Carbohydrate-Based Chiral Ligands for the Enantioselective Addition of Diethylzinc to Aldehydes
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Reactions Conditions in the Enantioselective Addition of Diethylzinc to Benzaldehyde
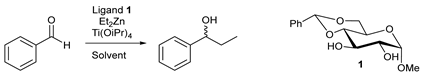
| Entry | Ligand (%) | Ti(OiPr)4 (eq.) | Et2Zn b (eq.) | Solvent | T (°C) | Conversion (%) d | ee (%) e |
|---|---|---|---|---|---|---|---|
| 1 | - | 1.4 | 3 | Hexane c | 0 | 100 | 0 |
| 2 | 10 | 1.4 | 3 | Hexane c | 0 | 91 | 32 (S) |
| 3 | 20 | 1.4 | 3 | Hexane c | 0 | 86 | 56 (S) |
| 4 | 30 | 1.4 | 3 | Hexane c | 0 | 52 | 55 (S) |
| 5 | 40 | 1.4 | 3 | Hexane c | 0 | 20 | 45 (S) |
| 6 | 20 | - | 3 | Hexane c | 0 | 3 | 0 |
| 7 | 20 | 0.8 | 3 | Hexane c | 0 | 42 | 47 (S) |
| 8 | 20 | 1 | 3 | Hexane c | 0 | 81 | 52 (S) |
| 9 | 20 | 1.2 | 3 | Hexane c | 0 | 72 | 55 (S) |
| 10 | 20 | 1.6 | 3 | Hexane c | 0 | 88 | 48 (S) |
| 11 | 20 | 2 | 3 | Hexane c | 0 | 98 | 46 (S) |
| 12 | 20 | 1.4 | 3 | Hexane c | 25 | 100 | 38 (S) |
| 13 | 20 | 1.4 | 3 | Hexane c | −28 | 60 | 44 (S) |
| 14 | 20 | 1.4 | 3 | Hexane c | −76 | 12 | 46 (S) |
| 15 | 20 | 1.4 | 3 | Toluene c | 0 | 70 | 53 (S) |
| 16 | 20 | 1.4 | 3 | DCM c | 0 | 70 | 49 (S) |
| 17 | 20 | 1.4 | 2 | Hexane c | 0 | 65 | 54 (S) |
| 18 | 20 | 1.4 | 2.5 | Hexane c | 0 | 98 | 52 (S) |
2.2. Evaluation of Chiral Diol Ligands Based on α-, β-D-Methylglucopyranoside and α-, β-D-Methylgalactopyranoside in the Enantioselective Addition of Diethylzinc to Benzaldehyde
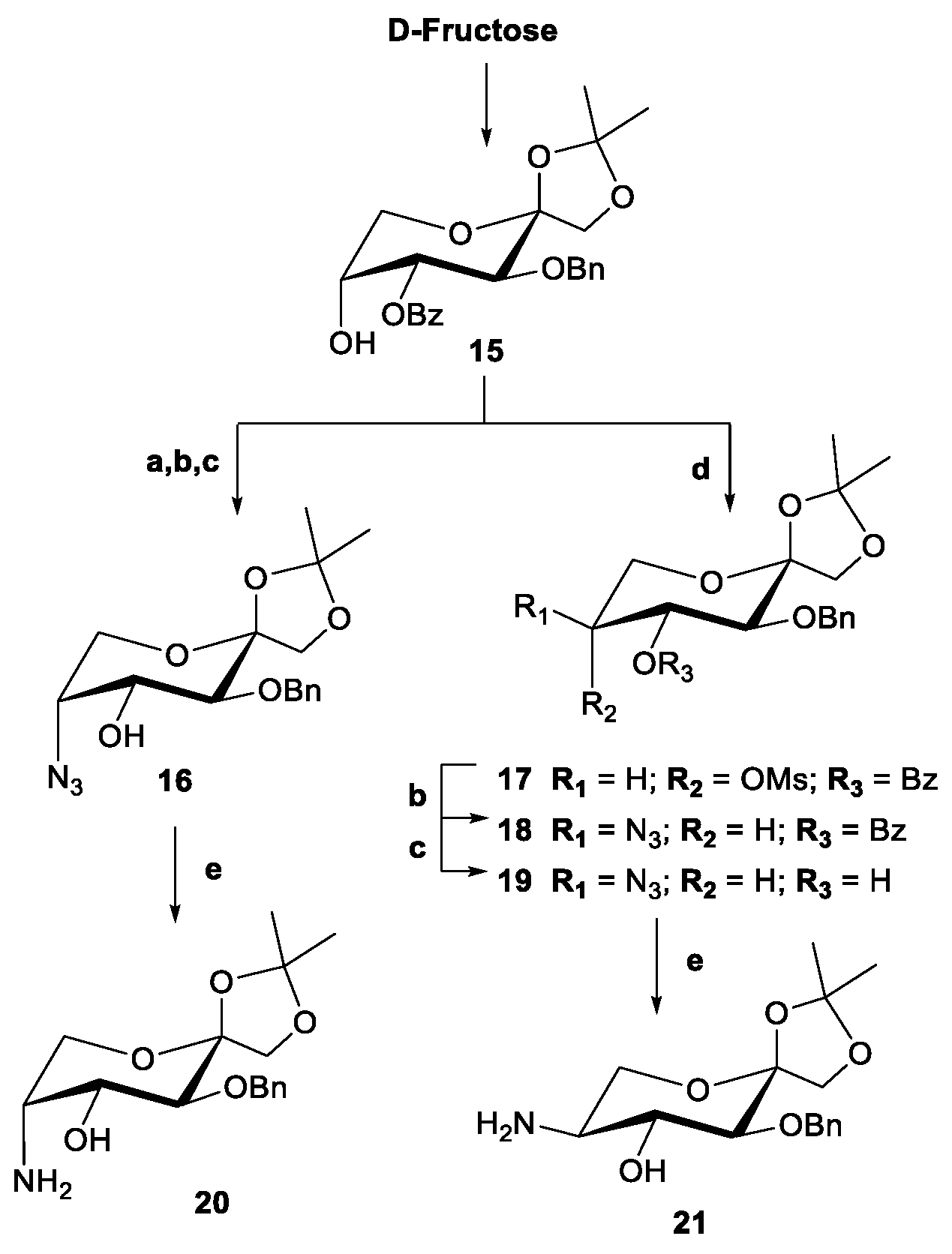
2.3. Evaluation of Chiral Diol Ligands Based on D-Fructose in the Enantioselective Addition of Diethylzinc to Benzaldehyde
2.4. Synthesis and Evaluation of β-Amino Alcohol Ligands Based on D-Fructose in the Enantioselective Addition of Diethylzinc to Benzaldehyde
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noyori, R.; Kitamura, M. Enantioselective Addition of Organometallic Reagents to Carbonyl Compounds: Chirality Transfer, Multiplication, and Amplification. Angew. Chem. Int. Ed. Engl. 1991, 30, 49–69. [Google Scholar] [CrossRef]
- Blaser, H.U. The Chiral Pool as a Source of Enantioselective Catalysts and Auxiliaries. Chem. Rev. 1992, 92, 935–952. [Google Scholar] [CrossRef]
- Soai, K.; Niwa, S. Enantioselective Addition of Organozinc Reagents to Aldehydes. Chem. Rev. 1992, 92, 833–856. [Google Scholar] [CrossRef]
- Pu, L.; Yu, H.B.i.n. Catalytic asymmetric organozinc additions to carbonyl compounds. Chem. Rev. 2001, 101, 757–824. [Google Scholar] [CrossRef] [PubMed]
- Hatano, M.; Ishihara, K. Catalytic enantioselective organozinc addition toward optically active tertiary alcohol synthesis. Chem. Rec. 2008, 8, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, T.; Soai, K.; Kobayashi, S. Enantioface-differentiating (asymmetric) addition of alkyllithium to aldehydes by using (2S, 2′S)-2-hydroxymethyl-1-[(1-methylpyrrolidin-2-yl)methyl] pyrrolidine as chiral ligand. Chem. Lett. 1978, 7, 219–222. [Google Scholar] [CrossRef]
- Soai, K.; Mukaiyama, T. Effects of solvents on the enantioface-differentiating (asymmetric) addition of butyllithium to benzaldehyde using (2S, 2′S)-2-hydroxymethyl-1-[(1-methylpyrrolidin-2-yl)methyl]pyrrolidine as a chiral ligand. Chem. Lett. 1978, 7, 491–492. [Google Scholar] [CrossRef]
- Sato, T.; Soai, K.; Suzuki, K.; Mukaiyama, T. Enantioface-differentiating (asymmetric) addition of dialkylmagnesium to aldehydes by using the lithium salt of (2S, 2′S)-2-hydroxymethyl-1-[(1-methylpyrpolidin-2-yl)methyl]pyrrolidine as a chiral ligand. Chem. Lett. 1978, 7, 601–604. [Google Scholar] [CrossRef]
- Oguni, N.; Omi, T.; Yamamoto, Y.; Nakamura, A. Enantioselective addition of diethylzinc to arylaldehydes catalyzed by chiral cobalt (II) and palladium (II) complexes. Chem. Lett. 1983, 12, 841–842. [Google Scholar] [CrossRef]
- Oguni, N.; Omi, T. Enantioselective addition of diethylzinc to benzaldehyde catalyzed by a small amount of chiral 2-amino-1-alcohols. Tetrahedron Lett. 1984, 25, 2823–2824. [Google Scholar] [CrossRef]
- Kitamura, M.; Suga, S.; Kawai, K.; Noyori, R. Catalytic Asymmetric Induction. Highly Enantioselective Addition of Dialkylzincs to Aldehydes. J. Am. Chem. Soc. 1986, 108, 6071–6072. [Google Scholar] [CrossRef] [PubMed]
- Vicario, J.L.; Badia, D.; Carrillo, L.; Reyes, E.; Etxebarria, J. α-Amino Acids, β-Amino Alcohols and Related Compounds as Chiral Auxiliaries, Ligands and Catalysts in the Asymmetric Aldol Reaction. Curr. Org. Chem. 2005, 9, 219–235. [Google Scholar] [CrossRef]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino Alcohols and Their Heterocyclic Derivatives as Chiral Auxiliaries in Asymmetric Synthesis. Chem. Rev. 1996, 96, 835–875. [Google Scholar] [CrossRef] [PubMed]
- Sappino, C.; Mari, A.; Mantineo, A.; Moliterno, M.; Palagri, M.; Tatangelo, C.; Suber, L.; Bovicelli, P.; Ricelli, A.; Righi, G. New chiral amino alcohol ligands for catalytic enantioselective addition of diethylzincs to aldehydes. Org. Biomol. Chem. 2018, 16, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.J. Titanium-Catalyzed Enantioselective Additions of Alkyl Groups to Aldehydes: Mechanistic Studies and New Concepts in Asymmetric Catalysis. Acc. Chem. Res. 2003, 36, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Duthaler, R.O.; Hafner, A. Chiral Titanium Complexes for Enantioselective Addition of Nucleophiles to Carbonyl Groups. Chem. Rev. 1992, 92, 807–832. [Google Scholar] [CrossRef]
- Cherng, Y.J.; Fang, J.M.; Lu, T.J. Pinane-type tridentate reagents for enantioselective reactions: Reduction of ketones and addition of diethylzinc to aldehydes. J. Org. Chem. 1999, 64, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Omote, M.; Kominato, A.; Sugawara, M.; Sato, K.; Ando, A.; Kumadaki, I. A novel axially dissymmetric ligand with chiral 2,2,2-trifluoro-1-hydroxyethyl groups. Tetrahedron Lett. 1999, 40, 5583–5585. [Google Scholar] [CrossRef]
- Yang, X.W.; Sheng, J.H.; Da, C.S.; Wang, H.S.; Su, W.; Wang, R.; Chan, A.S. Polymer-supported BINOL ligand for the titanium-catalyzed diethylzinc addition to aldehydes: A remarkable positive influence of the support on the enantioselectivity of the catalyst. J. Org. Chem. 2000, 65, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Kostova, K.; Genov, M.; Philipova, I.; Dimitrov, V. New bis-steroidal axially chiral diols as ligands for the asymmetric addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2000, 11, 3253–3256. [Google Scholar] [CrossRef]
- Huang, W.S.; Pu, L. The first highly enantioselective catalytic diphenylzinc additions to aldehydes: Synthesis of chiral diarylcarbinols by asymmetric catalysis. J. Org. Chem. 1999, 64, 4222–4223. [Google Scholar] [CrossRef]
- Balsells, J.; Walsh, P.J. Design of diastereomeric serf-inhibiting catalysts for control of turnover frequency and enantioselectivity. J. Am. Chem. Soc. 2000, 122, 3250–3251. [Google Scholar] [CrossRef]
- Yus, M.; Ramón, D.J.; Prieto, O. Synthesis of C2-symmetrical bis(1,2-hydroxy sulfonamide) ligands and application in the enantioselective addition of dialkylzinc to aldehydes. Tetrahedron Asymmetry 2002, 13, 1573–1579. [Google Scholar] [CrossRef]
- Bauer, T.; Tarasiuk, J.; Paniczek, K. Highly enantioselective diethylzinc addition to aldehydes catalyzed by d-glucosamine derivatives. Tetrahedron Asymmetry 2002, 13, 77–82. [Google Scholar] [CrossRef]
- Huang, L.N.; Hui, X.P.; Xu, P.F. Asymmetric addition of diethylzinc to benzaldehyde catalyzed by silica-immobilized titanium(IV) complexes of N-sulfonylated amino alcohols. J. Mol. Catal. A Chem. 2006, 258, 216–220. [Google Scholar] [CrossRef]
- Henderson, A.S.; Bower, J.F.; Galan, M.C. Carbohydrates as enantioinduction components in stereoselective catalysis. Org. Biomol. Chem. 2016, 14, 4008–4017. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Mishra, N.; Kumar, S.; Jaiswal, M.K.; Tiwari, V.K. Growing Impact of Carbohydrate-Based Organocatalysts. ChemistrySelect 2022, 7, e202201314. [Google Scholar] [CrossRef]
- Wojaczyńska, E.; Steppeler, F.; Iwan, D.; Scherrmann, M.C.; Marra, A. Synthesis and Applications of Carbohydrate-Based Organocatalysts. Molecules 2021, 26, 7291. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.; Smoliński, S. Enantioselective addition of diethylzinc to aldehydes catalyzed by d-glucosamine derivatives: Highly pronounced effect of trifluoromethylsulfonamide. Appl. Catal. A Gen. 2010, 375, 247–251. [Google Scholar] [CrossRef]
- Bauer, T.; Hakim, Z.Y. Synthesis of Novel 3-Deoxy-3-thio Derivatives of d-Glucosamine and Their Application as Ligands for the Enantioselective Addition of Diethylzinc to Benzaldehyde. Int. J. Mol. Sci. 2024, 25, 5542. [Google Scholar]
- Emmerson, D.P.; Villard, R.; Mugnaini, C.; Batsanov, A.; Howard, J.A.; Hems, W.P.; Tooze, R.P.; Davis, B.G. Precise structure activity relationships in asymmetric catalysis using carbohydrate scaffolds to allow ready fine tuning: Dialkylzinc–aldehyde additions. Org. Biomol. Chem. 2003, 1, 3826–3838. [Google Scholar] [CrossRef] [PubMed]
- Michigami, K.; Hayashi, M. Enantioselective alkylation of aldehydes using dialkylzincs catalyzed by simple chiral diols derived from naturally occurring monosaccharides. Tetrahedron 2013, 69, 4221–4225. [Google Scholar] [CrossRef]
- Mata, Y.; Diéguez, M.; Pàmies, O.; Woodward, S. Screening of a modular sugar-based phosphite ligand library in the asymmetric nickel-catalyzed trialkylaluminum addition to aldehydes. J. Org. Chem. 2006, 71, 8159–8165. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, H.; Hu, X.; Bai, C.; Zheng, Z. Structural probing of d-fructose derived ligands for asymmetric addition of diethylzinc to aldehydes. Tetrahedron Asymmetry 2003, 14, 297–304. [Google Scholar] [CrossRef]
- Cho, B.T.; Kim, N. Catalytic enantioselective reaction. Part 9. 1,2-O-Isopropylidene-5-deoxy-5-N,N-dialkyl (or -N-monoalkyl)amino-α-D-xylofuranose derivatives as highly effective chiral catalysts for enantioselective addition of diethylzinc to aliphatic and aromatic aldehydes. J. Chem. Soc. Perkin Trans. 1996, 1, 2901–2907. [Google Scholar] [CrossRef]
- Tae Cho, B.; Kim, N. Enantioselective addition of diethylzinc to aldehydes using γ-aminoalcohols derived from α-D-xylose as new chiral catalysts. Tetrahedron Lett. 1994, 35, 4115–4118. [Google Scholar] [CrossRef]
- Izquierdo, I.; Plaza, M.T.; Yáñez, V. Polyhydroxylated pyrrolidines: Synthesis from d-fructose of new tri-orthogonally protected 2,5-dideoxy-2,5-iminohexitols. Tetrahedron 2007, 63, 1440–1447. [Google Scholar] [CrossRef]
- Izquierdo Cubero, I.; Plaza López-Espinosa, M.T.; Robles Díaz, R.; Franco Montalbán, F. A practical route to partially protected pyrrolidines as precursors for the stereoselective synthesis of alexines. Carbohydr. Res. 2001, 330, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, I.; Plaza, M.T.; Rodríguez, M.; Franco, F.; Martos, A. Polyhydroxylated pyrrolidines, III. Synthesis of new protected 2,5-dideoxy-2,5-iminohexitols from d-fructose. Tetrahedron 2005, 61, 11697–11704. [Google Scholar] [CrossRef]
- Panchadhayee, R.; Kumar Misra, A. Efficient iodine-catalyzed preparation of benzylidene acetals of carbohydrate derivatives. J. Carbohydr. Chem. 2008, 27, 148–155. [Google Scholar] [CrossRef]
- Schneider, J.; Yuan, C.L.; Flowers, H.M. Synthesis of 2-O-benzyl- and 2,3- and 2,6-di-O-benzyl-D-galactose. Carbohydr. Res. 1974, 36, 159–166. [Google Scholar] [CrossRef]
- Raju, R.; Castillo, B.F.; Richardson, S.K.; Thakur, M.; Severins, R.; Kronenberg, M.; Howell, A.R. Synthesis and evaluation of 3’’- and 4’’-deoxy and -fluoro analogs of the immunostimulatory glycolipid, KRN7000. Bioorganic Med. Chem. Lett. 2009, 19, 4122–4125. [Google Scholar] [CrossRef] [PubMed]
- Bundle, D.R. Displacement of sugar chlorosulphates by bromide, azide, and acetate; a convenient synthesis of methyl 3,6-dideoxy-β-D-ribo-hexopyranoside. J. Chem. Soc. Perkin Trans. 1979, 1, 2751–2755. [Google Scholar] [CrossRef]
- Roën, A.; Padrón, J.I.; Vázquez, J.T. Hydroxymethyl rotamer populations in disaccharides. J. Org. Chem. 2003, 68, 4615–4630. [Google Scholar] [CrossRef] [PubMed]
- Debost, J.L.; Gelas, J.; Horton, D.; Mols, O. Preparative acetonation of pyranoid, vicinal trans-glycols under kinetic control: Methyl 2,3:4,6-di-O-isopropylidene-α- and -β-d-glucopyranoside. Carbohydr. Res. 1984, 125, 329–335. [Google Scholar] [CrossRef]
- Demchenko, A.V.; Pornsuriyasak, P.; De Meo, C. Acetal protecting groups in the organic laboratory: Synthesis of methyl 4,6-O-benzylidene-α-D-glucopyranoside. J. Chem. Educ. 2006, 83, 782–784. [Google Scholar] [CrossRef]
- Brady, R.F. Cyclic acetals of ketoses: Part III. Re-investigation of the synthesis of the isomeric DI-O-isopropylidene-β-d-fructopyranoses. Carbohydr. Res. 1970, 15, 35–40. [Google Scholar] [CrossRef]
- Veleti, S.K.; Lindenberger, J.J.; Thanna, S.; Ronning, D.R.; Sucheck, S.J. Synthesis of a poly-hydroxypyrolidine-based inhibitor of Mycobacterium tuberculosis GlgE. J. Org. Chem. 2014, 79, 9444–9450. [Google Scholar] [CrossRef] [PubMed]
- García-Moreno, M.I.; Rodríguez-Lucena, D.; Ortiz Mellet, C.; García Fernández, J.M. Pseudoamide-Type Pyrrolidine and Pyrrolizidine Glycomimetics and Their Inhibitory Activities against Glycosidases. J. Org. Chem. 2004, 69, 3578–3581. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W.; Hahn, S.; Flath, F.J. Studies on ketoses, 11. Pyranoid exo- and endo-glycals from D-fructose and L-sorbose: Practical routes for their acquisition and some ensuing reactions. Liebigs Ann. 1995, 1995, 2081–2088. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Doleschal, W.; Hahn, S. Studies on Ketoses, 2. Spirocyclic Dihydropyranones from D-Fructose. Liebigs Ann. Der Chem. 1985, 1985, 2454–2464. [Google Scholar] [CrossRef]
- Nortey, S.O.; Wu, W.N.; Maryanoff, B.E. Synthesis of hydroxylated derivatives of topiramate, a novel antiepileptic drug based on D-fructose: Investigation of oxidative metabolites. Carbohydr. Res. 1997, 304, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.B.; Li, Y.F.; Li, Z.J. Acyl chloride/DABCO-promoted acetal migration of 1,2:4,5-di-O-isopropylidene-d-fructopyranose. Carbohydr. Res. 2007, 342, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Dekany, G.; Lundt, I.; Niedermair, F.; Bichler, S.; Spreitz, J.; Sprenger, F.K.; Stütz, A.E. 1,5-Anhydro-d-fructose from d-fructose. Carbohydr. Res. 2007, 342, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, B.E.; Costanzo, M.J.; Nortey, S.O.; Greco, M.N.; Shank, R.P.; Schupsky, J.J.; Ortegon, M.P.; Vaught, J.L. Structure-activity studies on anticonvulsant sugar sulfamates related to topiramate. Enhanced potency with cyclic sulfate derivatives. J. Med. Chem. 1998, 41, 1315–1343. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, Z.; Fang, J.; Zhou, J.; Xiang, Y. d-Fructose-derived β-amino alcohol catalyzed direct asymmetric aldol reaction in the presence of p-nitrophenol. RSC Adv. 2013, 3, 21084–21091. [Google Scholar] [CrossRef]
- Simao, A.C.; Tatibouët, A.; Rauter, A.P.; Rollin, P. Selective iodination of vicinal cis-diols on ketopyranose templates. Tetrahedron Lett. 2010, 51, 4602–4604. [Google Scholar] [CrossRef]
- Simao, A.C.; Silva, S.; Rauter, A.P.; Rollin, P.; Tatibouët, A. Controlled Garegg Conditions for Selective Iodination on Pyranose Templates. Eur. J. Org. Chem. 2011, 2011, 2286–2292. [Google Scholar] [CrossRef]
- Paquette, L.A.; Zhou, R. Synthesis of enantiopure C2-symmetric VERDI disulfonamides and their application to the catalytic enantioselective addition of diethylzinc to aromatic and aliphatic aldehydes. J. Org. Chem. 1999, 64, 7929–7934. [Google Scholar] [CrossRef]
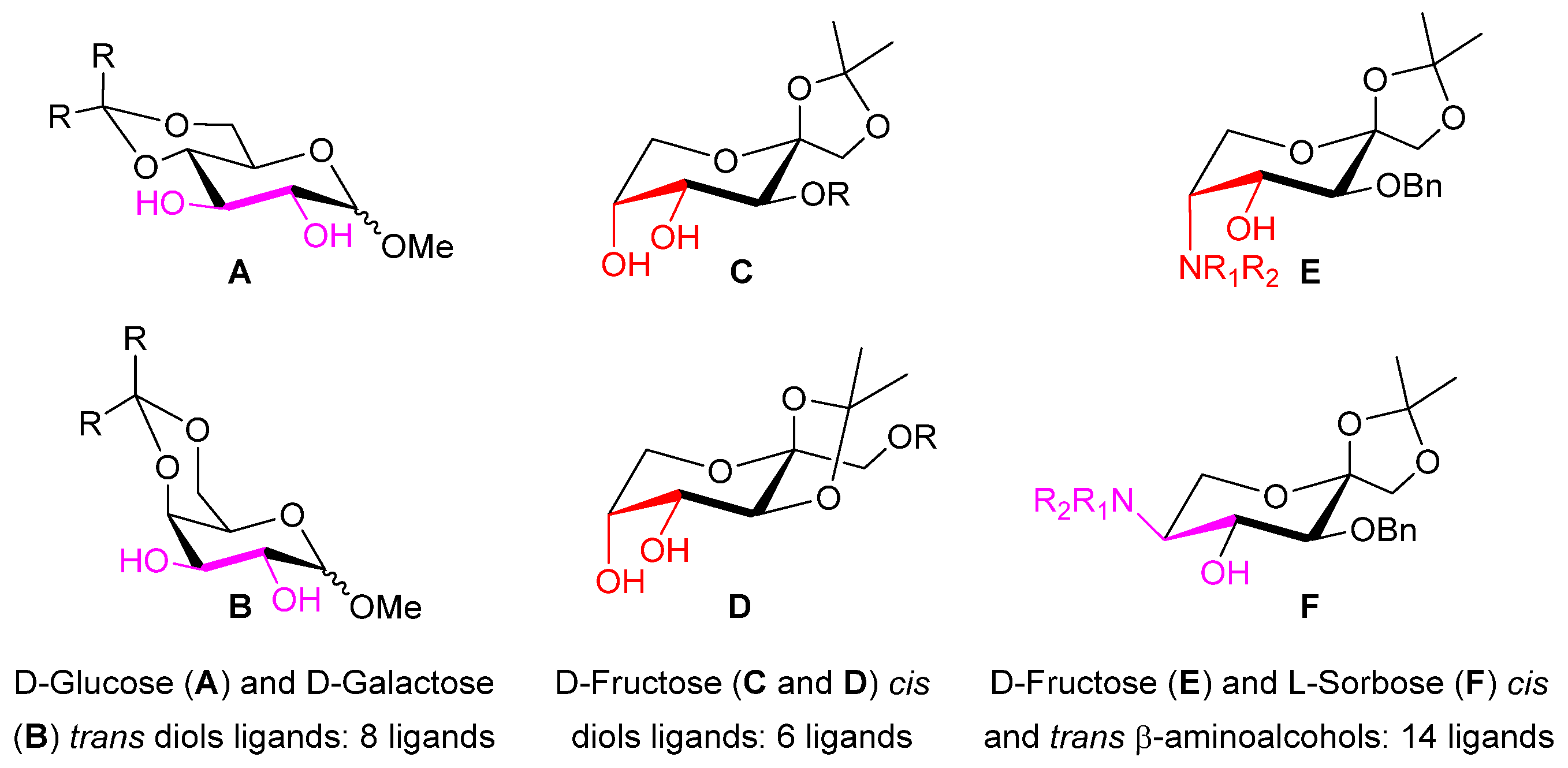
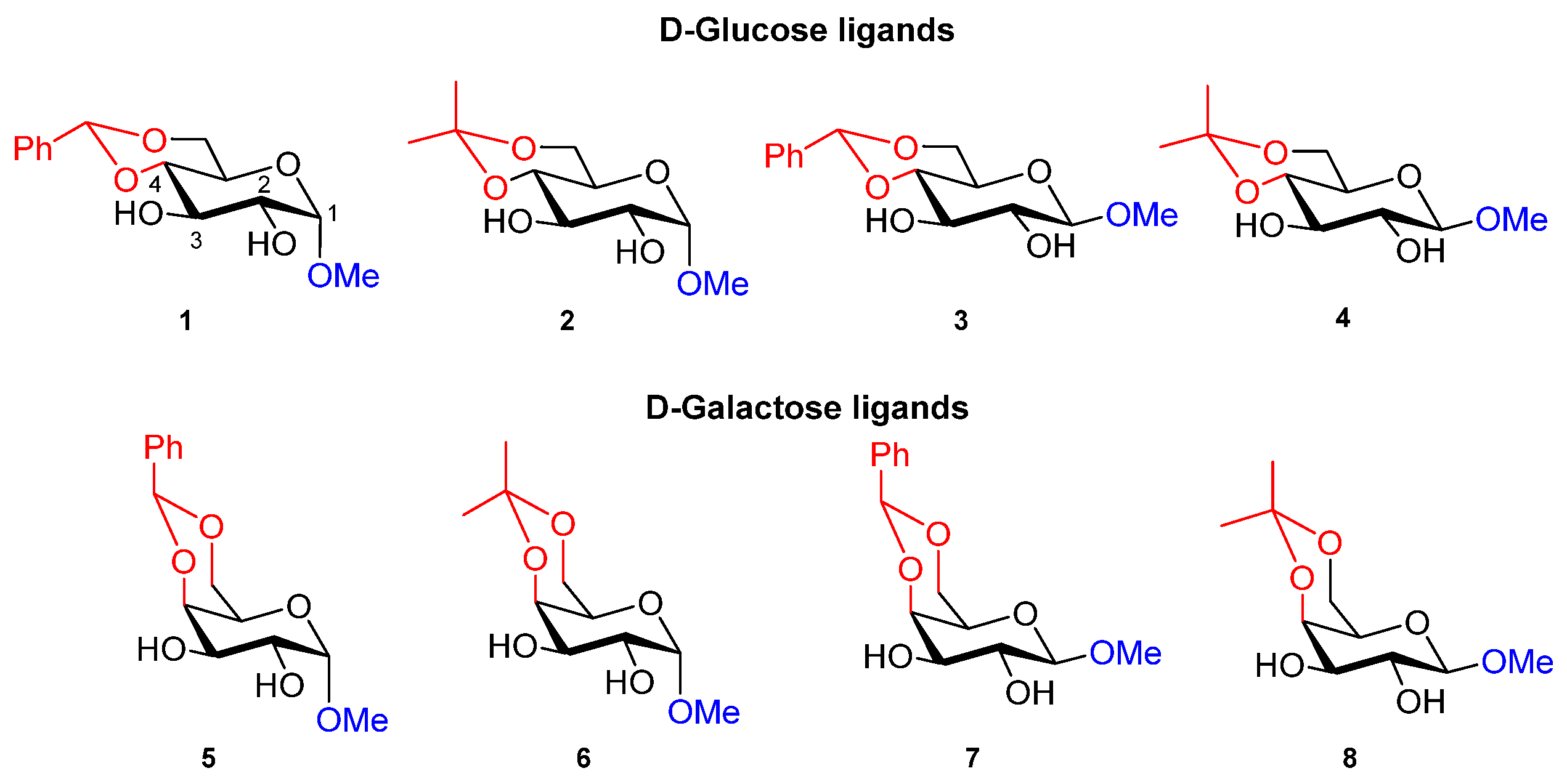

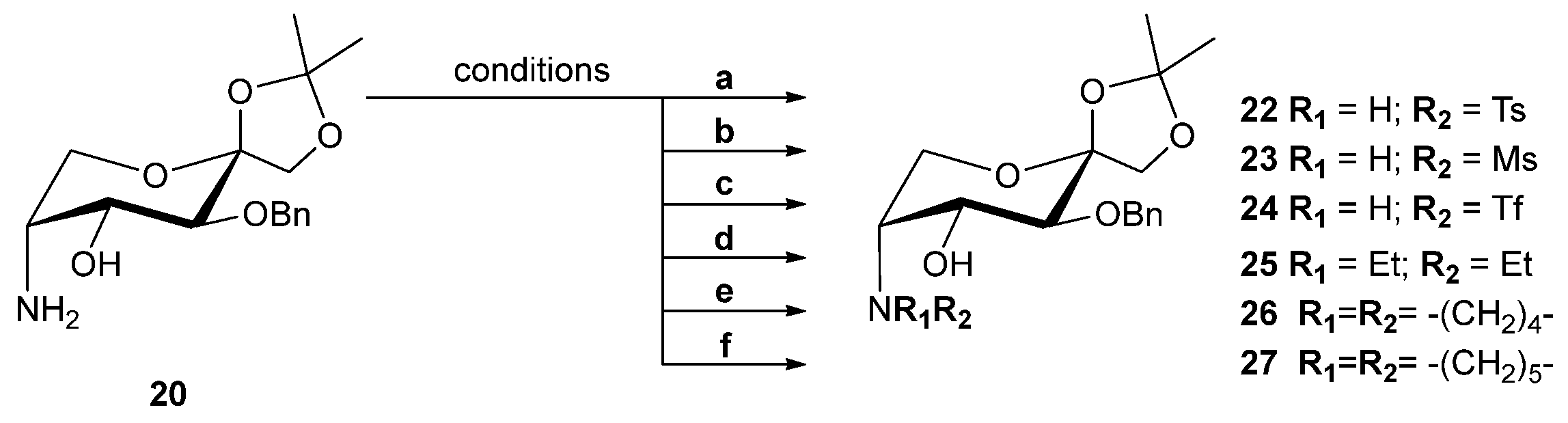
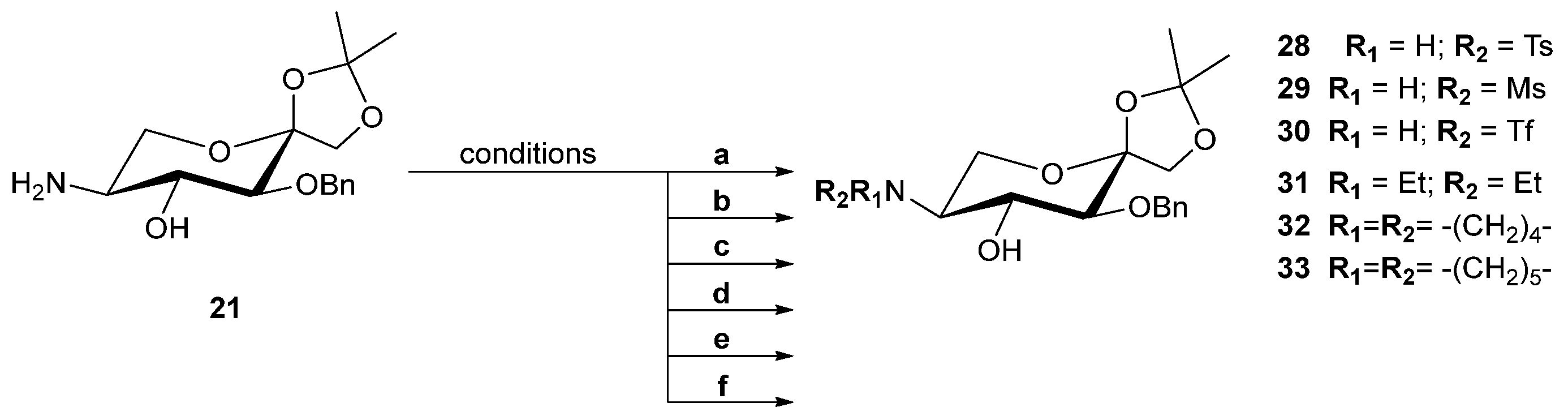
| Ligand | Conversion (%) b | ee (%) b | Configuration c |
|---|---|---|---|
| 1 | 86 | 56 | (S) |
| 2 | 75 | 46 | (S) |
| 3 | 77 | 35 | (S) |
| 4 | 90 | 40 | (S) |
| 5 | 89 | 21 | (R) |
| 6 | 90 | 4 | (R) |
| 7 | 80 | 40 | (S) |
| 8 | 89 | 14 | (S) |
| Ligand | Conversion (%) b | ee (%) b | Configuration c |
|---|---|---|---|
| 9 | 100 | 72 | (S) |
| 10 | 100 | 53 | (S) |
| 11 | 80 | 6 | (S) |
| 12 | 99 | 15 | (S) |
| 13 | 98 | 11 | (S) |
| 14 | 96 | 12 | (S) |
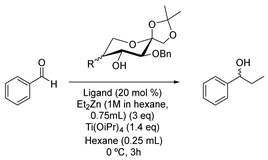 | ||||||
|---|---|---|---|---|---|---|
| Entry | Ligand | R | β-Amino Alcohol | Conversion [b] | ee (%) [b] | Configuration [c] |
| 1 | 20 |  | E | 91 | 49 | S |
| 2 | 22 | 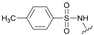 | E | 100 | 92 | S |
| 3 | 23 | 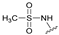 | E | 98 | 40 | S |
| 4 | 24 |  | E | 100 | 52 | S |
| 5 | 25 | 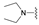 | E | 100 | 50 | S |
| 6 | 26 | 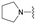 | E | 100 | 49 | S |
| 7 | 27 | 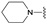 | E | 100 | 55 | S |
| 8 | 21 |  | Z | 78 | 2 | R |
| 9 | 28 | 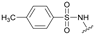 | Z | 91 | 30 | R |
| 10 | 29 | 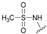 | Z | 98 | 1 | S |
| 11 | 30 | 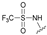 | Z | 100 | 4 | R |
| 12 | 31 | 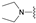 | Z | 100 | 3 | S |
| 13 | 32 | 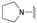 [c]
[c] | Z | 100 | 1 | R |
| 14 | 33 | 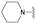 [c]
[c] | Z | 100 | 19 | S |
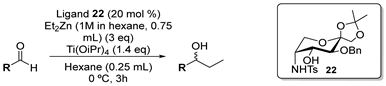 | |||||
|---|---|---|---|---|---|
| Entry | R | Conversion (%) [b] | Yield (%) | ee (%) [b] | Configuration [c] |
| 1 |  | 100 | 80 | 92 | S |
| 2 |  | 98 | 81 | 92 | S |
| 3 | 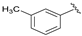 | 100 | 90 | 96 | S |
| 4 | 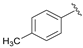 | 95 | 64 | 83 | S |
| 5 |  | 95 | 78 | 92 | S |
| 6 | 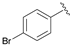 | 100 | 89 | 85 | S |
| 7 | 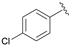 | 90 | 68 | 98 | S |
| 8 | 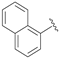 | 99 [e] | 84 | 92 [e] | S |
| 9 | 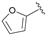 | 100 | 62 | 13 | R |
| 10 | 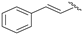 | 95 | 85 | 72 | R |
| 11 | 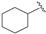 | 70 | 59 | 35 | S |
| 12 | 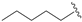 | 81 | 57 | 62 | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Delgado, F.J.; Lo Re, D.; Franco, F.; Tamayo, J.A. Carbohydrate-Based Chiral Ligands for the Enantioselective Addition of Diethylzinc to Aldehydes. Pharmaceuticals 2025, 18, 1088. https://doi.org/10.3390/ph18081088
López-Delgado FJ, Lo Re D, Franco F, Tamayo JA. Carbohydrate-Based Chiral Ligands for the Enantioselective Addition of Diethylzinc to Aldehydes. Pharmaceuticals. 2025; 18(8):1088. https://doi.org/10.3390/ph18081088
Chicago/Turabian StyleLópez-Delgado, F. Javier, Daniele Lo Re, F. Franco, and J. A. Tamayo. 2025. "Carbohydrate-Based Chiral Ligands for the Enantioselective Addition of Diethylzinc to Aldehydes" Pharmaceuticals 18, no. 8: 1088. https://doi.org/10.3390/ph18081088
APA StyleLópez-Delgado, F. J., Lo Re, D., Franco, F., & Tamayo, J. A. (2025). Carbohydrate-Based Chiral Ligands for the Enantioselective Addition of Diethylzinc to Aldehydes. Pharmaceuticals, 18(8), 1088. https://doi.org/10.3390/ph18081088






