Abstract
Background/Objectives: Suicide is a complex issue resulting in approximately 700,000 deaths annually. Individuals with mood disorders or schizophrenia are at an increased risk. Pharmacological interventions, such as lithium, clozapine, and ketamine, show promise in reducing suicidality. Methods: A systematic search was conducted across Google Scholar, Scopus, and PubMed to identify studies evaluating the effects of lithium, clozapine, and ketamine on suicidality. Peer-reviewed articles published between 2014 and 2024 that focused on adult populations were included. After screening 1297 records, 49 studies met the eligibility criteria: 14 on lithium, 23 on ketamine, and 12 on clozapine. Results: Multiple studies highlight lithium’s significant anti-suicidal effects in patients with bipolar disorder, showing superior suicide risk reduction compared to valproate and other mood stabilizers. Ketamine has been shown to rapidly reduce suicidal ideation, with effects observable within hours and lasting up to a week. While most studies support its short-term efficacy, findings regarding its long-term benefits and the impact of repeated dosing remain inconsistent. Clozapine has consistently demonstrated a reduction in suicide risk for individuals with schizophrenia. Large-scale cohort studies report a significant decrease in suicide attempts and mortality when compared to other antipsychotics. Conclusions: Lithium, ketamine, and clozapine were proven to be effective in reducing suicidality. However, limited data, adherence challenges, and methodological differences across studies highlight the need for more robust, large-scale experimental research. Effective suicide prevention is an extremely complex topic and also requires consideration of healthcare and social system factors.
1. Introduction
Suicide is a complex phenomenon that arises from the interplay of biological, psychological, and social factors. According to the World Health Organization, approximately 700,000 people die by suicide each year worldwide, with many more attempting suicide or experiencing serious suicidal thoughts [1,2]. Suicide is especially prevalent among individuals with mood disorders, such as major depressive disorder (MDD) and bipolar disorder (BD), as well as those diagnosed with schizophrenia spectrum disorders [3]. As a result, ensuring broad access to mental health care and providing effective treatment are essential pillars of suicide prevention [2].
While effective treatment of mental disorders is generally believed to reduce suicidal behavior indirectly, only a few medications have demonstrated a direct anti-suicidal effect. Among pharmacological interventions, three agents—lithium, clozapine, and ketamine—have consistently emerged as promising treatments with significant anti-suicidal properties [4,5].
1.1. Lithium
Lithium salts have been widely used in the treatment of mood disorders for over 70 years. They are effective in reducing the frequency, severity, and duration of mood episode relapses, thereby improving long-term stability and quality of life in patients. Today, lithium remains one of the first-line medications for preventing recurrences in both type I and type II bipolar disorder.
Although concerns have been raised about its safety, particularly with respect to hypothyroidism, electrocardiographic changes, and potential nephrotoxicity, lithium is generally considered safe when managed with appropriate medical supervision. Maternal use of lithium during the first trimester has been associated with an increased risk of cardiac malformations, including Ebstein’s anomaly.
A key aspect of lithium therapy is that dosing must be individualized based on serum levels; therefore, regular blood tests are required throughout treatment [6,7]. Despite decades of use, the exact mechanism of lithium’s action remains incompletely understood. It is known to exert complex effects on both intracellular and extracellular signaling pathways [8]. Notably, lithium appears to reduce aggression and impulsivity—traits that may contribute to its unique anti-suicidal properties [9].
1.2. Ketamine
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist that has long been recognized as a safe anesthetic agent with notable analgesic properties [10]. Esketamine, which contains only the (S)-enantiomer of ketamine, is primarily administered as a nasal spray [11]. In contrast, arketamine—the (R)-enantiomer—is less potent as both an NMDA receptor antagonist and an anesthetic, and it has not been approved for clinical use [12].
Beyond anesthesia, ketamine’s therapeutic applications have expanded to include pain management, procedural sedation, and, increasingly, psychiatric treatment [13]. Growing evidence supports its rapid antidepressant effects, even in patients with treatment-resistant depression, following a single subanesthetic dose [14]. This rapid onset of action sets ketamine apart from conventional antidepressants, which typically require weeks to achieve noticeable effects [15].
The antidepressant mechanisms of ketamine are thought to be multifaceted. These include NMDA receptor inhibition on γ-aminobutyric acid (GABA) interneurons, leading to disinhibition of glutamatergic transmission, and subsequent activation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors through increased glutamate release [16]. Esketamine has been approved by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of treatment-resistant major depressive disorder [17].
Interestingly, lithium and ketamine share overlapping neurobiological pathways involved in neuroplasticity and antidepressant effects. Ketamine rapidly enhances AMPA receptor activity and increases brain-derived neurotrophic factor (BDNF), which activates the mTOR signaling pathway and inhibits glycogen synthase kinase-3 beta (GSK-3β). Lithium also activates the mTOR pathway and inhibits GSK-3β, contributing to synaptic growth and plasticity. Preclinical studies suggest that lithium may potentiate ketamine’s antidepressant effects in models resistant to ketamine alone [18]. However, clinical evidence remains mixed. While lithium may not enhance ketamine’s efficacy in all patients, it could offer benefits in specific subgroups, particularly those with bipolar disorder [19,20]. Nonetheless, combining lithium and ketamine should be approached with caution due to the potential for additive adverse reactions [19].
1.3. Clozapine
Clozapine is the only antipsychotic approved by the FDA specifically for patients with treatment-resistant schizophrenia, defined as persistent symptoms despite adequate trials of at least two other antipsychotic medications [21]. It is widely regarded as the gold-standard treatment in this population due to its superior efficacy [21,22]. Long-term studies have demonstrated that clozapine reduces overall mortality in individuals with schizophrenia, largely due to decreased suicide rates and improved symptom control, which may help prevent medical complications and psychosocial deterioration [21]. Despite its clear benefits, clozapine is associated with a complex and potentially serious side effect profile. These include agranulocytosis (severe neutropenia), myocarditis, metabolic syndrome, seizures, and other adverse effects [23,24]. As a result, rigorous hematological monitoring—specifically regular assessments of white blood cell and neutrophil counts—is mandatory. Broader clinical surveillance is also required to detect and manage other complications early. Due to these challenges, clozapine remains underused or is introduced late in the treatment course, which may negatively affect patient outcomes [23].
Importantly, clozapine is the only antipsychotic approved by the FDA to reduce recurrent suicidal behavior in individuals with schizophrenia [25]. The landmark InterSePT trial—a two-year randomized study—found that clozapine reduced suicide attempts by 24–26% compared to olanzapine in patients with schizophrenia or schizoaffective disorder who were at high risk of suicide. Significant outcomes included fewer suicide attempts, hospitalizations, and rescue interventions [26]. In comparison to other antipsychotics, clozapine is more effective in alleviating core schizophrenia symptoms as well as comorbid depressive symptoms, both of which may contribute to its anti-suicidal effects [25]. Some studies also suggest that clozapine’s positive effects on sleep may mediate its impact on suicidality [27]. Basic research has revealed that clozapine acts through complex, multifactorial mechanisms, with no single pathway fully explaining its efficacy [27,28]. Its anti-suicidal properties may involve several neurobiological systems. These include modulation of the norepinephrine system, high affinity for 5-HT2A receptors, and influence on NMDA receptor expression [25,28]. Given that suicide is often conceptualized as self-directed aggression, clozapine’s anti-aggressive effects may also play a role. Furthermore, clozapine has been shown to reduce substance use—a known and significant risk factor for suicide [25].
This review aims to synthesize the literature from the past decade, focusing on the efficacy of lithium, clozapine, and ketamine in reducing suicidality. We explored the last 10 years of research, aiming to provide a comprehensive overview of the current evidence base, highlight potential mechanisms of action, and discuss both limitations and implications for clinical practice and future research.
2. Methods
2.1. Search Strategy
Databases Google Scholar, Scopus, and PubMed were searched using a query designed to capture articles investigating the effects of lithium, clozapine, ketamine, and esketamine on suicidality (suicidal ideation, suicidal behavior, or suicide risk). The main search query combined Mesh terms and Title/Abstract keywords:
((“Lithium” [Mesh] OR lithium [Title/Abstract]) OR (“Clozapine” [Mesh] OR clozapine [Title/Abstract]) OR (“Ketamine” [Mesh] OR ketamine [Title/Abstract]) OR (“Ketamine, (S)-” [Supplementary Concept] OR esketamine [Title/Abstract]))
AND
(“Suicide” [Mesh] OR “Suicidal Ideation” [Mesh] OR suicide [Title/Abstract] OR suicidal [Title/Abstract] OR suicidality [Title/Abstract] OR “anti-suicidal effect” [Title/Abstract] OR “antisuicidal effect” [Title/Abstract])
Only articles published in the last 10 years (2014–2024) were included.
2.2. Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (1) peer-reviewed publications in English; (2) original research articles; and (3) primary focus on the effects of lithium, clozapine, or ketamine on suicide risk, suicidal behavior, and/or suicidal ideation. Only studies involving adult populations (aged 18 years or older) were considered.
Exclusion criteria were as follows: case reports, editorials, letters to the editor, commentaries, and conference abstracts that did not contain original data. Studies were also excluded if they did not explicitly assess or report on outcomes related to suicidal ideation, suicide attempts, or overall suicidality. Research focusing on pediatric populations was not included in this review.
2.3. Data Extraction and Synthesis
Three authors (J.O., N.O., P.W.) independently screened the results of the systematic search. Each title and, when available, abstract were reviewed to determine relevance based on the predefined inclusion and exclusion criteria. Any discrepancies were resolved through discussion and consensus. Additionally, the reference lists of the included studies were screened to identify further relevant publications.
The initial search yielded 1297 records. After the removal of 719 duplicates, 578 unique records remained for screening. Of these, 413 were excluded based on title and abstract screening, leaving 165 full-text reports for retrieval. Ten reports could not be accessed, resulting in 155 studies assessed for eligibility. Following full-text review, 106 studies were excluded for reasons such as them being case reports, ecological or autopsy studies, or otherwise not meeting the inclusion criteria. Ultimately, 43 unique studies were included in the final analysis: 13 on lithium, 23 on ketamine, and 7 on clozapine (Figure 1).
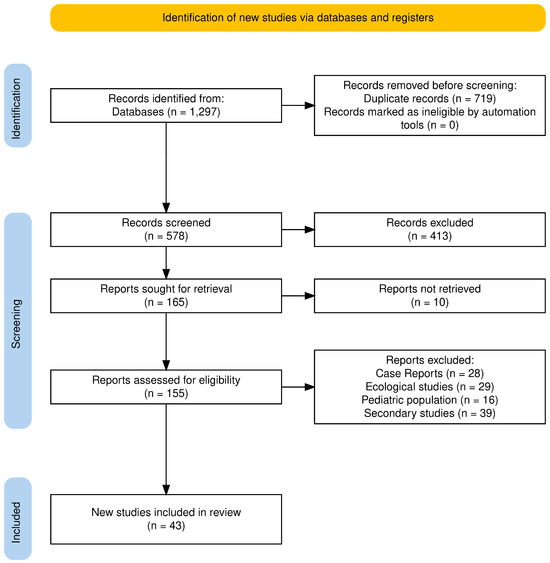
Figure 1.
PRISMA 2020 flow diagram.
All studies reporting suicidal outcomes—such as suicidal ideation, suicide attempts, or completed suicide—were considered eligible for analysis. Data were extracted on key variables, including study design, sample size (including control groups, if applicable), inclusion and exclusion criteria, duration of follow-up, medication dosage (when reported), and primary study outcomes. All numerical results, regardless of effect size, were included.
Risk of bias was assessed collectively by all authors, with consideration given to factors such as study design, sample size, heterogeneity of findings, reported effect sizes, and methodological quality as described in each publication.
The extracted data were summarized and synthesized in Table 1, Table 2 and Table 3, corresponding to lithium, ketamine, and clozapine. No additional data visualization tools were employed. The preparation of this literature review was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines [29].

Table 1.
Studies on lithium and suicidality.

Table 2.
Studies on ketamine and suicidality.

Table 3.
Studies on clozapine and suicidality.
3. Results
3.1. Lithium and Suicidality
3.1.1. Bipolar Patients
Individuals with BD face a markedly elevated risk of suicide, with estimates suggesting a 20- to 30-fold increase in suicide mortality compared to the general population [70]. As lithium is primarily prescribed in the treatment of BD, this patient group is particularly relevant for examining its anti-suicidal effects. In this review, six studies focusing specifically on BD populations were identified (Table 1).
A large population-based study conducted in Sweden between 2005 and 2013, involving 51,535 patients, found that lithium treatment significantly reduced the incidence of suicide-related events by 14% compared to periods without treatment. In contrast, valproate showed no protective effect [40]. The authors estimated that 12% of suicide-related events could have been prevented if patients had consistently adhered to lithium therapy, emphasizing the need for prioritizing lithium in treatment guidelines and ensuring proper patient support and monitoring [40].
Another long-term observational study from Hong Kong assessed all-cause and suicide-specific mortality in BD patients treated over a 16-year period with mood stabilizers such as lithium, valproate, quetiapine, and olanzapine [39]. The findings revealed that olanzapine and risperidone were associated with significantly higher all-cause mortality compared to lithium. Lithium and valproate had comparable mortality risks, reinforcing lithium’s relative safety and potential benefits in minimizing premature mortality [39].
A Taiwanese cohort study followed 25,787 patients with bipolar disorder over 16 years, comparing the impact of various mood stabilizers (lithium, valproate, lamotrigine, and carbamazepine) [37]. The study concluded that mood stabilizers—particularly lithium—were significantly associated with reduced risks of all-cause mortality, suicide, and natural death, with lithium demonstrating the lowest mortality risk among the agents studied [37].
Elena Toffol et al. analyzed 826 patients from a Finnish nationwide registry, focusing on high-risk BD individuals [35]. The study demonstrated that lithium was significantly associated with reduced suicide mortality and a 49% decrease in all-cause mortality. Conversely, treatment with antidepressants, valproic acid, and benzodiazepines was linked to increased risk of suicide attempts and mortality. However, the study’s limitations include non-standardized treatment protocols and the absence of structured diagnostic interviews [35].
A randomized controlled trial explored the anti-suicidal effects of lithium compared to valproate in high-risk bipolar patients, with a specific focus on age-related outcomes [30]. It found that patients aged 42 years or older on lithium have a significantly lower risk of suicidal behavior compared to patients > 42 years old on valproate or younger patients on either medication. The findings indicate that treatment with lithium may reduce suicide risk in bipolar patients > 42 years old, highlighting the importance of age-dependent differences in response to lithium. Nevertheless, the relatively small sample size (94 participants) may limit the generalizability of the findings [30].
3.1.2. Anti-Suicidal Effect Studied in U.S. Veterans
Several studies have explored the potential anti-suicidal properties of lithium among U.S. veteran populations, a group characterized by high rates of mental health disorders and elevated suicide risk.
A small cohort study investigated the use of the Veterans Crisis Line and hospitalization rates among veterans treated with lithium [38]. The results indicated a statistically significant reduction in hospitalizations for both suicide attempts and suicidal ideation after six months of lithium treatment. Specifically, hospitalization for suicide attempts dropped from 4.1% to 0%, and admissions for suicidal ideation decreased from 13.3% to 1.0%. However, due to the limited sample size, the authors advised that these findings should be interpreted as hypothesis-generating rather than conclusive [38].
Eric Smith and colleagues conducted three studies examining lithium’s impact on mortality in veterans. Two of these studies analyzed similar cohorts, comparing lithium and valproate in terms of general and suicide-specific mortality outcomes [38,42]. One study reported that lithium was associated with a reduction in non-suicide mortality during active treatment compared to valproate [38]. The other found no significant difference in suicide mortality between lithium and valproate during treatment. However, both studies noted that lithium discontinuation was linked to a heightened risk of suicide within 180 days, suggesting that while lithium may offer protective effects during treatment, abrupt discontinuation can increase vulnerability—particularly in high-risk individuals [42].
A third study by Smith and colleagues examined veterans both with and without bipolar disorder, using high-dimensional propensity score matching to explore the association between lithium and suicide risk [41]. Among veterans with bipolar disorder, lithium initiation was linked to an increased one-year suicide risk, largely driven by elevated risk following discontinuation. In contrast, among those without bipolar disorder, lithium was associated with a significantly reduced suicide risk, underscoring the importance of diagnosis-specific effects and the consequences of treatment interruption [41].
Two studies were published based on randomized, placebo-controlled clinical trials. The authors chose different methodologies: intent-to-treat [32] and per-protocol [36] approaches. The two studies evaluate lithium’s potential in reducing suicidality among U.S. veterans with bipolar disorder or major depressive disorder, offering complementary insights through different techniques.
In the per-protocol study [36], patients receiving lithium had a lower 12-month suicidality risk (18.8%) compared to those receiving placebo (24.3%), yielding a risk ratio of 0.78. However, the results were inconclusive due to low adherence rates (only 17%), emphasizing the crucial role of treatment compliance in achieving benefit [36].
Conversely, the intention-to-treat trial [32] found no significant difference in repeat suicide-related events between the lithium (25.5%) and placebo (23.5%) groups. The hazard ratio was 1.10, indicating no clear advantage for lithium when added to standard care. Notably, discontinuation of any medication, regardless of group, was associated with a heightened risk of suicide-related outcomes [32]. Together, these studies underscore the potential of lithium as a protective agent, particularly when adherence is maintained. However, they also highlight the challenges in real-world implementation, especially in achieving consistent medication adherence and mitigating risks associated with treatment discontinuation.
3.1.3. Other Populations
Ole Brus et al. examined the impact of lithium on suicide and readmission risks in patients with unipolar depression who had undergone electroconvulsive therapy (ECT) [34]. The study found that lithium significantly reduced both the risk of suicide (p = 0.014) and the risk of readmission (HR 0.84). The number needed to treat (NNT) to prevent one readmission was 16. These findings support the Swedish guidelines, which advocate for lithium use as a prophylactic measure following ECT. This suggests that lithium could play a more prominent role in the post-ECT management of these patients [34]. However, the study’s findings may not be generalizable to broader populations due to the specific cohort and treatment settings involved [34].
The final study identified was a randomized clinical trial [31] which involved only 56 patients, far below the target of over 200, leading to a statistically underpowered study. The results indicated that lithium, when added to usual care, did not significantly reduce deliberate self-harm after 12 months. This lack of effect is likely attributable to the insufficient sample size, which undermined the statistical power of the study.
Lithium shows strong and consistent evidence for long-term suicide prevention in bipolar disorder, outperforming other mood stabilizers like valproate and lamotrigine. However, high discontinuation and low adherence rates, ranging from 76.5% to as low as 17%, pose significant clinical challenges. These issues are linked to both the treatment regimen and the nature of bipolar disorder itself. Additionally, reduced suicide rates may be partly attributed to increased healthcare contact during lithium monitoring, suggesting a role of social factors beyond the drug’s direct effects.
Lithium levels in some of the studies were consistent between 0.6 and 0.8 mEq/dL. However, in as many as eight studies, they were not reported at all. The studies published between 2014 and 2024 also analyzed quite diverse populations (veterans, BD patients, European countries, Asian countries, the US). Quite a large group size (thousands of participants) was gathered by registry-based cohort-type studies. However, randomized controlled trials (RCTs) have recruited much smaller numbers of participants. Only two RCTs had about 500 patients, and the other two were underpowered and involved fewer than 100 patients. This rather high heterogeneity of the results makes it difficult to formulate more general conclusions.
3.2. Ketamine and Suicidality
We found 23 studies published between 2014 and 2024 that assessed ketamine’s anti-suicidal effects (Table 2). Out of these, twelve required subjects to have MDD, one required subjects to have bipolar disorder, one required subjects to have borderline personality disorder, and five did not require a specific psychiatric disorder as an inclusion criterion. The majority administered an intravenous (IV) 0.5 mg/kg dose of ketamine. Two studies assessed a 0.2 mg/kg dose, and another compared three doses of ketamine: 0.1, 0.5, and 1.0 mg/kg. Two studies administered a fixed dose of intranasal (IN) ketamine. Ten studies used a 0.02–0.05 mg/kg midazolam dose as a control, and the remaining nine used a saline solution.
Six studies assessed ketamine’s anti-suicidal effects in patients with major depressive disorder using saline as a control. Zolghadriha et al. and Domany et al. noted a rapid and significant reduction in suicidal ideation after the administration of a single IV ketamine infusion, with effects observed by 60 min and 90 min post-treatment, respectively [50,59]. An acute anti-suicidal effect of ketamine was also reported by Burger et al. Hu et al. investigated the impact of a single ketamine infusion adjunct to newly initiated antidepressant therapy [61,62]. Ketamine augmentation of escitalopram significantly lowered suicidal ideation between 2 h and 72 h post-infusion. A study by Ahmed et al. gave patients two infusions of 0.5 mg/kg ketamine 7 days apart and noted a significant reduction in suicide ideation one week after the second infusion [5]. However, contradictory findings have been observed. Ionescu et al. reported no significant advantage between repeated doses of ketamine in reducing suicidal ideation [56]. The contrasting results may be due to a couple of reasons. Most importantly, the study group had a greater level of chronicity and treatment resistance, with most patients failing five or more antidepressant trials, nearly 50% not responding to ECT treatment, and the current MDD episode being on average of 9.6 years long.
Six studies examined the effect of intravenous ketamine on suicidal ideation in patients with MDD using midazolam as a control. Grunebaum et al. found that a single ketamine infusion was associated with a significantly greater reduction in suicidal thoughts by 24 h post-treatment as assessed by the clinician-rated scale for suicidal ideation (SSI) [43,48]. This improvement was maintained during the 6-week follow-up; however, during this period medication changes to co-administered antidepressants were permissible. These findings are supported by Sinyor et al., who administered s ketamine infusions over 12 days [52]. The study found that ketamine infusions achieved a greater and longer-lasting (up to 42 days) reduction in suicidal ideation compared to the midazolam control. Both studies used ketamine as an adjunctive therapy and did not require subjects to have treatment-resistant depression. Five studies assessed ketamine’s anti-suicidal efficacy in treatment-resistant depression using midazolam as an active control. Phillips et al. found lower suicide item Montgomery–Åsberg Depression Rating Scale (MARDS-SI) scores 2 h and 7 days after a single ketamine infusion, and scores did not differ at 24 h [45]. Further reductions in MARDS-SI were seen during the administration of six open-label ketamine infusions over the course of two weeks, which ceased once ketamine infusions were reduced to once weekly. These weekly infusions were sufficient to sustain reductions in suicidal ideation. Both Price et al. and TP Su et al. observed a significant decrease in suicidal cognition 24 h after the administration of a single 0.5 mg/kg ketamine infusion [53,54]. In addition, TP Su et al. observed significant anti-suicidal effects of ketamine up to 5 days [53]. Similarly to Ionescu et al., TP-Su et al. noted that individuals whose current depressive episode lasted >24 months or who failed >4 antidepressant treatments did not benefit from ketamine’s anti-suicidal effects [51,53,56]. Feeney et al. found that among individuals with clinically significant suicidal ideation, those who had received ketamine had a significant reduction in suicidal ideation at 30 days post-infusion in comparison to those who had received midazolam [58]. However, a weakening effect was observed as early as day 3 post-infusion.
Grunebaum et al. evaluated the effect of a single 0.5 mg/kg infusion of ketamine on patients with bipolar depression and suicidal thoughts [43]. At day 1 post-infusion, a greater decrease in suicidal ideation was observed after the ketamine infusion than after midazolam. The difference was not statistically significant, which may be explained by the small sample size of 16 patients.
Ketamine’s anti-suicidal effect was also examined in patients with bipolar personality disorder and current suicidal ideation. However, Fineberg et al. found no significant decrease in suicidal ideation [44].
Three studies assessed the effects of intravenous ketamine in patients without a specific psychiatric diagnosis as an inclusion criterion. Murrough et al. and Abbar et al. investigated the efficacy of ketamine in patients with suicidal ideation and varying comorbid mental disorders [46,57]. Abbar et al. investigated the efficacy of two ketamine infusions administered 24 h apart vs. a saline placebo [46], whereas in the study conducted by Murrough et al., patients received a single IV infusion of ketamine or midazolam as a control [57]. It is worth noting that the exclusion criteria in both studies included psychotic disorders and substance dependance. Murrough et al. also excluded patients with a current intent to make a suicide attempt [57]. Abbar et al. found that a greater percentage of patients receiving ketamine achieved full remission of suicidal ideation 2 h after the first infusion and 72 h following the second infusion compared to the placebo group (43.8% vs. 7.3% and 63.0% vs. 31.6%, respectively) [46]. This effect was observed up to 6 weeks post-infusion; however, it was no longer statistically significant at 6 weeks. In Murrough et al.’s study, ketamine had a significant effect on suicidal ideation 48 h post-treatment, although this was not observed at 24 h [57].
Fan et al. found a significant decrease in suicidal ideation on day 1 and day 3 post-infusion of ketamine in patients with a new cancer diagnosis [55].
We found four studies assessing the anti-suicidal effects of esketamine. Three of which compared an 84 mg esketamine nasal spray to a placebo control in patients with mild depressive disorder and active suicidal ideation. All three studies repeated intakes of intranasal esketamine over a four-week period. One of these was a randomized controlled trial (RCT) conducted by Canuso et al. which found no differences between treatment groups at the end of the double-blind treatment or at post-treatment follow-up; however, a significant improvement on the MARDS suicidal item was observed 4 h post first dose [47]. Both Fu et al. and Ionescu et al. found no significant difference in suicidal ideation 24 h after first dosage [49,56]. Zeng et al. assessed esketamine as an ECT anesthetic in the treatment of MDD characterized by an inadequate response to at least two antidepressant therapies [60]. ECT was conducted three times per week, totaling eight sessions. Propofol was used as the control. The trial found no significant differences in anti-suicidal effects between the two groups.
Two studies analyzed the anti-suicidal effects of intranasal ketamine. Domany et al. administered a 40 mg IN dose of ketamine to patients requiring hospitalization due to suicide risk in an emergency department setting [59]. A significant reduction in suicidal ideation was observed in 4 h as measured by MARDS-SI; however, it was not confirmed by the self-reported BSS scale. Similar findings were seen by Jones et al. in patients with unipolar and bipolar depression with or without concomitant alcohol abuse [63]. Jones et al. found no significant change in SSI scores compared to a placebo; however, a significant improvement was seen in MARDS-SI [63].
Several meta-analyses have reported rapid and significant reductions in suicidal thoughts after the administration of a single ketamine infusion, with effects being observed within 2 h post-infusion [71]. The anti-suicidal duration of a single ketamine dose varies among studies between 24 h and 7 days post-infusion [71]. Contrasting results concerning repeated ketamine doses have been described, with Feng et al. observing no profit in the duration and efficacy of anti-suicidal ideation and Shen et al. revealing an additional benefit into the remission of suicidal behavior [72,73]. This calls for additional research in order to observe long-term effects and establish treatment protocols. The rapid anti-suicidal effect of ketamine may be implemented into the management of acute suicidality in an emergency setting. Ketamine could potentially reduce suicide mortality rates; however, further studies assessing its effect on actual suicidal behavior are required.
A significant convenience in comparing results is the consistency of ketamine dosage across the studies analyzed (mostly 0.5 mg/kg by infusion), but there was also some dose discrepancy. Most of the studies were RCTs, which reduces the risk of bias in research studies. However, the number of participants receiving active treatment was generally less than 100, indicating a rather underpowered study population. Only two RCTs had slightly more than 100 participants in the actively treated group, which is also hard to consider as groundbreaking.
However, ketamine demonstrated rapid but short-lasting anti-suicidal effects, making it a promising option for acute suicidal crises, particularly in depressive disorders where no other drug has shown such immediate impact. Unlike traditional antidepressants, which take weeks to become effective and lack proven anti-suicidal properties, ketamine reduces suicidal ideation within hours. However, its benefits are transient, and long-term efficacy remains uncertain. Further large-scale, real-world studies are needed before ketamine can be widely recommended in treatment guidelines.
3.3. Clozapine and Suicidality
The findings from studies published 2014–2024 consistently demonstrate that clozapine significantly reduces the risk of suicide and suicide attempts among patients with schizophrenia (Table 3). Multiple large-scale cohort studies, including those by Weitoft, van der Zalm, and Taipale, found that clozapine use was associated with a substantial reduction in suicide risk compared to other antipsychotics, with hazard ratios ranging from 0.21 to 0.66 [64,65,66,67].
Weitoft et al. examined 26,046 schizophrenia patients in Sweden between 2006 and 2009. The study found that clozapine users had a 55% lower risk of suicide (OR = 0.45; 95% CI: 0.20–0.98) and a 56% lower risk of attempted suicide (OR = 0.44; 95% CI: 0.28–0.70) compared to those using haloperidol [64].
Taipale et al. conducted a large-scale study in Finland (n = 61,889) and Sweden (n = 29,823) to compare the effects of different antipsychotics on suicide risk. Clozapine use was associated with a significant reduction in suicide risk, with hazard ratios (HRs) of 0.64 (95% CI: 0.49–0.84) in Finland and 0.66 (95% CI: 0.43–0.99) in Sweden. No other antipsychotic showed a similar protective effect. Conversely, benzodiazepines and Z-drugs were linked to an increased risk, with HRs ranging from 1.29 to 1.62 [66]
Van der Zalm et al. analyzed mortality among 22,110 Danish patients with first-time nonaffective psychotic disorders and a broader cohort of 50,881 individuals with prior diagnoses. Clozapine users had a lower suicide risk compared to those using other antipsychotics, with an adjusted HR of 1.76 (95% CI: 0.72–4.32) in the incidence cohort and 2.20 (95% CI: 1.35–3.59) in the prevalence cohort [65]. Interestingly, patients who discontinued clozapine within the first year were at significantly higher risk of suicide (HR = 0.65; 95% CI: 0.46–0.91), suggesting that cessation marks a high-risk period [65].
The protective effect was further supported by a study from Taiwan, which highlighted a dose-dependent reduction in suicide risk, where every 10-day increase in clozapine use led to a further decrease in risk [25]. Chen et al. (2024) conducted a nationwide cohort study in Taiwan using data from the National Health Insurance Research Database (2001–2019) to assess the impact of clozapine on all-cause, natural, and suicide mortality in patients with schizophrenia [25]. The study included 43,025 schizophrenia inpatients, of which 5800 received clozapine. Patients using clozapine had a 41% lower risk of all-cause mortality (HR = 0.59, 95% CI: 0.53–0.66). Suicide mortality risk was also reduced by 60% (HR = 0.40, 95% CI: 0.26–0.61) [25].
A small, single-center study by Gürcan et al. found that clozapine treatment significantly reduced suicide attempts in schizophrenia patients with a history of suicidality [69]. The study included 122 outpatients diagnosed with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders; DSM-IV criteria) and assessed their suicide attempt rates before and after clozapine treatment. Prior to clozapine, 39.3% of patients had attempted suicide, whereas after treatment, this number dropped to just 7.4%. Notably, among 39 patients (32%) who had attempted suicide before starting clozapine, none reported further suicide attempts after initiating the medication [69].
Hassan found no significant difference between clozapine and other antipsychotics in reducing suicidal ideation, suggesting that its protective effect might be more related to behavioral stabilization rather than direct mood improvement [68]. The main disadvantage of that study was small sample size; among 103 participants, 30% experienced suicidal ideation at baseline, and clozapine was used only by 28 individuals (27.2%) [68].
Clozapine significantly reduces suicide risk in patients with schizophrenia and schizoaffective disorder, outperforming other antipsychotics. Its anti-suicidal effect is dose-dependent and diminished by treatment discontinuation. Its benefits may stem from both superior symptom control and increased clinical monitoring due to required safety protocols, a factor also noted with lithium.
Data on the efficacy of clozapine, published between 2014 and 2024, came largely from huge cohort studies conducted in Scandinavian countries with long follow-up. However, the lack of experimental studies (RCTs) makes it difficult to generalize the results more broadly. There are also a small number of studies from other areas of the world and studies with a control group used for comparison. In addition, only one study included analysis of used dosages used and how they might cause the observed anti-suicidal effect.
4. Discussion
In this literature review, we looked at scientific studies on suicidal outcomes with the use of lithium, ketamine, and clozapine dating from 2014 to 2014.
Lithium is strongly supported as effective in long-term suicide prevention, especially in bipolar disorder, showing greater efficacy than other mood stabilizers like valproate or lamotrigine. However, discontinuation and poor adherence—ranging from 76.5% to as low as 17%—remain major concerns, linked to both treatment demands and disease characteristics [38,41]. Regular monitoring may help reduce suicide risk by increasing patient–clinician contact, a factor to consider in future guidelines [33,37]. Research from 2014 to 2024 covered diverse populations across regions and diagnoses. Large registry-based cohort studies included thousands, while RCTs had much smaller samples, only two with around 500 participants and two with under 100, limiting generalizability due to high heterogeneity.
Ketamine shows rapid but short-lived anti-suicidal effects, often reducing suicidal thoughts within hours or days—faster than any other known drug. This makes it promising for acute suicidal crises, especially since no drug has been proven to reduce suicidal behavior in depression. Unlike antidepressants, which take weeks to work and lack anti-suicidal efficacy, ketamine acts quickly. However, its effects fade over time, requiring repeated use or combination therapies. Evidence on long-term benefits is mixed, and large real-world studies are still needed before it can be widely recommended. Most ketamine studies used a consistent dose (0.5 mg/kg IV), aiding comparison, though some variation existed. The majority were RCTs, theoretically reducing bias, but sample sizes were small, usually under 100 participants, limiting their strength.
Clozapine has been consistently associated with reduced suicide risk in patients with schizophrenia and schizoaffective disorder. Cohort studies indicate that its use significantly lowers both suicide attempts and completed suicides compared to other antipsychotics. However, discontinuation of clozapine increases suicide risk, similar to lithium. The anti-suicidal effects appear to be dose-dependent, further reinforcing the importance of sustained treatment adherence. Additionally, the stringent monitoring required due to the risk of clozapine-induced blood disorders ensures more frequent clinical follow-ups. This increased medical oversight allows for earlier detection and intervention in cases of suicidality, potentially contributing to the reduced suicide rates observed in clozapine-treated patients. A similar protective effect has been suggested for lithium, where regular monitoring may facilitate timely management of suicidal risk [74]. Most data on clozapine’s efficacy come from large, long-term Scandinavian cohort studies. However, the lack of RCTs and limited studies from other regions or with control groups limit broader applicability. Only one study analyzed how dosage may influence its anti-suicidal effects.
4.1. Limitations
Despite promising evidence, several limitations remain. There are still question marks over how well the three aforementioned medications can work in the general population of real-world clinical settings. First, most of the data come primarily from observational studies. Only a few large experimental studies have been conducted. Ketamine has the highest number of RCTs, but they were conducted on fairly small groups. The specific location of a significant number of studies (studies on lithium conducted mainly in the U.S., studies on clozapine mainly in Scandinavian countries) also makes it difficult to extrapolate results to other populations. Perhaps the results are not so replicable in completely different healthcare systems and national suicide prevention strategies. Likewise, there is a lack of studies from middle- and/or low-income countries.
Also, in studies based on large registries of patients with long follow-up, variability of diagnoses and instability over time can be a problem. In some studies, recruited patients were diagnosed on the basis of the International Classification of Diseases 9th revision (ICD-9) and then ICD 10; also, DSM revisions have changed quite significantly over the years.
In addition, heterogeneity in outcome measurements also plays a significant role. Studies use various scales and/or different endpoints (suicide deaths, suicide attempt, hospitalization after suicide attempt), making cross-trial comparisons challenging. Safety and tolerability concerns also play a crucial role in treatment feasibility, with clozapine’s significant side-effect profile limiting its broader use, the need for close monitoring of lithium levels at the beginning of treatment, and ketamine necessitating stringent monitoring. Real-world adherence and tolerability may differ from controlled trial conditions, impacting the effectiveness of these treatments outside research settings. Additionally, mechanistic uncertainties persist, as the precise biological pathways through which these medications reduce suicidality remain difficult to isolate in clinical populations with complex psychiatric conditions.
All of these issues make it difficult to generalize the conclusions of these studies and make it more difficult to establish an accurate cause-and-effect mechanism. Without large longitudinal and/or randomized studies, we will not be able to answer questions about causality.
4.2. Future Directions
Future research directions on pharmacological prevention of suicide should include larger-scale, multi-center RCTs examining the long-term outcomes. However easy it is to say so, under practical conditions the organization of such a study can be significantly hampered.
First, death by suicide even in countries with the highest number of them averages about 15–20 deaths per 100,000 population per year [1]. This means that assembling a large enough general population cohort that can be followed for a reliable period of time may require recruiting hundreds of thousands or millions of participants. One can try to circumvent this obstacle by focusing on selected high-risk groups.
However, another major challenge is the paradigm shift in recruiting patients for clinical trials of psychiatric drugs. To date, a significant number of trials of antidepressants and antipsychotics exclude patients at increased risk of suicide [75]. Excluding patients at high risk of suicide may limit the comprehensiveness and generalizability of the findings. However, this approach raises important ethical considerations, as certain pharmacological treatments may in some circumstances elevate suicide risk—a factor that is not always identifiable at the initiation of therapy [76].
It is also necessary to study new drugs or those that have shown unexpected anti-suicidal effects in past studies, such as aripiprazole and others [77]. Also, many types of psychotherapy or electroconvulsive therapy have shown anti-suicidal effects in studies and thus are worthy of further investigation, including in clinical trials of combining psychiatric drugs with different treatment modalities [4,75].
In many developed regions, suicide in the pediatric population (<18 years old) is emerging as a growing problem, becoming one of the leading causes of death in this age population [1]. Unfortunately, we have very scant data on suicide prevention in this age group. Most of the studies analyzed in this article included only adult patients.
Finally, it may not be fully possible to enforce suicide prevention as simply just a drug treatment. Suicide is a complex phenomenon involving both numerous individual and broader social and economic variables [75]. Comprehensive suicide prevention in the general population and specific risk groups should also be based on controlling access to methods, school programs, and ensuring access to mental health care [2].
5. Conclusions
While lithium, ketamine, and clozapine each show varying degrees of reducing suicidal behavior, their effectiveness seems influenced by adherence, patient characteristics, and health system factors. Lithium for BD and clozapine for schizophrenia offer more robust long-term protective effects, while ketamine provides rapid but transient relief, particularly useful in acute settings. However, the current evidence base, largely reliant on observational studies and limited by methodological inconsistencies, highlights the need for larger, well-designed trials to guide clinical practice and inform future treatment guidelines.
Author Contributions
Conception and design, P.M.W.; administrative support, P.M.W. and P.Z.; collection and assembly of data, P.M.W., J.O., and N.O.; data analysis and interpretation, P.M.W., J.O., and N.O.; manuscript writing, P.M.W., J.O., N.O., and P.Z.; final approval of manuscript, P.M.W., J.O., N.O., and P.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Suicide Worldwide in 2019. Available online: https://www.who.int/publications-detail-redirect/9789240026643 (accessed on 25 May 2023).
- World Health Organization. Live Life: An Implementation Guide for Suicide Prevention in Countries; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002662-9. [Google Scholar]
- Song, Y.; Rhee, S.J.; Lee, H.; Kim, M.J.; Shin, D.; Ahn, Y.M. Comparison of Suicide Risk by Mental Illness: A Retrospective Review of 14-Year Electronic Medical Records. J. Korean Med. Sci. 2020, 35, e402. [Google Scholar] [CrossRef]
- Wilkinson, S.T.; Trujillo Diaz, D.; Rupp, Z.W.; Kidambi, A.; Ramirez, K.L.; Flores, J.M.; Avila-Quintero, V.J.; Rhee, T.G.; Olfson, M.; Bloch, M.H. Pharmacological and Somatic Treatment Effects on Suicide in Adults: A Systematic Review and Meta-Analysis. Focus 2023, 21, 197–208. [Google Scholar] [CrossRef]
- Ahmed, G.K.; Elserogy, Y.M.; Elfadl, G.M.A.; Ghada Abdelsalam, K.; Ali, M.A. Antidepressant and Anti-Suicidal Effects of Ketamine in Treatment-Resistant Depression Associated with Psychiatric and Personality Comorbidities: A Double-Blind Randomized Trial. J. Affect. Disord. 2023, 325, 127–134. [Google Scholar] [CrossRef]
- Chokhawala, K.; Lee, S.; Saadabadi, A. Lithium. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Tondo, L.; Alda, M.; Bauer, M.; Bergink, V.; Grof, P.; Hajek, T.; Lewitka, U.; Licht, R.W.; Manchia, M.; Müller-Oerlinghausen, B.; et al. Clinical Use of Lithium Salts: Guide for Users and Prescribers. Int. J. Bipolar Disord. 2019, 7, 16. [Google Scholar] [CrossRef]
- Malhi, G.S.; Tanious, M.; Das, P.; Coulston, C.M.; Berk, M. Potential Mechanisms of Action of Lithium in Bipolar Disorder. Current Understanding. CNS Drugs 2013, 27, 135–153. [Google Scholar] [CrossRef]
- Murphy, N.; Pham, G.; Weyland, A.; Engelhardt, J.; Kypriotakis, G.; Thomas, Y.T.; Kosten, T.R.; Moukaddam, N.; Mathew, S.J.; Swann, A.C. Lithium Reduces Impulsive Decision Making in Transdiagnostic Patients at High Risk for Suicide Attempt Recurrence: A Randomized, Double Blind, Placebo-Controlled, Crossover Study. J. Affect. Disord. Rep. 2024, 17, 100833. [Google Scholar] [CrossRef]
- Sinner, B.; Graf, B.M. Ketamine. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 313–333. [Google Scholar] [CrossRef]
- Das, J. Repurposing of Drugs-The Ketamine Story. J. Med. Chem. 2020, 63, 13514–13525. [Google Scholar] [CrossRef]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A Tale of Two Enantiomers. J. Psychopharmacol. 2021, 35, 109–123. [Google Scholar] [CrossRef]
- Gao, M.; Rejaei, D.; Liu, H. Ketamine Use in Current Clinical Practice. Acta Pharmacol. Sin. 2016, 37, 865–872. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant Effects of Ketamine in Depressed Patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Kang, M.J.Y.; Hawken, E.; Vazquez, G.H. The Mechanisms Behind Rapid Antidepressant Effects of Ketamine: A Systematic Review with a Focus on Molecular Neuroplasticity. Front. Psychiatry 2022, 13, 860882. [Google Scholar] [CrossRef]
- Zanos, P.; Gould, T.D. Mechanisms of Ketamine Action as an Antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Sapkota, A.; Khurshid, H.; Qureshi, I.A.; Jahan, N.; Went, T.R.; Sultan, W.; Alfonso, M. Efficacy and Safety of Intranasal Esketamine in Treatment-Resistant Depression in Adults: A Systematic Review. Cureus 2021, 13, e17352. [Google Scholar] [CrossRef]
- Price, J.B.; Yates, C.G.; Morath, B.A.; Van De Wakker, S.K.; Yates, N.J.; Butters, K.; Frye, M.A.; McGee, S.L.; Tye, S.J. Lithium Augmentation of Ketamine Increases Insulin Signaling and Antidepressant-like Active Stress Coping in a Rodent Model of Treatment-Resistant Depression. Transl. Psychiatry 2021, 11, 598. [Google Scholar] [CrossRef]
- Costi, S.; Soleimani, L.; Glasgow, A.; Brallier, J.; Spivack, J.; Schwartz, J.; Levitch, C.F.; Richards, S.; Hoch, M.; Stade, E.C.; et al. Lithium Continuation Therapy Following Ketamine in Patients with Treatment Resistant Unipolar Depression: A Randomized Controlled Trial. Neuropsychopharmacology 2019, 44, 1812–1819. [Google Scholar] [CrossRef]
- Wilkowska, A.; Szałach, Ł.; Cubała, W.J. Ketamine in Bipolar Disorder: A Review. Neuropsychiatr. Dis. Treat. 2020, 16, 2707–2717. [Google Scholar] [CrossRef]
- Wagner, E.; Siafis, S.; Fernando, P.; Falkai, P.; Honer, W.G.; Röh, A.; Siskind, D.; Leucht, S.; Hasan, A. Efficacy and Safety of Clozapine in Psychotic Disorders—A Systematic Quantitative Meta-Review. Transl. Psychiatry 2021, 11, 487. [Google Scholar] [CrossRef]
- Haidary, H.A.; Padhy, R.K. Clozapine. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kar, N.; Barreto, S.; Chandavarkar, R. Clozapine Monitoring in Clinical Practice: Beyond the Mandatory Requirement. Clin. Psychopharmacol. Neurosci. 2016, 14, 323–329. [Google Scholar] [CrossRef]
- Oloyede, E.; Dzahini, O.; Abolou, Z.; Gee, S.; Whiskey, E.; Malhotra, D.; Hussain, M.; Osborne, I.; Casetta, C.; McGuire, P.; et al. Clinical Impact of Reducing the Frequency of Clozapine Monitoring: Controlled Mirror-Image Cohort Study. Br. J. Psychiatry J. Ment. Sci. 2023, 223, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Chen, P.-H.; Pan, C.-H.; Su, S.-S.; Tsai, S.-Y.; Chen, C.-C.; Kuo, C.-J. Clozapine and Its Protective Effect on All-Cause, Natural, and Suicide Mortality in Patients with Schizophrenia: A Nationwide Cohort Study in Taiwan. Schizophr. Res. 2024, 268, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. Clozapine Treatment for Suicidality in SchizophreniaInternational Suicide Prevention Trial (InterSePT). Arch. Gen. Psychiatry 2003, 60, 82. [Google Scholar] [CrossRef]
- Vayalapalli, A.; McCall, W.V.; McEvoy, J.P.; Miller, B.J. Improved Insomnia Is One Pathway Underlying the Anti-suicidal Properties of Clozapine. Suicide Life. Threat. Behav. 2024, 54, 972–981. [Google Scholar] [CrossRef]
- Kerwin, R.W.; Bolonna, A.A. Is Clozapine Antisuicidal? Expert Rev. Neurother. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dervic, K.; Sher, L.; Galfalvy, H.C.; Grunebaum, M.; Burke, A.K.; Sullivan, G.; Sublette, M.E.; Mann, J.J.; Oquendo, M.A. Antisuicidal Effect of Lithum in Bipolar Disorder: Is There an Age-Specific Effect? J. Affect. Disord. 2023, 341, 8–11. [Google Scholar] [CrossRef]
- Girlanda, F.; Cipriani, A.; Agrimi, E.; Appino, M.G.; Barichello, A.; Beneduce, R.; Bighelli, I.; Bisoffi, G.; Bisogno, A.; Bortolaso, P.; et al. Effectiveness of Lithium in Subjects with Treatment-Resistant Depression and Suicide Risk: Results and Lessons of an Underpowered Randomised Clinical Trial. BMC Res. Notes 2014, 7, 731. [Google Scholar] [CrossRef]
- Szmulewicz, A.; Madenci, A.; Ferguson, R.; Liang, M.H.; Lew, R.; Katz, I.R.; Hernán, M.A. Estimating the Per-Protocol Effect of Lithium on Suicidality in a Randomized Trial of Individuals with Depression or Bipolar Disorder. J. Psychopharmacol. 2023, 37, 539–544. [Google Scholar] [CrossRef]
- Stark, K. Impact of Lithium on Suicidality in the Veteran Population. Fed. Pract. 2022, 39, 130–135. [Google Scholar] [CrossRef]
- Brus, O.; Cao, Y.; Hammar, Å.; Landén, M.; Lundberg, J.; Nordanskog, P.; Nordenskjöld, A. Lithium for Suicide and Readmission Prevention after Electroconvulsive Therapy for Unipolar Depression: Population-Based Register Study. BJPsych Open 2019, 5, e46. [Google Scholar] [CrossRef] [PubMed]
- Toffol, E.; Hätönen, T.; Tanskanen, A.; Lönnqvist, J.; Wahlbeck, K.; Joffe, G.; Tiihonen, J.; Haukka, J.; Partonen, T. Lithium Is Associated with Decrease in All-Cause and Suicide Mortality in High-Risk Bipolar Patients: A Nationwide Registry-Based Prospective Cohort Study. J. Affect. Disord. 2015, 183, 159–165. [Google Scholar] [CrossRef]
- Katz, I.R.; Rogers, M.P.; Lew, R.; Thwin, S.S.; Doros, G.; Ahearn, E.; Ostacher, M.J.; DeLisi, L.E.; Smith, E.G.; Ringer, R.J.; et al. Lithium Treatment in the Prevention of Repeat Suicide-Related Outcomes in Veterans with Major Depression or Bipolar Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 24. [Google Scholar] [CrossRef]
- Chen, P.-H.; Tsai, S.-Y.; Chen, P.-Y.; Pan, C.-H.; Su, S.-S.; Chen, C.-C.; Kuo, C.-J. Mood Stabilizers and Risk of All-Cause, Natural, and Suicide Mortality in Bipolar Disorder: A Nationwide Cohort Study. Acta Psychiatr. Scand. 2023, 147, 234–247. [Google Scholar] [CrossRef]
- Smith, E.G.; Austin, K.L.; Kim, H.M.; Eisen, S.V.; Kilbourne, A.M.; Miller, D.R.; Zivin, K.; Hannemann, C.; Sauer, B.C.; Valenstein, M. Mortality Associated with Lithium and Valproate Treatment of US Veterans Health Administration Patients with Mental Disorders. Br. J. Psychiatry 2015, 207, 55–63. [Google Scholar] [CrossRef]
- Chan, J.K.N.; Wong, C.S.M.; Fang, C.Z.; Hung, S.C.; Lo, H.K.Y.; Chang, W.C. Mortality Risk and Mood Stabilizers in Bipolar Disorder: A Propensity-Score-Weighted Population-Based Cohort Study in 2002–2018. Epidemiol. Psychiatr. Sci. 2024, 33, e31. [Google Scholar] [CrossRef]
- Song, J.; Sjölander, A.; Joas, E.; Bergen, S.E.; Runeson, B.; Larsson, H.; Landén, M.; Lichtenstein, P. Suicidal Behavior During Lithium and Valproate Treatment: A Within-Individual 8-Year Prospective Study of 50,000 Patients with Bipolar Disorder. Am. J. Psychiatry 2017, 174, 795–802. [Google Scholar] [CrossRef]
- Smith, E.G.; Austin, K.L.; Kim, H.M.; Miller, D.R.; Sauer, B.C.; Valenstein, M. Suicide Death over the First Year of Lithium versus Valproate Treatment in Cohorts with and without Bipolar Disorder. J. Psychiatr. Res. 2022, 147, 349–356. [Google Scholar] [CrossRef]
- Smith, E.G.; Austin, K.L.; Kim, H.M.; Miller, D.R.; Eisen, S.V.; Christiansen, C.L.; Kilbourne, A.M.; Sauer, B.C.; McCarthy, J.F.; Valenstein, M. Suicide Risk in Veterans Health Administration Patients with Mental Health Diagnoses Initiating Lithium or Valproate: A Historical Prospective Cohort Study. BMC Psychiatry 2014, 14, 357. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Ellis, S.P.; Keilp, J.G.; Moitra, V.K.; Cooper, T.B.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; Oquendo, M.A.; et al. Ketamine versus Midazolam in Bipolar Depression with Suicidal Thoughts: A Pilot Midazolam-Controlled Randomized Clinical Trial. Bipolar Disord. 2017, 19, 176–183. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Choi, E.Y.; Shapiro-Thompson, R.; Dhaliwal, K.; Neustadter, E.; Sakheim, M.; Null, K.; Trujillo-Diaz, D.; Rondeau, J.; Pittaro, G.F.; et al. A Pilot Randomized Controlled Trial of Ketamine in Borderline Personality Disorder. Neuropsychopharmacology 2023, 48, 991–999. [Google Scholar] [CrossRef]
- Phillips, J.L.; Norris, S.; Talbot, J.; Hatchard, T.; Ortiz, A.; Birmingham, M.; Owoeye, O.; Batten, L.A.; Blier, P. Single and Repeated Ketamine Infusions for Reduction of Suicidal Ideation in Treatment-Resistant Depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2020, 45, 606–612. [Google Scholar] [CrossRef]
- Abbar, M.; Demattei, C.; El-Hage, W.; Llorca, P.-M.; Samalin, L.; Demaricourt, P.; Gaillard, R.; Courtet, P.; Vaiva, G.; Gorwood, P.; et al. Ketamine for the Acute Treatment of Severe Suicidal Ideation: Double Blind, Randomised Placebo Controlled Trial. BMJ 2022, 376, e067194. [Google Scholar] [CrossRef]
- Canuso, C.M.; Singh, J.B.; Fedgchin, M.; Alphs, L.; Lane, R.; Lim, P.; Pinter, C.; Hough, D.; Sanacora, G.; Manji, H.; et al. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Psychiatry 2018, 175, 620–630. [Google Scholar] [CrossRef]
- Grunebaum, M.F.; Galfalvy, H.C.; Choo, T.-H.; Keilp, J.G.; Moitra, V.K.; Parris, M.S.; Marver, J.E.; Burke, A.K.; Milak, M.S.; Sublette, M.E.; et al. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am. J. Psychiatry 2018, 175, 327–335. [Google Scholar] [CrossRef]
- Fu, D.-J.; Ionescu, D.F.; Li, X.; Lane, R.; Lim, P.; Sanacora, G.; Hough, D.; Manji, H.; Drevets, W.C.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Major Depressive Disorder Symptoms in Patients Who Have Active Suicidal Ideation with Intent: Double-Blind, Randomized Study (ASPIRE I). J. Clin. Psychiatry 2020, 81, 19m13191. [Google Scholar] [CrossRef]
- Zolghadriha, A.; Anjomshoaa, A.; Jamshidi, M.R.; Taherkhani, F. Rapid and Sustained Antidepressant Effects of Intravenous Ketamine in Treatment-Resistant Major Depressive Disorder and Suicidal Ideation: A Randomized Clinical Trial. BMC Psychiatry 2024, 24, 341. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Fu, D.-J.; Qiu, X.; Lane, R.; Lim, P.; Kasper, S.; Hough, D.; Drevets, W.C.; Manji, H.; Canuso, C.M. Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients with Major Depressive Disorder Who Have Active Suicide Ideation with Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II). Int. J. Neuropsychopharmacol. 2020, 24, 22–31. [Google Scholar] [CrossRef]
- Sinyor, M.; Williams, M.; Belo, S.; Orser, B.; Vincent, M.; Mah, L.; Zarate, C.; Castel, S.; Levitt, A.J.; Schaffer, A. Ketamine Augmentation for Major Depressive Disorder and Suicidal Ideation: Preliminary Experience in an Inpatient Psychiatry Setting. J. Affect. Disord. 2018, 241, 103–109. [Google Scholar] [CrossRef]
- Su, T.-P.; Li, C.-T.; Lin, W.-C.; Wu, H.-J.; Tsai, S.-J.; Bai, Y.-M.; Mao, W.-C.; Tu, P.-C.; Chen, L.-F.; Li, W.-C.; et al. A Randomized, Double-Blind, Midazolam-Controlled Trial of Low-Dose Ketamine Infusion in Patients with Treatment-Resistant Depression and Prominent Suicidal Ideation. Int. J. Neuropsychopharmacol. 2023, 26, 331–339. [Google Scholar] [CrossRef]
- Price, R.B.; Iosifescu, D.V.; Murrough, J.W.; Chang, L.C.; Al Jurdi, R.K.; Iqbal, S.Z.; Soleimani, L.; Charney, D.S.; Foulkes, A.L.; Mathew, S.J. Effects of Ketamine on Explicit and Implicit Suicidal Cognition: A Randomized Controlled Trial in Treatment-Resistant Depression. Depress. Anxiety 2014, 31, 335–343. [Google Scholar] [CrossRef]
- Fan, W.; Yang, H.; Sun, Y.; Zhang, J.; Li, G.; Zheng, Y.; Liu, Y. Ketamine Rapidly Relieves Acute Suicidal Ideation in Cancer Patients: A Randomized Controlled Clinical Trial. Oncotarget 2016, 8, 2356. [Google Scholar] [CrossRef]
- Ionescu, D.F.; Bentley, K.H.; Eikermann, M.; Taylor, N.; Akeju, O.; Swee, M.B.; Pavone, K.J.; Petrie, S.R.; Dording, C.; Mischoulon, D.; et al. Repeat-Dose Ketamine Augmentation for Treatment-Resistant Depression with Chronic Suicidal Ideation: A Randomized, Double Blind, Placebo Controlled Trial. J. Affect. Disord. 2019, 243, 516–524. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant Efficacy of Ketamine in Treatment-Resistant Major Depression: A Two-Site Randomized Controlled Trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Feeney, A.; Hock, R.S.; Freeman, M.P.; Flynn, M.; Hoeppner, B.; Iosifescu, D.V.; Trivedi, M.H.; Sanacora, G.; Mathew, S.J.; Debattista, C.; et al. The Effect of Single Administration of Intravenous Ketamine Augmentation on Suicidal Ideation in Treatment-Resistant Unipolar Depression: Results from a Randomized Double-Blind Study. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2021, 49, 122–132. [Google Scholar] [CrossRef]
- Domany, Y.; Shelton, R.C.; McCullumsmith, C.B. Ketamine for Acute Suicidal Ideation. An Emergency Department Intervention: A Randomized, Double-blind, Placebo-controlled, Proof-of-concept Trial. Depress. Anxiety 2020, 37, 224–233. [Google Scholar] [CrossRef]
- Zeng, Q.-B.; Zou, D.-C.; Huang, X.-B.; Shang, D.-W.; Huang, X.; Yang, X.-H.; Ning, Y.-P.; Balbuena, L.; Xiang, Y.-T.; Zheng, W. Efficacy and Safety of Esketamine versus Propofol in Electroconvulsive Therapy for Treatment-Resistant Depression: A Randomized, Double-Blind, Controlled, Non-Inferiority Trial. J. Affect. Disord. 2025, 368, 320–328. [Google Scholar] [CrossRef]
- Hu, Y.-D.; Xiang, Y.-T.; Fang, J.-X.; Zu, S.; Sha, S.; Shi, H.; Ungvari, G.S.; Correll, C.U.; Chiu, H.F.K.; Xue, Y.; et al. Single i.v. Ketamine Augmentation of Newly Initiated Escitalopram for Major Depression: Results from a Randomized, Placebo-Controlled 4-Week Study. Psychol. Med. 2016, 46, 623–635. [Google Scholar] [CrossRef]
- Burger, J.; Capobianco, M.; Lovern, R.; Boche, B.; Ross, E.; Darracq, M.A.; McLay, R. A Double-Blinded, Randomized, Placebo-Controlled Sub-Dissociative Dose Ketamine Pilot Study in the Treatment of Acute Depression and Suicidality in a Military Emergency Department Setting. Mil. Med. 2016, 181, 1195–1199. [Google Scholar] [CrossRef]
- Jones, G.H.; Vecera, C.M.; Ruiz, A.C.; Wu, H.E.; McInturff, S.I.; Orejarena, M.J.; Smith, K.A.; Soares, J.C.; Zarate, C.A.; Lane, S.D.; et al. A Randomized, Double-Blind, Placebo-Controlled Pilot Trial of the Acute Antisuicidal and Antidepressant Effects of Intranasal (R,S)-Ketamine in Severe Unipolar and Bipolar Depression with and Without Comorbid Alcohol Use Disorder. J. Clin. Psychiatry 2024, 85, 23m14974. [Google Scholar] [CrossRef]
- Ringbäck Weitoft, G.; Berglund, M.; Lindström, E.A.; Nilsson, M.; Salmi, P.; Rosén, M. Mortality, Attempted Suicide, Re-hospitalisation and Prescription Refill for Clozapine and Other Antipsychotics in Sweden—A Register-based Study. Pharmacoepidemiol. Drug Saf. 2014, 23, 290–298. [Google Scholar] [CrossRef]
- Van Der Zalm, Y.; Foldager, L.; Termorshuizen, F.; Sommer, I.E.; Nielsen, J.; Selten, J. Clozapine and Mortality: A Comparison with Other Antipsychotics in a Nationwide Danish Cohort Study. Acta Psychiatr. Scand. 2021, 143, 216–226. [Google Scholar] [CrossRef]
- Taipale, H.; Lähteenvuo, M.; Tanskanen, A.; Mittendorfer-Rutz, E.; Tiihonen, J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons with Schizophrenia. Schizophr. Bull. 2021, 47, 23–30. [Google Scholar] [CrossRef]
- Taipale, H.; Tanskanen, A.; Mehtälä, J.; Vattulainen, P.; Correll, C.U.; Tiihonen, J. 20-year Follow-up Study of Physical Morbidity and Mortality in Relationship to Antipsychotic Treatment in a Nationwide Cohort of 62,250 Patients with Schizophrenia (FIN20). World Psychiatry 2020, 19, 61–68. [Google Scholar] [CrossRef]
- Hassan, A.; De Luca, V.; Dai, N.; Asmundo, A.; Di Nunno, N.; Monda, M.; Villano, I. Effectiveness of Antipsychotics in Reducing Suicidal Ideation: Possible Physiologic Mechanisms. Healthcare 2021, 9, 389. [Google Scholar] [CrossRef]
- Gürcan, G.; Şenol, Ş.H.; Yağcıoğlu, A.E.A.; Ertuğrul, A. Effect of Clozapine on Suicidality in Patients with Schizophrenia at a University Hospital in Turkey. Schizophr. Res. 2024, 268, 161–164. [Google Scholar] [CrossRef]
- Miller, J.N.; Black, D.W. Bipolar Disorder and Suicide: A Review. Curr. Psychiatry Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Chen, C.-C.; Zhou, N.; Hu, N.; Feng, J.-G.; Wang, X.-B. Acute Effects of Intravenous Sub-Anesthetic Doses of Ketamine and Intranasal Inhaled Esketamine on Suicidal Ideation: A Systematic Review and Meta-Analysis. Neuropsychiatr. Dis. Treat. 2023, 19, 587–599. [Google Scholar] [CrossRef]
- Feng, W.; Chen, C.; Zeng, Y.; Zhang, B. Efficacy of Single and Repeated Ketamine Administration for Suicidal Ideation in Adults with Psychiatric Disorders: A Meta-Analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 136, 111152. [Google Scholar] [CrossRef]
- Shen, Z.; Gao, D.; Lv, X.; Wang, H.; Yue, W. A Meta-Analysis of the Effects of Ketamine on Suicidal Ideation in Depression Patients. Transl. Psychiatry 2024, 14, 248. [Google Scholar] [CrossRef]
- Masdrakis, V.G.; Baldwin, D.S. Prevention of Suicide by Clozapine in Mental Disorders: Systematic Review. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2023, 69, 4–23. [Google Scholar] [CrossRef]
- Yao, Z.; McCall, W.V. Designing Clinical Trials to Assess the Impact of Pharmacological Treatment for Suicidal Ideation/Behavior: Issues and Potential Solutions. Pharm. Med. 2023, 37, 221–232. [Google Scholar] [CrossRef]
- Stone, M.; Laughren, T.; Jones, M.L.; Levenson, M.; Holland, P.C.; Hughes, A.; Hammad, T.A.; Temple, R.; Rochester, G. Risk of Suicidality in Clinical Trials of Antidepressants in Adults: Analysis of Proprietary Data Submitted to US Food and Drug Administration. BMJ 2009, 339, b2880. [Google Scholar] [CrossRef] [PubMed]
- Lenze, E.J.; Mulsant, B.H.; Blumberger, D.M.; Karp, J.F.; Newcomer, J.W.; Anderson, S.J.; Dew, M.A.; Butters, M.A.; Stack, J.A.; Begley, A.E.; et al. Efficacy, Safety, and Tolerability of Augmentation Pharmacotherapy with Aripiprazole for Treatment-Resistant Depression in Late Life: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2015, 386, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).