A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II
Abstract
1. Introduction
- PCSK9 inhibitors, such as alirocumab and evolocumab, monoclonal antibodies that prevent LDL receptor (LDLR) degradation and have demonstrated substantial LDL-C reduction and documented cardiovascular benefits;
- siRNA therapies, exemplified by inclisiran, which leverage ribonucleic acid (RNA) interference to silence PCSK9 gene expression and provide long-lasting LDL-C lowering with infrequent dosing;
- ACL inhibitors, such as bempedoic acid, which act upstream of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, offering LDL-C reduction with a low risk of muscle-related side effects;
- MTP inhibitors, such as lomitapide, which provide an LDLR–independent mechanism for lowering LDL-C in homozygous familial hypercholesterolemia (HoFH);
- ANGPTL3 inhibitors, including evinacumab, which have demonstrated efficacy in reducing LDL-C and TGs, particularly in patients with severely impaired LDLR function.
2. Pharmacological Treatments
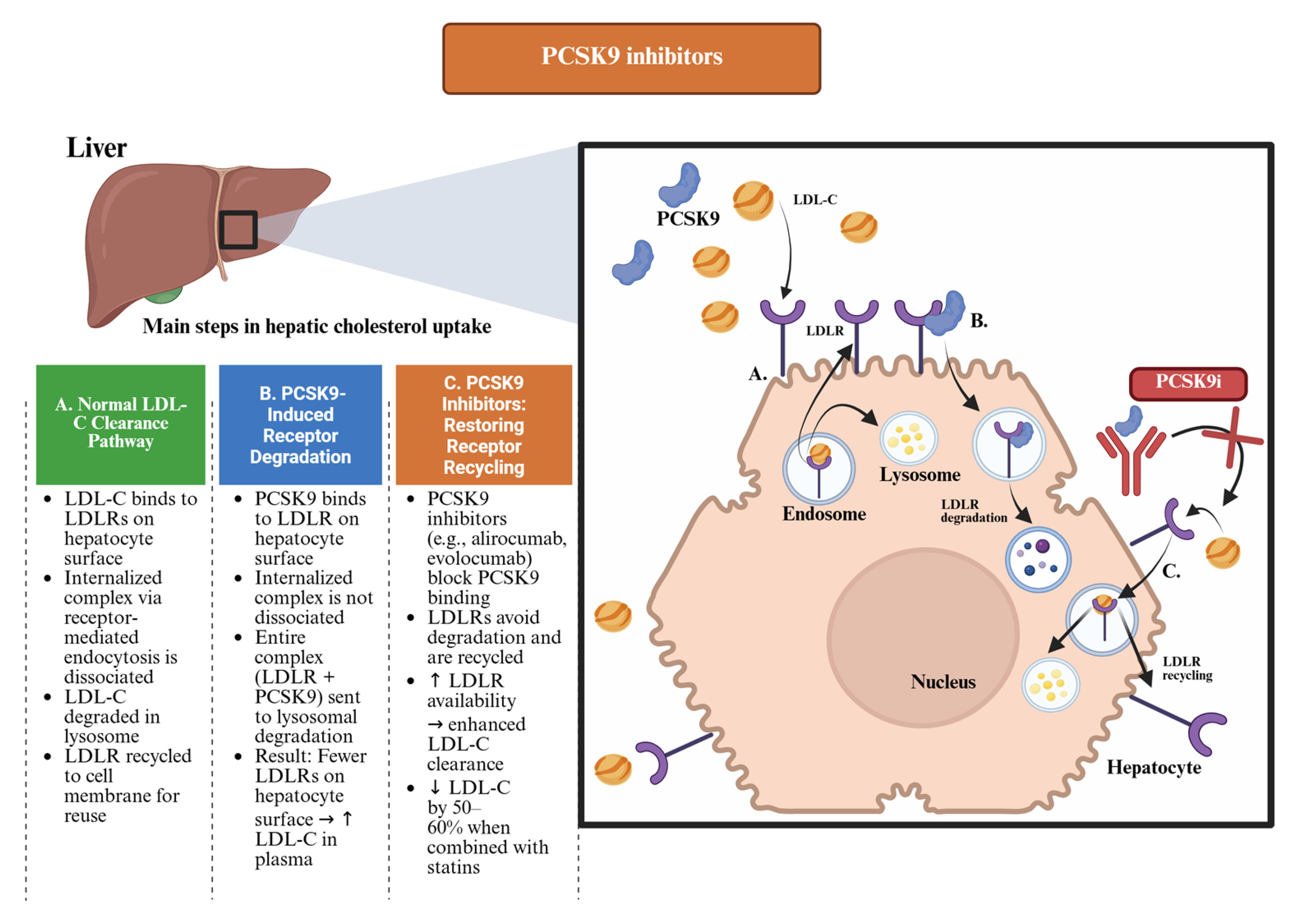
2.1. PCSK9 Inhibitors
2.1.1. Mechanism of Action
2.1.2. Clinical Indications
2.1.3. Safety Profile and Adverse Effects
2.1.4. Contraindications and Precautions
2.1.5. Dosage and Administration
2.1.6. Drug Interactions
2.1.7. Pharmacogenetic Considerations
2.1.8. Special Populations—Pregnancy, Pediatrics, Elderly
2.2. siRNA Therapies
2.2.1. Mechanism of Action
2.2.2. Clinical Indications
2.2.3. Adverse Effects and Safety Profile
2.2.4. Contraindications and Precautions
2.2.5. Dosage and Administration
2.2.6. Drug Interactions
2.2.7. Pharmacogenetic Considerations
2.2.8. Special Populations—Pregnancy, Pediatrics, Elderly
2.3. An Overview of the Divergent Fates of the LDLR: A Tale of Two Ligands—And One Silencer
- When bound to LDL-C, it is recycled and preserved.
- When bound to PCSK9, it is degraded and lost.
- When PCSK9 is silenced—as with inclisiran—the receptor is free to function optimally.
2.4. Bempedoic Acid
2.4.1. Mechanism of Action
2.4.2. Therapeutic Indications
2.4.3. Safety and Adverse Effects
2.4.4. Contraindications
2.4.5. Dosage and Administration
2.4.6. Drug–Drug Interactions of Bempedoic Acid
2.4.7. Pharmacogenetic Considerations
2.4.8. Special Populations—Pregnancy, Pediatrics, Elderly
2.5. Lomitapide
2.5.1. Mechanism of Action
2.5.2. Indications and Clinical Efficacy
2.5.3. Adverse Effects and Safety Profile
2.5.4. Contraindications
2.5.5. Dosage and Administration
2.5.6. Drug Interactions
2.5.7. Pharmacogenetic Considerations
2.5.8. Special Populations
2.6. Angiopoietin-like Protein 3 Inhibitors
2.6.1. Mechanism of Action
2.6.2. Therapeutic Indications
2.6.3. Safety and Adverse Effects
2.6.4. Contraindications
2.6.5. Dosage and Administration
2.6.6. Drug–Drug Interactions
2.6.7. Pharmacogenetic Considerations
2.6.8. Special Populations
2.7. Pharmacovigilance Aspects
3. Overview of the Major Guidelines Recommendations on Lipid-Lowering Therapies
4. Future Perspectives in Cholesterol-Lowering Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ACL | Adenosine triphosphate-citrate lyase |
| ACSVL1 | Very-long-chain acyl-CoA synthetase 1 |
| AHA | American Heart Association |
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| ANGPTL3 | Angiopoietin-like protein 3 |
| ANP | Atrial Natriuretic Peptide |
| ApoB | Apolipoprotein B |
| ASCVD | Atherosclerotic cardiovascular disease |
| ASGPR | Asialoglycoprotein receptor |
| AST | Aspartate aminotransferas |
| BCRP | Breast Cancer Resistance Protein |
| CETP | Cholesteryl Ester Transfer Protein |
| CRP | C-reactive protein |
| CVD | Cardiovascular diseases |
| CYP3A4 | Cytochrome P450 3A4 |
| DNA | Deoxyribonucleic acid |
| eGFR | Estimated glomerular filtration rate |
| EAS | European Atherosclerosis Society |
| EL | Endothelial lipase |
| EGF-A | Epidermal growth factor-like repeat A |
| ESC | European Society of Cardiology |
| EU | European Union |
| FDA | Food and Drug Administration |
| FH | Familial hypercholesterolemia |
| HDL | High-density lipoprotein |
| GalNAc | N-acetylgalactosamine |
| hs-CRP | High-sensitivity C-reactive protein |
| HDL-C | High-density lipoprotein cholesterol |
| HeFH | Heterozygous familial hypercholesterolemia |
| HMG-CoA | 3-hydroxy-3-methylglutaryl coenzyme A |
| HoFH | Homozygous familial hypercholesterolemia |
| INR | International Normalized Ratio |
| LA | Ligand-binding type A |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | Low-density lipoprotein receptor |
| Lp(a) | Lipoprotein(a) |
| LPL | Lipoprotein lipase |
| mRNA | messenger ribonucleic acid |
| MTP | Microsomal triglyceride transfer protein |
| NICE | National Institute for Health and Care Excellence |
| OAT | Organic anion transporter |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| P-gp | P-glycoprotein |
| RISC | RNA-induced silencing complex |
| RNA | Ribonucleic acid |
| RNAi | RNA interference |
| siRNAs | Small interfering RNAs |
| SRE | Sterol regulatory element |
| SREBP | Sterol Regulatory Element-Binding Protein |
| TGs | Triglycerides |
| UGT | UDP-glucuronosyltransferase |
| US | United States |
| VEGF | Vascular endothelial growth factor |
| VLDLs | Very-low-density lipoproteins |
| VLP | Virus-like particle |
Appendix A
| Interacting Drug/Class | Type of Interaction | Effect | Clinical Recommendation |
|---|---|---|---|
| Statins | Pharmacodynamic (additive effect) | Additive LDL-C lowering effect | Commonly co-administered; no adjustment needed |
| Ezetimibe | Pharmacodynamic (additive effect) | Enhanced LDL-C reduction | Safe and often used in combination |
| Immunosuppressants (e.g., cyclosporine) | Theoretical (immune modulation) | Potential modulation of antibody metabolism (no known interaction) | Monitor if used concurrently, though no evidence of harm |
| Anticoagulants/Antiplatelets | Theoretical (safety concern) | Very rare bleeding events reported in trials (no causal link) | Use with vigilance in high-risk patients |
| Vaccines/Biologics | Theoretical (immune system impact) | Limited data; no immunosuppression by PCSK9 inhibitors | No adjustments necessary; standard vaccination practices apply |
| Other monoclonal antibodies | Theoretical (anti-drug antibody risk) | No known interactions | Monitor for unexpected immune responses if used concurrently |
| Pregnancy/lactation drugs | None known (monoclonal IgG1-based) | Large molecules; minimal placental transfer early in pregnancy | Use only if clearly needed; no DDI concerns |
| Interacting Drug/Class | Type of Interaction | Effect | Clinical Recommendation |
|---|---|---|---|
| Statins | Pharmacodynamic (additive effect) | Additive LDL-C lowering effect | Co-administration is common and safe |
| Ezetimibe | Pharmacodynamic (additive effect) | Further LDL-C reduction | Safe; often used in combination |
| Antiplatelet/anticoagulants | Theoretical (injection site bleeding) | Slight ↑ risk of local bleeding due to subcutaneous administration | Monitor injection site; otherwise safe |
| CYP450 substrates/inhibitors | No interaction | Inclisiran is not metabolized by or affecting CYP enzymes | No adjustment needed |
| Renally excreted drugs | No interaction | Although inclisiran is partially cleared renally, no accumulation observed | No adjustment needed in mild/moderate renal impairment |
| Other siRNA or RNA-based therapies | Theoretical (immune stimulation) | Potential for unknown immune or off-target RNA effects | Monitor when used concurrently, though data are limited |
| Immunosuppressants (e.g., cyclosporine) | Theoretical (PK/PD interactions) | No known interaction; limited clinical data | Caution advised; monitor if used together |
| Interacting Drug | Type of Interaction | Effect | Clinical Recommendation |
|---|---|---|---|
| Simvastatin | Pharmacokinetic (increased exposure) | ↑ Simvastatin levels (dose-dependent) → risk of myopathy/rhabdomyolysis | Avoid doses >20 mg/day when co-administered |
| Pravastatin | Pharmacokinetic (increased exposure) | ↑ Pravastatin levels (dose-dependent) → risk of myopathy | Avoid doses >40 mg/day |
| Atorvastatin/Rosuvastatin | Minimal interaction | No clinically meaningful interaction | No clinically relevant interaction expected, although monitoring is advisable in elderly or polytreated patients |
| Colchicine | Additive toxicity (e.g., myopathy) | Potential ↑ risk of myopathy when used with statins and bempedoic acid | Monitor closely for muscle symptoms |
| Fibrates (e.g., gemfibrozil) | Pharmacodynamic/toxicity overlap | ↑ Risk of myopathy when combined with statins and bempedoic acid | Caution advised; monitor for muscle-related side effects |
| Uricosuric agents (e.g., probenecid) | Pharmacodynamic | Opposing effects on uric acid (bempedoic acid may ↑ uric acid) | Monitor serum uric acid levels |
| Cyclosporine | Unclear mechanism | Potential ↑ bempedoic acid exposure | Use caution; consider monitoring bempedoic acid-related effects |
| Interacting Drug/Class | Type of Interaction | Effect | Clinical Recommendations |
|---|---|---|---|
| Strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin, itraconazole) | CYP3A4 inhibition | ↑ Lomitapide plasma concentration → risk of hepatotoxicity | Contraindicated |
| Moderate CYP3A4 inhibitors (e.g., fluconazole, diltiazem, verapamil) | CYP3A4 inhibition | ↑ Lomitapide exposure | Reduce lomitapide dose; monitor liver enzymes |
| Weak CYP3A4 inhibitors (e.g., atorvastatin) | CYP3A4 inhibition | Mild ↑ in lomitapide levels | May require dose adjustment or close monitoring |
| CYP3A4 inducers (e.g., rifampin, carbamazepine, St. John’s Wort) | CYP3A4 induction | ↓ Lomitapide levels → reduced efficacy | Avoid if possible |
| Statins (especially simvastatin, lovastatin) | Additive hepatotoxicity risk | ↑ Risk of transaminase elevations | Use lowest effective statin dose; monitor LFTs closely |
| Warfarin | Lomitapide inhibits CYP2C9 | ↑ INR and bleeding risk | Monitor INR more frequently after starting or adjusting lomitapide |
| Oral contraceptives (estrogen-containing) | Hepatotoxicity risk overlap | ↑ Risk of liver enzyme elevation | Monitor LFTs; consider non-estrogen contraceptive |
| Vitamin E and fat-soluble vitamins | Fat malabsorption due to lomitapide | Deficiency risk (esp. A, D, E, K) | Recommend daily multivitamin supplement with fat-soluble vitamins |
| P-gp substrates (e.g., digoxin, colchicine) | Lomitapide inhibits P-gp | ↑ Substrate levels → potential toxicity | Use caution; monitor drug levels or adverse effects |
| Alcohol | Additive hepatic toxicity | ↑ Risk of hepatotoxicity | Advise limiting or avoiding alcohol |
| Drug/Class | LDL-C | HDL-C | TG | Other Clinically Relevant Effect * |
|---|---|---|---|---|
| Statins (moderate–high intensity) | 30–60% [99] | 5–10% [99] | 10–40% (greater fall when baseline TG is high) [99] | ↓ hs-CRP ≈ 30–40% [99] |
| Ezetimibe | 18–25% [99] | 2–3% [151] | 5–10% [151] | ↓ hs-CRP ≈ 8–10% [151] |
| PCSK9 mAbs (alirocumab/evolocumab) | 50–60% (on top of statin) [152] | 10–15% [152] | 15–30% [152] | ↓ Lp(a) ≈ 20–30% [14] |
| Inclisiran (siRNA) | 45–52% [156] | 5–8% [156] | 10–30% (real-world data) [156] | ↓ Lp(a) ≈ 20–25% [156] |
| Bempedoic acid | 17–23% [73] | ≈5% (small fall) [78] | ≈0% (neutral) [78] | ↓ hs-CRP ≈ 25–30% [83] |
| Lomitapide | 40–60% (HoFH trials up to −70%) [102] | ~0% (no consistent change) [102] | 30–65% (dose-dependent) [102] | ↓ Apo B ≈ 50–56% [96] |
| Drug/Class | Renal Elimination | Dose Adjustment in Mild/Moderate Impairment (eGFR 30–89) | Dose Adjustment in Severe Impairment (eGFR < 30) | Observations |
|---|---|---|---|---|
| Statins | Variable (some renal excretion) | Usually no adjustment for atorvastatin, fluvastatin, pitavastatin. Yes for others. | Yes for rosuvastatin, simvastatin, pravastatin—reduce dose. | Choose statin based on renal profile; monitor CK and renal function. |
| Ezetimibe | Minimal (mainly fecal) | No adjustment needed. | No adjustment needed. | Safe in all stages of CKD; may be used in dialysis patients. |
| PCSK9 Inhibitors (alirocumab, evolocumab) | Minimal renal clearance | No adjustment needed. | No adjustment needed. | No clinically significant change in exposure. Safe in severe CKD. |
| Inclisiran | Partial renal clearance (~20%) | No adjustment in mild/moderate impairment. | Use with caution; no dosage adjustment recommended but data limited. | Exposure may increase slightly in severe CKD; monitor as needed. |
| Bempedoic Acid | Renal + hepatic elimination | No adjustment in mild/moderate impairment. | Caution advised; no adjustment officially required, but monitor. | Limited data in eGFR <30; risk of ↑ uric acid. |
| Lomitapide | Minimal renal excretion | No adjustment recommended. | Avoid in severe renal impairment due to lack of data. | Contraindicated or not recommended in severe CKD; monitor LFTs and lipid response. |
| Medicine/Class | Hepatic Metabolism | Mild Impairment (Child-Pugh A) | Moderate Impairment (Child-Pugh B) | Severe Impairment (Child-Pugh C) | Observations |
|---|---|---|---|---|---|
| Statins (varies by type) | Extensive (CYP3A4/2C9) or glucuronidation | Use with caution, start low | Avoid or reduce dose, especially for simvastatin, lovastatin | Contraindicated in many cases | Statins are hepatically cleared; monitor LFTs. Atorvastatin and pravastatin are relatively safer. |
| Ezetimibe | Hepatic via glucuronidation | No adjustment, but monitor LFTs | Use with caution | Not recommended | Increased exposure in hepatic impairment; avoid in moderate–severe cases. |
| PCSK9 Inhibitors (alirocumab, evolocumab) | Not hepatically metabolized (catabolized like proteins) | No adjustment | No adjustment | Limited data, but generally safe | Safe due to non-hepatic metabolism; monitor for immune reactions. |
| Inclisiran | Minimal hepatic metabolism (ASGPR-mediated hepatic uptake) | No adjustment | No adjustment, but monitor | Not recommended | Lacks data in Child-Pugh C; generally well-tolerated in mild/moderate impairment. |
| Bempedoic Acid | Prodrug activated in liver via ACSVL1 | Use with caution | Not recommended | Not recommended | Limited data; avoid in advanced liver disease due to unknown risk. |
| Lomitapide | Extensive CYP3A4 metabolism | Start at low dose | Contraindicated | Contraindicated | High hepatotoxicity risk; black box warning for liver injury. Monitor transaminases and hepatic fat. |
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef]
- The Global Cardiovascular Risk Consortium. Global Effect of Modifiable Risk Factors on Cardiovascular Disease and Mortality. N. Engl. J. Med. 2023, 389, 1273–1285. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Nițu, E.-T.; Jianu, N.; Merlan, C.; Foica, D.; Sbârcea, L.; Buda, V.; Suciu, M.; Lombrea, A.; Movilă, D.E. A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part I. Life 2025, 15, 1185. [Google Scholar] [CrossRef]
- Kazibwe, R.; Jehopio, J.; Schaich, C.L.; Rikhi, R.; Mirzai, S.; Chevli, P.A.; Namutebi, J.H.; Chebrolu, S.; O’Connor, S.; Yeboah, J.; et al. Atherogenic Dyslipidemia and Incident Cardiovascular Events in High-Risk Hypertension. Prog. Cardiovasc. Dis. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Cortés, C.; López, C.; Paublini, H.; Arroyo Bote, S.; López-González, Á.A.; Ramírez-Manent, J.I. Relationship between Atherogenic Dyslipidaemia and Lipid Triad with Different Scales of Overweight and Obesity in 418,343 Spanish Workers. J. Nutr. Metab. 2022, 2022, 9946255. [Google Scholar] [CrossRef]
- Lorenzatti, A.J.; Toth, P.P. New Perspectives on Atherogenic Dyslipidaemia and Cardiovascular Disease. Eur. Cardiol. Rev. 2020, 15, e04. [Google Scholar] [CrossRef]
- Recommendations|Cardiovascular Disease: Risk Assessment and Reduction, Including Lipid Modification|Guidance|NICE. Available online: https://www.nice.org.uk/guidance/ng238 (accessed on 29 July 2025).
- Visseren, F.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Nair, T. Role of PCSK9 Inhibitors in the Management of Dyslipidaemia. Indian Heart J. 2024, 76, S44–S50. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Papakonstantinou, E.J.; Carreño, J.; Echavarria Uceta, R.; Guzman, E.; Sourlas, A. PCSK9 Targeting in the Management of Hypercholesterolaemia. EMJ Cardiol. 2023, 11, 87–97. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Hegele, R.A.; Fazio, S.; Cannon, C.P. The Evolving Future of PCSK9 Inhibitors. J. Am. Coll. Cardiol. 2018, 72, 314–329. [Google Scholar] [CrossRef]
- Abduljabbar, M.H. PCSK9 Inhibitors: Focus on Evolocumab and Its Impact on Atherosclerosis Progression. Pharmaceuticals 2024, 17, 1581. [Google Scholar] [CrossRef] [PubMed]
- Page, M.M.; Watts, G.F. PCSK9 Inhibitors—Mechanisms of Action. Aust. Prescr. 2016, 39, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ilut, S.; Pirlog, B.O.; Pirlog, R.; Nutu, A.; Vacaras, V.; Armean, S.M. Recent Advances on the Roles of PCSK-9 Inhibitors in the Management of Acute Ischemic Stroke Patients. Int. J. Mol. Sci. 2022, 23, 10221. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Bai, J.; Zhou, J.; Zou, Y.; Yu, M. Adverse Event Profiles of PCSK9 Inhibitors Alirocumab and Evolocumab: Data Mining of the FDA Adverse Event Reporting System. Br. J. Clin. Pharmacol. 2022, 88, 5317–5325. [Google Scholar] [CrossRef]
- Xiao, Y.; Ba, Z.; Pang, S.; Liu, D.; Wang, H.; Liang, H.; Wang, Y.; Yuan, J. PCSK9 Inhibitor: Safe Alternative to Fill the Treatment Gap in Statin-Limited Conditions? Rev. Cardiovasc. Med. 2022, 23, 380. [Google Scholar] [CrossRef]
- Moşteoru, S.; Gaiţă, D.; Banach, M. An Update on PCSK9 Inhibitors- Pharmacokinetics, Drug Interactions, and Toxicity. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 1199–1205. [Google Scholar] [CrossRef]
- Hindi, N.N.; Alenbawi, J.; Nemer, G. Pharmacogenomics Variability of Lipid-Lowering Therapies in Familial Hypercholesterolemia. J. Pers. Med. 2021, 11, 877. [Google Scholar] [CrossRef]
- Meng, F.H.; Liu, S.; Xiao, J.; Zhou, Y.X.; Dong, L.W.; Li, Y.F.; Zhang, Y.Q.; Li, W.H.; Wang, J.Q.; Wang, Y.; et al. New Loss-of-Function Mutations in PCSK9 Reduce Plasma LDL Cholesterol. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1219–1233. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H.; Hobbs, H.H. Sequence Variations in PCSK9, Low LDL, and Protection against Coronary Heart Disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Tandirerung, F.J. Does Genotype Affect the Efficacy of PCSK9 Inhibitors in the Treatment of Familial Hypercholesterolemia? Cardiovasc. Drugs Ther. 2025, 39, 405–413. [Google Scholar] [CrossRef]
- Jeswani, B.M.; Sharma, S.; Rathore, S.S.; Nazir, A.; Bhatheja, R.; Kapoor, K. PCSK9 Inhibitors: The Evolving Future. Health Sci. Rep. 2024, 7, e70174. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, A.; Hong, J.C.; Khera, R.; Virani, S.S.; Krumholz, H.M.; Nasir, K. Updated Cost-Effectiveness Assessments of PCSK9 Inhibitors from the Perspectives of the Health System and Private Payers: Insights Derived from the FOURIER Trial. JAMA Cardiol. 2017, 2, 1369–1374. [Google Scholar] [CrossRef]
- Health Advances Insights: Health Advances. Available online: https://www.healthadvances.com/insights/blog/pcsk9-inhibitors-battle-for-reimbursement-germanys-decision-and-implications-in-other-countries (accessed on 26 July 2025).
- Amgen and Harvard Pilgrim Agree To First Cardiovascular Outcomes-Based Refund Contract for Repatha® (Evolocumab)|Amgen. Available online: https://www.amgen.com/newsroom/press-releases/2017/05/amgen-and-harvard-pilgrim-agree-to-first-cardiovascular-outcomesbased-refund-contract-for-repatha-evolocumab (accessed on 26 July 2025).
- Cigna Reaches Pay-for-Performance Deals for PCSK9 Inhibitors. Available online: https://www.ajmc.com/view/cigna-reaches-pay-for-performance-deals-for-pcsk9-inhibitors (accessed on 26 July 2025).
- Department of Health. The Pharmaceutical Price Regulation Scheme: Twelfth Report to Parliament; Department of Health: London, UK, 2014. [Google Scholar]
- Radu, C.; Serban, D.E.; Chiriac, N.D. Delayed Access to Innovative Medicines in Romania: A Comprehensive Analysis of the Reimbursement Processes (2015–2024). Front. Public Health 2025, 13, 1592419. [Google Scholar] [CrossRef]
- Olatunji, G.; Kokori, E.; Yusuf, I.A.; Akinmoju, O.; Egbunu, E.; Muogbo, I.; Lema, K.; Kanagala, S.G.; Owolabi, S.; Abdulbasit, M.; et al. Inclisiran SiRNA Technology in the Management of Dyslipidemia: A Narrative Review of Clinical Trials. Curr. Probl. Cardiol. 2024, 49, 102419. [Google Scholar] [CrossRef]
- Ebenezer, O.; Oyebamiji, A.K.; Olanlokun, J.O.; Tuszynski, J.A.; Wong, G.K.S. Recent Update on SiRNA Therapeutics. Int. J. Mol. Sci. 2025, 26, 3456. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Hong, L.; Wang, H.; Li, B.; Zhang, M.; Li, J.; Yang, L.; Liu, F. Inclisiran: A New Generation of Lipid-Lowering SiRNA Therapeutic. Front. Pharmacol. 2023, 14, 1260921. [Google Scholar] [CrossRef]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.A.; Awidi, A. SiRNA: Mechanism of Action, Challenges, and Therapeutic Approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Di Fusco, S.A.; Maggioni, A.P.; Bernelli, C.; Perone, F.; De Marzo, V.; Conte, E.; Musella, F.; Uccello, G.; De Luca, L.; Gabrielli, D.; et al. Inclisiran: A New Pharmacological Approach for Hypercholesterolemia. Rev. Cardiovasc. Med. 2022, 23, 375. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Bajaj, A.; Brousseau, M.E.; Taub, P.R. Harnessing RNA Interference for Cholesterol Lowering: The Bench-to-Bedside Story of Inclisiran. J. Am. Heart Assoc. 2024, 13, 32031. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Muñoz Estrella, A.; Sourlas, A.; Silverio, D.; Hilario, E.; Montan, P.D.; Guzman, E. Inclisiran: A New Promising Agent in the Management of Hypercholesterolemia. Diseases 2018, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Kuzmich, N.; Andresyuk, E.; Porozov, Y.; Tarasov, V.; Samsonov, M.; Preferanskaya, N.; Veselov, V.; Alyautdin, R. PCSK9 as a Target for Development of a New Generation of Hypolipidemic Drugs. Molecules 2022, 27, 434. [Google Scholar] [CrossRef]
- Di Giacomo-Barbagallo, F.; Andreychuk, N.; Scicali, R.; Gonzalez-Lleó, A.; Piro, S.; Masana, L.; Ibarretxe, D. Inclisiran, Reasons for a Novel Agent in a Crowded Therapeutic Field. Curr. Atheroscler. Rep. 2025, 27, 25. [Google Scholar] [CrossRef]
- Cowart, K.; Singleton, J.; Carris, N.W. Inclisiran for the Treatment of Hyperlipidemia and for Atherosclerotic Cardiovascular Disease Risk Reduction: A Narrative Review. Clin. Ther. 2023, 45, 1099–1104. [Google Scholar] [CrossRef]
- Wołowiec, Ł.; Osiak, J.; Wołowiec, A.; Wijata, A.; Grześk, E.; Kozakiewicz, M.; Banach, J.; Nowaczyk, A.; Nowaczyk, J.; Grześk, G. Inclisiran—Safety and Effectiveness of Small Interfering RNA in Inhibition of PCSK-9. Pharmaceutics 2023, 15, 323. [Google Scholar] [CrossRef]
- Cicala, G.; Rottura, M.; Gianguzzo, V.M.; Cristiano, F.; Drago, S.F.A.; Pallio, G.; Irrera, N.; Imbalzano, E.; Spina, E.; Arcoraci, V. Safety of Inclisiran: A Disproportionality Analysis from the EudraVigilance Database. Pharmaceuticals 2024, 17, 1365. [Google Scholar] [CrossRef]
- Wright, R.S.; Koenig, W.; Landmesser, U.; Leiter, L.A.; Raal, F.J.; Schwartz, G.G.; Lesogor, A.; Maheux, P.; Stratz, C.; Zang, X.; et al. Safety and Tolerability of Inclisiran for Treatment of Hypercholesterolemia in 7 Clinical Trials. J. Am. Coll. Cardiol. 2023, 82, 2251–2261. [Google Scholar] [CrossRef]
- Nishikido, T. Clinical Potential of Inclisiran for Patients with a High Risk of Atherosclerotic Cardiovascular Disease. Cardiovasc. Diabetol. 2023, 22, 20. [Google Scholar] [CrossRef]
- Ray, K.K.; Troquay, R.P.T.; Visseren, F.L.J.; Leiter, L.A.; Scott Wright, R.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A.; et al. Long-Term Efficacy and Safety of Inclisiran in Patients with High Cardiovascular Risk and Elevated LDL Cholesterol (ORION-3): Results from the 4-Year Open-Label Extension of the ORION-1 Trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef]
- Harbi, M.H. Current Usage of Inclisiran for Cardiovascular Diseases: Overview of Current Clinical Trials. Front. Pharmacol. 2025, 16, 1449712. [Google Scholar] [CrossRef]
- Amaeze, O.; Isoherranen, N.; Shum, S. The ADME Characteristics of SiRNA Therapeutics and the Opportunity to Predict Disposition in Pregnant Women. Drug Metab. Dispos. 2024, 53, DMD-MR-2023-001383. [Google Scholar] [CrossRef]
- Humphreys, S.C.; Davis, J.A.; Iqbal, S.; Kamel, A.; Kulmatycki, K.; Lao, Y.; Liu, X.; Rodgers, J.; Snoeys, J.; Vigil, A.; et al. Considerations and Recommendations for Assessment of Plasma Protein Binding and Drug-Drug Interactions for SiRNA Therapeutics. Nucleic Acids Res. 2022, 50, 6020–6037. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Arya, V.; Reynolds, K.S.; Rogers, H. Clinical Pharmacology of RNA Interference–Based Therapeutics: A Summary Based on Food and Drug Administration–Approved Small Interfering RNAs. Drug Metab. Dispos. 2023, 51, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Delabays, B.; Trajanoska, K.; Walonoski, J.; Mooser, V. Cardiovascular Pharmacogenetics: From Discovery of Genetic Association to Clinical Adoption of Derived Test. Pharmacol. Rev. 2024, 76, 791–827. [Google Scholar] [CrossRef] [PubMed]
- Mulder, J.W.C.M.; Galema-Boers, A.M.H.; Roeters van Lennep, J.E. First Clinical Experiences with Inclisiran in a Real-World Setting. J. Clin. Lipidol. 2023, 17, 818–827. [Google Scholar] [CrossRef]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The Current State and Future Directions of RNAi-Based Therapeutics. Nat. Rev. Drug Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef]
- Leqvio|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/leqvio (accessed on 7 June 2025).
- Raal, F.; Durst, R.; Bi, R.; Talloczy, Z.; Maheux, P.; Lesogor, A.; Kastelein, J.J.P. Efficacy, Safety, and Tolerability of Inclisiran in Patients With Homozygous Familial Hypercholesterolemia: Results From the ORION-5 Randomized Clinical Trial. Circulation 2024, 149, 354–362. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Di Angelantonio, E.; Gao, P.; Pennells, L.; Kaptoge, S.; Caslake, M.; Thompson, A.; Butterworth, A.S.; Sarwar, N.; Wormser, D.; Saleheen, D.; et al. Lipid-Related Markers and Cardiovascular Disease Prediction. JAMA 2012, 307, 2499–2506. [Google Scholar] [CrossRef]
- Karnati, S.A.; Wee, A.; Shirke, M.M.; Harky, A. Racial Disparities and Cardiovascular Disease: One Size Fits All Approach? J. Card. Surg. 2020, 35, 3530–3538. [Google Scholar] [CrossRef]
- Rogula, S.; Błażejowska, E.; Gąsecka, A.; Szarpak, Ł.; Jaguszewski, M.J.; Mazurek, T.; Filipiak, K.J. Inclisiran—Silencing the Cholesterol, Speaking up the Prognosis. J. Clin. Med. 2021, 10, 2467. [Google Scholar] [CrossRef]
- Mizuta, M.H.; Santos, R.D. Functional Testing of Familial Hypercholesterolemia-Related Variants: From Bench to Clinics. JACC Basic. Transl. Sci. 2025, 10, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Tichý, L.; Freiberger, T.; Blaha, V.; Satny, M.; Hubacek, J.A. Genetics of Familial Hypercholesterolemia: New Insights. Front. Genet. 2020, 11, 574474. [Google Scholar] [CrossRef] [PubMed]
- Pećin, I.; Hartgers, M.L.; Hovingh, G.K.; Dent, R.; Reiner, E. Prevention of Cardiovascular Disease in Patients with Familial Hypercholesterolaemia: The Role of PCSK9 Inhibitors. Eur. J. Prev. Cardiol. 2017, 24, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.Y.; Liu, X.; Yang, Z.; Zhu, X.; Liu, M.; Du, M.; Pan, X.; Wang, Y. PCSK9 Promotes LDLR Degradation by Preventing SNX17-Mediated LDLR Recycling. Circulation 2025, 151, 1512–1526. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Deng, Y.M.; Liu, X.R.; Cao, J.P.; Zhou, M.; Tang, Y.L.; Xiong, W.H.; Jiang, Z.S.; Tang, Z.H.; Liu, L.S. PCSK9: A New Participant in Lipophagy in Regulating Atherosclerosis? Clin. Chim. Acta 2019, 495, 358–364. [Google Scholar] [CrossRef]
- Momtazi-Borojeni, A.A.; Sabouri-Rad, S.; Gotto, A.M.; Pirro, M.; Banach, M.; Awan, Z.; Barreto, G.E.; Sahebkar, A. PCSK9 and Inflammation: A Review of Experimental and Clinical Evidence. Eur. Heart J. Cardiovasc. Pharmacother. 2019, 5, 237–245. [Google Scholar] [CrossRef]
- Dec, A.; Niemiec, A.; Wojciechowska, E.; Maligłówka, M.; Bułdak, Ł.; Bołdys, A.; Okopień, B. Inclisiran—A Revolutionary Addition to a Cholesterol-Lowering Therapy. Int. J. Mol. Sci. 2023, 24, 6858. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef]
- Agarwala, A.; Goldberg, A.C. Bempedoic Acid: A Promising Novel Agent for LDL-C Lowering. Future Cardiol. 2020, 16, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Gupta, M.; Devanabanda, A.R.; Bandyopadhyay, D.; Aronow, W.S.; Ray, K.K.; Mamas, M.; Ghosh, R.K. Bempedoic Acid and Its Role in Contemporary Management of Hyperlipidemia in Atherosclerosis. Ann. Med. 2022, 54, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Sirtori, C.R.; Carugo, S.; Banach, M.; Corsini, A. Bempedoic Acid: For Whom and When. Curr. Atheroscler. Rep. 2022, 24, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.; Natale, F.; Molinari, R.; Franzese, R.; Mollo, N.; Cimmino, G. Bempedoic Acid and Statins in Lipid-Lowering Strategy: Which Came First, the Egg or the Chicken? Future Pharmacol. 2023, 3, 392–406. [Google Scholar] [CrossRef]
- Biolo, G.; Vinci, P.; Mangogna, A.; Landolfo, M.; Schincariol, P.; Fiotti, N.; Mearelli, F.; Di Girolamo, F.G. Mechanism of Action and Therapeutic Use of Bempedoic Acid in Atherosclerosis and Metabolic Syndrome. Front. Cardiovasc. Med. 2022, 9, 1028355. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E.; Farnier, M.; Fras, Z.; Latkovskis, G.; Laufs, U.; Paneni, F.; Parini, P.; Pirro, M.; Reiner, Ž.; et al. Bempedoic Acid in the Management of Lipid Disorders and Cardiovascular Risk. 2023 Position Paper of the International Lipid Expert Panel (ILEP). Prog. Cardiovasc. Dis. 2023, 79, 2–11. [Google Scholar] [CrossRef]
- Yarrarapu, S.N.S.; Goyal, A.; Venkata, V.S.; Panchal, V.; Sivasubramanian, B.P.; Du, D.T.; Jakulla, R.S.; Pamulapati, H.; Afaq, M.A.; Owens, S.; et al. Comprehensive Review of Statin-Intolerance and the Practical Application of Bempedoic Acid. J. Cardiol. 2024, 84, 22–29. [Google Scholar] [CrossRef]
- Burke, A.C.; Telford, D.E.; Huff, M.W. Bempedoic Acid: Effects on Lipoprotein Metabolism and Atherosclerosis. Curr. Opin. Lipidol. 2019, 30, 1–9. [Google Scholar] [CrossRef]
- Agarwala, A.; Quispe, R.; Goldberg, A.C.; Michos, E.D. Bempedoic Acid for Heterozygous Familial Hypercholesterolemia: From Bench to Bedside. Drug Des. Devel Ther. 2021, 15, 1955–1963. [Google Scholar] [CrossRef]
- Ferri, N.; Colombo, E.; Corsini, A. Bempedoic Acid, the First-in-Class Oral ATP Citrate Lyase Inhibitor with Hypocholesterolemic Activity: Clinical Pharmacology and Drug–Drug Interactions. Pharmaceutics 2024, 16, 1371. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Bays, H.; Catapano, A.L.; Goldberg, A.; Ray, K.K.; Saseen, J.J. Role of Bempedoic Acid in Clinical Practice. Cardiovasc. Drugs Ther. 2021, 35, 853–864. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Yang, H.X.; Zhang, M.; Long, S.Y.; Tuo, Q.H.; Tian, Y.; Chen, J.X.; Zhang, C.P.; Liao, D.F. Cholesterol in LDL Receptor Recycling and Degradation. Clin. Chim. Acta 2020, 500, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Bhandari, M.; Vishwakarma, P.; Singh, A.; Perrone, M.A.; Sethi, R. Bempedoic Acid: An Emerging Therapy for Uncontrolled Low-Density Lipoprotein (LDL) Cholesterol. J. Cardiovasc. Dev. Dis. 2023, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Jacomelli, I.; Monzo, L.; Panattoni, G.; Lanzillo, C.; Rebecchi, M.; Calò, L. Bempedoic Acid: What Prospective Uses? Eur. Heart J. Suppl. 2023, 25, C109–C111. [Google Scholar] [CrossRef]
- Yang, J. Bempedoic Acid for the Treatment of Hypercholesterolemia. Expert. Rev. Cardiovasc. Ther. 2020, 18, 373–380. [Google Scholar] [CrossRef]
- Lin, Y.; Parco, C.; Karathanos, A.; Krieger, T.; Schulze, V.; Chernyak, N.; Icks, A.; Kelm, M.; Brockmeyer, M.; Wolff, G. Clinical Efficacy and Safety Outcomes of Bempedoic Acid for LDL-C Lowering Therapy in Patients at High Cardiovascular Risk: A Systematic Review and Meta-Analysis. BMJ Open 2022, 12, e048893. [Google Scholar] [CrossRef]
- Marrs, J.C.; Anderson, S.L. Bempedoic Acid for the Treatment of Dyslipidemia. Drugs Context 2020, 9. [Google Scholar] [CrossRef]
- Lincoff, A.M.; Ray, K.K.; Sasiela, W.J.; Haddad, T.; Nicholls, S.J.; Li, N.; Cho, L.; Mason, D.; Libby, P.; Goodman, S.G.; et al. Comparative Cardiovascular Benefits of Bempedoic Acid and Statin Drugs. J. Am. Coll. Cardiol. 2024, 84, 152–162. [Google Scholar] [CrossRef]
- De Filippo, O.; D’Ascenzo, F.; Iannaccone, M.; Bertaina, M.; Leone, A.; Borzillo, I.; Ravetti, E.; Solano, A.; Pagliassotto, I.; Nebiolo, M.; et al. Safety and Efficacy of Bempedoic Acid: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Cardiovasc. Diabetol. 2023, 22, 324. [Google Scholar] [CrossRef]
- Bays, H.E.; Bloedon, L.A.T.; Lin, G.; Powell, H.A.; Louie, M.J.; Nicholls, S.J.; Lincoff, A.M.; Nissen, S.E. Safety of Bempedoic Acid in Patients at High Cardiovascular Risk and with Statin Intolerance. J. Clin. Lipidol. 2024, 18, e59–e69. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Cincione, I. Evaluating Pharmacokinetics of Bempedoic Acid in the Treatment of Hypercholesterolemia. Expert. Opin. Drug Metab. Toxicol. 2021, 17, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ray, K.K.; Catapano, A.L.; Ference, T.B.; Burgess, S.; Neff, D.R.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; Di Angelantonio, E.; et al. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Cheng, H. Application of Mendelian Randomization in the Discovery of Risk Factors for Coronary Heart Disease from 2009 to 2023: A Bibliometric Review. Clin. Cardiol. 2024, 47, e24154. [Google Scholar] [CrossRef] [PubMed]
- Khasawneh, L.Q.; Al-Mahayri, Z.N.; Ali, B.R. Mendelian Randomization in Pharmacogenomics: The Unforeseen Potentials. Biomed. Pharmacother. 2022, 150, 112952. [Google Scholar] [CrossRef]
- Duarte Lau, F.; Giugliano, R.P. Adenosine Triphosphate Citrate Lyase and Fatty Acid Synthesis Inhibition: A Narrative Review. JAMA Cardiol. 2023, 8, 879–887. [Google Scholar] [CrossRef]
- Burke, A.C.; Telford, D.E.; Sutherland, B.G.; Edwards, J.Y.; Sawyez, C.G.; Barrett, P.H.R.; Newton, R.S.; Pickering, J.G.; Huff, M.W. Bempedoic Acid Lowers Low-Density Lipoprotein Cholesterol and Attenuates Atherosclerosis in Low-Density Lipoprotein Receptor–Deficient (LDLR+/− and LDLR−/−) Yucatan Miniature Pigs. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1178–1190. [Google Scholar] [CrossRef]
- Stefanutti, C. Lomitapide–a Microsomal Triglyceride Transfer Protein Inhibitor for Homozygous Familial Hypercholesterolemia. Curr. Atheroscler. Rep. 2020, 22, 38. [Google Scholar] [CrossRef]
- Nilemdo|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nilemdo (accessed on 23 June 2025).
- FDA. Highlights of Prescribing Information; FDA: Silver Spring, MD, USA, 2015. [Google Scholar]
- Raschi, E.; Casula, M.; Cicero, A.F.G.; Corsini, A.; Borghi, C.; Catapano, A. Beyond Statins: New Pharmacological Targets to Decrease LDL-Cholesterol and Cardiovascular Events. Pharmacol. Ther. 2023, 250, 108507. [Google Scholar] [CrossRef]
- Blom, D.J.; Cuchel, M.; Ager, M.; Phillips, H. Target Achievement and Cardiovascular Event Rates with Lomitapide in Homozygous Familial Hypercholesterolaemia. Orphanet J. Rare Dis. 2018, 13, 96. [Google Scholar] [CrossRef]
- Goulooze, S.C.; Cohen, A.F.; Rissmann, R. Lomitapide. Br. J. Clin. Pharmacol. 2015, 80, 179–181. [Google Scholar] [CrossRef]
- D’Erasmo, L.; Steward, K.; Cefalù, A.B.; Di Costanzo, A.; Boersma, E.; Bini, S.; Arca, M.; Van Lennep, J.R. Efficacy and Safety of Lomitapide in Homozygous Familial Hypercholesterolaemia: The Pan-European Retrospective Observational Study. Eur. J. Prev. Cardiol. 2022, 29, 832–841. [Google Scholar] [CrossRef]
- Iannuzzo, G.; Calcaterra, I.L.; Gentile, M.; Stanzione, C.; de Ruberto, F.; di Taranto, M.D.; Cardiero, G.; Fortunato, G.; Di Minno, M. New Insights into the Management of Homozygous Familial Hypercholesterolemia Patients Treated with Lomitapide: A Single-Center Experience. Front. Endocrinol. 2024, 15, 1515846. [Google Scholar] [CrossRef]
- Munkhsaikhan, U.; Kwon, Y.I.; Sahyoun, A.M.; Galán, M.; Gonzalez, A.A.; Ait-Aissa, K.; Abidi, A.H.; Kassan, A.; Kassan, M. The Beneficial Effect of Lomitapide on the Cardiovascular System in LDLr−/− Mice with Obesity. Antioxidants 2023, 12, 1287. [Google Scholar] [CrossRef]
- Harada-Shiba, M.; Haruna, S.; Kogawa, N. Real-World Safety and Efficacy of Lomitapide in Homozygous Familial Hypercholesterolemia: Interim Report of Special-Use Survey in Japan. Future Cardiol. 2024, 20, 67–80. [Google Scholar] [CrossRef]
- Arca, M.; D’Erasmo, L.; Cuchel, M.; Blom, D.J.; Cegla, J.; Duell, P.B.; Santos, R.D.; O’Brien, S. Long-Term Experience with Lomitapide Treatment in Patients with Homozygous Familial Hypercholesterolemia: Over 10 Years of Efficacy and Safety Data. J. Clin. Lipidol. 2025, in press. [Google Scholar] [CrossRef]
- Drug Approval Package: Juxtapid (Lomitapide) NDA 203858. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/2038858_juxtapid_toc.cfm (accessed on 25 June 2025).
- Tuteja, S.; Duffy, D.; Dunbar, R.L.; Movva, R.; Gadi, R.; Bloedon, L.T.; Cuchel, M. Pharmacokinetic Interactions of the Microsomal Triglyceride Transfer Protein Inhibitor, Lomitapide, with Drugs Commonly Used in the Management of Hypercholesterolemia. Pharmacotherapy 2014, 34, 227–239. [Google Scholar] [CrossRef]
- CHMP. Annex I Summary of Product Characteristics; CHMP: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Rayan, R.A.; Patel, P.; Sharma, S. Lomitapide; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- D’Erasmo, L.; Giammanco, A.; Suppressa, P.; Pavanello, C.; Iannuzzo, G.; Di Costanzo, A.; Tramontano, D.; Minicocci, I.; Bini, S.; Vogt, A.; et al. Efficacy of Long-Term Treatment of Autosomal Recessive Hypercholesterolemia With Lomitapide: A Subanalysis of the Pan-European Lomitapide Study. Front. Genet. 2022, 13, 937750. [Google Scholar] [CrossRef]
- Katsiki, N.; Mantzoros, C.S. Statins in Relation to Adiponectin: A Significant Association with Clinical Implications. Atherosclerosis 2016, 253, 270–272. [Google Scholar] [CrossRef][Green Version]
- Lomitapide-DS-Amryt2022. Available online: https://ec.europa.eu/health/documents/community-register/2023/20230526159083/anx_159083_en.pdf (accessed on 25 June 2025).
- Sosnowska, B.; Adach, W.; Surma, S.; Rosenson, R.S.; Banach, M. Evinacumab, an ANGPTL3 Inhibitor, in the Treatment of Dyslipidemia. J. Clin. Med. 2023, 12, 168. [Google Scholar] [CrossRef]
- Wang, X.; Musunuru, K. Angiopoietin-Like 3: From Discovery to Therapeutic Gene Editing. JACC Basic. Transl. Sci. 2019, 4, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Pirruccello, J.P.; Do, R.; Peloso, G.M.; Guiducci, C.; Sougnez, C.; Garimella, K.V.; Fisher, S.; Abreu, J.; Barry, A.J.; et al. Exome Sequencing, ANGPTL3 Mutations, and Familial Combined Hypolipidemia. N. Engl. J. Med. 2010, 363, 2220–2227. [Google Scholar] [CrossRef]
- Noto, D.; Cefalù, A.B.; Valenti, V.; Fayer, F.; Pinotti, E.; Ditta, M.; Spina, R.; Vigna, G.; Yue, P.; Kathiresan, S.; et al. Prevalence of ANGPTL3 and APOB Gene Mutations in Subjects With Combined Hypolipidemia. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 3. [Google Scholar] [CrossRef] [PubMed]
- Minicocci, I.; Montali, A.; Robciuc, M.R.; Quagliarini, F.; Censi, V.; Labbadia, G.; Gabiati, C.; Pigna, G.; Sepe, M.L.; Pannozzo, F.; et al. Mutations in the ANGPTL3 Gene and Familial Combined Hypolipidemia: A Clinical and Biochemical Characterization. J. Clin. Endocrinol. Metab. 2012, 97, E1266–E1275. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.D.; Fazio, S. From Lipids to Inflammation: New Approaches to Reducing Atherosclerotic Risk. Circ. Res. 2016, 118, 732–749. [Google Scholar] [CrossRef]
- Gusarova, V.; Alexa, C.A.; Wang, Y.; Rafique, A.; Kim, J.H.; Buckler, D.; Mintah, I.J.; Shihanian, L.M.; Cohen, J.C.; Hobbs, H.H.; et al. ANGPTL3 Blockade with a Human Monoclonal Antibody Reduces Plasma Lipids in Dyslipidemic Mice and Monkeys. J. Lipid Res. 2015, 56, 1308–1317. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Ghosh, A.; Al-Khairi, I.; Madiraju, S.R.M.; Abubaker, J.; Prentki, M. The Multi-Faces of Angptl8 in Health and Disease: Novel Functions beyond Lipoprotein Lipase Modulation. Prog. Lipid Res. 2020, 80, 101067. [Google Scholar] [CrossRef]
- Kersten, S. New Insights into Angiopoietin-like Proteins in Lipid Metabolism and Cardiovascular Disease Risk. Curr. Opin. Lipidol. 2019, 30, 205–211. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.P.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.-C.; Gipe, D.A.; et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188, Erratum in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef]
- Mach, F.; Visseren, F.L.J.; Cater, N.B.; Salhi, N.; Soronen, J.; Ray, K.K.; Delgado, V.; Jukema, J.W.; Laufs, U.; Zamorano, J.-L.; et al. Addressing Residual Risk beyond Statin Therapy: New Targets in the Management of Dyslipidaemias–A Report from the European Society of Cardiology Cardiovascular Round Table. J. Clin. Lipidol. 2024, 18, e685–e700. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; Waring, A.A.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. What Function of Nanoparticles Is the Primary Factor for Their Hyper-Toxicity? Adv. Colloid. Interface Sci. 2023, 314, 102881. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761181 (accessed on 25 June 2025).
- Wiegman, A.; Greber-Platzer, S.; Ali, S.; Reijman, M.D.; Brinton, E.A.; Charng, M.J.; Srinivasan, S.; Baker-Smith, C.; Baum, S.; Brothers, J.A.; et al. Evinacumab for Pediatric Patients With Homozygous Familial Hypercholesterolemia. Circulation 2024, 149, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Evkeeza|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/evkeeza#news-on (accessed on 25 June 2025).
- Zimodro, J.M.; Rizzo, M.; Gouni-Berthold, I. Current and Emerging Treatment Options for Hypertriglyceridemia: State-of-the-Art Review. Pharmaceuticals 2025, 18, 147. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Gaudet, D.; Ballantyne, C.M.; Baum, S.J.; Bergeron, J.; Kershaw, E.E.; Moriarty, P.M.; Rubba, P.; Whitcomb, D.C.; Banerjee, P.; et al. Evinacumab in Severe Hypertriglyceridemia with or without Lipoprotein Lipase Pathway Mutations: A Phase 2 Randomized Trial. Nat. Med. 2023, 29, 729–737. [Google Scholar] [CrossRef]
- Mohamed, F.; Mansfield, B.S.; Raal, F.J. ANGPTL3 as a Drug Target in Hyperlipidemia and Atherosclerosis. Curr. Atheroscler. Rep. 2022, 24, 959–967. [Google Scholar] [CrossRef]
- Mormone, A.; Tortorella, G.; Esposito, F.; Caturano, A.; Marrone, A.; Cozzolino, D.; Galiero, R.; Marfella, R.; Sasso, F.C.; Rinaldi, L. Advances in Pharmacological Approaches for Managing Hypercholesterolemia: A Comprehensive Overview of Novel Treatments. Biomedicines 2024, 12, 432. [Google Scholar] [CrossRef]
- Stefanutti, C.; Chan, D.C.; Di Giacomo, S.; Morozzi, C.; Watts, G.F. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals 2022, 15, 1389. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Kastelein, J.J.P.; Rubba, P.; Duell, P.B.; Koseki, M.; Stroes, E.; Ali, S.; Banerjee, P.; et al. The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia. JACC Adv. 2023, 2, 100648. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Q.; Yi, Y.; Wang, J.; Chen, P.; Luo, F.; Fang, Z. ANGPTL3 as a Target for Treating Lipid Disorders in Type 2 Diabetes Patients. Lipids Health Dis. 2024, 23, 356. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; Hamlin, R.; Pordy, R.; Dong, Y.; et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 2307–2319. [Google Scholar] [CrossRef]
- Watts, G.F.; Chan, D.C.; Raal, F.J. Inhibition of ANGPTL3 as a Target for Treating Dyslipidemias. In Clinical Lipidology: A Companion to Braunwald’s Heart Disease; Elsevier: Amsterdam, The Netherlands, 2024; pp. 253–267-e1. [Google Scholar] [CrossRef]
- Krzemińska, J.; Młynarska, E.; Radzioch, E.; Wronka, M.; Rysz, J.; Franczyk, B. Management of Familial Hypercholesterolemia with Special Emphasis on Evinacumab. Biomedicines 2022, 10, 3273. [Google Scholar] [CrossRef]
- Evinacumab Interactions Checker—Drugs.Com. Available online: https://www.drugs.com/drug-interactions/evinacumab.html (accessed on 25 June 2025).
- Stitziel, N.O.; Khera, A.V.; Wang, X.; Bierhals, A.J.; Vourakis, A.C.; Sperry, A.E.; Natarajan, P.; Klarin, D.; Emdin, C.A.; Zekavat, S.M.; et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2054–2063. [Google Scholar] [CrossRef]
- Aghasizadeh, M.; Nosrati, M.; Saberi-Karimian, M.; Safarian, H.; Assadian, P.; Akbarpour, E.; Sahebkar, A.; Avan, A.; Ferns, G.A.; Kazemi, T.; et al. Association of ANGPTL3 Polymorphisms with High-Density Lipoprotein Cholesterol Uptake Capacity in Patients with Cardiovascular Disease. J. Clin. Lab. Anal. 2021, 35, e23980. [Google Scholar] [CrossRef]
- Stankov, S.; Vitali, C.; Rader, D.J. Gain-of-Function Variants in Lipid Genes Enhance Biological Insight and Point Toward Therapeutic Opportunities. Circulation 2022, 146, 740–742. [Google Scholar] [CrossRef]
- Details for: EVKEEZA—Drug and Health Products Portal. Available online: https://dhpp.hpfb-dgpsa.ca/dhpp/resource/103038 (accessed on 25 June 2025).
- Azadi, S.M.; Fadaei, R.; Omid-Shafaat, R.; Hosseini, J.; Moradi, N. Elevated Angiopoietin-like Protein 3 Serum Levels in Diabetic Nephropathy Patients and Its Association with Renal Function and Lipid Profile. BMC Nephrol. 2023, 24, 172. [Google Scholar] [CrossRef]
- Béliard, S.; Mourre, F.; Valéro, R. Hyperlipidaemia in Diabetes: Are There Particular Considerations for next-Generation Therapies? Diabetologia 2024, 67, 974–984. [Google Scholar] [CrossRef]
- Swarnakar, R.; Sahu, D.; Bahinipati, J.; Pradhan, T.; Meher, D.; Sarangi, R.; Mahapatra, S. The Significance of ANGPTL3 and ANGPTL4 Proteins in the Development of Dyslipidemia in Type 2 Diabetes Mellitus. J. Family Med. Prim. Care 2025, 14, 947–953. [Google Scholar] [CrossRef]
- Fornari, E.; Stefanutti, C.; Mancioppi, V.; Watts, G.F.; Pisciotta, L.; Morandi, A.; Maffeis, C. Safety and Effectiveness of Evinacumab in an Infant with Homozygous Familial Hypercholesterolemia: A New Renaissance for the Very Young? J. Clin. Lipidol. 2025, 19, 689–694. [Google Scholar] [CrossRef]
- Olatunji, G.; Kokori, E.; Moradeyo, A.A.; Lema, K.; Ayo, O.R.; Obasanjo, O.M.; Mustapha, M.J.; Stanley, A.C.; Aderinto, N. A Mini-Review of Efficacy, Safety, and Influence of Novel Evinacumab on Familial Hypercholesterolemia. Egypt. J. Intern. Med. 2024, 36, 69. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Arca, M.; Celant, S.; Olimpieri, P.P.; Colatrella, A.; Tomassini, L.; D’Erasmo, L.; Averna, M.; Zambon, A.; Catapano, A.L.; Russo, P. Real-World Effectiveness of PCSK9 Inhibitors in Reducing LDL-C in Patients With Familial Hypercholesterolemia in Italy: A Retrospective Cohort Study Based on the AIFA Monitoring Registries. J. Am. Hear. Assoc. 2023, 12, e026550. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; López-Sendón, J.L.; Averna, M.; Bigot, G.; Banach, M.; Letierce, A.; Loy, M.; Samuel, R.; Manvelian, G.; Batsu, I.; et al. Safety and Efficacy of Alirocumab in a Real-Life Setting: The ODYSSEY APPRISE Study. Eur. J. Prev. Cardiol. 2021, 28, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; López-Sendon, J.L.; Averna, M.; Cariou, B.; Loy, M.; Manvelian, G.; Batsu, I.; Poulouin, Y.; Gaudet, D. Treatment Adherence and Effect of Concurrent Statin Intensity on the Efficacy and Safety of Alirocumab in a Real-Life Setting: Results from ODYSSEY APPRISE. Arch. Med. Sci. 2022, 18, 285–292. [Google Scholar] [CrossRef]
- Ray, K.K.; Kallend, D.; Leiter, L.A.; Raal, F.J.; Koenig, W.; Jaros, M.J.; Schwartz, G.G.; Landmesser, U.; Conde, L.G.; Scott Wright, R. Effect of Inclisiran on Lipids in Primary Prevention: The ORION-11 Trial. Eur. Heart J. 2022, 43, 5047–5057. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Estrella, A.M.; Skavdis, A.; Genao, E.P.; Martinez, I.; Guzman, E. Inclisiran for the Treatment of Cardiovascular Disease: A Short Review on the Emerging Data and Therapeutic Potential. Ther. Clin. Risk Manag. 2020, 16, 1031–1037. [Google Scholar] [CrossRef]
- Blom, D.J.; Fayad, Z.A.; Kastelein, J.J.P.; Larrey, D.; Makris, L.; Schwamlein, C.; Bloeden, L.; Underberg, J. LOWER, a Registry of Lomitapide-Treated Patients with Homozygous Familial Hypercholesterolemia: Rationale and Design. J. Clin. Lipidol. 2016, 10, 273–282. [Google Scholar] [CrossRef]
- Leucker, T.M.; Blaha, M.J.; Jones, S.R.; Vavuranakis, M.A.; Williams, M.S.; Lai, H.; Schindler, T.H.; Latina, J.; Schulman, S.P.; Gerstenblith, G. Effect of Evolocumab on Atherogenic Lipoproteins during the Peri- And Early Postinfarction Period: A Placebo-Controlled, Randomized Trial. Circulation 2020, 142, 419–421. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Luo, F.; Yu, L.; Xian, X.; Shan, B.; Das, A. Editorial: New and Emerging Lipid-Lowering Therapies for Reducing Cardiovascular Risk: Beyond Statins. Front. Cardiovasc. Med. 2024, 11, 1364170. [Google Scholar] [CrossRef]
- Michaeli, D.T.; Michaeli, J.C.; Albers, S.; Boch, T.; Michaeli, T. Established and Emerging Lipid-Lowering Drugs for Primary and Secondary Cardiovascular Prevention. Am. J. Cardiovasc. Drugs 2023, 23, 477–495. [Google Scholar] [CrossRef]

| Feature | LDL-C | PCSK9 | Inclisiran |
|---|---|---|---|
| Molecule type | Lipoprotein particle (contains ApoB-100) | Secreted protein regulator (serine protease) | Chemically modified small interfering RNA (siRNA) |
| Binds to LDLR | Yes, via LA repeats (extracellular) | Yes, via EGF-A domain (extracellular or intracellular) | No direct binding; acts upstream at the mRNA level |
| Binding site on LDLR | LA (ligand-binding) domain (R4–R7) | EGF-A domain | None |
| Ca2+ dependence | Required for structural stability of LA repeats | Ca2+ required to fold EGF-A, but not in binding interface | Not applicable |
| Effect of endosomal pH | Acidic pH weakens interaction → dissociation | Acidic pH strengthens interaction → complex stabilized | Prevents PCSK9 protein formation → no interaction occurs |
| LDLR fate | Recycled to membrane | Degraded in lysosome | Preserved (prevents degradation indirectly) |
| LDL-C clearance | Enhanced | Inhibited | Enhanced |
| Mechanism of action | Delivers cholesterol to liver cells for degradation | Tags LDLR for lysosomal degradation | Silences hepatic PCSK9 gene expression (RNA interference) |
| Therapeutic role | Target of LDLR function (not a therapy) | Targeted by inhibitors (e.g., monoclonal antibodies) | Therapeutic agent (e.g., inclisiran) |
| Route of action | Natural physiological ligand | Endogenous negative regulator | Synthetic therapeutic siRNA |
| Time of action | Fast (minutes) | Moderate (hours) | Long-term (weeks to months per dose) |
| Clinical outcome | Lowers plasma LDL-C when cleared by LDLR | Raises plasma LDL-C by reducing LDLR availability | Lowers LDL-C by increasing LDLR availability |
| Example drugs | Not applicable | Evolocumab, alirocumab | Inclisiran |
| Therapy Class | ESC/EAS | ACC/AHA | NICE Guidelines |
|---|---|---|---|
| Statins | First-line therapy across all risk categories. High-intensity statins recommended for high and very-high-risk patients. | First-line therapy. High-intensity statins preferred. No fixed LDL-C target; emphasis on ≥50% LDL-C reduction. | First-line therapy. High-intensity statins recommended. Target: ≥40% non-HDL-C reduction. |
| Ezetimibe | Second-line if LDL-C target not achieved on statins. Combination strongly recommended. | Added if LDL-C remains ≥70 mg/dL in very high-risk patients on maximal statins. | Added if target LDL-C reduction not achieved. Approved for combination or monotherapy if statin intolerant. |
| PCSK9 Inhibitors | Third-line after statin + ezetimibe in very-high-risk patients. Recommended if LDL-C ≥55 mg/dL persists. | Reasonable if LDL-C ≥70 mg/dL on maximal statin + ezetimibe in very high-risk individuals. | Approved for FH or very-high-risk patients who do not achieve ≥40% non-HDL-C reduction. Strict eligibility and cost control. |
| Inclisiran | Recognized as an alternative to PCSK9 inhibitors in high/very-high-risk patients; recommended in updated position statements. | Mentioned as promising but limited outcome data; not incorporated into formal treatment pathways. | Approved for primary hypercholesterolemia or mixed dyslipidemia in adults if LDL-C goals unmet on maximally tolerated therapy. |
| Bempedoic Acid | Considered as adjunctive therapy in patients with statin intolerance or inadequate LDL-C reduction; acknowledged in 2023 updates. | Emerging option for statin-intolerant patients or those requiring further LDL-C lowering; outcome data evolving. | Approved for use in statin-intolerant patients or as add-on therapy. Cost-effectiveness considerations apply. |
| ANGPTL3 Inhibitors (Evinacumab) | Approved in HoFH with inadequate LDL-C response to other treatments; reserved for specialist care in severe cases. | Recognized as adjunctive treatment in HoFH; availability varies. | Not routinely commissioned; considered only in HoFH under specialist supervision and exceptional circumstances. |
| Lomitapide | Approved in HoFH; recommended under expert supervision due to hepatic fat accumulation risk and monitoring requirements. | Approved for HoFH; reserved for specialized lipid clinics. | Commissioned only for HoFH in highly selected patients; requires specialist management. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianu, N.; Nițu, E.-T.; Merlan, C.; Nour, A.; Buda, S.; Suciu, M.; Luca, S.A.; Sbârcea, L.; Andor, M.; Buda, V. A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II. Pharmaceuticals 2025, 18, 1150. https://doi.org/10.3390/ph18081150
Jianu N, Nițu E-T, Merlan C, Nour A, Buda S, Suciu M, Luca SA, Sbârcea L, Andor M, Buda V. A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II. Pharmaceuticals. 2025; 18(8):1150. https://doi.org/10.3390/ph18081150
Chicago/Turabian StyleJianu, Narcisa, Ema-Teodora Nițu, Cristina Merlan, Adina Nour, Simona Buda, Maria Suciu, Silvia Ana Luca, Laura Sbârcea, Minodora Andor, and Valentina Buda. 2025. "A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II" Pharmaceuticals 18, no. 8: 1150. https://doi.org/10.3390/ph18081150
APA StyleJianu, N., Nițu, E.-T., Merlan, C., Nour, A., Buda, S., Suciu, M., Luca, S. A., Sbârcea, L., Andor, M., & Buda, V. (2025). A Comprehensive Review of the Latest Approaches to Managing Hypercholesterolemia: A Comparative Analysis of Conventional and Novel Treatments: Part II. Pharmaceuticals, 18(8), 1150. https://doi.org/10.3390/ph18081150











