Development, Validation and Application of the Dried Blood Spot Analysis Method for the Determination of Ustekinumab in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
2.1. Method Validation
2.1.1. Selectivity, Recovery, and Dilution Integrity
2.1.2. Calibration Model, Accuracy, Precision, and Limits of Quantification
2.1.3. Hematocrit Effect
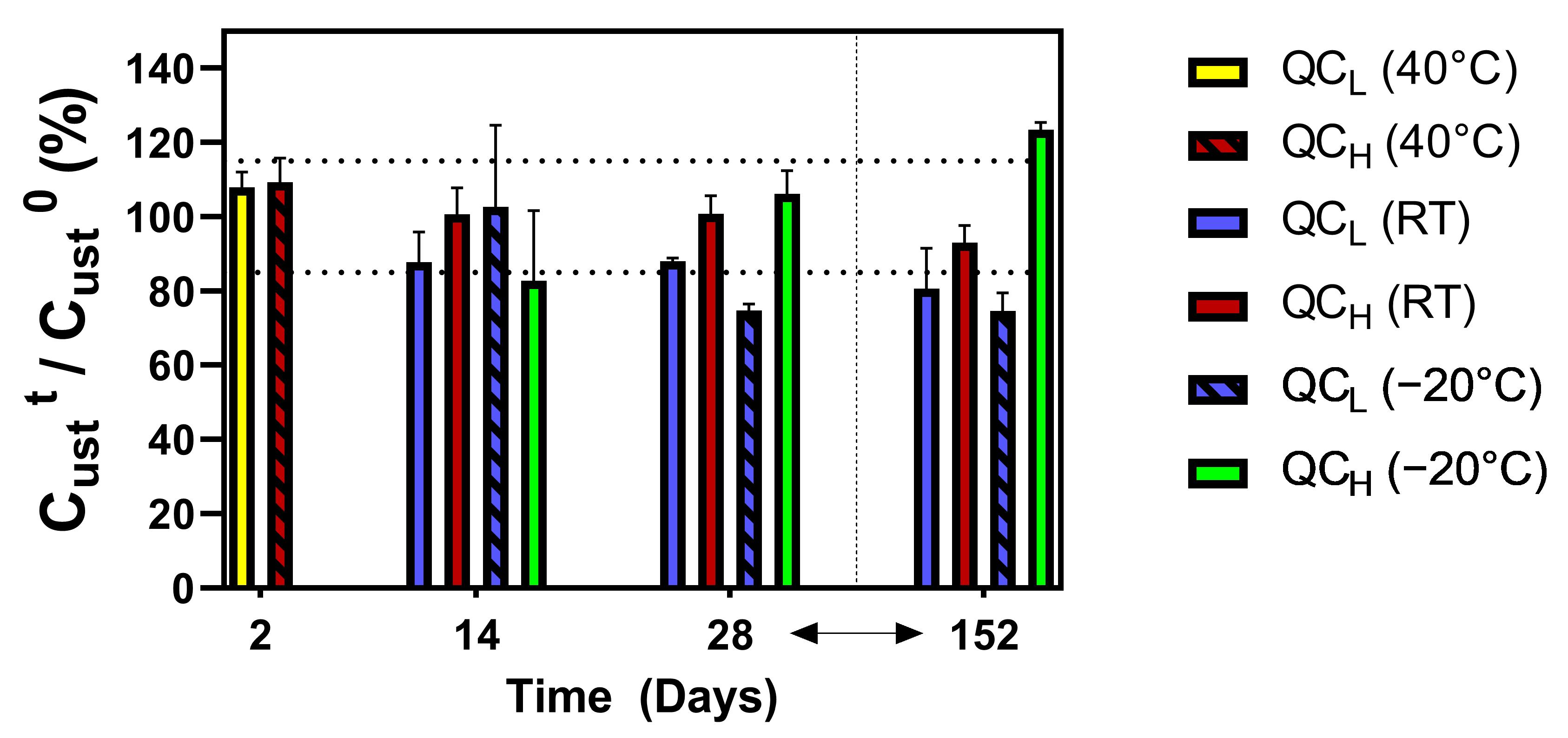
2.1.4. Stability
2.2. Clinical Validation
2.2.1. Patient Characteristics
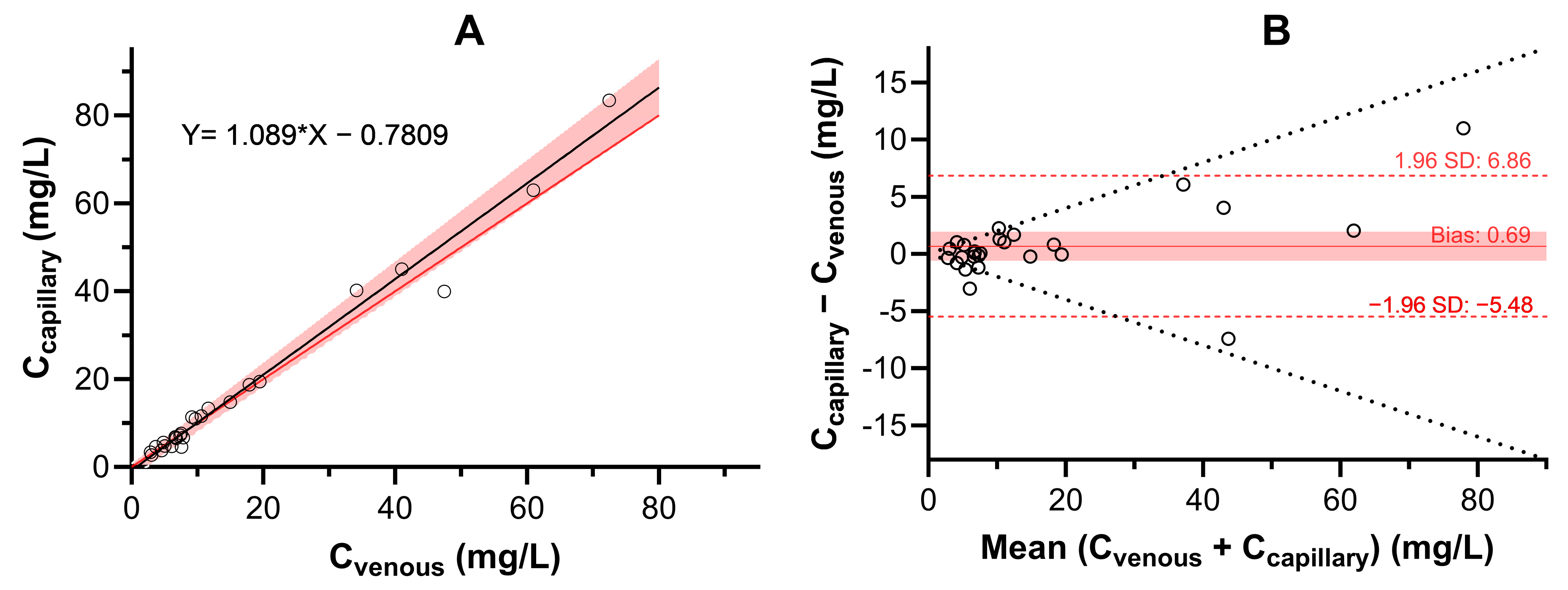
2.2.2. Correlation Between Serum and Dried Blood Spot Venous Ustekinumab Concentrations
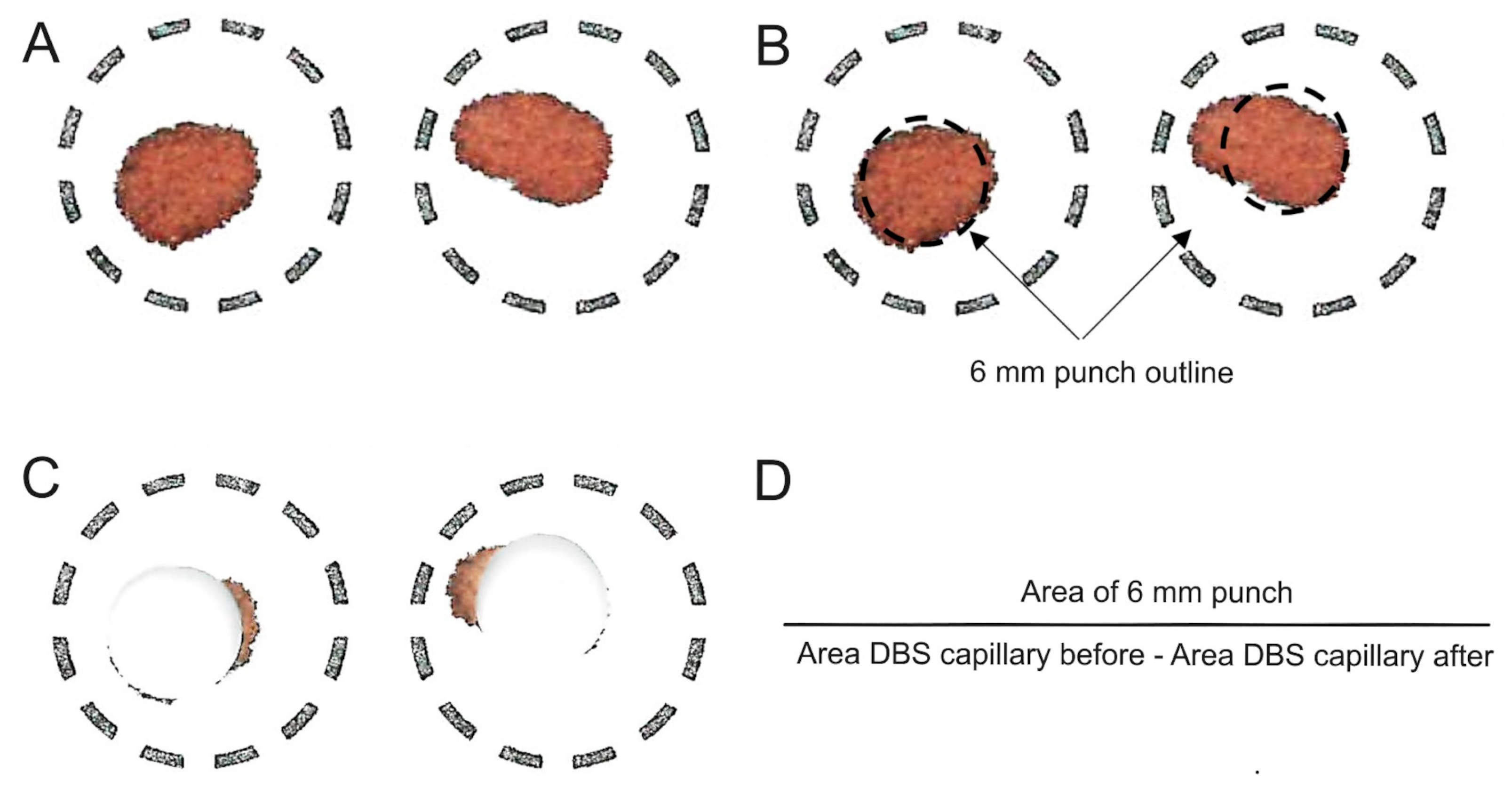
2.2.3. Correction of the Ustekinumab Concentration in Capillary DBS Due to Irregular Spot Shape
2.2.4. Correlation Between Ustekinumab Concentration in Venous and Capillary Dried Blood Spots
3. Discussion
4. Materials and Methods
4.1. Chemical and Materials
4.2. DBS Method
4.3. DBS Method Validation
4.3.1. Selectivity, Recovery, and Dilution Integrity
4.3.2. Calibration Model, Accuracy, Precision, and Limits of Quantification
4.3.3. Hematocrit Effect
4.3.4. Stability
4.4. Patients’ Samples
4.5. Clinical Validation
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 95% CI | 95% Confidence Interval |
| ADAs | Anti-drug antibodies |
| bmin | Intercept obtained by nonlinear regression |
| BSA | Bovine serum albumin |
| BW | Body weight |
| CD | Crohn’s disease |
| CHCT 0.4 | UST concentration in DBS obtained with 0.4 hematocrit |
| CHCTi | UST concentration in DBS obtained with specific blood hematocrit |
| CRP | C-reactive protein |
| DBS | Dried blood spots |
| dbs c | Uncorrected dried blood spot area |
| dbs c corr | Corrected dried blood spot area |
| DBSc | Capillary dried blood spots |
| DBSv | Venous dried blood spots |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMA | European Medicines Agency |
| FC | Fecal calprotectin |
| HCT | Hematocrit |
| HCTi | Individual hematocrit |
| IATDMCT | International association for Therapeutic Drug Monitoring and Clinical Toxicology |
| IBD | Inflammatory bowel disease |
| IL | Interleukin |
| kmin | Slope obtained by nonlinear regression |
| Li-heparin | Lithium heparin |
| LLOQ | Lower Limit of Quantification |
| LoA | Limits of Agreement |
| mAb | Monoclonal antibody |
| MAPE | Mean absolute percentage error |
| MPPE | Mean predictive percent error |
| nM | Number of men |
| nW | Number of women |
| PK | Pharmacokinetics |
| Q1 | First quartile (25th percentile) |
| Q3 | Third quartile (75th percentile) |
| QCH | Quality control high concentration |
| QCL | Quality control low concentration |
| QCM | Quality control medium concentration |
| RSD | Relative standard deviation |
| RT | Room temperature |
| S-alb | Serum concentration of albumin |
| TDM | Therapeutic drug monitoring |
| UC | Ulcerative colitis |
| ULOQ | Upper limit of quantification |
| UST | Ustekinumab |
References
- Benson, J.M.; Peritt, D.; Scallon, B.J.; Heavner, G.A.; Shealy, D.J.; Giles-Komar, J.M.; Mascelli, M.A. Discovery and Mechanism of Ustekinumab: A Human Monoclonal Antibody Targeting Interleukin-12 and Interleukin-23 for Treatment of Immune-Mediated Disorders. mAbs 2011, 3, 535–545. [Google Scholar] [CrossRef]
- Nia, J.K.; Lebwohl, M.G. Ustekinumab. In Therapy for Severe Psoriasis; Elsevier: Amsterdam, The Netherlands, 2016; pp. 127–137. [Google Scholar] [CrossRef]
- STELARA® (Ustekinumab) for Plaque Psoriasis Treatment. Available online: https://www.stelarahcp.com/plaque-psoriasis (accessed on 13 September 2021).
- Bruner, L.P.; White, A.M.; Proksell, S. Inflammatory Bowel Disease. Prim. Care Clin. Off. Pract. 2023, 50, 411–427. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.R.; Schellens, J.H.M.; Beijnen, J.H. Clinical Pharmacokinetics of Therapeutic Monoclonal Antibodies. Clin. Pharmacokinet. 2010, 49, 633–659. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Marano, C.; O’Brien, C.; Szapary, P.; Zhang, H.; Johanns, J.; Leong, R.W.; Hisamatsu, T.; Van Assche, G.; et al. Ustekinumab Pharmacokinetics and Exposure Response in a Phase 3 Randomized Trial of Patients With Ulcerative Colitis. Clin. Gastroenterol. Hepatology 2020, 8, 2244–2255.e9. [Google Scholar] [CrossRef]
- Adedokun, O.J.; Xu, Z.; Gasink, C.; Jacobstein, D.; Szapary, P.; Johanns, J.; Gao, L.L.; Davis, H.M.; Hanauer, S.B.; Feagan, B.G.; et al. Pharmacokinetics and Exposure Response Relationships of Ustekinumab in Patients With Crohn’s Disease. Gastroenterology 2018, 154, 1660–1671. [Google Scholar] [CrossRef]
- Papamichael, K.; Cheifetz, A.S. Therapeutic Drug Monitoring in Inflammatory Bowel Disease: For Every Patient and Every Drug? Curr. Opin. Gastroenterol. 2019, 34, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Hanžel, J.; Zdovc, J.; Kurent, T.; Sever, N.; Javornik, K.; Tuta, K.; Koželj, M.; Smrekar, N.; Novak, G.; Štabuc, B.; et al. Peak Concentrations of Ustekinumab After Intravenous Induction Therapy Identify Patients With Crohn’s Disease Likely to Achieve Endoscopic and Biochemical Remission. Clin. Gastroenterol. Hepatol. 2021, 19, 111–118.e10. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic Drug Monitoring with Biologic Agents in Immune Mediated Inflammatory Diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Detrez, I.; Schops, G.; Lefrère, J.; Tops, S.; Van Assche, G.; Vermeire, S.; Van Moerkercke, W.; Ferrante, M.; Gils, A. Golimumab Dried Blood Spot Analysis (GOUDA): A Prospective Trial Showing Excellent Correlation with Venepuncture Samples and More Detailed Pharmacokinetic Information. AAPS J. 2019, 21, 10. [Google Scholar] [CrossRef]
- Bloem, K.; Schaap, T.; Boshuizen, R.; Kneepkens, E.L.; Wolbink, G.J.; Vries, A.D.; Rispens, T. Capillary Blood Microsampling to Determine Serum Biopharmaceutical Concentration: Mitra((R)) Microsampler vs. Dried Blood Spot. Bioanalysis 2018, 10, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Berends, S.E.; Bloem, K.; de Vries, A.; Schaap, T.; Rispens, T.; Strik, A.S.; Talwar, R.; Löwenberg, M.; D’Haens, G.R.; Mathôt, R.A. Monitoring of Adalimumab Concentrations at Home in Patients with Inflammatory Bowel Disease Using Dried Blood Samples. Ther. Drug Monit. 2020, 42, 289–294. [Google Scholar] [CrossRef]
- Van den Berghe, N.; Verstockt, B.; Vandeput, E.; Ballet, V.; Gils, A.; Ferrante, M.; Vermeire, S.; Thomas, D. Development and Validation of Dried Blood Spot Sampling as a Tool to Identify the Best Time Point to Measure Predictive Ustekinumab Serum Concentrations in Patients with Crohn’s Disease. J. Crohn’s Colitis 2020, 14 (Suppl. S1), S502. [Google Scholar] [CrossRef]
- Kneepkens, E.L.; Pouw, M.F.; Wolbink, G.J.; Schaap, T.; Nurmohamed, M.T.; de Vries, A.; Rispens, T.; Bloem, K. Dried Blood Spots from Finger Prick Facilitate Therapeutic Drug Monitoring of Adalimumab and Anti-Adalimumab in Patients with Inflammatory Diseases. Br. J. Clin. Pharmacol. 2017, 83, 2474–2484. [Google Scholar] [CrossRef]
- Berends, S.E.; D’Haens, G.R.A.M.G.; Schaap, T.; de Vries, A.; Rispens, T.; Bloem, K.; Mathot, R.A.A.; Mathôt, R.A.A. Dried Blood Samples Can Support Monitoring of Infliximab Concentrations in Patients with Inflammatory Bowel Disease: A Clinical Validation. Br. J. Clin. Pharmacol. 2019, 85, 1544–1551. [Google Scholar] [CrossRef]
- Bian, S.; Van den Berghe, N.; Vandersmissen, L.; Tops, S.; Vermeire, S.; Ferrante, M.; Gils, A.; Thomas, D. Evaluating an Easy Sampling Method Using Dried Blood Spots to Determine Vedolizumab Concentrations. J. Pharm. Biomed. Anal. 2020, 185, 113224. [Google Scholar] [CrossRef]
- Mingas, P.-D.; Zdovc, J.; Grabnar, I.; Vovk, T. The Evolving Role of Microsampling in Therapeutic Drug Monitoring of Monoclonal Antibodies in Inflammatory Diseases. Molecules 2021, 26, 1787. [Google Scholar] [CrossRef]
- Capiau, S.; Veenhof, H.; Koster, R.A.; Bergqvist, Y.; Boettcher, M.; Halmingh, O.; Keevil, B.G.; Koch, B.C.P.; Linden, R.; Pistos, C.; et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: Development and Validation of Dried Blood Spot-Based Methods for Therapeutic Drug Monitoring. Ther. Drug Monit. 2019, 41, 409–430. [Google Scholar] [CrossRef]
- GUTHRIE, R.; SUSI, A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics 1963, 32, 338–343. [Google Scholar] [CrossRef]
- Verstockt, B.; Dreesen, E.; Noman, M.; Outtier, A.; Van Den Berghe, N.; Aerden, I.; Compernolle, G.; Van Assche, G.; Gils, A.; Vermeire, S.; et al. Ustekinumab Exposure-Outcome Analysis in Crohn’s Disease Only in Part Explains Limited Endoscopic Remission Rates. J. Crohn’s Colitis 2019, 13, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Zdovc, J.A.; Hanžel, J.; Kurent, T.; Sever, N.; Koželj, M.; Smrekar, N.; Novak, G.; Štabuc, B.; Dreesen, E.; Thomas, D.; et al. Ustekinumab Dosing Individualization in Crohn’s Disease Guided by a Population Pharmacokinetic–Pharmacodynamic Model. Pharmaceutics 2021, 13, 1587. [Google Scholar] [CrossRef]
- Wilhelm, A.J.; den Burger, J.C.G.; Swart, E.L. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clin. Pharmacokinet. 2014, 53, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Voudoukis, E.; Karmiris, K.; Oustamanolakis, P.; Theodoropoulou, A.; Sfiridaki, A.; Paspatis, G.A.; Koutroubakis, I.E. Association between Thrombocytosis and Iron Deficiency Anemia in Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1212–1216. [Google Scholar] [CrossRef]
- Emmons, G.; Rowland, M. Pharmacokinetic Considerations as to When to Use Dried Blood Spot Sampling. Bioanalysis 2010, 2, 1791–1796. [Google Scholar] [CrossRef]
- Veenhof, H.; Koster, R.A.; Junier, L.A.T.; Berger, S.P.; Bakker, S.J.L.; Touw, D.J. Volumetric Absorptive Microsampling and Dried Blood Spot Microsampling vs. Conventional Venous Sampling for Tacrolimus Trough Concentration Monitoring. Clin. Chem. Lab. Med. 2020, 58, 1687–1695. [Google Scholar] [CrossRef]
- Klak, A.; Pauwels, S.; Vermeersch, P. Preanalytical Considerations in Therapeutic Drug Monitoring of Immunosuppressants with Dried Blood Spots. Diagnosis 2019, 6, 57–68. [Google Scholar] [CrossRef]
- Papp, K.A.; Langley, R.G.; Lebwohl, M.; Krueger, G.G.; Szapary, P.; Yeilding, N.; Guzzo, C.; Hsu, M.-C.; Wang, Y.; Li, S.; et al. Efficacy and Safety of Ustekinumab, a Human Interleukin-12/23 Monoclonal Antibody, in Patients with Psoriasis: 52-Week Results from a Randomised, Double-Blind, Placebo-Controlled Trial (PHOENIX 2). Lancet 2008, 371, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.L.; Kimball, A.B.; Papp, K.A.; Yeilding, N.; Guzzo, C.; Wang, Y.; Li, S.; Dooley, L.T.; Gordon, K.B. Efficacy and Safety of Ustekinumab, a Human Interleukin-12/23 Monoclonal Antibody, in Patients with Psoriasis: 76-Week Results from a Randomised, Double-Blind, Placebo-Controlled Trial (PHOENIX 1). Lancet 2008, 371, 1665–1674. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Feagan, B.G.; Gasink, C.; Jacobstein, D.; Zou, B.; Johanns, J.; Adedokun, O.J.; Sands, B.E.; Rutgeerts, P.; et al. IM-UNITI: Three-Year Efficacy, Safety, and Immunogenicity of Ustekinumab Treatment of Crohn’s Disease. J. Crohn’s Colitis 2020, 14, 23–32. [Google Scholar] [CrossRef]
- Scott, F.I.; Lichtenstein, G.R. Therapeutic Drug Monitoring of Anti-TNF Therapy in Inflammatory Bowel Disease. Curr. Treat. Options Gastroenterol. 2014, 12, 59–75. [Google Scholar] [CrossRef]
- Koster, R.A.; Alffenaar, J.W.C.; Botma, R.; Greijdanus, B.; Touw, D.J.; Uges, D.R.A.; Kosterink, J.G.W. What Is the Right Blood Hematocrit Preparation Procedure for Standards and Quality Control Samples for Dried Blood Spot Analysis? Bioanalysis 2015, 7, 345–351. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. European Medicines Agency. Guideline on Bioanalytical Method Validation. EMA Guid. Doc. 2012, 2011, 1–23. [Google Scholar]

| Linear Response Range (mg/L) | Intercept | Slope | r2 | |
|---|---|---|---|---|
| Day 1 | 3–12 (mg/L) | 0.2011 | 0.0955 | 0.9858 |
| Day 2 | 0.3188 | 0.0969 | 0.9984 | |
| Day 3 | 0.1527 | 0.0892 | 0.9912 | |
| Intra-Day Precision | Intra-Day Accuracy | ||||||
|---|---|---|---|---|---|---|---|
| n = 5 | Level | Nominal (mg/L) | Mean (mg/L) | RSD (%) | n = 5 | Level | % |
| LLOQ | 3.0 | 2.7 | 6.6 | LLOQ | 88.5 | ||
| QCL | 5.0 | 5.1 | 9.1 | QCL | 103 | ||
| QCM | 8.0 | 6.7 | 13.4 | QCM | 84.2 | ||
| QCH | 10.0 | 11.1 | 1.2 | QCH | 111 | ||
| Inter-Day Precision | Inter-Day Accuracy | ||||||
| n = 15 | Level | Nominal (mg/L) | Mean (mg/L) | RSD (%) | n = 15 | Level | % |
| LLOQ | 3.0 | 2.7 | 6.3 | LLOQ | 90.1 | ||
| QCL | 5.0 | 5.3 | 11.2 | QCL | 106 | ||
| QCM | 8.0 | 8.1 | 6.3 | QCM | 102 | ||
| QCH | 10.0 | 9.4 | 6.4 | QCH | 94.4 | ||
| QC | HCT | Nominal (mg/L) | Mean (mg/L) | RSD (%) | CHCTi/CHCT 0.4 (%) |
|---|---|---|---|---|---|
| QCL | 0.25 | 5.0 | 5.8 | 3.59 | 112 |
| QCL | 0.4 | 5.0 | 5.1 | 9.14 | 100 |
| QCL | 0.55 | 5.0 | 5.5 | 6.48 | 107 |
| QCH | 0.25 | 10.0 | 12.2 | 5.72 | 107 |
| QCH | 0.4 | 10.0 | 11.1 | 1.23 | 100 |
| QCH | 0.55 | 10.0 | 11.6 | 1.18 | 105 |
| Age a | [years], median (Q1–Q3) | 52.4 (42.0–57.9) |
| Sex | (nM/nW) | 5/5 |
| BW a | [kg], median (Q1–Q3) | 76.5 (67.5–92.5) |
| CRP a,b | [mg/L], median (Q1–Q3) | 9.5 (6.5–12.5) |
| HCT | [], median (Q1–Q3) | 0.40 (0.39–0.44) |
| FC a,c | [mg/kg], median (Q1–Q3) | 130 (44.5–215) |
| S-alb a | [g/L], median (Q1–Q3) | 43.0 (41.0–46.0) |
| Dose initial a | [mg], median (Q1–Q3) | 390 (390–520) |
| Dose maintenance d | [mg/week], (number of patients) | 90 mg/week 8, (9) 90 mg/week 4, (1) |
| Week of therapy d,e | [week], median (Q1–Q3) | 4.0 (2.0–8.6) |
| Predicting Model | Deming Regression | Bland–Altman Analysis | Predictive Performance | ||||
|---|---|---|---|---|---|---|---|
| Slope (95% CI) | Intercept (95% CI) | Bias (mg/L) (95% CI) | 95% LoA (mg/L) | MPPE (%) | MAPE (%) | ||
| 1 | kmin = 1.15 | 0.999 (0.84, 1.16) | −0.541 (−3.38, 2.30) | −0.554 (−2.45, 1.35) | −11.04, 9.94 | −2.19 | 14.44 |
| 2 | kmin = 1.17; bmin = 1.04 | 0.979 (0.82, 1.13) | 0.523 (−2.26, 3.31) | −1.71E−5 (−1.83, 1.83) | −10.4, 10.4 | 2.94 | 15.54 |
| 3 | 2.016 (1.54, 2.49) | −2.646 (−10.6, 5.34) | 22.85 (13.4, 32.3) | −31.1, 76.8 | 83.74 | 83.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingas, P.-D.; Aguiar Zdovc, J.; Grabnar, I.; Drobne, D.; Vovk, T. Development, Validation and Application of the Dried Blood Spot Analysis Method for the Determination of Ustekinumab in Patients with Inflammatory Bowel Disease. Pharmaceuticals 2025, 18, 1253. https://doi.org/10.3390/ph18091253
Mingas P-D, Aguiar Zdovc J, Grabnar I, Drobne D, Vovk T. Development, Validation and Application of the Dried Blood Spot Analysis Method for the Determination of Ustekinumab in Patients with Inflammatory Bowel Disease. Pharmaceuticals. 2025; 18(9):1253. https://doi.org/10.3390/ph18091253
Chicago/Turabian StyleMingas, Panagiotis-Dimitrios, Jurij Aguiar Zdovc, Iztok Grabnar, David Drobne, and Tomaž Vovk. 2025. "Development, Validation and Application of the Dried Blood Spot Analysis Method for the Determination of Ustekinumab in Patients with Inflammatory Bowel Disease" Pharmaceuticals 18, no. 9: 1253. https://doi.org/10.3390/ph18091253
APA StyleMingas, P.-D., Aguiar Zdovc, J., Grabnar, I., Drobne, D., & Vovk, T. (2025). Development, Validation and Application of the Dried Blood Spot Analysis Method for the Determination of Ustekinumab in Patients with Inflammatory Bowel Disease. Pharmaceuticals, 18(9), 1253. https://doi.org/10.3390/ph18091253







