1. Introduction
Cystic fibrosis (CF) is an autosomal recessive genetic disorder that affects 162,428 (144,606–186,620) people across 94 countries worldwide [
1]. It is determined by variations in the gene sequence encoding for the CF transmembrane regulator (CFTR), a channel protein that acts as chloride and bicarbonate transporter [
2]. To date, more than 2100 mutations have been identified (Cystic Fibrosis Mutation Database. Hospital for Sick Children, Toronto.
http://www.genet.sickkids.on.ca/, accessed on 23 September 2024). However, the most frequent variant found in 85% of patients is F508del [
3]. Although loss of function in CFTR protein leads to multisystemic dysfunction that involves the upper and lower airway, liver, pancreas, and gastrointestinal and reproductive tracts, lung disease is the most common cause of morbidity and mortality [
4]. In fact, lung function is progressively impaired, being affected by chronic infections and multiple pulmonary exacerbations [
5,
6]. In this
scenario, a pathological cascade including dehydrated airway surface liquid, reduced mucociliary clearance and dense secretions primarily lead to chronic pulmonary inflammation, irreversible lung architecture modification, respiratory failure and death [
7]. Pharmacological therapies are mainly symptomatic and include mucolytics, airway hydrators, antibiotics and pancreatic enzymes [
8,
9,
10,
11]. However, in the last decade, “CFTR modulators” (also named “caftor”) have been developed and introduced into clinical practice, attempting to partially restore defective CFTR protein function [
8,
12]. In fact, these agents are able to counteract the intracellular dysfunction of CFTR protein, allowing significant improvement in terms of respiratory function, nutrition and quality of life for people with CF (pwCF) [
8,
12]. Actually, CFTR modulators can be distinguished into two groups defined as potentiators and correctors [
13]. Potentiators (i.e., ivacaftor, IVA) increase anion transport by potentiating the channel-open frequency of CFTR protein at the cell surface [
13]. Conversely, correctors (i.e., lumacaftor, LUM; tezacaftor, TEZ; elexacaftor, ELX) facilitate anion transport by correcting misfolding errors and promoting the CFTR protein migration to the cell surface [
13]. Therefore, combinations of potentiators/correctors such as ivacaftor/lumacaftor, ivacaftor/tezacaftor and elexacaftor/tezacaftor/ivacaftor are often used in therapy to synergistically improve anion transport through F508del-CFTR protein via different mechanisms of action [
13]. Recently, a novel oral drug formulation that combines ELX/TEZ/IVA (ETI) has been approved in both Europe (Kaftrio
®) and the United States (Trikafta
®) for treating CF patients aged 2 years and older carrying at least one F508del mutation [
9,
12,
13,
14,
15]. Clinical trials for non-F508del patients are currently underway. The safety and efficacy of this triple combination have already been evaluated in several clinical studies showing a significant functional improvement and a positive outcome in patients bearing the F508del and other variants [
13,
16,
17,
18,
19]. However, it is worth noting that high inter-individual variability in plasma levels of patients treated with “caftors” has been reported [
9,
20,
21]. This variability is further enhanced by the drug–drug interactions (DDI) that involve CFTR modulators. In fact, these drugs are primarily metabolized in the liver through cytochrome P450 (CYP450) enzymes, mainly CYP3A4 and CYP3A5, which are inhibited by several drugs concomitantly administered to CF patients such as triazole antifungal agents, antivirals and macrolide antibiotics (i.e., clarithromycin and erythromycin) [
12,
22]. Moreover, physiological aspects that make children different from adults in terms of organ maturation and developmental changes represent an additional factor contributing to a higher inter-individual variability in pediatric patients compared to adults. Therefore, although therapeutic drug monitoring (TDM) is not currently indicated for CFTR modulators during routine clinical practice, there is an open debate on its clinical utility [
4,
8]. In fact, since their market authorization, different pharmacokinetic (PK) and pharmacodynamic (PD) studies have been conducted on both pediatric and adult CF patients [
8,
19,
23,
24,
25,
26]. However, more evidence is still needed to better elucidate the PK/PD behavior of these drugs in a real-world setting [
4]. There is also an emerging adverse effect profile registered among CF patients, in particular concerning psychological effects such as anxiety, low mood, “brain fog” and insomnia, although it is not completely clear whether or not these effects could be ascribable to elexacaftor/tezacaftor/ivacaftor [
4,
27,
28,
29,
30,
31]. Therefore, monitoring plasma concentrations of CFTR modulators could be useful for dosing optimization in order to guarantee therapeutic drug exposures and limit adverse reactions [
4,
8]. Moreover, population PK (popPK) studies based on TDM results may represent a valid approach aimed at improving our knowledge on PK properties and individualized dose regimens in specific patient populations including pediatric subjects. To date, several bioanalytical methods based on liquid chromatography tandem mass spectrometry (LC-MS/MS) have been proposed for the quantification of different CFTR modulators in plasma and dried blood spot samples, although there are currently no commercially available assays [
23,
25,
32,
33,
34,
35,
36,
37,
38,
39,
40]. However, examination of the existing literature often reveals complex chromatographic conditions and long sample preparation processes. In fact, an ideal analytical method should be robust, straightforward, reliable, and cost-effective.
Here, we have developed and validated a new sensitive LC-MS/MS method for simultaneous plasma-level determination of ivacaftor (IVA), tezacaftor (TEZ) and elexacaftor (ELX). Our method is characterized by an easy sample preparation and a wider calibration range (0.1–20 µg/mL), which is the same for each tested compound and allows the measurement of “caftors” in a low volume of plasma (50 µL). This method has been fully validated according to ICH guideline M10 on bioanalytical method validation and study sample analysis [
41], and it has been applied to pediatric patients affected by cystic fibrosis under steady-state treatment with Kaftrio
®. The aim of our study is to propose a viable assay for the implementation of “caftor” therapeutic drug monitoring in the bioanalytical laboratory during routine clinical practice. In fact, TDM for CFTR modulators could be useful not only to improve our knowledge about the PK/PD of these drugs in special populations but also to allow individualized dosing strategies and provide more cost-effective management, particularly considering the high cost burden of these medications to the health system.
3. Discussion
To date, different pharmacokinetic (PK) and pharmacodynamic (PD) studies have been conducted on both pediatric and adult CF patients [
8,
19,
23,
24,
25,
26]. However, what constitutes the therapeutic range for the CFTR modulators still remains an open question [
8]. Actually, our knowledge is limited and sometimes derives from the SmPCs or from sparse real-life studies in which data from both children and adults are included [
35]. These aspects highlight the necessity of obtaining more evidence in order to better elucidate the PK/PD behavior of these drugs in a real-world setting [
4]. Another important aspect that underlays the utility of monitoring “caftor” plasma concentrations is represented by the adverse reactions that involve also psychological effects [
4,
27,
28,
29,
30,
31]. However, in two previously published reports, a reduction in ETI dose led to improved mental health [
28,
49]. Finally, although the use of “caftors” in pregnancy is “off-label”, emerging evidence suggests that the continuation of therapy with CFTR modulators is able to prevent a clinical decline during pregnancy [
50,
51]. Considering the altered PK profile that characterizes this critical time, further studies aimed at evaluating the PK/PD parameters of ivacaftor/tezacaftor/elexacaftor in pregnancy and breastfeeding are needed [
52].
Based on these considerations, TDM of CFTR modulators could be useful for improving dosing optimization in different clinical scenarios, ensuring therapeutic drug exposures and limiting adverse reactions [
4,
8]. Recently, a retrospective analysis conducted on adult patients with cystic fibrosis, who were assuming ETI combination, has highlighted the value of utilizing a TDM program, underlying the need for further PK studies [
53].
Our aim was to develop and validate a new LC-MS/MS method for simultaneous plasma-level determination of ivacaftor (IVA), tezacaftor (TEZ) and elexacaftor (ELX). The method presented here has been fully validated following ICH guideline M10 on bioanalytical method validation and study sample analysis [
41], and it has been applied to pediatric patients affected by cystic fibrosis under steady-state treatment with Kaftrio
®.
Currently, there are no commercially available assays for “caftor” determination in biological matrices. Therefore, several bioanalytical methods based on liquid chromatography tandem mass spectrometry (LC-MS/MS) have been proposed to detect CFTR modulators in plasma samples [
23,
25,
32,
33,
34,
35,
36,
37,
40]. In
Supplementary Table S3, we report the analytical characteristics of our method compared to those already published. Although the run time and the use of deuterated internal standard (IS) do not represent a significant difference compared to other published reports, a wider calibration range (0.1–20 µg/mL), which is the same for each tested compound, allows the measurement of “caftor” plasma levels in a larger concentration window. In fact, as reported in
Table 5, the median (IQR) level of elexacaftor 4 h after daily morning intake of Kaftrio
® was 17.85 (13.27–24.10) µg/mL. Thanks to the wide calibration range, we were able to measure ELX concentrations without diluting samples. This aspect could be particularly useful for studies aimed at exploring the PK of CFTR modulators in both children and adults for whom the high-interindividual variability could be responsible for significant oscillations in drug plasma levels.
Sensitivity can be considered as another important advantage of our method since we are able to quantify drug concentrations at the LLOQ level by using a very small injection volume (1 µL). Moreover, low injection volumes in mass spectrometry offer several advantages, primarily related to improved chromatographic resolution, enhanced sensitivity, and reduced sample consumption. In fact, by reducing the volume of sample injected into the separation column, band broadening is reduced, leading to sharper, better-defined peaks and improved resolution of closely eluting compounds. This aspect can be particularly beneficial in complex samples where many compounds show narrow retention times. Furthermore, lower injection volumes can lead to higher sensitivity, by increasing the concentration of analytes reaching the detector, especially when micro- or nano-flow chromatography techniques are used [
54].
Finally, to further validate our bioanalytical method, we performed system suitability assessment according to the United States Pharmacopeia (USP) [
42]. In detail, the Resolution (Rs), Capacity Factor (k’), Peak Symmetry (As) and Tailing Factor (T) resulted within the acceptable ranges [
43,
44].
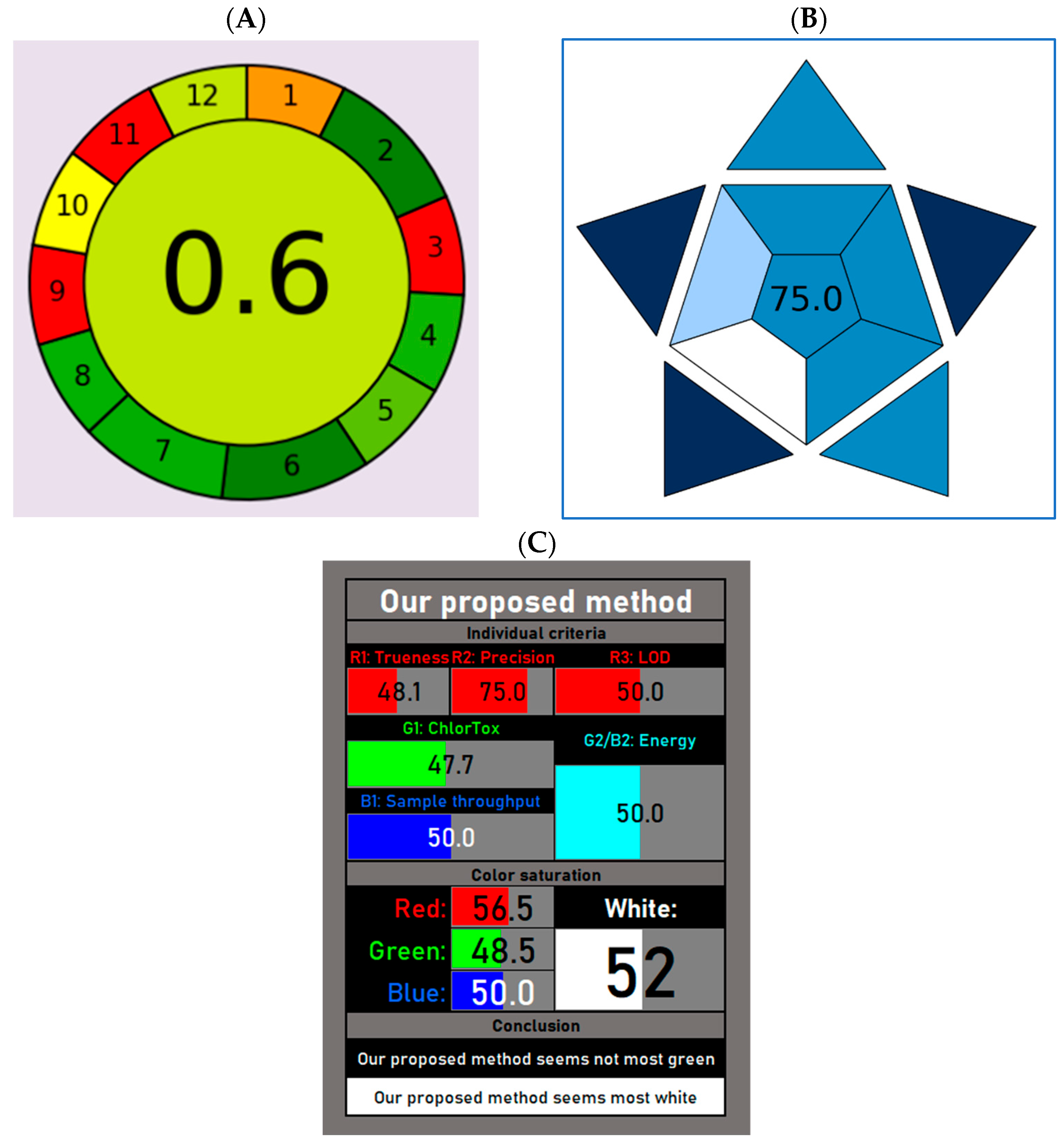
In our method, sample preparation is simple and does not require multiple steps (i.e., LLE, SPE and/or derivatization). Moreover, it is carried out from 50 µL of plasma. This low sample volume is favorably suitable to both adults and pediatric patients with CF. In fact, for studies involving pediatric patients, ethical and physiological concerns do not allow the collection of large volumes of blood through multiple repeated tests. Additionally, the use of small sample volumes and the absence of derivatization steps are two important aspects in the overall greenness evaluation and represent the greenest hallmarks of our method. Conversely, off-line sampling and the energy consumption by the LC-MS/MS instrument are the most hazardous subsections in our AGREE score. However, considering that blood samples from both hospitalized and outpatients cannot be collected in the TDM laboratory, a different sampling procedure could not be performed. Similarly, the analytical technique used in our study (i.e., LC-MS/MS) still represents the gold standard for TDM analysis, and alternative immunometric methods based on immunoenzymatic reactions are not advisable due to their low sensitivity and specificity. However, the overall AGREE score calculated for our method (0.60) can be classified as “acceptable green”, and it is in line with previously published LC-MS/MS methods [
55], including one report aimed to simultaneously quantify ivacaftor, tezacaftor, and elexacaftor in plasma samples of CF patients [
35].
A higher score has been calculated when evaluating the blueness of our developed method. To this aim, we have used the Blue Applicability Grade Index (BAGI), a metric that incorporates 10 criteria to assess both the practicability and greenness of different assays [
46]. In fact, this tool is used for evaluating the practicability of a method in analytical chemistry and the attributable score ranges from 25 to 100 (the higher the score, the more practical the method). For a method to be considered “practical,” the final rating score should exceed 60. Here, we have calculated a BAGI score of 75, thus denoting the good practicability of our method.
Finally, the white analytical chemistry (WAC) expands the concept of green analytical chemistry (GAC) and highlights the importance of achieving the best possible compromise between greenness and functionality [
47]. In fact, WAC refers to the Red–Green–Blue (RGB) model of a light color, in which greenness is one of the three primary components of whiteness [
56]. Red is assigned to analytical performances such as the accuracy, precision and sensitivity, whereas blue is assigned to the practical and economic aspects [
46]. The overall quality of the method is expressed by whiteness. Here, we have used an improved version of the RGB model, called RGBfast, which also includes the ChlorTox Scale, a recently developed greenness indicator [
48,
57]. As depicted in
Figure 3C, our method seems “whiter” compared to a similar published report [
35], despite limited greenness. However, in accordance with a previously provided definition, a whiter method is one that is more comprehensive and better overall, but at the same time not necessarily greener. In fact, certain methods may be highly green but not very functional in the red or blue areas, and so would not represent the desired compromise [
48].
The clinical applicability of our validated method was tested by measuring ETI plasma concentrations in pediatric patients followed at our center during routine clinical practice. In particular, blood sampling was performed before (C
trough, T0) and 4 h after (T4) the morning intake of Kaftrio
®. Our data for IVA revealed a median (IQR) C
trough value of 0.79 (0.37–1.34) µg/mL, which agreed with the drug registration documents (Trikafta
® mean ± standard deviation, SD = 0.63 ± 0.26 µg/mL) [
18] and with previously published studies on both children (mean ± SD= 0.71 ± 0.20 µg/mL) and adult patients (mean ± SD = 0.90 ± 0.37 µg/mL) [
35]. For both TEZ and ELX, we found some discrepancies between our results and other reports including the medication SmPC (Kaftrio
®) [
22]. For example, our results for TEZ C
trough (median, IQR = 1.37, 0.72–1.87 µg/mL) confirm the minimum concentration (C
min) values shown in a study by Vonk S.E.M. and colleagues (median, range = 1.3, 0.5–2.8 µg/mL) (2024) [
58], but not those described in the registration documents (Trikafta
® mean ± SD = 2.10 ± 0.82 µg/mL) [
18]. Conversely, C
trough values measured in our study for ELX (median, IQR = 8.73, 5.82–12.94 µg/mL) were higher than those reported in both SmPCs (Trikafta
® mean ± SD = 4.05 ± 2.07 µg/mL) [
18] and previous studies (median, range = 2.8, 0.9–7.9 µg/mL) [
58]. However, a recent study conducted in a real-world cohort of adults with cystic fibrosis has reported an AUC
0–24h for elexacaftor and tezacaftor ranging from 58.7 to 422.9 mg⋅h/L and 38.0 to 207.7 mg⋅h/L, respectively [
26].
Reasons behind these discrepancies may be attributed to differences in the nature of these studies. In fact, our method has been applied to samples collected from pediatric patients (age range: 7–17 years) in a real-life setting, which significantly differs from the fixed conditions established during the registration studies. In particular, plasma samples tested with our validated method were obtained from CF patients who showed clinical stability or were experiencing respiratory exacerbations during the follow-up visits in a clinical practice scenario. Therefore, the inter-individual variability together with the underlying health conditions could be important factors affecting the PK response to these drugs.
In this context, it is also important to consider possible drug–drug interactions as a consequence of multiple concomitant medications. In fact, the cytochrome P450 3A (CYP3A) system is the main metabolizing route for all three drugs. Many drugs commonly used in CF patients act as inhibitors or inducers of both CYP3A4 and CYP3A5 isoforms (for example, rifampicin, azole antifungals or macrolides antibiotics) leading to DDIs that could potentially alter “caftor” plasma concentrations [
8]. Similarly, the bioavailability of CFTR modulators is significantly affected by the concomitant ingestion of food containing a high percentage of fat [
8]. In fact, it has been reported that the AUC of elexacaftor is increased by 1.9- to 2.5-fold when assumed with a fatty meal, whereas exposure to TEZ is not affected by the simultaneous ingestion of fatty food (Kaftrio
®) [
22]. Therefore, a real-world setting could be characterized by different sources of variabilities that in a randomized clinical trial (RCT) are significantly reduced. Patients tested in this study were concomitantly receiving therapies with pancreatic enzymes, bronchodilators, antibiotics (including rifampin, carbapenems, macrolides, beta-lactams, lipopeptides, fluoroquinolones) and proton pump inhibitors. Among these medications, the Kaftrio
® dosing regimen should be adjusted when clarithromycin (macrolide antibiotic) and/or rifampin are co-administered [
22]. Therefore, this aspect underlies not only the utility of monitoring ETI concentrations in a real-life setting but also the different sources of variability that make routine clinical practice different from RCTs.
Additionally, children are physiologically different from adults in terms of organ maturation and developmental changes. This aspect adds a further source of inter-individual variability that could partially explain the differences observed in our C
trough values compared to the studies involving both adults and pediatric subjects [
35,
58]. However, it is also worth noting that within the pediatric population, changes in body weight (< or ≥30 Kg) require a different dosage (Kaftrio
® SmPC) [
22]. Therefore, an age range of 7–17 years is not enough to guarantee dosage homogeneity, introducing an additional variable that could affect plasma concentrations. Perhaps, it is possible that tezacaftor and elexacaftor could be more susceptible to these “real-life” sources of variability, thus explaining the observed discrepancies.
In order to better elucidate the PK properties of ivacaftor in a real-life pediatric setting, an additional blood sampling at 4 h (T4) following Kaftrio
® intake (considered as the time to reach the maximum plasma concentration, T
max, for IVA) was included in our TDM protocol. After estimating plasma concentrations of ivacaftor at 12 h, an AUC
0–12h was calculated for each patient. As reported in
Table 5, our results for IVA C
max (median, IQR = 1.49, 1.07–2.43) and AUC
0–12h (median, IQR = 11.43, 7.93–19.45 μg/mL*h) agreed with the drug registration documents (C
max, mean ± SD =1.24 ± 0.34 µg/mL and AUC
0–12h, mean ± SD =11.7 ± 4.01 μg/mL*h, Kaftrio
®) [
22] and with previously published studies on both children and adult patients [
23,
24,
26,
33,
53,
59,
60].
Similarly, a significant correlation (
p < 0.001) was found between C
trough (T0) and AUC
0–24h values for ivacaftor (
Figure 4A), confirming previous published evidence [
58]. This strong correlation was further confirmed by the analysis of residuals that showed a low %CV between measured and predicted AUC
0–24h values of 0.02 ± 13.12 (mean ± SD) (
Figure 4B). Moreover, by using the derived regression equation, we were able to back-calculate the C
trough values for IVA. The median (IQR) value for the calculated C
trough was 0.80 (0.42–1.32) µg/mL. This median value established with the regression equation (
Figure 4A) presented high correspondence with our observed C
trough (median, IQR = 0.79, 0.37–1.34 µg/mL) and with the C
min ranges found in the registration documents (Trikafta
® mean ± SD = 0.63 ± 0.26 µg/mL) [
18]. These results agree with those reported by Vonk S.E.M and colleagues (2024) on the back-calculated C
trough values for IVA (mean ± 1.96 SD = 0.7 ± 0.1–1.2 µg/mL), and suggest that C
trough could be a good predictor of the systemic exposure to ivacaftor [
58]. In fact, although the AUC represents the gold standard for determining drug exposure, it requires multiple blood sampling during one dosing interval. As a consequence, this practice is invasive and poorly feasible in both clinical studies and during the routine clinical practice, especially when neonates and children are involved. Therefore, measuring only one C
trough sample instead of performing multiple blood sampling could represent an important advantage in evaluating the systemic exposure to CFTR modulators. In our study, we were not able to assess the correlation between C
trough and AUC
0–24h also for tezacaftor and elexacaftor. In fact, based on a T
max of 3 h for TEZ and 6 h for ELX [
8], we were not able to calculate the AUC
0–24h. However, Vonk S.E.M. and colleagues (2024) reported a significant correlation between C
min and the AUC
0–24h values for tezacaftor and elexacaftor [
58]. Therefore, considering the agreement between our data for IVA and those previously reported [
58], a similar result should also be predictable in our pediatric setting for TEZ and ELX.
Both short- and long-term stability were evaluated on prepared QC samples kept at room temperature in the autosampler for a maximum of 9 days. After 24 h from QC sample preparation and first assessment (Time 0), stability was around 100% for all QC samples. After 9 days from day 0, stability decreased exclusively for the L-, M- and H-QC of ivacaftor (73.54–78.27%), probably due to different chemical properties of ivacaftor compared to tezacaftor and elexacaftor. However, a similar result has been already observed in a recently published method in which the authors evaluated the stability of compounds in EDTA plasma after four freeze–thaw cycles, and for ten days in the autosampler after sample preparation. Following ten days in the autosampler, the observed remaining % for the H-QC of ivacaftor was 88%, compared to the H-QC of tezacaftor and elexacaftor (both 99.8%) [
40].
Finally, n = 3 T0 and T4 patients’ samples were re-tested after a storage at −80 °C for a period of five months. ELX/TEZA/IVA concentrations showed a stability higher than 85% of the first measurement, meeting the criteria of the remaining percentages being between 85% and 115%. Therefore, in order to overcome the absence of mass spectrometry facilities, centers involved in PK studies could collect and store samples at −80 °C before sending them to TDM laboratories for the evaluation of ELX/TEZA/IVA plasma levels.
One limitation of this study may be the absence in our LC-MS/MS method of active metabolite determination. In fact, elexacaftor, ivacaftor and tezacaftor are metabolized through CYP3A isoforms, and the concomitant assumption of potent CYP3A inhibitors or inducers would lead to changes in active metabolite concentrations requiring an adjustment of caftor dosage [
37]. However, considering that both elexacaftor M23-445 and M1-TEZ have similar potencies to their parent compounds, whereas M1-IVA has shown a 1/6 potency of IVA [
8], our aim was to primarily focus on the measurement of ELX/TEZA/IVA plasma concentrations. Moreover, due to the lack of therapeutic ranges for the active metabolites, future studies will be required to assess the utility of TDM for these compounds.
Similarly, the clinical applicability of this method has been tested on plasma samples of pediatric patients aged from 7 to 17 years. Considering the recent approval of ETI combination for treating CF patients aged 2 years and older, a further limitation of our study could be represented by the lack of PK data collected from younger children in a real-world clinical setting. Therefore, future reports will be needed in the next future to fill this knowledge gap.