Polycystic Ovary Syndrome Attenuates TSH-Lowering Effect of Metformin in Young Women with Subclinical Hypothyroidism
Abstract
1. Introduction
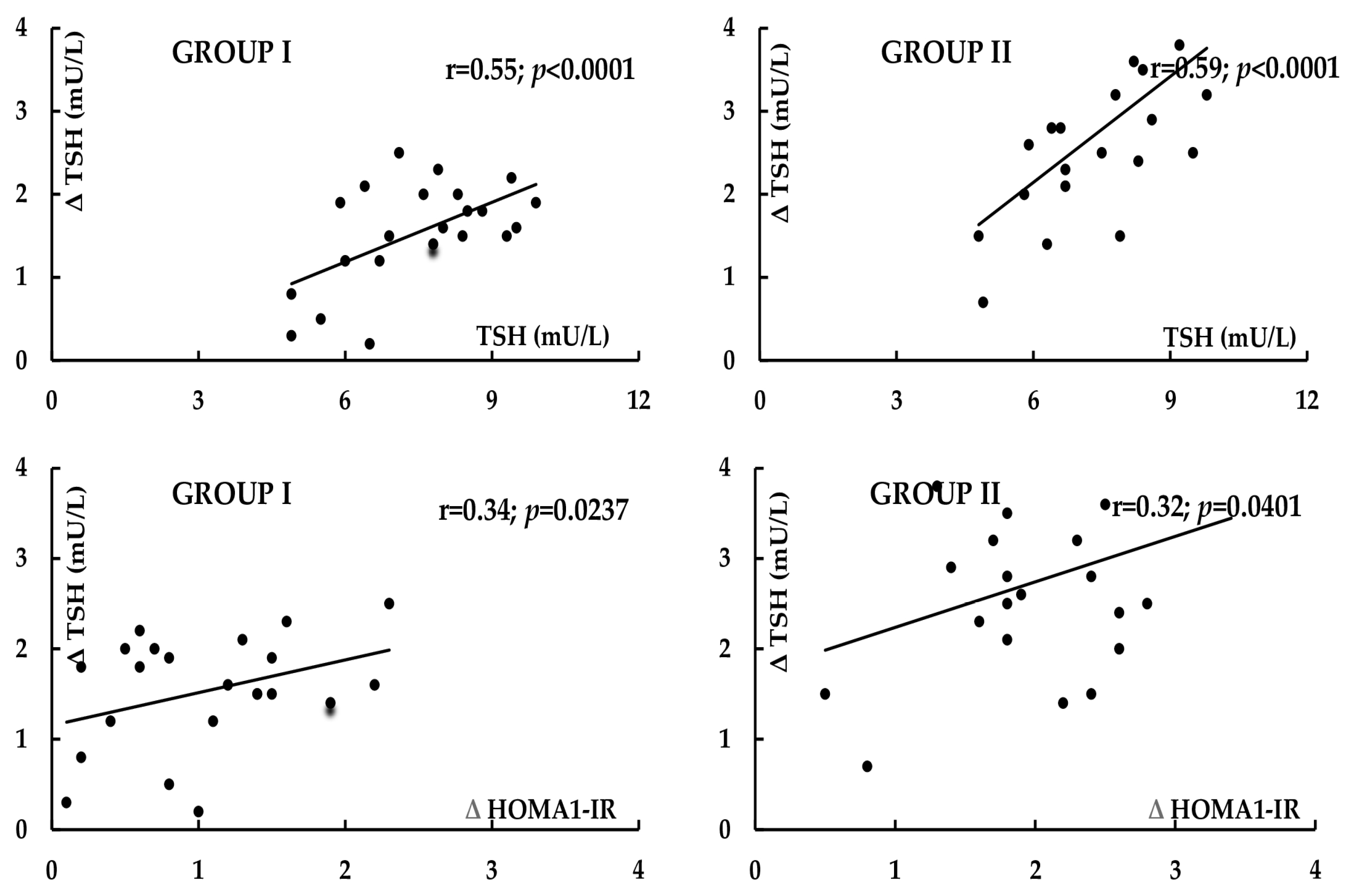
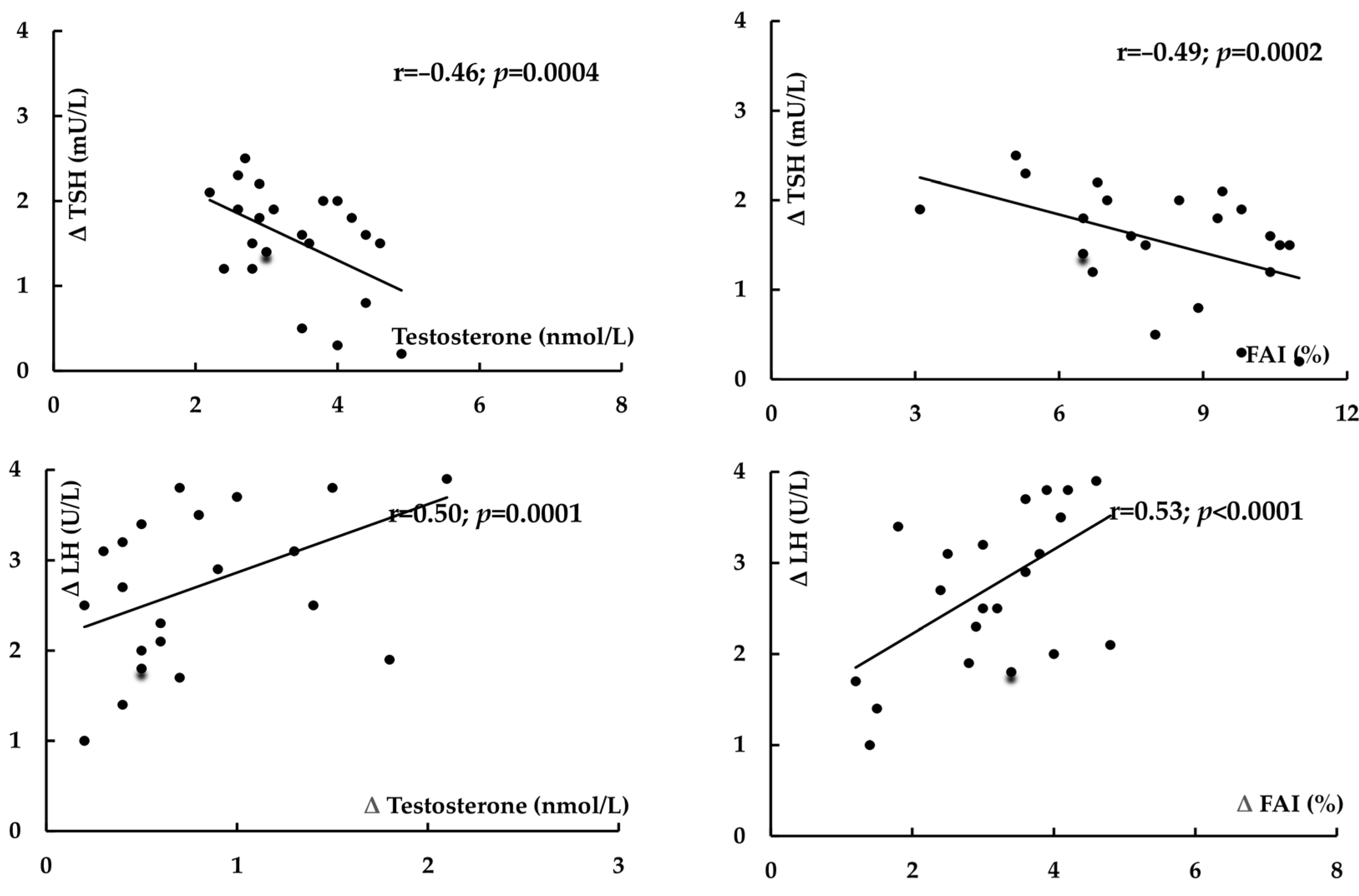
2. Results
3. Discussion
4. Materials and Methods
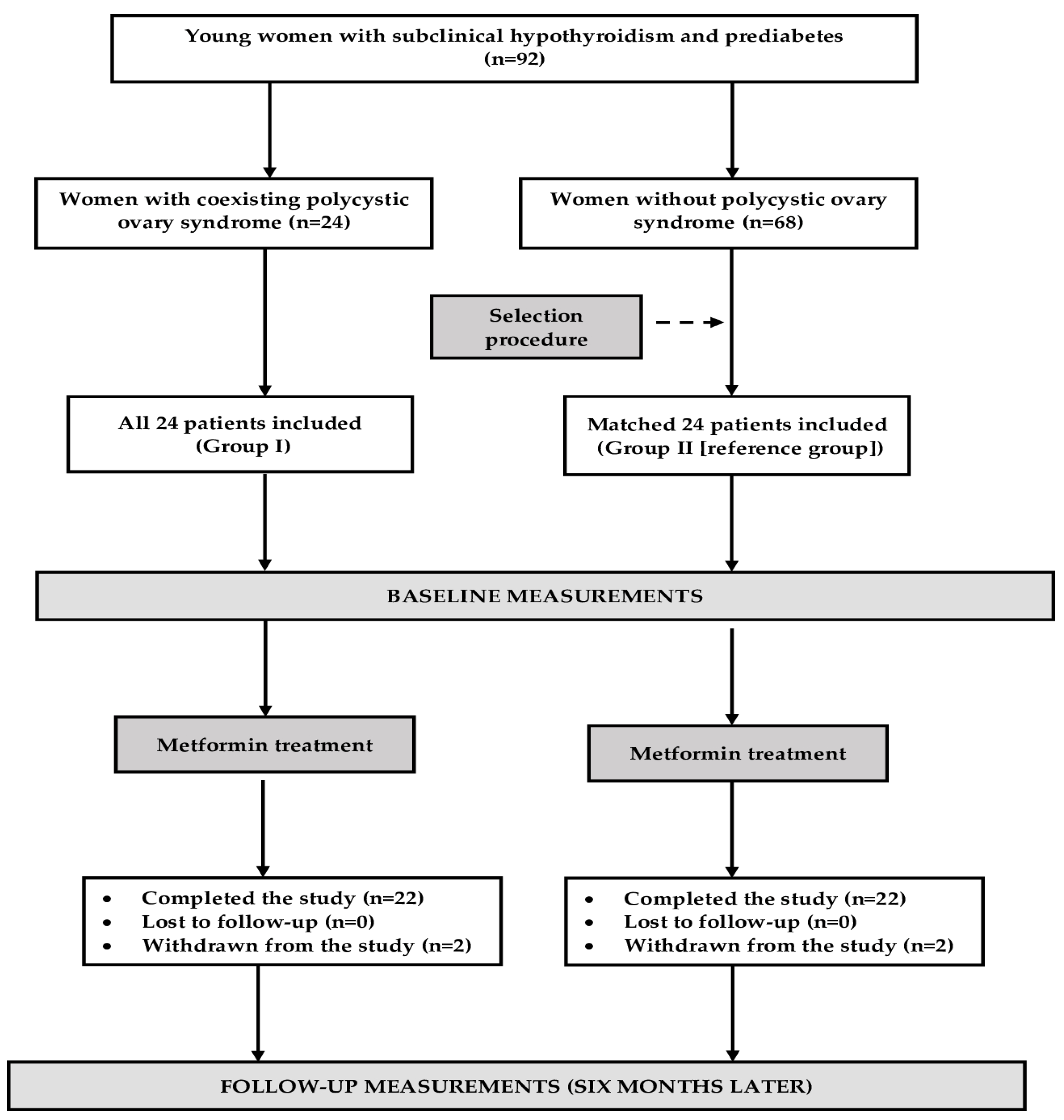
4.1. Study Population
4.2. Study Design
4.3. Laboratory Assays
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, R.R.; Jin, H.; Gao, K.; Twamley, E.W.; Ou, J.J.; Shao, P. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with worst-episode schizophrenia: A double-blind, randomized, placebo-controlled study. Am. J. Psychiatry 2012, 169, 813–821. [Google Scholar] [CrossRef]
- Bo, Q.J.; Wang, Z.M.; Li, X.B.; Ma, X.; Wang, C.Y.; de Leon, J. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: A systematic review. Psychiatry Res. 2016, 237, 257–263. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, T.; Sheng, Y.; Li, R.; Xu, H. The effects of letrozole and metformin combined with targeted nursing care on ovarian function, LH, and FSH in infertile patients with polycystic ovary syndrome. J. Healthc. Eng. 2022, 2022, 3712166. [Google Scholar] [CrossRef]
- Zahra, M.; Shah, M.; Ali, A.; Rahim, R. Effects of metformin on endocrine and metabolic parameters in patients with polycystic ovary syndrome. Horm. Metab. Res. 2017, 49, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Di Minno, A.; Tortora, A.; Ambrosino, P.; Lupoli, G.A.; Di Minno, M.N. Effects of treatment with metformin on TSH levels: A meta-analysis of literature studies. J. Clin. Endocrinol. Metab. 2014, 99, E143–E148. [Google Scholar] [CrossRef]
- Haroon, S.M.; Khan, K.; Maqsood, M.; Iqbal, S.; Aleem, M.; Khan, T.U. Exploring the effect of metformin to lower thyroid-stimulating hormone in euthyroid and hypothyroid type-2 diabetic patients. Cureus 2021, 13, e13283. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Condorelli, R.A.; Barbagallo, F.; Aversa, A.; Calogero, A.E.; La Vignera, S. TSH lowering effects of metformin: A possible mechanism of action. J. Endocrinol. Investig. 2021, 44, 1547–1550. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Impact of metformin on hypothalamic-pituitary-thyroid axis activity in women with autoimmune and non-autoimmune subclinical hypothyroidism: A pilot study. Pharmacol. Rep. 2024, 76, 195–206. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Effect of metformin on hypothalamic-pituitary-thyroid axis activity in elderly antipsychotic-treated women with type 2 diabetes and subclinical hypothyroidism: A preliminary study. J. Clin. Pharmacol. 2018, 58, 586–592. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. The association between vitamin D status and the impact of metformin on hypothalamic-pituitary-thyroid axis activity in women with subclinical hypothyroidism. Pharmaceutics 2024, 16, 1093. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Rosuvastatin potentiates the thyrotropin-lowering effect of metformin in men with non-autoimmune subclinical hypothyroidism and prediabetes. J. Clin. Pharm. Ther. 2022, 47, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okrzesik, J.; Okopień, B. Different effects of metformin on the hypothalamic-pituitary-thyroid axis in bromocriptine- and cabergoline-treated patients with Hashimoto’s thyroiditis and glucose metabolism abnormalities. Exp. Clin. Endocrinol. Diabetes. 2015, 123, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Szkróbka, W.; Okopień, B. Sex-dependent effect of metformin on hypothalamic-pituitary-thyroid axis activity in patients with subclinical hypothyroidism. Pharmacol. Rep. 2016, 68, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2021, 106, e1071–e1083. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, Y.; Qiang, C.; Zhang, C. Effect of TSH on oocyte maturation of PCOS patients with normal thyroid function in IVF. Reprod. Biol. Endocrinol. 2022, 20, 132. [Google Scholar] [CrossRef]
- Ding, X.; Yang, L.V.; Wang, J.; Tang, R.; Chen, Q.V.; Pan, J.; Yang, H.; Chen, X.V.; Chen, Z.V.; Mu, L. Subclinical hypothyroidism in polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2018, 9, 700. [Google Scholar] [CrossRef]
- Du, D.; Li, X. The relationship between thyroiditis and polycystic ovary syndrome: A meta-analysis. Int. J. Clin. Exp. Med. 2013, 6, 880–889. [Google Scholar]
- Roa Dueñas, O.H.; Van der Burgh, A.C.; Ittermann, T.; Ligthart, S.; Ikram, M.A.; Peeters, R.; Chaker, L. Thyroid function and the risk of prediabetes and type 2 diabetes. J. Clin. Endocrinol. Metab. 2022, 107, 1789–1798. [Google Scholar] [CrossRef]
- Yang, L.; Lv, X.; Yue, F.; Wei, D.; Liu, W.; Zhang, T. Subclinical hypothyroidism and the risk of metabolic syndrome: A meta-analysis of observational studies. Endocr. Res. 2016, 41, 158–165. [Google Scholar] [CrossRef]
- Lim, S.S.; Kakoly, N.S.; Tan, J.W.; Fitzgerald, G.; Bahri Khomami, M.; Joham, A.E.; Cooray, S.D.; Misso, M.L.; Norman, R.J.; Harrison, C.L.; et al. Metabolic syndrome in polycystic ovary syndrome: A systematic review, meta-analysis and meta-regression. Obes. Rev. 2019, 20, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Paparodis, R.D.; Bosdou, J.K.; Bothou, C.; Macut, D.; Goulis, D.G.; Livadas, S. Risk of type 2 diabetes mellitus in polycystic ovary syndrome is associated with obesity: A meta-analysis of observational studies. Endocrine 2021, 74, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Notaro, A.L.; Neto, F.T. The use of metformin in women with polycystic ovary syndrome: An updated review. J. Assist. Reprod. Genet. 2022, 39, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.M.; Gottwald-Hostalek, U.; Andag-Silva, A. Update on the therapeutic role of metformin in the management of polycystic ovary syndrome: Effects on pathophysiologic process and fertility outcomes. Womens Health 2025, 21, 17455057241311759. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, F.L.; Li, S.C. Statin is a reasonable treatment option for patients with polycystic ovary syndrome: A meta-analysis of randomized controlled trials. Exp. Clin Endocrinol. Diabetes 2012, 120, 367–375. [Google Scholar] [CrossRef]
- Hamad, I.N.; Kadhim, S.A.; Fawzi, H.A.; Swadi, A. Effects of combined metformin and cabergoline versus metformin alone on ovarian and hormonal activities in Iraqi patients with PCOS and hyperprolactinemia: A randomized clinical trial. J. Med. Life 2023, 16, 1615–1621. [Google Scholar] [CrossRef]
- Morteza Taghavi, S.; Rokni, H.; Fatemi, S. Metformin decreases thyrotropin in overweight women with polycystic ovarian syndrome and hypothyroidism. Diab. Vasc. Dis. Res. 2011, 8, 47–48. [Google Scholar] [CrossRef]
- Morgante, G.; Musacchio, M.C.; Orvieto, R.; Massaro, M.G.; De Leo, V. Alterations in thyroid function among the different polycystic ovary syndrome phenotypes. Gynecol. Endocrinol. 2013, 29, 967–969. [Google Scholar] [CrossRef]
- Rotondi, M.; Cappelli, C.; Magri, F.; Botta, R.; Dionisio, R.; Iacobello, C.; De Cata, P.; Nappi, R.E.; Castellano, M.; Chiovato, L. Thyroidal effect of metformin treatment in patients with polycystic ovary syndrome. Clin. Endocrinol. 2011, 75, 378–381. [Google Scholar] [CrossRef]
- Garcia-Beltran, C.; Bassols, J.; Carreras-Badosa, G.; López Bermejo, A.; Ibáñez, L.; de Zegher, F. Raised thyroid-stimulating hormone in girls with polycystic ovary syndrome: Effects of randomized interventions. Horm. Res. Paediatr. 2023, 96, 458–464. [Google Scholar] [CrossRef]
- Trouva, A.; Alvarsson, M.; Calissendorff, J.; Åsvold, B.O.; Vanky, E.; Hirschberg, A.L. Thyroid status during pregnancy in women with polycystic ovary syndrome and the effect of metformin. Front. Endocrinol. 2022, 13, 772801. [Google Scholar] [CrossRef]
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98. [Google Scholar] [CrossRef]
- Nicholls, A.R.; Holt, R.I. Growth hormone and insulin-like growth factor-1. Front. Horm. Res. 2016, 47, 101–114. [Google Scholar]
- Knobel, M. Etiopathology, clinical features, and treatment of diffuse and multinodular nontoxic goiters. J. Endocrinol. Investig. 2016, 39, 357–373. [Google Scholar] [CrossRef]
- Tsatsoulis, A. The role of insulin resistance/hyperinsulinism on the rising trend of thyroid and adrenal nodular disease in the current environment. J. Clin. Med. 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Ittermann, T.; Markus, M.R.; Schipf, S.; Derwahl, M.; Meisinger, C.; Völzke, H. Metformin inhibits goitrogenous effects of type 2 diabetes. Eur. J. Endocrinol. 2013, 169, 9–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, X.; Wu, D.; Hu, C.; Xu, T.; Liu, Y.; Liu, C. Role of metformin in the treatment of patients with thyroid nodules and insulin resistance: A systematic review and meta-analysis. Thyroid 2019, 29, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Impaired prolactin-lowering effects of metformin in women with polycystic ovary syndrome. J. Clin. Med. 2023, 12, 5474. [Google Scholar] [CrossRef]
- Brown, E.D.; Obeng-Gyasi, B.; Hall, J.E.; Shekhar, S. The thyroid hormone axis and female reproduction. Int. J. Mol. Sci. 2023, 24, 9815. [Google Scholar] [CrossRef]
- Petríková, J.; Lazúrová, I.; Yehuda, S. Polycystic ovary syndrome and autoimmunity. Eur. J. Intern. Med. 2010, 21, 369–371. [Google Scholar] [CrossRef]
- Tosca, L.; Froment, P.; Rame, C.; McNeilly, J.R.; McNeilly, A.S.; Maillard, V.; Dupont, J. Metformin decreases GnRH- and activin-induced gonadotropin secretion in rat pituitary cells: Potential involvement of adenosine 5’ monophosphate-activated protein kinase (PRKA). Biol. Reprod. 2011, 84, 351–362. [Google Scholar] [CrossRef]
- Ueno, M. Molecular anatomy of the brain endothelial barrier: An overview of the distributional features. Curr. Med. Chem. 2007, 14, 1199–1206. [Google Scholar] [CrossRef]
- Labuzek, K.; Suchy, D.; Gabryel, B.; Bielecka, A.; Liber, S.; Okopień, B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010, 62, 956–965. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef]
- Jalil, A.T.; Zair, M.A.; Hanthal, Z.R.; Naser, S.J.; Aslandook, T.; Abosaooda, M.; Fadhil, A. Role of the AMP-activated protein kinase in the pathogenesis of polycystic ovary syndrome. Indian J. Clin. Biochem. 2024, 39, 450–458. [Google Scholar] [CrossRef]
- Li, T.; Zhang, T.; Cui, T.; Yang, Y.; Liu, R.; Chen, Y.; Yin, C. Involvement of endogenous testosterone in hepatic steatosis in women with polycystic ovarian syndrome. J. Steroid Biochem. Mol. Biol. 2020, 204, 105752. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.J. Non-thyroidal illness syndrome is a manifestation of hypothalamic-pituitary dysfunction, and in view of current evidence, should be treated with appropriate replacement therapies. Crit. Care. Clin. 2006, 22, 57–86. [Google Scholar] [CrossRef] [PubMed]
- Ortega-González, C.; Cardoza, L.; Coutiño, B.; Hidalgo, R.; Arteaga-Troncoso, G.; Parra, A. Insulin sensitizing drugs increase the endogenous dopaminergic tone in obese insulin-resistant women with polycystic ovary syndrome. J. Endocrinol. 2005, 184, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Toney, T.W.; Lookingland, K.J.; Moore, K.E. Role of testosterone in the regulation of tuberoinfundibular dopaminergic neurons in the male rat. Neuroendocrinology 1991, 54, 23–29. [Google Scholar] [CrossRef]
- Tahapary, D.L.; Pratisthita, L.B.; Fitri, N.A.; Marcella, C.; Wafa, S.; Kurniawan, F.; Rizka, A.; Tarigan, T.J.; Harbuwono, D.S.; Purnamasari, D.; et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab. Syndr. 2022, 16, 102581. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Chien, H.Y.; Chen, S.M.; Li, W.C. Dopamine receptor agonists mechanism of actions on glucose lowering and their connections with prolactin actions. Front. Clin. Diabetes Healthc. 2023, 4, 935872. [Google Scholar] [CrossRef]
- Wilson, C.M.; McPhaul, M.J. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol. Cell. Endocrinol. 1996, 120, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Davison, S.L.; Davis, S.R. Androgens in women. J. Steroid Biochem. Mol. Biol. 2003, 85, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Kłapcińska, B.; Poprzecki, S.; Danch, A.; Sobczak, A.; Kempa, K. Selenium levels in blood of Upper Silesian population: Evidence of suboptimal selenium status in a significant percentage of the population. Biol. Trace Elem. Res. 2005, 108, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Trofimiuk-Müldner, M.; Konopka, J.; Sokołowski, G.; Dubiel, A.; Kieć-Klimczak, M.; Kluczyński, Ł.; Motyka, M.; Rzepka, E.; Walczyk, J.; Sokołowska, M.; et al. Current iodine nutrition status in Poland (2017): Is the Polish model of obligatory iodine prophylaxis able to eliminate iodine deficiency in the population? Public Health Nutr. 2020, 23, 2467–2477. [Google Scholar] [CrossRef]
- French, D. Clinical utility of laboratory developed mass spectrometry assays for steroid hormone testing. J. Mass Spectrom. Adv. Clin. Lab. 2023, 28, 13–19. [Google Scholar] [CrossRef]
| Variable | Group I * | Group II ** | p-Value |
|---|---|---|---|
| Number (n) | 22 | 22 | - |
| Age (years) | 32 ± 8 | 34 ± 8 | 0.4117 |
| Reasons for hypothyroidism (autoimmune/non-autoimmune) (%) | 50/50 | 55/45 | 0.5014 |
| Smokers (%)/number of cigarettes a day (n)/smoking duration (months) | 36/9 ± 6/129 ± 41 | 41/9 ± 7/125 ± 46 | 0.5238 |
| Body mass index (kg/m2) | 24.9 ± 4.6 | 23.9 ± 4.3 | 0.4605 |
| Systolic blood pressure (mmHg) | 127 ± 20 | 124 ± 19 | 0.6127 |
| Diastolic blood pressure (mmHg) | 84 ± 7 | 83 ± 6 | 0.6136 |
| Variable | Group I * | Group II ** | p-Value |
|---|---|---|---|
| Glucose (mg/dL) [3.89–5.55] | |||
| Baseline | 6.22 ± 0.34 | 6.29 ± 0.38 | 0.5231 |
| Follow-up | 5.98 ± 0.37 | 5.72 ± 0.31 | 0.0154 |
| p-value (follow-up vs. baseline) | 0.0304 | <0.0001 | - |
| HOMA1-IR [<2.0] | |||
| Baseline | 4.1 ± 1.3 | 3.9 ± 1.4 | 0.6260 |
| Follow-up | 3.1 ± 1.2 | 2.0 ± 1.4 | 0.0078 |
| p-value (follow-up vs. baseline) | 0.0013 | 0.0001 | - |
| HbA1c (%) [4.0–5.6] | |||
| Baseline | 6.0 ± 0.2 | 6.1 ± 0.2 | 0.1147 |
| Follow-up | 5.6 ± 0.2 | 5.3 ± 0.2 | 0.0001 |
| p-value (follow-up vs. baseline) | <0.0001 | <0.0001 | - |
| TSH (mU/L) [0.4–4.5] | |||
| Baseline | 7.5 ± 1.4 | 7.6 ± 1.3 | 0.8073 |
| Follow-up | 6.0 ± 1.5 | 4.9 ± 1.4 | 0.0158 |
| p-value (follow-up vs. baseline) | 0.0014 | <0.0001 | - |
| Free thyroxine (pmol/L) [10.2–21.3] | |||
| Baseline | 14.5 ± 2.5 | 14.2 ± 2.3 | 0.6808 |
| Follow-up | 14.9 ± 2.9 | 15.0 ± 2.8 | 0.9079 |
| p-value (follow-up vs. baseline) | 0.6267 | 0.3063 | - |
| Free triiodothyronine (pmol/L) [2.1–6.4] | |||
| Baseline | 3.4 ± 0.7 | 3.2 ± 0.7 | 0.3488 |
| Follow-up | 3.6 ± 0.8 | 3.4 ± 0.9 | 0.5847 |
| p-value (follow-up vs. baseline) | 0.5102 | 0.5752 | - |
| Total thyroxine (nmol/L) [60–150] | |||
| Baseline | 98 ± 20 | 102 ± 25 | 0.6536 |
| Follow-up | 106 ± 23 | 110 ± 30 | 0.7358 |
| p-value (follow-up vs. baseline) | 0.3594 | 0.5070 | |
| Total triiodothyronine (nmol/L) [1.2–3.1] | |||
| Baseline | 1.8 ± 0.3 | 1.8 ± 0.4 | 1.0000 |
| Follow-up | 1.9 ± 0.4 | 2.0 ± 0.5 | 0.6156 |
| p-value (follow-up vs. baseline) | 0.4662 | 0.2664 | - |
| LH (U/L) [2.3–8.4] | |||
| Baseline | 6.5 ± 2.5 | 3.1 ± 1.5 | <0.0001 |
| Follow-up | 3.7 ± 2.0 | 3.2 ± 1.7 | 0.3767 |
| p-value (follow-up vs. baseline) | 0.0002 | 0.8371 | - |
| FSH (U/L) [3.0–9.5] | |||
| Baseline | 3.7 ± 1.9 | 3.4 ± 1.4 | 0.5542 |
| Follow-up | 3.5 ± 1.5 | 3.7 ± 1.8 | 0.6909 |
| p-value (follow-up vs. baseline) | 0.7003 | 0.5405 | - |
| LH/FSH ratio | |||
| Baseline | 1.8 ± 0.7 | 0.9 ± 0.5 | <0.0001 |
| Follow-up | 1.1 ± 0.6 | 0.9 ± 0.4 | 0.2004 |
| p-value (follow-up vs. baseline) | 0.0009 | 1.0000 | - |
| Testosterone (nmol/L) [0.7–2.4] | |||
| Baseline | 3.4 ± 0.7 | 1.3 ± 0.4 | <0.0001 |
| Follow-up | 2.6 ± 0.8 | 1.4 ± 0.4 | <0.0001 |
| p-value (follow-up vs. baseline) | 0.0010 | 0.4117 | - |
| SHBG (nmol/L) [25–120] | |||
| Baseline | 42 ± 13 | 46 ± 12 | 0.2950 |
| Follow-up | 52 ± 15 | 58 ± 16 | 0.2065 |
| p-value (follow-up vs. baseline) | 0.0228 | 0.0074 | - |
| FAI (%) [<5%] | |||
| Baseline | 8.1 ± 1.5 | 2.8 ± 1.2 | <0.0001 |
| Follow-up | 5.0 ± 2.0 | 2.4 ± 1.1 | <0.0001 |
| p-value (follow-up vs. baseline) | <0.0001 | 0.2556 | |
| DHEA-S (μmol/L) [2.2–10.8] | |||
| Baseline | 10.5 ± 3.8 | 5.9 ± 1.9 | <0.0001 |
| Follow-up | 9.2 ± 4.1 | 6.1 ± 2.1 | 0.0030 |
| p-value (follow-up vs. baseline) | 0.2816 | 0.7421 | - |
| Androstenedione (nmol/L) [1.4–7.8] | |||
| Baseline | 8.2 ± 2.2 | 3.8 ± 1.4 | <0.0001 |
| Follow-up | 7.3 ± 2.4 | 3.7 ± 1.5 | <0.0001 |
| p-value (follow-up vs. baseline) | 0.2019 | 0.8203 | - |
| Estradiol (pmol/L) [175–640] | |||
| Baseline | 392 ± 105 | 280 ± 85 | 0.0004 |
| Follow-up | 378 ± 112 | 295 ± 92 | 0.0103 |
| p-value (follow-up vs. baseline) | 0.6710 | 0.5773 | - |
| Prolactin (ng/mL) [5–28] | |||
| Baseline | 19 ± 9 | 15 ± 9 | 0.1479 |
| Follow-up | 15 ± 8 | 13 ± 8 | 0.4117 |
| p-value (follow-up vs. baseline) | 0.1268 | 0.4403 | - |
| ACTH (pg/mL) [14–68] | |||
| Baseline | 36 ± 18 | 42 ± 14 | 0.2240 |
| Follow-up | 40 ± 15 | 37 ± 16 | 0.5256 |
| p-value (follow-up vs. baseline) | 0.4278 | 0.2763 | - |
| IGF-1 (ng/mL) [90–320] | |||
| Baseline | 220 ± 70 | 207 ± 73 | 0.5498 |
| Follow-up | 200 ± 68 | 218 ± 64 | 0.3711 |
| p-value (follow-up vs. baseline) | 0.3419 | 0.5979 | - |
| Variable | Group I * | Group II ** | p-Value |
|---|---|---|---|
| Δ Glucose | −4 ± 6 | −9 ± 8 | 0.0238 |
| Δ HbA1c | −9 ± 4 | −13 ± 4 | 0.0019 |
| Δ HOMA1-IR | −24 ± 20 | −49 ± 31 | 0.0028 |
| Δ TSH | −20 ± 12 | −36 ± 18 | 0.0012 |
| Δ Free thyroxine | 3 ± 10 | 6 ± 12 | 0.3728 |
| Δ Free triiodothyronine | 6 ± 9 | 6 ± 14 | 1.0000 |
| Δ Total thyroxine | 8 ± 11 | 9 ± 13 | 0.7843 |
| Δ Total triiodothyronine | 6 ± 20 | 11 ± 24 | 0.4570 |
| Δ LH | −43 ± 28 | 3 ± 15 | <0.0001 |
| Δ FSH | −5 ± 25 | 9 ± 29 | 0.0937 |
| Δ LH/FSH ratio | −39 ± 31 | 0 ± 25 | <0.0001 |
| Δ Testosterone | −24 ± 26 | 8 ± 20 | <0.0001 |
| Δ SHBG | 24 ± 20 | 26 ± 20 | 0.7418 |
| Δ FAI | −38 ± 29 | −14 ± 26 | 0.0061 |
| Δ DHEA-S | −12 ± 32 | 3 ± 28 | 0.1055 |
| Δ Androstenedione | −11 ± 29 | −3 ± 24 | 0.3246 |
| Δ Estradiol | −4 ± 28 | 5 ± 30 | 0.3175 |
| Δ Prolactin | −21 ± 20 | −13 ± 22 | 0.2139 |
| Δ ACTH | 11 ± 28 | 12 ± 24 | 0.8994 |
| Δ IGF-1 | −9 ± 30 | 5 ± 29 | 0.1230 |
| Variable | Autoimmune Hypothyroidism | Non-Autoimmune Hypothyroidism | p-Value |
|---|---|---|---|
| Δ Glucose | −5 ± 10 | −4 ± 8 | 0.7983 |
| Δ HbA1c | −8 ± 2 | −8 ± 3 | 1.0000 |
| Δ HOMA1-IR | −21 ± 23 | −27 ± 25 | 0.5646 |
| Δ TSH | −18 ± 16 | −22 ± 15 | 0.5520 |
| Δ Free thyroxine | 4 ± 11 | 2 ± 13 | 0.7010 |
| Δ Free triiodothyronine | 5 ± 11 | 7 ± 12 | 0.6880 |
| Δ Total thyroxine | 5 ± 14 | 11 ± 15 | 0.3440 |
| Δ Total triiodothyronine | 4 ± 18 | 8 ± 22 | 0.6457 |
| Δ LH | −39 ± 32 | −46 ± 29 | 0.5968 |
| Δ LH/FSH ratio | −37 ± 40 | −41 ± 36 | 0.8078 |
| Δ Testosterone | −27 ± 21 | −21 ± 29 | 0.5845 |
| Δ FAI | −42 ± 30 | −34 ± 34 | 0.5650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Ott, J.; Burgio, S.; Zaami, S.; Okopień, B. Polycystic Ovary Syndrome Attenuates TSH-Lowering Effect of Metformin in Young Women with Subclinical Hypothyroidism. Pharmaceuticals 2025, 18, 1149. https://doi.org/10.3390/ph18081149
Krysiak R, Kowalcze K, Ott J, Burgio S, Zaami S, Okopień B. Polycystic Ovary Syndrome Attenuates TSH-Lowering Effect of Metformin in Young Women with Subclinical Hypothyroidism. Pharmaceuticals. 2025; 18(8):1149. https://doi.org/10.3390/ph18081149
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, Johannes Ott, Sofia Burgio, Simona Zaami, and Bogusław Okopień. 2025. "Polycystic Ovary Syndrome Attenuates TSH-Lowering Effect of Metformin in Young Women with Subclinical Hypothyroidism" Pharmaceuticals 18, no. 8: 1149. https://doi.org/10.3390/ph18081149
APA StyleKrysiak, R., Kowalcze, K., Ott, J., Burgio, S., Zaami, S., & Okopień, B. (2025). Polycystic Ovary Syndrome Attenuates TSH-Lowering Effect of Metformin in Young Women with Subclinical Hypothyroidism. Pharmaceuticals, 18(8), 1149. https://doi.org/10.3390/ph18081149









