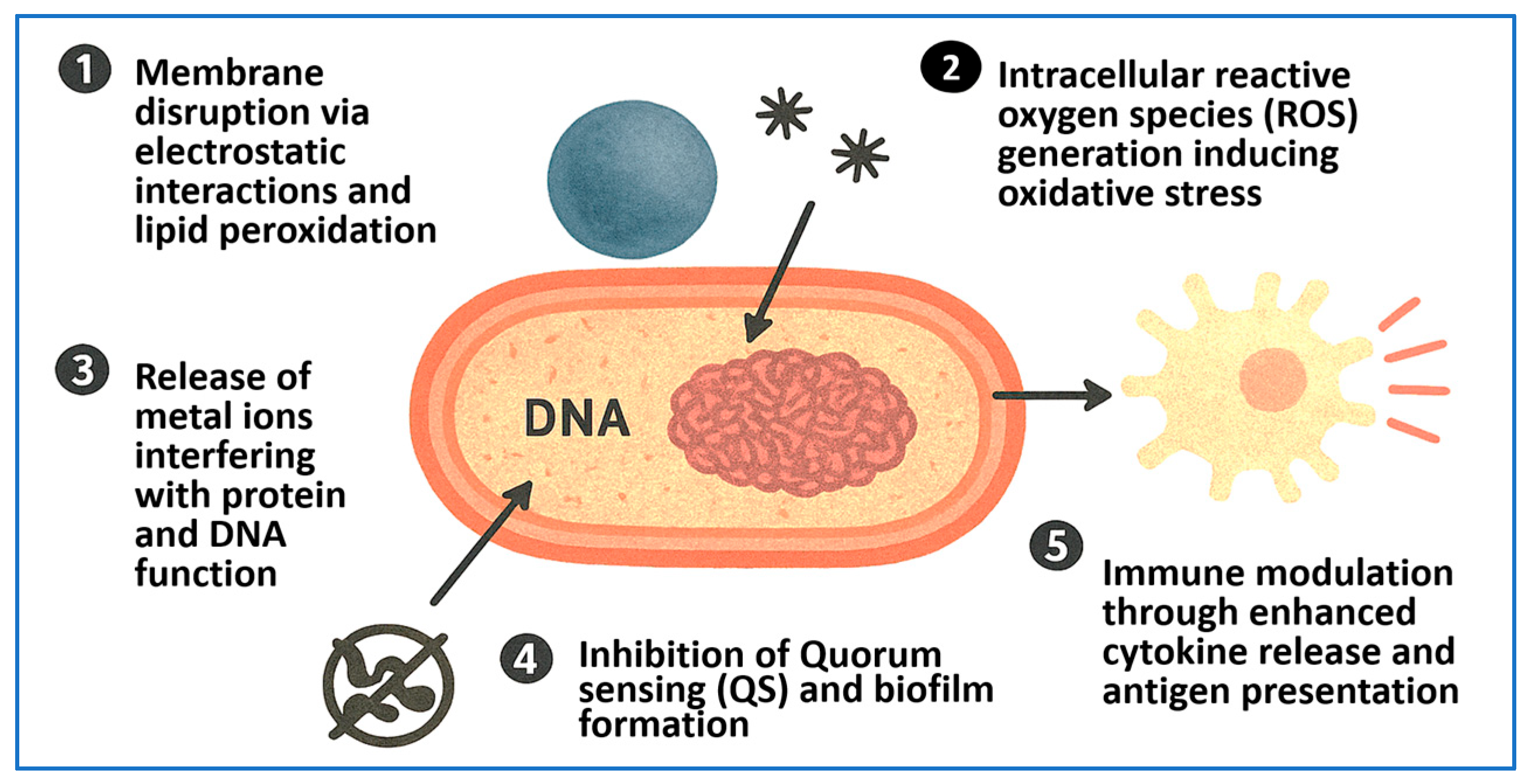
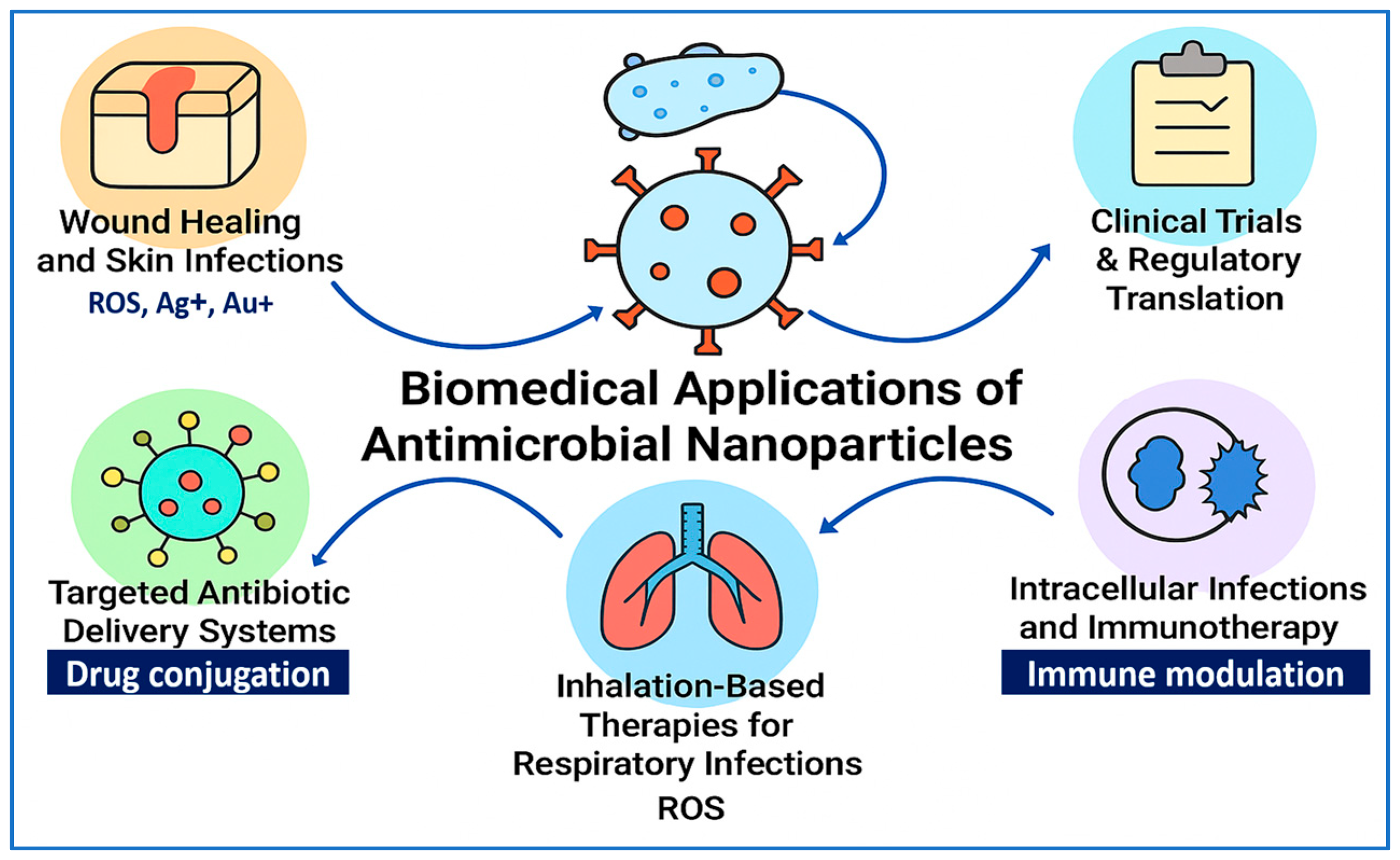
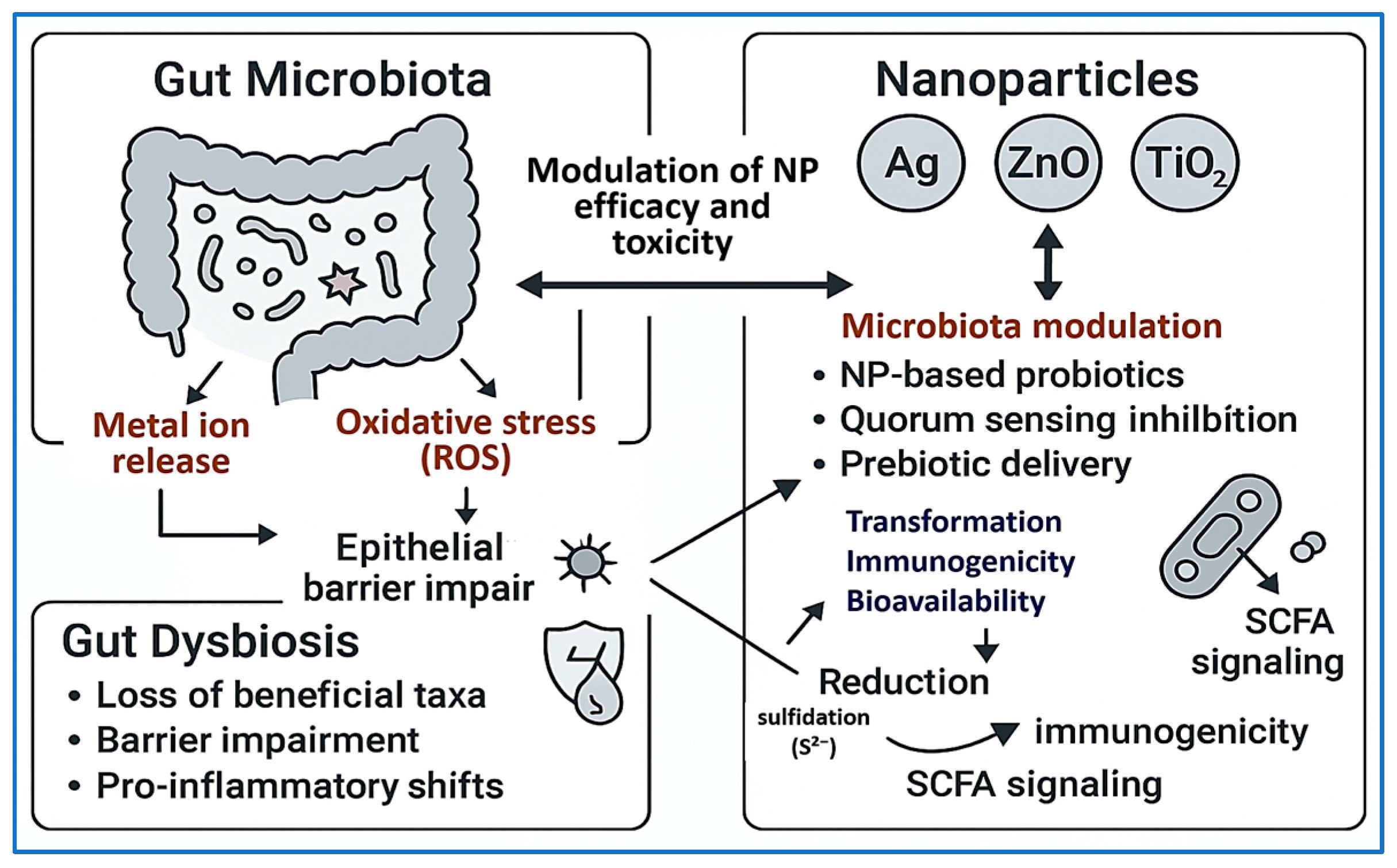
| Synthesis Method | Size (nm) | Surface Area (m2/g) | Temp/Time | Precursors | Morphology | Application | Reference |
|---|---|---|---|---|---|---|---|
| AgNPs | |||||||
| Green synthesis using sumac (Rhus coriaria) extract | ~4 | 41.8 | 25 °C/12 h | Sumac extract + silver nitrate (AgNO3) | Spherical, homogeneous distribution, face-centered cubic crystalline structure | Antibacterial activity against B. cereus, B. subtilis, Enterococcus faecalis, P. aeruginosa, and C. albicans; protection against plasmid DNA damage | [39] |
| Green synthesis using Ocimum sanctum (Tulsi) extract | ~18–25 | 35.6 | 60–70 °C/2 h | AgNO3, aqueous tulsi leaf extract | Spherical (TEM, SEM) | Antibacterial and anticancer potential | [75] |
| Green synthesis using Candida parapsilosis (yeast) isolated from Sudanese soil | ~10–25 | 48 | Not specified | Yeast biomass extract + AgNO3 | Spherical (HRTEM-confirmed) | Antibacterial against MDR bacteria; inhibition zones up to 29 mm; MIC/MBC 0.3125 mg/mL; synergistic antibiotic enhancement (up to 9.84-fold) | [76] |
| Chemical reduction optimized via Face-Centered Central Composite Design (FCCCD) with varying pH, AgNO3, sodium citrate (TSC), and NaBH4 concentrations | Majority < 10.30 nm (67.66 % of particles) | 53 | Ambient conditions/Reaction time not explicitly stated | AgNO3, TSC, NaBH4 | Spherical and hemispherical | Antimicrobial and antifungal activity against S. aureus, E. coli, E. coli AmpC-resistant, and C. albicans; reduced cytotoxicity profile | [77] |
| Green synthesis using Zingiber officinale (ginger) extract | 41.98 | 34.8 | Ambient/Not specified | AgNO3, ginger extract (gingerol source) | Spherical | Antibacterial and antibiofilm activity against biofilm-associated Enterococcus spp. from urinary tract clinical isolates | [78] |
| ZnO NPs | |||||||
| Sol–gel method | 16–28 | 52.2 | 80 °C/4 h | Zinc acetate, NaOH | Hexagonal rods | Antibacterial textiles | [79] |
| Green synthesis (Moringa oleifera leaf extract) | 55 | 15.43 | ~Room Temp/unspecified duration | Aqueous extract of Moringa oleifera leaves | Spherical | Hepatoprotective effect in CCl4-treated albino rats | [80] |
| Green synthesis (using Scenedesmus obliquus algae extract) | 17–34 | 34.9 | 70 °C/2 h | Zinc nitrate hexahydrate, algae extract | Spherical | Phosphorus adsorption; antibacterial vs. E. coli, S. aureus | [81] |
| Green synthesis (Allium cepa (onion) extract) | 8.13 (avg.) | 33.3 | 60 °C/1 h (plus annealing at 400 °C for 2 h) | ZnSO4·7H2O, onion extract, NaOH | Spherical, aggregated (1–16 nm TEM) | Phosphorus adsorption (opt. at pH 3), antibacterial activity vs. E. coli and S. aureus | [82] |
| Green synthesis using Trifolium pratense (red clover) extract | 20–80 | 14.29 | 60 °C/3 h (stirring) + drying and calcination at 400 °C | Zinc acetate dihydrate, red clover extract, NaOH | Spherical to irregular | Antibacterial activity against E. coli, S. aureus, and C. albicans | [83] |
| CuO NPs/CuO/ZnO NPs | |||||||
| Green synthesis using Syzygium aromaticum (clove) extract | 14.8 | Not reported | 80 °C/3 h (stirring) + drying at 100 °C, annealed at 400 °C | CuCl2·2H2O, clove extract | Spherical to aggregated | Structural and dielectric material development | [84] |
| Precipitation + polymer surface modification using PEG, PVP, and chitosan | 13–50 | Not reported | Room temp/24 h + drying at 60 °C | CuCl2·2H2O, NaOH, PEG/PVP/Chitosan (modifier) | Spherical and quasi-spherical | Antibacterial activity against E. coli, S. aureus, B. subtilis, C. albicans | [85] |
| Green synthesis using Tribulus terrestris leaf extract | 18.9 | Not re-ported | 80 °C/2 h (stirring) + calcination at 500 °C | CuSO4·5H2O, plant extract | Spherical | Antimicrobial activity against S. aureus, E. coli; strong antioxidant activity (DPPH, ABTS) | [86] |
| Co-precipitation + multi-walled carbon nanotube (MWCNT) surface modification | 13.44–21.96 | 33.83 | 70 °C/3 h + calcination at 400 °C | AgNO3, Cu(NO3)2·3H2O, Zn(NO3)2·6H2O, NaOH, MWCNT (5%) | Irregular, spherical, and semi-porous | Photocatalytic degradation of methylene blue (96.2 %) under sunlight; antibacterial activity vs. S. aureus, E. coli | [87] |
| Co-precipitation + annealing | 15–30 | 41.7 | 500 °C/2 h | Cu(NO3)2, Zn(NO3)2, NH4OH | Aggregated clusters | Water disinfection | [88] |
| Physical vapor deposition (thermal evaporation) + post-annealing | ~42 (CuO)/~39 (ZnO) | Not re-ported | Annealing at 350 °C for 1 h | High-purity Cu and Zn metals evaporated on glass substrate | Uniform thin film with granular nanostructure | Gas sensing (ammonia and acetone detection) | [89] |
| TiO2 NPs | |||||||
| Sol–gel method | 15–25 | ~112 | 80 °C/6 h | Titanium isopropoxide, ethanol, water, HCl | Spherical anatase | Antibacterial activity against E. coli, S. aureus, and C. albicans under UV illumination | [90] |
| Green synthesis using Carica papaya leaves | ~50 | 78.9 | Calcined at 400 °C/3 h | Titanium isopropoxide (TTIP), Carica papaya leaf extract | Spherical, smooth anatase NPs | Effective antibacterial activity against E. coli, S. aureus, and B. subtilis | [91] |
| Sol–gel (chemical) | ~14–25 | Not reported | Calcination at 400 °C | Titanium isopropoxide, ethanol | Spherical | Strong antibacterial activity against E. coli and S. aureus, and antifungal against C. albicans. Also effective against HSV-1 virus and in photocatalysis under UV light | [92] |
| Sol–gel synthesis (TiO2/Ag nanocomposite) | ~10–20 | Not specified | 450 °C/2 h (calcination) | Titanium isopropoxide, AgNO3 | Spherical with Ag clusters | Broad-spectrum antibacterial activity against E. coli and S. aureus | [93] |
| Sol–gel synthesis (commonly used in cited dental applications) | ~15–30 | Not reported | ~400–500 °C (calcination) | Titanium isopropoxide, solvents | Spherical, anatase | Antibacterial coatings on dental implants, restorative materials, and endodontic sealers | [94] |
| NP Type | Target Pathogens | MIC Range (µg/mL) | Cytotoxicity/Biocompatibility | Reference |
|---|---|---|---|---|
| AgNPs | ||||
| Actinobacteria-mediated, protein-capped | E. coli, K. pneumoniae, P. aeruginosa, S. aureus | 8–128; 64–256 | Dose-dependent cytotoxicity: IC50 (MTT) 16.3 µg/mL (RAW 264.7), 12.0 µg/mL (MCF-7); higher toxicity to cancer cells; ROS increase (MCF-7: 1.47–3.13×, RAW 264.7: 1.02–2.58×); LDH leakage up to ~45 % | [101] |
| Salvia pratensis aerial and root extracts | Broad-spectrum antibacterial and antifungal activity; strongest against Penicillium spp. | Bacteria: < 0.0039; Fungi (Penicillium): < 0.0391 | Fully biocompatible with tested eukaryotic cells; no hemolysis at ≤150 µg/mL | [102] |
| Green-synthesized using Achillea millefolium extract | E. coli, P. aeruginosa, S. aureus, C. albicans | Bacteria: 3.12–25; Fungi: 6.25–50 | No significant cytotoxicity against Vero and HaCaT cell lines at concentrations ≤100 µg/mL; >90% cell viability maintained | [103] |
| Green-synthesized using Ocimum sanctum leaf extract) | Not primarily antimicrobial-focused; study evaluated genotoxicity protection—AgNPs generally active against Gram-positive bacteria, Gram-negative bacteria, and fungi per the phytosynthetic AgNP literature | Not determined in this study | Showed protective effect against cyclophosphamide-induced DNA damage in human lymphocytes; no acute cytotoxicity reported in vitro | [104] |
| Green-synthesized using Streptomyces antimycoticus L-1 | S. aureus, B. subtilis, P. aeruginosa, E. coli, S. typhimurium | 6.25–100 ppm (zone of inhibition: 9.5–21.7 mm) | IC50 (Caco-2 cells) = 5.7 ± 0.2 ppm; 100 ppm considered safe for fabric coating; retained antibacterial activity after 10 wash cycles | [105] |
| ZnO NPs | ||||
| ZnO NPs immobilized in poly(allylamine hydrochloride)/alginate multilayers with vaterite on titanium coating | S. aureus, S. epidermidis, C. albicans | Not specified; >90% microbial viability reduction | Zn2+ release below cytotoxic limit for MC3T3-E1 preosteoblast cells; biocompatible for implant applications | [55] |
| Green-synthesized ZnO NPs using Casuarina equisetifolia leaf extract under UV-A and UV-C light | B. subtilis, P. fluorescens, P. aeruginosa | 3.12–25 | Reduced HepG2 cell viability to 36.97 %; highly biocompatible toward brine shrimp and human RBCs | [106] |
| Green-synthesized ZnO NPs using Chelidonium majus extract | S. aureus (NCTC 4163, clinical), P. aeruginosa (NCTC 6749, clinical), E. coli (ATCC 25922, clinical), C. albicans (ATCC 10231, clinical), Aspergillus niger (ATCC 16404), Trichophyton rubrum (ATCC 28188) | Bacteria: 3.12–25; Fungi: 6.25–50 | Demonstrated high efficiency against human non-small cell lung cancer A549 cells; no specific normal cell cytotoxicity data reported in Abstract | [107] |
| ZnO-NPs reinforced in sodium alginate/hydroxyapatite scaffolds | E. coli, S. aureus | 6.25–50 | >70 % porosity; neutral pH; enhanced apatite deposition; high bioactivity; suitable for bone regeneration; no cytotoxicity reported | [108] |
| Green-synthesized ZnO nanoparticles (Rosmarinus officinalis L. extract; size 53–67 nm) | S. aureus (Gram-positive), E. coli (Gram-negative) | ~81.4, 40.7, 20.35, and 10.17 µg/mL | Reported as biocompatible and non-toxic to humans in prior studies; no direct cytotoxicity assay performed in this study | [109] |
| CuO NPs | ||||
| Green-synthesized using Trichoderma harzianum; ~15–40 nm | A. baumannii clinical isolates | 12.5–50 | Low cytotoxicity toward human fibroblast cells at ≤50 µg/mL; also showed anticancer activity against HepG2 cells | [110] |
| Green-synthesized using metabolites of Aspergillus niger G3-1, spherical, 14–47.4 nm | Sitophilus granarius, Rhyzopertha dominica | 50–100 ppm (dose-dependent mortality: 55–94.4 % for S. granarius, 70–90% for R. dominica) | Low phytotoxicity; 50 ppm promoted wheat growth, photosynthetic pigments, and antioxidant enzyme activity without affecting carbohydrate or protein content | [111] |
| Biosynthesized via Aspergillus terreus BR.1 from Allium sativum root | Different pathogenic bacteria and Candida species | 25–50 | Selective cytotoxicity: IC50 for MCF7 (159.2 µg/mL) and PC3 (116.2 µg/mL) cancer cells; higher IC50 for normal cells (Vero: 220.6 µg/mL, Wi38: 229.5 µg/mL); stable and non-toxic at lower concentrations | [112] |
| Biosynthesized via Streptomyces rochei cell-free filtrate | Multidrug-resistant S. aureus, including MRSA clinical isolates | 6.5 (CuO-NPs, in combination with cefoxitin) | Non-toxic to human HFB-4 cells at ≤8 µg/mL (100 % viability); normal cell morphology observed | [113] |
| Mycosynthesized CuO-NPs | P. aeruginosa | MIC: 25 MBC: 50 | Minimal toxicity toward normal human skin cells; selective cytotoxicity against HepG2 cancer cells; enhanced wound healing efficacy when combined with curcumin (CUR) | [114] |
| TiO2 NPs | ||||
| Aloe vera-mediated in PVA:SA nanocomposite films | B. cereus, S. aureus, E. coli | 12.5–50 | Biocompatible with human skin fibroblasts; suitable for wound dressing applications | [115] |
| Green-synthesized using Withania somnifera root extract | E. coli, P. aeruginosa, MRSA, L. monocytogenes, Serratia marcescens, C. albicans | 6.25–50 | Significant biofilm inhibition (43–71 % at 0.5× MIC for formation; 24–64 % for mature biofilms); cytotoxic against HepG2 liver cancer cells in vitro | [116] |
| TiO2 nano-colloids (sonochemically synthesized) | P. aeruginosa, other Gram-positive and Gram-negative bacteria; Newcastle disease virus (NDV) | 2.24–21.21 | Not specified; demonstrated antiviral and antibacterial activity at non-toxic doses in assays | [117] |
| Biosynthesized using Streptomyces vinaceusdrappus AMG31) | Enterococcus faecalis, E. coli, Penicillium glabrum, Aspergillus niger, C. albicans | Gram-positive: MIC 12.5; MBC 25; Gram-negative (E. coli): MIC 6.25; MBC 12.5 | Minimal hemolysis (1.9 % at 1000 µg/mL); selective cytotoxicity toward cancer cells (Caco-2 IC50 74.1 µg/mL, PANC-1 IC50 71.04 µg/mL) with lower toxicity to normal WI38 cells (IC50 153.1 µg/mL); hemocompatible and moderate wound healing effect | [118] |
| Vanillic acid–conjugated TiO2 NPs (VA–TiO2 NPs) | S. aureus, Streptococcus mutans, Enterococcus faecalis, C. albicans | 60 | Concentration-dependent apoptosis in human oral carcinoma KB cells at 15–120 µg/mL; promising for dental applications; not explicitly stated for normal cell safety | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbehiry, A.; Abalkhail, A. Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers. Pharmaceuticals 2025, 18, 1195. https://doi.org/10.3390/ph18081195
Elbehiry A, Abalkhail A. Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers. Pharmaceuticals. 2025; 18(8):1195. https://doi.org/10.3390/ph18081195
Chicago/Turabian StyleElbehiry, Ayman, and Adil Abalkhail. 2025. "Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers" Pharmaceuticals 18, no. 8: 1195. https://doi.org/10.3390/ph18081195
APA StyleElbehiry, A., & Abalkhail, A. (2025). Antimicrobial Nanoparticles Against Superbugs: Mechanistic Insights, Biomedical Applications, and Translational Frontiers. Pharmaceuticals, 18(8), 1195. https://doi.org/10.3390/ph18081195






