Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications
Abstract
1. Introduction
2. Research Strategy
3. Preparation of Drug Nanoparticles
3.1. Nanomilling of Drug Particles
3.1.1. Milling Technologies
3.1.2. Challenges in the Preparation of Nanoparticles Using Milling
3.1.3. Processing of Nanosuspension into Solid Form
3.2. Precipitation of Drug Nanoparticles
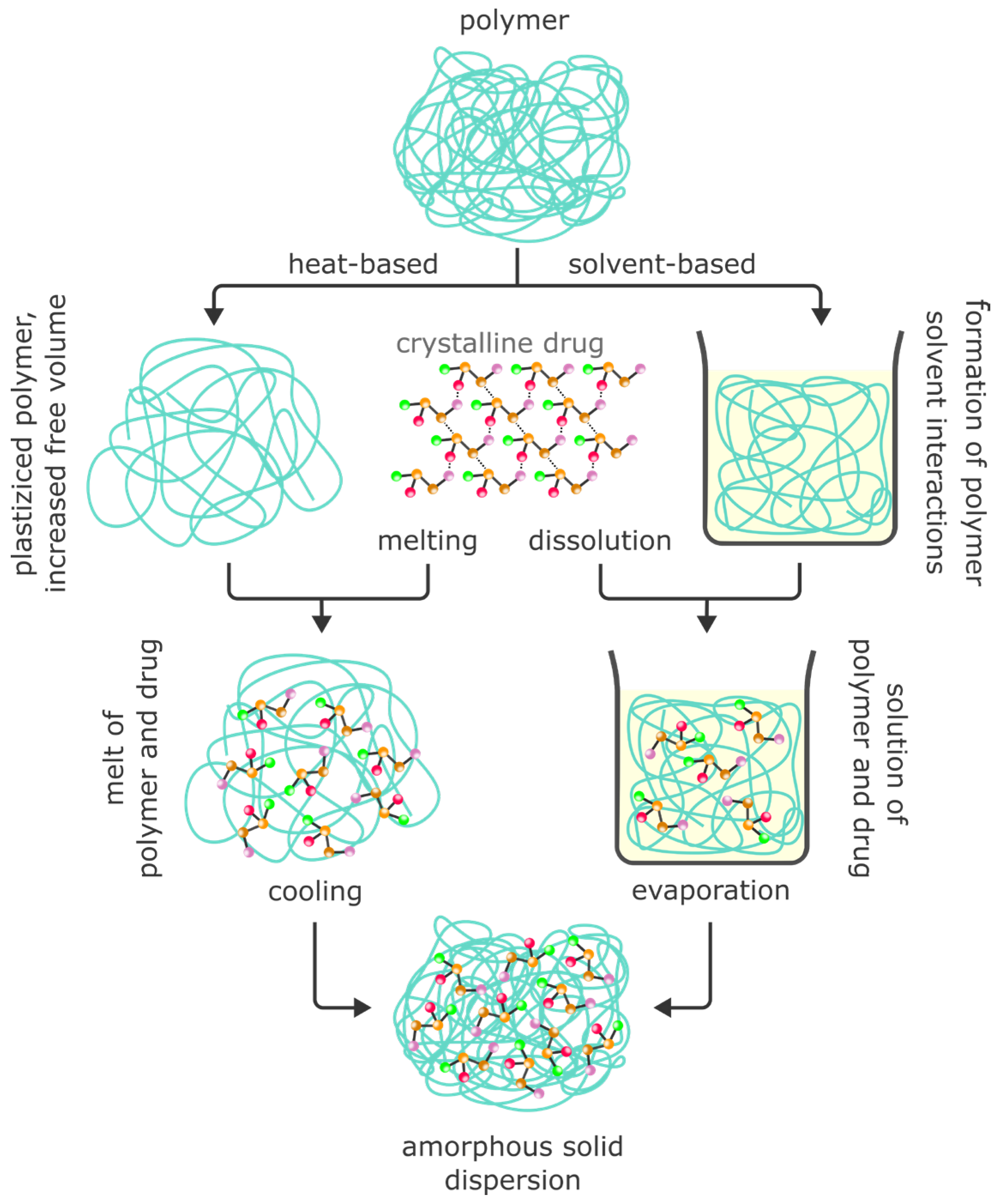
4. Solid Dispersions
- (1)
- Eutectic mixtures;
- (2)
- Solid solutions;
- (3)
- Crystalline dispersions.
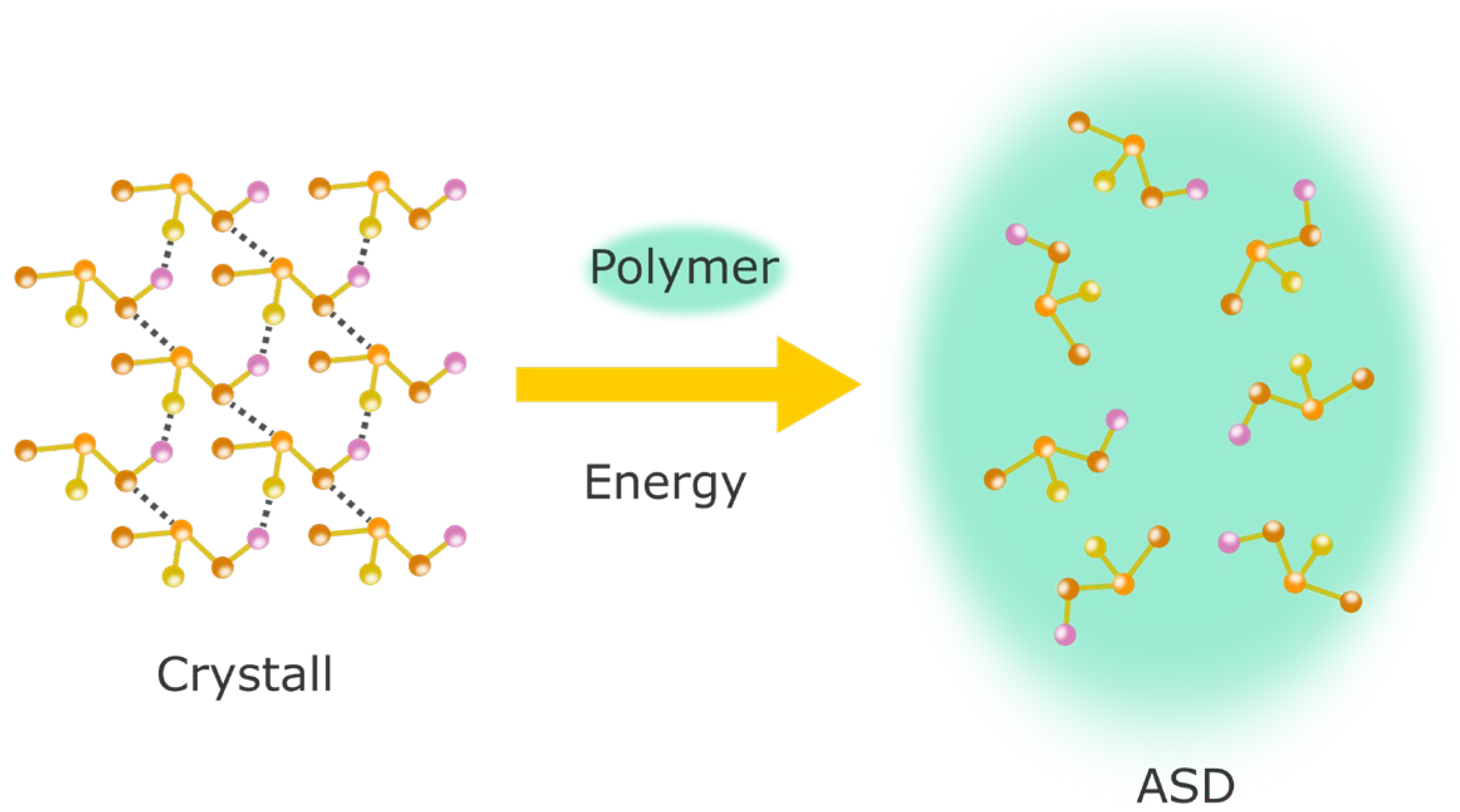
4.1. Amorphous Solid Dispersions (ASDs)
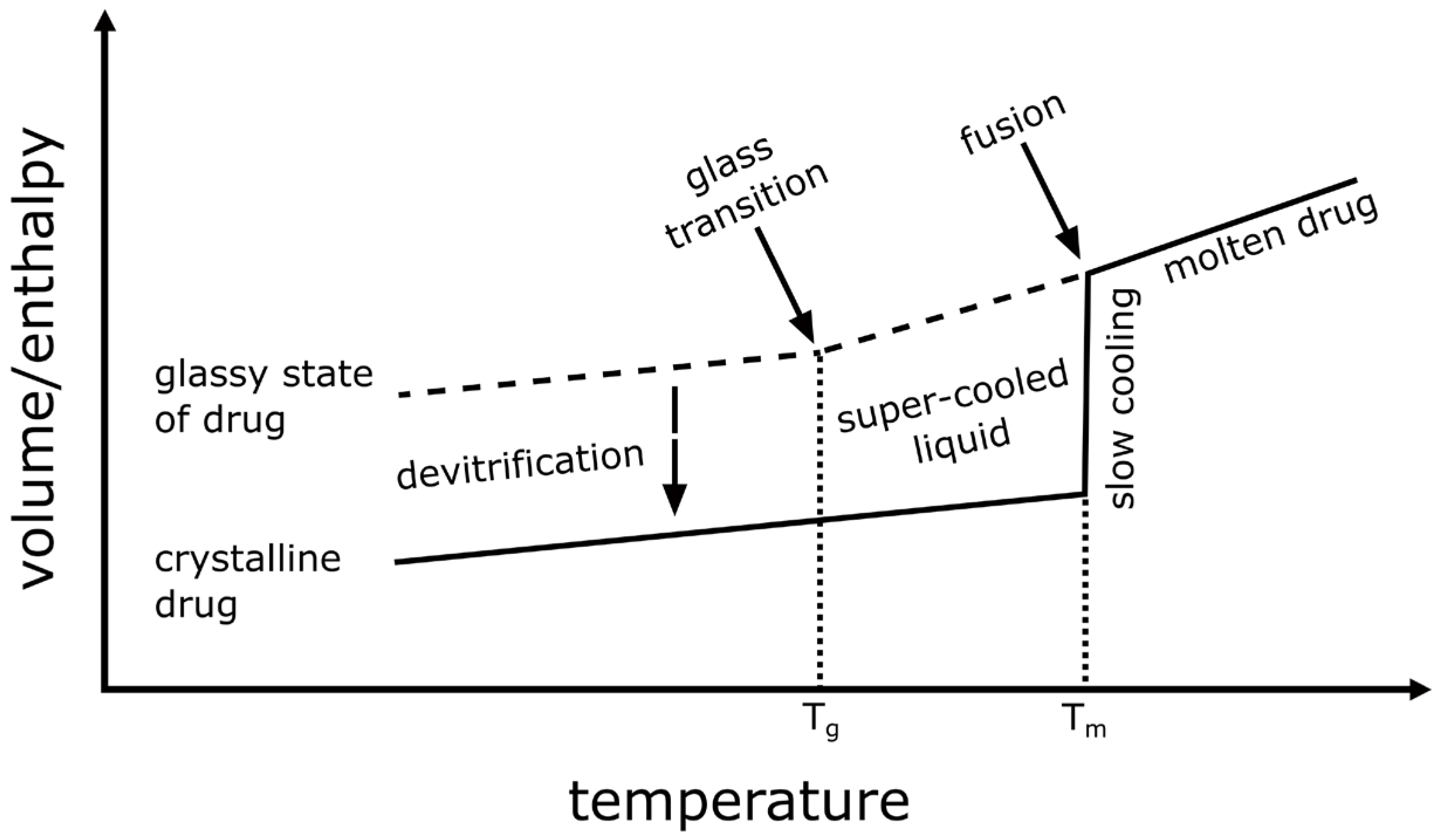
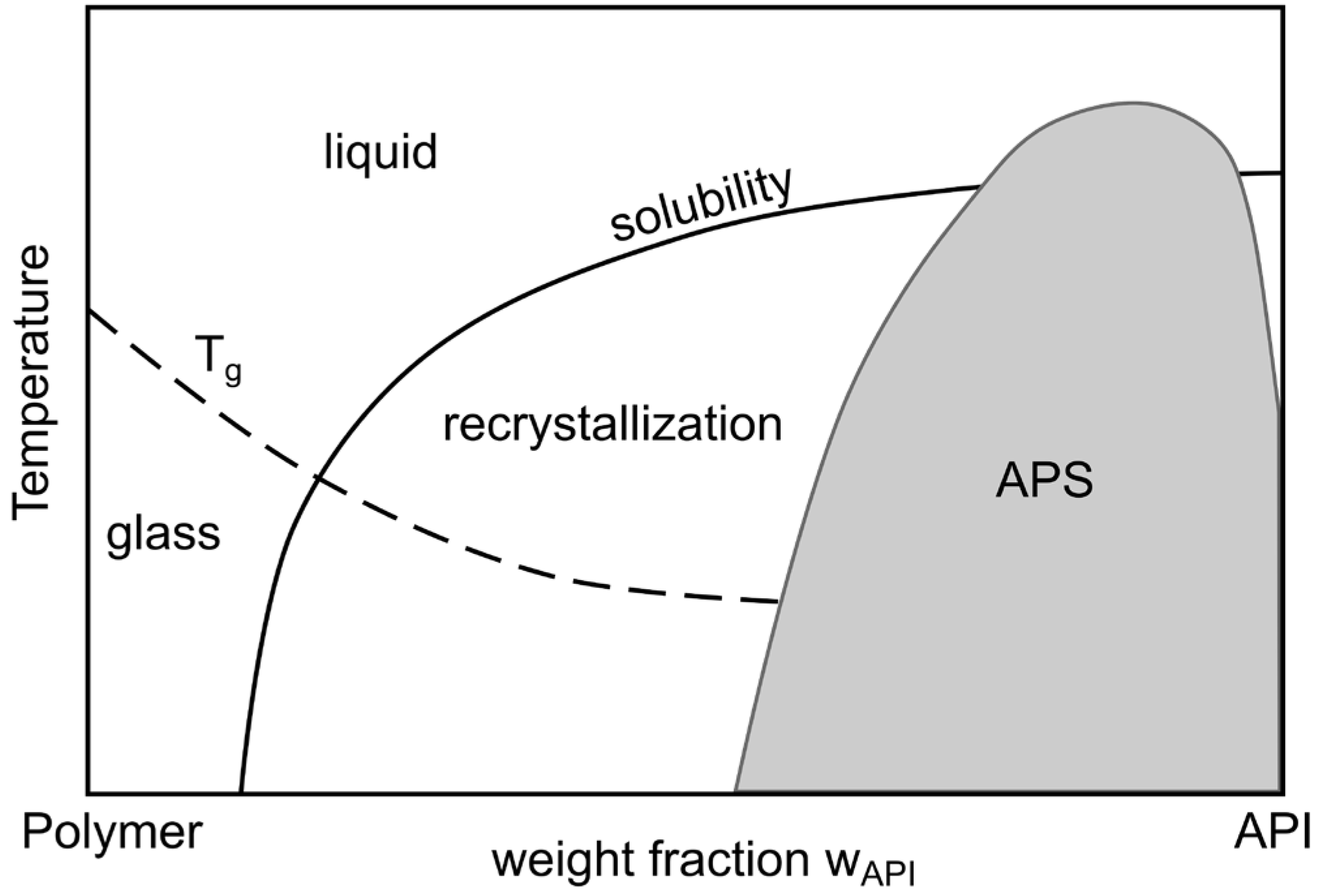
4.1.1. Theoretical Background to the Preparation of ASDs
4.1.2. Spray Drying of Protein-Based ASDs
4.1.3. Hot-Melt Extrusion
- a.
- HME with carbon dioxide
- b.
- HME and 3D printing using Fused Deposition Modeling (FDM)
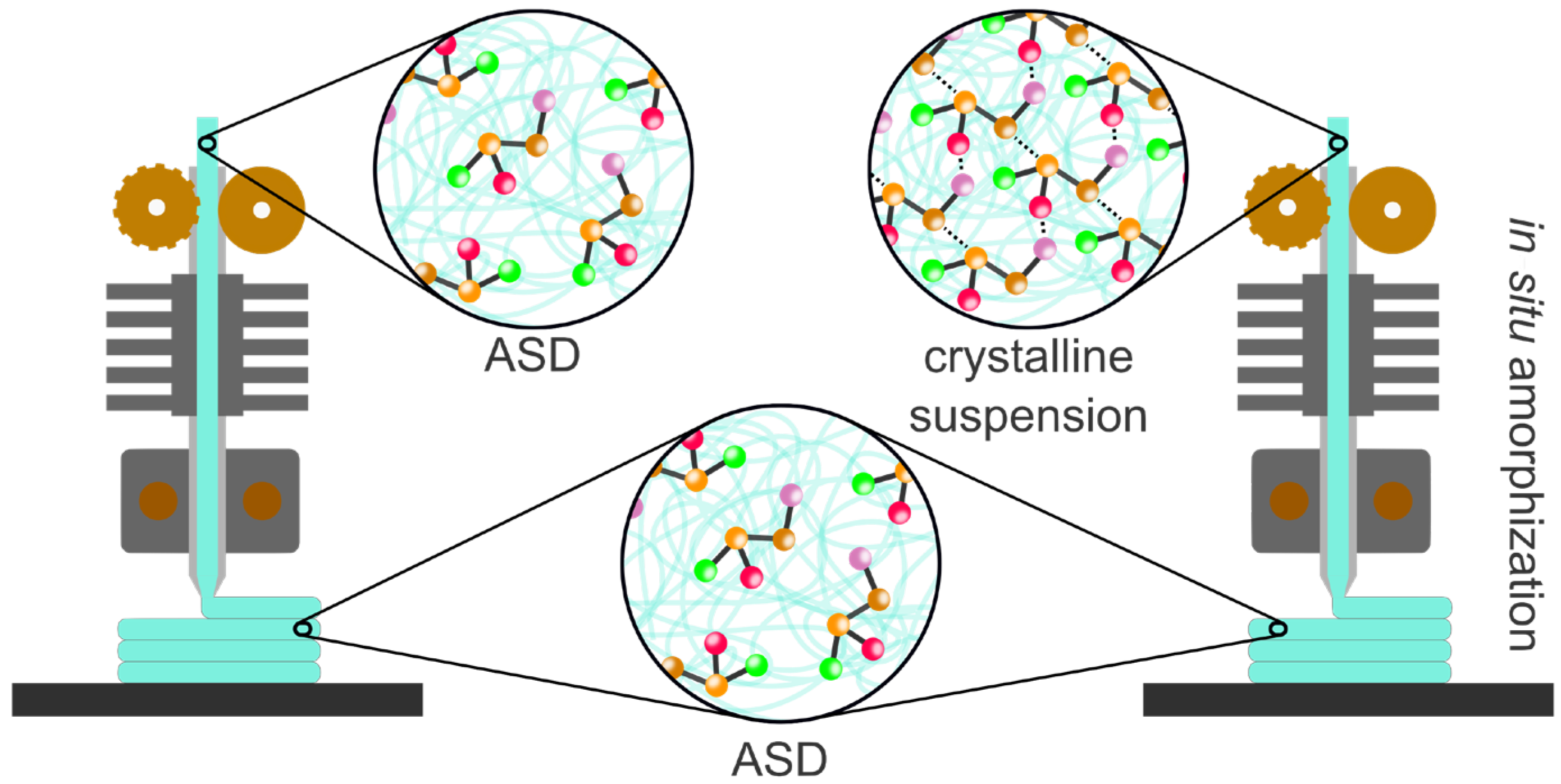
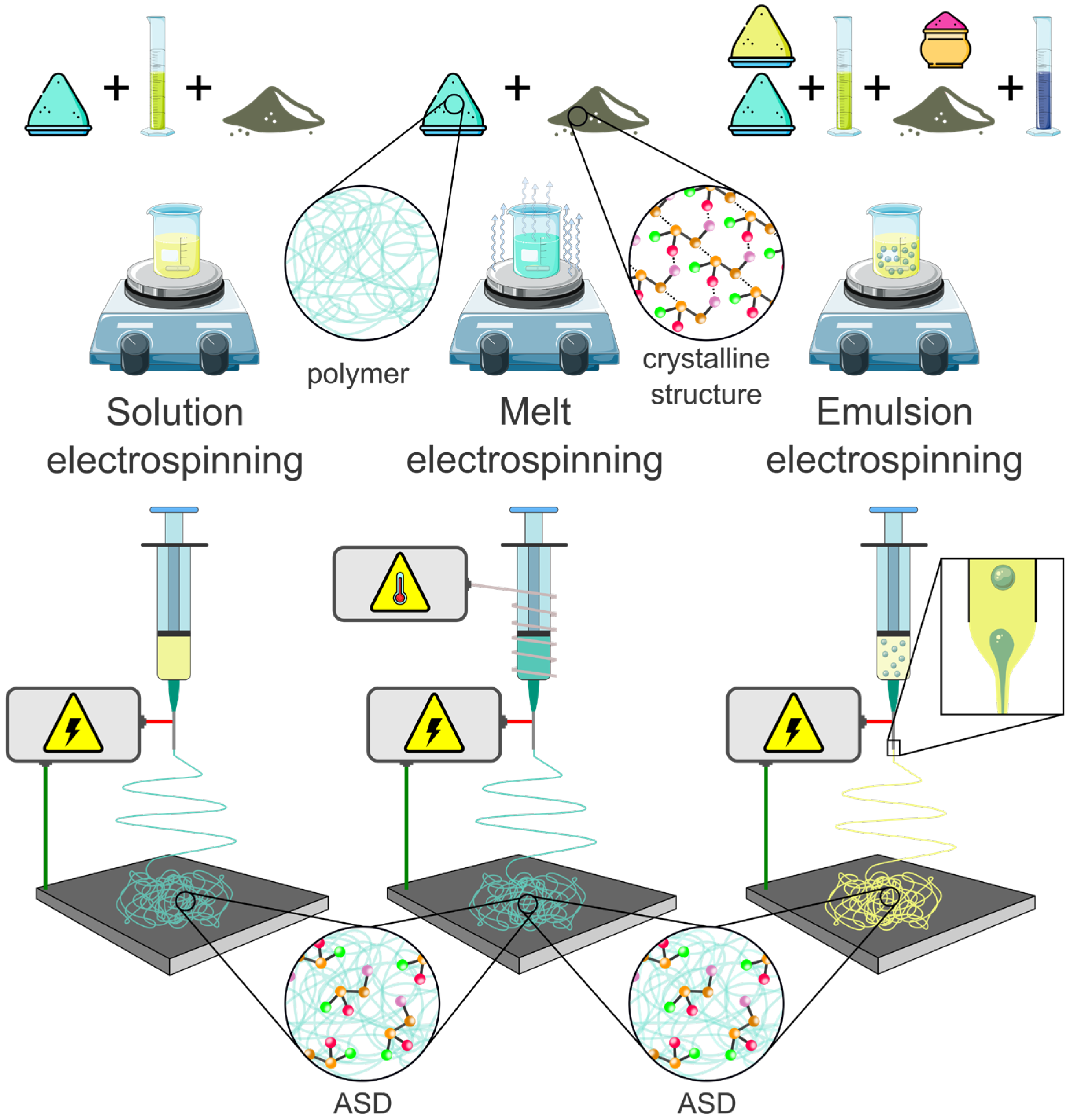
4.1.4. Electrospinning of ASDs
- a.
- Solution electrospinning (SES)
- b.
- Melt electrospinning (MES)
- c.
- Emulsion electrospinning (EES)
- d.
- Current trends in electrospinning
4.2. Crystalline Dispersions
4.3. Mesoporous Systems and Aerogels
5. Lipid-Based Formulations
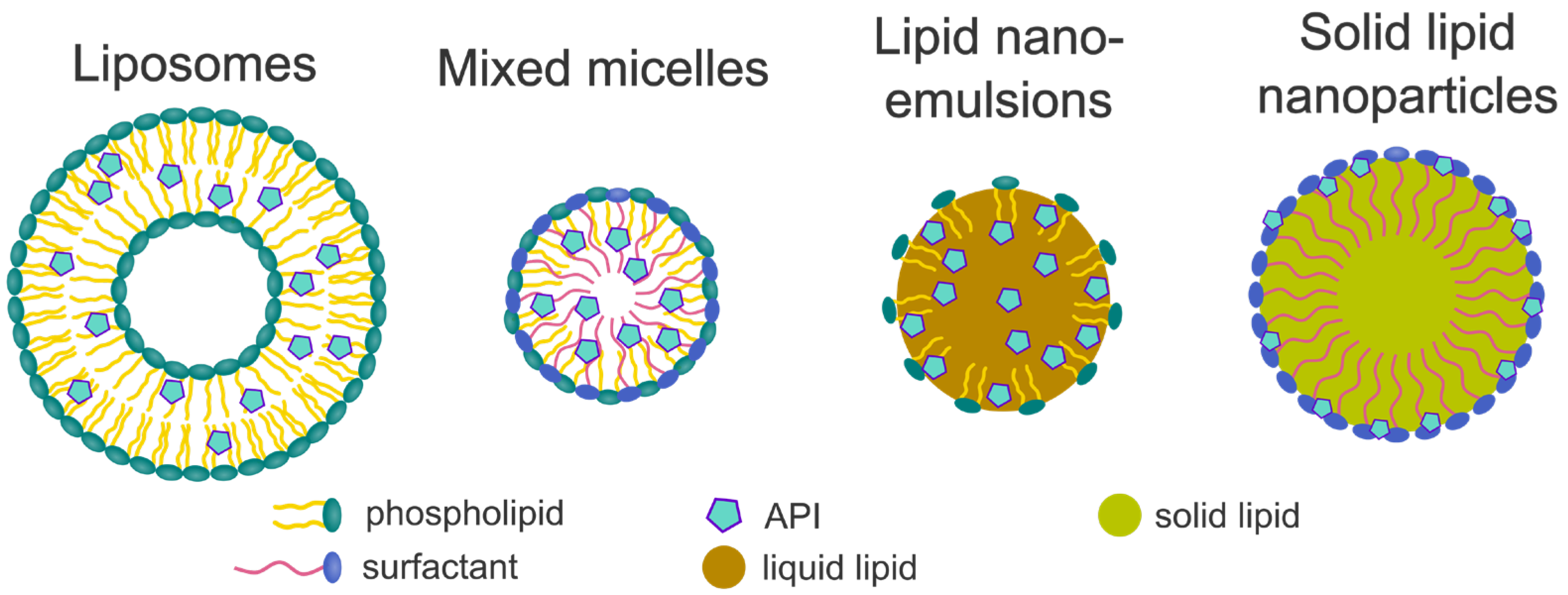
5.1. Liposomes
5.1.1. Composition of Liposomes
5.1.2. Preparation of Liposomal Formulations Containing Poorly Water-Soluble Drugs
5.2. Mixed Micelles
5.2.1. Liquid MM Formulations
5.2.2. Solid MM Formulations
5.3. Lipid Nanoemulsions
5.3.1. Nanoemulsions
5.3.2. Self-(Micro/Nano)emulsifying Formulations
5.4. Solid-Lipid-Based Formulations
6. Major Findings and Future Perspectives
6.1. Major Findings
6.2. Future Prospective
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stielow, M.; Witczynska, A.; Kubryn, N.; Fijalkowski, L.; Nowaczyk, J.; Nowaczyk, A. The bioavailability of drugs-The current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef] [PubMed]
- Lipp, R. The innovator pipeline: Bioavailability challenges and advanced oral drug delivery opportunities. Am. Pharm. Rev. 2013, 16, 10–16. [Google Scholar]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J. Strategies to address low drug solubility in discovery and development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Yalkowsky, S.H.; Valvani, S.C. Solubility and partitioning I: Solubility of nonelectrolytes in water. J. Pharm. Sci. 1980, 69, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Yalkowsky, S.H. Estimation of the aqueous solubility I: Application to organic nonelectrolytes. J. Pharm. Sci. 2001, 90, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, C.A.S.; Charman, W.N.; Porter, C.J.H. Computational prediction of formulation strategies for beyond-rule-of-5 compounds. Adv. Drug. Deliv. Rev. 2016, 101, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solution. J. Am. Chem. Soc. 1897, 19, 930–934. [Google Scholar] [CrossRef]
- Wurster, D.E.; Taylor, P.W. Dissolution Rates. J. Pharm. Sci. 1965, 54, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, J.-U.A.H.; Müller, R.H. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomed. 2008, 3, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Vermant, J.; Martens, J.A.; Froyen, L.; Humbeeck, J.V.; Van den Mooter, G.; Augustijns, P. Solubility increases associated with crystalline drug nanoparticles: Methodologies and significance. Mol. Pharm. 2010, 7, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- International Standard ISO 80004-1:2023; Nanotechnologies—Vocabulary—Part 1: Core Vocabulary. ISO: Geneva, Switzerland, 2023.
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Rabinow, B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004, 3, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.B.; Uday, B.K. Nanoparticle Technology for Drug Delivery; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Moschwitzer, J.P. Drug nanocrystals in the commercial pharmaceutical development process. Int. J. Pharm. 2013, 453, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Liversidge, G.G.; Conzentino, P. Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats. Int. J. Pharm. 1995, 125, 309–313. [Google Scholar] [CrossRef]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug. Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Juhnke, M.; Weichert, R. Zerkleinerung weicher Materialien ohne Verunreinigung der Produkte durch die Mahlkörper. Chem. Ing. Tech. 2005, 77, 90–94. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, C.F.; Kwade, A. Process engineering with planetary ball mills. Chem. Soc. Rev. 2013, 42, 7660–7667. [Google Scholar] [CrossRef] [PubMed]
- Juhnke, M.; Berghausen, J.; Timpe, C. Accelerated Formulation Development for nanomilled active pharmaceutical ingredients using a screening approach. Chem. Eng. Technol. 2010, 33, 1412–1418. [Google Scholar] [CrossRef]
- Koehler, J.K.; Schmager, S.; Bender, V.; Steiner, D.; Massing, U. Preparation of nanosized pharmaceutical formulations by dual centrifugation. Pharmaceuticals 2023, 16, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Bögershausen, A.; Rischer, M.; Schubert, R.; Massing, U. Dual centrifugation—A new technique for nanomilling of poorly soluble drugs and formulation screening by an DoE-approach. Int. J. Pharm. 2017, 530, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Liebich, L.; Bogershausen, A.; Massing, U.; Hoffmann, S.; Mende, S.; Rischer, M. Rapid development of API nano-formulations from screening to production combining dual centrifugation and wet agitator bead milling. Int. J. Pharm. 2019, 565, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Breitung-Faes, S.; Kwade, A. Breakage, temperature dependency and contamination of Lactose during ball milling in ethanol. Adv. Powder Technol. 2016, 27, 1700–1709. [Google Scholar] [CrossRef]
- Mahlberg, L.; Steiner, D. Patient-individual dosing of poorly water-soluble drugs—Printing drug-containing, oily inks on structured film templates. In Proceedings of the 14th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Vienna, Austria, 18–21 March 2024. Poster Presentation. [Google Scholar]
- da Igreja, P.; Erve, A.; Thommes, M. Melt milling as manufacturing method for solid crystalline suspensions. Eur. J. Pharm. Biopharm. 2021, 158, 245–253. [Google Scholar] [CrossRef]
- da Igreja, P.; Klump, D.; Bartsch, J.; Thommes, M. Reduction of submicron particle agglomeration via melt foaming in solid crystalline suspension. J. Dispers. Sci. Technol. 2024, 45, 307–316. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Busmann, E.; Grandeury, A.; Juhnke, M.; Bunjes, H.; Kwade, A. Challenges in nanogrinding of active pharmaceutical ingredients. Chem. Eng. Technol. 2014, 37, 840–846. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient; Danish Technical Press: Vanløse, Denmark, 1967. [Google Scholar]
- Peppersack, C.; Flach, F.; Prziwara, P.; Damm, C.; Breitung-Faes, S.; Peukert, W.; Kwade, A. Conceptual stabilizer selection for nanomilling based on dispersibility parameters. Adv. Powder Technol. 2023, 34, 104197. [Google Scholar] [CrossRef]
- Bitterlich, A.; Laabs, C.; Krautstrunk, I.; Dengler, M.; Juhnke, M.; Grandeury, A.; Bunjes, H.; Kwade, A. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur. J. Pharm. Biopharm. 2015, 92, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Konnerth, C.; Damm, C.; Schmidt, J.; Peukert, W. Mechanical activation of trans-stilbene during wet grinding. Adv. Powder Technol. 2014, 25, 1808–1816. [Google Scholar] [CrossRef]
- Li, M.; Yaragudi, N.; Afolabi, A.; Dave, R.; Bilgili, E. Sub-100nm drug particle suspensions prepared via wet milling with low bead contamination through novel process intensification. Chem. Eng. Sci. 2015, 130, 207–220. [Google Scholar] [CrossRef]
- Flach, F.; Konnerth, C.; Peppersack, C.; Schmidt, J.; Damm, C.; Breitung-Faes, S.; Peukert, W.; Kwade, A. Impact of formulation and operating parameters on particle size and grinding media wear in wet media milling of organic compounds—A case study for pyrene. Adv. Powder Technol. 2016, 27, 2507–2519. [Google Scholar] [CrossRef]
- Flach, F.; Breitung-Faes, S.; Kwade, A. Grinding media wear induced agglomeration of electrosteric stabilized particles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 140–151. [Google Scholar] [CrossRef]
- Flach, F.; Breitung-Faes, S.; Kwade, A. Tailoring product formulation properties to reduce grinding media wear. Chem. Eng. Sci. 2019, 207, 69–78. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, H. Preparation and analysis of a polyacrylate grinding aid for grinding calcium carbonate (GCC) in an ultrafine wet grinding process. Powder Technol. 2014, 254, 470–479. [Google Scholar] [CrossRef]
- Schonfeld, B.; Sundermann, J.; Keller, B.L.; Westedt, U.; Heinzerling, O. Transformation of ABT-199 nanocrystal suspensions into a redispersible drug product-impact of vacuum drum drying, spray drying and tableting on re-nanodispersibility. Pharmaceutics 2024, 16, 782. [Google Scholar] [CrossRef] [PubMed]
- Ouranidis, A.; Gkampelis, N.; Vardaka, E.; Karagianni, A.; Tsiptsios, D.; Nikolakakis, I.; Kachrimanis, K. Overcoming the Solubility Barrier of Ibuprofen by the Rational Process Design of a Nanocrystal Formulation. Pharmaceutics 2020, 12, 969. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Keck, C.M.; Muller, R.H. Solidification of hesperidin nanosuspension by spray drying optimized by design of experiment (DoE). Drug Dev. Ind. Pharm. 2018, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kesisoglou, F.; Panmai, S.; Wu, Y. Nanosizing--oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007, 59, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.L.; Pedersen, G.P.; Kristensen, H.G. Preparation of redispersible dry emulsions by spray drying. Int. J. Pharm. 2001, 212, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Czyz, S.; Wewers, M.; Finke, J.H.; Kwade, A.; van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H. Spray drying of API nanosuspensions: Importance of drying temperature, type and content of matrix former and particle size for successful formulation and process development. Eur. J. Pharm. Biopharm. 2020, 152, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wanning, S.; Suverkrup, R.; Lamprecht, A. Pharmaceutical spray freeze drying. Int. J. Pharm. 2015, 488, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Touzet, A.; Pfefferle, F.; Lamprecht, A.; Pellequer, Y. Formulation of ketoconazole nanocrystal-based cryopellets. AAPS PharmSciTech 2020, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Danjo, K. Design of self-dispersible dry nanosuspension through wet milling and spray freeze-drying for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2013, 50, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, H.; Serajuddin, A.T.M. Development of fully redispersible dried nanocrystals by using sucrose laurate as stabilizer for increasing surface area and dissolution rate of poorly water-soluble drugs. J. Pharm. Sci. 2022, 111, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Moreno, J.; Bilgili, E.; Dave, R. Fast dissolution of poorly water soluble drugs from fluidized bed coated nanocomposites: Impact of carrier size. Int. J. Pharm. 2016, 513, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.; Czyz, S.; Finke, J.H.; John, E.; Van Eerdenbrugh, B.; Juhnke, M.; Bunjes, H.; Kwade, A. Influence of formulation parameters on redispersibility of naproxen nanoparticles from granules produced in a fluidized bed process. Pharmaceutics 2020, 12, 363. [Google Scholar] [CrossRef] [PubMed]
- Wewers, M.; Finke, J.H.; Czyz, S.; Van Eerdenbrugh, B.; John, E.; Buch, G.; Juhnke, M.; Bunjes, H.; Kwade, A. Evaluation of the formulation parameter-dependent redispersibility of API nanoparticles from fluid bed granules. Pharmaceutics 2022, 14, 1688. [Google Scholar] [CrossRef]
- Sahnen, F.; Kamps, J.P.; Langer, K. Conversion of indomethacin nanosuspensions into solid dosage forms via fluid bed granulation and compaction. Eur. J. Pharm. Biopharm. 2020, 154, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.E.; Bose, S. Spray granulation: Importance of process parameters on in vitro and in vivo behavior of dried nanosuspensions. Eur. J. Pharm. Biopharm. 2013, 85, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Schenck, D.; Ghosh, I.; Hollywood, A.; Maulit, E.; Ruegger, C. Application of spray granulation for conversion of a nanosuspension into a dry powder form. Eur. J. Pharm. Sci. 2012, 47, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Krampe, R.; Visser, J.C.; Frijlink, H.W.; Breitkreutz, J.; Woerdenbag, H.J.; Preis, M. Oromucosal film preparations: Points to consider for patient centricity and manufacturing processes. Expert Opin. Drug Deliv. 2016, 13, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Boddu, S.H.S.; Bhandare, R.; Ahmad, S.S.; Nair, A.B. Orodispersible films: Current innovations and emerging trends. Pharmaceutics 2023, 15, 2753. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. Redispersion of nanoparticle-loaded orodispersible films: Preservation of particle fineness. Chem. Ing. Tech. 2017, 89, 1034–1040. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Instant ODFs—Development of an intermediate, nanoparticle-based product platform for individualized medication. Eur. J. Pharm. Biopharm. 2018, 126, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. Efficient production of nanoparticle-loaded orodispersible films by process integration in a stirred media mill. Int. J. Pharm. 2016, 511, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, A.; Peltonen, L. Production of itraconazole nanocrystal-based polymeric film formulations for immediate drug release. Pharmaceutics 2020, 12, 960. [Google Scholar] [CrossRef] [PubMed]
- Van Nguyen, K.; Nguyen, H.T.; Nghiem, L.H.T.; Van Can, M.; Tran, T.H. Nanosized-loratadine embedded orodispersible films for enhanced bioavailability: Scalable preparations and characterizations. AAPS PharmSciTech 2022, 23, 78. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Tidau, M.; Finke, J.H. Embedding of poorly water-soluble drugs in orodispersible films-comparison of five formulation strategies. Pharmaceutics 2022, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Elele, E.; Shen, Y.; Susarla, R.; Khusid, B.; Keyvan, G.; Michniak-Kohn, B. Electrodeless electrohydrodynamic drop-on-demand encapsulation of drugs into porous polymer films for fabrication of personalized dosage units. J. Pharm. Sci. 2012, 101, 2523–2533. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. SOFTs—Structured orodispersible film templates. Eur. J. Pharm. Biopharm. 2019, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Palo, M.; Kolakovic, R.; Laaksonen, T.; Maattanen, A.; Genina, N.; Salonen, J.; Peltonen, J.; Sandler, N. Fabrication of drug-loaded edible carrier substrates from nanosuspensions by flexographic printing. Int. J. Pharm. 2015, 494, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H. Development of Nanosuspension Formulations Compatible with Inkjet Printing for the Convenient and Precise Dispensing of Poorly Soluble Drugs. Pharmaceutics 2022, 14, 449. [Google Scholar] [CrossRef] [PubMed]
- Carou-Senra, P.; Rodriguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Inkjet Printing of Pharmaceuticals. Adv. Mater. 2024, 36, e2309164. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhao, T.; Fang, J.; Song, J.; Dong, H.; Liu, J.; Li, S.; Zhao, M. Recent developments in the use of nanocrystals to improve bioavailability of APIs. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1958. [Google Scholar] [CrossRef] [PubMed]
- Shahidulla, S.M.; Miskan, R.; Sultana, S. Nanosuspensions in pharmaceutical sciences: A comprehensive review. Int. J. Health Sci. Res. 2023, 13, 332–342. [Google Scholar] [CrossRef]
- Ran, Q.; Wang, M.; Kuang, W.; Ouyang, J.; Han, D.; Gao, Z.; Gong, J. Advances of combinative nanocrystal preparation technology for improving the insoluble drug solubility and bioavailability. Crystals 2022, 12, 1200. [Google Scholar] [CrossRef]
- Schikarski, T.; Trzenschiok, H.; Avila, M.; Peukert, W. Impact of solvent properties on the precipitation of active pharmaceutical ingredients. Powder Technol. 2023, 415, 118032. [Google Scholar] [CrossRef]
- Melzig, S.; Finke, J.H.; Schilde, C.; Vierheller, A.; Dietzel, A.; Kwade, A. Fluid mechanics and process design of high-pressure antisolvent precipitation of fenofibrate nanoparticles using a customized microsystem. Chem. Eng. J. 2019, 371, 554–564. [Google Scholar] [CrossRef]
- Melzig, S.; Finke, J.H.; Schilde, C.; Kwade, A. Formation of long-term stable amorphous ibuprofen nanoparticles via antisolvent melt precipitation (AMP). Eur. J. Pharm. Biopharm. 2018, 131, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Shegokar, R.; Müller, R.H. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int. J. Pharm. 2010, 399, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Muller, R.H.; Moschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Raghava Srivalli, K.M.; Mishra, B. Drug nanocrystals: A way toward scale-up. Saudi Pharm. J. 2016, 24, 386–404. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Obi, N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Tekade, A.R.; Yadav, J.N. A Review on solid dispersion and carriers used therein for solubility enhancement of poorly water soluble drugs. Adv. Pharm. Bull. 2020, 10, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Tejaa, S.B.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, A.K. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2013, 4, 70–94. [Google Scholar]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Luebbert, C.; Huxoll, F.; Sadowski, G. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017, 22, 296. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Meiland, P.; Larsen, B.S.; Knopp, M.M.; Tho, I.; Rades, T. A new method to determine drug-polymer solubility through enthalpy of melting and mixing. Int. J. Pharm. 2022, 629, 122391. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.S.; Meiland, P.; Tzdaka, E.; Tho, I.; Rades, T. A unifying approach to drug-in-polymer solubility prediction: Streamlining experimental workflow and analysis. Eur. J. Pharm. Biopharm. 2024, 203, 114478. [Google Scholar] [CrossRef] [PubMed]
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Bohr, A.; Rades, T.; Grohganz, H.; Löbmann, K. Whey proteins as stabilizers in amorphous solid dispersions. Eur. J. Pharm. Sci. 2019, 128, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Bulduk, B.; Widmer, T.; Wiborg, O.; Sanchez-Felix, M.; Löbmann, K. Protein based amorphous solid dispersion: A case study investigating different whey proteins at high drug loading. Pharm. Res. 2023, 40, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Bulduk, B.; Anlahr, J.; Müllers, W.; Löbmann, K. Enhanced dissolution rate of nimodipine through β-lactoglobulin based formulation. Int. J. Pharm. 2023, 635, 122693. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Margrethe Brekstad Kjellin, M.; Schaal, Z.; Zhang, T.; Löbmann, K.; Leng, D. A comparative study between a protein based amorphous formulation and other dissolution rate enhancing approaches: A case study with rifaximin. Pharmaceutics 2022, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, X.; Foderà, V.; Larsson, P.; Schaal, Z.; Bergström, C.A.S.; Löbmann, K.; Kabedev, A. Analysis of stabilization mechanisms in β-lactoglobulin-based amorphous solid dispersions by experimental and computational approaches. Eur. J. Pharm. Sci. 2024, 192, 106639. [Google Scholar] [CrossRef] [PubMed]
- el-Egakey, M.A.; Soliva, M.; Speiser, P. Hot extruded dosage forms. I. Technology and dissolution kinetics of polymeric matrices. Pharm. Acta Helv. 1971, 46, 31–52. [Google Scholar] [PubMed]
- Gottschalk, T.; Özbay, C.; Feuerbach, T.; Thommes, M. Predicting throughput and melt temperature in pharmaceutical hot melt extrusion. Pharmaceutics 2022, 14, 1757. [Google Scholar] [CrossRef] [PubMed]
- Ghebre-Sellassie, I.; Martin, C.E.; Zhang, F.; DiNunzio, J. Pharmaceutical Extrusion Technology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Hot-melt extrusion: From theory to application in pharmaceutical formulation. AAPS PharmSciTech 2016, 17, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Repka, M.A.; Bandari, S.; Kallakunta, V.R.; Vo, A.Q.; McFall, H.; Pimparade, M.B.; Bhagurkar, A.M. Melt extrusion with poorly soluble drugs—An integrated review. Int. J. Pharm. 2018, 535, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Winck, J.; Gottschalk, T.; Thommes, M. Predicting residence time and melt temperature in pharmaceutical hot melt extrusion. Pharmaceutics 2023, 15, 1417. [Google Scholar] [CrossRef] [PubMed]
- Winck, J.; Daalmann, M.; Berghaus, A.; Thommes, M. In-line monitoring of solid dispersion preparation in small scale extrusion based on UV–vis spectroscopy. Pharm. Dev. Technol. 2022, 27, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- LaFountaine, J.S.; McGinity, J.W.; Williams, R.O. Challenges and strategies in thermal processing of amorphous solid dispersions: A review. AAPS PharmSciTech 2016, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Kasturirangan, A.; Koh, C.A.; Teja, A.S. Glass-transition temperatures in CO2 + polymer systems: Modeling and experiment. Ind. Eng. Chem. Res. 2011, 50, 158–162. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F.; Alessi, P.; Cortesi, A.; Eva, F.; Elvassore, N. Polymer plasticization using supercritical carbon dioxide: experiment and modeling. Ind. Eng. Chem. Res. 2003, 42, 3022–3029. [Google Scholar] [CrossRef]
- Handge, U.A.; Altstädt, V. Viscoelastic properties of solutions of polystyrene melts and carbon dioxide: Analysis of a transient shear rheology approach. J. Rheol. 2012, 56, 743–766. [Google Scholar] [CrossRef][Green Version]
- Kiran, E.; Sarver, J.A.; Hassler, J.C. Solubility and diffusivity of CO2 and N2 in polymers and polymer swelling, glass transition, melting, and crystallization at high pressure: A critical review and perspectives on experimental methods, data, and modeling. J. Supercrit. Fluids 2022, 185, 105378. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Heymans, K.; Adriaensen, J.; Liu, D.; Tomasko, D.; Arien, A.; Peeters, J.; Van den Mooter, G.; Brewster, M.E. Hot stage extrusion of p-amino salicylic acid with EC using CO2 as a temporary plasticizer. Int. J. Pharm. 2006, 327, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.P.; Abu-Diak, O.; Kusmanto, F.; Hornsby, P.; Hui, Z.; Jones, D.S. Physicochemical characterization and drug-release properties of celecoxib hot-melt extruded glass solutions. J. Pharm. Pharmacol. 2010, 62, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Sauceau, M.; Nyúl, K.; Rodier, E.; Vajna, B.; Marosi, G.; Fages, J. Use of supercritical CO2-aided and conventional melt extrusion for enhancing the dissolution rate of an active pharmaceutical ingredient. Polym. Adv. Technol. 2012, 23, 909–918. [Google Scholar] [CrossRef]
- Lyons, J.G.; Hallinan, M.; Kennedy, J.E.; Devine, D.M.; Geever, L.M.; Blackie, P.; Higginbotham, C.L. Preparation of monolithic matrices for oral drug delivery using a supercritical fluid assisted hot melt extrusion process. Int. J. Pharm. 2007, 329, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Decorte, A.; Li, H.; Tomasko, D.; Arien, A.; Peeters, J.; Rombaut, P.; Van den Mooter, G.; Brewster, M.E. The effect of pressurized carbon dioxide as a plasticizer and foaming agent on the hot melt extrusion process and extrudate properties of pharmaceutical polymers. J. Supercrit. Fluids 2006, 38, 383–391. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Heymans, K.; Adriaensen, J.; Liu, D.; Tomasko, D.L.; Arien, A.; Peeters, J.; Rombaut, P.; Van den Mooter, G.; et al. The effect of supercritical CO2 as a reversible plasticizer and foaming agent on the hot stage extrusion of itraconazole with EC 20cps. J. Supercrit. Fluids 2007, 40, 153–162. [Google Scholar] [CrossRef]
- Rahimi, S.K.; O’Donnell, K.; Haight, B.; Machado, A.; Martin, C.; Meng, F.; Listro, T.; Zhang, F. Supercritical-CO2 foam extrusion of hydroxypropyl methyl cellulose acetate succinate/itraconazole amorphous solid dispersions: Processing-structure-property relations. J. Pharm. Sci. 2021, 110, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Almutairy, B.; Sarabu, S.; Almotairy, A.; Ashour, E.; Bandari, S.; Batra, A.; Tewari, D.; Durig, T.; Repka, M.A. Processability of AquaSolve™ LG polymer by hot-melt extrusion: Effects of pressurized CO2 on physicomechanical properties and API stability. J. Drug Deliv. Sci. Technol. 2019, 52, 165–176. [Google Scholar] [CrossRef]
- Verreck, G.; Decorte, A.; Heymans, K.; Adriaensen, J.; Cleeren, D.; Jacobs, A.; Liu, D.; Tomasko, D.; Arien, A.; Peeters, J.; et al. The effect of pressurized carbon dioxide as a temporary plasticizer and foaming agent on the hot stage extrusion process and extrudate properties of solid dispersions of itraconazole with PVP-VA 64. Eur. J. Pharm. Sci. 2005, 26, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ashour, E.A.; Kulkarni, V.; Almutairy, B.; Park, J.B.; Shah, S.P.; Majumdar, S.; Lian, Z.; Pinto, E.; Bi, V.; Durig, T.; et al. Influence of pressurized carbon dioxide on ketoprofen-incorporated hot-melt extruded low molecular weight hydroxypropylcellulose. Drug Dev. Ind. Pharm. 2016, 42, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Klueppelberg, J.; Handge, U.A.; Thommes, M.; Winck, J. Influence of carbon dioxide on the phase behavior of pharmaceutical drug-polymer dispersions. Macromol. Chem. Phys. 2025, 226, 2400359. [Google Scholar] [CrossRef]
- Council of the European Union. Council conclusions on personalised medicine for patients. Off. J. Eur. Union 2015, 431, 2–5. [Google Scholar]
- Nashed, N.; Lam, M.; Nokhodchi, A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int. J. Pharm. 2021, 596, 120237. [Google Scholar] [CrossRef] [PubMed]
- Roche, A.; Sanchez-Ballester, N.M.; Bataille, B.; Delannoy, V.; Soulairol, I. Fused deposition modelling 3D printing and solubility improvement of BCS II and IV active ingredients—A narrative review. J. Control. Release 2024, 365, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Khalid, G.M.; Billa, N. Solid dispersion formulations by FDM 3D printing—A review. Pharmaceutics 2022, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Anaya, B.J.; Cerda, J.R.; D’Atri, R.M.; Yuste, I.; Luciano, F.C.; Kara, A.; Ruiz, H.K.; Ballesteros, M.P.; Serrano, D.R. Engineering of 3D printed personalized polypills for the treatment of the metabolic syndrome. Int. J. Pharm. 2023, 642, 123194. [Google Scholar] [CrossRef] [PubMed]
- Parulski, C.; Gresse, E.; Jennotte, O.; Felten, A.; Ziemons, E.; Lechanteur, A.; Evrard, B. Fused deposition modeling 3D printing of solid oral dosage forms containing amorphous solid dispersions: How to elucidate drug dissolution mechanisms through surface spectral analysis techniques? Int. J. Pharm. 2022, 626, 122157. [Google Scholar] [CrossRef] [PubMed]
- Buyukgoz, G.G.; Kossor, C.G.; Davé, R.N. Enhanced supersaturation via Fusion-Assisted Amorphization during FDM 3D printing of crystalline poorly soluble drug loaded filaments. Pharmaceutics 2021, 13, 1857. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Investigation of the degradation and in-situ amorphization of the enantiomeric drug escitalopram oxalate during Fused Deposition Modeling (FDM) 3D printing. Eur. J. Pharm. Sci. 2023, 185, 106423. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Fused Deposition Modeling (FDM) 3D printing of the thermo-sensitive peptidomimetic drug enalapril maleate. Pharmaceutics 2022, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Ponsar, H.; Wiedey, R.; Quodbach, J. Hot-melt extrusion process fluctuations and their impact on critical quality attributes of filaments and 3D-printed dosage forms. Pharmaceutics 2020, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a simple mechanical screening method for predicting the feedability of a pharmaceutical FDM 3D printing filament. Pharm. Res. 2018, 35, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7, 702. [Google Scholar] [CrossRef]
- Cooley, J.F. Apparatus for Electrically Dispersing Fluids. U.S. Patent US1962599A, 2 April 1902. [Google Scholar]
- Morton, W.J. Method of Dispersing Fluids. U.S. Patent US590500A, 29 July 1902. [Google Scholar]
- Formhals, A. Process and Apparatus for Preparing Artificial Threads. U.S. Patent US1975504A, 2 October 1934. [Google Scholar]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G.; et al. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Li, J.-J.; Williams, G.R.; Zhao, M. Electrospun amorphous solid dispersions of poorly water-soluble drugs: A review. J. Control. Release 2018, 292, 91–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.D.; Dalton, P.D.; Hutmacher, D.W. Melt electrospinning today: An opportune time for an emerging polymer process. Prog. Polym. Sci. 2016, 56, 116–166. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Balogh, A.; Farkas, B.; Faragó, K.; Farkas, A.; Wagner, I.; van Assche, I.; Verreck, G.; Nagy, Z.K.; Marosi, G. Melt-blown and electrospun drug-loaded polymer fiber mats for dissolution enhancement: A comparative study. J. Pharm. Sci. 2015, 104, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.K.; Balogh, A.; Drávavölgyi, G.; Ferguson, J.; Pataki, H.; Vajna, B.; Marosi, G. Solvent-free melt electrospinning for preparation of fast dissolving drug delivery system and comparison with solvent-based electrospun and melt extruded systems. J. Pharm. Sci. 2013, 102, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Meng, Z. Melt electrospinning vs. solution electrospinning: A comparative study of drug-loaded poly (ε-caprolactone) fibres. Mater. Sci. Eng. C 2017, 74, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of core-sheath composite nanofibers by emulsion electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Yarin, A.L. Coaxial electrospinning and emulsion electrospinning of core–shell fibers. Polym. Adv. Technol. 2011, 22, 310–317. [Google Scholar] [CrossRef]
- Kamali, H.; Farzadnia, P.; Movaffagh, J.; Abbaspour, M. Optimization of curcumin nanofibers as fast dissolving oral films prepared by emulsion electrospinning via central composite design. J. Drug Deliv. Sci. Technol. 2022, 75, 103714. [Google Scholar] [CrossRef]
- Shibata, T.; Yoshimura, N.; Kobayashi, A.; Ito, T.; Hara, K.; Tahara, K. Emulsion-electrospun polyvinyl alcohol nanofibers as a solid dispersion system to improve solubility and control the release of probucol, a poorly water-soluble drug. J. Drug Deliv. Sci. Technol. 2022, 67, 102953. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Williams, G.R.; Bligh, S.W.A.; White, K.; Zhu, L.-M.; Chatterton, N.P. Self-assembled liposomes from amphiphilic electrospun nanofibers. Soft Matter 2011, 7, 8239. [Google Scholar] [CrossRef]
- Friedl, J.D.; Walther, M.; Vestweber, P.K.; Wächter, J.; Knoll, P.; Jörgensen, A.M.; Bernkop-Schnürch, A.; Windbergs, M. SEDDS-loaded mucoadhesive fiber patches for advanced oromucosal delivery of poorly soluble drugs. J. Control. Release 2022, 348, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Ji, Y.; Ding, Y.; Huang, C.; He, H.; Yu, D.-G. Electrospun self-emulsifying core-shell nanofibers for effective delivery of paclitaxel. Front. Bioeng. Biotechnol. 2023, 11, 1112338. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Yang, C.; Jin, M.; Williams, G.R.; Zou, H.; Wang, X.; Bligh, S.W.A. Medicated Janus fibers fabricated using a Teflon-coated side-by-side spinneret. Colloids Surf. B Biointerfaces 2016, 138, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ge, R.; Zhao, P.; Williams, G.R.; Yu, D.-G.; Bligh, S.A. Exploring wettability difference-driven wetting by utilizing electrospun chimeric Janus microfiber comprising cellulose acetate and polyvinylpyrrolidone. Mat. Des. 2023, 226, 111652. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- van Duong, T.; van den Mooter, G. The role of the carrier in the formulation of pharmaceutical solid dispersions. Part I: Crystalline and semi-crystalline carriers. Expert Opin. Drug Deliv. 2016, 13, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Justen, A.; Kurth, C.; Schaldach, G.; Thommes, M. Preparation of micron and submicron particles via spray drying and electrostatic precipitation. Chem. Eng. Technol. 2023, 46, 343–349. [Google Scholar] [CrossRef]
- Thommes, M.; Ely, D.R.; Carvajal, M.T.; Pinal, R. Improvement of the dissolution rate of poorly soluble drugs by solid crystal suspensions. Mol. Pharm. 2011, 8, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A review of pharmaceutical cocrystal preparation routes and applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Franco, P.; Pessolano, E.; Belvedere, R.; Petrella, A.; Marco, I. Supercritical impregnation of mesoglycan into calcium alginate aerogel for wound healing. J. Supercrit. Fluids 2020, 157, 104711. [Google Scholar] [CrossRef]
- Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- Chin, S.-F.; Jimmy, F.B.; Pang, S.-C. Fabrication of cellulose aerogel from sugarcane bagasse as drug delivery carriers. J. Phys. Sci. 2016, 27, 159–168. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Albayati, T.M.; Ali, N.S.; Salih, I.K. Drug loading methods and kinetic release models using of mesoporous silica nanoparticles as a drug delivery system: A review. S. Afr. J. Chem. Eng. 2024, 50, 261–280. [Google Scholar] [CrossRef]
- Khalbas, A.H.; Albayati, T.M.; Saady, N.M.C.; Zendehboudi, S.; Salih, I.K.; Tofah, M.L. Insights into drug loading techniques with mesoporous silica nanoparticles: Optimization of operating conditions and assessment of drug stability. J. Drug Deliv. Sci. Technol. 2024, 96, 105698. [Google Scholar] [CrossRef]
- Zarinwall, A.; Maurer, V.; Pierick, J.; Oldhues, V.M.; Porsiel, J.C.; Finke, J.H.; Garnweitner, G. Amorphization and modified release of ibuprofen by post-synthetic and solvent-free loading into tailored silica aerogels. Drug Deliv. 2022, 29, 2086–2099. [Google Scholar] [CrossRef] [PubMed]
- Bugnone, C.A.; Ronchetti, S.; Manna, L.; Banchero, M. An emulsification/internal setting technique for the preparation of coated and uncoated hybrid silica/alginate aerogel beads for controlled drug delivery. J. Supercrit. Fluids 2018, 142, 1–9. [Google Scholar] [CrossRef]
- Kazemzadeh, P.; Sayadi, K.; Toolabi, A.; Sayadi, J.; Zeraati, M.; Chauhan, N.P.S.; Sargazi, G. Structure-property relationship for different mesoporous silica nanoparticles and its drug delivery applications: A review. Front. Chem. 2022, 10, 823785. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Chatterjee, B.; Kalave, S.; Pandya, M. Role of fine silica as amorphous solid dispersion carriers for enhancing drug load and preventing recrystallization- A comprehensive review. Curr. Drug Deliv. 2023, 20, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Chen, D.; Xu, W.; Tao, Y.; Pan, Y.; Meng, K.; Shabbir, M.A.B.; Liu, Q.; Huang, L.; et al. Solid lipid nanoparticles with enteric coating for improving stability, palatability, and oral bioavailability of enrofloxacin. Int. J. Nanomed. 2019, 14, 1619–1631. [Google Scholar] [CrossRef] [PubMed]
- Ulker, Z.; Erkey, C. An emerging platform for drug delivery: Aerogel based systems. J. Control. Release 2014, 177, 51–63. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in drug delivery: From design to application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Vinjamur, M.; Mukhopadhyay, M. In vitro release kinetics of drugs from silica aerogels loaded by different modes and conditions using supercritical CO2. J. Supercrit. Fluids 2021, 170, 105142. [Google Scholar] [CrossRef]
- Singh, N.; Vinjamur, M.; Mukhopadhyay, M. Influence of drug properties on loadings and release kinetics of drugs from silica aerogels loaded in supercritical CO2. J. Supercrit. Fluids 2022, 181, 105510. [Google Scholar] [CrossRef]
- Singh, N.; Vinjamur, M.; Mukhopadhyay, M. Insights into adsorptive drug loading on silica aerogels from supercritical CO2. Langmuir 2022, 38, 13075–13083. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Feasibility study of hydrophilic and hydrophobic silica aerogels as drug delivery systems. J. Non-Cryst. Solids 2004, 350, 54–60. [Google Scholar] [CrossRef]
- Smirnova, I.; Suttiruengwong, S.; Seiler, M.; Arlt, W. Dissolution rate enhancement by adsorption of poorly soluble drugs on hydrophilic silica aerogels. Pharm. Dev. Technol. 2004, 9, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Suttiruengwong, S.; Arlt, W. Aerogels: Tailor-made Carriers for Immediate and Prolonged Drug Release. KONA Powder Part. J. 2005, 23, 86–97. [Google Scholar] [CrossRef]
- Uejo, F.; Limwikrant, W.; Moribe, K.; Yamamoto, K. Dissolution improvement of fenofibrate by melting inclusion in mesoporous silica. Asian J. Pharm. Sci. 2013, 8, 329–335. [Google Scholar] [CrossRef]
- Baumgartner, A.; Dobaj, N.; Planinšek, O. Investigating the influence of processing conditions on dissolution and physical stability of solid dispersions with fenofibrate and mesoporous silica. Pharmaceutics 2024, 16, 575. [Google Scholar] [CrossRef] [PubMed]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug delivery formulations of ordered and nonordered mesoporous silica: Comparison of three drug loading methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-C.; Ng, W.K.; Hu, J.; Letchmanan, K.; Ng, J.; Tan, R.B.H. Solvent-free direct formulation of poorly-soluble drugs to amorphous solid dispersion via melt-absorption. Adv. Powder Technol. 2017, 28, 1316–1324. [Google Scholar] [CrossRef]
- Aerts, C.A.; Verraedt, E.; Depla, A.; Follens, L.; Froyen, L.; van Humbeeck, J.; Augustijns, P.; van den Mooter, G.; Mellaerts, R.; Martens, J.A. Potential of amorphous microporous silica for ibuprofen controlled release. Int. J. Pharm. 2010, 397, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zůza, D.; Šoltys, M.; Mužík, J.; Lizoňová, D.; Lhotka, M.; Ulbrich, P.; Kašpar, O.; Štěpánek, F. Silica particles with three levels of porosity for efficient melt amorphisation of drugs. Microporous Mesoporous Mater. 2019, 274, 61–69. [Google Scholar] [CrossRef]
- Mužík, J.; Lizoňová, D.; Zadražil, A.; Štěpánek, F. Drug amorphisation by fluid bed hot-melt impregnation of mesoporous silica carriers. Chem. Eng. J. 2020, 392, 123754. [Google Scholar] [CrossRef]
- Hussain, T.; Waters, L.J.; Parkes, G.M.B.; Shahzad, Y. Microwave processed solid dispersions for enhanced dissolution of gemfibrozil using non-ordered mesoporous silica. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 428–435. [Google Scholar] [CrossRef]
- Ahern, R.J.; Hanrahan, J.P.; Tobin, J.M.; Ryan, K.B.; Crean, A.M. Comparison of fenofibrate-mesoporous silica drug-loading processes for enhanced drug delivery. Eur. J. Pharm. Sci. 2013, 50, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Zolotov, S.A.; Demina, N.B.; Zolotova, A.S.; Shevlyagina, N.V.; Buzanov, G.A.; Retivov, V.M.; Kozhukhova, E.I.; Zakhoda, O.Y.; Dain, I.A.; Filatov, A.R.; et al. Development of novel darunavir amorphous solid dispersions with mesoporous carriers. Eur. J. Pharm. Sci. 2021, 159, 105700. [Google Scholar] [CrossRef] [PubMed]
- Baán, A.; Adriaensens, P.; Lammens, J.; Delgado Hernandez, R.; Vanden Berghe, W.; Pieters, L.; Vervaet, C.; Kiekens, F. Dry amorphisation of mangiferin, a poorly water-soluble compound, using mesoporous silica. Eur. J. Pharm. Biopharm. 2019, 141, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Bahl, D.; Bogner, R.H. Amorphization of indomethacin by co-grinding with neusilin US2: Amorphization kinetics, physical stability and mechanism. Pharm. Res. 2006, 23, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Grobelny, P.; Kazakevich, I.; Zhang, D.; Bogner, R. Amorphization of itraconazole by inorganic pharmaceutical excipients: Comparison of excipients and processing methods. Pharm. Dev. Technol. 2015, 20, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Mellaerts, R.; Jammaer, J.A.G.; van Speybroeck, M.; Chen, H.; van Humbeeck, J.; Augustijns, P.; van den Mooter, G.; Martens, J.A. Physical state of poorly water soluble therapeutic molecules loaded into SBA-15 ordered mesoporous silica carriers: A case study with itraconazole and ibuprofen. Langmuir 2008, 24, 8651–8659. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, T.; Salonen, J.; Tuura, J.; Kumar, N.; Salmi, T.; Murzin, D.Y.; Hamdy, M.S.; Mul, G.; Laitinen, L.; Kaukonen, A.M.; et al. Evaluation of mesoporous TCPSi, MCM-41, SBA-15, and TUD-1 materials as API carriers for oral drug delivery. Drug Deliv. 2007, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 115–121. [Google Scholar] [CrossRef]
- Kiss, T.; Katona, G.; Mérai, L.; Janovák, L.; Deák, Á.; Kozma, G.; Kónya, Z.; Ambrus, R. Development of a hydrophobicity-controlled delivery system containing levodopa methyl ester hydrochloride loaded into a mesoporous silica. Pharmaceutics 2021, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Vraníková, B.; Niederquell, A.; Šklubalová, Z.; Kuentz, M. Relevance of the theoretical critical pore radius in mesoporous silica for fast crystallizing drugs. Int. J. Pharm. 2020, 591, 120019. [Google Scholar] [CrossRef] [PubMed]
- Hentzschel, C.M.; Alnaief, M.; Smirnova, I.; Sakmann, A.; Leopold, C.S. Enhancement of griseofulvin release from liquisolid compacts. Eur. J. Pharm. Biopharm. 2012, 80, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Hentzschel, C.M.; Alnaief, M.; Smirnova, I.; Sakmann, A.; Leopold, C.S. Tableting properties of silica aerogel and other silicates. Drug Dev. Ind. Pharm. 2012, 38, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Liu, L.; Bai, L.; An, H.; Zheng, L.; Yi, L. Preparation and characterization of carbon aerogel microspheres by an inverse emulsion polymerization. J. Non-Cryst. Solids 2011, 357, 793–797. [Google Scholar] [CrossRef]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Katsiotis, C.S.; Tikhomirov, E.; Leliopoulos, C.; Strømme, M.; Welch, K. Development of a simple paste for 3D printing of drug formulations containing a mesoporous material loaded with a poorly water-soluble drug. Eur. J. Pharm. Biopharm. 2024, 198, 114270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gou, K.; Guo, X.; Ke, J.; Li, S.; Li, H. Advances in regulating physicochemical properties of mesoporous silica nanocarriers to overcome biological barriers. Acta Biomater. 2021, 123, 72–92. [Google Scholar] [CrossRef] [PubMed]
- Barkat, A.; Beg, S.; Panda, S.K.; Alharbi, K.S.; Rahman, M.; Ahmed, F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin. Cancer Biol. 2021, 69, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Yu, Y.; Lu, R. Mesoporous silica nanoparticles as carrier to overcome bacterial drug resistant barriers. Int. J. Pharm. 2023, 631, 122529. [Google Scholar] [CrossRef] [PubMed]
- Charman, W.N. Lipids, lipophilic drugs, and oral drug delivery—Some emerging concepts. J. Pharm. Sci. 2000, 89, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Banerjee, A.; Önyüksel, H. Improvement of drug safety by the use of lipid-based nanocarriers. J. Control. Release 2012, 163, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Talegaonkar, S.; Bhattacharyya, A. Potential of lipid nanoparticles (SLNs and NLCs) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Crounse, R.G. Human Pharmacology of Griseofulvin: The Effect of Fat Intake on Gastrointestinal Absorption11From the Department of Dermatology, University of Miami Medical School, Miami, Florida. J. Investig. Dermatol. 1961, 37, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Gangavarapu, A.; Tapia-Lopez, L.V.; Sarkar, B.; Pena-Zacarias, J.; Badruddoza, A.Z.M.; Nurunnabi, M. Lipid nanoparticles for enhancing oral bioavailability. Nanoscale 2024, 16, 18319–18338. [Google Scholar] [CrossRef] [PubMed]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 years of oral lipid-based formulations: Provenance, progress and future perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Gudmundsson, O.S.; Hageman, M.J. Application of lipid-based formulations in drug discovery. J. Med. Chem. 2012, 55, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN227. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G.; Ryman, B.E. Liposomes as carriers of enzymes or drugs: A new approach to the treatment of storage diseases. Biochem. J. 1971, 124, 58. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Drug entrapment in liposomes. FEBS Lett. 1973, 36, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine (first of two parts). N. Engl. J. Med. 1976, 295, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. The carrier potential of liposomes in biology and medicine (second of two parts). N. Engl. J. Med. 1976, 295, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berestein, G.; Fainstein, V.; Hopfer, R.; Mehta, K.; Sullivan, M.P.; Keating, M.; Rosenblum, M.G.; Mehta, R.; Luna, M.; Hersh, E.M.; et al. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: A preliminary study. J. Infect. Dis. 1985, 151, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Peretz, T.; Sulkes, A.; Amselem, S.; Ben-Yosef, R.; Ben-Baruch, N.; Catane, R.; Biran, S.; Barenholz, Y. Systemic administration of doxorubicin-containing liposomes in cancer patients: A phase I study. Eur. J. Cancer Clin. Oncol. 1989, 25, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.N.; Martino, L.D.; Gradoni, L.; Giacchino, R.; Russo, R.; Gaeta, G.B.; Pempinello, R.; Scott, S.; Raimondi, F.; Cascio, A.; et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: A multi-centre trial. QJM 1994, 87, 75–81. [Google Scholar] [PubMed]
- James, N.D.; Coker, R.J.; Tomlinson, D.; Harris, J.R.; Gompels, M.; Pinching, A.J.; Stewart, J.S. Liposomal doxorubicin (Doxil): An effective new treatment for Kaposi’s sarcoma in AIDS. Clin. Oncol. 1994, 6, 294–296. [Google Scholar] [CrossRef] [PubMed]
- van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K. Liposomes for enhanced bioavailability of water-insoluble drugs: In vivo evidence and recent approaches. Pharmaceutics 2020, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Luiz, H.; Oliveira Pinho, J.; Gaspar, M.M. Advancing medicine with lipid-nased nanosystems-The successful case of liposomes. Biomedicines 2023, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Massing, U.; Cicko, S.; Ziroli, V. Dual asymmetric centrifugation (DAC)—A new technique for liposome preparation. J. Control. Release 2008, 125, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhou, S.; Li, J.; Wang, Y.; Su, Y.; Chi, D.; Wang, J.; Wang, X.; He, Z.; Lin, G.; et al. Simple weak-acid derivatives of paclitaxel for remote loading into liposomes and improved therapeutic effects. RSC Adv. 2020, 10, 27676–27687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, F.; Feng, J.; Chen, C.; He, Z.; Sun, M.; Sun, J. Transforming a toxic non-ionizable drug into an efficacious liposome via ionizable prodrug and remote loading strategies against malignant breast tumors. Mol. Pharm. 2023, 20, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Zhou, S.; Li, J.; Wang, J.; Chi, D.; Wang, X.; Lin, G.; He, Z.; Wang, Y. Remote loading paclitaxel–doxorubicin prodrug into liposomes for cancer combination therapy. Acta Pharm. Sin. B 2020, 10, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Wilson, F.P.; Hutchinson, L. A Contribution to the Bio-Chemistry of Haemolysis:-(a) Changes in solubility of the lipoids in presence of one another, and of certain unsaturated organic substances. (b) The balancing action of certain pairs of Haemolysers in preventing Haemolysis. (c) The protective action of serum proteins against Haemolysers. (d) The effects of oxydising and reducing agents upon Haemolysis. Biochem. J. 1909, 4, 346–368. [Google Scholar] [PubMed]
- Martin, G.P.; Marriott, C. Membrane damage by bile salts: The protective function of phospholipids. J. Pharm. Pharmacol. 1981, 33, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Qi, J.; Lu, Y.; Hu, F.; Yin, Z.; Wu, W. Lecithin in mixed micelles attenuates the cytotoxicity of bile salts in CaCo-2 cells. Toxicol. In Vitro 2013, 27, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Hara, I.; Kobayashi, T.; Hayashi, M. Effect of phosphatidyl choline on the hemolytic activities of bile salts. J. Oleo Sci. 1980, 29, 743–747. [Google Scholar] [CrossRef]
- Steffen, H.; Schmidt, D. Injektionslösungen. German Patent Application DE2730570C2, 19 February 1989. [Google Scholar]
- Alkan-Onyuksel, H.; Ramakrishnan, S.; Chai, H.B.; Pezzuto, J.M. A mixed micellar formulation suitable for the parenteral administration of taxol. Pharm. Res. 1994, 11, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Müller, B.W. Increasing drug solubility by means of bile salt-phosphatidylcholine-based mixed micelles. Eur. J. Pharm. Biopharm. 1998, 46, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Müller, B.W. Solubility and stability of lorazepam in bile salt/soya phosphatidylcholine-mixed micelles. Drug Dev. Ind. Pharm. 1999, 25, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Brajtburg, J.; Elberg, S.; Travis, S.J.; Kobayashi, G.S. Treatment of murine candidiasis and cryptococcosis with amphotericin B incorporated into egg lecithin-bile salt mixed micelles. Antimicrob. Agents Chemother. 1994, 38, 294–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, G. Safety of excipients in pediatric formulations-A call for toxicity studies in juvenile animals? Children 2015, 2, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Chougule, M.; Padhi, B.K.; Misra, A. Development of novel lyophilized mixed micelle amphotericin B formulation for treatment of systemic fungal infection. Curr. Drug Deliv. 2005, 2, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, X.; Shen, B.; Xu, H.; Shen, C.; Dai, L.; Bai, J.; Yuan, H.; Han, J. Application of spray granulation for conversion of mixed phospholipid-bile salt micelles to dry powder form: Influence of drug hydrophobicity on nanoparticle reagglomeration. Int. J. Nanomed. 2014, 9, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Shen, C.; Li, X.; Shen, B.; Yu, C.; Xu, P.; Xu, H.; Han, J.; Yuan, H. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv. 2015, 22, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xie, Y.; Qi, J.; Hu, F.; Lu, Y.; Li, S.; Wu, W. Bile salt/phospholipid mixed micelle precursor pellets prepared by fluid-bed coating. Int. J. Nanomed. 2013, 8, 1653–1663. [Google Scholar]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Coupland, J.N.; Elias, R.J. Effect of the lipophilicity of model ingredients on their location and reactivity in emulsions and solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 9–17. [Google Scholar] [CrossRef]
- Kupetz, E.; Bunjes, H. Lipid nanoparticles: Drug localization is substance-specific and achievable load depends on the size and physical state of the particles. J. Control. Release 2014, 189, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Hormann, K.; Zimmer, A. Drug delivery and drug targeting with parenteral lipid nanoemulsions—A review. J. Control. Release 2016, 223, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Damm, C.; Romeis, S.; Peukert, W. Formation of nanoemulsions in stirred media mills. Chem. Eng. Sci. 2013, 102, 300–308. [Google Scholar] [CrossRef]
- Steiner, D.; Bunjes, H. Influence of process and formulation parameters on the preparation of solid lipid nanoparticles by dual centrifugation. Int. J. Pharm. X 2021, 3, 100085. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Ghisleni, D.D.; Pinto, T.J.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—A review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Goke, K.; Bunjes, H. Drug solubility in lipid nanocarriers: Influence of lipid matrix and available interfacial area. Int. J. Pharm. 2017, 529, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shang, Z.; Gao, C.; Du, M.; Xu, S.; Song, H.; Liu, T. Nanoemulsion for solubilization, stabilization, and in vitro release of pterostilbene for oral delivery. AAPS PharmSciTech 2014, 15, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.D.; Johnston, J.A.; Matza, L.S.; Curtis, S.E.; Havel, H.A.; Sweetana, S.A.; Gelhorn, H.L. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer. Adherence 2016, 10, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Vingkar, S.K. Formulation, antimalarial activity and biodistribution of oral lipid nanoemulsion of primaquine. Int. J. Pharm. 2008, 347, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Wang, Y.W.; Huang, M.T.; Ho, C.T.; Huang, Q. Enhancing anti-inflammation activity of curcumin through O/W nanoemulsions. Food Chem. 2008, 108, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Amiji, M.M. Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J. Nanosci. Nanotechnol. 2006, 6, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, H.; Guan, S. Enhancement of the oral bioavailability of breviscapine by nanoemulsions drug delivery system. Drug Dev. Ind. Pharm. 2015, 41, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Huang, Q. Improving the oral bioavailability of curcumin using novel organogel-based nanoemulsions. J. Agric. Food Chem. 2012, 60, 5373–5379. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Poudel, B.K.; Marasini, N.; Yang, K.Y.; Kim, J.W.; Kim, J.O.; Choi, H.-G.; Yong, C.S. Enhanced solubility and oral bioavailability of itraconazole by combining membrane emulsification and spray drying technique. Int. J. Pharm. 2012, 434, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Dollo, G.; Le Corre, P.; Guerin, A.; Chevanne, F.; Burgot, J.L.; Leverge, R. Spray-dried redispersible oil-in-water emulsion to improve oral bioavailability of poorly soluble drugs. Eur. J. Pharm. Sci. 2003, 19, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Schumann, L.V.; Bunjes, H. Processing of Lipid Nanodispersions into Solid Powders by Spray Drying. Pharmaceutics 2022, 14, 2464. [Google Scholar] [CrossRef] [PubMed]
- Groves, M.J.; Mustafa, R.M.A. Measurement of the ‘spontaneity’ of self-emulsifiable oils. J. Pharm. Pharmacol. 1974, 26, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Pouton, C.W. Self-emulsifying drug delivery systems: Assessment of the efficiency of emulsification. Int. J. Pharm. 1985, 27, 335–348. [Google Scholar] [CrossRef]
- Gershanik, T.; Benita, S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm. 2000, 50, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Grune, L.; Bunjes, H. Self-dispersing formulations for the delivery of poorly soluble drugs—Miscibility of phosphatidylcholines with oils and fats. Eur. J. Pharm. Biopharm. 2020, 151, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Grune, L.; Bunjes, H. Solubility of poorly soluble drugs in phosphatidylcholine-based drug delivery systems: Comparison of the loading capacity in the bulk formulation and its dispersed state. Pharmaceuticals 2024, 17, 400. [Google Scholar] [CrossRef] [PubMed]
- Froelich, A.; Osmalek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Kuentz, M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov. Today Technol. 2012, 9, e97–e104. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Yi, T.; Liu, Y.; Huan, D.; He, J.K. Preparation and characterization of self-microemulsifying oral fast dissolving films of total ginkgo flavonoid. Chin. Tradit. Herb. Drugs 2011, 42, 1517–1522. [Google Scholar]
- Xiao, L.; Yi, T.; Liu, Y. A new self-microemulsifying mouth dissolving film to improve the oral bioavailability of poorly water soluble drugs. Drug Dev. Ind. Pharm. 2013, 39, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.; Mader, K. Preparation and characterization of a self-emulsifying pellet formulation. Eur. J. Pharm. Biopharm. 2007, 66, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. A proof of concept for 3D printing of solid lipid-based formulations of poorly water-soluble drugs to control formulation dispersion kinetics. Pharm. Res. 2019, 36, 102. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.W.; Dumont, C.; Caisse, P.; Simon, G.P.; Boyd, B.J. A 3D-printed polymer-lipid-hybrid tablet towards the development of bespoke SMEDDS formulations. Pharmaceutics 2021, 13, 2107. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergstrom, C.A.S. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 120304. [Google Scholar] [CrossRef] [PubMed]
- Meirinho, S.; Rodrigues, M.; Santos, A.O.; Falcao, A.; Alves, G. Self-emulsifying drug delivery systems: An alternative approach to improve brain bioavailability of poorly water-soluble drugs through intranasal administration. Pharmaceutics 2022, 14, 1487. [Google Scholar] [CrossRef] [PubMed]
- Lucks, J.S.; Müller, R.H.; König, B. Solid lipid nanoparticles (SLN)—An alternative parenteral drug delivery system. Eur. J. Pharm. Biopharm. 1992, 38, 33. [Google Scholar]
- Kumar, R.; Singh, A.; Sharma, K.; Dhasmana, D.; Garg, N.; Siril, P.F. Preparation, characterization and in vitro cytotoxicity of Fenofibrate and Nabumetone loaded solid lipid nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 106, 110184. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Gonzalez-Mira, E.; Garcia, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidone-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B. Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H.; Steiniger, F.; Richter, W. Visualizing the structure of triglyceride nanoparticles in different crystal modifications. Langmuir 2007, 23, 4005–4011. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Awad, T.S.; Kristbergsson, K.; McClements, D.J.; Weiss, J. Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN). J. Colloid Interface Sci. 2009, 334, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Westesen, K.; Bunjes, H.; Koch, M.H.J. Physiochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control. Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Müller, R.H.; Mäder, K.; Gohla, S. Solid lipid nanoparticles (SNL) for controlled drug delivery—A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Illing, A.; Unruh, T. Investigation on the flow behavior of dispersions of solid triglyceride nanoparticles. Int. J. Pharm. 2004, 284, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bayon-Cordero, L.; Alkorta, I.; Arana, L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.; Gallego, L.; Alkorta, I. Incorporation of antibiotics into solid lipid nanoparticles: A promising approach to reduce antibiotic resistance emergence. Nanomaterials 2021, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, D.; Sarpietro, M.G.; Castelli, F.; Lauro, M.R.; Torrisi, C.; Russo, S.; Puglia, C. Development of solid lipid nanoparticles as dry powder: Characterization and formulation considerations. Molecules 2023, 28, 1545. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.P.; Gaspar, M.M.; Eleuterio, C.V.; Grenha, A.; Blanco, M.; Goncalves, L.M.D.; Taboada, P.; Almeida, A.J.; Remunan-Lopez, C. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol. Pharm. 2017, 14, 2977–2990. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible Films: A Delivery Platform for Solid Lipid Nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.; Ojewole, E.; Kalhapure, R.; Govender, T. In vitro comparative evaluation of monolayered multipolymeric films embedded with didanosine-loaded solid lipid nanoparticles: A potential buccal drug delivery system for ARV therapy. Drug Dev. Ind. Pharm. 2014, 40, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100. [Google Scholar] [PubMed]
- Hirun, N.; Mahadlek, J.; Limmatvapirat, S.; Sriamornsak, P.; Yonemochi, E.; Furuishi, T.; Kraisit, P. Fabrication and characterization of pectin films containing solid lipid nanoparticles for buccal delivery of fluconazole. Int. J. Mol. Sci. 2024, 25, 5413. [Google Scholar] [CrossRef] [PubMed]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Casadei, M.A.; Cerreto, F.; Cesa, S.; Giannuzzo, M.; Feeney, M.; Marianecci, C.; Paolicelli, P. Solid lipid nanoparticles incorporated in dextran hydrogels: A new drug delivery system for oral formulations. Int. J. Pharm. 2006, 325, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Eugster, R.; Luciani, P. Liposomes: Bridging the gap from lab to pharmaceuticals. Curr. Opin. Colloid Interface Sci. 2025, 75, 101875. [Google Scholar] [CrossRef]
- Tomberg, T.; Hämäläinen, I.; Strachan, C.J.; van Veen, B. Dynamic phase behavior of amorphous solid dispersions revealed with in situ stimulated Raman scattering microscopy. Mol. Pharm. 2024, 21, 6444–6457. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zheng, W.; Huanle, X.; Defang, O. FormulationAI: A novel web-based platform for drug formulation design driven by artificial intelligence. Brief. Bioinform. 2024, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Dong, J.; Ouyang, D. AI-directed formulation strategy design initiates rational drug development. J. Control. Release 2025, 378, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Gao, H.; Ouyang, D. PharmSD: A novel AI-based computational platform for solid dispersion formulation design. Int. J. Pharm. 2021, 604, 120705. [Google Scholar] [CrossRef] [PubMed]
- Bekker, J.; Davis, J. Learning from positive and unlabeled data: A survey. Mach. Learn. 2020, 109, 719–760. [Google Scholar] [CrossRef]
| Advantages | Disadvantages | |
|---|---|---|
| SES |
|
|
| MES |
|
|
| EES |
|
|
| Technology | Principle | BCS Class | Benefits | Drawbacks |
|---|---|---|---|---|
| Nanomilling of drug particles | Nanonization | II (IV) |
|
|
| Precipitation of nanoparticles | Nanonization | II (IV) |
|
|
| Spray drying with protein carriers | ASD formation | II |
|
|
| HME | ASD formation | II |
|
|
| HME with CO2 | ASD formation | II |
|
|
| HME coupled FDM | ASD formation | II |
|
|
| Electrospinning | ASD formation | II |
|
|
| Mesoporous systems and aerogels | Drug amorphization | II (IV) |
|
|
| Liposomes | Drug solubilization | II, IV |
|
|
| Mixed micelles | Drug solubilization | II, IV |
|
|
| Lipid nanoemulsions | Dissolution in lipids | II, IV |
|
|
| Solid-lipid-based formulations | Dissolution in lipids | II, IV |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quodbach, J.; Preis, E.; Karkossa, F.; Winck, J.; Finke, J.H.; Steiner, D. Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications. Pharmaceuticals 2025, 18, 1089. https://doi.org/10.3390/ph18081089
Quodbach J, Preis E, Karkossa F, Winck J, Finke JH, Steiner D. Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications. Pharmaceuticals. 2025; 18(8):1089. https://doi.org/10.3390/ph18081089
Chicago/Turabian StyleQuodbach, Julian, Eduard Preis, Frank Karkossa, Judith Winck, Jan Henrik Finke, and Denise Steiner. 2025. "Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications" Pharmaceuticals 18, no. 8: 1089. https://doi.org/10.3390/ph18081089
APA StyleQuodbach, J., Preis, E., Karkossa, F., Winck, J., Finke, J. H., & Steiner, D. (2025). Novel Strategies for the Formulation of Poorly Water-Soluble Drug Substances by Different Physical Modification Strategies with a Focus on Peroral Applications. Pharmaceuticals, 18(8), 1089. https://doi.org/10.3390/ph18081089









