Evaluation of Antiepileptic Drugs’ Stability in Oral Fluid Samples
Abstract
1. Introduction
2. Results and Discussion
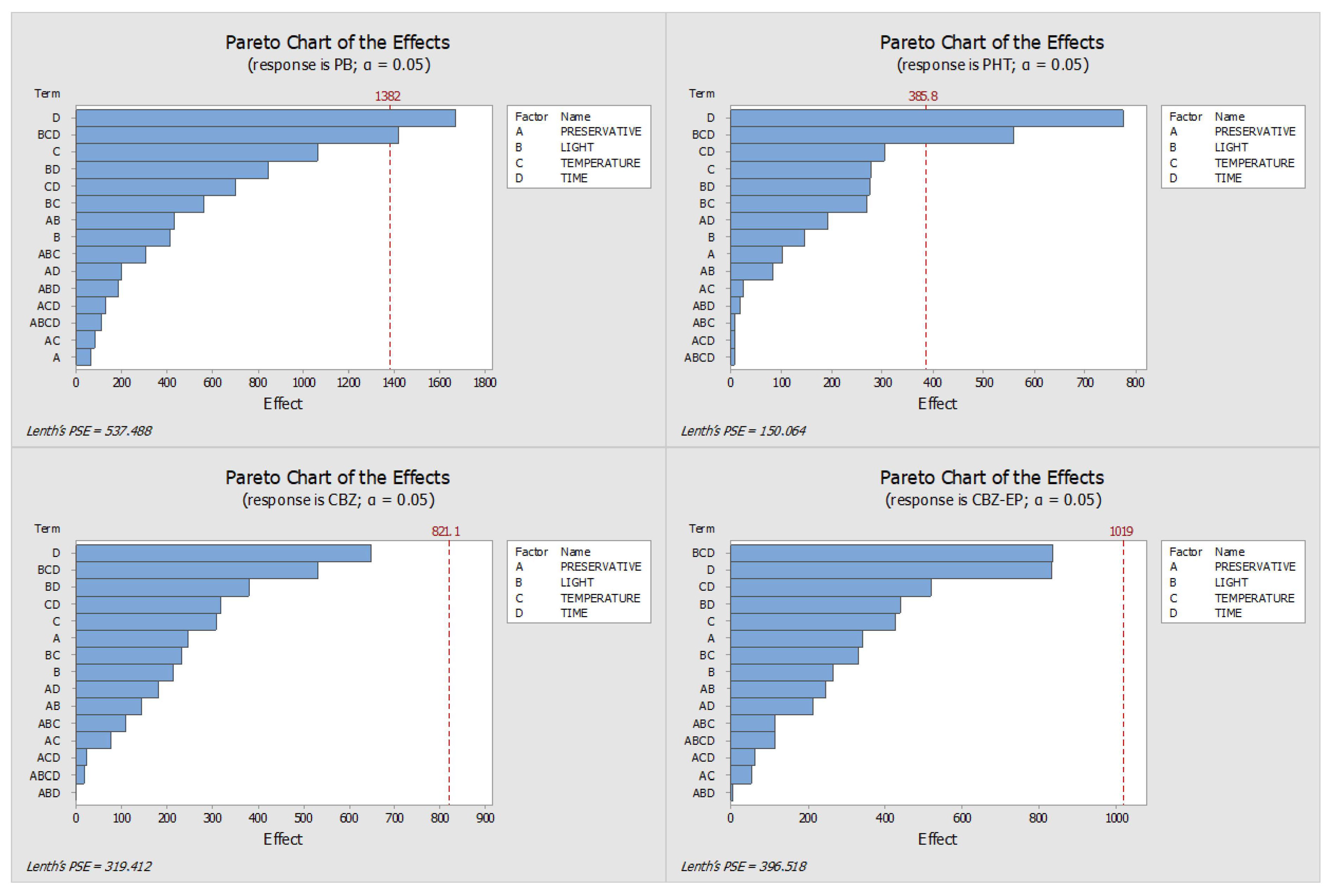
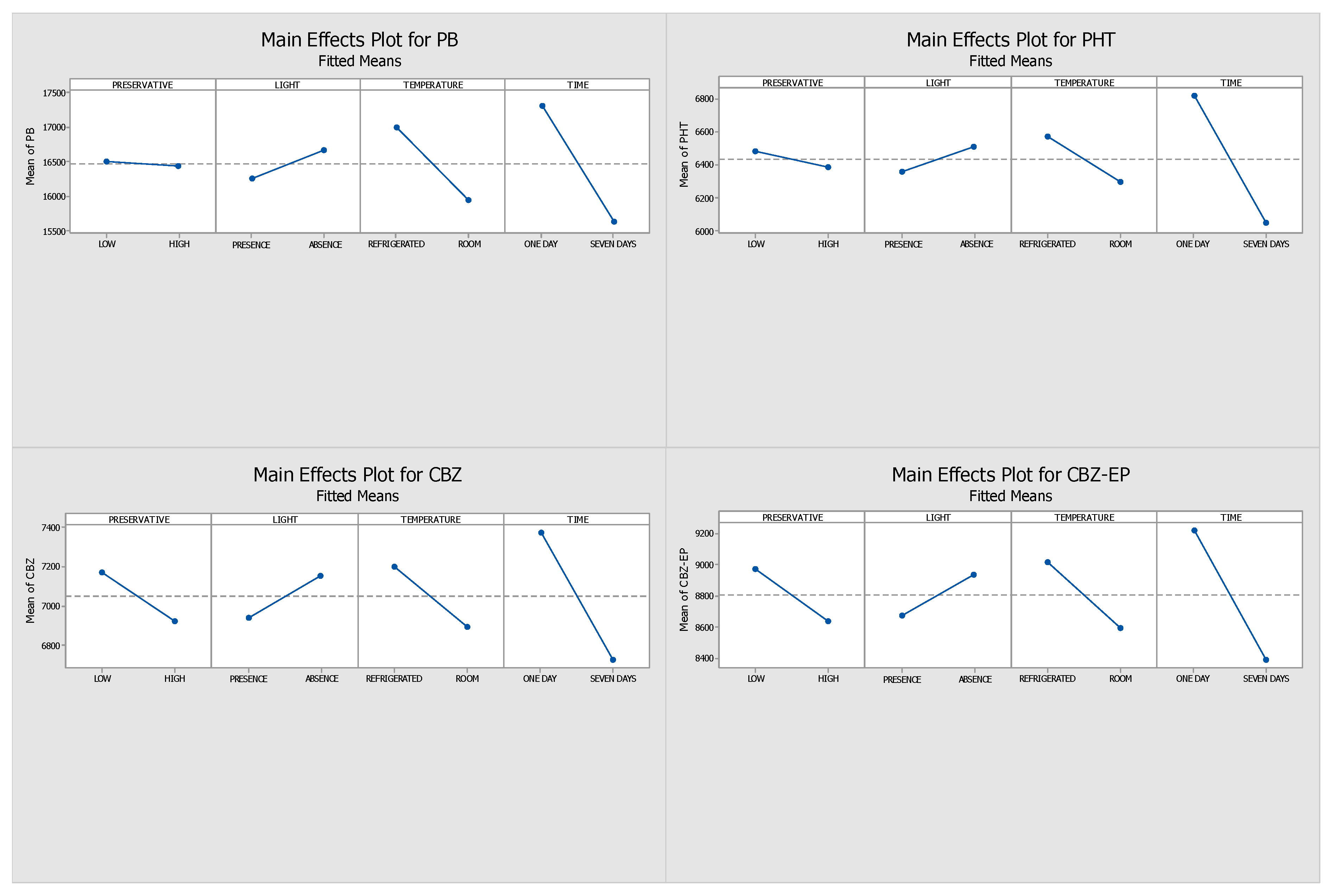
2.1. Optimisation of the Stability Protocol
2.2. Stability Study Without Preservative
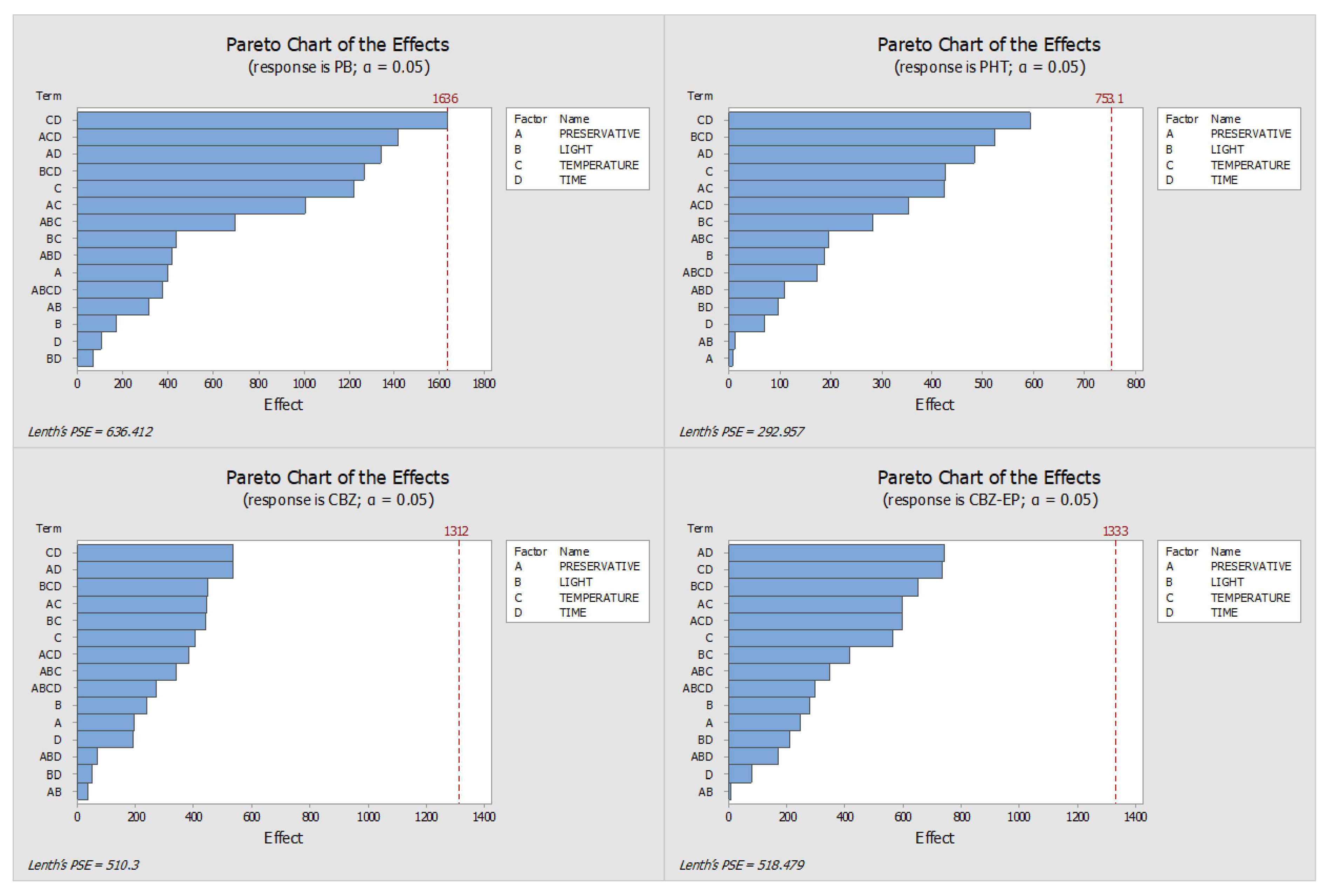
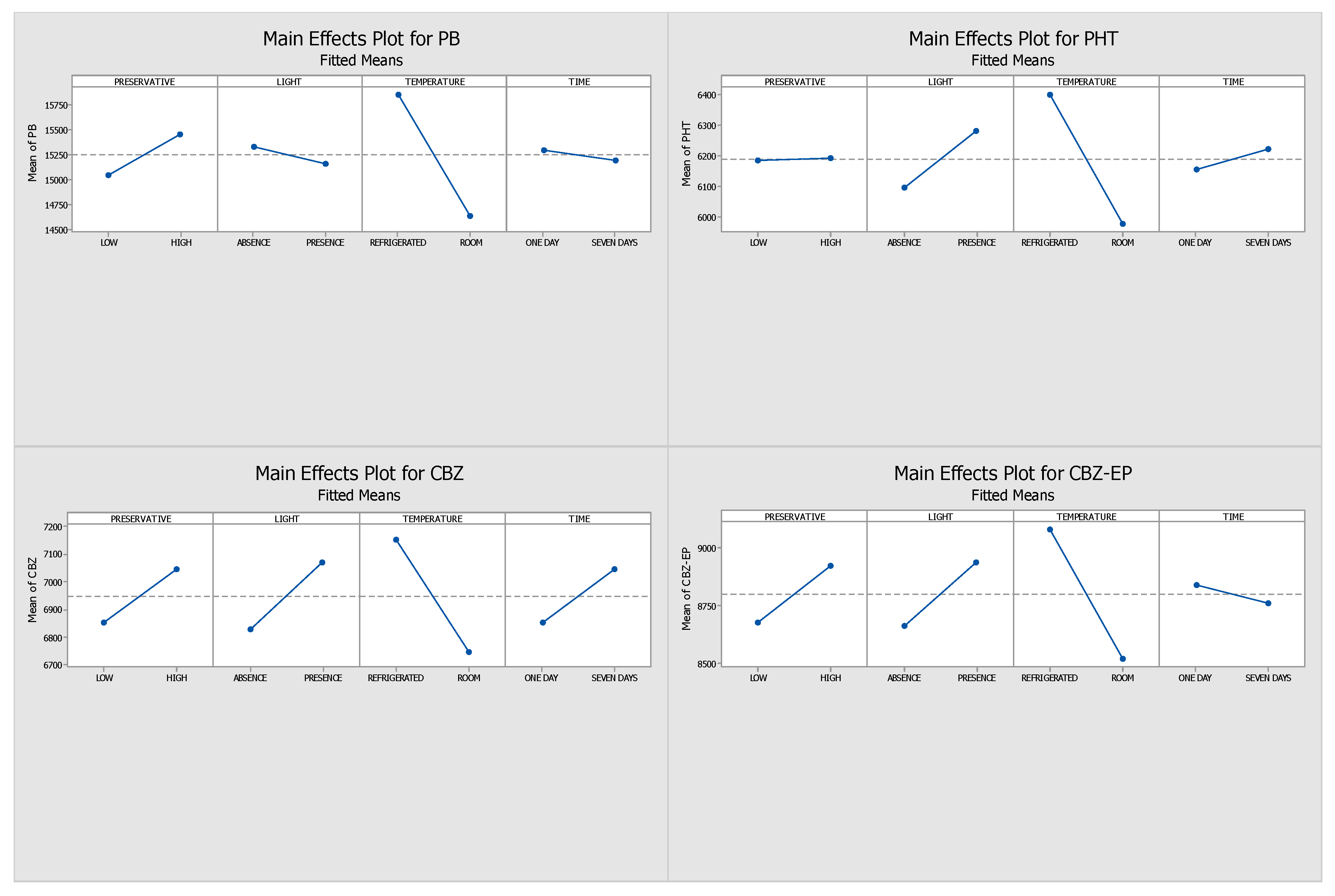
2.3. Final Conditions for the Long-Term Stability Assay
2.4. Long-Term Sample Stability
3. Materials and Methods
3.1. Reagents and Standards
3.2. Biological Specimens
3.3. HPLC-DAD Conditions
3.4. Sample Preparation
3.5. Design of Experiments
3.6. Long-Term Stability Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEDs | Antiepileptic drugs |
| TDM | Therapeutic drug monitoring |
| DSS | Dried saliva spots |
| GC-MS/MS | Gas chromatography–tandem mass spectrometry |
| HPLC-DAD | High-performance liquid chromatography with diode array detection |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LC-MS | Liquid chromatography–mass spectrometry |
| ELISA | Enzyme-linked immunoassay |
| mCOP-PCR | Modified competitive oligonucleotide priming-polymerase chain reaction |
| SERS | Surface-enhanced Raman scattering |
| CBZ | Carbamazepine |
| CBZ-EP | Carbamazepine-10,11-epoxide |
| PHT | Phenytoin |
| PB | Phenobarbital |
| DOE | Design of experiments |
References
- Falco-Walter, J. Epilepsy-Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Global Health: Epilepsy. Semin. Neurol. 2018, 38, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Giussani, G.; Sander, J.W. The Natural History and Prognosis of Epilepsy. Epileptic Disord. 2015, 17, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Johannessen Landmark, C.; Johannessen, S.I.; Patsalos, P.N. Therapeutic Drug Monitoring of Antiepileptic Drugs: Current Status and Future Prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef] [PubMed]
- Neumann, J.; Beck, O.; Dahmen, N.; Böttcher, M. Potential of Oral Fluid as a Clinical Specimen for Compliance Monitoring of Psychopharmacotherapy. Ther. Drug Monit. 2018, 40, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, H.; Abdel-Rehim, M. Saliva as an Alternative Specimen to Plasma for Drug Bioanalysis. A Review. TrAC—Trends Anal. Chem. 2016, 83, 70–79. [Google Scholar] [CrossRef]
- Patrick, M.; Parmiter, S.; Mahmoud, S.H. Feasibility of Using Oral Fluid for Therapeutic Drug Monitoring of Antiepileptic Drugs. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Carona, A.; Bicker, J.; Silva, R.; Silva, A.; Santana, I.; Sales, F.; Falcão, A.; Fortuna, A. HPLC Method for the Determination of Antiepileptic Drugs in Human Saliva and Its Application in Therapeutic Drug Monitoring. J. Pharm. Biomed. Anal. 2021, 197. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Queiroz, J.A. Current Technologies and Considerations for Drug Bioanalysis in Oral Fluid. Bioanalysis 2009, 1, 637–667. [Google Scholar] [CrossRef] [PubMed]
- Patsalos, P.N.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs by Use of Saliva. Ther. Drug Monit. 2013, 35, 4–29. [Google Scholar] [CrossRef] [PubMed]
- de Campos, E.G.; da Costa, B.R.B.; dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative Matrices in Forensic Toxicology: A Critical Review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.C. Stability of 26 Sedative Hypnotics in Six Toxicological Matrices at Different Storage Conditions. J. Anal. Toxicol. 2016, 40, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, V.N.; Stoykova, S.; Runiov, A.; Dimitrova, T.; Aleksandrova, D.; Tsakovski, S.; Mitewa, M. Stability of Diazepam in Blood Samples at Different Storage Conditions and in the Presence of Alcohol. Forensic Sci. Int. 2012, 215, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, X.L.; Zhang, M.; Wang, J.; Zeng, S.; Min, J.Z. Potential Use of a Dried Saliva Spot (DSS) in Therapeutic Drug Monitoring and Disease Diagnosis. J. Pharm. Anal. 2022, 12, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, C.; Gonçalves, J.; Soares, S.; Rosado, T.; Araujo, A.R.T.S.; Passarinha, L.A.; Barroso, M.; Gallardo, E. Evaluation of Antipsychotic Drugs’ Stability in Oral Fluid Samples. Molecules 2023, 28, 2030. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.; Soares, S.; Gonçalves, J.; Rosado, T.; Fernández, N.; Rodilla, M.J.; Passarinha, A.L.; Barroso, M.; Gallardo, E. Stability of Cocaine, Opiates, and Metabolites in Dried Saliva Spots. Molecules 2022, 27, 641. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.; Rosado, T.; Barroso, M.; Passarinha, L.; Gallardo, E. Optimization and Validation of a Procedure Using the Dried Saliva Spots Approach for the Determination of Tobacco Markers in Oral Fluid. J. Pharm. Biomed. Anal. 2022, 212, 114648. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. New Method for the Monitoring of Antidepressants in Oral Fluid Using Dried Spot Sampling. Pharmaceuticals 2021, 14, 1284. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.; Rosado, T.; Barroso, M.; Gallardo, E. Determination of Antiepileptic Drugs Using Dried Saliva Spots. J. Anal. Toxicol. 2019, 43, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Nakajima, D.; Ishikawa, M.; Konno, R.; Nakamura, R.; Ohara, O.; Kawashima, Y. Evaluation of the Suitability of Dried Saliva Spots for In-Depth Proteome Analyses for Clinical Applications. J. Proteome Res. 2022, 21, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, A.; Abdel-Rehim, M. Dried Saliva Spot as a Sampling Technique for Saliva Samples. Biomed. Chromatogr. 2014, 28, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.L.B.; dos Santos, M.K.; Limberger, R.P. Development and Validation of a Method Using Dried Oral Fluid Spot to Determine Drugs of Abuse. J. Forensic Sci. 2019, 64, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Plank, A.-C.; Maschke, J.; Rohleder, N.; Fasching, P.A.; Beckmann, M.W.; Kornhuber, J.; Eichler, A.; Moll, G.H.; Kratz, O. Comparison of C-Reactive Protein in Dried Blood Spots and Saliva of Healthy Adolescents. Front. Immunol. 2021, 12, 795580. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, Y.O.S.; Nishio, H.; Niba, E.T.E.; Okamoto, K.; Shintaku, H.; Takeshima, Y.; Saito, T.; Shinohara, M.; Awano, H. Detection of Spinal Muscular Atrophy Patients Using Dried Saliva Spots. Genes 2021, 12, 1621. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, R.; Paul, R.; Abdelkader, A. Rapid Detection of Amitriptyline in Dried Blood and Dried Saliva Samples with Surface-Enhanced Raman Spectroscopy. Sensors 2022, 22, 8257. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.N.; Shi, F.; Huang, G.; He, Y.; Yu, S.; Liu, L.; Li, Y.; Wen, H. Evaluation of Metabolite Stability in Dried Blood Spot Stored at Different Temperatures and Times. Sci. Rep. 2024, 14, 30964. [Google Scholar] [CrossRef] [PubMed]
- Hassib, S.T.; Hashem, H.M.A.; Mahrouse, M.A.; Mostafa, E.A. Development and Bio-Analytical Validation of Chromatographic Determination Method of Rufinamide in Presence of its Metabolite in Human Plasma. J. Chromatogr. Sci. 2021, 59, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Seyfinejad, B.; Ozkan, S.A.; Jouyban, A. Ultrasound-Assisted Electromembrane Extraction of Clonazepam from Plasma and Determination Using Capillary Electrophoresis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1181, 122928. [Google Scholar] [CrossRef] [PubMed]
- Milosheska, D.; Roškar, R. Simple HPLC-UV Method for Therapeutic Drug Monitoring of 12 Antiepileptic Drugs and Their Main Metabolites in Human Plasma. Molecules 2023, 28, 7830. [Google Scholar] [CrossRef] [PubMed]
- Vosough, M.; Ghafghazi, S.; Sabetkasaei, M. Chemometrics Enhanced HPLC-DAD Performance for Rapid Quantification of Carbamazepine and Phenobarbital in Human Serum Samples. Talanta 2014, 119, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Garduño, Ó.; González-Esquivel, D.F.; Escalante-Membrillo, C.; Fernández, Á.; Rojas-Tomé, I.S.; Jung Cook, H.; Castro, N. Comparison of a High-Performance Liquid Chromatography Method for Quantification of Carbamazepine with Chemiluminescent Microparticle Immunoassay. Biomed. Chromatogr. 2016, 30, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Heideloff, C.; Bunch, D.R.; Wang, S. A Novel HPLC Method for Quantification of 10 Antiepileptic Drugs or Metabolites in Serum/Plasma Using a Monolithic Column. Ther. Drug Monit. 2010, 32, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.M.; Bodhankar, S.L. Simultaneous Determination of Lamotrigine, Phenobarbitone, Carbamazepine and Phenytoin in Human Serum by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2005, 39, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Tavares Luiz, M.; Santos Rosa Viegas, J.; Palma Abriata, J.; Viegas, F.; Testa Moura de Carvalho Vicentini, F.; Lopes Badra Bentley, M.V.; Chorilli, M.; Maldonado Marchetti, J.; Tapia-Blácido, D.R. Design of Experiments (DoE) to Develop and to Optimize Nanoparticles as Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Tye, H. Application of Statistical “design of Experiments” Methods in Drug Discovery. Drug Discov. Today 2004, 9, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Wang, L.; Zhu, A.; Cai, X.; Li, P.; Li, W. Design of Experiment Techniques for the Optimization of Chromatographic Analysis Conditions: A Review. Electrophoresis 2022, 43, 1882–1898. [Google Scholar] [CrossRef] [PubMed]
- Stimpfl, T.; Muller, K.; Gergov, M.; Lebeau, M.; Polettini, A.; Sporkert, F.; Weinmann, W. Recomendations on Sample Collection. TIAFT Bulletin XXIX—Number 1. Available online: https://www.tiaft.org/data/uploads/documents/tiaft-sta-recommendations-on-sample-collection.pdf (accessed on 10 September 2024).
- Nielsen, M.K.K.; Johansen, S.S. Determination of Olanzapine in Whole Blood Using Simple Protein Precipitation and Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Toxicol. 2009, 33, 212–217. [Google Scholar] [CrossRef] [PubMed][Green Version]


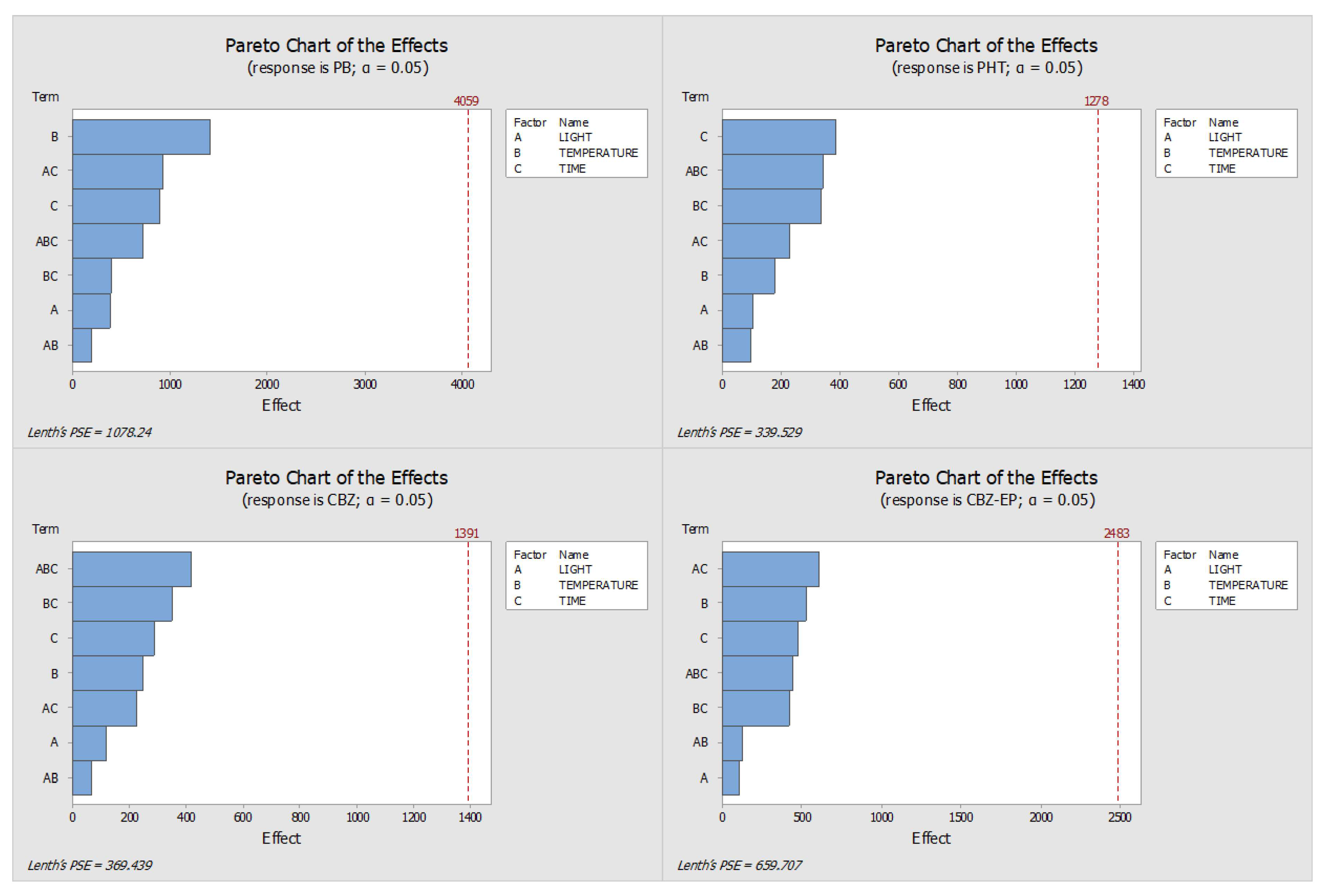
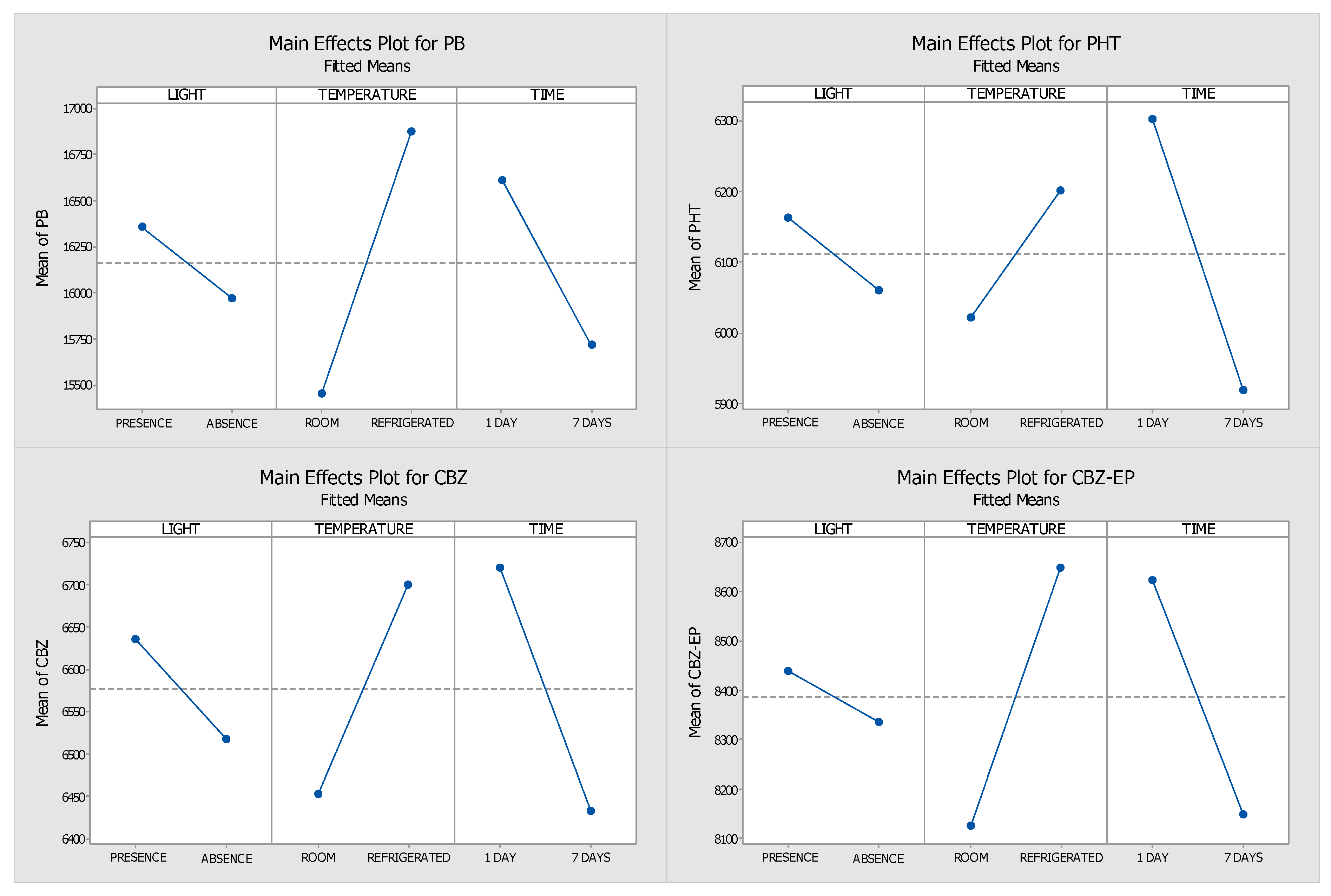
| 1st Day | 7th Day | |||
|---|---|---|---|---|
| Analyte | A | B | A | B |
| PB | 19,324 | 19,814 | 18,747 | 19,610 |
| PHT | 7553 | 7801 | 7277 | 7344 |
| CBZ | 8002 | 8027 | 7659 | 6636 |
| CBZ-EP | 9806 | 10,190 | 9344 | 9672 |
| With Ascorbic Acid | No Preservative | ||||
|---|---|---|---|---|---|
| Analyte | Days | Relative Loss (%) | CV (%) | Relative Loss (%) | CV (%) |
| PB | 1 | 0 | 5.3 | 0 | 1.3 |
| 7 | 11 | 0.9 | −8 | 5.8 | |
| 14 | 3 | 1.9 | −16 | 1.2 | |
| 21 | −2 | 4.3 | −21 | 10.6 | |
| 28 | −9 | 2.8 | −26 | 2.6 | |
| 35 | −29 | 0.1 | −34 | 1.9 | |
| 42 | −30 | 2.1 | −39 | 7.3 | |
| 49 | −36 | 1.9 | −45 | 8.3 | |
| 56 | −43 | 5.7 | −51 | 2.3 | |
| 63 | −49 | 10.1 | −55 | 5.2 | |
| PHT | 1 | 0 | 2.6 | 0 | 5.7 |
| 7 | 14 | 2.3 | −9 | 2.6 | |
| 14 | 5 | 8.2 | −17 | 1.1 | |
| 21 | −1 | 9.3 | −22 | 1.2 | |
| 28 | −9 | 5.7 | −29 | 9.3 | |
| 35 | −24 | 4.5 | −35 | 7.4 | |
| 42 | −37 | 2.9 | −38 | 12.9 | |
| 49 | −42 | 6.0 | −48 | 7.1 | |
| 56 | −46 | 4.0 | −54 | 8.2 | |
| 63 | −51 | 6.6 | −60 | 5.2 | |
| CBZ | 1 | 0 | 4.8 | 0 | 1.1 |
| 7 | 13 | 0.4 | −7 | 4.6 | |
| 14 | 1 | 1.6 | −19 | 1.3 | |
| 21 | −4 | 8.7 | −24 | 3.8 | |
| 28 | −13 | 4.3 | −31 | 1.1 | |
| 35 | −27 | 5.4 | −37 | 9.6 | |
| 42 | −34 | 10.2 | −42 | 1.8 | |
| 49 | −35 | 7.8 | −48 | 6.3 | |
| 56 | −45 | 0.5 | −53 | 2.2 | |
| 63 | −47 | 0.1 | −58 | 3.6 | |
| CBZ-EP | 1 | 0 | 1.0 | 0 | 1.3 |
| 7 | 15 | 1.1 | −8 | 1.5 | |
| 14 | 6 | 0.5 | −18 | 5.3 | |
| 21 | −5 | 2.6 | −22 | 3.9 | |
| 28 | −11 | 0.4 | −30 | 7.6 | |
| 35 | −29 | 0.3 | −36 | 7.8 | |
| 42 | −35 | 0.7 | −41 | 0.8 | |
| 49 | −39 | 9.5 | −45 | 4.5 | |
| 56 | −41 | 1.1 | −53 | 2.5 | |
| 63 | −45 | 1.3 | −57 | 5.2 | |
| With Ascorbic Acid | No Preservative | ||||
|---|---|---|---|---|---|
| Analyte | Days | Relative Loss (%) | CV (%) | Relative Loss (%) | CV (%) |
| PB | 1 | 0 | 5.4 | 0 | 2.6 |
| 7 | −8 | 1.8 | 6 | 1.1 | |
| 14 | −17 | 5.2 | 10 | 8.1 | |
| 21 | −20 | 6.7 | −1 | 4.7 | |
| 28 | −23 | 1.2 | −11 | 8.9 | |
| 35 | −38 | 3.7 | −24 | 5.6 | |
| 42 | −45 | 8.8 | −33 | 3.5 | |
| 49 | −66 | 4.8 | −43 | 1.7 | |
| 56 | −69 | 3.4 | −55 | 2.2 | |
| 63 | −64 | 8.1 | −69 | 3.1 | |
| PHT | 1 | 0 | 4.3 | 0 | 4.7 |
| 7 | −10 | 1.6 | 7 | 9.5 | |
| 14 | −16 | 4.9 | 11 | 6.1 | |
| 21 | −19 | 2.2 | −2 | 7.2 | |
| 28 | −22 | 2.6 | −10 | 2.6 | |
| 35 | −34 | 3.5 | −22 | 1.7 | |
| 42 | −44 | 9.2 | −34 | 5.1 | |
| 49 | −69 | 8.4 | −44 | 1.3 | |
| 56 | −71 | 5.6 | −53 | 2.1 | |
| 63 | −60 | 1.0 | −65 | 4.2 | |
| CBZ | 1 | 0 | 7.8 | 0 | 5.9 |
| 7 | −10 | 3.6 | 8 | 4.9 | |
| 14 | −19 | 0.6 | 13 | 1.6 | |
| 21 | −20 | 1.3 | −1 | 8.8 | |
| 28 | −23 | 3.2 | −12 | 6.5 | |
| 35 | −36 | 2.4 | −24 | 3.7 | |
| 42 | −49 | 1.6 | −36 | 0.5 | |
| 49 | −67 | 0.9 | −47 | 4.4 | |
| 56 | −73 | 8.7 | −58 | 5.2 | |
| 63 | −62 | 6.7 | −67 | 5.6 | |
| CBZ-EP | 1 | 0 | 8.5 | 0 | 1.3 |
| 7 | −9 | 7.9 | 8 | 7.7 | |
| 14 | −15 | 6.4 | 14 | 2.9 | |
| 21 | −18 | 4.3 | 2 | 6.5 | |
| 28 | −21 | 4.1 | −9 | 5.3 | |
| 35 | −39 | 8.0 | −21 | 2.1 | |
| 42 | −46 | 10.0 | −32 | 9.2 | |
| 49 | −64 | 10.1 | −44 | 10.4 | |
| 56 | −68 | 8.4 | −55 | 5.1 | |
| 63 | −60 | 3.2 | −68 | 3.4 | |
| Run Order | Preservative Concentration | Time | Temperature | Light |
|---|---|---|---|---|
| 1 | Low | One Day | 4 °C | Absence |
| 2 | High | One Day | 4 °C | Absence |
| 3 | Low | One Day | 4 °C | Presence |
| 4 | High | One Day | 4 °C | Presence |
| 5 | Low | One Day | 25 °C | Absence |
| 6 | High | One Day | 25 °C | Absence |
| 7 | Low | One Day | 25 °C | Presence |
| 8 | High | One Day | 25 °C | Presence |
| 9 | Low | Seven Days | 4 °C | Absence |
| 10 | High | Seven Days | 4 °C | Absence |
| 11 | Low | Seven Days | 4 °C | Presence |
| 12 | High | Seven Days | 4 °C | Presence |
| 13 | Low | Seven Days | 25 °C | Absence |
| 14 | High | Seven Days | 25 °C | Absence |
| 15 | Low | Seven Days | 25 °C | Presence |
| 16 | High | Seven Days | 25 °C | Presence |
| Run Order | Time | Temperature | Light |
|---|---|---|---|
| 1 | 1 Day | 25 °C | Absence |
| 2 | 1 Day | 25 °C | Presence |
| 3 | 1 Day | 4 °C | Absence |
| 4 | 1 Day | 4 °C | Presence |
| 5 | 7 Days | 25 °C | Absence |
| 6 | 7 Days | 25 °C | Presence |
| 7 | 7 Days | 4 °C | Absence |
| 8 | 7 Days | 4 °C | Presence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinho, J.; Simão, A.Y.; Rosado, T.; Gallardo, E. Evaluation of Antiepileptic Drugs’ Stability in Oral Fluid Samples. Pharmaceuticals 2025, 18, 1049. https://doi.org/10.3390/ph18071049
Martinho J, Simão AY, Rosado T, Gallardo E. Evaluation of Antiepileptic Drugs’ Stability in Oral Fluid Samples. Pharmaceuticals. 2025; 18(7):1049. https://doi.org/10.3390/ph18071049
Chicago/Turabian StyleMartinho, João, Ana Y. Simão, Tiago Rosado, and Eugenia Gallardo. 2025. "Evaluation of Antiepileptic Drugs’ Stability in Oral Fluid Samples" Pharmaceuticals 18, no. 7: 1049. https://doi.org/10.3390/ph18071049
APA StyleMartinho, J., Simão, A. Y., Rosado, T., & Gallardo, E. (2025). Evaluation of Antiepileptic Drugs’ Stability in Oral Fluid Samples. Pharmaceuticals, 18(7), 1049. https://doi.org/10.3390/ph18071049









