Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Modulate Chemoradiotherapy Response in Cervical Cancer Spheroids
Abstract
1. Introduction
2. Results
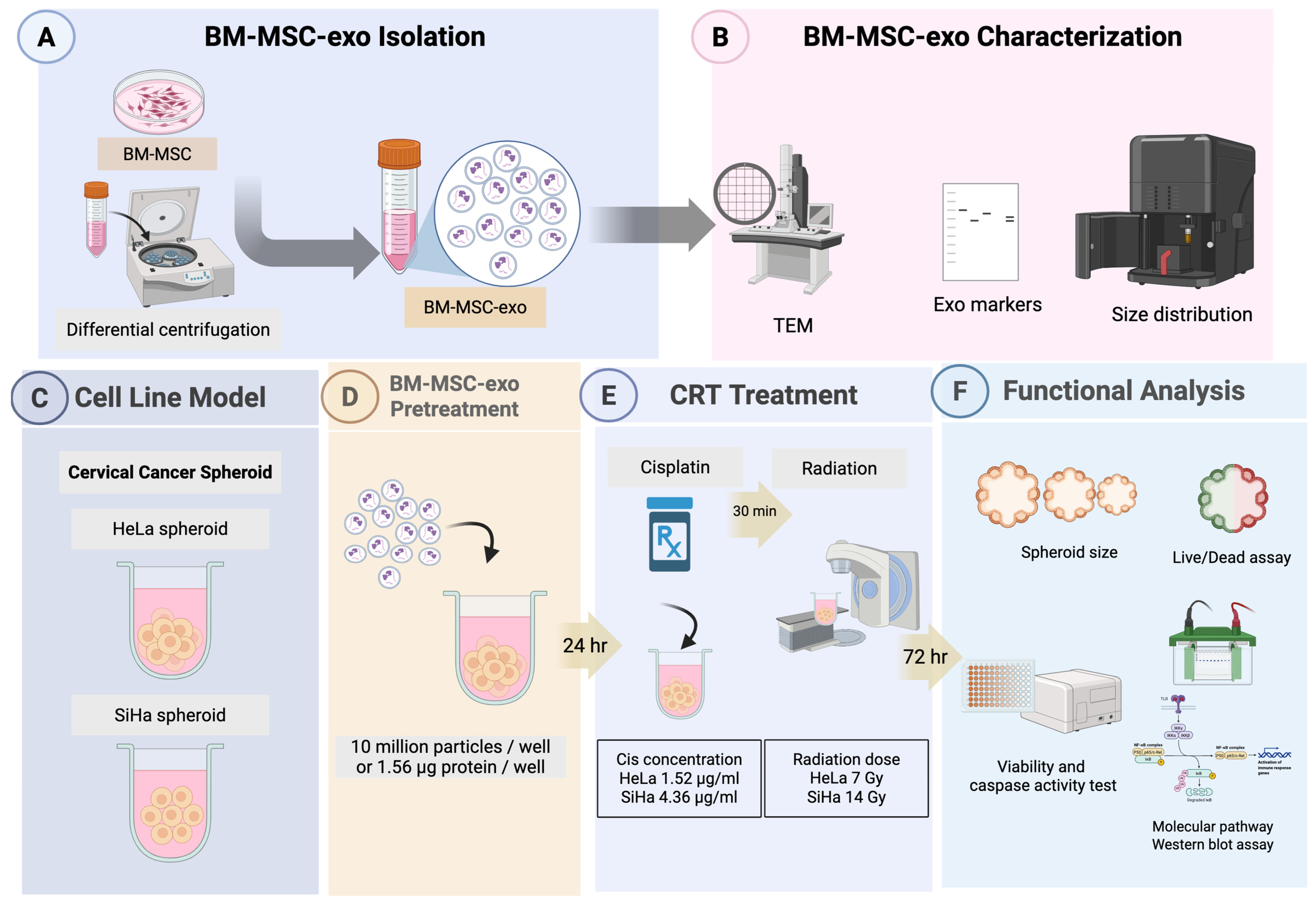
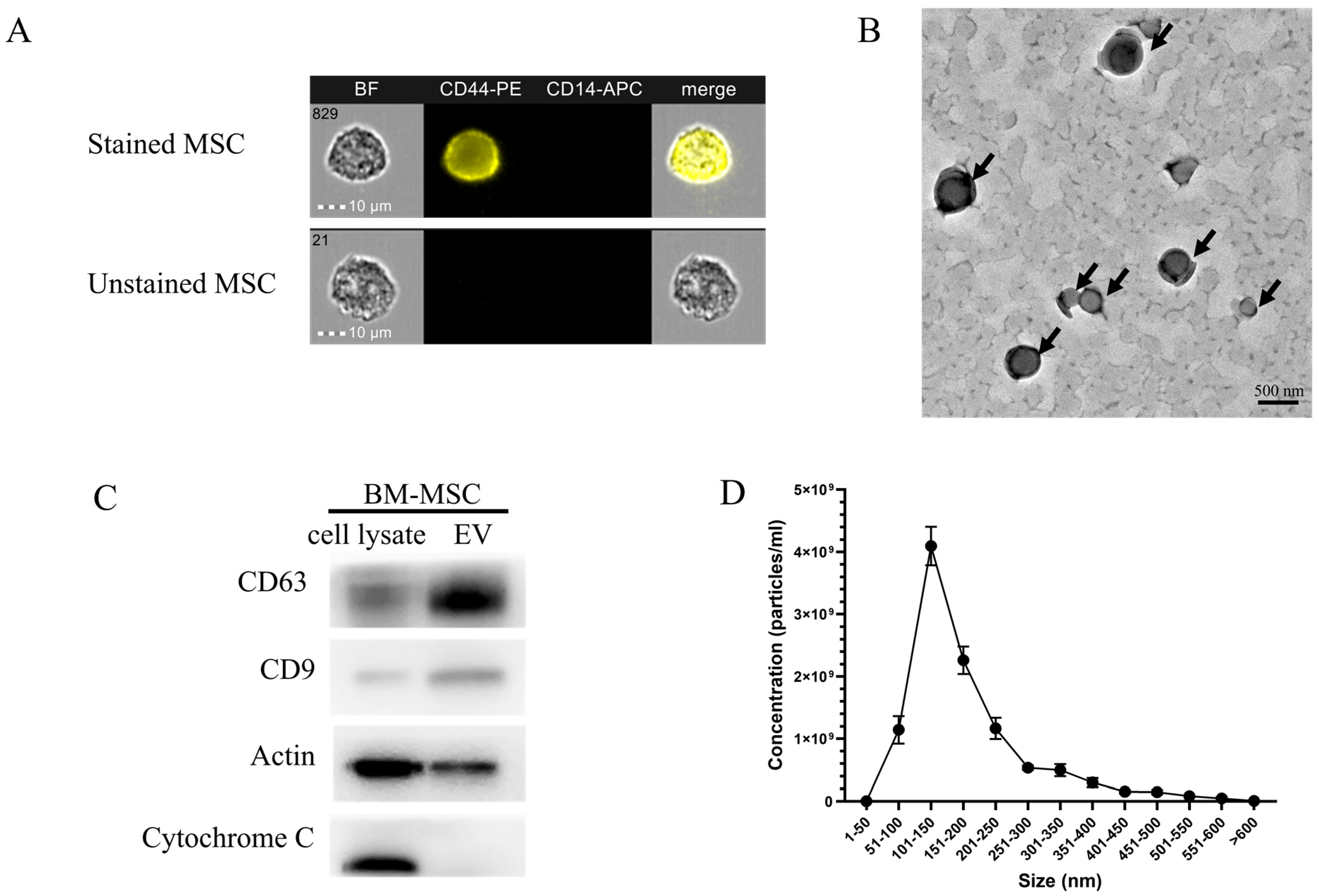
2.1. Characterization and Quantification of Bone Marrow Mesenchymal Stem Cell-Derived Exosomes (BM-MSCs-Exo)
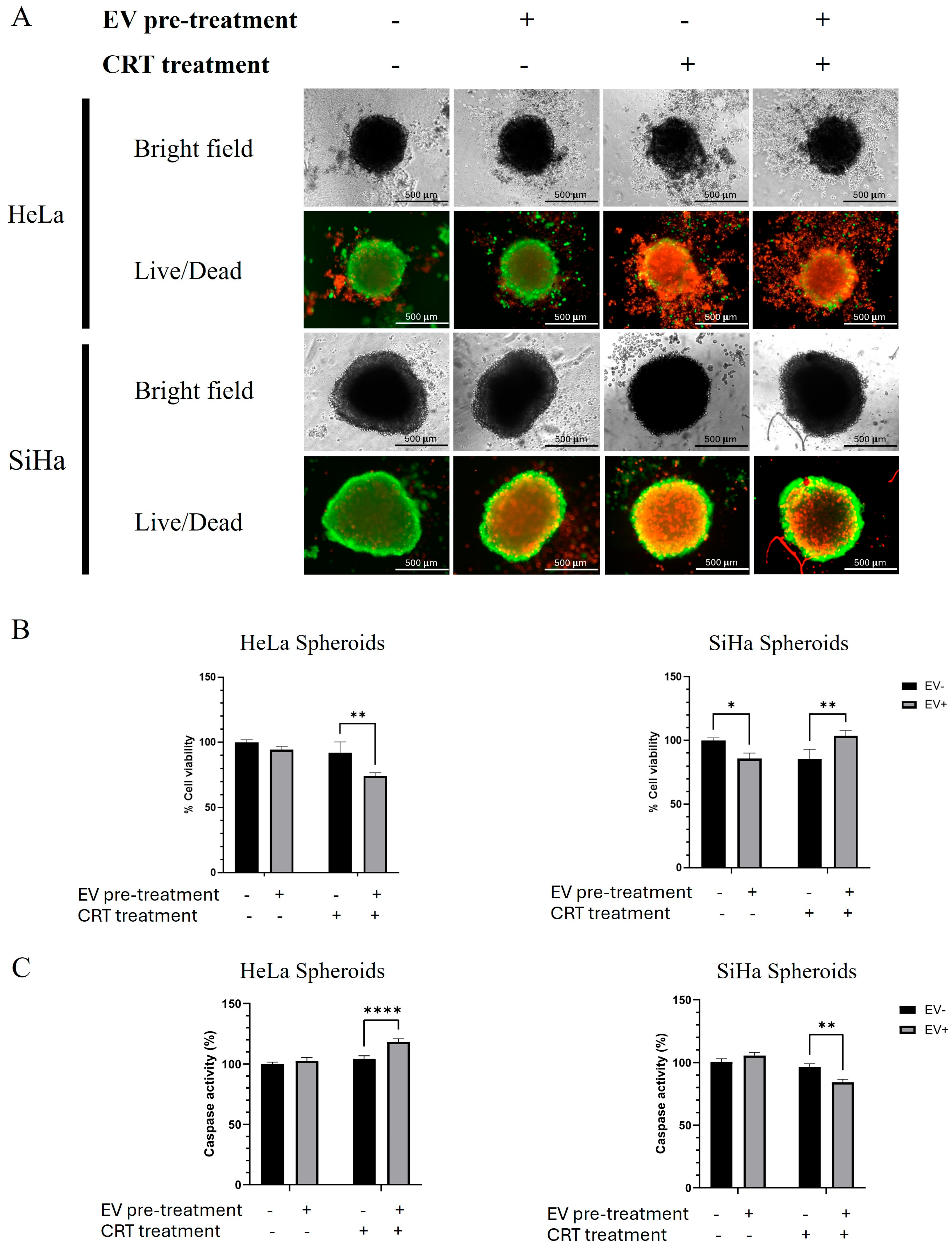
2.2. The Effect of Crt Combined with Bm-Mscs-Exo Pre-Treatment
2.2.1. BM-MSCs-Exo Pre-Treatment Decreased the Size of Hela Spheroids but Had No Effect on SiHa Spheroids After CRT Treatment
2.2.2. The Combination of CRT with BM-MSCs-Exo Pre-Treatment Showed an Enhancing Effect on HeLa but Not on SiHa Spheroids
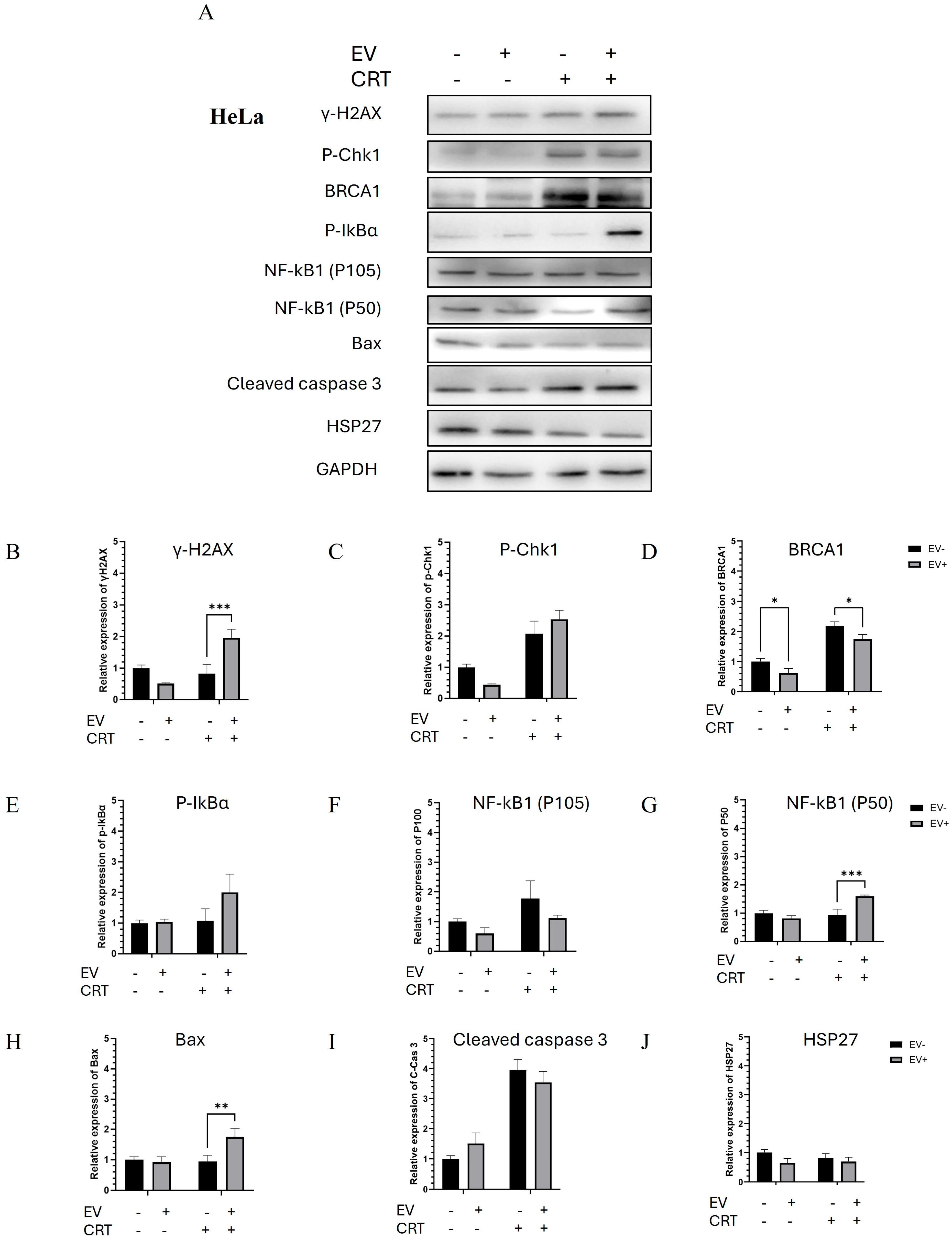
2.2.3. Pre-Treatment with BM-MSCs-Exo Enhanced the CRT Treatment of HeLa Spheroids Through the Activation of Apoptotic Molecules
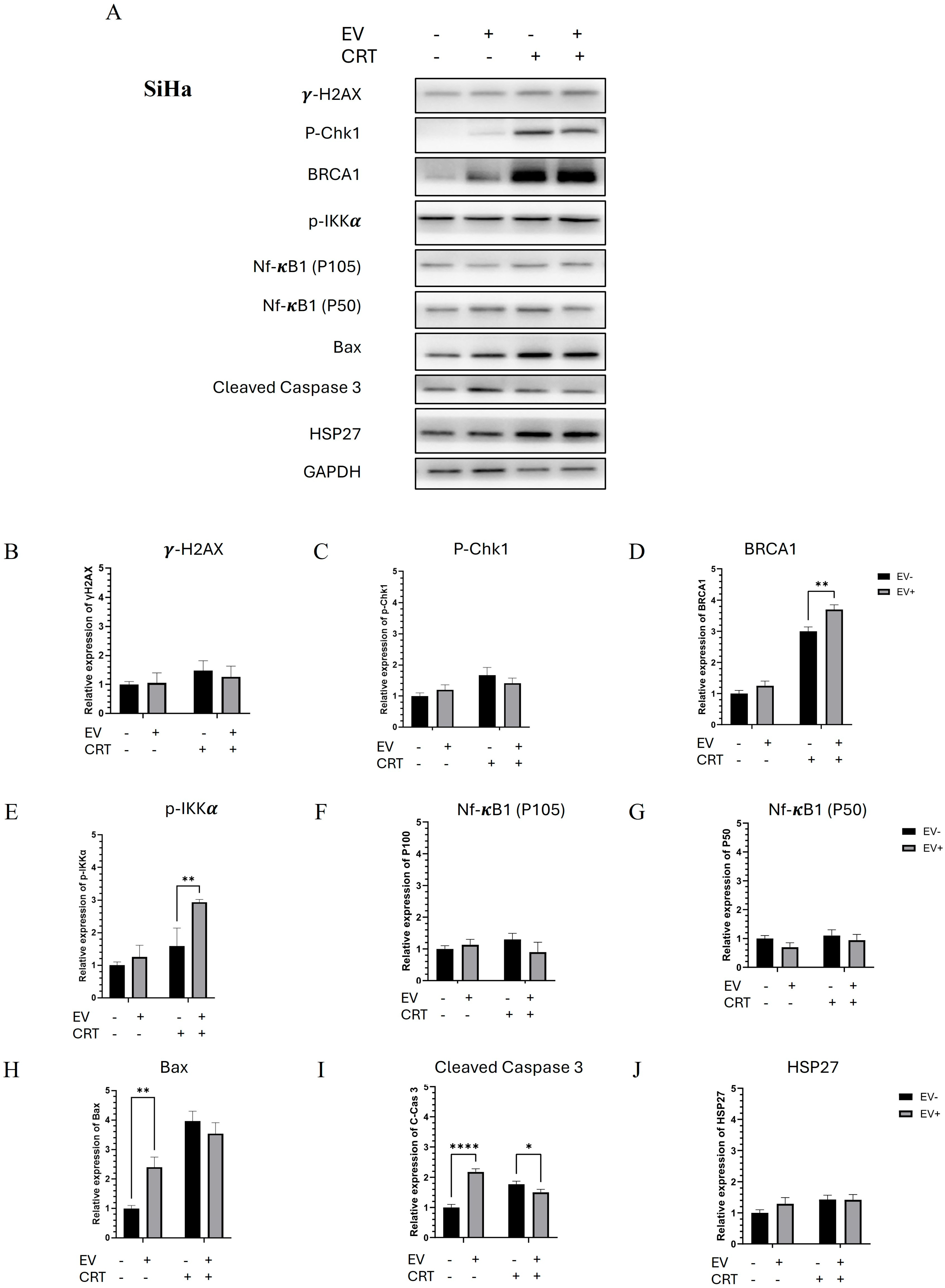
2.2.4. Pre-Treatment with BM-MSCs-Exo Enhanced the Resistance of SiHa Spheroids to CRT by Downregulating NF-κB and Apoptotic Molecules
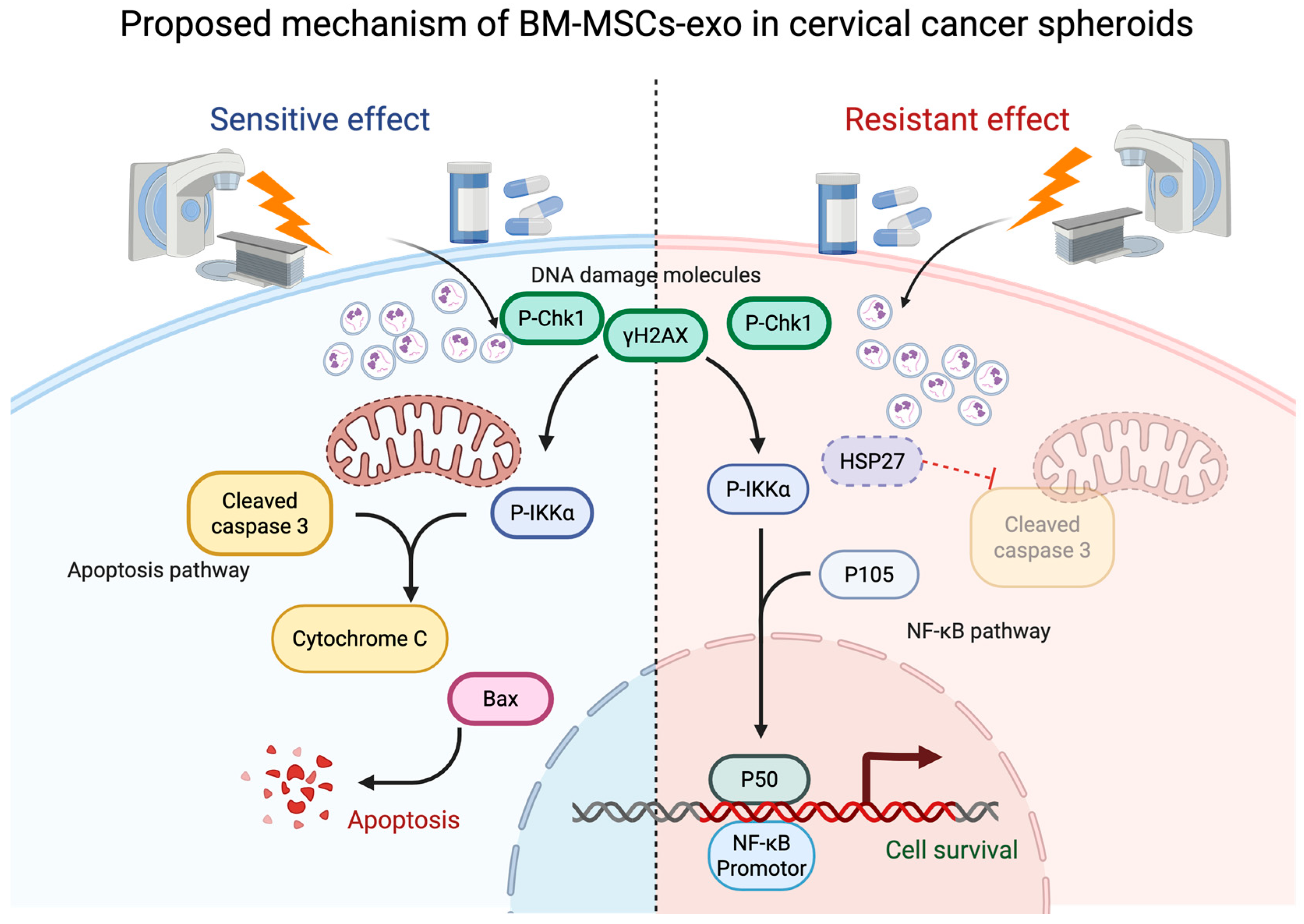
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Exosome Isolation
4.2. BM-MSC-Exo Characterization
4.2.1. BM-MSC-Exo Visualization Using TEM
4.2.2. Western Blot Analysis of Exosome Markers
4.2.3. Concentration and Size Distribution of Exosomes Evaluated Using NTA
4.3. Pre-Treatment with BM-MSCs-Exo Followed by CRT Treatment in Cervical Cancer Spheroids
4.4. Viability and Caspase Activity Test
4.5. Molecular Activities of BM-MSCs-Exo Pre-Treatment Combined with CRT Treatment and Western Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSCC | cervical squamous cell carcinoma |
| HPV | human papilloma virus |
| BM-MSCs | bone marrow mesenchymal stem cells |
| BM-MSC-exo | bone marrow mesenchymal stem cell-derived exosome |
| MSCs | mesenchymal stem cells |
| MSC-exo | mesenchymal stem cell-derived exosome |
| CRT | chemoradiotherapy |
| DSBs | double-strand breaks |
| SDS-PAGE | SDS polyacrylamide gel electrophoresis |
| NTA | nanoparticle tracking analysis |
| TEM | transmission electron microscope |
| PVDF membranes | polyvinylidene difluoride membranes |
| TBS-T | Tris-buffered saline with 0.1% (v/v) Tween-20 |
| PBS | phosphate-buffered saline |
Appendix A
| Name of Antibody (Cat No.) | Molecular Weight (kDa) | Dilution |
|---|---|---|
| Antibody for BM-MSCs-exo | ||
| CD9 Rabbit mAb (13174) | 22–24 | 1:1000 |
| CD63 Rabbit mAb (52090) | 25–60 | 1:1000 |
| β-Actin Antibody (4967) | 45 | 1:1000 |
| Cytochrome c Rabbit mAb (11940) | 14 | 1:1000 |
| Antibody for molecular mechanism | ||
| DNA Damage Pathway | ||
| Phospho-BRCA1 (Ser1524) Antibody (9009) | 220 | 1:1000 |
| Phospho-Chk1 (Ser345) Rabbit mAb (2348) | 56 | 1:1000 |
| Phospho-Histone H2A.X (Ser139) Rabbit mAb (9718) | 15 | 1:1000 |
| NF-κB Pathway | ||
| Phospho-IKKα/β (Ser176/180) Rabbit mAb (2697) | 85 | 1:1000 |
| Phospho-IκBα (Ser32) Rabbit mAb (2859) | 40 | 1:1000 |
| NF-κB1 p105/p50 Rabbit mAb (13586) | 50 (Active Form) 120 (Precursor) | 1:1000 |
| Apoptosis Pathway | ||
| Cleaved Caspase-3 (Asp175) Rabbit mAb (9664) | 19 | 1:1000 |
| Bax (D2E11) Rabbit mAb (5023) | 20 | 1:1000 |
| Phospho-HSP27 (Ser82) Rabbit mAb (9709) | 27 | 1:1000 |
| Internal Control | ||
| GAPDH Rabbit mAb (2118) | 37 | 1:1000 |
| Secondary Antibody | ||
| Anti-rabbit IgG, HRP-linked Antibody (7074) | 1:1000 |
References
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Paino, F.; Regad, T.; Papaccio, G.; Desiderio, V.; Tirino, V. Concise review: Cancer cells, cancer stem cells, and mesenchymal stem cells: Influence in cancer development. Stem Cells Transl. Med. 2017, 6, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Yu, J.-W.; Ni, X.-C.; Wu, J.-G.; Wang, S.-L.; Jiang, B.-J. Bone marrow-derived mesenchymal stem cells increase drug resistance in CD133-expressing gastric cancer cells by regulating the PI3K/AKT pathway. Tumor Biol. 2016, 37, 14637–14651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tu, H.; Yang, Y.; Fang, L.; Wu, Q.; Li, J. Mesenchymal stem cell-derived extracellular vesicles: Roles in tumor growth, progression, and drug resistance. Stem Cells Int. 2017, 2017, 1758139. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Zhang, B.; Zhang, X.; Xue, J.; Yuan, X.; Yan, Y.; Wang, M.; Zhu, W.; Qian, H.; Xu, W. Exosomes derived from human mesenchymal stem cells confer drug resistance in gastric cancer. Cell Cycle 2015, 14, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Withers, H.R. Biological basis for radiation therapy for cancer. Lancet 1992, 339, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Akhunzianov, A.A.; Rozhina, E.V.; Filina, Y.V.; Rizvanov, A.A.; Miftakhova, R.R. Resistance to Radiotherapy in Cancer. Diseases 2025, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-K.; Park, S.-R.; Jung, B.-K.; Jeon, Y.-K.; Lee, Y.-S.; Kim, M.-K.; Kim, Y.-G.; Jang, J.-Y.; Kim, C.-W.; Shi, S. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE 2013, 8, e84256. [Google Scholar] [CrossRef] [PubMed]
- Shams, F.; Pourjabbar, B.; Hashemi, N.; Farahmandian, N.; Golchin, A.; Nuoroozi, G.; Rahimpour, A. Current progress in engineered and nano-engineered mesenchymal stem cells for cancer: From mechanisms to therapy. Biomed. Pharmacother. 2023, 167, 115505. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, J.; Chen, M.; Chen, F.; Tu, W. Exosomal microRNA-146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2-mediated PI3K/Akt axis. Int. J. Mol. Med. 2020, 46, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, S.; Zhu, S.; Yi, M.; Luo, S.; Wu, K. MiRNA-mediated EMT and CSCs in cancer chemoresistance. Exp. Hematol. Oncol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, Y.; Xu, Y.; Li, G.; Li, Z.; Liu, T. Mesenchymal stem cell-derived exosomes in cancer therapy resistance: Recent advances and therapeutic potential. Mol. Cancer. 2022, 21, 179. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xu, B.; Li, J.; Yang, X.; Gu, J.; Yao, X.; Sun, X. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J. Exp. Clin. Cancer Res. 2021, 40, 38. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, Z.; Malekzadeh, M.; Sharifi, M.; Hashemibeni, B. The role of miR-16 and miR-34a family in the regulation of cancers: A review. Heliyon 2025, 11, e42733. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Ahmad, Z.A.; Dass, S.A.; Shamsuddin, S.; Kumaran, N.M.; Balakrishnan, V. Growth and Invasion of 3D Spheroid Tumor of HeLa and CasKi Cervical Cancer Cells. Oncologie 2021, 23, 279–291. [Google Scholar] [CrossRef]
- Molika, P.; Leetanaporn, K.; Chiangjong, W.; Choochuen, P.; Navakanitworakul, R. Proteomic Analysis Reveals Cadherin, Actin, and Focal Adhesion Molecule-Mediated Formation of Cervical Cancer Spheroids. Cells 2024, 13, 2004. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Nat. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Gardiner, C.; Brooks, A.S.; Tannetta, D.S.; Ferguson, D.J.P.; Hole, P.; Carr, B.; Redman, C.W.G.; Harris, A.L.; Dobson, P.J.; et al. Sizing and phenotyping of cellular vesicles using Nanoparticle Tracking Analysis. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Skladanowski, A.; Bozko, P. H2AX phosphorylation: Its role in DNA damage response and cancer therapy. J. Nucleic Acids 2010, 2010, 920161. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhu, F.; Cho, Y.-Y.; Tang, F.; Zykova, T.; Ma, W.-Y.; Bode, A.M.; Dong, Z. Cell apoptosis: Requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol. Cell 2006, 23, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Sedelnikova, O.A.; Pilch, D.R.; Redon, C.; Bonner, W.M. Histone H2AX in DNA damage and repair. Cancer Biol. Ther. 2003, 2, 233–235. [Google Scholar] [PubMed]
- Du, Y. NF-kB Signaling Pathway. Chemo-Radiat.-Resist. Cancer Ther. 2022, 13, 590764154. [Google Scholar]
- Rosen, E.M.; Fan, S.; Pestell, R.G.; Goldberg, I.D. BRCA1 gene in breast cancer. J. Cell Physiol. 2003, 196, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Brodie, S.G. Roles of BRCA1 and its interacting proteins. Bioessays 2000, 22, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, S.; Meng, Q.; Ma, Y.; Katiyar, P.; Schlegel, R.; Rosen, E.M. BRCA1 interaction with human papillomavirus oncoproteins. J. Biol. Chem. 2005, 280, 33165–33177. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Liu, S.; Cui, M.; Kanazawa, N. Effect of BRCA1 on the concurrent chemoradiotherapy resistance of cervical squamous cell carcinoma based on transcriptome sequencing analysis. Biomed. Res. Int. 2020, 2020, 3598417. [Google Scholar] [CrossRef] [PubMed]
- Movahed, Z.G.; Mansouri, K.; Mohsen, A.H.; Matin, M.M. Bone marrow mesenchymal stem cells enrich breast cancer stem cell population via targeting metabolic pathways. Med. Oncol. 2025, 42, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhou, A.; Cheng, W.; Zhao, Y.; Liu, C.; Zeng, Y.; Lin, L.; Zhou, Z.; Peng, Y.; Chen, P. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Inhibit Apoptosis of Pulmonary Microvascular Endothelial Cells in COPD Mice Through miR-30b/Wnt5a Pathway. Int. J. Nanomed. 2025, 20, 1191–1211. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-J.; Chen, Y.; Xu, Y.-Q.; Lin, M.-A.; Wen, H.; Chen, Y.-T.; Pan, P.-L. Regulation of miR-30b in cancer development, apoptosis, and drug resistance. Open Life Sci. 2022, 17, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Zhang, J.; Ma, X.; Dong, M.; Sun, B.; Xin, Y. Roles and regulatory mechanisms of miR-30b in cancer, cardiovascular disease, and metabolic disorders. Exp. Ther. Med. 2021, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tian, Q.; Liu, R.; Xu, K.; Shi, S.; Zhang, X.; Gao, L.; Yin, X.; Xu, S.; Wang, P. Inhibitory role of bone marrow mesenchymal stem cells-derived exosome in non-small-cell lung cancer: microRNA-30b-5p, EZH2 and PI3K/AKT pathway. J. Cell Mol. Med. 2023, 27, 3526–3538. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Afzal, M.; Goyal, K.; Ganesan, S.; Kumari, M.; Sunitha, S.; Dash, A.; Saini, S.; Rana, M.; Gupta, G.; et al. MSC-derived extracellular vesicles: Precision miRNA delivery for overcoming cancer therapy resistance. Regen. Ther. 2025, 29, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Collino, F.; Deregibus, M.C.; Grange, C.; Tetta, C.; Camussi, G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013, 22, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.S.; Korkolopoulou, P.; Piperi, C. Exploring the Interaction of Tumor-Derived Exosomes and Mesenchymal Stem Cells in Tumor Biology. Int. J. Mol. Sci. 2025, 26, 3095. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, F.; Tian, D.; Wang, T.; Lu, T.; Huang, X.; Zhang, P.; Qin, L. miR-199a-3p enhances cisplatin sensitivity of ovarian cancer cells by targeting ITGB8. Oncol. Rep. 2018, 39, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Nittayaboon, K.; Leetanaporn, K.; Sangkhathat, S.; Roytrakul, S.; Navakanitworakul, R. Proteomic Analysis of Butyrate-Resistant Colorectal Cancer-Derived Exosomes Reveals Potential Resistance to Anti-Cancer Drugs. Discov. Med. 2024, 36, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Jeanmard, N.; Bissanum, R.; Sriplung, H.; Charoenlappanit, S.; Roytrakul, S.; Navakanitworakul, R.; Mishra, S.K. Proteomic profiling of urinary extracellular vesicles differentiates breast cancer patients from healthy women. PLoS ONE 2023, 18, e0291574. [Google Scholar] [CrossRef] [PubMed]
- Monnamorn, L.; Seree-Aphinan, C.; Molika, P.; Vichitkunakorn, P.; Pattanapanyasat, K.; Khwannimit, B.; Navakanitworakul, R. The concentration of large extracellular vesicles differentiates early septic shock from infection. Front. Med. 2021, 8, 724371. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nittayaboon, K.; Molika, P.; Bissanum, R.; Leetanaporn, K.; Chumsuwan, N.; Navakanitworakul, R. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Modulate Chemoradiotherapy Response in Cervical Cancer Spheroids. Pharmaceuticals 2025, 18, 1050. https://doi.org/10.3390/ph18071050
Nittayaboon K, Molika P, Bissanum R, Leetanaporn K, Chumsuwan N, Navakanitworakul R. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Modulate Chemoradiotherapy Response in Cervical Cancer Spheroids. Pharmaceuticals. 2025; 18(7):1050. https://doi.org/10.3390/ph18071050
Chicago/Turabian StyleNittayaboon, Kesara, Piyatida Molika, Rassanee Bissanum, Kittinun Leetanaporn, Nipha Chumsuwan, and Raphatphorn Navakanitworakul. 2025. "Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Modulate Chemoradiotherapy Response in Cervical Cancer Spheroids" Pharmaceuticals 18, no. 7: 1050. https://doi.org/10.3390/ph18071050
APA StyleNittayaboon, K., Molika, P., Bissanum, R., Leetanaporn, K., Chumsuwan, N., & Navakanitworakul, R. (2025). Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Modulate Chemoradiotherapy Response in Cervical Cancer Spheroids. Pharmaceuticals, 18(7), 1050. https://doi.org/10.3390/ph18071050








