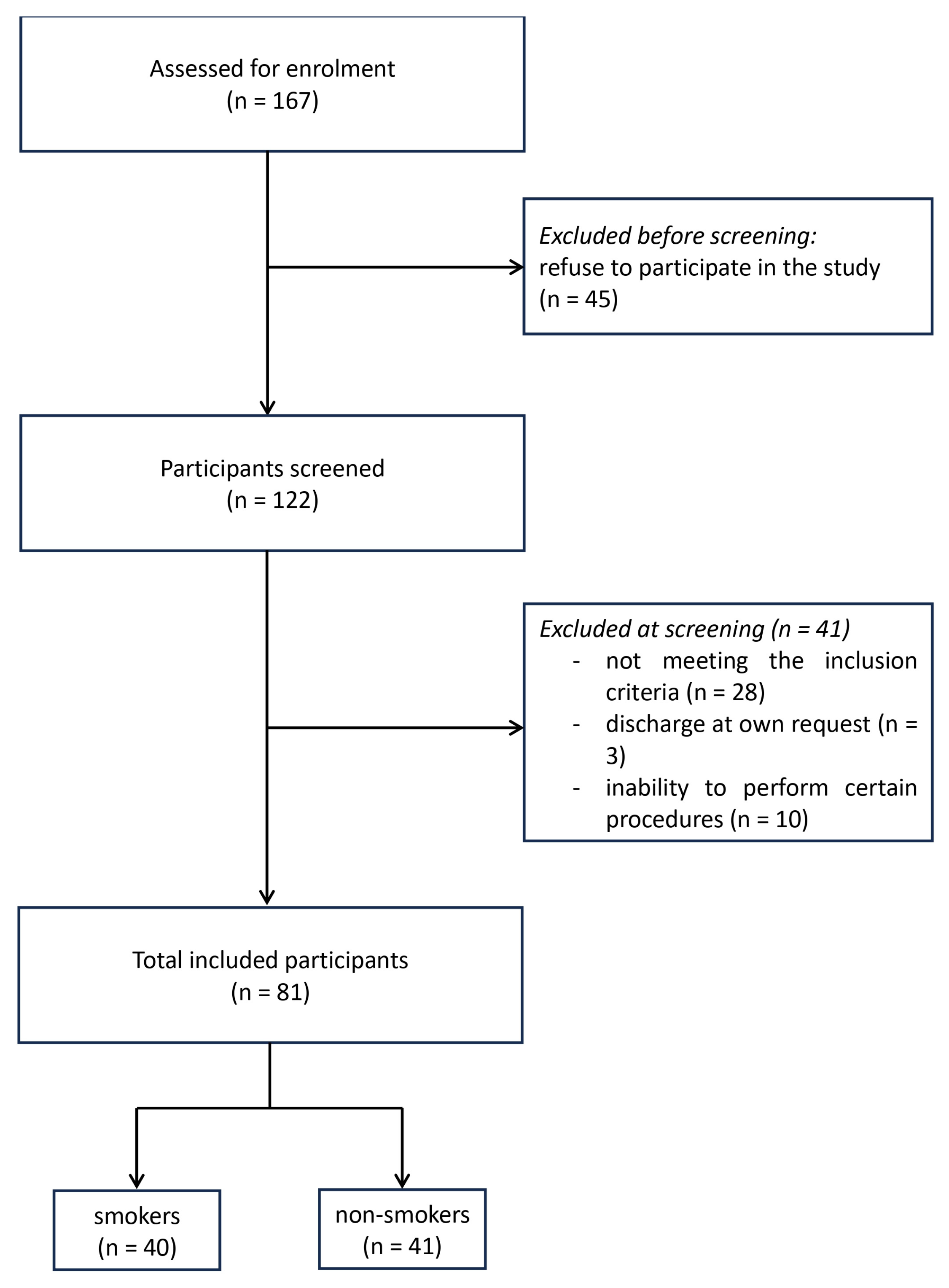
Nicotine Misuse and Treatment of Schizophrenia Exacerbations in Men: An Observational Study in Poland
Abstract
1. Introduction
2. Results
2.1. Past Course of Disease
2.2. Course of Hospitalization
2.3. Pharmacotherapy in the Context of CYP1A2 Metabolism
2.4. PANSS, MADRS
2.5. Extrapyramidal Symptom Scales
2.6. Quality of Life
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Drugs Metabolized Mainly by CYP1A2 | Drugs Metabolized Partlyby CYP1A2 | Drugs Metabolized by Pathways Other than CYP1A2 |
|---|---|---|
| clozapine olanzapine | haloperidol levomepromazine perazine | amisulpride aripiprazole chlorprothixene flupentixol quetiapine risperidone zuclopenthixol |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| mental disorders in family | 18 (45.0%) | 17 (41.5%) | 35 (43.2%) | 0.7480 |
| alcoholism in family | 15 (37.5%) | 20 (48.8%) | 35 (43.2%) | 0.3055 |
| domestic violence | 14 (35.0%) | 17 (41.5%) | 31 (38.3%) | 0.5496 |
| additional somatic treatment | 13 (32.5%) | 22 (53.7%) | 35 (43.2%) | 0.0546 |
| hyperlipidaemia | 8 (20.0%) | 10 (24.4%) | 18 (22.2%) | 0.6347 |
| metabolic syndrome | 5 (12.5%) | 9 (22.0%) | 14 (17.3%) | 0.2607 |
| arterial hypertension | 4 (10.0%) | 11 (26.8%) | 15 (18.5%) | 0.0512 |
| RS (n = 15) | HS (n = 25) | NS (n = 41) | p | |
|---|---|---|---|---|
| Age of first onset | 0.5339 | |||
| mean (SD) | 22.5 (6.0) | 22.9 (4.5) | 24.3 (6.6) | |
| range | 16.0–40.0 | 16.0–31.0 | 13.0–45.0 | |
| median | 21.0 | 23.0 | 23.0 | |
| 95% CI | [19.2; 25.8] | [21.0; 24.7] | [22.2; 26.4] | |
| Number of hospitalizations | 0.0171 | |||
| mean (SD) | 9.9 (8.5) | 13.1 (9.4) | 9.1 (12.8) | |
| range | 1.0–30.0 | 2.0–38.0 | 1.0–70.0 | |
| median | 8.0 | 11.01 | 5.01 | |
| 95% CI | [5.3; 14.6] | [9.2; 17.0] | [5.1; 13.1] |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| olanzapine | 23 (57.5%) | 13 (31.7%) | 36 (44.4%) | 0.0195 |
| clozapine | 10 (25.0%) | 18 (43.9%) | 28 (34.6%) | 0.0737 |
| risperidone | 9 (22.5%) | 4 (9.8%) | 13 (16.0%) | 0.1182 |
| chlorprothixene | 3 (7.5%) | 1 (2.4%) | 4 (4.9%) | 0.2932 |
| zuclopenthixol | 9 (22.5%) | 8 (19.5%) | 17 (21.0%) | 0.7413 |
| quetiapine | 1 (2.5%) | 5 (12.2%) | 6 (7.4%) | 0.0958 |
| aripiprazole | 4 (10.0%) | 8 (19.5%) | 12 (14.8%) | 0.2283 |
| levomepromazine | 3 (7.5%) | 2 (4.9%) | 5 (6.2%) | 0.6240 |
| haloperidol | 2 (5.0%) | 1 (2.4%) | 3 (3.7%) | 0.5417 |
| flupenthixol | 0 (0.0%) | 3 (7.3%) | 3 (3.7%) | 0.0813 |
| other | 0 (0.0%) | 3 (7.3%) | 3 (3.7%) | 0.0813 |
| NM+ (n = 14) | M (n = 26) | Overall (n = 40) | p | ||
|---|---|---|---|---|---|
| S | 0.1690 | ||||
| mean (SD) | 575.0 (357.2) | 754.7 (380.0) | 691.8 (377.7) | ||
| range | 100.0–1100.0 | 400.0–1800.0 | 100.0–1800.0 | ||
| median | 550.0 | 600.0 | 600.0 | ||
| 95% CI | [368.8; 781.2] | [601.2; 908.2] | [571.0; 812.6] | ||
| NM+ (n = 21) | M (n = 20) | Overall (n = 41) | p | ||
| NS | 0.7083 | ||||
| mean (SD) | 905.4 (468.1) | 855.7 (369.1) | 881.1 (418.2) | ||
| range | 200.0–1600.0 | 200.0–1440.0 | 200.0–1600.0 | ||
| median | 900.0 | 875.0 | 900.0 | ||
| 95% CI | [692.4; 1118.5] | [682.9; 1028.4] | [749.2; 1013.1] | ||
| NM (n = 7) | M+ (n = 33) | Overall (n = 40) | p | ||
|---|---|---|---|---|---|
| S | 0.0049 | ||||
| mean (SD) | 320.3 (228.4) | 562.9 (210.3) | 520.5 (230.2) | ||
| range | 100.0–775.0 | 353.0–1354.0 | 100.0–1354.0 | ||
| median | 250.0 | 536.0 | 474.0 | ||
| 95% CI | [109.1; 531.5] | [488.4; 637.5] | [446.8; 594.1] | ||
| NM (n = 7) | M+ (n = 34) | Overall (n = 41) | p | ||
| NS | 0.0376 | ||||
| mean (SD) | 571.1 (335.1) | 945.0 (408.6) | 881.1 (418.2) | ||
| range | 200.0–1141.0 | 200.0–1600.0 | 200.0–1600.0 | ||
| median | 650.0 | 950.0 | 900.0 | ||
| 95% CI | [261.3; 881.0] | [802.4; 1087.5] | [749.2; 1013.1] | ||
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| PANNS overall | 0.2901 | |||
| mean (SD) | 115.0 (17.0) | 118.0 (15.9) | 116.5 (16.4) | |
| range | 80.0–149.0 | 75.0–150.0 | 75.0–150.0 | |
| median | 115.0 | 118.0 | 117.0 | |
| 95% CI | [109.6; 120.4] | [112.9; 123.0] | [112.9; 120.1] | |
| General psychopathology | 0.1497 | |||
| mean (SD) | 33.0 (6.2) | 34.8 (6.0) | 33.9 (6.1) | |
| range | 20.0–46.0 | 22.0–46.0 | 20.0–46.0 | |
| median | 32.5 | 36.0 | 34.0 | |
| 95% CI | [31.0; 35.0] | [32.9; 36.7] | [32.6; 35.3] | |
| Positive symptoms | 0.9322 | |||
| mean (SD) | 16.5 (4.1) | 16.4 (4.2) | 16.5 (4.1) | |
| range | 9.0–25.0 | 7.0–23.0 | 7.0–25.0 | |
| median | 16.0 | 17.0 | 16.0 | |
| 95% CI | [15.2; 17.8] | [15.1; 17.7] | [15.5; 17.4] | |
| Negative symptoms | 0.0137 | |||
| mean (SD) | 22.2 (4.8) | 25.5 (5.4) | 23.9 (5.3) | |
| range | 13.0–30.0 | 17.0–36.0 | 13.0–36.0 | |
| median | 22.0 | 25.0 | 24.0 | |
| 95% CI | [20.7; 23.7] | [23.8; 27.2] | [22.7; 25.1] | |
| Dizorganization coefficient | 0.6001 | |||
| mean (SD) | 13.5 (3.1) | 13.1 (3.4) | 13.3 (3.2) | |
| range | 8.0–20.0 | 6.0–21.0 | 6.0–21.0 | |
| median | 13.0 | 13.0 | 13.0 | |
| 95% CI | [12.5; 14.5] | [12.0; 14.2] | [12.6; 14.0] | |
| Activity coefficient | 0.5115 | |||
| mean (SD) | 17.1 (6.9) | 16.2 (6.3) | 16.6 (6.6) | |
| range | 6.0–26.0 | 4.0–26.0 | 4.0–26.0 | |
| median | 18.5 | 16.0 | 17.0 | |
| 95% CI | [14.8; 19.3] | [14.2; 18.2] | [15.2; 18.1] | |
| Affective coefficient | 0.2944 | |||
| mean (SD) | 7.9 (3.8) | 8.6 (3.6) | 8.3 (3.7) | |
| range | 3.0–15.0 | 3.0–18.0 | 3.0–18.0 | |
| median | 7.0 | 8.0 | 7.0 | |
| 95% CI | [6.7; 9.1] | [7.5; 9.8] | [7.5; 9.1] | |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| PANNS overall | 0.2879 | |||
| mean (SD) | 70.0 (13.2) | 74.0 (16.6) | 72.0 (15.1) | |
| range | 49.0–108.0 | 42.0–109.0 | 42.0–109.0 | |
| median | 68.5 | 71.0 | 69.0 | |
| 95% CI | [65.8; 74.2] | [68.7; 79.2] | [68.7; 75.3] | |
| General psychopathology | 0.6266 | |||
| mean (SD) | 20.0 (4.8) | 21.1 (6.4) | 20.6 (5.7) | |
| range | 11.0–30.0 | 11.0–38.0 | 11.0–38.0 | |
| median | 20.0 | 20.0 | 20.0 | |
| 95% CI | [18.4; 21.5] | [19.1; 23.2] | [19.3; 21.8] | |
| Positive symptoms | 0.6001 | |||
| mean (SD) | 7.2 (2.6) | 7.7 (3.2) | 7.4 (2.9) | |
| range | 4.0–13.0 | 4.0–15.0 | 4.0–15.0 | |
| median | 6.0 | 7.0 | 7.0 | |
| 95% CI | [6.3; 8.0] | [6.7; 8.7] | [6.8; 8.1] | |
| Negative symptoms | 0.6199 | |||
| mean (SD) | 18.8 (4.9) | 19.8 (6.4) | 19.3 (5.7) | |
| range | 10.0–30.0 | 9.0–34.0 | 9.0–34.0 | |
| median | 18.0 | 19.0 | 18.0 | |
| 95% CI | [17.2; 20.3] | [17.7; 21.8] | [18.0; 20.5] | |
| Dizorganization coefficient | 0.4414 | |||
| mean (SD) | 9.4 (2.6) | 9.2 (3.4) | 9.3 (3.0) | |
| range | 5.0–16.0 | 3.0–17.0 | 3.0–17.0 | |
| median | 9.0 | 8.0 | 9.0 | |
| 95% CI | [8.6; 10.2] | [8.1; 10.3] | [8.6; 10.0] | |
| Activity coefficient | 0.4498 | |||
| mean (SD) | 6.3 (1.9) | 6.6 (2.0) | 6.4 (2.0) | |
| range | 4.0–12.0 | 4.0–15.0 | 4.0–15.0 | |
| median | 6.0 | 6.0 | 6.0 | |
| 95% CI | [5.7; 6.9] | [5.9; 7.2] | [6.0; 6.9] | |
| Affective coefficient | 0.0258 | |||
| mean (SD) | 4.6 (1.8) | 5.4 (1.9) | 5.0 (1.9) | |
| range | 3.0–11.0 | 3.0–13.0 | 3.0–13.0 | |
| median | 4.0 | 5.0 | 5.0 | |
| 95% CI | [4.0; 5.1] | [4.8; 6.0] | [4.6; 5.4] |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| MADRS admission | 0.2434 | |||
| mean (SD) | 18.7 (10.3) | 22.3 (12.4) | 20.5 (11.5) | |
| range | 0.0–47.0 | 2.0–48.0 | 0.0–48.0 | |
| median | 16.5 | 19.0 | 18.0 | |
| 95% CI | [15.4; 22.0] | [18.4; 26.2] | [18.0; 23.0] | |
| MADRS discharge | 0.0005 | |||
| mean (SD) | 6.0 (4.6) | 10.1 (5.7) | 8.1 (5.5) | |
| range | 0.0–16.0 | 0.0–26.0 | 0.0–26.0 | |
| median | 5.5 | 10.0 | 7.0 | |
| 95% CI | [4.5; 7.4] | [8.4; 11.9] | [6.9; 9.3] |
| Predictor | β | 95% CI | p |
|---|---|---|---|
| HS status | −2.97 | [−5.10; −0.84] | 0.0069 |
| RS status | −3.16 | [−5.63; −0.69] | 0.0129 |
References
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Crow, T.J. Molecular Pathology of Schizophrenia: More than One Disease Process? Br. Med. J. 1980, 280, 66–68. [Google Scholar] [CrossRef]
- Bilder, R.M.; Mukherjee, S.; Rieder, R.O.; Pandurangi, A.K. Symptomatic and Neuropsychological Components of Defect States. Schizophr. Bull. 1985, 11, 409–419. [Google Scholar] [CrossRef]
- Liddle, P.F. The Symptoms of Chronic Schizophrenia: A Re-Examination of the Positive-Negative Dichotomy. Br. J. Psychiatry 1987, 151, 145–151. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.W.; Schulz, S.C. What We Know: Findings That Every Theory of Schizophrenia Should Explain. Schizophr. Bull. 2009, 35, 493–508. [Google Scholar] [CrossRef]
- Os, J.V.; Gilvarry, C.; Bale, R.; Horn, E.V.; Tattan, T.; White, I.; Murray, R.; on behalf of the UK700 Group. A Comparison of the Utility of Dimensional and Categorical Representations of Psychosis. Psychol. Med. 1999, 29, 595–606. [Google Scholar] [CrossRef]
- Cho, W.; Shin, W.-S.; An, I.; Bang, M.; Cho, D.-Y.; Lee, S.-H. Biological Aspects of Aggression and Violence in Schizophrenia. Clin. Psychopharmacol. Neurosci. 2019, 17, 475–486. [Google Scholar] [CrossRef]
- Mosolov, S.N.; Yaltonskaya, P.A. Primary and Secondary Negative Symptoms in Schizophrenia. Front. Psychiatry 2022, 12, 766692. [Google Scholar] [CrossRef] [PubMed]
- Peralta, V.; Cuesta, M.J. How Many and Which Are the Psychopathological Dimensions in Schizophrenia? Issues Influencing Their Ascertainment. Schizophr. Res. 2001, 49, 269–285. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders Fourth Edition Text Revision (DSM-IV-TR); American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Drake, R.; Whitaker, A.; Gates, C.; Cotton, P. Suicide among Schizophrenics: A Review. Compr. Psychiatry 1985, 26, 90–100. [Google Scholar] [CrossRef]
- Caldwell, C.B.; Gottesman, I.I. Schizophrenia—A High-Risk Factor for Suicide: Clues to Risk Reduction. Suicide Life Threat. Behav. 1992, 22, 479–493. [Google Scholar] [CrossRef]
- Teshima, A.; Laverty, A.; Filippidis, F. Burden of Current and Past Smoking across 28 European Countries in 2017: A Cross-Sectional Analysis. Tob. Induc. Dis. 2022, 20, 56. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- He, H.; Pan, Z.; Wu, J.; Hu, C.; Bai, L.; Lyu, J. Health Effects of Tobacco at the Global, Regional, and National Levels: Results From the 2019 Global Burden of Disease Study. Nicotine Tob. Res. 2022, 24, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in Relation to Smoking: 50 Years’ Observations on Male British Doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Hatsukami, D.K.; Mitchell, J.E.; Dahlgren, L.A. Prevalence of Smoking among Psychiatric Outpatients. Am. J. Psychiatry 1986, 143, 993–997. [Google Scholar] [CrossRef]
- De Leon, J. Smoking and Vulnerability for Schizophrenia. Schizophr. Bull. 1996, 22, 405–409. [Google Scholar] [CrossRef]
- Reichler, H.; Baker, A.; Lewin, T.; Carr, V. Smoking among In-Patients with Drug-Related Problems in an Australian Psychiatric Hospital. Drug Alcohol Rev. 2001, 20, 231–237. [Google Scholar] [CrossRef]
- Dani, J.A.; Harris, R.A. Nicotine Addiction and Comorbidity with Alcohol Abuse and Mental Illness. Nat. Neurosci. 2005, 8, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.; Richmond, R.; Haile, M.; Lewin, T.J.; Carr, V.J.; Taylor, R.L.; Jansons, S.; Wilhelm, K. A Randomized Controlled Trial of a Smoking Cessation Intervention Among People with a Psychotic Disorder. Am. J. Psychiatry 2006, 163, 1934–1942. [Google Scholar] [CrossRef]
- Glassman, A.H.; Helzer, J.E.; Covey, L.S.; Cottler, L.B.; Stetner, F.; Tipp, J.E.; Johnson, J. Smoking, Smoking Cessation, and Major Depression. JAMA 1990, 264, 1546–1549. [Google Scholar] [CrossRef]
- Hays, J.T.; Ebbert, J.O.; Sood, A. Treating Tobacco Dependence in Light of the 2008 US Department of Health and Human Services Clinical Practice Guideline. Mayo Clin. Proc. 2009, 84, 730–736. [Google Scholar] [CrossRef]
- Jackson, J.G.; Diaz, F.J.; Lopez, L.; De Leon, J. A Combined Analysis of Worldwide Studies Demonstrates an Association between Bipolar Disorder and Tobacco Smoking Behaviors in Adults. Bipolar Disord. 2015, 17, 575–597. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Flynn, K. Smoking and Schizophrenia. Schizophr. Res. 1992, 8, 93–102. [Google Scholar] [CrossRef]
- Williams, J.M.; Foulds, J. Successful Tobacco Dependence Treatment in Schizophrenia. Am. J. Psychiatry 2007, 164, 222–227. [Google Scholar] [CrossRef]
- George, T.P.; Vessicchio, J.C.; Sacco, K.A.; Weinberger, A.H.; Dudas, M.M.; Allen, T.M.; Creeden, C.L.; Potenza, M.N.; Feingold, A.; Jatlow, P.I. A Placebo-Controlled Trial of Bupropion Combined with Nicotine Patch for Smoking Cessation in Schizophrenia. Biol. Psychiatry 2008, 63, 1092–1096. [Google Scholar] [CrossRef]
- Kelly, D.L.; McMahon, R.P.; Wehring, H.J.; Liu, F.; Mackowick, K.M.; Boggs, D.L.; Warren, K.R.; Feldman, S.; Shim, J.-C.; Love, R.C.; et al. Cigarette Smoking and Mortality Risk in People with Schizophrenia. Schizophr. Bull. 2011, 37, 832–838. [Google Scholar] [CrossRef]
- Chambers, R.A.; Krystal, J.H.; Self, D.W. A Neurobiological Basis for Substance Abuse Comorbidity in Schizophrenia. Biol. Psychiatry 2001, 50, 71–83. [Google Scholar] [CrossRef]
- Zammit, S.; Allebeck, P.; Dalman, C.; Lundberg, I.; Hemmingsson, T.; Lewis, G. Investigating the Association Between Cigarette Smoking and Schizophrenia in a Cohort Study. Am. J. Psychiatry 2003, 160, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.; Reichenberg, A.; Grotto, I.; Yasvitzky, R.; Rabinowitz, J.; Lubin, G.; Nahon, D.; Knobler, H.Y.; Davidson, M. Higher Rates of Cigarette Smoking in Male Adolescents Before the Onset of Schizophrenia: A Historical-Prospective Cohort Study. Am. J. Psychiatry 2004, 161, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Murray, R.; Asher, L.; Leonardi-Bee, J. The Effects of Tobacco Smoking, and Prenatal Tobacco Smoke Exposure, on Risk of Schizophrenia: A Systematic Review and Meta-Analysis. Nicotine Tob. Res. 2020, 22, 3–10. [Google Scholar] [CrossRef]
- Wootton, R.E.; Richmond, R.C.; Stuijfzand, B.G.; Lawn, R.B.; Sallis, H.M.; Taylor, G.M.J.; Hemani, G.; Jones, H.J.; Zammit, S.; Davey Smith, G.; et al. Evidence for Causal Effects of Lifetime Smoking on Risk for Depression and Schizophrenia: A Mendelian Randomisation Study. Psychol. Med. 2020, 50, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Lönn, S.L.; Sundquist, J.; Sundquist, K. Smoking and Schizophrenia in Population Cohorts of Swedish Women and Men: A Prospective Co-Relative Control Study. Am. J. Psychiatry 2015, 172, 1092–1100. [Google Scholar] [CrossRef]
- Wolfe, R.M.; Reeves, L.E.; Gibson, L.E.; Cooper, S.; Ellman, L.M. Attenuated Positive Psychotic Symptoms in Relation to Cigarette Smoking in a Nonclinical Population. Nicotine Tob. Res. 2017, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Riala, K.; Hakko, H.; Isohanni, M.; Pouta, A.; Räsänen, P. Is Initiation of Smoking Associated with the Prodromal Phase of Schizophrenia? J. Psychiatry Neurosci. 2005, 30, 26–32. [Google Scholar] [CrossRef]
- Dalack, G.W.; Healy, D.J.; Meador-Woodruff, J.H. Nicotine Dependence in Schizophrenia: Clinical Phenomena and Laboratory Findings. Am. J. Psychiatry 1998, 155, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Bacher, I.; Rabin, R.A.; Woznica, A.A.; Sacco, K.A.; George, T. Nicotinic Receptor Mechanisms in Neuropsychiatric Disorders: Therapeutic Implications. Prim. Psychiatry 2010, 17, 35–41. [Google Scholar]
- Ding, J.B.; Hu, K. Cigarette Smoking and Schizophrenia: Etiology, Clinical, Pharmacological, and Treatment Implications. Schizophr. Res. Treat. 2021, 2021, 7698030. [Google Scholar] [CrossRef]
- Uludag, K.; Zhao, M. A Narrative Review on the Association between Smoking and Schizophrenia Symptoms. JCBP 2023, 1, 1014. [Google Scholar] [CrossRef]
- Nowak, D.; Antczak, A.; Krol, M.; Pietras, T.; Shariati, B.; Bialasiewicz, P.; Jeczkowski, K.; Kula, P. Increased Content of Hydrogen Peroxide in the Expired Breath of Cigarette Smokers. Eur. Respir. J. 1996, 9, 652–657. [Google Scholar] [CrossRef]
- Quaak, M.; Van Schayck, C.P.; Knaapen, A.M.; Van Schooten, F.J. Genetic Variation as a Predictor of Smoking Cessation Success. A Promising Preventive and Intervention Tool for Chronic Respiratory Diseases? Eur. Respir. J. 2009, 33, 468–480. [Google Scholar] [CrossRef]
- Liston, H.L.; Markowitz, J.S.; DeVane, C.L. Drug Glucuronidation in Clinical Psychopharmacology. J. Clin. Psychopharmacol. 2001, 21, 500–515. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P.; Benowitz, N.L. Metabolism and Disposition Kinetics of Nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Ziedonis, D. Addressing Tobacco among Individuals with a Mental Illness or an Addiction. Addict. Behav. 2004, 29, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Haslemo, T.; Eikeseth, P.H.; Tanum, L.; Molden, E.; Refsum, H. The Effect of Variable Cigarette Consumption on the Interaction with Clozapine and Olanzapine. Eur. J. Clin. Pharmacol. 2006, 62, 1049–1053. [Google Scholar] [CrossRef]
- Nakajima, M.; Yokoi, T. Interindividual Variability in Nicotine Metabolism: C-Oxidation and Glucuronidation. Drug Metab. Pharmacokinet. 2005, 20, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Haring, C.; Meise, U.; Humpel, C.; Saria, A.; Fleischhacker, W.W.; Hinterhuber, H. Dose-Related Plasma Levels of Clozapine: Influence of Smoking Behaviour, Sex and Age. Psychopharmacology 1989, 99, S38–S40. [Google Scholar] [CrossRef]
- Haring, C.; Fleischhacker, W.W.; Schett, P.; Humpel, C.; Barnas, C.; Saria, A. Influence of Patient-Related Variables on Clozapine Plasma Levels. Am. J. Psychiatry 1990, 147, 1471–1475. [Google Scholar] [CrossRef]
- Van Der Weide, J.; Steijns, L.S.; Van Weelden, M.J. The Effect of Smoking and Cytochrome P450 CYP1A2 Genetic Polymorphism on Clozapine Clearance and Dose Requirement. Pharmacogenetics 2003, 13, 169–172. [Google Scholar] [CrossRef]
- Derenne, J.L.; Baldessarini, R.J. Clozapine Toxicity Associated with Smoking Cessation: Case Report. Am. J. Ther. 2005, 12, 469–471. [Google Scholar] [CrossRef]
- Wagner, E.; McMahon, L.; Falkai, P.; Hasan, A.; Siskind, D. Impact of Smoking Behavior on Clozapine Blood Levels—A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2020, 142, 456–466. [Google Scholar] [CrossRef]
- Seppälä, N.H.; Leinonen, E.V.J.; Lehtonen, M.; Kivistö, K.T. Clozapine Serum Concentrations Are Lower in Smoking than in Non-Smoking Schizophrenic Patients. Pharmacol. Toxicol. 1999, 85, 244–246. [Google Scholar] [CrossRef]
- Rostami-Hodjegan, A.; Amin, A.M.; Spencer, E.P.; Lennard, M.S.; Tucker, G.T.; Flanagan, R.J. Influence of Dose, Cigarette Smoking, Age, Sex, and Metabolic Activity on Plasma Clozapine Concentrations: A Predictive Model and Nomograms to Aid Clozapine Dose Adjustment and to Assess Compliance in Individual Patients. J. Clin. Psychopharmacol. 2004, 24, 70–78. [Google Scholar] [CrossRef]
- Smith, R.L.; Wollmann, B.M.; Kyllesø, L.; Tran, T.T.A.; Tveito, M.; Molden, E. Effect of Valproic Acid on the Metabolic Spectrum of Clozapine in Patients with Schizophrenia. J. Clin. Psychopharmacol. 2022, 42, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Herráiz, A.G.; Ramos, S.I.; Gervasini, G.; Vizcaíno, S.; Benítez, J. Role of the Smoking-Induced Cytochrome P450 (CYP)1A2 and Polymorphic CYP2D6 in Steady-State Concentration of Olanzapine. J. Clin. Psychopharmacol. 2003, 23, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Gex-Fabry, M.; Balant-Gorgia, A.E.; Balant, L.P. Therapeutic Drug Monitoring of Olanzapine: The Combined Effect of Age, Gender, Smoking, and Comedication. Ther. Drug Monit. 2003, 25, 46–53. [Google Scholar] [CrossRef]
- Tsuda, Y.; Saruwatari, J.; Yasui-Furukori, N. Meta-Analysis: The Effects of Smoking on the Disposition of Two Commonly Used Antipsychotic Agents, Olanzapine and Clozapine. BMJ Open 2014, 4, e004216. [Google Scholar] [CrossRef] [PubMed]
- Bidzan, L. Zależność Pomiędzy Paleniem Tytoniu a Współistnieniem Zaburzeń Psychicznych. Przegląd Lek. 2009, 66, 525–528. [Google Scholar]
- Wehring, H.J.; Liu, F.; McMahon, R.P.; Mackowick, K.M.; Love, R.C.; Dixon, L.; Kelly, D.L. Clinical Characteristics of Heavy and Non-Heavy Smokers with Schizophrenia. Schizophr. Res. 2012, 138, 285–289. [Google Scholar] [CrossRef][Green Version]
- Goff, D.C.; Henderson, D.C.; Amico, E. Cigarette Smoking in Schizophrenia: Relationship to Psychopathology and Medication Side Effects. Am. J. Psychiatry 1992, 149, 1189–1194. [Google Scholar] [CrossRef]
- Hickling, L.M.; Perez-Iglesias, R.; Ortiz-García de la Foz, V.; Balanzá-Martínez, V.; McGuire, P.; Crespo-Facorro, B.; Ayesa-Arriola, R. Tobacco Smoking and Its Association with Cognition in First Episode Psychosis Patients. Schizophr. Res. 2018, 192, 269–273. [Google Scholar] [CrossRef]
- Patkar, A.A.; Gopalakrishnan, R.; Lundy, A.; Leone, F.T.; Certa, K.M.; Weinstein, S.P. Relationship Between Tobacco Smoking and Positive and Negative Symptoms in Schizophrenia. J. Nerv. Ment. Dis. 2002, 190, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.C.; Gurpegui, M.; Diaz, F.J.; Leon, J.D. Nicotine Dependence and Symptoms in Schizophrenia: Naturalistic Study of Complex Interactions. Br. J. Psychiatry 2005, 186, 215–221. [Google Scholar] [CrossRef]
- Salokangas, R.K.R.; Honkonen, T.; Stengård, E.; Koivisto, A.-M.; Hietala, J. Cigarette Smoking in Long-Term Schizophrenia. Eur. Psychiatry 2006, 21, 219–223. [Google Scholar] [CrossRef]
- Menza, M.A.; Grossman, N.; Van Horn, M.; Cody, R.; Forman, N. Smoking and Movement Disorders in Psychiatric Patients. Biol. Psychiatry 1991, 30, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ziedonis, D.M.; Kosten, T.R.; Glazer, W.M.; Frances, R.J. Nicotine Dependence and Schizophrenia. Psychiatr. Serv. 1994, 45, 204–206. [Google Scholar] [CrossRef]
- Salokangas, R.K.R.; Saarijärvi, S.; Taiminen, T.; Lehto, H.; Niemi, H.; Ahola, V.; Syvälahti, E. Effect of Smoking on Neuroleptics in Schizophrenia. Schizophr. Res. 1997, 23, 55–60. [Google Scholar] [CrossRef]
- Taiminen, T.J.; Salokangas, R.K.R.; Saarijärvi, S.; Niemi, H.; Lehto, H.; Ahola, V.; Syvälahti, E. Smoking and Cognitive Deficits in Schizophrenia: A Pilot Study. Addict. Behav. 1998, 23, 263–266. [Google Scholar] [CrossRef]
- Lyon, E.R. A Review of the Effects of Nicotine on Schizophrenia and Antipsychotic Medications. Psychiatr. Serv. 1999, 50, 1346–1350. [Google Scholar] [CrossRef]
- Salokangas, R.K.R. Gender and the Use of Neuroleptics in Schizophrenia. Schizophr. Res. 2004, 66, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dobrinas, M.; Cornuz, J.; Oneda, B.; Kohler Serra, M.; Puhl, M.; Eap, C.B. Impact of Smoking, Smoking Cessation, and Genetic Polymorphisms on CYP1A2 Activity and Inducibility. Clin. Pharmacol. Ther. 2011, 90, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Lua, A.C.; Wu, L.S.-H.; Wu, B.-J.; Lee, S.-M.; Liu, C.-Z. Cigarette Smoking Has a Differential Effect on the Plasma Level of Clozapine in Taiwanese Schizophrenic Patients Associated with the CYP1A2 Gene −163A/C Single Nucleotide Polymorphism. Psychiatr. Genet. 2016, 26, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Na Takuathung, M.; Hanprasertpong, N.; Teekachunhatean, S.; Koonrungsesomboon, N. Impact of CYP1A2 Genetic Polymorphisms on Pharmacokinetics of Antipsychotic Drugs: A Systematic Review and Meta-Analysis. Acta Psychiatr. Scand. 2019, 139, 15–25. [Google Scholar] [CrossRef]
- Krishnadas, R.; Jauhar, S.; Telfer, S.; Shivashankar, S.; McCreadie, R.G. Nicotine Dependence and Illness Severity in Schizophrenia. Br. J. Psychiatry 2012, 201, 306–312. [Google Scholar] [CrossRef]
- De Beaurepaire, R.; Rat, P.; Beauverie, P.; Houery, M.; Niel, P.; Castéra, S.; Dagorne, O.; Espaze, R.; Giroult, P.; Mahuzier, G.; et al. Is Smoking Linked to Positive Symptoms in Acutely Ill Psychiatric Patients? Nord. J. Psychiatry 2012, 66, 225–231. [Google Scholar] [CrossRef]
- Matthews, A.M.; Wilson, V.B.; Mitchell, S.H. The Role of Antipsychotics in Smoking and Smoking Cessation. CNS Drugs 2011, 25, 299–315. [Google Scholar] [CrossRef]
- Burki, T.K. Smoking and Mental Health. Lancet Respir. Med. 2016, 4, 437. [Google Scholar] [CrossRef]
- Salín-Pascual, R.J.; Rosas, M.; Jimenez-Genchi, A.; Rivera-Meza, B.L. Antidepressant Effect of Transdermal Nicotine Patches in Nonsmoking Patients with Major Depression. J. Clin. Psychiatry 1996, 57, 387–389. [Google Scholar] [PubMed]
- Smith, R.C.; Singh, A.; Infante, M.; Khandat, A.; Kloos, A. Effects of Cigarette Smoking and Nicotine Nasal Spray on Psychiatric Symptoms and Cognition In Schizophrenia. Neuropsychopharmacology 2002, 27, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Allan, R.; Fleming, M.; Atkinson, J. Mood and Smoking in Schizophrenia. Psychiatr. Ment. Health Nurs. 2008, 15, 722–727. [Google Scholar] [CrossRef]
- Decina, P.; Caracci, G.; Sandik, R.; Berman, W.; Mukherjee, S.; Scapicchio, P. Cigarette Smoking and Neuroleptic-Induced Parkinsonism. Biol. Psychiatry 1990, 28, 502–508. [Google Scholar] [CrossRef]
- Huang, H.; Dong, M.; Zhang, L.; Zhong, B.-L.; Ng, C.H.; Ungvari, G.S.; Yuan, Z.; Meng, X.; Xiang, Y.-T. Psychopathology and Extrapyramidal Side Effects in Smoking and Non-Smoking Patients with Schizophrenia: Systematic Review and Meta-Analysis of Comparative Studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 476–482. [Google Scholar] [CrossRef]
- Schmitz, N.; Kruse, J.; Kugler, J. Disabilities, Quality of Life, and Mental Disorders Associated with Smoking and Nicotine Dependence. Am. J. Psychiatry 2003, 160, 1670–1676. [Google Scholar] [CrossRef]
- Dixon, L.; Medoff, D.R.; Wohlheiter, K.; DiClemente, C.; Goldberg, R.; Kreyenbuhl, J.; Adams, C.; Lucksted, A.; Davin, C. Correlates of Severity of Smoking Among Persons with Severe Mental Illness. Am. J. Addict. 2007, 16, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Medici, C.R.; Vestergaard, C.H.; Hjorth, P.; Hansen, M.V.; Shanmuganathan, J.W.; Viuff, A.G.; Munk-Jørgensen, P. Quality of Life and Clinical Characteristics in a Nonselected Sample of Patients with Schizophrenia. Int. J. Soc. Psychiatry 2016, 62, 12–20. [Google Scholar] [CrossRef]
- Stubbs, B.; Mitchell, A.J.; De Hert, M.; Correll, C.U.; Soundy, A.; Stroobants, M.; Vancampfort, D. The Prevalence and Moderators of Clinical Pain in People with Schizophrenia: A Systematic Review and Large Scale Meta-Analysis. Schizophr. Res. 2014, 160, 1–8. [Google Scholar] [CrossRef]
- Stubbs, B.; Thompson, T.; Acaster, S.; Vancampfort, D.; Gaughran, F.; Correll, C.U. Decreased Pain Sensitivity among People with Schizophrenia: A Meta-Analysis of Experimental Pain Induction Studies. Pain 2015, 156, 2121–2131. [Google Scholar] [CrossRef]
- Butler, M.A.; Iwasaki, M.; Guengerich, F.P.; Kadlubar, F.F. Human Cytochrome P-450PA (P-450IA2), the Phenacetin O-Deethylase, Is Primarily Responsible for the Hepatic 3-Demethylation of Caffeine and N-Oxidation of Carcinogenic Arylamines. Proc. Natl. Acad. Sci. USA 1989, 86, 7696–7700. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J. Psychopharmacology: Atypical Antipsychotic Dosing: The Effect of Smoking and Caffeine. Psychiatr. Serv. 2004, 55, 491–493. [Google Scholar] [CrossRef]
- Thorgeirsson, T.E.; Gudbjartsson, D.F.; Surakka, I.; Vink, J.M.; Amin, N.; Geller, F.; Sulem, P.; Rafnar, T.; Esko, T.; Walter, S.; et al. Sequence Variants at CHRNB3–CHRNA6 and CYP2A6 Affect Smoking Behavior. Nat. Genet. 2010, 42, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.E.; Petty, R.G.; Turetsky, B.I.; Gur, R.C. Schizophrenia throughout Life: Sex Differences in Severity and Profile of Symptoms. Schizophr. Res. 1996, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Chue, P. Sex Differences in Schizophrenia, a Review of the Literature. Acta Psychiatr. Scand. Suppl. 2000, 401, 3–38. [Google Scholar] [CrossRef]
- Friedman, J.H. Viewpoint: Challenges in Our Understanding of Neuroleptic Induced Parkinsonism. Park. Relat. Disord. 2014, 20, 1325–1328. [Google Scholar] [CrossRef]
- Seeman, M.V. Gender Differences in the Prescribing of Antipsychotic Drugs. Am. J. Psychiatry 2004, 161, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
- Bergiannaki, J.D.; Kostaras, P. Pharmacokinetic and pharmacodynamic effects of psychotropic medications: Differences between sexes. Psychiatriki 2016, 27, 118–126. [Google Scholar] [CrossRef]
- Benowitz, N.; Lessovschlaggar, C.; Swan, G.; Jacobiii, P. Female Sex and Oral Contraceptive Use Accelerate Nicotine Metabolism. Clin. Pharmacol. Ther. 2006, 79, 480–488. [Google Scholar] [CrossRef]
- Johnstone, E.; Benowitz, N.; Cargill, A.; Jacob, R.; Hinks, L.; Day, I.; Murphy, M.; Walton, R. Determinants of the Rate of Nicotine Metabolism and Effects on Smoking Behavior. Clin. Pharmacol. Ther. 2006, 80, 319–330. [Google Scholar] [CrossRef]
- Davis, J.M. Dose Equivalence of the Antipsychotic Drugs. In Catecholamines and Schizophrenia; Elsevier: Oxford, UK, 1975; pp. 65–73. ISBN 9780080182421. [Google Scholar]
- Practice Guideline for the Treatment of Patients with Schizophrenia. American Psychiatric Association. Am. J. Psychiatry 1997, 154, 1–63. [CrossRef]
- Woods, S.W. Chlorpromazine Equivalent Doses for the Newer Atypical Antipsychotics. J. Clin. Psychiatry 2003, 64, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Chue, P.; Eerdekens, M.; Augustyns, I.; Lachaux, B.; Molcan, P.; Eriksson, L.; Pretorius, H.; David, A.S. Comparative Efficacy and Safety of Long-Acting Risperidone and Risperidone Oral Tablets. Eur. Neuropsychopharmacol. 2005, 15, 111–117. [Google Scholar] [CrossRef]
- Lindenmayer, J.P. Long-Acting Injectable Antipsychotics: Focus on Olanzapine Pamoate. Neuropsychiatr. Dis. Treat. 2010, 6, 261–267. [Google Scholar] [CrossRef]
- Detke, H.C.; Zhao, F.; Garhyan, P.; Carlson, J.; McDonnell, D. Dose Correspondence between Olanzapine Long-Acting Injection and Oral Olanzapine: Recommendations for Switching. Int. Clin. Psychopharmacol. 2011, 26, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.K.; Doose, D.R.; Nair, N.P. The Bioavailability and Pharmacokinetics of Oral and Depot Intramuscular Haloperidol in Schizophrenic Patients. J. Clin. Pharmacol. 1987, 27, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Alldredge, B.K. Seizure Risk Associated with Psychotropic Drugs: Clinical and Pharmacokinetic Considerations. Neurology 1999, 53, S68–S75. [Google Scholar] [PubMed]
- Bozikas, V.P.; Papakosta, M.; Niopas, I.; Karavatos, A.; Mirtsou-Fidani, V. Smoking Impact on CYP1A2 Activity in a Group of Patients with Schizophrenia. Eur. Neuropsychopharmacol. 2004, 14, 39–44. [Google Scholar] [CrossRef]
- Urichuk, L.; Prior, T.I.; Dursun, S.; Baker, G. Metabolism of Atypical Antipsychotics: Involvement of Cytochrome P450 Enzymes and Relevance for Drug-Drug Interactions. Curr. Drug Metab. 2008, 9, 410–418. [Google Scholar] [CrossRef]


| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| Marital status | 0.5163 | |||
| single | 35 (87.5%) | 32 (78.0%) | 67 (82.7%) | |
| divorced | 2 (5.0%) | 3 (7.3%) | 5 (6.2%) | |
| married | 3 (7.5%) | 6 (14.6%) | 9 (11.1%) | |
| Education | 0.7148 | |||
| primary school | 10 (25.0%) | 7 (17.1%) | 17 (21.0%) | |
| vocational training | 14 (35.0%) | 13 (31.7%) | 27 (33.3%) | |
| secondary school | 13 (32.5%) | 18 (43.9%) | 31 (38.3%) | |
| higher education | 3 (7.5%) | 3 (7.3%) | 6 (7.4%) | |
| Residence | 0.6270 | |||
| countryside | 2 (5.0%) | 5 (12.2%) | 7 (8.6%) | |
| city with <50 k inhabitants | 8 (20.0%) | 7 (17.1%) | 15 (18.5%) | |
| city with 50–100 k inhabitants | 1 (2.5%) | 2 (4.9%) | 3 (3.7%) | |
| city with >100 k inhabitants | 29 (72.5%) | 27 (65.9%) | 56 (69.1%) | |
| Home environment | 0.6361 | |||
| living alone | 6 (15.0%) | 5 (12.2%) | 11 (13.6%) | |
| living with family of origin | 28 (70.0%) | 30 (73.2%) | 58 (71.6%) | |
| living with family of procreation | 4 (10.0%) | 6 (14.6%) | 10 (12.3%) | |
| other (living with housemates) | 1 (2.5%) | 0 (0.0%) | 1 (1.2%) | |
| other (supported housing) | 1 (2.5%) | 0 (0.0%) | 1 (1.2%) | |
| Family of origin | 0.5825 | |||
| incomplete | 20 (50.0%) | 18 (43.9%) | 38 (46.9%) | |
| complete | 20 (50.0%) | 23 (56.1%) | 43 (53.1%) | |
| Criminal record | 0.3052 | |||
| convicted | 31 (77.5%) | 35 (85.4%) | 66 (81.5%) | |
| never convicted | 9 (22.5%) | 5 (12.2%) | 14 (17.3%) | |
| proceeding in progress | 0 (0.0%) | 1 (2.4%) | 1 (1.2%) |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| Age at first onset | 0.4584 | |||
| mean (SD) | 22.8 (5.0) | 24.3 (6.6) | 23.5 (5.9) | |
| range | 16.0–40.0 | 13.0–45.0 | 13.0–45.0 | |
| median | 23.0 | 23.0 | 23.0 | |
| 95% CI | [21.2; 24.3] | [22.2; 26.4] | [22.2; 24.8] | |
| Number of exacerbations | 0.0065 | |||
| mean (SD) | 12.8 (9.2) | 9.5 (12.8) | 11.1 (11.2) | |
| range | 1.0–42.0 | 1.0–70.0 | 1.0–70.0 | |
| median | 13.0 | 6.0 | 8.0 | |
| 95% CI | [9.8; 15.7] | [5.5; 13.6] | [8.7; 13.6] | |
| Number of hospitalizations | 0.0105 | |||
| mean (SD) | 11.9 (9.1) | 9.1 (12.8) | 10.5 (11.2) | |
| range | 1.0–38.0 | 1.0–70.0 | 1.0–70.0 | |
| median | 10.5 | 5.0 | 7.0 | |
| 95% CI | [9.0; 14.8] | [5.1; 13.1] | [8.0; 13.0] |
| S (n = 40) | NS (n = 41) | Overall (n = 81) | p | |
|---|---|---|---|---|
| CPZE | 0.0305 | |||
| mean (SD) | 689.3 (379.7) | 881.1 (418.2) | 786.4 (408.7) | |
| range | 100.0–1800.0 | 200.0–1600.0 | 100.0–1800.0 | |
| median | 600.0 | 900.0 | 680.0 | |
| 95% CI | [567.9; 810.7] | [749.2; 1013.1] | [696.0; 876.8] | |
| CPZE high dose | 0.0212 | |||
| n (%) | 8 (20%) | 18 (43.9%) | 26 (32.1%) |
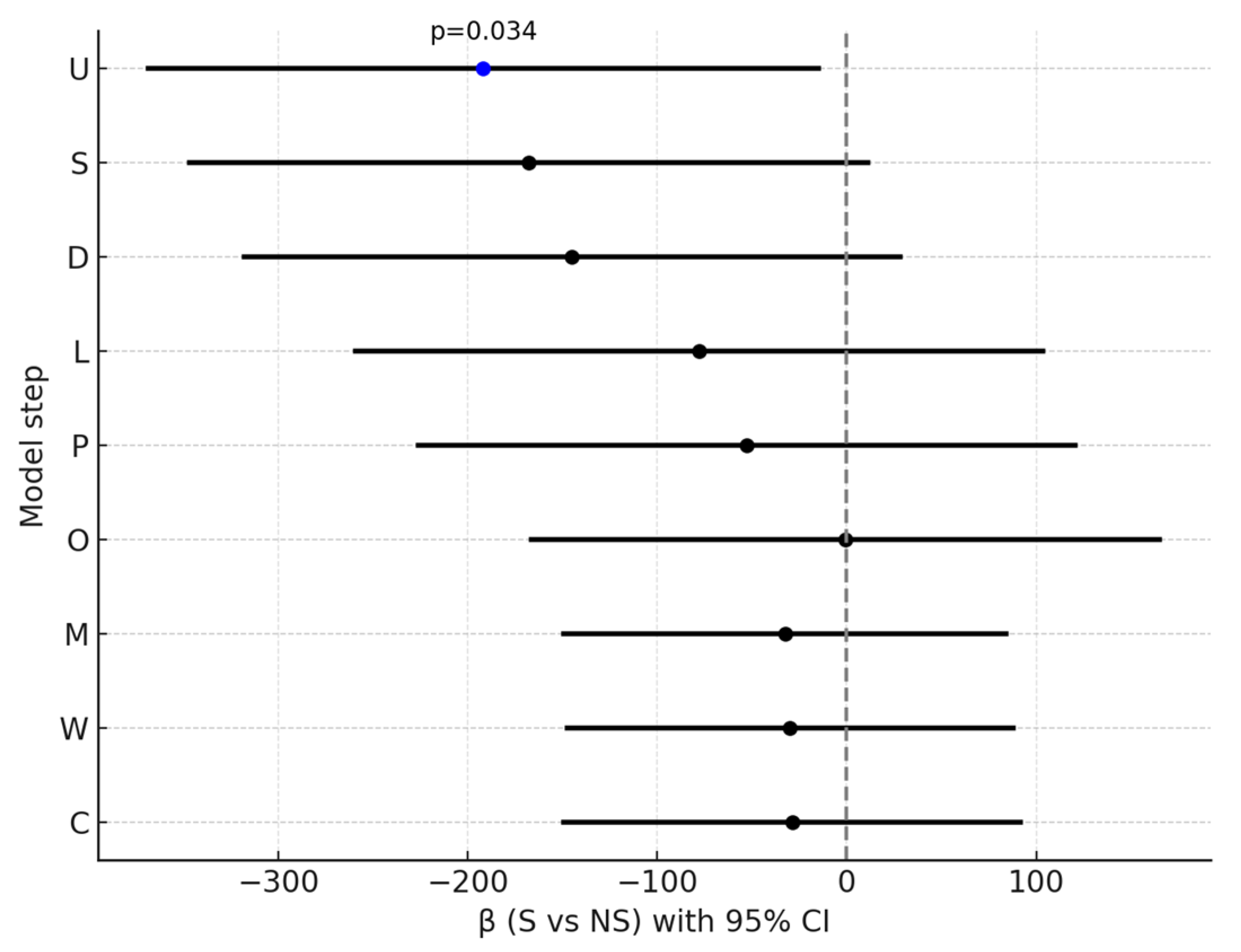
| Step | Model | β (S vs. NS) | 95% CI | p | R2 |
|---|---|---|---|---|---|
| U | unadjusted | −191.85 | [−368.62; −15.07] | 0.034 | 0.056 |
| S | +baseline severity | −167.72 | [−346.72; 11.28] | 0.066 | 0.091 |
| D | +duration of illness | −144.95 | [−318.26; 28.35] | 0.100 | 0.168 |
| L | +LAI | −77.96 | [−259.38; 103.46] | 0.395 | 0.213 |
| P | +polytherapy | −52.77 | [−226.04; 120.51] | 0.546 | 0.299 |
| O | +olanzapine | −0.46 | [−166.23; 165.31] | 0.996 | 0.390 |
| M | +CYP1A2 drugs | −32.56 | [−149.30; 84.17] | 0.580 | 0.703 |
| W | +other psychiatric medications | −29.91 | [−147.73; 87.92] | 0.614 | 0.704 |
| C | +caffeine use | −28.85 | [−149.37; 91.68] | 0.635 | 0.704 |
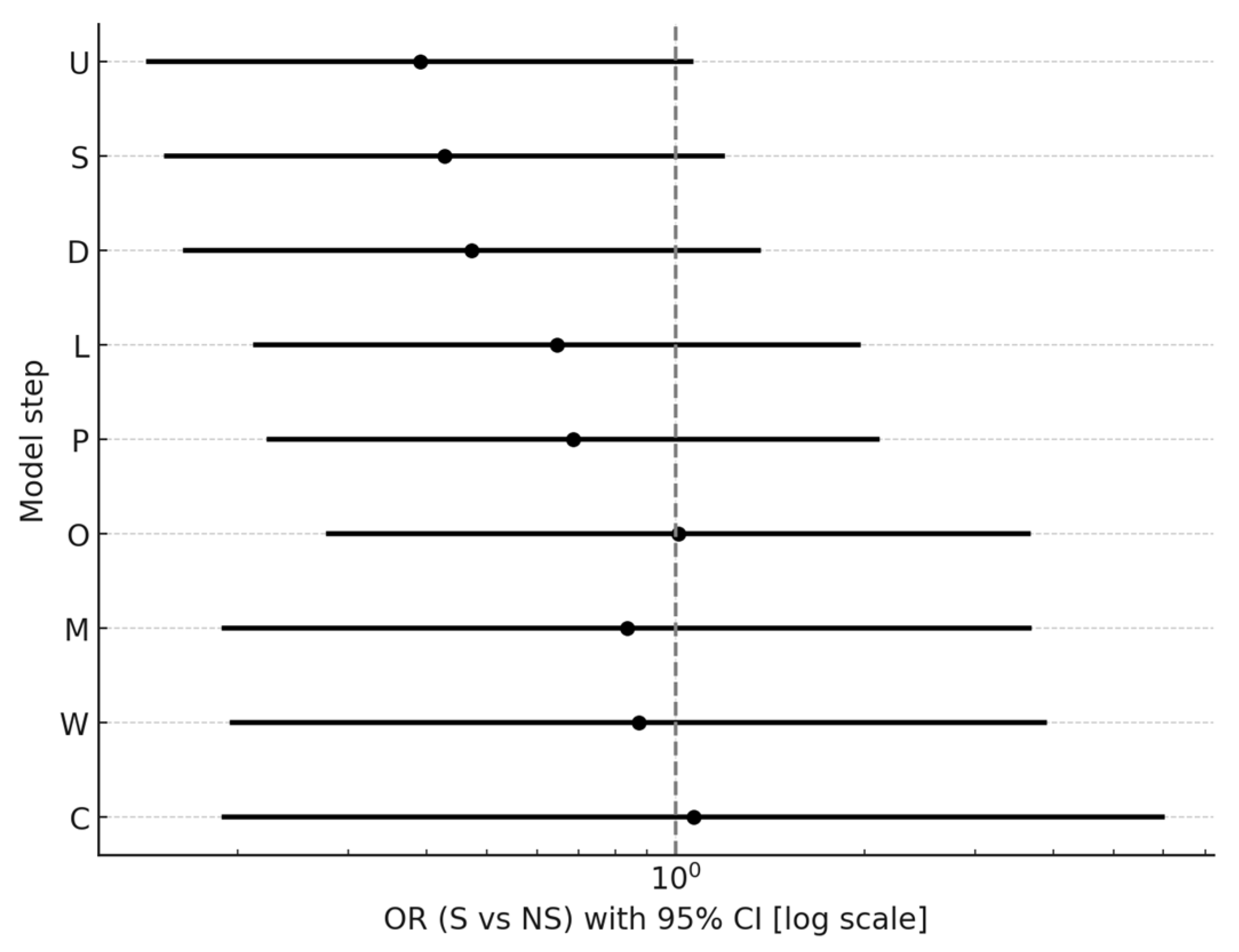
| Step | Model | OR | 95% CI | p | Pseudo R2 |
|---|---|---|---|---|---|
| U | unadjusted | 0.39 | [0.14; 1.06] | 0.065 | 0.036 |
| S | +baseline severity | 0.43 | [0.15; 1.19] | 0.103 | 0.057 |
| D | +duration of illness | 0.47 | [0.16; 1.35] | 0.162 | 0.095 |
| L | +LAI | 0.65 | [0.21; 1.95] | 0.438 | 0.125 |
| P | +polytherapy | 0.69 | [0.22; 2.10] | 0.507 | 0.137 |
| O | +olanzapine | 1.01 | [0.28; 3.65] | 0.988 | 0.286 |
| M | +CYP1A2 drugs | 0.84 | [0.19; 3.66] | 0.811 | 0.421 |
| W | +other psychiatric medications | 0.87 | [0.20; 3.88] | 0.857 | 0.444 |
| C | +caffeine use | 1.07 | [0.19; 5.99] | 0.941 | 0.447 |
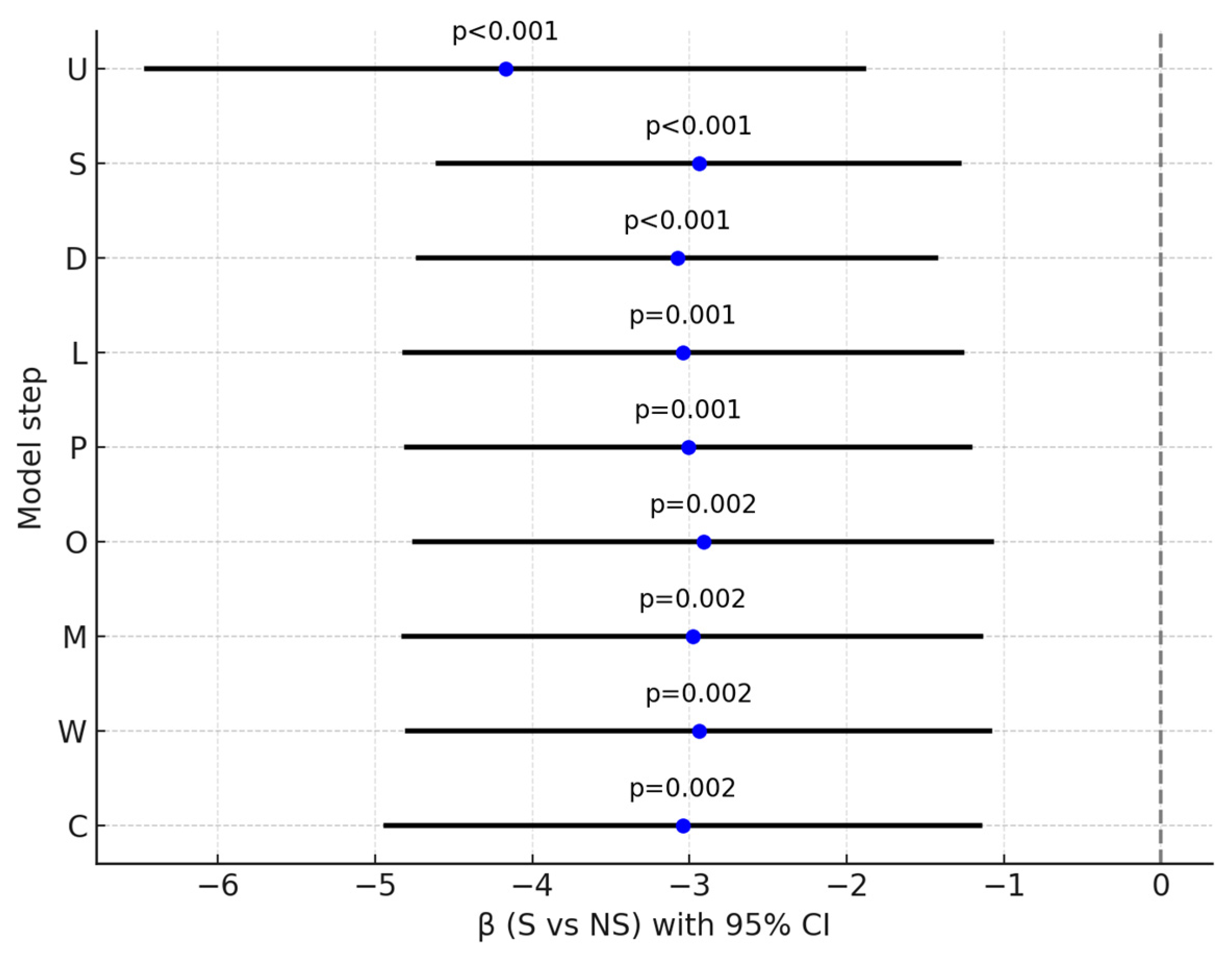
| Step | Model | β (S vs. NS) | 95% CI | p | R2 |
|---|---|---|---|---|---|
| U | unadjusted | −4.17 | [−6.45; −1.89] | <0.001 | 0.144 |
| S | +baseline severity | −2.94 | [−4.60; −1.28] | <0.001 | 0.576 |
| D | +duration of illness | −3.08 | [−4.72; −1.43] | <0.001 | 0.591 |
| L | +LAI | −3.04 | [−4.81; −1.27] | 0.001 | 0.591 |
| P | +polytherapy | −3.01 | [−4.80; −1.22] | 0.001 | 0.592 |
| O | +olanzapine | −2.91 | [−4.75; −1.08] | 0.002 | 0.593 |
| M | +CYP1A2 drugs | −2.98 | [−4.81; −1.15] | 0.002 | 0.601 |
| W | +other psychiatric medications | −2.94 | [−4.79; −1.09] | 0.002 | 0.602 |
| C | +caffeine use | −3.04 | [−4.93; −1.15] | 0.002 | 0.605 |
| S (n = 40) | NS (n = 41) | p | |
|---|---|---|---|
| remission in depression | 29 (82.9%) | 13 (39.4%) | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowski, J.; Bidzan, L.; Brzozowska, A. Nicotine Misuse and Treatment of Schizophrenia Exacerbations in Men: An Observational Study in Poland. Pharmaceuticals 2025, 18, 1366. https://doi.org/10.3390/ph18091366
Grabowski J, Bidzan L, Brzozowska A. Nicotine Misuse and Treatment of Schizophrenia Exacerbations in Men: An Observational Study in Poland. Pharmaceuticals. 2025; 18(9):1366. https://doi.org/10.3390/ph18091366
Chicago/Turabian StyleGrabowski, Jakub, Leszek Bidzan, and Aleksandra Brzozowska. 2025. "Nicotine Misuse and Treatment of Schizophrenia Exacerbations in Men: An Observational Study in Poland" Pharmaceuticals 18, no. 9: 1366. https://doi.org/10.3390/ph18091366
APA StyleGrabowski, J., Bidzan, L., & Brzozowska, A. (2025). Nicotine Misuse and Treatment of Schizophrenia Exacerbations in Men: An Observational Study in Poland. Pharmaceuticals, 18(9), 1366. https://doi.org/10.3390/ph18091366






