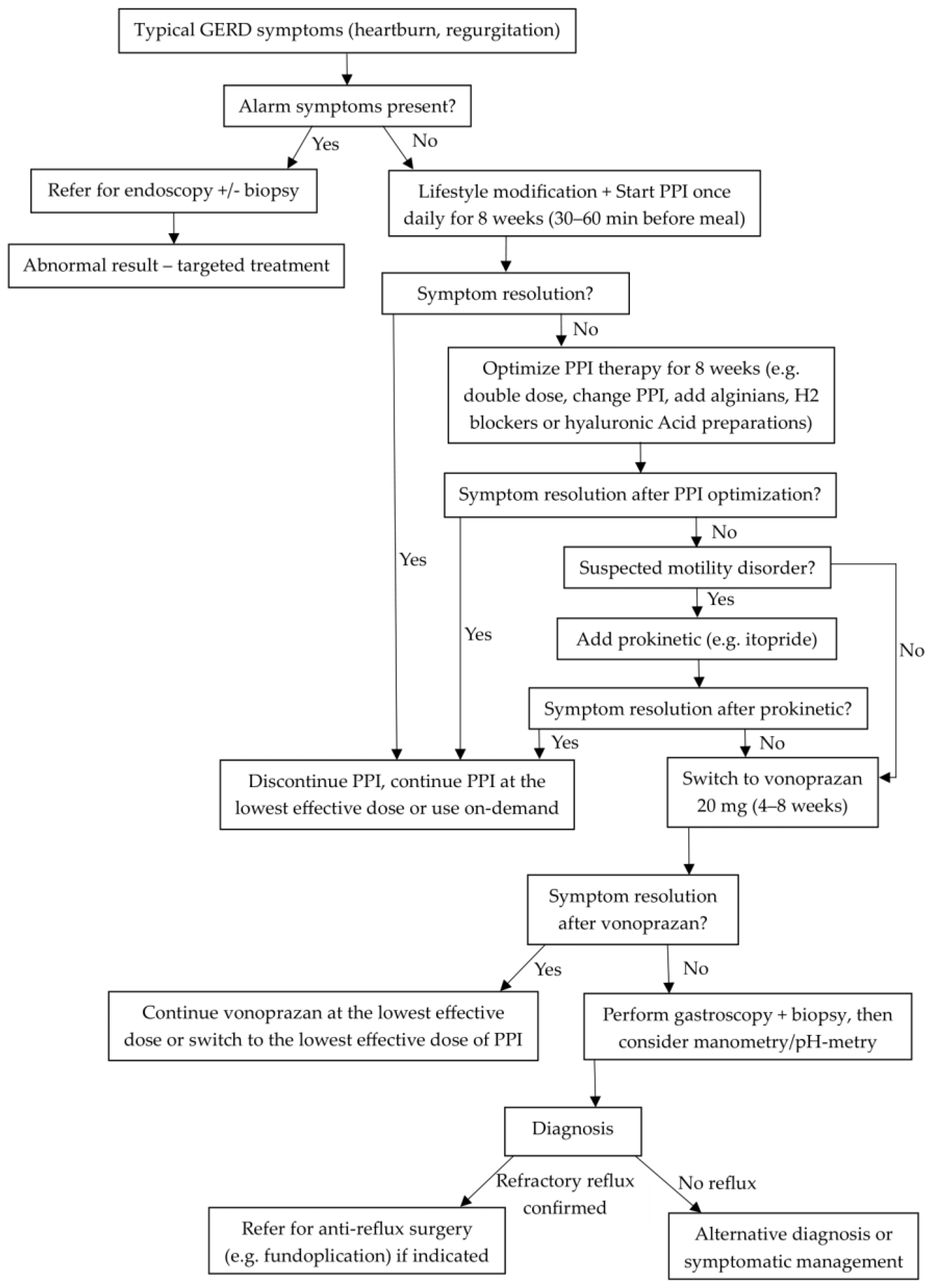
3.4.1. Efficacy in Reflux Esophagitis (RE) Healing
A prospective study by Hoshino et al. examined the efficacy of vonoprazan in treating PPI-resistant reflux esophagitis (RE). The study included 24 patients categorized with the Los Angeles (LA) classification, all of whom were unresponsive to at least 8 weeks of PPI therapy (lansoprazole 30 mg, rabeprazole 10 mg, or esomeprazole 20 mg daily in the morning). These patients, with endoscopically confirmed esophagitis, were treated with 20 mg of vonoprazan daily after breakfast for 4 weeks [
51].
Follow-up endoscopic evaluations after 4 weeks showed that 87.5% of the patients achieved successful healing of the esophageal changes, with the success rates varying by disease severity: 100% for grade A (3/3), 85.7% for grade B (6/7), 90.9% for grade C (10/11), and 66.7% for grade D (2/3). Patients with additional pathologies, such as hiatal hernia or scleroderma, were less likely to achieve complete acid suppression. One patient with both scleroderma and a hiatal hernia (grade C RE) exhibited gastric pH levels below four for 52.2% of the treatment duration, which points out the challenges in acid control in complex cases. However, vonoprazan was highly effective in most patients, especially those without additional complicating factors [
51].
Symptom relief, assessed using the Frequency Scale for Symptoms of Gastroesophageal Reflux Disease (FSSG), showed significant improvement within the first week of treatment and sustained benefits over 28 days (
p < 0.05 for all time points compared to baseline). Following the initial treatment phase, 21 patients were subjected to a maintenance regimen of vonoprazan 10 mg daily after breakfast for 8 weeks. At the end of the maintenance phase, in 76.2% (16/21) of the patients at gastroscopy, no signs of esophagitis were shown, which documents the vonoprazan’s efficacy in both short-term and long-term treatment of PPI-resistant RE [
51].
A smaller prospective study involved eight patients with persistent gastric mucosal injury after an 8-week course of standard PPI therapy (rabeprazole 10 mg/day, esomeprazole 20 mg/day, or lansoprazole 30 mg/day in the morning) and aimed to evaluate the efficacy of vonoprazan in treating gastroesophageal reflux disease (GERD) refractory to proton pump inhibitor (PPI) therapy. All the patients were extensive metabolizers of CYP2C19. Six patients were diagnosed with ineffective esophageal motility, all of whom had a hiatal hernia [
52].
The patients were assessed using high-resolution manometry (HRM) and 24 h MII-pH monitoring while on PPI therapy as baseline measurements. After completing the initial tests, the patients received 20 mg of vonoprazan daily for 4 weeks. Following this treatment period, the participants underwent an upper endoscopy and 24 h MII-pH monitoring. pH was monitored 5 cm above and 10 cm below the proximal edge of the LES [
52].
The result showed that 87.5% (7 out of 8) patients achieved complete mucosal lesions healing, characterized by the resolution of visible lesions, as confirmed by follow-up endoscopy after 4 weeks of vonoprazan treatment. The patient with Los Angeles (LA) grade C esophagitis did not achieve complete mucosal healing after 4 weeks of vonoprazan therapy, with persistent lesions (grade A) remaining. The patient continued vonoprazan therapy at 20 mg daily for an additional 4 weeks, ultimately achieving complete mucosal healing [
52].
Additionally, gastric pH control improved significantly, with the median 24 h gastric pH > 4 HTR increasing from 26.5% during PPI therapy to 78.0% after four weeks of vonoprazan treatment, suggesting superior gastric acid control with vonoprazan compared to PPIs [
52]. Moreover, nocturnal gastric acid breakthrough decreased from 87.5% (7 z 8) of the patients during PPI therapy to 50% after switching to vonoprazan, highlighting superior gastric acid control with vonoprazan compared to PPIs. Additionally, four of the eight patients experienced complete resolution of reflux symptoms (heartburn or regurgitation) after switching to vonoprazan [
52].
It should be emphasized that the doses of proton pump inhibitors (PPIs) used in some studies—such as esomeprazole 20 mg daily—were lower than the standard doses typically recommended for patients with refractory GERD. For optimal acid suppression, increasing the PPI dose (e.g., to esomeprazole 40 mg daily) or switching to a different PPI may be necessary. These factors should be considered when interpreting efficacy comparisons between PPIs and P-CABs such as vonoprazan [
4].
3.4.2. Efficacy in GERD Symptom Control and Long-Term Healing
In a retrospective analysis of 55 GERD patients (30 with non-erosive reflux disease [NERD] and 25 with erosive esophagitis [EE]), the effect of daily oral vonoprazan 10 mg was evaluated over the course of one year. Symptom assessment was performed using the Izumo scale, a validated questionnaire for gastrointestinal symptoms. After one month of treatment, 89% of the patients reported significant improvement in symptoms, including heartburn, regurgitation, throat discomfort, postprandial distress, epigastric pain, constipation, and diarrhea. Additionally, symptom improvement was statistically significant at all time points during the one-year follow-up, as assessed by the Izumo scale (
p < 0.001). Furthermore, 82% of these patients maintained this improvement throughout the entire year of vonoprazan therapy. Among all the patients who used the treatment for one year, 47% (26/55) reported sustained resolution and long-term relief from GERD symptoms. Notably, 95% of the patients with erosive esophagitis had maintained endoscopic healing in a follow-up endoscopy conducted at 12 months [
53].
In a similar retrospective study involving 88 GERD patients treated with 10 mg of vonoprazan daily, 86% experienced symptom improvement, including reductions in heartburn, regurgitation, throat discomfort, epigastric pain, postprandial distress, constipation, and diarrhea, with 57% achieving complete resolution of symptoms within one month. The patients with erosive esophagitis demonstrated a significantly higher rate of symptom resolution (71%) compared to those with non-erosive disease (47%) (
p = 0.025). The observed difference in treatment response may be attributed to the heterogeneous pathophysiology of NERD. NERD encompasses a wide range of underlying mechanisms, including esophageal hypersensitivity, functional heartburn, esophageal motility disorders, and both acid and non-acid reflux. Furthermore, impaired esophageal motility, which leads to delayed acid clearance, may further contribute to the persistence of symptoms despite adequate acid suppression. These factors underscore the necessity for individualized diagnostic approaches and tailored therapeutic strategies before considering the escalation to more aggressive treatment regimens [
54].
According to the results of the multivariate analysis conducted by the authors, older age (≥60 years,
p = 0.002), obesity (BMI ≥ 24,
p = 0.030), and erosive esophagitis (
p = 0.018) were identified as strong predictors of a better response to vonoprazan treatment [
43]. Older patients may have better compliance and reduced gastric sensitivity and/or motility, which contributes to better treatment outcomes [
55]. Obesity is associated with increased gastric acid secretion and higher visceral fat, which stimulate gastrin release and contribute to acid reflux. This increased acid production in obese patients makes their GERD symptoms more responsive to acid suppression therapies, as reducing excess acid directly alleviates their symptoms [
54,
56]. In contrast, alcohol consumption (
p = 0.011) and a history of Helicobacter pylori eradication (
p = 0.015) were found to be negative predictors. Alcohol (>20 g daily) can directly damage the esophageal mucosa, impair motility by slowing normal peristalsis, and lower LES pressure, which decreases the efficacy of acid suppression therapies by making it easier for stomach acid to flow back into the esophagus [
57]. In patients with a history of
Helicobacter pylori eradication, atrophic changes in the gastric lining can reduce acid secretion, further limiting the effectiveness of acid-suppressive therapies [
58].
In a prospective, randomized, double-blind trial involving 32 patients with erosive esophagitis and heartburn occurring at least once a week, patients were randomly assigned to receive either 20 mg of vonoprazan or 30 mg of lansoprazole daily before breakfast for 14 days. The study aimed to assess the onset and duration of symptom relief, with a particular focus on heartburn [
59].
By the first day of treatment, 37.5% (6/16) of the patients treated with vonoprazan experienced complete relief from their heartburn symptoms during the day for the next 7 consecutive days compared to only 18.8% (3/16) of the patients receiving lansoprazole. The difference in the onset of daytime heartburn relief between the two groups was statistically significant (
p < 0.05). The differences in relief were even more pronounced for nocturnal heartburn. On Day 1, 33.3% (5/15) of the patients treated with vonoprazan achieved complete relief from nocturnal heartburn for the following 7 days, whereas only 9.1% (1/11) of the patients receiving lansoprazole reported similar results, with the difference reaching statistical significance (
p < 0.01). These findings underscore vonoprazan’s superior efficacy in alleviating heartburn symptoms, particularly nocturnal symptoms, which are often more difficult to manage with traditional proton pump inhibitors like lansoprazole [
59].
In addition to its faster and more sustained relief of heartburn symptoms, vonoprazan also demonstrated significant improvements in sleep quality compared to lansoprazole. After 14 days of treatment, sleep quality, as assessed by the Pittsburgh Sleep Quality Index (PSQI), showed a significant improvement in patients treated with vonoprazan (
p < 0.05), while no notable change was observed in patients receiving lansoprazole [
59].
In a subsequent, randomized, double-blind, phase III trial, healing rates at 8 weeks were similar between vonoprazan 20 mg (92.4%) and lansoprazole 30 mg (91.3%) among 468 Asian patients with erosive esophagitis. Vonoprazan demonstrated numerically greater early efficacy, with 75.0% healing after 2 weeks compared to 67.8% for lansoprazole. Although these early differences did not reach statistical significance, vonoprazan showed better outcomes, particularly in patients with more severe disease (Los Angeles grades C/D). Importantly, adverse event rates were comparable between the groups (38.1% vs. 36.6%), supporting a similar safety profile [
60].
A prospective, randomized, double-blind controlled trial (RCT) by Laine et al. (2023) also confirmed vonoprazan’s 20 mg superiority in the early phases of treatment for erosive esophagitis [
61]. After 2 weeks, vonoprazan showed significantly better healing rates, defined as endoscopically confirmed resolution of erosive lesions, in patients with grade C/D esophagitis (70.2% for vonoprazan vs. 52.6% for lansoprazole 30 mg;
p = 0.0008) based on a result from 1024 participants. Additionally, heartburn relief in the maintenance phase, was similar between the two groups, with 80.6% of the patients on vonoprazan (20 mg) reporting 24 h heartburn-free days compared to 78.6% in the lansoprazole group (15 mg) based on the findings from 878 patients. The observed difference of 2.0% (95% CI: −2.6 to 6.7) did not reach statistical significance for superiority. However, non-inferiority was established, as the lower bound of the confidence interval was above the predefined margin of −15%.
In a randomized, multicenter clinical trial evaluating on-demand vonoprazan therapy for non-erosive reflux disease (NERD), vonoprazan demonstrated significantly higher efficacy in relieving heartburn compared to placebo. In the groups receiving vonoprazan (10 mg, 20 mg, and 40 mg), a significantly higher percentage of heartburn episodes were completely relieved within 3 h of dosing, with relief lasting for 24 h. Heartburn episodes were measured using daily diaries, where patients recorded the onset and intensity of symptoms. These episodes were then assessed for complete relief within 3 h and sustained relief for 24 h. The on-demand therapy lasted for 6 weeks, during which the patients took vonoprazan only when experiencing heartburn symptoms: with 10 mg vonoprazan, 56% of the heartburn episodes were fully relieved, with 20 mg—60.6%, and with 40 mg—70%. In contrast, only 27.3% of the heartburn episodes were completely relieved in the placebo group (
p < 0.0001). Vonoprazan also demonstrated significantly higher efficacy within the first hour post-dose compared to the placebo (
p < 0.0001) [
62].
A 2024 meta-analysis by Zhuang et al. compared the efficacy and safety of vonoprazan and proton pump inhibitors (PPIs) in treating Los Angeles grade C/D esophagitis [
63]. The analysis included 24 randomized controlled trials (RCTs) comparing three P-CABs (vonoprazan, tegoprazan, and keverprazan) and six PPIs (lansoprazole, esomeprazole, omeprazole, rabeprazole extended-release, pantoprazole, and dexlansoprazole). Surface Under the Cumulative Ranking Curve (SUCRA) scores were used to assess the relative efficacy of these treatments. SUCRA is a statistical measure used in meta-analysis to rank interventions based on their likelihood of being the most effective. A higher SUCRA score indicates a greater likelihood of a treatment being ranked as the best option [
63].
For initial treatment, vonoprazan 20 mg/day demonstrated a significantly lower risk of treatment failure compared to 30 mg/day of lansoprazole (RR = 2.72, 95% CI [1.10–6.73],
p = 0.03) and 20 mg/day of omeprazole (RR = 3.51, 95% CrI [1.31–12.14]). Significant superiority was observed specifically against lansoprazole and omeprazole, while comparisons with other PPIs did not show significant differences. Vonoprazan 20 mg/day reduced the absolute risk of treatment failure by 11–21% compared to PPIs, with treatment failure rates of 6% for vonoprazan versus 21% for overall PPIs. The ranking analysis confirmed that vonoprazan 20 mg q.d. (SUCRA = 0.89) was the top-performing treatment for grade C/D esophagitis, far outpacing all the PPI and P-CAB options. For instance, kaverprazan 20 mg/day ranked second with a SUCRA score of 0.87, followed by dexlansoprazole 60 mg/day (SUCRA = 0.66) and esomeprazole 40 mg/day (SUCRA = 0.61). For maintenance treatment, direct comparisons showed the superiority of vonoprazan 20 mg/day over lansoprazole 15 mg/day (RR = 8.39, 95% CI [2.06–34.24],
p = 0.001), but pooled network analysis did not demonstrate significant differences between vonoprazan and other PPIs. Nevertheless, vonoprazan 20 mg/day reduced the absolute risk of treatment failure by 4–18% compared to PPIs, with an estimated treatment failure rate of 16% for vonoprazan compared to 30% for other PPIs [
63].
Vonoprazan exhibited a favorable safety profile, with similar rates of adverse events (AEs), serious adverse events (SAEs), and drug withdrawal compared to PPIs. In short-term safety rankings, vonoprazan 20 mg q.d. (SUCRA = 0.59) performed better than 40 mg of esomeprazole q.d. (SUCRA = 0.42) and 60 mg of dexlansoprazole q.d. (SUCRA = 0.52), further confirming its relative safety in comparison to maximized-dose PPIs [
63].
Considering both efficacy and safety outcomes, 20 mg of vonoprazan q.d. demonstrated superior effectiveness and was relatively safe in the initial treatment of grade C/D esophagitis [
63].
In the 5-year VISION study, which aimed to evaluate the long-term efficacy and safety of vonoprazan, the results demonstrated that vonoprazan provided superior long-term control of recurrent erosive esophagitis (EE) compared to lansoprazole. The cumulative recurrence rate over 260 weeks was 10.8% (95% CI, 6.4–18.0%) for vonoprazan and 38.0% (95% CI, 25.5–54.1%) for lansoprazole (
p = 0.001). While initial healing rates were similar (96.4% for vonoprazan vs. 97.1% for lansoprazole,
p = 1.00), vonoprazan showed significantly better prevention of recurrence during long-term maintenance therapy [
64].
Although lifestyle modifications such as avoiding meals 2–3 h before bedtime, elevating the head of the bed, and weight reduction in overweight or obese patients are recommended for GERD management, these strategies were not consistently evaluated in most studies reviewed [
2]. Some studies did include fixed mealtimes and postural modifications, but these were not the primary focus [
52]. Additionally, reducing alcohol consumption and quitting smoking may also benefit patients with GERD. The limited reporting of non-pharmacologic strategies may restrict the interpretation of treatment resistance, highlighting the need for more comprehensive assessment in future studies [
2,
4].
Table 2,
Table 3 and
Table 4 summarize the clinical efficacy, patient outcomes, and meta-analytical findings regarding vonoprazan use in GERD management.