Abstract
Background: Meta-analyses on the prevalence and significance of thyroid incidentalomas at PET (TIP) are available only about [18F]FDG. Focal TIP at [18F]FDG PET is not rare and may be malignant lesions in about one-third of cases. The aim of this study is to perform a meta-analysis on the prevalence and clinical significance of TIP using other PET radiotracers beyond [18F]FDG. Methods: A comprehensive literature search of studies about TIP was carried out using four different databases, screened until 31 December 2024. Only original articles about TIP using radiopharmaceuticals other than [18F]FDG were selected. A proportion meta-analysis on the prevalence and clinical significance of TIP was carried out on a patient-based analysis using a random-effects model. Results: 21 studies (29,409 patients) were included in the meta-analysis. PET was performed using radiolabeled somatostatin analogues (SSA) [n = 5], choline [n = 6], prostate-specific membrane antigen (PSMA) [n = 7], or fibroblast activation protein inhibitors (FAPI) [n = 3]. The uptake pattern of TIP was described as focal, diffuse, or mixed/heterogeneous. The pooled prevalence of TIP was 5.6% for SSA-PET, 6.1% for choline-PET, 4.2% for PSMA-PET, and 3.6% for FAPI-PET. The final diagnosis of TIP with a diffuse pattern was a benign condition or represented a physiological uptake. Conversely, TIP with focal or mixed/heterogeneous pattern may represent a benign condition in most cases, but even a malignant lesion in 6–10% of cases. Conclusions: As for [18F]FDG, TIP using other radiopharmaceuticals is not rare. Most of them are benign, but those with focal or heterogeneous uptake patterns may represent a malignant lesion in some cases (even if the risk of malignancy is lower compared to [18F]FDG PET), thus requiring further evaluation. Further studies are warranted to better clarify the clinical impact of TIP detection.
Keywords:
thyroid; incidentaloma; incidental; PET; positron emission tomography; nuclear medicine; somatostatin; choline; PSMA; FAPI 1. Introduction
Incidental imaging findings or incidentalomas are unexpected lesions diagnosed in patients undergoing imaging for an unrelated reason. The prevalence of incidentalomas in various organs is increasing due to the enhanced use and sensitivity of different diagnostic imaging modalities [1,2]. Notably, incidental imaging findings could be clinically relevant, but their prevalence and clinical significance vary depending on the anatomical site and the imaging method used [1,2].
Advancements in molecular imaging could lead to a more frequent identification of incidentalomas compared to morphological imaging, as functional abnormalities may precede morphological changes [1,2,3]. As for molecular imaging, positron emission tomography (PET), administering different radiopharmaceuticals, is increasingly used for oncological and non-oncological indications [4]. PET can be coupled with computed tomography (PET/CT) or magnetic resonance imaging (PET/MRI) as hybrid imaging methods, and different PET radiopharmaceuticals evaluating several metabolic patterns or receptor expression can be used [4].
About thyroid incidentalomas, they are found on imaging studies performed for reasons other than thyroid diseases and represent a common scenario encountered by health care providers [5,6,7]. The initial workup for thyroid incidentalomas comprises a thorough history and physical examination, thyroid function tests, thyroid ultrasound, and fine-needle aspiration of any suspicious lesions [5].
A systematic review is the application of strategies that limit bias in the assembly, critical appraisal, and synthesis of all relevant studies on a specific topic. Meta-analysis is the statistical synthesis of data from separate but comparable studies, leading to a quantitative summary of the pooled results [8,9]. Systematic reviews and meta-analyses can be applied to nuclear medicine, radiology, and hybrid imaging [10,11,12,13,14,15,16,17,18,19,20,21].
Several systematic reviews and meta-analyses have already evaluated the prevalence and clinical significance of thyroid incidentalomas detected by PET (TIP) using fluorine-18 fluorodeoxyglucose ([18F]FDG), a radiolabeled glucose analogue commonly used to detect tumours and inflammatory/infectious diseases characterized by increased glucose metabolism [22,23,24,25,26,27,28,29]. Available evidence-based data on TIP at [18F]FDG PET demonstrate that they are not rare, with a pooled prevalence ranging from 1 to 3% of all [18F]FDG PET scans [30]. On the other hand, TIP with diffuse [18F]FDG uptake patterns are usually benign conditions, representing thyroiditis in most of the cases [31]; TIP with focal [18F]FDG uptake patterns may have a risk of malignancy ranging from 20% to 35%, thus requiring further evaluation [30].
In current clinical practice, PET/CT and PET/MRI can be performed with other radiopharmaceuticals beyond [18F]FDG evaluating different metabolic pathways or receptor status, for instance radiolabeled somatostatin analogues to evaluate tumours with increased somatostatin receptor (SSTR) expression, radiolabeled choline to evaluate tumours or conditions with increased cell membrane turnover, radiolabeled prostate-specific membrane antigen (PSMA) ligands to evaluate mainly prostate cancer and fibroblast activation protein inhibitors (FAPI) to assess the activity of cancer-associated fibroblasts (CAFs) in several tumours [4].
Interestingly, the prevalence and clinical significance of TIP with other PET radiopharmaceuticals beyond [18F]FDG have not been fully evaluated. Some examples of TIP using other radiopharmaceuticals beyond [18F]FDG are reported in Figure 1.
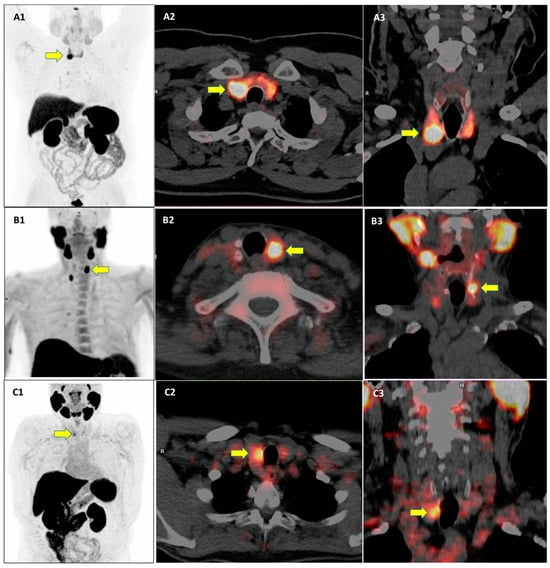
Figure 1.
Examples of three cases of thyroid incidental findings (yellow arrows) at PET/CT using different PET radiopharmaceuticals. PET/CT images using [68Ga]Ga-DOTA-peptides (A1–A3), radiolabeled choline (B1–B3), and radiolabeled prostate-specific membrane antigen (C1–C3) are provided.
The aim of this systematic review and meta-analysis is therefore to evaluate the current literature to establish the prevalence and the clinical significance of TIP using other PET radiopharmaceuticals beyond [18F]FDG through a pooled analysis. Our hypothesis is that the prevalence and clinical significance of TIP could be quite different based on the PET radiopharmaceutical used.
2. Methods
2.1. Review Question, Working Group, and Review Protocol
The first step of this review was to formulate a review question defining patients, intervention, and outcomes. The review question was the following: “Which are the prevalence and clinical significance of thyroid incidentalomas at PET using other radiopharmaceuticals beyond [18F]FDG?”.
The working group for this study was composed of one junior nuclear medicine physician (C.M.I.), six senior nuclear medicine physicians (D.A., A.R., A.P., M.C., G.P., G.T.) with experience in PET and nuclear medicine in thyroid diseases, one senior radiologist (A.P.), and one senior endocrinologist with a special interest in thyroid disorders (P.T.). Five co-authors have extensive experience in systematic reviews and meta-analyses of diagnostic imaging techniques, including previous pooled analyses on prevalence and clinical significance of incidental imaging findings (D.A., A.R., A.P., P.T., and G.T.).
A predefined protocol was followed to perform this study [32], and the review article was reported according to the PRISMA statement [33]. The protocol was not published on PROSPERO or other public databases, as this is not mandatory [33].
2.2. Search Strategy
A comprehensive literature search of studies about TIP was carried out by two review authors independently (C.M.I. and G.T.). Four different databases (PubMed/MEDLINE, EMBASE, Cochrane Library, Google Scholar) were screened until 31 December 2024. Based on the review question, the following search string was created combining several text words: (A) “thyroid” AND (B) “incidental” OR “incidentaloma*” OR “incidental*” OR “unexpected” OR “unusual” AND C) “PET” OR “positron”.
No filters or restrictions on publication dates or language were used during the systematic literature search. To obtain a more sensitive literature search, the references of the potentially eligible articles were also screened for additional studies.
2.3. Study Selection
Two review authors independently performed the study selection (C.M.I. and G.T.), applying the predefined inclusion and exclusion criteria. Regarding inclusion criteria, studies or subsets of studies were included only if they investigated the prevalence and significance of TIP using other radiopharmaceuticals beyond [18F]FDG. The exclusion criteria were: (a) articles outside the scope of this review or not providing information on the prevalence and clinical significance of TIP; (b) review articles, editorials, comments, letters, and conference proceedings in the topic of interest; (c) case reports in the topic of interest.
Titles and abstracts of the records retrieved using the predefined search string in the selected databases were screened. After the exclusion of non-eligible records, the full texts of the potentially eligible articles were downloaded and screened. Finally, studies were included in the review after a virtual consensus meeting among four co-authors (C.M.I., D.A., A.R., and G.T.).
Articles included in the systematic review were included in the quantitative analysis (meta-analysis) on the specific radiopharmaceutical, only if sufficient data to calculate the prevalence and clinical significance of TIP were available.
2.4. Data Extraction and Quality Assessment
Two review authors (C.M.I. and G.T.) independently performed the data extraction and the quality assessment. The following data were extracted from the selected articles using predefined data collection forms: basic study characteristics, patient characteristics, technical aspects, and outcome data. The overall quality of the studies included in this systematic review was assessed online using the NIH quality assessment tools [34].
2.5. Statistical Analysis
The pooled prevalence of TIP using other radiopharmaceuticals beyond [18F]FDG was calculated for each radiopharmaceutical through a patient-based proportion meta-analysis using a random-effects model, which considers the variability among studies. Subgroup analyses taking into account the radiopharmaceutical uptake pattern were performed if sufficient data were available.
About the clinical significance of TIP, the pooled risk of malignancy was calculated, taking into account the final histopathological diagnosis.
Pooled data were presented with 95% confidence interval (95%CI) values.
Heterogeneity was estimated through the I2 index [18]. OpenMeta[Analyst] (version 1.0 for Windows) was used as free open-source statistical software.
3. Results
3.1. Literature Search
Figure 2 summarizes the results of the literature search. A total of 700 records were identified using the selected databases. Titles and abstracts of these records were screened; 21 studies were finally included in the systematic review [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55], and no additional studies were found screening the reference list of the retrieved articles. Five studies evaluated the prevalence and clinical significance of TIP with radiolabeled somatostatin analogues [35,36,37,38,39], six with radiolabeled choline [40,41,42,43,44,45], seven with radiolabeled PSMA ligands [46,47,48,49,50,51,52], and three with radiolabeled FAPI [53,54,55].
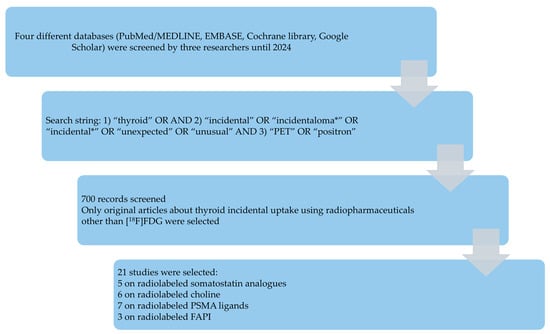
Figure 2.
Results of the literature search.
3.2. Qualitative Synthesis
Table 1 summarizes the main findings about original studies on TIP with other radiopharmaceuticals beyond [18F]FDG on 29,409 patients. The overall quality of the selected studies was assessed as moderate using the NIH quality assessment tool. Different radiopharmaceutical uptake patterns were described in TIP, including focal, diffuse, and heterogeneous/mixed patterns. Regarding the clinical significance of TIP based on the different radiopharmaceutical uptake pattern, when further evaluated, TIP with a diffuse uptake pattern usually represented a benign thyroid disease, whereas TIP with focal or heterogeneous uptake pattern could underlie a malignant thyroid disease in some cases [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].

Table 1.
Main findings of studies on incidental thyroid findings at PET (TIP) with different radiopharmaceuticals.
3.2.1. Radiolabeled Somatostatin Analogues
Five studies evaluated TIP at somatostatin receptor PET/CT or PET/MRI (using [68Ga]Ga-DOTA-peptides or [64Cu]Cu-DOTA-peptides), usually performed for the evaluation of neuroendocrine neoplasms [35,36,37,38,39]. The prevalence of TIP ranged from 2% to 11% of all PET scans. The prevalence of TIP with focal or heterogeneous uptake patterns ranged from 1% to 6% of all PET scans. When further evaluated, TIP with a diffuse uptake pattern at somatostatin receptor PET corresponded to thyroiditis, goitre, or absence of any clear thyroid disease due to physiological radiopharmaceutical uptake. Conversely, TIP with focal or heterogeneous uptake, even if most frequently benign, may represent malignant lesions in some cases. The overall risk of malignancy of TIP with focal or heterogenous uptake patterns ranged from 1% to 21%. The risk of malignancy of TIP with focal or heterogenous uptake pattern, further evaluated with thyroid imaging, ranged from 4% to 31%. At histopathological diagnosis, the malignant lesions described among TIP at somatostatin receptor PET corresponded to papillary thyroid carcinoma, medullary thyroid carcinoma, and metastases to the thyroid gland [35,36,37,38,39]. There was no significant difference in radiopharmaceutical uptake among benign and malignant TIP at somatostatin receptor PET [35,36,37,38,39].
3.2.2. Radiolabeled Choline
Six studies evaluated TIP at radiolabeled choline PET/CT or PET/MRI (using [11C]Choline or [18F]Fluorocholine), usually performed for the evaluation of prostate cancer or for localization of hyperfunctioning parathyroid glands in patients with hyperparathyroidism [40,41,42,43,44,45]. The prevalence of TIP ranged from 1% to 13% of all PET scans. The prevalence of TIP with focal or heterogeneous uptake patterns ranged from 0.3% to 9% of all PET scans. When further evaluated, TIP with a diffuse uptake pattern at radiolabeled choline PET corresponded to thyroiditis, goitre, or absence of any clear thyroid disease due to physiological radiopharmaceutical uptake. Conversely, TIP with focal or heterogeneous uptake, even if most frequently benign, may represent malignant lesions in some cases. The overall risk of malignancy of TIP with focal or heterogenous uptake patterns ranged from 0 to 22%. The risk of malignancy of TIP with focal or heterogenous uptake pattern, further evaluated with thyroid imaging, ranged from 0 to 25%. At histopathological diagnosis, the malignant lesions described among TIP at radiolabeled choline PET corresponded to papillary thyroid carcinoma in most of the cases and less frequently to lymphoma, squamous cell carcinoma, and metastases to the thyroid gland [40,41,42,43,44,45]. There was no significant difference in radiopharmaceutical uptake among benign and malignant TIP at radiolabeled choline PET [40,41,42,43,44,45].
3.2.3. Radiolabeled PSMA Ligands
Seven studies evaluated TIP at PET/CT or PET/MRI with radiolabeled PSMA ligands (using [68Ga]Ga-PSMA-11, [18F]F-DCFPyL, [18F]F-PSMA-JK-7, or [18F]F-PSMA-1007), usually performed for the evaluation of prostate cancer [46,47,48,49,50,51,52]. The prevalence of TIP ranged from 0.5% to 22% of all PET scans. The prevalence of TIP with focal or heterogeneous uptake patterns ranged from 0.5% to 6% of all PET scans. When further evaluated, TIP with diffuse uptake pattern at radiolabeled PSMA ligands PET corresponded to thyroiditis, goitre, or absence of any clear thyroid disease due to physiological radiopharmaceutical uptake. Conversely, TIP with focal or heterogeneous uptake, even if most frequently benign, may represent malignant lesions in some cases. The overall risk of malignancy of TIP with focal or heterogenous uptake patterns ranged from 0 to 25%. The risk of malignancy of TIP with focal or heterogenous uptake pattern, further evaluated with thyroid imaging, also ranged from 0 to 25%. At histopathological diagnosis, the malignant lesions described among TIP at radiolabeled PSMA ligands PET corresponded to papillary thyroid carcinoma in most of the cases and less frequently to follicular thyroid carcinoma, Hürthle cell carcinoma, and metastases to the thyroid gland [46,47,48,49,50,51,52]. A study showed that the prevalence of TIP may be different, taking into account the different radiolabeled PSMA ligands: a lower TIP prevalence was demonstrated using [18F]F-DCFPyL and [18F]F-PSMA-JK-7, and a higher TIP prevalence was found using [18F]F-PSMA-1007 [47]. Prevalence of TIP also varies, taking into account the different PET image analysis methods, being higher using visual analysis compared to semi-quantitative analysis [47]. Conversely, the prevalence of TIP did not differ significantly between observers [47]. The radiopharmaceutical uptake was significantly higher in patients with malignant findings than in patients with benign conditions [46,50].
3.2.4. Radiolabeled FAPI
Only three studies evaluated TIP at PET/CT or PET/MRI with radiolabeled FAPI (using [68Ga]Ga-FAPI-46, [68Ga]Ga-FAPI-04 or [18F]F-FAPI-04), performed for the evaluation of several tumours [53,54,55]. The prevalence of TIP ranged from 1% to 20% of all PET scans. Only TIPs with diffuse radiopharmaceutical uptake were described. When further evaluated, TIP with diffuse uptake pattern at radiolabeled FAPI PET corresponded to thyroiditis, goitre, or absence of any clear thyroid disease due to physiological radiopharmaceutical uptake in most of the cases. A single malignant lesion was described among patients with diffuse uptake of radiolabeled FAPI in the thyroid gland corresponding to a lymphoma at histopathology [53,54,55].
3.3. Quantitative Synthesis
Meta-analyses on the prevalence of TIP demonstrated the following pooled values: 5.6% (95%CI: 3.0–8.2%) for PET with radiolabeled somatostatin analogues, 6.1% (95%CI: 2.6–9.5%) for PET with radiolabeled choline, 4.2% (95%CI: 2.7–5.7%) for PET with radiolabeled PSMA ligands, and 3.6% (95%CI: 0–7.5%) for PET with radiolabeled FAPI.
A subgroup analysis on the prevalence of TIP for focal and heterogeneous radiopharmaceutical uptake patterns demonstrated the following pooled values: 3.5% (95%CI: 1.9–5.1%) for PET with radiolabeled somatostatin analogues, 1.7% (95%CI: 0.7–2.7%) for PET with radiolabeled choline, 1.9% (95%CI: 1.1–2.6%) for PET with radiolabeled PSMA ligands, and not calculable for PET with radiolabeled FAPI.
Meta-analyses on the overall malignancy risk of TIP with focal or heterogeneous uptake pattern demonstrated the following pooled values: 5.7% (95%CI: 0.5–10.9%) for PET with radiolabeled somatostatin analogues, 10.1% (95%CI: 5.1–15%) for PET with radiolabeled choline, 6.5% (95%CI: 2.5–10.5%) for PET with radiolabeled PSMA ligands, and not calculable for PET with radiolabeled FAPI. These values slightly increased, considering only TIP further evaluated as reported in Table 1.
Significant statistical heterogeneity was found in the meta-analyses on the prevalence and clinical significance of TIP with different PET radiopharmaceuticals (I2 > 50%).
4. Discussion
4.1. Literature Data
To the best of our knowledge, this is the first comprehensive review and meta-analysis providing a summary on the prevalence and clinical significance of TIP using other PET radiopharmaceuticals beyond [18F]FDG. We have included only original studies in our review, excluding case reports, which provide low-quality evidence due to several biases [56].
Our review found that most literature on TIP focuses on radiolabeled somatostatin analogues, choline, and PSMA, with fewer studies available on TIP using other PET radiopharmaceuticals.
The thyroid gland is a frequent site of incidental radiopharmaceutical uptake with PET radiopharmaceuticals [3]. In our analysis, we have found that the overall pooled prevalence of TIP with other radiopharmaceuticals beyond [18F]FDG varies between 4% and 6% (quite similar to that of TIP using [18F]FDG); therefore, these findings are not rare and should not be overlooked.
Similarly to [18F]FDG PET, whereas diffuse tracer uptake pattern in the thyroid gland represents a benign condition in nearly all patients with TIP using other PET radiopharmaceuticals, focal or heterogeneous tracer uptake pattern in the thyroid gland may represent a malignant lesion, even if in a minority of patients. The overall pooled risk of malignancy of TIP with focal or heterogeneous uptake patterns ranged from 6% to 10% for most of the PET radiopharmaceuticals evaluated in our analysis. Notably, this risk seems to be significantly lower compared to the risk of malignancy of TIP with focal uptake pattern using [18F]FDG (reported to be between 20% and 35% according to evidence-based data [30]). Potential reasons for this difference among [18F]FDG and other PET radiopharmaceuticals could be related to the different uptake mechanisms of these PET radiopharmaceuticals. [18F]FDG uptake, reflecting the glucose metabolism, is more associated with the biological aggressiveness of the lesions compared to other PET radiopharmaceuticals. This could explain the higher malignancy risk of TIP using [18F]FDG compared to other PET radiopharmaceuticals. However, it is not possible to discriminate between benign and malignant TIP only at visual or semi-quantitative analyses of PET images with different radiopharmaceuticals due to the overlap of radiopharmaceutical uptake among these two groups [30,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55].
Notably, due to the possible risk of malignancy of TIP with different PET radiopharmaceuticals, in particular in cases of focal or heterogeneous radiopharmaceutical uptake patterns, clinical evaluation, thyroid function test, and thyroid ultrasound should be suggested in the PET report to further evaluate TIP when they are detected [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55]. Interestingly, current evidence suggests that investigation and management of thyroid nodules should not be influenced by the mode of detection (incidental versus non-incidental thyroid nodule) [57].
It could be hypothesized that if malignant thyroid lesions are incidentally detected using other PET radiopharmaceuticals beyond [18F]FDG, these PET radiopharmaceuticals could be used to evaluate primary thyroid malignancies. However, even if literature data are available on the use of radiolabeled somatostatin analogues [58,59,60,61,62,63], choline [64,65,66,67,68], PSMA ligands [62,69,70,71,72,73,74] and FAPI [74,75,76,77,78,79] in thyroid cancer; currently, these PET methods are used only in research setting for this purpose [30], except the use of somatostatin receptor PET in medullary thyroid cancer according to existing guidelines [80].
4.2. Limitations and Suggestions for Future Research
Several limitations of our analysis should be acknowledged. First, there is still a limited number of studies assessing TIP with each PET radiopharmaceutical beyond [18F]FDG. Second, significant heterogeneity among the studies included in the meta-analyses was found, likely due to differences in patient characteristics, countries (with different iodine status influencing the prevalence of thyroid diseases), PET radiopharmaceuticals, imaging protocols, and indications among the included studies. In this regard, some PET radiopharmaceuticals evaluated are used mainly for prostate cancer, and this could create a possible gender bias. Third, even if we have excluded case reports from the analysis, we cannot exclude the presence of publication bias; funnel plots were not used to assess the presence of publication bias due to the low number of studies available for each PET radiopharmaceutical. Both heterogeneity and publication bias could have an impact on the results presented in this study.
As a first suggestion for future research, we recommend performing more studies on the prevalence and clinical significance of TIP with other tracers beyond [18F]FDG and, in particular, with FAPI ligands. Second, it could be interesting to evaluate the possible change in management related to TIP detection with PET imaging using different PET radiopharmaceuticals. Third, long-term clinical outcomes of patients with TIP could be assessed in future studies.
5. Conclusions
As for [18F]FDG PET, TIP using other PET radiopharmaceuticals is not rare and should not be overlooked. Most of these findings are benign, but those with focal or heterogeneous radiopharmaceutical uptake patterns may represent malignant lesions in some cases (even if the malignancy risk is lower compared to [18F]FDG PET), thus requiring further evaluation. The evidence-based data from this analysis should inform future guidelines on managing thyroid incidentalomas. However, further studies are needed to better understand the clinical impact of TIP detection using various PET radiopharmaceuticals.
Author Contributions
Conceptualization, G.T.; methodology, G.T.; software, G.T.; validation, A.R.; formal analysis, C.M.I. and G.T.; resources, C.M.I., D.A., A.R. and G.T.; data curation, C.M.I. and G.T.; writing—original draft preparation, C.M.I. and G.T.; writing—review and editing, C.M.I., D.A., A.R., A.P., M.C., G.P. and P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Sullivan, J.W.; Muntinga, T.; Grigg, S.; Ioannidis, J.P.A. Prevalence and outcomes of incidental imaging findings: Umbrella review. BMJ 2018, 361, k2387. [Google Scholar] [CrossRef] [PubMed]
- Alabousi, M.; Wilson, E.; Al-Ghetaa, R.K.; Patlas, M.N. General Review on the Current Management of Incidental Findings on Cross-Sectional Imaging: What Guidelines to Use, How to Follow Them, and Management and Medical-Legal Considerations. Radiol. Clin. N. Am. 2021, 59, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, R.; Scalia, G.; Strigari, L.; Ippolito, M.; Paolini, F.; Brunasso, L.; Sciortino, A.; Iacopino, D.G.; Maugeri, R.; Ferini, G.; et al. Nuclear medicine imaging modalities to detect incidentalomas and their impact on patient management: A systematic review. J. Cancer Res. Clin. Oncol. 2024, 150, 368. [Google Scholar] [CrossRef]
- Juweid, M.E.; Al-Qasem, S.F.; Khuri, F.R.; Gallamini, A.; Lohmann, P.; Ziellenbach, H.J.; Mottaghy, F.M. Beyond fluorodeoxyglucose: Molecular imaging of cancer in precision medicine. CA Cancer J. Clin. 2025, 75, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.B.; Perrier, N.D. The incidental thyroid nodule. CA Cancer J. Clin. 2018, 68, 97–105. [Google Scholar] [CrossRef]
- Sharbidre, K.G.; Lockhart, M.E.; Tessler, F.N. Incidental Thyroid Nodules on Imaging: Relevance and Management. Radiol. Clin. N. Am. 2021, 59, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.V.; Job, J.; Fiorillo, L.E.; Sipos, J. Thyroid Incidentalomas: Practice Considerations for Radiologists in the Age of Incidental Findings. Radiol. Clin. N. Am. 2020, 58, 1019–1031. [Google Scholar] [CrossRef]
- Signore, A.; Campagna, G. Evidence-based medicine: Reviews and meta-analysis. Clin. Transl. Imaging 2023, 11, 109–112. [Google Scholar] [CrossRef]
- Bhandari, M.; Giannoudis, P.V. Evidence-based medicine: What it is and what it is not. Injury 2006, 37, 302–306. [Google Scholar] [CrossRef]
- Puig, S.; Felder-Puig, R. Evidence-based radiology: A new approach to evaluate the clinical practice of radiology. Rofo 2006, 178, 671–679. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, J.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean J. Radiol. 2015, 16, 1175–1187. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.W.; Choi, S.H.; Huh, J.; Park, S.H. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean J. Radiol. 2015, 16, 1188–1196. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.J.; Lee, J. Diagnostic test accuracy: Application and practice using R software. Epidemiol. Health. 2019, 41, e2019007. [Google Scholar] [CrossRef]
- Sadeghi, R.; Zakavi, R.; Kakhki, V.R. How to apply the evidence-based medicine concept to nuclear medicine diagnostic studies—A review. Nucl. Med. Rev. Cent. East Eur. 2009, 12, 59–64. [Google Scholar] [PubMed]
- Cronin, P.; Kelly, A.M.; Altaee, D.; Foerster, B.; Petrou, M.; Dwamena, B.A. How to Perform a Systematic Review and Meta-analysis of Diagnostic Imaging Studies. Acad. Radiol. 2018, 25, 573–593. [Google Scholar] [CrossRef]
- Hong, J.U.; Kim, J.H.; Lee, K.H.; Lee, M.; Hyun, I.Y.; Cho, S.G.; Kim, Y.J.; Lee, H.Y.; Kim, G.R. Characteristics, trend, and methodological quality of systematic reviews and meta-analyses in nuclear medicine: A bibliometric analysis of studies published between 2005 and 2016. Medicine 2019, 98, e15785. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, K.H.; Ku, Y.J.; Cho, S.G.; Kim, Y.J.; Lee, H.Y.; Kim, J.H. Characteristics, Trends, and Quality of Systematic Review and Meta-Analysis in General Radiology between 2007 and 2015. Acad. Radiol. 2017, 24, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Alabousi, M.; Alabousi, A.; McGrath, T.A.; Cobey, K.D.; Budhram, B.; Frank, R.A.; Nguyen, F.; Salameh, J.P.; Dehmoobad Sharifabadi, A.; McInnes, M.D.F. Epidemiology of systematic reviews in imaging journals: Evaluation of publication trends and sustainability? Eur. Radiol. 2019, 29, 517–526. [Google Scholar] [CrossRef]
- Sardanelli, F.; Bashir, H.; Berzaczy, D.; Cannella, G.; Espeland, A.; Flor, N.; Helbich, T.; Hunink, M.; Malone, D.E.; Mann, R.; et al. The role of imaging specialists as authors of systematic reviews on diagnostic and interventional imaging and its impact on scientific quality: Report from the EuroAIM Evidence-based Radiology Working Group. Radiology 2014, 272, 533–540. [Google Scholar] [CrossRef]
- Jones, C.M.; Athanasiou, T. Diagnostic accuracy meta-analysis: Review of an important tool in radiological research and decision making. Br. J. Radiol. 2009, 82, 441–446. [Google Scholar] [CrossRef]
- Jones, C.M.; Ashrafian, H.; Skapinakis, P.; Arora, S.; Darzi, A.; Dimopoulos, K.; Athanasiou, T. Diagnostic accuracy meta-analysis: A review of the basic principles of interpretation and application. Int. J. Cardiol. 2010, 140, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Scappaticcio, L.; Piccardo, A.; Treglia, G.; Poller, D.N.; Trimboli, P. The dilemma of 18F-FDG PET/CT thyroid incidentaloma: What we should expect from FNA. A systematic review and meta-analysis. Endocrine 2021, 73, 540–549. [Google Scholar] [CrossRef]
- de Leijer, J.F.; Metman, M.J.H.; van der Hoorn, A.; Brouwers, A.H.; Kruijff, S.; van Hemel, B.M.; Links, T.P.; Westerlaan, H.E. Focal Thyroid Incidentalomas on 18F-FDG PET/CT: A Systematic Review and Meta-Analysis on Prevalence, Risk of Malignancy and Inconclusive Fine Needle Aspiration. Front. Endocrinol. 2021, 12, 723394. [Google Scholar] [CrossRef]
- Nayan, S.; Ramakrishna, J.; Gupta, M.K. The Proportion of Malignancy in Incidental Thyroid Lesions on 18-FDG PET Study: A Systematic Review and Meta-analysis. Otolaryngol. Head Neck Surg. 2014, 151, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Zhang, L.; Lu, Z.W.; Wie, W.J.; Zhang, Y.; Ji, Q.H. Risk of malignancy in focal thyroid lesions identified by (18)F-fluorodeoxyglucose positron emission tomography or positron emission tomography/computed tomography: Evidence from a large series of studies. Tumour Biol. 2014, 35, 6139–6147. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Bertagna, F.; Sadeghi, R.; Verburg, F.A.; Ceriani, L.; Giovanella, L. Focal thyroid incidental uptake detected by 18F-fluorodeoxyglucose positron emission tomography. Meta-analysis on prevalence and malignancy risk. Nuklearmedizin 2013, 52, 130–136. [Google Scholar] [CrossRef]
- Bertagna, F.; Treglia, G.; Piccardo, A.; Giubbini, R. Diagnostic and clinical significance of F-18-FDG-PET/CT thyroid incidentalomas. J. Clin. Endocrinol. Metab. 2012, 97, 3866–3875. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, K.K.; Bonnema, S.J.; Brix, T.H.; Hegedüs, L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: A systematic review. Thyroid 2012, 22, 918–925. [Google Scholar] [CrossRef]
- Shie, P.; Cardarelli, R.; Sprawls, K.; Fulda, K.G.; Taur, A. Systematic review: Prevalence of malignant incidental thyroid nodules identified on fluorine-18 fluorodeoxyglucose positron emission tomography. Nucl. Med. Commun. 2009, 30, 742–748. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Foppiani, L.; Treglia, G.; Ferrarazzo, G.; Massollo, M.; Bottoni, G.; Giovanella, L. PET/CT in thyroid nodule and differentiated thyroid cancer patients. The evidence-based state of the art. Rev. Endocr. Metab. Disord. 2019, 20, 47–64. [Google Scholar] [CrossRef]
- Albano, D.; Treglia, G.; Giovanella, L.; Giubbini, R.; Bertagna, F. Detection of thyroiditis on PET/CT imaging: A systematic review. Hormones 2020, 19, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, R.; Treglia, G. Systematic reviews and meta-analyses of diagnostic studies: A practical guideline. Clin. Transl. Imaging 2017, 5, 83–87. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseerf, L.; Tetzlaff, J.M.; Akli, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- NIH Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 31 December 2024).
- Sjöstedt, S.M.S.; Hahn, C.H.; Rasmussen, Å.K.; Oturai, P.S.; Cramon, P.K. Abnormal uptake related to the thyroid gland on somatostatin receptor-targeted PET imaging: Reported prevalence and rate of thyroid malignancy and parathyroid adenomas. Endocr. Connect. 2024, 13, e240419. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.; Fisher, J.C.; Rothberger, G.D.; Prescott, J.D.; Allendorf, J.D.; Patel, K.; Suh, I. Incidental 68Ga-DOTATATE uptake in thyroid nodules: Is guideline-directed management still appropriate? Surgery 2024, 175, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kohlenberg, J.D.; Panda, A.; Johnson, G.B.; Castro, M.R. Radiologic and clinicopathologic characteristics of thyroid nodules with focal 68Ga-DOTATATE PET activity. Nucl. Med. Commun. 2021, 42, 510–516. [Google Scholar] [CrossRef]
- Nockel, P.; Millo, C.; Keutgen, X.; Klubo-Gwiezdzinska, J.; Shell, J.; Patel, D.; Nilubol, N.; Herscovitch, P.; Sadowski, S.M.; Kebebew, E. The Rate and Clinical Significance of Incidental Thyroid Uptake as Detected by Gallium-68 DOTATATE Positron Emission Tomography/Computed Tomography. Thyroid 2016, 26, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Matyskiel, R.; Zemczak, A.; Strzelczyk, J.; Pawlak, D.; Królicki, L.; Kos-Kudła, B. How often do we see incidental 68Ga-DOTATATE thyroid uptake in PET/CT in patients with neuroendocrine tumours? Endokrynol. Pol. 2015, 66, 231–236. [Google Scholar] [CrossRef]
- Grünig, H.; Strobel, K.; Zander, A.; Pérez Lago, M.D.S.; Lima, T.; Wicke, C.; Fischli, S.; Bhure, U. Significance of incidental thyroid 18 F-fluorocholine uptake in patients with hyperparathyroidism imaged for localizing hyperfunctioning parathyroid glands. Nucl. Med. Commun. 2024, 45, 938–946. [Google Scholar] [CrossRef]
- Frota Lima, L.M.; Bogsrud, T.V.; Gharib, H.; Ryder, M.; Johnson, G.; Durski, J. Risk of malignancy in thyroid nodules with increased 11C-Choline uptake detected incidentally on PET/CT: A diagnostic accuracy study. Medicine 2024, 103, e39602. [Google Scholar] [CrossRef]
- Broos, W.A.M.; Knol, R.J.J.; Zant, F.M.V.; Schaper, N.C.; Wondergem, M. Incidental Findings on 18F-Fluorocholine PET/CT for Parathyroid Imaging. World J. Nucl. Med. 2022, 21, 192–199. [Google Scholar] [CrossRef]
- Roland, A.; Drouet, C.; Boulahdour, H.; Cochet, A.; De Bari, B. Unusual uptakes on 18F-fluorocholine positron emission tomography/computed tomography (PET/CT): A retrospective study of 368 prostate cancer patients referred for a biochemical recurrence or an initial staging. Quant. Imaging Med. Surg. 2021, 11, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Durmo, R.; Bertagna, F.; Giubbini, R. 18F-choline PET/CT incidental thyroid uptake in patients studied for prostate cancer. Endocrine 2019, 63, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Calabria, F.; Chiaravalloti, A.; Cicciò, C.; Gangemi, V.; Gullà, D.; Rocca, F.; Gallo, G.; Cascini, G.L.; Schillaci, O. PET/CT with 18F-choline: Physiological whole bio-distribution in male and female subjects and diagnostic pitfalls on 1000 prostate cancer patients: 18F-choline PET/CT bio-distribution and pitfalls. A southern Italian experience. Nucl. Med. Biol. 2017, 51, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Çerçi Koçar, İ.; Özcan, P.P.; Koç, Z.P.; Süle, M.; Akbay, E.; Gen, R.; Sezer, K. Retrospective analysis of thyroid incidentalomas detected by [68Ga]Ga-PSMA-11 PET/CT. Endocrine 2024, 86, 302–309. [Google Scholar] [CrossRef]
- Donswijk, M.L.; Piek, M.W.; Cheung, Z.; Wondergem, M.; Stokkel, M.P.M.; de Boer, J.P.; van der Ploeg, I.M.C. Incidence of PSMA PET thyroid incidentaloma depends on analysis method and tracer. Eur. Radiol. 2023, 33, 3377–3385. [Google Scholar] [CrossRef]
- Piek, M.W.; de Vries, L.H.; Donswijk, M.L.; de Keizer, B.; de Boer, J.P.; Lodewijk, L.; van Leeuwaarde, R.S.; Vriens, M.R.; Hartemink, K.J.; van der Ploeg, I.M.C. Retrospective analysis of PSMA PET/CT thyroid incidental uptake in adults: Incidence, diagnosis, and treatment/outcome in a tertiary cancer referral center and University Medical Center. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2392–2400. [Google Scholar] [CrossRef]
- Perry, E.; Talwar, A.; Sharma, S.; O’Connor, D.; Wong, L.M.; Taubman, K.; Sutherland, T.R. Non-prostate cancer tumours: Incidence on 18F-DCFPyL PSMA PET/CT and uptake characteristics in 1445 patients. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3277–3288. [Google Scholar] [CrossRef]
- Gossili, F.; Petersen, L.J.; Zacho, H.D. The frequency of thyroid incidental findings and risk of malignancy detected by 68Ga-labeled prostate-specific membrane antigen PET/CT in prostate cancer. Hell. J. Nucl. Med. 2020, 23, 240–245. [Google Scholar]
- Keidar, Z.; Gill, R.; Goshen, E.; Israel, O.; Davidson, T.; Morgulis, M.; Pirmisashvili, N.; Ben-Haim, S. 68Ga-PSMA PET/CT in prostate cancer patients—Patterns of disease, benign findings and pitfalls. Cancer Imaging 2018, 18, 39. [Google Scholar] [CrossRef]
- Osman, M.M.; Iravani, A.; Hicks, R.J.; Hofman, M.S. Detection of Synchronous Primary Malignancies with 68Ga-Labeled Prostate-Specific Membrane Antigen PET/CT in Patients with Prostate Cancer: Frequency in 764 Patients. J. Nucl. Med. 2017, 58, 1938–1942. [Google Scholar] [CrossRef] [PubMed]
- Pabst, K.M.; Kessler, L.; Ferdinandus, J.; Hamacher, R.; Bartel, T.; Siveke, J.T.; Nader, M.; Brandenburg, T.; Desaulniers, M.; Herrmann, K.; et al. [68Ga]Ga-FAPI versus 2-[18F]FDG PET/CT in patients with autoimmune thyroiditis: A case control study. EJNMMI Res. 2024, 14, 66. [Google Scholar] [CrossRef]
- Kou, Y.; Jiang, X.; Yao, Y.; Shen, J.; Jiang, X.; Chen, S.; Lu, H.; Wang, X.; Zhao, M.; Xiao, D.; et al. Physiological tracer distribution and benign lesion incidental uptake of Al18F-NOTA-FAPI-04 on PET/CT imaging. Nucl. Med. Commun. 2022, 43, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Liu, L.; Lei, L.; Wang, L.; Chen, Y. Clinical Significance of Diffusely Increased Uptake of 68Ga-FAPI in Thyroid Gland. Front. Med. 2021, 8, 782231. [Google Scholar] [CrossRef] [PubMed]
- Randles, R.; Finnegan, A. Guidelines for writing a systematic review. Nurse Educ. Today 2023, 125, 105803. [Google Scholar] [CrossRef]
- Chooi, J.E.; Ravindiran, A.; Balasubramanian, S.P. The influence of incidental detection of thyroid nodule on thyroid cancer risk and prognosis—A systematic review. Clin. Endocrinol. 2022, 96, 246–254. [Google Scholar] [CrossRef]
- Pishdad, R.; Treglia, G.; Mehta, A.; Santhanam, P. Somatostatin receptor imaging of thyroid tissue and differentiated thyroid cancer using gallium-68-labeled radiotracers-a review of clinical studies. Endocrine 2024, 85, 566–575. [Google Scholar] [CrossRef]
- Kunte, S.C.; Wenter, V.; Toms, J.; Lindner, S.; Unterrainer, M.; Eilsberger, F.; Jurkschat, K.; Wängler, C.; Wängler, B.; Schirrmacher, R.; et al. PET/CT imaging of differentiated and medullary thyroid carcinoma using the novel SSTR-targeting peptide [18F]SiTATE—First clinical experiences. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 900–912. [Google Scholar] [CrossRef]
- Almeida, L.S.; Santos, A.; Assumpção, L.; Costa, T.O.; Araujo, M.; Lima, M.; Zantut-Wittmann, D.E.; Etchebehere, E. 68 Ga-DOTATATE PET/CT Versus 18 F-FDG PET/CT in TENIS Syndrome: A Head-to-Head Comparison with Elevated and Suppressed TSH Levels in Papillary Thyroid Carcinoma—A Pilot Study. Clin. Nucl. Med. 2024, 49, 1004–1013. [Google Scholar] [CrossRef]
- Nazar, A.K.; Basu, S. Radiolabeled Somatostatin Analogs for Cancer Imaging. Semin. Nucl. Med. 2024, 54, 914–940. [Google Scholar] [CrossRef]
- Volpe, F.; Nappi, C.; Zampella, E.; Di Donna, E.; Maurea, S.; Cuocolo, A.; Klain, M. Current Advances in Radioactive Iodine-Refractory Differentiated Thyroid Cancer. Curr. Oncol. 2024, 31, 3870–3884. [Google Scholar] [CrossRef]
- Gild, M.L.; Kumar, S.; Fuchs, T.L.; Glover, A.; Sidhu, S.; Sywak, M.; Tsang, V.; Gill, A.J.; Robinson, B.G.; Schembri, G.; et al. The Clinical Utility of Gallium-68-DOTATATE Positron Emission Tomography Scanning in Medullary Thyroid Cancer. Endocr. Pract. 2024, 30, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bani, J.; Morland, D.; Hubelé, F.; Ignat, M.; Latge, A.; Bourahla, K.; Zalzali, M.; Vix, M.; Taïeb, D.; Imperiale, A. Dual-Time-Point 18F-Fluorocholine PET/CT Improves Characterization of Thyroid Nodules in Patients Referred for Primary Hyperparathyroidism: A Proof of Concept Study. Clin. Nucl. Med. 2021, 46, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Jamsek, J.; Hocevar, M.; Bergant, D.; Zaletel, K.; Rep, S.; Lezaic, L. Diagnostic value of [18F]Fluorocholine PET/CT in detection of primary medullary thyroid cancer. Ann. Nucl. Med. 2021, 35, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ciappuccini, R.; Licaj, I.; Lasne-Cardon, A.; Babin, E.; de Raucourt, D.; Blanchard, D.; Bastit, V.; Saguet-Rysanek, V.; Lequesne, J.; Peyronnet, D.; et al. 18F-Fluorocholine Positron Emission Tomography/Computed Tomography is a Highly Sensitive but Poorly Specific Tool for Identifying Malignancy in Thyroid Nodules with Indeterminate Cytology: The Chocolate Study. Thyroid 2021, 31, 800–809. [Google Scholar] [CrossRef]
- Ciappuccini, R.; Jeanne, C.; Bardet, S. Incidental focal thyroid uptake on 18F-Choline PET-CT: Need to rule out thyroid cancer. Endocrine 2018, 62, 729–730. [Google Scholar] [CrossRef]
- Piccardo, A.; Trimboli, P.; Puntoni, M.; Foppiani, L.; Treglia, G.; Naseri, M.; Bottoni, G.L.; Massollo, M.; Sola, S.; Ferrarazzo, G.; et al. Role of 18F-Choline Positron Emission Tomography/Computed Tomography to Detect Structural Relapse in High-Risk Differentiated Thyroid Cancer Patients. Thyroid 2019, 29, 549–556. [Google Scholar] [CrossRef]
- Rizzo, A.; Racca, M.; Dall’Armellina, S.; Delgado Bolton, R.C.; Albano, D.; Dondi, F.; Bertagna, F.; Annunziata, S.; Treglia, G. Potential Role of PSMA-Targeted PET in Thyroid Malignant Disease: A Systematic Review. Diagnostics 2023, 13, 564. [Google Scholar] [CrossRef]
- Eilsberger, F.; Luster, M.; Librizzi, D.; Rodepeter, F.; Holzer, K.; Pfestroff, A. Medullary Thyroid Carcinoma presenting as an Incidentaloma on Gallium-68-PSMA-PET/CT—Systematic Literature Review and Case Report. Nuklearmedizin 2022, 61, 458–461. [Google Scholar] [CrossRef]
- Verma, P.; Malhotra, G.; Meshram, V.; Chandak, A.; Sonavane, S.; Lila, A.R.; Bandgar, T.R.; Asopa, R.V. Prostate-Specific Membrane Antigen Expression in Patients with Differentiated Thyroid Cancer with Thyroglobulin Elevation and Negative Iodine Scintigraphy Using 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2021, 46, e406–e409. [Google Scholar] [CrossRef]
- Verma, P.; Malhotra, G.; Agrawal, R.; Sonavane, S.; Meshram, V.; Asopa, R.V. Evidence of Prostate-Specific Membrane Antigen Expression in Metastatic Differentiated Thyroid Cancer Using 68Ga-PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2018, 43, e265–e268. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Feng, Y.; Xu, L.; Li, W.; Guan, L.; Zuo, R.; Liu, S.; Pang, H.; Wang, Z. The value of gallium-68 prostate-specific membrane antigen PET/CT and 2-[18F]fluoro-2-deoxy-D-glucose PET/CT in the detection of thyroid cancer lesions: A prospective head-to-head comparison. Br. J. Radiol. 2023, 97, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Coerts, H.I.; de Keizer, B.; Verburg, F.A. Advances in the Development of Positron Emission Tomography Tracers for Improved Detection of Differentiated Thyroid Cancer. Cancers 2024, 16, 1401. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Albano, D.; Dondi, F.; Cioffi, M.; Muoio, B.; Annunziata, S.; Racca, M.; Bertagna, F.; Piccardo, A.; Treglia, G. Diagnostic yield of FAP-guided positron emission tomography in thyroid cancer: A systematic review. Front. Med. 2024, 11, 1381863. [Google Scholar] [CrossRef]
- Guglielmo, P.; Alongi, P.; Baratto, L.; Conte, M.; Abenavoli, E.M.; Buschiazzo, A.; Celesti, G.; Dondi, F.; Filice, R.; Gorica, J.; et al. FAPi-Based Agents in Thyroid Cancer: A New Step towards Diagnosis and Therapy? A Systematic Review of the Literature. Cancers 2024, 16, 839. [Google Scholar] [CrossRef]
- Hadjitheodorou, P.; Roll, W.; Alevroudis, E.; Kyrou, K.; Adamou, G.; Fesas, A.; Pourkhessalian, M.R.; Kalogirou, C.; Tsechelidis, I.; Vrachimis, A. Head-to-head comparison of 18F-FDG versus 18F-FAPI-74 PET in radioactive-iodine refractory differentiated thyroid cancer patients. Hell. J. Nucl. Med. 2025, 28, 2–7. [Google Scholar]
- Isik, E.G.; Has Simsek, D.; Gul, N.; Erturk, S.M.; Buyukkaya, F.; Soyluk Selcukbiricik, O.; Iscan, A.Y.; Özkan, Z.G.; Sanli, Y.; Mudun, A.; et al. Head-to-Head Comparison of 68Ga-FAPI-04 and 68Ga-DOTA-TATE PET/CT in Recurrent Medullary Thyroid Cancer. Clin. Nucl. Med. 2024, 50, e80–e86. [Google Scholar] [CrossRef]
- Pishdad, R.; Santhanam, P. Current and Emerging Radiotracers and Technologies for Detection of Advanced Differentiated Thyroid Cancer: A Narrative Review. Cancers 2025, 17, 425. [Google Scholar] [CrossRef]
- Giovanella, L.; Treglia, G.; Iakovou, I.; Mihailovic, J.; Verburg, F.A.; Luster, M. EANM practice guideline for PET/CT imaging in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 61–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).