The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer’s Disease: A Comprehensive Systematic Review
Abstract
1. Introduction
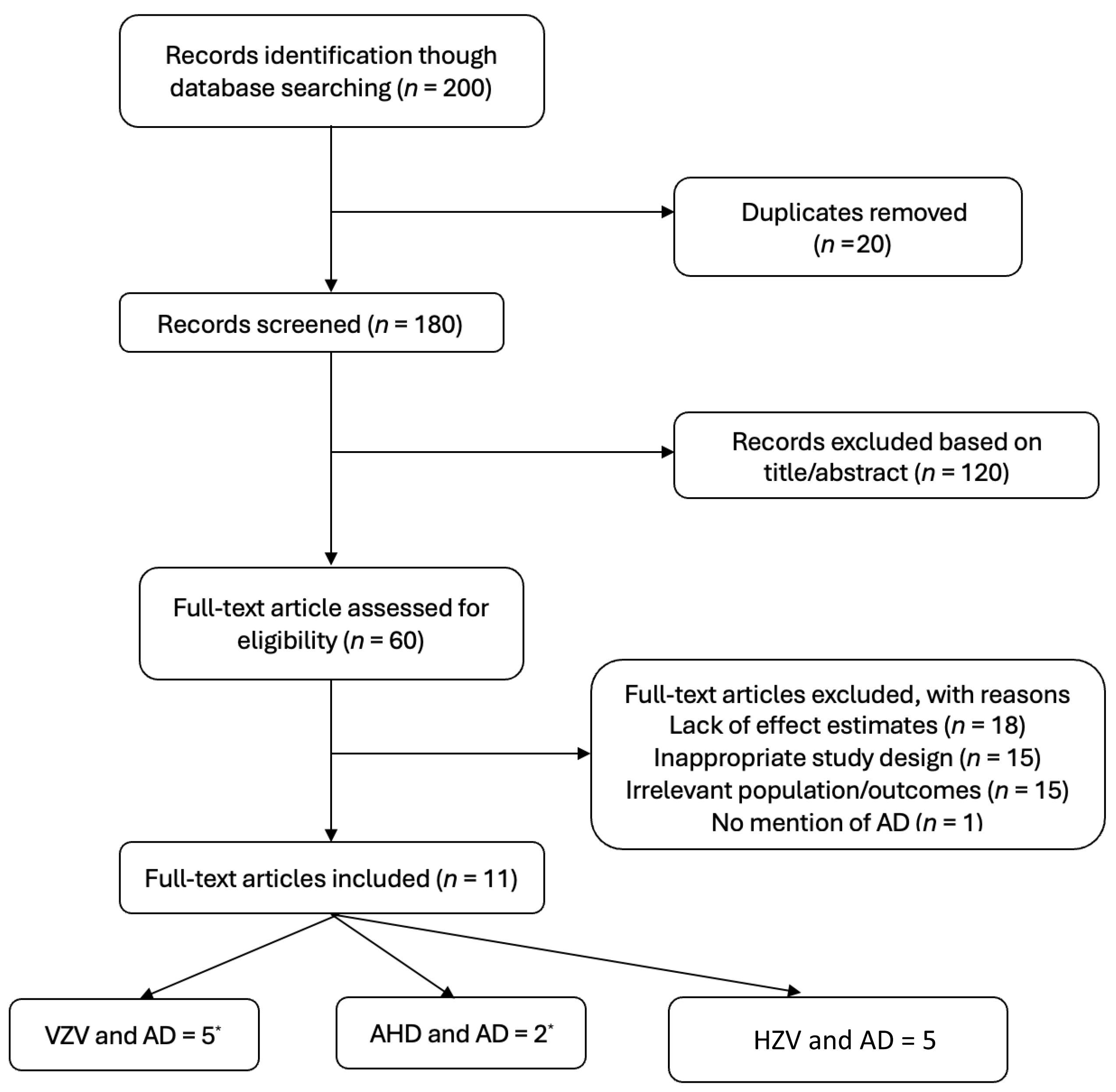
2. Methods
3. Eligibility Criteria
4. Data Extraction
5. Results
5.1. Varicella Zoster Virus Infection and Alzheimer’s Disease
5.2. Varicella Zoster Virus Vaccination and Risk of Alzheimer’s Disease
5.3. Antiherpetic Drug Use and Alzheimer’s Disease Risk
6. Discussion
6.1. Varicella Zoster Virus Infection and AD Risk
6.2. Varicella Zoster Virus Vaccination and AD Risk
6.3. Antiherpetic Drug Use and AD Risk
7. Policy Implications
8. Limitation
9. Critical Appraisal of Reviewed Studies According to the DREAM Study
9.1. Misclassification Bias
9.2. Selection Bias
9.3. Confounding Bias
9.4. Causality Bias
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| Aβ | Amyloid-beta |
| AHD | Antiherpetic Drug |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| DREAM | Drug Repurposing for Effective Alzheimer’s Medicines |
| HR | Hazard Ratio |
| HSV | Herpes Simplex Virus |
| HZ | Herpes Zoster |
| MeSH | Medical Subject Headings |
| OR | Odds Ratio |
| RCT | Randomized Controlled Trial |
| RR | Relative Risk |
| RZV | Recombinant Zoster Vaccine |
| SAIL | Secure Anonymised Information Linkage |
| TNF | Tumor Necrosis Factor |
| VHA | Veterans Health Administration |
| VZV | Varicella Zoster Virus |
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- ADI—Dementia Statistics. Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/ (accessed on 3 January 2025).
- Eid, A.; Mhatre, I.; Richardson, J.R. Gene-Environment Interactions in Alzheimer’s Disease: A Potential Path to Precision Medicine. Pharmacol. Ther. 2019, 199, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Abondio, P.; Bruno, R.; Ceraudo, L.; Paparazzo, E.; Citrigno, L.; Luiselli, D.; Bruni, A.C.; Passarino, G.; Colao, R.; et al. Alzheimer’s Disease as a Viral Disease: Revisiting the Infectious Hypothesis. Ageing Res. Rev. 2023, 91, 102068. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.R.; Torkzaban, B.; Ahooyi, T.M.; Khalili, K. Potential Role for Herpesviruses in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 855–869. [Google Scholar] [CrossRef]
- Piotrowski, S.L.; Tucker, A.; Jacobson, S. The Elusive Role of Herpesviruses in Alzheimer’s Disease: Current Evidence and Future Directions. Neuroimmune Pharmacol. Ther. 2023, 2, 253–266. [Google Scholar] [CrossRef]
- Anwar, M.M. The Emerging Mechanism behind Viral Infections and Extracellular Vesicles Hypotheses Leading to Neuroinflammation and Alzheimer’s Disease Pathology. Ibrain 2023, 9, 63–71. [Google Scholar] [CrossRef]
- Elhalag, R.H.; Motawea, K.R.; Talat, N.E.; Rouzan, S.S.; Reyad, S.M.; Elsayed, S.M.; Chébl, P.; Abowafia, M.; Shah, J. Herpes Zoster Virus Infection and the Risk of Developing Dementia: A Systematic Review and Meta-Analysis. Medicine 2023, 102, e34503. [Google Scholar] [CrossRef]
- Li, H.; Liu, C.-C.; Zheng, H.; Huang, T.Y. Amyloid, Tau, Pathogen Infection and Antimicrobial Protection in Alzheimer’s Disease—Conformist, Nonconformist, and Realistic Prospects for AD Pathogenesis. Transl. Neurodegener. 2018, 7, 34. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Williamson, E.; Shiekh, S.I.; Borjas-Howard, J.; Pearce, N.; Breuer, J.M.; Smeeth, L. No Evidence That Herpes Zoster Is Associated with Increased Risk of Dementia Diagnosis. Ann. Clin. Transl. Neurol. 2022, 9, 363–374. [Google Scholar] [CrossRef]
- Bubak, A.N.; Como, C.N.; Coughlan, C.M.; Johnson, N.R.; Hassell, J.E.; Mescher, T.; Niemeyer, C.S.; Mahalingam, R.; Cohrs, R.J.; Boyd, T.D.; et al. Varicella-Zoster Virus Infection of Primary Human Spinal Astrocytes Produces Intracellular Amylin, Amyloid-β, and an Amyloidogenic Extracellular Environment. J. Infect. Dis. 2020, 221, 1088–1097. [Google Scholar] [CrossRef]
- Tran, D.N.; Bakx, A.T.C.M.; Van Dis, V.; Aronica, E.; Verdijk, R.M.; Ouwendijk, W.J.D. No Evidence of Aberrant Amyloid β and Phosphorylated Tau Expression in Herpes Simplex Virus-infected Neurons of the Trigeminal Ganglia and Brain. Brain Pathol. 2022, 32, e13044. [Google Scholar] [CrossRef] [PubMed]
- Cairns, D.M.; Itzhaki, R.F.; Kaplan, D.L. Potential Involvement of Varicella Zoster Virus in Alzheimer’s Disease via Reactivation of Quiescent Herpes Simplex Virus Type 1. J. Alzheimer’s Dis. 2022, 88, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Pomirchy, M.; Bommer, C.; Pradella, F.; Michalik, F.; Peters, R.; Geldsetzer, P. Herpes Zoster Vaccination and Dementia Occurrence. JAMA 2025. [Google Scholar] [CrossRef] [PubMed]
- Eyting, M.; Xie, M.; Michalik, F.; Heß, S.; Chung, S.; Geldsetzer, P. A Natural Experiment on the Effect of Herpes Zoster Vaccination on Dementia. Nature 2025, 641, 438–446. [Google Scholar] [CrossRef]
- Curran, D.; Patterson, B.J.; Carrico, J.; Salem, A.; La, E.M.; Lorenc, S.; Hicks, K.A.; Poston, S.; Carpenter, C.F. Public Health Impact of Recombinant Zoster Vaccine for Prevention of Herpes Zoster in US Adults Immunocompromised Due to Cancer. Hum. Vaccines Immunother. 2013, 19, 2167907. [Google Scholar] [CrossRef]
- Van Lier, E.A.; De Melker, H.E. Herpes Zoster in the Netherlands; RIVM: Bilthoven, The Netherlands, 2018. [Google Scholar]
- Bae, S.; Yun, S.-C.; Kim, M.-C.; Yoon, W.; Lim, J.S.; Lee, S.-O.; Choi, S.-H.; Kim, Y.S.; Woo, J.H.; Kim, S.Y.; et al. Association of Herpes Zoster with Dementia and Effect of Antiviral Therapy on Dementia: A Population-Based Cohort Study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 987–997. [Google Scholar] [CrossRef]
- Linard, M.; Bezin, J.; Hucteau, E.; Joly, P.; Garrigue, I.; Dartigues, J.-F.; Pariente, A.; Helmer, C. Antiherpetic Drugs: A Potential Way to Prevent Alzheimer’s Disease? Alzheimer’s Res. Ther. 2022, 14, 3. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Desai, R.J.; Varma, V.R.; Gerhard, T.; Segal, J.; Mahesri, M.; Chin, K.; Nonnenmacher, E.; Gabbeta, A.; Mammen, A.M.; Varma, S.; et al. Targeting Abnormal Metabolism in Alzheimer’s Disease: The Drug Repurposing for Effective Alzheimer’s Medicines (DREAM) Study. Alzheimer’s Dement 2020, 6, e12095. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Choi, H.G.; Kim, J.-H.; Kim, J.H.; Kim, E.S.; Park, H.Y.; Min, K.-W.; Kwon, M.J. Associations between Proton Pump Inhibitors and Alzheimer’s Disease: A Nested Case–Control Study Using a Korean Nationwide Health Screening Cohort. Alzheimer’s Res. Ther. 2022, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Lopatko Lindman, K.; Hemmingsson, E.; Weidung, B.; Brännström, J.; Josefsson, M.; Olsson, J.; Elgh, F.; Nordström, P.; Lövheim, H. Herpesvirus Infections, Antiviral Treatment, and the Risk of Dementia—A Registry-based Cohort Study in Sweden. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12119. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.J.; Veres, K.; Sørensen, H.T.; Obel, N.; Henderson, V.W. Incident Herpes Zoster and Risk of Dementia. Neurology 2022, 99, e660–e668. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.; Park, M.; Kim, J. Increased Incidence of Dementia Following Herpesvirus Infection in The Korean Population. Medicine 2022, 101, e31116. [Google Scholar] [CrossRef]
- Lehrer, S.; Rheinstein, P.H. Vaccination Reduces Risk of Alzheimer’s Disease, Parkinson’s Disease, and Other Neurodegenerative Disorders. Discov. Med. 2022, 34, 97–101. [Google Scholar]
- Lophatananon, A.; Carr, M.; Mcmillan, B.; Dobson, C.; Itzhaki, R.; Parisi, R.; Ashcroft, D.M.; Muir, K.R. The Association of Herpes Zoster and Influenza Vaccinations with the Risk of Developing Dementia: A Population-Based Cohort Study within the UK Clinical Practice Research Datalink. BMC Public Health 2023, 23, 1903. [Google Scholar] [CrossRef]
- Scherrer, J.F.; Salas, J.; Wiemken, T.L.; Hoft, D.F.; Jacobs, C.; Morley, J.E. Impact of Herpes Zoster Vaccination on Incident Dementia: A Retrospective Study in Two Patient Cohorts. PLoS ONE 2021, 16, e0257405. [Google Scholar] [CrossRef]
- Schnier, C.; Janbek, J.; Lathe, R.; Haas, J. Reduced Dementia Incidence after Varicella Zoster Vaccination in Wales 2013–2020. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12293. [Google Scholar] [CrossRef]
- Harris, K.; Ling, Y.; Bukhbinder, A.S.; Chen, L.; Phelps, K.N.; Cruz, G.; Thomas, J.; Kim, Y.; Jiang, X.; Schulz, P.E. The Impact of Routine Vaccinations on Alzheimer’s Disease Risk in Persons 65 Years and Older: A Claims-Based Cohort Study Using Propensity Score Matching. J. Alzheimer’s Dis. 2023, 95, 703–718. [Google Scholar] [CrossRef]
- Chia, R.; Sabir, M.S.; Bandres-Ciga, S.; Saez-Atienzar, S.; Reynolds, R.H.; Gustavsson, E.; Walton, R.L.; Ahmed, S.; Viollet, C.; Ding, J.; et al. Genome Sequencing Analysis Identifies New Loci Associated with Lewy Body Dementia and Provides Insights into Its Genetic Architecture. Nat. Genet. 2021, 53, 294–303. [Google Scholar] [CrossRef]
- Kettunen, P.; Koistinaho, J.; Rolova, T. Contribution of CNS and Extra-CNS Infections to Neurodegeneration: A Narrative Review. J. Neuro Inflamm. 2024, 21, 152. [Google Scholar] [CrossRef]
- Braun, A.; Höfler, M.; Auer, S. Cost-Effectiveness of Prevention for People at Risk for Dementia: A Scoping Review and Qualitative Synthesis. J. Prev. Alzheimer’s Dis. 2024, 11, 402–413. [Google Scholar] [CrossRef]
| Sample Size | Study Design | Region | Year | Study |
|---|---|---|---|---|
| 229,594 | Retrospective cohort | South Korea | 2021 | Bae et al. [18] |
| 265,172 | Retrospective cohort | Sweden | 2021 | Lindman et al. [24] |
| 281,102 | Retrospective cohort | South Korea | 2022 | Shim et al. [26] |
| 1,483,195 | Retrospective cohort | Denmark | 2022 | Schmidt et al. [25] |
| 1,125,691 | Case–control | South Korea | 2021 | Choi et al. [23] |
| 143 | Retrospective cohort | United States | 2022 | Lehrer et al. [27] |
| 212,417 | Retrospective cohort | United States | 2023 | Harris et al. [31] |
| 228,223 | Case–control | United Kingdom | 2021 | Lophatananon et al. [28] |
| 136,016 (VHA); 172,790 (MarketScan) | Retrospective cohort | United States | 2021 | Scherrer et al. [29] |
| 336,341 | Retrospective cohort | Wales | 2022 | Schnier et al. [30] |
| 68,291 | Retrospective cohort | France | 2022 | Linard et al. [19] |
| Study | Year | Region | Sample Size | Age (Years) | Adjusted for Confounders? | Main Findings |
|---|---|---|---|---|---|---|
| Bae et al. [18] | 2021 | South Korea | 229,594 | ≥50 | Yes (age, sex, comorbidities) | HZ infection associated with increased AD risk (aHR = 1.12; 95% CI: 1.04–1.19). |
| Lindman et al. [24] | 2021 | Sweden | 265,172 | ≥50 | Yes (age, sex, comorbidities) | Untreated herpes infections increased AD risk (aHR = 1.50; 95% CI: 1.29–1.74). |
| Shim et al. [26] | 2022 | South Korea | 281,102 | ≥50 | Yes (age, sex, comorbidities) | HSV infection increased AD risk (aHR = 1.18; 95% CI: 1.16–1.20); VZV infection increased risk (aHR = 1.09; 95% CI: 1.07–1.11). |
| Schmidt et al. [25] | 2022 | Denmark | 1,483,195 | ≥40 | Yes (age, sex, comorbidities) | HZ infection associated with decreased AD risk (HR = 0.93; 95% CI: 0.90–0.97). |
| Choi et al. [23] | 2021 | South Korea | 1,125,691 | ≥60 | Yes (age, sex, comorbidities) | No association between HZ infection and AD (OR = 0.90; 95% CI: 0.84–0.97). |
| Study | Year | Region | Sample Size | Age (Years) | Vaccine Type | Adjusted for Confounders? | Main Findings |
|---|---|---|---|---|---|---|---|
| Lehrer et al. [27] | 2022 | United States | 143 | ≥60 | Zostavax | Yes (age, sex, comorbidities) | Zostavax associated with 15% reduction in AD risk. |
| Harris et al. [31] | 2023 | United States | 212,417 | ≥65 | Zostavax, Shingrix | Yes (age, sex, comorbidities) | Zostavax reduced AD risk (RR = 0.92; 95% CI: 0.90–0.94); Shingrix reduced AD risk (RR = 0.27; 95% CI: 0.25–0.29). |
| Lophatananon et al. [28] | 2021 | United Kingdom | 228,223 | ≥70 | Zostavax | Yes (age, sex, comorbidities) | Vaccination reduced AD risk (OR = 0.91; 95% CI: 0.89–0.92). |
| Scherrer et al. [29] | 2021 | United States | 136,016 (VHA); 172,790 (MarketScan) | ≥65 | Zostavax | Yes (age, sex, comorbidities) | Vaccination reduced AD risk (aHR = 0.75, 95% CI: 0.71–0.80 in VHA cohort; HR = 0.70, 95% CI: 0.55–0.88 in MarketScan cohort). |
| Schnier et al. [30] | 2022 | Wales | 336,341 | ≥70 | Zostavax | Yes (age, sex, comorbidities) | Vaccination reduced AD risk (aHR = 0.81; 95% CI: 0.77–0.86). |
| Study | Year | Region | Sample Size | Age (Years) | AHD Type | Adjusted for Confounders? | Main Findings |
|---|---|---|---|---|---|---|---|
| Lindman et al. [24] | 2021 | Sweden | 265,172 | ≥50 | Acyclovir, Valacyclovir | Yes (age, sex, comorbidities) | Antiviral treatment reduced AD risk (aHR = 0.89; 95% CI: 0.86–0.92). |
| Linard et al. [19] | 2022 | France | 68,291 | ≥65 | Acyclovir, Valacyclovir | Yes (age, sex, comorbidities) | AHD use reduced AD risk (HR = 0.85; 95% CI: 0.75–0.96). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, A.; Koen, E.; Helmantel, M.; Rafie, K.; Dolga, A.M.; van Munster, B.C.; Hak, E. The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer’s Disease: A Comprehensive Systematic Review. Pharmaceuticals 2025, 18, 722. https://doi.org/10.3390/ph18050722
Alghamdi A, Koen E, Helmantel M, Rafie K, Dolga AM, van Munster BC, Hak E. The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer’s Disease: A Comprehensive Systematic Review. Pharmaceuticals. 2025; 18(5):722. https://doi.org/10.3390/ph18050722
Chicago/Turabian StyleAlghamdi, Ali, Emma Koen, Manon Helmantel, Karim Rafie, Amalia M. Dolga, Barbara C. van Munster, and Eelko Hak. 2025. "The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer’s Disease: A Comprehensive Systematic Review" Pharmaceuticals 18, no. 5: 722. https://doi.org/10.3390/ph18050722
APA StyleAlghamdi, A., Koen, E., Helmantel, M., Rafie, K., Dolga, A. M., van Munster, B. C., & Hak, E. (2025). The Association Between Herpes Zoster, Antiherpetic Therapies, and Alzheimer’s Disease: A Comprehensive Systematic Review. Pharmaceuticals, 18(5), 722. https://doi.org/10.3390/ph18050722







