Advances and Challenges in the Development of New and Novel Treatment Strategies for Eosinophilic Esophagitis (EoE)
Abstract
1. Introduction
2. Literature Search Strategy
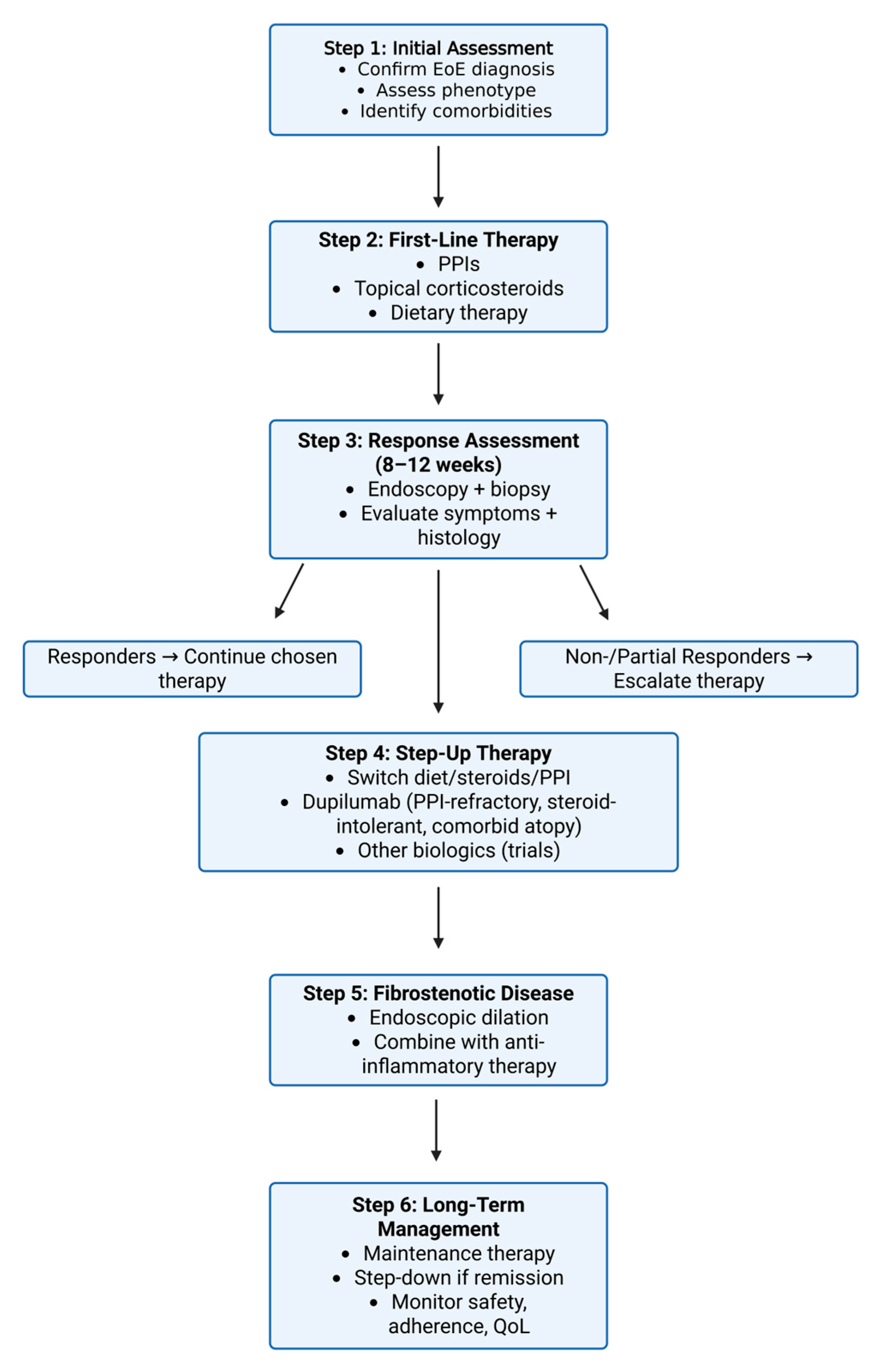
3. Current Therapeutic Approaches
3.1. Dietary Modifications
3.2. Proton Pump Inhibitors
3.3. Corticosteroids
3.4. Anti IL-4/IL-13 Agent
3.5. Anti IL-13 Agents
3.6. Anti IL-5 Agents
3.7. Anti-Siglec, Anti-S1P, Anti-c-KIT
4. Drugs Acting on the Thymic Stromal Lymphopoietin (TSLP)
4.1. Small Molecules
4.2. Microbiome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonsalves, N.P.; Aceves, S.S. Diagnosis and treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2020, 145, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.W.; Lee, K.; Shin, J.I.; Cho, S.H.; Turner, S.; Shin, J.U.; Yeniova, A.Ö.; Koyanagi, A.; Jacob, L.; Smith, L.; et al. Global incidence and prevalence of eosinophilic esophagitis, 1976-2022: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 3270–3284.e77. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, D.; Marella, S.; Idelman, G.; Chang, J.W.; Chehade, M.; Hogan, S.P. Eosinophilic esophagitis: Immune mechanisms and therapeutic targets. Clin. Exp. Allergy 2022, 52, 1142–1156. [Google Scholar] [CrossRef]
- Muir, A.; Falk, G.W. Eosinophilic esophagitis: A review. JAMA 2021, 326, 1310–1318. [Google Scholar] [CrossRef]
- Kottyan, L.C.; Parameswaran, S.; Weirauch, M.T.; Rothenberg, M.E.; Martin, L.J. The Genetic Etiology of Eosinophilic Esophagitis. J. Allergy Clin. Immunol. 2020, 145, 9. [Google Scholar] [CrossRef]
- Furuta, G.T.; Katzka, D.A. Eosinophilic esophagitis. N. Engl. J. Med. 2015, 373, 1640–1648. [Google Scholar] [CrossRef]
- Chen, P.; Anderson, L.; Zhang, K.; Weiss, G.A. Eosinophilic gastritis/gastroenteritis. Curr. Gastroenterol. Rep. 2021, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Bredenoord, A.J.; Patel, K.; Schoepfer, A.M.; Dellon, E.S.; Chehade, M.; Aceves, S.S.; Spergel, J.M.; Shumel, B.; Deniz, Y.; Rowe, P.J.; et al. Disease burden and unmet need in eosinophilic esophagitis. Am. J. Gastroenterol. 2022, 117, 1231–1241. [Google Scholar] [CrossRef]
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Amil Dias, J.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358. [Google Scholar] [CrossRef]
- Fernandez-Becker, N.Q. Eosinophilic esophagitis: Incidence, diagnosis, management, and future directions. Gastroenterol. Clin. North Am. 2021, 50, 825–841. [Google Scholar] [CrossRef]
- de Bortoli, N.; Visaggi, P.; Penagini, R.; Annibale, B.; Svizzero, F.B.; Barbara, G.; Bartolo, O.; Battaglia, E.; Di Sabatino, A.; De Angelis, P.; et al. The 1st EoETALY consensus on the diagnosis and management of eosinophilic esophagitis—Definition, clinical presentation and diagnosis. Dig. Liver Dis. 2024, 56, 951–963. [Google Scholar] [CrossRef]
- Dhar, A.; Haboubi, H.N.; Attwood, S.E.; Auth, M.K.H.; Dunn, J.M.; Sweis, R.; Morris, D.; Epstein, J.; Novelli, M.R.; Hunter, H.; et al. British Society of Gastroenterology [BSG] and British Society of Paediatric Gastroenterology, Hepatology and Nutrition [BSPGHAN] joint consensus guidelines on the diagnosis and management of eosinophilic oesophagitis in children and adults. Gut 2022, 71, 1459–1487. [Google Scholar] [CrossRef]
- Dellon, E.S.; Khoury, P.; Muir, A.B.; Liacouras, C.A.; Safroneeva, E.; Atkins, D.; Collins, M.H.; Gonsalves, N.; Falk, G.W.; Spergel, J.M.; et al. A Clinical Severity Index for Eosinophilic Esophagitis: Development, Consensus, and Future Directions. Gastroenterology 2022, 163, 59–76. [Google Scholar] [CrossRef]
- Menard-Katcher, C.; Aceves, S. Pathophysiology and Clinical Impact of Esophageal Remodeling and Fibrosis in Eosinophilic Esophagitis. Immunol. Allergy Clin. North Am. 2024, 44, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, C.A.; Farha, N.; Hermanns, C.; Maruggi, C.; Falloon, K.; Thanawala, S.; Harnegie, M.P.; Brown, J.M.; Ivanov, A.I.; Dellon, E.S.; et al. Systematic Review: Variability in Definitions of Fibrostenosis in Eosinophilic Oesophagitis. Aliment. Pharmacol. Ther. 2025, 62, 277–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safroneeva, E.; Straumann, A.; Schoepfer, A.M. Latest insights on the relationship between symptoms and biologic findings in adults with eosinophilic esophagitis. Gastrointest. Endosc. Clin. N. Am. 2018, 28, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.C.; Thakkar, K.P.; Ketchem, C.J.; Eluri, S.; Reed, C.C.; Dellon, E.S. A gap in care leads to progression of fibrosis in eosinophilic esophagitis patients. Clin. Gastroenterol. Hepatol. 2022, 20, 1701–1708. [Google Scholar] [CrossRef]
- Schoepfer, A.; Safroneeva, E.; Straumann, A. Eosinophilic esophagitis: Impact of latest insights into pathophysiology on therapeutic strategies. Dig. Dis. 2016, 34, 462–468. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Dellon, E.S.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Sun, X.; Hamilton, J.D.; et al. Efficacy and safety of dupilumab up to 52 weeks in adults and adolescents with eosinophilic oesophagitis [LIBERTY EoE TREET study]: A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 990–1004. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Morey, R.; Desai, N.K.; Lan, L.; Williams, J.; Dellon, E.S. Budesonide oral suspension improves outcomes in patients with eosinophilic esophagitis: Results from a phase 3 trial. Clin. Gastroenterol. Hepatol. 2022, 20, 525–534.e10. [Google Scholar] [CrossRef]
- Vincenzo Savarino, E.; Fassan, M.; de Bortoli, N.; Romano, C.; Di Sabatino, A.; Penagini, R.; Racca, F.; Sarnelli, G.; Oliva, S. Italian EoExpert panel recommendation for disease control, switching criteria, and follow-up in eosinophilic esophagitis from pediatric to adult age. Ther. Adv. Gastroenterol. 2025, 18, 17562848251337515. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirano, I.; Chan, E.S.; Rank, M.A.; Sharaf, R.N.; Stollman, N.H.; Stukus, D.R.; Wang, K.; Greenhawt, M.; Falck-Ytter, Y.T.; Chachu, K.A.; et al. AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis. Gastroenterology 2020, 158, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Franciosi, J.P.; Gordon, M.; Sinopoulou, V.; Dellon, E.S.; Gupta, S.K.; Reed, C.C.; Gutiérrez-Junquera, C.; Venkatesh, R.D.; Erwin, E.A.; Egiz, A.; et al. Medical treatment of eosinophilic esophagitis. Cochrane Database Syst. Rev. 2023, 7, CD004065. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, C.; Kavallar, A.M.; Aldrian, D.; Lindner, A.K.; Müller, T.; Vogel, G.F. Efficacy of Elimination Diets in Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 2197–2210.e3. [Google Scholar] [CrossRef] [PubMed]
- Molina-Infante, J.; Modolell, I.; Alcedo, J.; Garcia-Romero, R.; Casabona-Frances, S.; Prieto-Garcia, A.; Modolell, I.; Gonzalez-Cordero, P.L.; Perez-Martinez, I.; Martin-Lorente, J.L.; et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2-4-6 study. J. Allergy Clin. Immunol. 2018, 141, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, K.L.; Gonsalves, N.; Dellon, E.S.; Katzka, D.A.; Abonia, J.P.; Aceves, S.S.; Arva, N.C.; Besse, J.A.; Bonis, P.A.; Caldwell, J.M.; et al. One-food versus six-food elimination diet therapy for the treatment of eosinophilic oesophagitis: A multicentre, randomised, open-label trial. Lancet Gastroenterol. Hepatol. 2023, 8, 408–421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rank, M.A.; Sharaf, R.N.; Furuta, G.T.; Aceves, S.S.; Greenhawt, M.; Spergel, J.M.; Falck-Ytter, Y.T.; Dellon, E.S.; AGA Institute; Joint Task Force on Allergy-Immunology Practice Parameters collaborators. Technical Review on the Management of Eosinophilic Esophagitis: A Report from the AGA Institute and the Joint Task Force on Allergy-Immunology Practice Parameters. Gastroenterology 2020, 158, 1789–1810.e15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nasser, J.; Mehravar, S.; Pimentel, M.; Lim, J.; Mathur, R.; Boustany, A.; Rezaie, A. Elemental Diet as a Therapeutic Modality: A Comprehensive Review. Dig. Dis. Sci. 2024, 69, 3344–3360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barni, S.; Arasi, S.; Mastrorilli, C.; Pecoraro, L.; Giovannini, M.; Mori, F.; Liotti, L.; Saretta, F.; Castagnoli, R.; Caminiti, L.; et al. Pediatric eosinophilic esophagitis: A review for the clinician. Ital. J. Pediatr. 2021, 47, 230. [Google Scholar] [CrossRef]
- Rodríguez-Alcolado, L.; Navarro, P.; Arias-González, L.; Grueso-Navarro, E.; Lucendo, A.J.; Laserna-Mendieta, E.J. Proton-Pump Inhibitors in Eosinophilic Esophagitis: A Review Focused on the Role of Pharmacogenetics. Pharmaceutics 2024, 16, 487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Min, S.; Chang, J.W. From Past to Present: The Evolution of Pharmacologic Therapies for Eosinophilic Esophagitis. Gastroenterol. Hepatol. 2025, 21, 298–303. [Google Scholar] [PubMed] [PubMed Central]
- Laserna-Mendieta, E.J.; Casabona, S.; Guagnozzi, D.; Savarino, E.; Perelló, A.; Guardiola-Arévalo, A.; Barrio, J.; Pérez-Martínez, I.; Krarup, A.L.; Alcedo, J.; et al. Efficacy of proton pump inhibitor therapy for eosinophilic oesophagitis in 630 patients: Results from the EoE connect registry. Aliment. Pharmacol. Ther. 2020, 52, 798–807. [Google Scholar] [CrossRef]
- Jensen, E.T.; Huang, K.Z.; Chen, H.X.; Landes, L.E.; McConnell, K.A.; Almond, M.A.; Safta, A.M.; Johnston, D.T.; Durban, R.; Jobe, L.; et al. Longitudinal growth outcomes following first line treatment for pediatric eosinophilic esophagitis patients HHS Public Access. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 50–55. [Google Scholar] [CrossRef]
- Cameron, B.A.; Xue, A.Z.; Kiran, A.; LaFata, S.; Ocampo, A.A.; McCallen, J.; Lee, C.J.; Borinsky, S.A.; Redd, W.D.; Cotton, C.C.; et al. Esophageal Candidiasis Is Strongly Associated with Treatment Response to Topical Steroids in Eosinophilic Esophagitis and Could Be a Marker of Adherence. Gastro Hep Adv. 2024, 3, 612–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellon, E.S.; Woosley, J.T.; Arrington, A.; McGee, S.J.; Covington, J.; Moist, S.E.; Gebhart, J.H.; Tylicki, A.E.; Shoyoye, S.O.; Martin, C.F.; et al. Efficacy of Budesonide vs Fluticasone for Initial Treatment of Eosinophilic Esophagitis in a Randomized Controlled Trial. Gastroenterology 2019, 157, 65–73.e5. [Google Scholar] [CrossRef]
- Alsohaibani, F.I.; Peedikayil, M.C.; Alzahrani, M.A.; Azzam, N.A.; Almadi, M.A.; Dellon, E.S.; Al-Hussaini, A.A. Eosinophilic esophagitis: Current concepts in diagnosis and management. Saudi J. Gastroenterol. 2024, 30, 210–227. [Google Scholar] [CrossRef]
- Takeda Pharmaceuticals America, Inc. EOHILIA [Budesonide Oral Suspension] Prescribing Information. US Food and Drug Administration. 2024. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/213976s000lbl.pdf (accessed on 22 February 2024).
- Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Schlag, C.; Biedermann, L.; Vaquero, C.S.; Ciriza de Los Rios, C.; Schmoecker, C.; Madisch, A.; et al. International EOS-2 Study Group. Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo-Controlled Trial of Patients with Eosinophilic Esophagitis. Gastroenterology 2020, 159, 1672–1685.e5. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, L.; Schlag, C.; Straumann, A.; Lucendo, A.J.; Miehlke, S.; Vieth, M.; Santander, C.; Ciriza de Los Rios, C.; Schmöcker, C.; Madisch, A.; et al. Efficacy and Safety of Budesonide Orodispersible Tablets for Eosinophilic Esophagitis up to 3 Years: An Open-Label Extension Study. Clin. Gastroenterol. Hepatol. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Barwick, E.; Perananthan, V.; Parkash, N.; Lucas, S.; Chauhan, A.; Yu, C.; Tan, K.; Sepe, G.; Suleiman, M.; et al. Research Communication: Real-World Effectiveness of Budesonide Orodispersible Tablets for Eosinophilic Oesophagitis: The Importance of Patience and Education. Aliment. Pharmacol. Ther. 2025, 62, 204–207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellon, E.S.; Collins, M.H.; Katzka, D.A.; Mukkada, V.A.; Falk, G.W.; Zhang, W.; Goodwin, B.; Terreri, B.; Boules, M.; Desai, N.K.; et al. Effect of randomized treatment withdrawal of budesonide oral suspension on clinically relevant efficacy outcomes in patients with eosinophilic esophagitis: A post hoc analysis. Ther. Adv. Gastroenterol. 2024, 17, 17562848241307602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lancho Muñoz, A.; López Tobaruela, J.M.; Iglesias Conejero, P.; Redondo Cerezo, E. Successful management of eosinophilic esophagitis with mometasone: An unusual therapeutic approach. Rev. Esp. Enferm. Dig. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves First Treatment for Eosinophilic Esophagitis, a Chronic Immune Disorder. Available online: https://www.fda.gov/media/161413/download (accessed on 7 September 2023).
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Aceves, S.S.; Dellon, E.S.; Greenhawt, M.; Hirano, I.; Liacouras, C.A.; Spergel, J.M. Clinical guidance for the use of dupilumab in eosinophilic esophagitis: A yardstick. Ann. Allergy Asthma Immunol. 2023, 130, 371–378. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef]
- Bowyer, K.; Swisher, A.R.; Jiang, N.; Liang, J. Dupilumab adverse reactions in eosinophilic esophagitis treatment: A Food and Drug Administration Adverse Event Reporting System database analysis. Dis. Esophagus 2025, 38, doaf055. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Rind, D.; Chapman, R.; Kumar, V.; Kahn, S.; Carlson, J. Economic evaluation of dupilumab for moderate-to-severe atopic dermatitis: A cost-utility analysis. J. Drugs Dermatol. 2018, 17, 750–756. [Google Scholar]
- Rossi, C.M.; Santacroce, G.; Lenti, M.V.; di Sabatino, A. Eosinophilic esophagitis in the era of biologics. Expert Rev. Gastroenterol. Hepatol. 2024, 18, 271–281. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.P.; Lee, J.T. Insights into the Implications of Coexisting Type 2 Inflammatory Diseases. J. Inflamm. Res. 2021, 14, 4259–4266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellon, E.S.; Rothenberg, M.E.; Collins, M.H.; Hirano, I.; Chehade, M.; Bredenoord, A.J.; Lucendo, A.J.; Spergel, J.M.; Aceves, S.; Sun, X.; et al. Dupilumab in adults and adolescents with eosinophilic esophagitis. N. Engl. J. Med. 2022, 387, 2317–2330. [Google Scholar] [CrossRef]
- Hirano, I.; Collins, M.H.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.M.; Straumann, A.; Safroneeva, E.; Grimm, M.; Smith, H.; et al. RPC4046, a monoclonal antibody against IL13, reduces histologic and endoscopic activity in patients with eosinophilic esophagitis. Gastroenterology 2019, 156, 592–603.e10. [Google Scholar] [CrossRef]
- Dellon, E.S.; Collins, M.H.; Rothenberg, M.E.; Assouline-Dayan, Y.; Evans, L.; Gupta, S.; Schoepfer, A.; Straumann, A.; Safroneeva, E.; Rodriguez, C.; et al. Long-term efficacy and tolerability of RPC4046 in an open-label extension trial of patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2021, 19, 473–483.e1. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Wen, T.; Greenberg, A.; Alpan, O.; Enav, B.; Hirano, I.; Nadeau, K.; Kaiser, S.; Peters, T.; Perez, A.; et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol. 2015, 135, 500–507. [Google Scholar] [CrossRef]
- Hillas, G.; Fouka, E.; Papaioannou, A.I. Antibodies targeting the interleukin-5 signaling pathway used as add-on therapy for patients with severe eosinophilic asthma: A review of the mechanism of action, efficacy, and safety of the subcutaneously administered agents, mepolizumab and benralizumab. Expert. Rev. Respir. Med. 2020, 14, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Straumann, A.; Conus, S.; Grzonka, P.; Kita, H.; Kephart, G.; Bussmann, C.; Beglinger, C.; Smith, D.A.; Patel, J.; Byrne, M.; et al. Anti-interleukin-5 antibody treatment [mepolizumab] in active eosinophilic oesophagitis: A randomised, placebo-controlled, double-blind trial. Gut 2010, 59, 21–30. [Google Scholar] [CrossRef]
- Spergel, J.M.; Rothenberg, M.E.; Collins, M.H.; Furuta, G.T.; Markowitz, J.E.; Fuchs, G.; O’gOrman, M.A.; Abonia, J.P.; Young, J.; Henkel, T.; et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2012, 129, 456–463.e3. [Google Scholar] [CrossRef]
- Stein, M.L.; Collins, M.H.; Villanueva, J.M.; Kushner, J.P.; Putnam, P.E.; Buckmeier, B.K.; Filipovich, A.H.; Assa’ad, A.H.; Rothenberg, M.E. Anti-IL-5 [mepolizumab] therapy for eosinophilic esophagitis. J. Allergy Clin. Immunol. 2006, 118, 1312–1319. [Google Scholar] [CrossRef]
- Ridolo, E.; Barone, A.; Ottoni, M.; Peveri, S.; Montagni, M.; Nicoletta, F. The new therapeutic frontiers in the treatment of eosinophilic esophagitis: Biological drugs. Int. J. Mol. Sci. 2024, 25, 1702. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Dellon, E.S.; Collins, M.H.; Bredenoord, A.J.; Hirano, I.; Peterson, K.A.; Brooks, L.; Caldwell, J.M.; Fjällbrant, H.; Grindebacke, H.; et al. Eosinophil Depletion with Benralizumab for Eosinophilic Esophagitis. N. Engl. J. Med. 2024, 390, 2252–2263. [Google Scholar] [CrossRef]
- Pyne, A.L.; Uchida, A.M.; Hazel, M.W.; Stubben, C.J.; Chang, J.W.; Bailey, D.D.; Gonsalves, N.; Allen-Brady, K.; Peterson, K.A.; Pletneva, M.A. Effect of benralizumab on histopathology and inflammatory signatures in a clinical cohort of eosinophilic esophagitis. Dis. Esophagus 2025, 38, doae031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korver, W.; Wong, A.; Gebremeskel, S.; Negri, G.L.; Schanin, J.; Chang, K.; Leung, J.; Benet, Z.; Luu, T.; Brock, E.C.; et al. The Inhibitory Receptor Siglec-8 Interacts with FcεRI and Globally Inhibits Intracellular Signaling in Primary Mast Cells Upon Activation. Front. Immunol. 2022, 13, 833728. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dellon, E.S.; Chehade, M.; Genta, R.M.; Leiman, D.A.; Peterson, K.A.; Spergel, J.; Wechsler, J.; Bortey, E.; Chang, A.T.; Hirano, I. Results from KRYPTOS, a phase 2/3 study of lirentelimab [AK002] in adults and adolescents with EoE [S4496]. Am. J. Gastroenterol. 2022, 117, E316–E317. [Google Scholar] [CrossRef]
- Study Details|A Study of CDX-0159 in Patients with Eosinophilic Esophagitis. Available online: https://www.clinicaltrials.gov/study/NCT05774184 (accessed on 27 June 2025).
- Sandborn, W.J.; Peyrin-Biroulet, L.; Zhang, J.; Chiorean, M.; Vermeire, S.; Lee, S.D.; Kühbacher, T.; Yacyshyn, B.; Cabell, C.H.; Naik, S.U.; et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology 2020, 158, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Dellon, E.S.; Collins, M.H.; Bredenoord, A.J.; Philpott, H.; Biedermann, L.; Dulcine, M.; Nguyen-Cleary, T.; Su, C.; Yu, J.; Tan, H.; et al. Etrasimod as a treatment for eosinophilic oesophagitis [VOYAGE]: A double-blind, placebo-controlled, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2025, 10, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, M.; Kabata, H.; Irie, M.; Fukunaga, K. Current summary of clinical studies on anti-TSLP antibody, Tezepelumab, in asthma. Allergol. Int. 2023, 72, 24–30. [Google Scholar] [CrossRef]
- Rochman, Y.; Kotliar, M.; Ben-Baruch Morgenstern, N.; Barski, A.; Wen, T.; Rothenberg, M.E. TSLP shapes the pathogenic responses of memory CD4+ T cells in eosinophilic esophagitis. Sci. Signal. 2023, 16, eadg6360. [Google Scholar] [CrossRef]
- Hoy, S.M. Tezepelumab: First approval. Drugs 2022, 82, 461–468. [Google Scholar] [CrossRef] [PubMed]
- NL-OMON53940. [N.D.]. A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled Phase 3 Efficacy and Safety Study of Tezepelumab in Patients with Eosinophilic Esophagitis [CROSSING]. Available online: https://Trialsearch.Who.Int/Trial2.Aspx?TrialID=NL-OMON53940 (accessed on 1 August 2023).
- Hill, D.A.; Grundmeier, R.W.; Ramos, M.; Spergel, J.M. Eosinophilic esophagitis is a late manifestation of the allergic March. J. Allergy Clin. Immunol. Pract. 2018, 6, 1528–1533. [Google Scholar] [CrossRef]
- Virchow, J.C. Eosinophilic esophagitis: Asthma of the Esophagus? Dig. Dis. 2014, 32, 54–60. [Google Scholar] [CrossRef]
- Soendergaard, M.B.; Håkansson, K.E.J.; Hansen, S.; Bjerrum, A.S.; Schmid, J.M.; Johansson, S.L.; Rasmussen, L.M.; Bonnesen, B.; Vijdea, R.; von Bülow, A.; et al. Biologic Prescription Patterns and Biomarker Determinants of First-Line Dupilumab in Danish Adult Patients with Severe Asthma and Comorbid Atopic Dermatitis. Clin. Exp. Allergy 2025. [Google Scholar] [CrossRef] [PubMed]
- Altman, M.C.; Janczyk, T.; Murphy, R.C.; Jayavelu, N.D.; Calatroni, A.; Kattan, M.; Gill, M.A.; Stokes, J.; Liu, A.H.; Khurana Hershey, G.K.; et al. Inner City Asthma Consortium and Childhood Asthma in Urban Settings Network. Inflammatory Pathways in Residual Asthma Exacerbations Among Mepolizumab-Treated Urban Children: A Secondary Analysis of a Randomized Clinical Trial. JAMA Pediatr. 2025, 179, 957–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buendía, J.A.; Zuluaga, A.F. Exploratory analysis of the economically justifiable price of reslizumab for asthma severe in Colombia. BMC Public. Health 2025, 25, 2116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tripp, C.S.; Cuff, C.; Campbell, A.L.; Hendrickson, B.A.; Voss, J.; Melim, T.; Wu, C.; Cherniack, A.D.; Kim, K. RPC4046, A Novel Anti-interleukin-13 Antibody, Blocks IL-13 Binding to IL-13 α1 and α2 Receptors: A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation First-in-Human Study. Adv. Ther. 2017, 34, 1364–1381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moraes Dos Santos, F.; Rodríguez Martínez, C.; Giovini, V.; Espinoza, M.A.; Balmaceda, C.; Romero, J. Cost-effectiveness of mepolizumab vs anti-interleukin-5/5r biologic therapies for the treatment of adults with severe asthma with an eosinophilic phenotype: A Chilean healthcare system perspective. J. Med. Econ. 2025, 28, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Sánchez Suárez, M.; Martín Roldan, A.; Rojo-Tolosa, S.; Jiménez-Morales, A.; Morales-García, C. Factors influencing the real-world effectiveness of benralizumab in uncontrolled asthma. Sci. Rep. 2025, 15, 21777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pérez-Codesido, S.; Allali, D.; Seebach, J.; Harr, T. Allergologie: Ce qui a changé en 2022 [Allergology: What’s new in 2022]. Rev. Med. Suisse 2023, 19, 196–198. (In French) [Google Scholar] [CrossRef] [PubMed]
- Lugogo, N.L.; Akuthota, P.; Sumino, K.; Mathur, S.K.; Burnette, A.F.; Lindsley, A.W.; Llanos, J.P.; Marchese, C.; Ambrose, C.S.; Emmanuel, B. Effectiveness and Safety of Tezepelumab in a Diverse Population of US Patients with Severe Asthma: Initial Results of the PASSAGE Study. Adv. Ther. 2025, 42, 3334–3353, Erratum in Adv. Ther. 2025, 42, 4724–4725. https://doi.org/10.1007/s12325-025-03316-2. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, W.; Gao, Y.; Zhang, B.; Zuo, W. Off-Label Use of Monoclonal Antibodies for Eosinophilic Esophagitis in Humans: A Scoping Review. Biomedicines 2024, 12, 2576. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Straumann, A.; Hoesli, S.; Bussmann, C.; Stuck, M.; Perkins, M.; Collins, L.P.; Payton, M.; Pettipher, R.; Hunter, M.; Steiner, J.; et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 2013, 68, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Attwood, S.E.A.; Lewis, C.J.; Bronder, C.S.; Morris, C.D.; Armstrong, G.R.; Whittam, J. Eosinophilic oesophagitis: A novel treatment using Montelukast. Gut 2003, 52, 181–185. [Google Scholar] [CrossRef]
- Alexander, J.A.; Ravi, K.; Enders, F.T.; Geno, D.M.; Kryzer, L.A.; Mara, K.C.; Smyrk, T.C.; Katzka, D.A. Montelukast does not maintain symptom remission after topical steroid therapy for eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2017, 15, 214–221.e2. [Google Scholar] [CrossRef]
- Taherali, F.; Taub, M.; Varum, F.; Bravo, R.; Basit, A.W. Investigating accumulation of budesonide and tacrolimus in an ex vivo porcine oesophageal model: Translational potential for local application of drugs to treat eosinophilic oesophagitis. Eur. J. Pharm. Sci. 2025, 209, 107086. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. ENTYVIO [Vedolizumab] for Injection, for Intravenous Use. 2014. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125476s025s030lbl.pdf (accessed on 10 November 2022).
- Nhu, Q.M.; Chiao, H.; Moawad, F.J.; Bao, F.; Konijeti, G.G. The anti-α4β7 integrin therapeutic antibody for inflammatory bowel disease, vedolizumab, ameliorates eosinophilic esophagitis: A novel clinical observation. Am. J. Gastroenterol. 2018, 113, 1261–1263. [Google Scholar] [CrossRef] [PubMed]
- Taft, T.H.; Mutlu, E.A. The potential role of vedolizumab in concomitant eosinophilic esophagitis and Crohn’s disease. Clin. Gastroenterol. Hepatol. 2018, 16, 1840–1841. [Google Scholar] [CrossRef]
- Zuo, W.; Sun, Y.; Liu, R.; Du, L.; Yang, N.; Sun, W.; Wang, P.; Tang, X.; Liu, Y.; Ma, Y.; et al. Management guideline for the off-label use of medicine in China [2021] Expert. Rev. Clin. Pharmacol. 2022, 15, 1253–1268. [Google Scholar] [CrossRef]
- Abonia, J.P.; Rudman Spergel, A.K.; Hirano, I.; Shoda, T.; Zhang, X.; Martin, L.J.; Mukkada, V.A.; Putnam, P.E.; Blacklidge, M.; Neilson, D.; et al. Consortium of Eosinophilic Gastrointestinal Disease Researchers. Losartan Treatment Reduces Esophageal Eosinophilic Inflammation in a Subset of Eosinophilic Esophagitis. J. Allergy Clin. Immunol. Pr. 2024, 12, 2427–2438.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, M.; Wang, J.X.; Zhou, Y.; Kennedy, K.V.; Teranishi, R.; Itami, T.; Ishikawa, S.; Hara, T.; Winters, H.; McMillan, E.A.; et al. FOXM1 Modulation Alleviates Epithelial Remodeling and Inflammation in Eosinophilic Esophagitis. bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Barchi, A.; Massimino, L.; Mandarino, F.V.; Yacoub, M.R.; Albarello, L.; Savarino, E.V.; Ungaro, F.; Danese, S.; Passaretti, S.; Bredenoord, A.J.; et al. Clinical, Histologic, and Safety Outcomes with Long-term Maintenance Therapies for Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Kaymak, T.; Kaya, B.; Wuggenig, P.; Nuciforo, S.; Göldi, A.; Swiss EoE Cohort Study Group (SEECS); Oswald, F.; Roux, J.; Noti, M.; Melhem, H.; et al. IL-20 subfamily cytokines impair the oesophageal epithelial barrier by diminishing filaggrin in eosinophilic oesophagitis. Gut 2023, 72, 821–833. [Google Scholar] [CrossRef]
- Angerami Almeida, K.; de Queiroz Andrade, E.; Burns, G.; Hoedt, E.C.; Mattes, J.; Keely, S.; and Collison, A. The microbiota in eosinophilic esophagitis: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Massimino, L.; Barchi, A.; Mandarino, F.V.; Spanò, S.; Lamparelli, L.A.; Vespa, E.; Passaretti, S.; Peyrin-Biroulet, L.; Savarino, E.V.; Jairath, V.; et al. A multi-omic analysis reveals the esophageal dysbiosis as the predominant trait of eosinophilic esophagitis. J. Transl. Med. 2023, 21, 46. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, N.; Wang, Z. Eosinophilic esophagitis and esophageal microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1206343. [Google Scholar] [CrossRef]
- Busing, J.D.; Buendia, M.; Choksi, Y.; Hiremath, G.; Das, S.R. Microbiome in Eosinophilic Esophagitis—Metagenomic, Metatranscriptomic, and Metabolomic Changes: A Systematic Review. Front. Physiol. 2021, 12, 731034. [Google Scholar] [CrossRef]
- Holvoet, S.; Doucet-Ladevèze, R.; Perrot, M.; Barretto, C.; Nutten, S.; Blanchard, C. Beneficial effect of Lactococcus lactis NCC 2287 in a murine model of eosinophilic esophagitis. Allergy 2016, 71, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Facchin, S.; Bonazzi, E.; Tomasulo, A.; Bertin, L.; Lorenzon, G.; Maniero, D.; Zingone, F.; Cardin, R.; Barberio, B.; Ghisa, M.; et al. Could modulating the esophageal microbiome be the answer for eosinophilic esophagitis treatment? Expert Rev. Gastroenterol. Hepatol. 2025, 19, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Kohley, A.; Attwal, S.; Jones, S.M.; Akmyradov, C.; Chandler, P.; Tootle, C.; Nawaz, S.; Ayers, T.; Kawatu, D.; Pesek, R.D. Impact of Atopic Status on Clinical Presentation and Treatment Response in Pediatric Patients with Eosinophilic Esophagitis. J. Allergy Clin. Immunol. Pr. 2024, 12, 3358–3362. [Google Scholar] [CrossRef] [PubMed]
- Redd, W.D.; Ocampo, A.A.; Xue, Z.; Chang, N.C.; Thakkar, K.P.; Reddy, S.B.; Greenberg, S.B.; Lee, C.J.; Ketchem, C.J.; Eluri, S.; et al. Eosinophilic esophagitis patients with multiple atopic conditions: Clinical characteristics and treatment response to topical steroids. Ann. Allergy Asthma Immunol. 2023, 131, 109–115.e2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
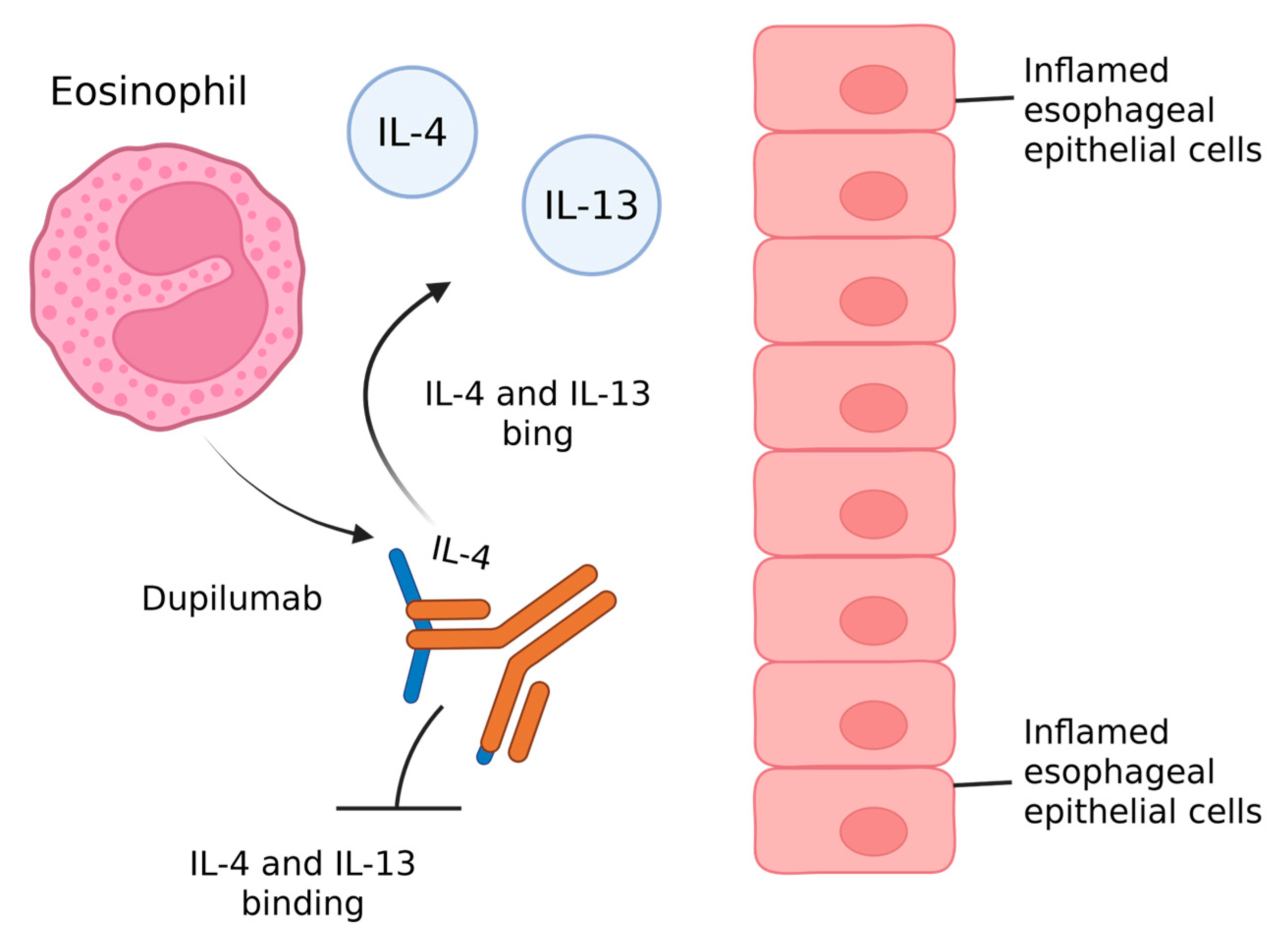
| Esophageal Strictures | Histological Remission | Symptom Relief | |
|---|---|---|---|
| PPIs [29,30,31] | No | Plausible | Yes |
| Corticosteroids [36,37,38] | No | Plausible | Yes |
| Diet [25] | No | Plausible | Yes |
| Dupilumab [43,44] | No | Plausible | Yes |
| Dilation [12] | Yes | No | Yes |
| Biologics | Approved for EoE | Approved for Asthma | Mechanism of Action |
|---|---|---|---|
| Dupilumab [73] | yes | yes | Anti-IL-4/ IL-13 |
| Mepolizumab [74,77] | investigational | yes | Anti-IL-5 |
| Reslizumab [75] | investigational | yes | Anti-IL-5 |
| Cendakimab [76] | investigational | investigational | Anti-IL-13 |
| Dectrekumab [57] | investigational | no data | Anti-IL-13 |
| Benralizumab [78] | investigational | yes | Anti-IL-5 |
| Lirentelimab [79] | investigational | investigational | Anti-Siglec-8 |
| Etrasimod [66] | investigational | no data | Anti-S1P |
| Tezepelumab [79] | investigational | yes | Anti-TLSP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olic, I.; Zivkovic, P.M.; Zaja, I.; Pavlovic, N.; Kumric, M.; Bozic, J. Advances and Challenges in the Development of New and Novel Treatment Strategies for Eosinophilic Esophagitis (EoE). Pharmaceuticals 2025, 18, 1359. https://doi.org/10.3390/ph18091359
Olic I, Zivkovic PM, Zaja I, Pavlovic N, Kumric M, Bozic J. Advances and Challenges in the Development of New and Novel Treatment Strategies for Eosinophilic Esophagitis (EoE). Pharmaceuticals. 2025; 18(9):1359. https://doi.org/10.3390/ph18091359
Chicago/Turabian StyleOlic, Ivna, Piero Marin Zivkovic, Ivan Zaja, Nikola Pavlovic, Marko Kumric, and Josko Bozic. 2025. "Advances and Challenges in the Development of New and Novel Treatment Strategies for Eosinophilic Esophagitis (EoE)" Pharmaceuticals 18, no. 9: 1359. https://doi.org/10.3390/ph18091359
APA StyleOlic, I., Zivkovic, P. M., Zaja, I., Pavlovic, N., Kumric, M., & Bozic, J. (2025). Advances and Challenges in the Development of New and Novel Treatment Strategies for Eosinophilic Esophagitis (EoE). Pharmaceuticals, 18(9), 1359. https://doi.org/10.3390/ph18091359









