Assessing Omega-3 Therapy and Its Cardiovascular Benefits: What About Icosapent Ethyl? A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Language
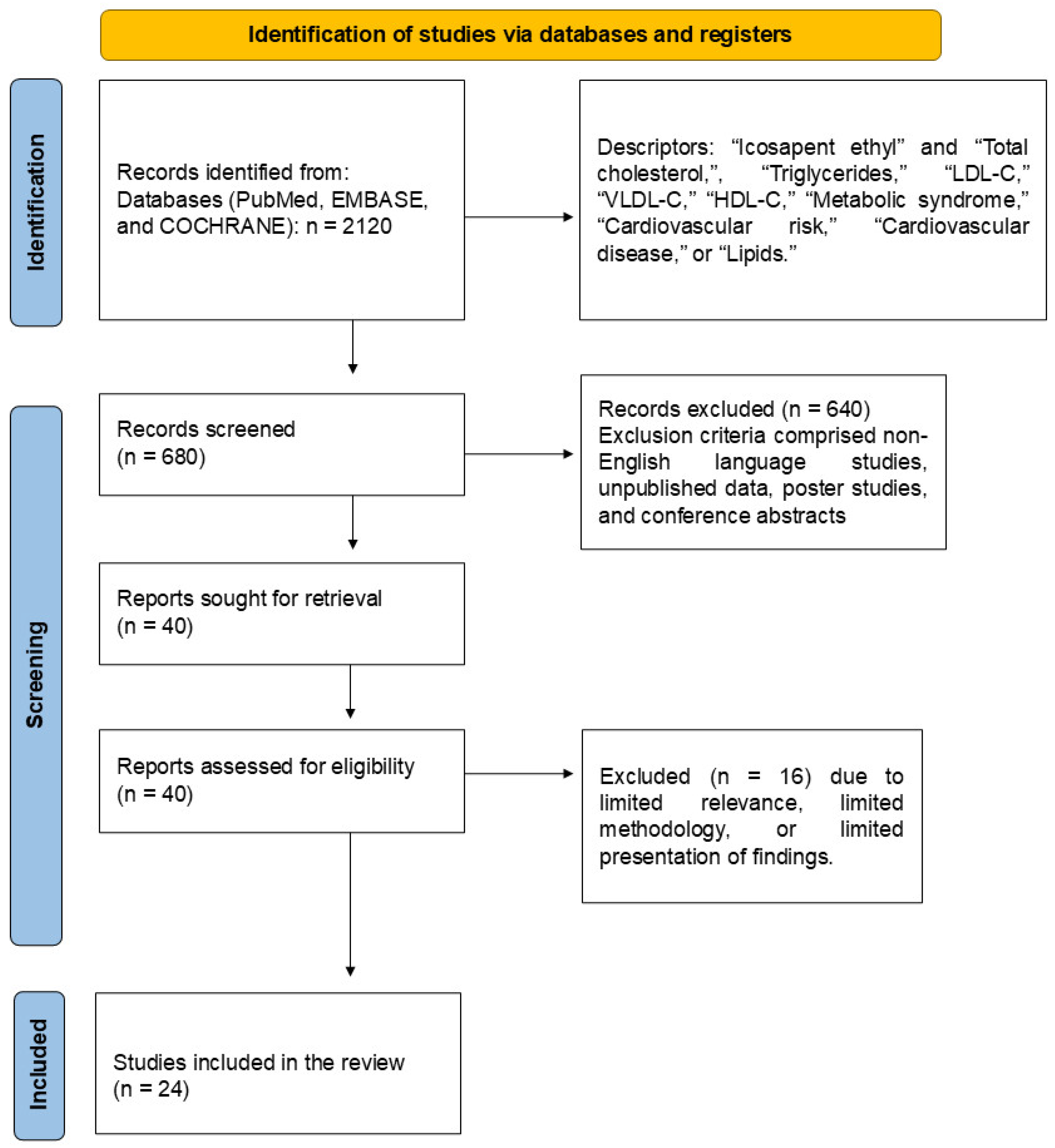
2.3. Literature Search and Databases
2.4. Study Selection
- (P) Patients older than 18 years old;
- (I) The intervention group ought to receive IPE;
- (C) For comparison groups, we included studies that evaluated subjects who received a placebo;
- (O) The outcome of interest was total cholesterol or TG or LDL-C or very low-density lipoprotein (VLDL) or high-density lipoprotein cholesterol (HDL-C);
- (S) We included studies with no blinding, single-blinded, or double-blinded randomized control and crossover designs. This investigation was limited to articles published in peer-reviewed journals and written in English. We excluded conference papers, master’s dissertations, doctoral theses, descriptive studies, case studies, editorials, and reviews. The time range for the studies included the years between 1999 and 2024.
2.5. Data Extraction
2.6. Search and Selection of Relevant Articles
2.7. Data Items
2.8. Quality Assessment
2.9. Assessment of the Risk of Bias in Individual Studies and Across Studies
2.10. Certainty Assessment (Levels of Evidence)
2.11. Qualitative Analysis (Systematic Review)
2.12. Synthesis of Results and Summary Measures
3. Results
3.1. GRADE (Levels of Evidence)
- Total cholesterol: Moderate certainty;
- TG: Moderate certainty;
- HDL-C: Moderate certainty;
- LDL-C: Moderate certainty.
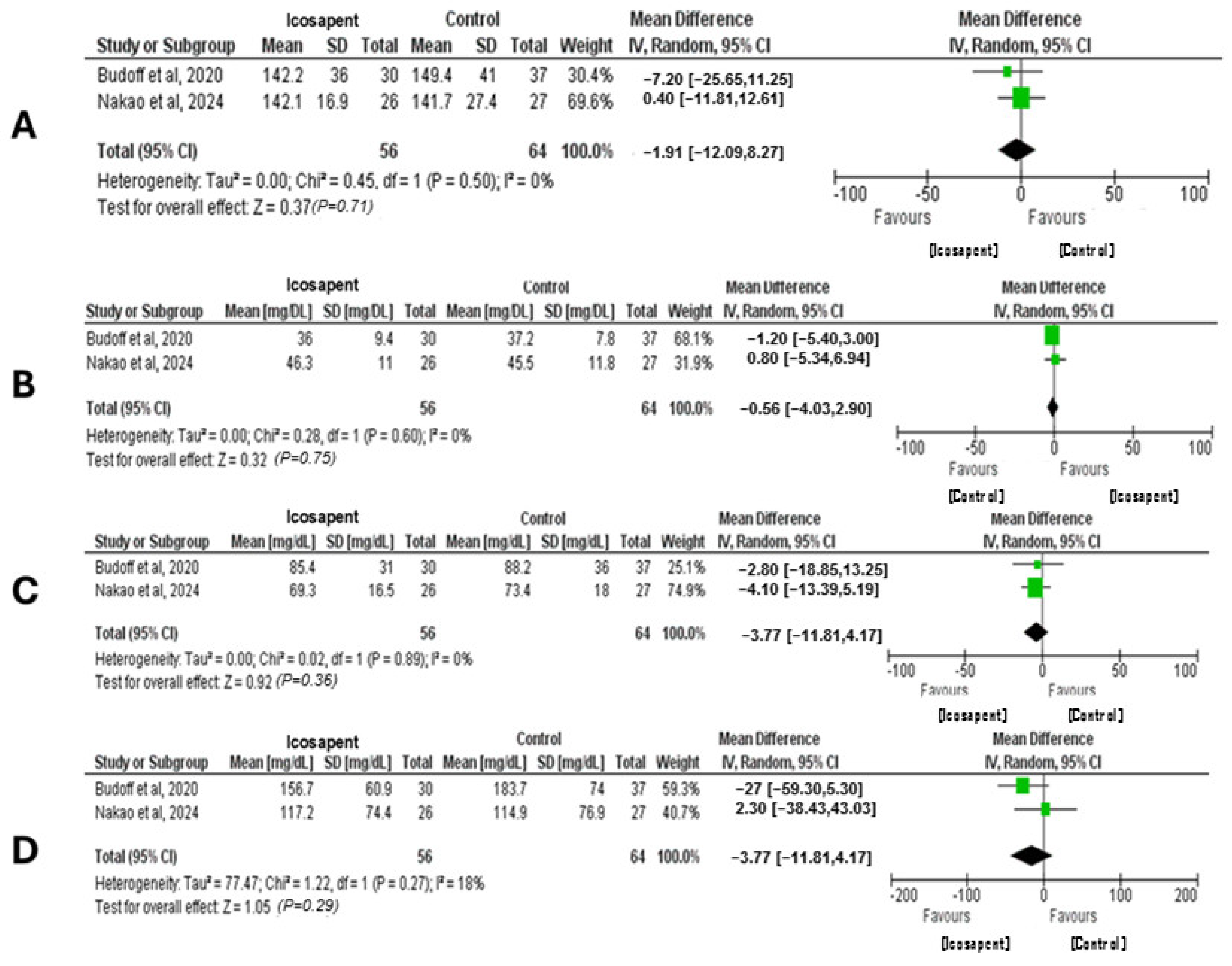
3.2. Quantitative Analysis (Meta-Analysis)
4. Lipid-Lowering Drugs
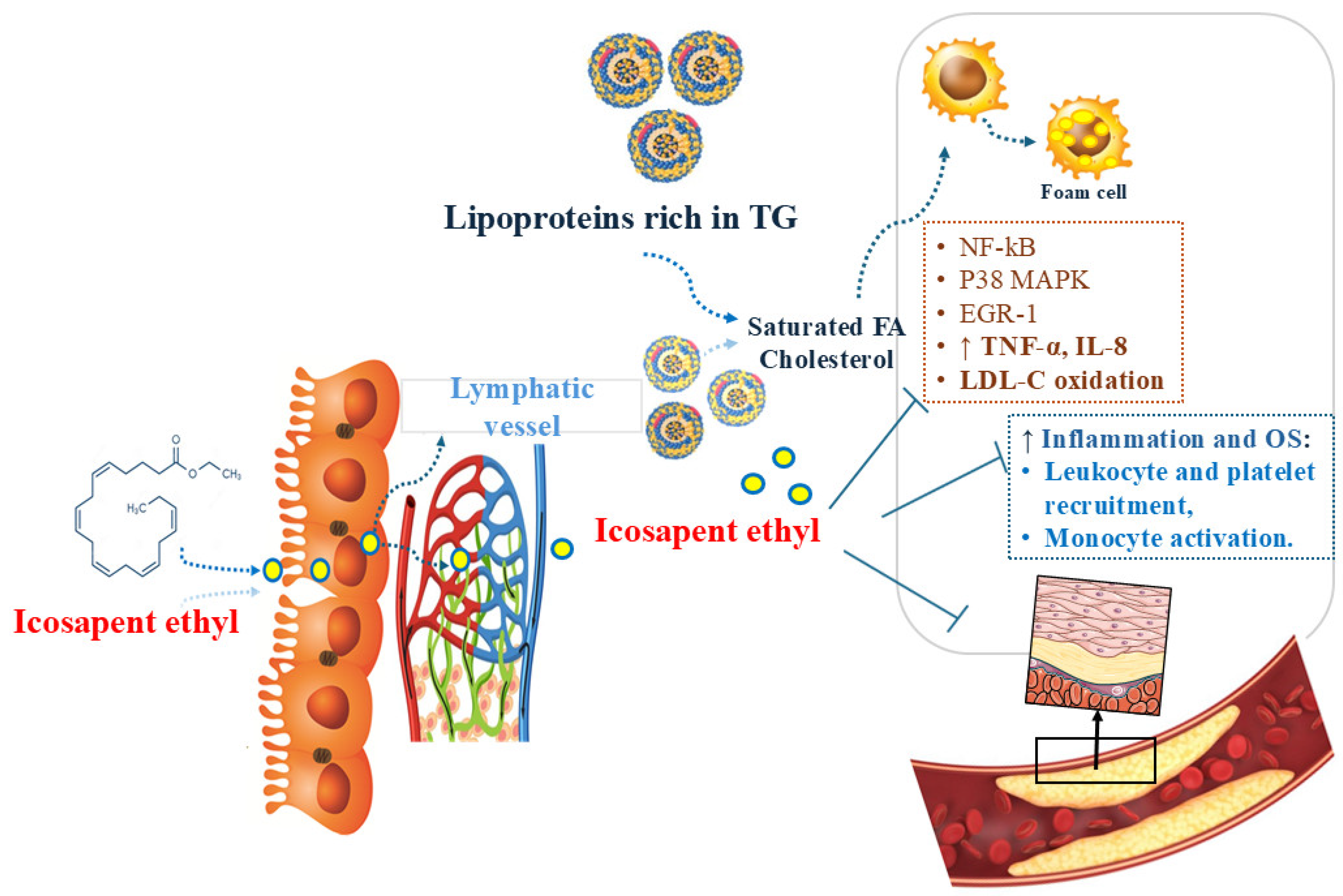
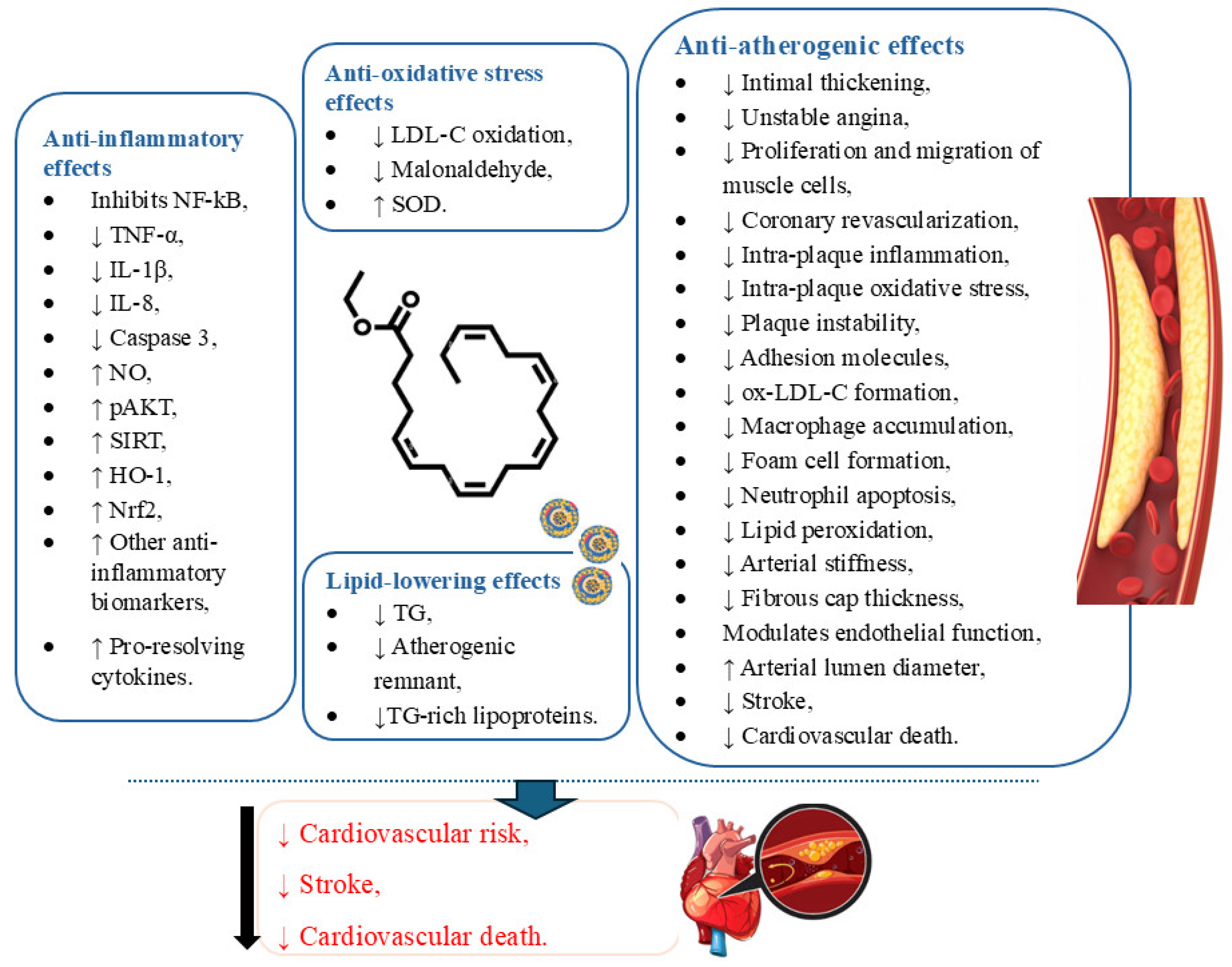
5. Icosapent Ethyl
5.1. Icosapent Ethyl and Inflammation
5.2. Icosapent Ethyl and Oxidative Stress
5.3. Clinical Trials Performed with Icosapent Ethyl
Interpretations of the Meta-Analysis from the Included Clinical Trials Performed with Icosapent Ethyl Assessment
5.4. Limitations and Future Research Endeavors Based on the Included Studies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huston, J.; Schaffner, H.; Cox, A.; Sperry, A.; McGee, S.; Lor, P.; Langley, L.; Skrable, B.; Ashchi, M.; Bisharat, M.; et al. A Critical Review of Icosapent Ethyl in Cardiovascular Risk Reduction. Am. J. Cardiovasc. Drugs 2023, 23, 393–406. [Google Scholar] [CrossRef]
- Fayyaz, S.; Islam, M.; Ahmed, A.; Saeed, H. Evaluation of Anti-Hyperlipidemic Activity of the Seeds Extracts of Ficus carica: In Vitro and In Silico Approaches. Cell Biochem. Funct. 2024, 42, e4124. [Google Scholar] [CrossRef]
- Savo, M.T.; De Amicis, M.; Cozac, D.A.; Cordoni, G.; Corradin, S.; Cozza, E.; Amato, F.; Lassandro, E.; Da Pozzo, S.; Tansella, D.; et al. Comparative Prognostic Value of Coronary Calcium Score and Perivascular Fat Attenuation Index in Coronary Artery Disease. J. Clin. Med. 2024, 13, 5205. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Rodrigues, V.D.; Laurindo, L.F.; Cherain, L.M.A.; de Lima, E.P.; Boaro, B.L.; da Silva Camarinha Oliveira, J.; Chagas, E.F.B.; Catharin, V.C.S.; Dos Santos Haber, J.F.; et al. Targeting AMPK with Irisin: Implications for metabolic disorders, cardiovascular health, and inflammatory conditions—A systematic review. Life Sci. 2025, 360, 123230. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12, 2096. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Moretti, R.C., Jr.; Torres Pomini, K.; Laurindo, L.F.; Sloan, K.P.; Sloan, L.A.; Castro, M.V.M.; Baldi, E., Jr.; Ferraz, B.F.R.; de Souza Bastos Mazuqueli Pereira, E.; et al. Glycolipid Metabolic Disorders, Metainflammation, Oxidative Stress, and Cardiovascular Diseases: Unraveling Pathways. Biology 2024, 13, 519. [Google Scholar] [CrossRef]
- Akhtar, M.; Dawood, M.H.; Khan, M.; Raza, M.; Akhtar, M.; Jahan, S.; Bates, M.; Challa, A.; Ahmed, R.; Naveed, A.K.; et al. Mortality Patterns of Coronary Artery Diseases and Atrial Fibrillation in Adults in the United States from 1999 to 2022: An Analysis Using CDC Wonder. Am. J. Med. Sci. 2025, in press. [CrossRef]
- Ain, Q.; Nawaz, A.; Khan, M.; Sikonja, J.; Batool, H.; Zaheer, R.; Khan, M.I.; Ajmal, M.; Sadiq, F.; Groselj, U. Dyslipidaemia among children and adolescents in Pakistan: A five-year retrospective cohort study based on laboratory data. Lipids Health Dis. 2025, 24, 110. [Google Scholar] [CrossRef]
- Bäck, M. Icosapent ethyl in cardiovascular prevention: Resolution of inflammation through the eicosapentaenoic acid—Resolvin E1—ChemR23 axis. Pharmacol. Ther. 2023, 247, 108439. [Google Scholar] [CrossRef]
- Tofano, R.J.; Pescinni-Salzedas, L.M.; Chagas, E.F.B.; Detregiachi, C.R.P.; Guiguer, E.L.; Araujo, A.C.; Bechara, M.D.; Rubira, C.J.; Barbalho, S.M. Association of Metabolic Syndrome and Hyperferritinemia in Patients at Cardiovascular Risk. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3239–3248. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Dugré, N.; Lindblad, A.J.; Perry, D.; Allan, G.M.; Braschi, É.; Falk, J.; Froentjes, L.; Garrison, S.R.; Kirkwood, J.E.M.; Korownyk, C.S.; et al. Lipid-Lowering therapies for cardiovascular disease prevention and management in primary care: PEER umbrella systematic review of systematic reviews. Can. Fam. Physician Med. Fam. Can. 2023, 69, 701–711. [Google Scholar] [CrossRef]
- Chen, Y.F.; Li, S.; Wang, M.J.; Wu, M.Y.; Du, Z.C.; Wei, L.P. Inclisiran Treatment for Cardiovascular Disease Risk Reduction: A Systematic Review and Meta-Analysis. J. Coll. Physicians Surg. Pak. JCPSP 2024, 34, 1090–1095. [Google Scholar] [CrossRef]
- Tauil, R.B.; Golono, P.T.; de Lima, E.P.; de Alvares Goulart, R.; Guiguer, E.L.; Bechara, M.D.; Nicolau, C.C.T.; Yanaguizawa Junior, J.L.; Fiorini, A.M.R.; Méndez-Sánchez, N.; et al. Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols. Pharmaceuticals 2024, 17, 1354. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Wu, Z.; Huang, X. A significant presence in atherosclerotic cardiovascular disease: Remnant cholesterol: A review. Medicine 2024, 103, e38754. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Proietti, E.; Perrone, G.; Demarco, V.; Galoppi, P.; Stefanutti, C.; Pisciotta, L. Characteristics, Physiopathology and Management of Dyslipidemias in Pregnancy: A Narrative Review. Nutrients 2024, 16, 2927. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Adreak, N.; Sharma, A. Medications for Lipid Control: Statins vs Newer Drugs. Can. J. Cardiol. 2024, 40, S26–S34. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Guo, X.; Zhu, N.; Niu, L.; Ding, X.; Xie, Z.; Chen, X.; Yang, F. Oleic Acid and Eicosapentaenoic Acid Reverse Palmitic Acid-induced Insulin Resistance in Human HepG2 Cells via the Reactive Oxygen Species/JUN Pathway. Genom. Proteom. Bioinform. 2021, 19, 754–771. [Google Scholar] [CrossRef]
- Burger, P.M.; Bhatt, D.L.; Dorresteijn, J.A.N.; Koudstaal, S.; Mosterd, A.; Martens, F.; Steg, P.G.; Visseren, F.L.J. Effects of icosapent ethyl according to baseline residual risk in patients with atherosclerotic cardiovascular disease: Results from REDUCE-IT. Eur. Heart J. Cardiovasc. Pharmacother. 2024, 10, 488–499. [Google Scholar] [CrossRef]
- Streja, E.; Feingold, K.R. Evaluation and Treatment of Dyslipidemia in the Elderly. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; Copyright © 2000–2024; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ahmed, F.; Chester, T.; Sarwar, S. Idiopathic and Metabolic Triggers Leading to Recurrent Acute Pancreatitis in a 30-Year-Old Woman. Hosp. Pharm. 2025, 00185787251321072. [Google Scholar] [CrossRef]
- Aggarwal, R.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Dunbar, R.L.; Ketchum, S.B.; Tardif, J.C.; Martens, F.; Ballantyne, C.M.; et al. Cardiovascular Outcomes with Icosapent Ethyl by Baseline Low-Density Lipoprotein Cholesterol: A Secondary Analysis of the REDUCE-IT Randomized Trial. J. Am. Heart Assoc. 2025, 14, e038656. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef]
- Kaur, G.; Mason, R.P.; Steg, P.G.; Bhatt, D.L. Omega-3 fatty acids for cardiovascular event lowering. Eur. J. Prev. Cardiol. 2024, 31, 1005–1014. [Google Scholar] [CrossRef]
- Gaba, P.; Bhatt, D.L.; Mason, R.P.; Miller, M.; Verma, S.; Steg, P.G.; Boden, W.E. Benefits of icosapent ethyl for enhancing residual cardiovascular risk reduction: A review of key findings from REDUCE-IT. J. Clin. Lipidol. 2022, 16, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Vrablik, M.; Corsini, A.; Tůmová, E. Beta-Blockers for Atherosclerosis Prevention: A Missed Opportunity? Curr. Atheroscler. Rep. 2022, 24, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bakbak, E.; Krishnaraj, A.; Bhatt, D.L.; Quan, A.; Park, B.; Bakbak, A.I.; Bari, B.; Terenzi, K.A.; Pan, Y.; Fry, E.J.; et al. Icosapent ethyl modulates circulating vascular regenerative cell content: The IPE-PREVENTION CardioLink-14 trial. Med 2024, 5, 718–734.e4. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Sutariya, B.; Montenegro, D.M.; Chukwu, M.; Ehsan, P.; Aburumman, R.N.; Muthanna, S.I.; Menon, S.R.; Vithani, V.; Penumetcha, S.S. Emphasis on Icosapent Ethyl for Cardiovascular Risk Reduction: A Systematic Review. Cureus 2022, 14, e32346. [Google Scholar] [CrossRef]
- Curfman, G.; Shehada, E. Icosapent ethyl: Scientific and legal controversies. Open Heart 2021, 8, e001616. [Google Scholar] [CrossRef]
- Trivedi, K.; Le, V.; Nelson, J.R. The case for adding eicosapentaenoic acid (icosapent ethyl) to the ABCs of cardiovascular disease prevention. Postgrad. Med. 2021, 133, 28–41. [Google Scholar] [CrossRef]
- Zhang, W.; Gan, D.; Huo, S.; Chen, P. Unraveling the discrepancies between REDUCE-IT and STRENGTH trials with omega-3 fatty acids: New analytical approaches. Front. Nutr. 2024, 11, 1490953. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Ferrières, J.; Waters, M.; Mortensen, M.B.; Lan, N.S.R.; Wong, N.D. Global eligibility and cost effectiveness of icosapent ethyl in primary and secondary cardiovascular prevention. Front. Cardiovasc. Med. 2023, 10, 1220017. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ (Clin. Res. ed.) 2021, 372, n71. [Google Scholar] [CrossRef]
- Moher, D.; Liberat, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2009, 151, 264–269. [Google Scholar]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Meader, N.; King, K.; Llewellyn, A.; Norman, G.; Brown, J.; Rodgers, M.; Moe-Byrne, T.; Higgins, J.P.; Sowden, A.; Stewart, G. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst. Rev. 2014, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ (Clin. Res. ed.) 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G.; Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Ando, M.; Sanaka, T.; Nihei, H. Eicosapentanoic Acid Reduces Plasma Levels of Remnant Lipoproteins and Prevents in Vivo Peroxidation of LDL in Dialysis Patients. J. Am. Soc. Nephrol. 1999, 10, 2177–2184. [Google Scholar] [CrossRef]
- Cawood, A.L.; Ding, R.; Napper, F.L.; Young, R.H.; Williams, J.A.; Ward, M.J.; Gudmundsen, O.; Vige, R.; Payne, S.P.; Ye, S.; et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Braeckman, R.A.; Ballantyne, C.M.; Kastelein, J.J.; Otvos, J.D.; Stirtan, W.G.; Soni, P.N. Icosapent ethyl, a pure EPA omega-3 fatty acid: Effects on lipoprotein particle concentration and size in patients with very high triglyceride levels (the MARINE study). J. Clin. Lipidol. 2012, 6, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Stein, E.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am. J. Cardiol. 2012, 110, 984–992. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Elajami, T.K.; Ashfaque, H.; Saleh, M.; Bistrian, B.R.; Welty, F.K. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients with Coronary Artery Disease: A Randomized Clinical Trial. J. Am. Heart Assoc. 2017, 6, e006981. [Google Scholar] [CrossRef]
- Sezai, A.; Unosawa, S.; Taoka, M.; Osaka, S.; Obata, K.; Kanno, S.; Sekino, H.; Tanaka, M. Long-Term Comparison of Ethyl Icosapentate vs. Omega-3-Acid Ethyl in Patients with Cardiovascular Disease and Hypertriglyceridemia (DEFAT Trial). Circ. J. Off. J. Jpn. Circ. Soc. 2019, 83, 1368–1376. [Google Scholar] [CrossRef]
- Miller, M.; Ballantyne, C.M.; Bays, H.E.; Granowitz, C.; Doyle, R.T., Jr.; Juliano, R.A.; Philip, S. Effects of Icosapent Ethyl (Eicosapentaenoic Acid Ethyl Ester) on Atherogenic Lipid/Lipoprotein, Apolipoprotein, and Inflammatory Parameters in Patients with Elevated High-Sensitivity C-Reactive Protein (from the ANCHOR Study). Am. J. Cardiol. 2019, 124, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.; Vors, C.; Harris, W.S.; Jackson, K.H.; Tchernof, A.; Couture, P.; Lamarche, B. Comparing the serum TAG response to high-dose supplementation of either DHA or EPA among individuals with increased cardiovascular risk: The ComparED study. Br. J. Nutr. 2019, 121, 1223–1234. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Steg, P.G.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. REDUCE-IT USA: Results From the 3146 Patients Randomized in the United States. Circulation 2020, 141, 367–375. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Dhingra, N.K.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; et al. Icosapent Ethyl Reduces Ischemic Events in Patients with a History of Previous Coronary Artery Bypass Grafting: REDUCE-IT CABG. Circulation 2021, 144, 1845–1855. [Google Scholar] [CrossRef]
- Budoff, M.J.; Bhatt, D.L.; Kinninger, A.; Lakshmanan, S.; Muhlestein, J.B.; Le, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: Final results of the EVAPORATE trial. Eur. Heart J. 2020, 41, 3925–3932. [Google Scholar] [CrossRef]
- Budoff, M.J.; Muhlestein, J.B.; Bhatt, D.L.; Le Pa, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Kinninger, A.; Lakshmanan, S.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: A prospective, placebo-controlled randomized trial (EVAPORATE): Interim results. Cardiovasc. Res. 2021, 117, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.E.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; Doyle, R.T., Jr.; et al. Reduction in Revascularization with Icosapent Ethyl: Insights from REDUCE-IT Revascularization Analyses. Circulation 2021, 143, 33–44. [Google Scholar] [CrossRef]
- Peterson, B.E.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; Doyle, R.T., Jr.; et al. Treatment with Icosapent Ethyl to Reduce Ischemic Events in Patients with Prior Percutaneous Coronary Intervention: Insights From REDUCE-IT PCI. J. Am. Heart Assoc. 2022, 11, e022937. [Google Scholar] [CrossRef]
- Gaba, P.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Juliano, R.A.; Jiao, L.; Doyle, R.T., Jr.; et al. Prevention of Cardiovascular Events and Mortality with Icosapent Ethyl in Patients with Prior Myocardial Infarction. J. Am. Coll. Cardiol. 2022, 79, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Bays, H.E.; Ballantyne, C.M.; Underberg, J.A.; Kastelein, J.J.P.; Johnson, J.B.; Ferguson, J.J. A Head-to-Head Comparison of a Free Fatty Acid Formulation of Omega-3 Pentaenoic Acids Versus Icosapent Ethyl in Adults with Hypertriglyceridemia: The ENHANCE-IT Study. J. Am. Heart Assoc. 2022, 11, e024176. [Google Scholar] [CrossRef]
- Selvaraj, S.; Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Juliano, R.A.; Jiao, L.; Tardif, J.C.; Ballantyne, C.M. Impact of Icosapent Ethyl on Cardiovascular Risk Reduction in Patients with Heart Failure in REDUCE-IT. J. Am. Heart Assoc. 2022, 11, e024999. [Google Scholar] [CrossRef] [PubMed]
- Olshansky, B.; Bhatt, D.L.; Miller, M.; Steg, P.G.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; et al. Cardiovascular Benefits of Icosapent Ethyl in Patients with and without Atrial Fibrillation in REDUCE-IT. J. Am. Heart Assoc. 2023, 12, e026756. [Google Scholar] [CrossRef]
- Miller, M.; Bhatt, D.L.; Steg, P.G.; Brinton, E.A.; Jacobson, T.A.; Jiao, L.; Tardif, J.C.; Ballantyne, C.M.; Budoff, M.; Mason, R.P. Potential effects of icosapent ethyl on cardiovascular outcomes in cigarette smokers: REDUCE-IT smoking. Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 129–137. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Qu, Y.; Zhang, S.; Chen, Y.; Chen, X.; Qi, X.; Liu, P.; Liu, S.; Jiang, S.; et al. Icosapent ethyl therapy for very high triglyceride levels: A 12-week, multi-center, placebo-controlled, randomized, double-blinded, phase III clinical trial in China. Lipids Health Dis. 2023, 22, 71. [Google Scholar] [CrossRef]
- Sayah, N.; Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Jiao, L.; Pineda, A.L.; Doyle, R.T., Jr.; Tardif, J.C.; et al. Icosapent ethyl following acute coronary syndrome: The REDUCE-IT trial. Eur. Heart J. 2024, 45, 1173–1176. [Google Scholar] [CrossRef]
- Szarek, M.; Bhatt, D.L.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Tardif, J.C.; Ballantyne, C.M.; Mason, R.P.; Ketchum, S.B.; Lira Pineda, A.; et al. Lipoprotein(a) Blood Levels and Cardiovascular Risk Reduction with Icosapent Ethyl. J. Am. Coll. Cardiol. 2024, 83, 1529–1539. [Google Scholar] [CrossRef]
- Nakao, K.; Noguchi, T.; Miura, H.; Asaumi, Y.; Morita, Y.; Takeuchi, S.; Matama, H.; Sawada, K.; Doi, T.; Hosoda, H.; et al. Effect of Eicosapentaenoic Acid/Docosahexaenoic Acid on Coronary High-Intensity Plaques Detected Using Noncontrast T1-weighted Imaging: The AQUAMARINE EPA/DHA Randomized Study. J. Atheroscler. Thromb. 2024, 31, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed]
- Zimodro, J.M.; Mucha, M.; Berthold, H.K.; Gouni-Berthold, I. Lipoprotein Metabolism, Dyslipidemia, and Lipid-Lowering Therapy in Women: A Comprehensive Review. Pharmaceuticals 2024, 17, 913. [Google Scholar] [CrossRef]
- Apple, S.J.; Clark, R.; Daich, J.; Gonzalez, M.L.; Ostfeld, R.J.; Toth, P.P.; Bittner, V.; Martin, S.S.; Rana, J.S.; Nasir, K.; et al. Closing the Gaps in Care of Dyslipidemia: Revolutionizing Management with Digital Health and Innovative Care Models. Rev. Cardiovasc. Med. 2023, 24, 350. [Google Scholar] [CrossRef] [PubMed]
- Biteli, P.; Barbalho, S.M.; Detregiachi, C.R.P.; Dos Santos Haber, J.F.; Chagas, E.F.B. Dyslipidemia influences the effect of physical exercise on inflammatory markers on obese women in post-menopause: A randomized clinical trial. Exp. Gerontol. 2021, 150, 111355. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Bueno Ottoboni, A.M.M.; Fiorini, A.M.R.; Guiguer, É.L.; Nicolau, C.C.T.; Goulart, R.A.; Flato, U.A.P. Grape juice or wine: Which is the best option? Crit. Rev. Food Sci. Nutr. 2020, 60, 3876–3889. [Google Scholar] [CrossRef]
- Kounatidis, D.; Tentolouris, N.; Vallianou, N.G.; Mourouzis, I.; Karampela, I.; Stratigou, T.; Rebelos, E.; Kouveletsou, M.; Stamatopoulos, V.; Tsaroucha, E.; et al. The Pleiotropic Effects of Lipid-Modifying Interventions: Exploring Traditional and Emerging Hypolipidemic Therapies. Metabolites 2024, 14, 388. [Google Scholar] [CrossRef]
- Mirzai, S.; Chevli, P.A.; Rikhi, R.; Shapiro, M.D. Familial Hypercholesterolemia: From Clinical Suspicion to Novel Treatments. Rev. Cardiovasc. Med. 2023, 24, 311. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Y.-J.; Qian, J.; Li, J.-J. Landscape of Statin as a Cornerstone in Atherosclerotic Cardiovascular Disease. Rev. Cardiovasc. Med. 2023, 24, 373. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Xu, H. A systematic review of the drug-drug interaction between Statins and Quinolones. BMC Pharmacol. Toxicol. 2024, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Bytyçi, I.; Penson, P.E.; Mikhailidis, D.P.; Wong, N.D.; Hernandez, A.V.; Sahebkar, A.; Thompson, P.D.; Mazidi, M.; Rysz, J.; Pella, D.; et al. Prevalence of statin intolerance: A meta-analysis. Eur. Heart J. 2022, 43, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Siemens, R.; Pryjma, M.; Buchkowsky, S.; Barry, A.R. Real-World effectiveness of monoclonal antibody inhibitors of PCSK9 in patients with heterozygous familial hypercholesterolemia: A retrospective cohort study. Pharmacotherapy 2024, 44, 730–737. [Google Scholar] [CrossRef]
- Li, J.J. Interpretation of Chinese Expert Consensus on the Diagnosis and Management Strategy of Patients with Statin Intolerance: A guiding file for helping to lipid management for Chinese population. J. Geriatr. Cardiol. JGC 2024, 21, 713–715. [Google Scholar] [CrossRef]
- Mercep, I.; Strikic, D.; Hrabac, P.; Pecin, I.; Reiner, Ž. PCSK9 inhibition: From effectiveness to cost-effectiveness. Front. Cardiovasc. Med. 2024, 11, 1339487. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.H. Expanding the therapeutic landscape: Ezetimibe as non-statin therapy for dyslipidemia. Korean J. Intern. Med. 2023, 38, 797–809. [Google Scholar] [CrossRef]
- Lin, L.; Luo, S.; Cai, K.; Huang, H.; Liang, H.; Zhong, L.; Xu, Y. The Effectiveness and Safety of Intensive Lipid-Lowering with Different Rosuvastatin-Based Regimens in Patients at High Cardiovascular Disease Risk: A Nonblind, Randomized, Controlled Trial. Rev. Cardiovasc. Med. 2023, 24, 222. [Google Scholar] [CrossRef] [PubMed]
- ▼Icosapent ethyl for CV risk reduction. Drug Ther. Bull. 2024, 62, 135–140. [CrossRef]
- Sattar, Y.; Alraies, M.C. Cardiovascular outcomes and coronary artery disease prevention secondary to icosapent ethyl: A meta-analysis of randomized clinical trials. Ann. Med. Surg. 2024, 86, 4941–4943. [Google Scholar] [CrossRef]
- Brinton, E.A.; Mason, R.P. Prescription omega-3 fatty acid products containing highly purified eicosapentaenoic acid (EPA). Lipids Health Dis. 2017, 16, 23. [Google Scholar] [CrossRef]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid:arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Subramanian, S. Therapy for Hyperlipidemia. Med. Clin. N. Am. 2024, 108, 881–894. [Google Scholar] [CrossRef]
- Mason, R.P.; Jacob, R.F. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim. Biophys. Acta 2015, 1848, 502–509. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Dawoud, H.; Bhatt, D.L.; Mason, R.P. Eicosapentaenoic Acid Improves Endothelial Nitric Oxide Bioavailability Via Changes in Protein Expression During Inflammation. J. Am. Heart Assoc. 2024, 13, e034076. [Google Scholar] [CrossRef] [PubMed]
- Asulin, M.; Gorodetzer, N.; Fridman, R.; Shelly Ben-Shushan, R.; Cohen, Z.; Beyer, A.M.; Chuyun, D.; Gutterman, D.D.; Szuchman-Sapir, A. 5,6-diHETE lactone (EPA-L) mediates hypertensive microvascular dilation by activating the endothelial GPR-PLC-IP(3) signaling pathway. Biochem. Biophys. Res. Commun. 2024, 700, 149585. [Google Scholar] [CrossRef]
- Morita, S.; Sasaki, H.; Kaneda, Y.; Rogi, T.; Izumo, T.; Nakai, M. Effects of Combining Docosahexaenoic Acid and Eicosapentaenoic Acid with Sesame Lignan on Vascular Endothelial Function. J. Nutr. Sci. Vitaminol. 2023, 69, 370–376. [Google Scholar] [CrossRef]
- Khattib, A.; Shmet, M.; Ashkar, R.; Hayek, T.; Khatib, S. Novel bioactive lipids enhanced HDL-mediated cholesterol efflux from macrophages through the ABCA1 receptor pathway. Chem. Phys. Lipids 2024, 258, 105367. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Sasaki, J.; Ishibashi, S.; Birou, S.; Daida, H.; Dohi, S.; Egusa, G.; Hiro, T.; Hirobe, K.; Iida, M.; et al. Executive summary of the Japan Atherosclerosis Society (JAS) guidelines for the diagnosis and prevention of atherosclerotic cardiovascular diseases in Japan -2012 version. J. Atheroscler. Thromb. 2013, 20, 517–523. [Google Scholar] [CrossRef]
- Bays, H.E.; Jones, P.H.; Orringer, C.E.; Brown, W.V.; Jacobson, T.A. National Lipid Association Annual Summary of Clinical Lipidology 2016. J. Clin. Lipidol. 2016, 10, S1–S43. [Google Scholar] [CrossRef]
- Pearson, G.J.; Thanassoulis, G.; Anderson, T.J.; Barry, A.R.; Couture, P.; Dayan, N.; Francis, G.A.; Genest, J.; Grégoire, J.; Grover, S.A.; et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021, 37, 1129–1150. [Google Scholar] [CrossRef]
- Katkenov, N.; Mukhatayev, Z.; Kozhakhmetov, S.; Sailybayeva, A.; Bekbossynova, M.; Kushugulova, A. Systematic Review on the Role of IL-6 and IL-1β in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2024, 11, 206. [Google Scholar] [CrossRef]
- Hong, C.G.; Florida, E.; Li, H.; Parel, P.M.; Mehta, N.N.; Sorokin, A.V. Oxidized low-density lipoprotein associates with cardiovascular disease by a vicious cycle of atherosclerosis and inflammation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 9, 1023651. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Sherratt, S.C.R.; Eckel, R.H. Omega-3-fatty acids: Do they prevent cardiovascular disease? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101681. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, D.; Lei, Q.; Lu, A.; He, X. Roles of Resolvins in Chronic Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 14883. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Zamorano, J.L.; Parhofer, K.G. Reducing residual cardiovascular risk in Europe: Therapeutic implications of European medicines agency approval of icosapent ethyl/eicosapentaenoic acid. Pharmacol. Ther. 2022, 237, 108172. [Google Scholar] [CrossRef]
- Chait, A.; Eckel, R.H.; Vrablik, M.; Zambon, A. Lipid-lowering in diabetes: An update. Atherosclerosis 2024, 394, 117313. [Google Scholar] [CrossRef]
- Abbas, N.A.T.; El-Sayed, S.S.; Abd El-Fatah, S.S.; Sarhan, W.M.; Abdelghany, E.M.A.; Sarhan, O.; Mahmoud, S.S. Mechanistic aspects of ameliorative effects of Eicosapentanoic acid ethyl ester on methotrexate-evoked testiculopathy in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 357–369. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem. Biophys. Res. Commun. 2018, 496, 335–338. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Bhatt, D.L.; Mason, R.P. A biological rationale for the disparate effects of omega-3 fatty acids on cardiovascular disease outcomes. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102450. [Google Scholar] [CrossRef]
- Abdelsameea, A.A.; Alsemeh, A.E.; Alabassery, N.; Samy, W.; Fawzy, A.; Abbas, N.A.T. Icosapent ethyl alleviates acetic acid-induced ulcerative colitis via modulation of SIRT1 signaling pathway in rats. Int. Immunopharmacol. 2023, 115, 109621. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Q.; Liao, X.; Elbelt, U.; Weylandt, K.H. The effects of omega-3 fatty acids in type 2 diabetes: A systematic review and meta-analysis. Prostaglandins Leukot. Essent. Fat. Acids 2022, 182, 102456. [Google Scholar] [CrossRef] [PubMed]
- Selvi, M.K.; Sowmya, B.; Kannan, T.; Latha, M.; Jena, I.; Arun Kumar, V.; Vijayaraj, P. Chapter 2—Advances in personalized food and nutrition. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 31–60. [Google Scholar]
- Niforou, A.; Konstantinidou, V.; Naska, A. Genetic Variants Shaping Inter-individual Differences in Response to Dietary Intakes-A Narrative Review of the Case of Vitamins. Front. Nutr. 2020, 7, 558598. [Google Scholar] [CrossRef] [PubMed]
- Laurindo, L.F.; Pomini, K.T.; de Lima, E.P.; Laurindo, L.F.; Rodrigues, V.D.; da Silva Camarinha Oliveira, J.; Araújo, A.C.; Guiguer, E.L.; Rici, R.E.G.; Maria, D.A.; et al. Isoorientin: Unveiling the hidden flavonoid’s promise in combating cancer development and progression—A comprehensive review. Life Sci. 2025, 360, 123280. [Google Scholar] [CrossRef]
- Michaeli, D.T.; Michaeli, J.C.; Boch, T.; Michaeli, T. Cost-Effectiveness of Icosapent Ethyl, Evolocumab, Alirocumab, Ezetimibe, or Fenofibrate in Combination with Statins Compared to Statin Monotherapy. Clin. Drug Investig. 2022, 42, 643–656. [Google Scholar] [CrossRef]
- Weintraub, W.S.; Bhatt, D.L.; Zhang, Z.; Dolman, S.; Boden, W.E.; Bress, A.P.; Bellows, B.K.; Derington, C.G.; Philip, S.; Steg, G.; et al. Cost-Effectiveness of Icosapent Ethyl in REDUCE-IT USA: Results From Patients Randomized in the United States. J. Am. Heart Assoc. 2024, 13, e032413. [Google Scholar] [CrossRef]
- Lachaine, J.; Charron, J.N.; Gregoire, J.C.; Hegele, R.A.; Leiter, L.A. Cost-Effectiveness of Icosapent Ethyl (IPE) for the Reduction of the Risk of Ischemic Cardiovascular Events in Canada. Clin. Outcomes Res. 2023, 15, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, W.S.; Bhatt, D.L.; Zhang, Z.; Dolman, S.; Boden, W.E.; Bress, A.P.; King, J.B.; Bellows, B.K.; Tajeu, G.S.; Derington, C.G.; et al. Cost-Effectiveness of Icosapent Ethyl for High-risk Patients with Hypertriglyceridemia Despite Statin Treatment. JAMA Netw. Open 2022, 5, e2148172. [Google Scholar] [CrossRef]
- Yang, X.H.; Tu, Q.M.; Li, L.; Guo, Y.P.; Wang, N.S.; Jin, H.M. Triglyceride-Lowering therapy for the prevention of cardiovascular events, stroke, and mortality in patients with diabetes: A meta-analysis of randomized controlled trials. Atherosclerosis 2024, 394, 117187. [Google Scholar] [CrossRef]
| Ref. | Model/Country | Population | Intervention/Comparison | Outcomes | Side Effects |
|---|---|---|---|---|---|
| [42] | Clinical study, 7-month/Japan. | In dialysis treatment, 38 subjects, 5♀, 33♂, 38–65 y. | The patients were randomly allocated to either the control group or the treatment group, which received a highly purified EPA in EE form (ethyl all-cis-5,8,11,14,17-icosapentanoate) at 1800 mg/day. The study was conducted in the baseline observation, treatment (3 months), and washout period (3 months). | Treatment with EPA significantly reduced the levels of both remnant lipoproteins and ox-LDL-C by 52% and 38%, respectively. Furthermore, the gel filtration chromatography of lipoproteins showed that the treatment also normalized other potential abnormalities in lipoproteins. | One patient reported mild headache and diarrhea, but these symptoms soon disappeared without any treatment. |
| [43] | Randomized, double-blind, placebo-controlled/United Kingdom. | 121 subjects, 68♂, 32♀,41–91 y, were destined to undergo carotid endarterectomy. | Sixty-one subjects were randomized to receive control capsules (olive oil), and the other sixty subjects received n-3 PUFA EE capsules twice daily for 21 days until surgery. | Plaques from patients in the n-3 PUFA group contained fewer foam cells than those in the control group. EPA content in plaque was inversely associated with plaque instability, inflammation, and the number of T cells; plaques showed significantly lower levels of IL-6 and intercellular adhesion molecule. | No side effects were reported. |
| [44] | Phase three, multi-center, placebo-controlled, randomized, double-blind, 12-week study/United States of America. | 177 subjects, 133♂, 44♀, with very high TG levels (≥500 mg/dL and ≤2000 mg/dL). | Subjects were randomized into three groups: IPE 4 g/day, IPE 2 g/day, and placebo. After 12 weeks, nuclear magnetic resonance spectroscopy measured lipoprotein particle concentrations and sizes. | The IPE 4 g/day group showed a significant reduction in concentrations of VLDL (−27.9%), total LDL-C (−16.3%), small LDL-C (−25.6%), and total HDL-C (−7.4%), as well as a reduction in VLDL particle size (−8.6%). | No side effects were reported. |
| [45] | Phase three, multi-center, placebo-controlled, randomized, double-blinded, 12-week clinical trial/United States of America. | 702 subjects, 431♂, 271♀, >18 y in high-risk statin-treated patients with high TG levels (>200 and <500 mg/dL). | Subjects were randomized to 3 groups: one receiving AMR101 (ω-3 fatty acid agent containing > 96% pure IPE) 4 g/day for 12 weeks, another receiving AMR101 2 g/day for 12 weeks, or a placebo group. | AMR101 4 and 2 g/day significantly decreased TG levels by 21.5% and 10.1%, respectively, and non-HDL-C by 13.6% and 5%, respectively. Additionally, AMR101 4 g/day promoted more significant decreases in TG and non-HDL-C in patients with higher-efficacy statin regimens and more substantial reductions in TG in patients with higher baseline TG levels. | The reported side effects included diarrhea, nausea, nasopharyngitis, arthralgia, and belching. |
| [46] | Randomized, controlled, parallel, 30-month clinical trial/United States of America. | 285 subjects, 21–80 y, and with stable coronary artery disease on statins. | Subjects were randomized into ω-3 EE (1.86 g of EPA and 1.5 g of DHA per day, n = 143) or the no ω-3 group (control, n = 142) for 30 months. | Controls showed significant progression of fibrous plaque, whereas the ω-3 EE group presented no modifications. Among the subjects on low-intensity statin therapy, ω-3 EE promoted the attenuation of fibrous plaque progression. Patients on high-intensity statin therapy showed no changes in plaque in either treatment group. | No serious musculoskeletal events in the ω-3 EE group compared with the control. |
| [47] | Randomized, single-blinded, 3-year clinical trial/Japan. | 87 subjects, 59♂, 28♀, 58–80 y with untreated hypertriglyceridemia (TG level ≥ 150 mg/dL) and who had undergone cardiac surgery. | Subjects were randomized to the EPA group, which received 1.8 g of IPE 3×/day, or the EPA + DHA group, which received 2 g of IPE + DHA once daily for 3 years. | Six months after completing the intervention, the results showed that TG, remnant-like particles cholesterol, ox-LDL-C, and cystatin-C levels were significantly lower in the EPA + DHA group than in the EPA group. | In the EPA group, n = 2 reported arrhythmia, and n = 1 HF; in the EPA + DHA group, 1 patient reported HF. |
| [48] | Phase three, randomized, double-blind, placebo-controlled clinical study/United States of America. | 702 participants, 136♂, 110♀ with a history of high CV risk, TG 200–499 mg/dL, LDL-C 40–99 mg/dL controlled on statin therapy and elevated hsCRP (hsCRP ≥ 2 mg/L). | Participants were randomized to IPE 4 g/day (n = 126) and placebo (n = 120). | IPE 4 g/day significantly reduced fasting TG by 19.9% (p < 0.0001). Apolipoprotein B, apolipoprotein C-III, and markers of oxidation and inflammation, including hsCRP, ox-LDL-C, and Lp-PLA2, were significantly decreased with IPE 4 g/day compared to placebo (p = 0.0213 to <0.0001). | Nausea, diarrhea, nasopharyngitis, and arthralgia. |
| [49] | Double-blind, controlled, crossover, randomized study/Canada. | 154 subjects, 85♀, 36♂, 36–68 y, with WC ≥ 80 cm for ♀, ≥94 cm for ♂ and serum hsCRP concentrations > 1 and <10 mg/L. | The study was divided into 3 phases, each lasting 10 weeks, separated by 9-week washouts. In total, 3 × 1 g of 90% purified long-chain n-3 PUFA capsule/day was provided throughout the 3 phases: (1) 2.7 g/day DHA; (2) 2.7 g/day EPA; (3) 0 g/day DHA + EPA (3 g/day of corn oil). The objective was to compare the effect of DHA and EPA on the plasma concentrations of hsCRP. | The overall mean reduction in serum TG concentrations was more significant with DHA than with EPA (45 and 32%, respectively; p < 0.001). | No side effects were reported. |
| [50] | Phase three B, international, multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group trial/United States of America. | 3146 subjects, 1015♀, 2131♂, 59–71 y statin-treated patients with TG ≥ 135 and <500 mg/dL, LDL-C > 40 and ≤100 mg/dL, and a history of atherosclerosis or DM with additional CV risk factors. | Patients were randomized into the placebo group (n = 1598) and the IPE group (n = 1548), receiving 4 g/day of IPE. Subjects were followed for an average of 4.9 years. | Results showed that all the following factors were significantly reduced in the IPE group: CV death (6.7% to 4.7%; p = 0.007), MI (8.8% to 6.7%; p = 0.01), stroke (4.1% to 2.6%; p = 0.02), and all-cause mortality (9.8% to 7.2% p = 0.004). | Diarrhea, constipation, dysphagia, belching, AF, and bleeding are treatment-emergent adverse events of any type. |
| [51] | Phase three B, double-blind study/United States of America (leading country). | 8179 patients, with a stable dose of statin for at least 4 weeks at baseline, LDL-C between 41 mg/dL and 100 mg/dL, and TG between 135 mg/dL and 500 mg/dL. | Among the 8179 randomized patients, those who underwent coronary artery revascularization were selected and randomly assigned to receive either IPE 4 g/day (n = 897) or placebo (n = 940). | There was a significant reduction in the primary outcome; the HR was 0.76, with a p-value of 0.004. In the secondary outcome, HR = 0.69 and p = 0.001. In the first outcome, plus subsequent or recurrent ischemic events, the HR was 0.64 with p = 0.0002, compared to placebo. | Bleeding or AF requiring hospitalization for at least 24 h are the main adverse effects. |
| [52] | Prospective, placebo-controlled, randomized, double-blind study/United States of America. | 80 subjects, 36♂, 31♀, 51–63 y, with coronary atherosclerosis by CCTA (≥1 angiographic stenosis with ≥20% narrowing), on stable statin therapy with LDL-C levels of 40–115 mg/dL and persistently high levels of TG (135–499 mg/dL). | Patients were randomized 1:1 into the IPE 4 g/day (n = 30) and placebo groups (n = 37); additionally, they underwent an MDCT examination to assess plaque volume in CCTA at 0, 9, and 18 months. | At 9 months, there was no significant change in LAP between groups (p = 0.469). There was a slowdown in total non-calcified plaque (sum of LAP, fibrofatty, and fibrous plaque) (p = 0.010), total plaque (non-calcified + calcified plaque) (15% vs. 26%, p = 0.0004), fibrous plaque (17% vs. 40%, p = 0.011), and calcified plaque (−1% vs. 9%, p = 0.001). | The article reported no adverse effects. |
| [53] | Multi-center randomized, double-blind, placebo-controlled trial/United States of America. | Eighty subjects, 31♀, 37♂, 51–63 y, with known coronary atherosclerosis, elevated fasting TG levels (135–499 mg/dL), and LDL-C levels between ≥40 and ≤115 mg/dL, as well as on stable statin therapy. | Participants were divided into an IPE or a placebo group to evaluate the effect of IPE 4 g/day on coronary plaque progression, as determined by CCTA, compared to an oil placebo mineral. Patients underwent three MDCT exams: the first at the beginning of the study, an intermediate one at 9 months, and a final one at 18 months. | Significant reductions in LAP in the IPE group. Total plaque (−9% with IPE vs. +11% with placebo, p = 0.002), total non-calcified plaque (−19% vs. +9%, p = 0.0005), fibrofatty (−34% vs. +32%, p = 0.0002), fibrous (−20% vs. 1%, p = 0.003), and calcified plaque (−1% vs. +15%, p = 0.053. | No side effects were reported. |
| [54] | Phase three B, double-blind, randomized, placebo-controlled trial/United States of America (leading country). | 8179 patients, with a stable statin dose for at least 4 weeks at baseline, LDL-C between 41 mg/dL and 100 mg/dL, and TG between 135 mg/dL and 499 mg/dL. | Patients were randomized into two groups: IPE 4 g/day (n = 4000) and placebo (n = 4000), and underwent the intervention for an average of 4.8 years. | IPE significantly reduced the need for PCI, with an HR of 0.68 and p < 0.0001, and the need for CABG surgery, with an HR of 0.61 and p = 0.0005. | Minor bleeding events, with a tendency toward increased significant bleeding, and a slightly increased risk of AF. |
| [55] | Randomized double-blind, multi-center, placebo-controlled trial/United States of America (leading country). | 8179 patients, with a stable dose of statin for at least 4 weeks at baseline, LDL-C between 41 mg/dL and 100 mg/dL, and TG between 135 mg/dL and 499 mg/dL. | Patients randomized by REDUCE-IT who underwent PCI were distributed into the IPE 4 g/day and placebo groups, followed for a median of 4.8 years. | 34% reduction in the primary composite outcome and a 34% reduction in the primary secondary composite outcome of CV death, non-fatal MI, or non-fatal stroke, presenting HR = 0.66. | Documented AF that required emergency treatment or hospitalization. |
| [56] | Randomized double-blind, multi-center, placebo-controlled trial/United States of America (leading country). | 8179 patients, with a stable dose of statin for at least 4 weeks at baseline, LDL-C between 41 mg/dL and 100 mg/dL, and TG between 135 mg/dL and 499 mg/dL. | Among those randomized, 3693 patients with a history of previous MI were assigned to either IPE 4 g/day (n = 1870) or placebo (n = 1823) groups, followed for an average of 4.8 years. | Significant reduction in the incidence of CV death, MI, stroke, coronary revascularization, or hospitalization for unstable angina from 26.1% to 20.2% compared to placebo (HR: 0.74; p = 0.00001). The secondary outcome (CV death, MI, or stroke) was reduced from 18.0% to 13.3% (HR: 0.71; p = 0.00006). | The article reported no adverse effects. |
| [57] | Open, randomized, 2-way crossover clinical trial/United States of America. | 100 subjects, 43♂, 57♀, mean 60.3 y, with fasting TG of 150–499 mg/dL. | The trial consisted of two 28-day treatment periods, separated by an interval of at least 28 days. Subjects were randomized to EPA-EE (first period) and EPA + DPA-FFA (second period) or vice versa, taking two capsules twice daily with meals. | EPA-EE increased hsCRP by 8.5% (p = 0.034). EPA + DPA-FFA increased DHA by 1.7%; EPA-EE decreased DHA by 3.3% (p = 0.011). | Subjects receiving EPA + DPA-FFA reported nausea, diarrhea, belching, and arthralgia. Subjects receiving EPA-EE reported diarrhea, arthralgia, and constipation. |
| [58] | Randomized double-blind, multi-center, placebo-controlled trial/United States of America (leading country). | 8179 patients, with a stable dose of statin for at least 4 weeks at baseline, LDL-C between 41 mg/dL and 100 mg/dL, and TG between 135 mg/dL and 499 mg/dL. | Patients were randomized into IPE (n = 703) and placebo (n = 743). | In patients with HF, IPE reduced TG (33.5 mg/dL, or 15.4% p < 0.0001) and hsCRP (35.1% p < 0.0001) from baseline up to 2 years compared to placebo. | The article reported no adverse effects. |
| [59] | Phase three B, international, multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group trial/United States of America (leading country). | 8179 subjects, 59–71 y statin-treated patients with TG levels between ≥135 and <499 mg/dL, LDL-C > 41 and ≤100 mg/dL, and a history of atherosclerosis or DM with additional CV risk factors. | Patients were randomized into the placebo group (n = 4000) and the IPE group (n = 4000), which received 4 g/day of IPE (2 g twice daily with meals). Subjects were followed for an average of 4.9 years. | AF hospitalization event rates were higher in patients with prior AF (12.5% versus 6.3%, IPE versus placebo; p = 0.007) than those without prior AF (2.2% versus 1.6%, IPE versus placebo; p = 0.09). | Diarrhea, constipation, dysphagia, belching, AF, and bleeding are treatment-emergent adverse events of any type. |
| [60] | Phase three B, international, multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group trial/United States of America (leading country). | 8179 subjects, 59–71 y statin-treated patients with TG levels between ≥135 and <499 mg/dL, LDL-C > 41 and ≤100 mg/dL, and a history of atherosclerosis or DM with additional CV risk factors. | Patients were randomized into two groups: the placebo group (n = 4000) and the IPE group (n = 4000), which received 4 g/day of IPE (2 g twice daily with meals). Subjects were followed for an average of 4.9 years. Then, in the post hoc analysis, the subjects were classified into three groups: current smokers (n = 1241), former smokers (n = 3672), and never smokers (n = 3264). | Significant reductions in time to CV death, non-fatal MI, non-fatal stroke, coronary revascularization, or hospitalization for unstable angina (p < 0.0001) and in total events (p < 0.0001) were observed in current and former smokers who used IPE. The estimated rates of first occurrences were 23.0% in former smokers, in contrast to 25.7% in never smokers on placebo. Also, there were reductions in CV death as well as non-fatal MI or stroke (p < 0.0001) in both current and former smoker groups. | Diarrhea, constipation, dysphagia, belching, AF, and bleeding are treatment-emergent adverse events of any type. |
| [61] | Randomized, double-blind, multi-center, placebo-controlled clinical trial, phase three/China. | 373 subjects, 93♀, 280♂, median 48.9 y, with reduced TG levels by 5.6–22.6 mmol/L with stable diet and physical activity. | Subjects were randomized into three groups: (1) IPE 2 g/day: one IPE, 1 g and one placebo capsule 2×/day; (2) IPE 4 g/day group, two IPE capsules, each with a dosage of 1 g 2×/day; and placebo group, two placebo capsules 2×/day/6–8 weeks, and if mean TG levels were 5.6–22.6 mmol/L after screening, these participants were selected for treatment phase/12 weeks. | There was a reduced TG level by 28.4%, 12.0%, and 6.2% in the groups receiving placebo, IPE 2 g/day, and IPE 4 g/day, respectively. IPE at a dose of 4 g/day reduced total cholesterol levels by 19.9% compared to baseline (p < 0.001), and IPE at a dosage of 2 g/day led to a reduction in TG level of 5.0% (p = 0.361). | Diarrhea and urticaria. |
| [62] | Phase three B, international, multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group trial/United States of America (leading country). | 8179 subjects, 59–71 y statin-treated patients with TG levels between ≥135 and <499 mg/dL, LDL-C > 41 and ≤100 mg/dL, and a history of atherosclerosis or DM with additional CV risk factors. | Patients were randomized into two groups: the placebo group (n = 4000) and the IPE group (n = 4000), which received 4 g/day of IPE (2 g twice daily with meals). Subjects were followed for an average of 4.9 years. Then, in the post hoc analysis, the effects of IPE use in patients with recent (<12 months) ACS (n = 840) were evaluated. | IPE reduced the incidence of the first primary composite outcome by 37% (p = 0.002) and of the first key secondary composite outcome by 36% (p = 0.01). Also, IPE lowered CV death and non-fatal MI by 36% (p = 0.03) and lowered urgent or emergent revascularization in 44% (p = 0.009). | Diarrhea, constipation, dysphagia, belching, AF, and bleeding are treatment-emergent adverse events of any type. |
| [63] | Phase three B, international, multi-center, prospective, randomized, double-blinded, placebo-controlled, parallel-group trial/United States of America (leading country). | 8179 subjects, 59–71 y statin-treated patients with TG levels between ≥135 and <499 mg/dL, LDL-C > 41 and ≤100 mg/dL, and a history of atherosclerosis or DM with additional CV risk factors. | Patients were randomized into the placebo group (n = 4000) and IPE group (n = 4000), which received 4 g/day of IPE (2 g 2×/day with meals). Subjects were followed for an average of 4.9 years. Then, the post hoc analysis evaluated the CV benefit of IPE associated with different Lp(a) levels. | Lp(a) was significantly related to the first and total major adverse CV events (p < 0.0001). IPE significantly reduced the first major adverse CV events in subgroups with ≥50 or <50 mg/dL concentrations. | Diarrhea, constipation, dysphagia, belching, AF, and bleeding are treatment-emergent adverse events of any type. |
| [64] | Single-center, triple-arm, randomized, controlled, 12-month, open-label trial/Japan. | 84 subjects, 13♀, 71♂, medium 68.2 y, and with established coronary artery disease, on statin therapy and LDL-C levels < 100 mg/dL. | Subjects were randomly allocated to one of three groups: the 2 g/day EPA/DHA group, the 4 g/day EPA/DHA group, or the no-treatment group. After 12 months of treatment, atherosclerotic plaque was assessed by CMR. | There was a reduction in the PMR of −0.15 in the 2 g/day and 4 g/day EPA/DHA groups compared to the baseline; in the untreated group, no changes were observed. | Not reported. |
| [27] | Prospective, 3-month, open-label, randomized study/Canada. | 70 subjects, 56♂, 14♀, 56–77 y in statin treatment, with TG ≥ 1.50 and <5.6 mmol/L and either ASCVD or DM2 with additional CV risk factors. | Subjects were randomized to the IPE (4 g/day) group or the usual care group. Then, vascular regenerative cells with ALDHhi were isolated from blood samples collected at baseline and 3-month follow-up visits and characterized using lineage-specific cell surface markers. | IPE increased the mean frequency of ALDHhi side scatter low CD133+ cells (p = 0.02), decreased overall ALDHhi side scatter low cell frequency, reduced ROS, and increased ALDHhi side scatter granulocyte precursor cell content. | Gastrointestinal events, n = 2, underwent PCI; one was hospitalized for acute kidney injury. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, N.M.; Oliveira, M.V.B.; Quesada, K.; Haber, J.F.d.S.; José Tofano, R.; Rubira, C.J.; Zutin, T.L.M.; Direito, R.; Pereira, E.d.S.B.M.; de Oliveira, C.M.; et al. Assessing Omega-3 Therapy and Its Cardiovascular Benefits: What About Icosapent Ethyl? A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 601. https://doi.org/10.3390/ph18040601
Machado NM, Oliveira MVB, Quesada K, Haber JFdS, José Tofano R, Rubira CJ, Zutin TLM, Direito R, Pereira EdSBM, de Oliveira CM, et al. Assessing Omega-3 Therapy and Its Cardiovascular Benefits: What About Icosapent Ethyl? A Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(4):601. https://doi.org/10.3390/ph18040601
Chicago/Turabian StyleMachado, Nathália Mendes, Maria Vitória Barroso Oliveira, Karina Quesada, Jesselina Francisco dos Santos Haber, Ricardo José Tofano, Claudio José Rubira, Tereza Lais Menegucci Zutin, Rosa Direito, Eliana de Souza Bastos Mazuqueli Pereira, Camila Marcondes de Oliveira, and et al. 2025. "Assessing Omega-3 Therapy and Its Cardiovascular Benefits: What About Icosapent Ethyl? A Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 4: 601. https://doi.org/10.3390/ph18040601
APA StyleMachado, N. M., Oliveira, M. V. B., Quesada, K., Haber, J. F. d. S., José Tofano, R., Rubira, C. J., Zutin, T. L. M., Direito, R., Pereira, E. d. S. B. M., de Oliveira, C. M., Goulart, R. d. A., Valenti, V. E., Sloan, K. P., Sloan, L. A., Laurindo, L. F., & Barbalho, S. M. (2025). Assessing Omega-3 Therapy and Its Cardiovascular Benefits: What About Icosapent Ethyl? A Systematic Review and Meta-Analysis. Pharmaceuticals, 18(4), 601. https://doi.org/10.3390/ph18040601










