Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders
Abstract
1. Introduction
2. Sigma-1 Receptor: Biology and Mechanism of Action
2.1. S1R as a Chaperone Protein and Inter-Organelle Signaling Modulator
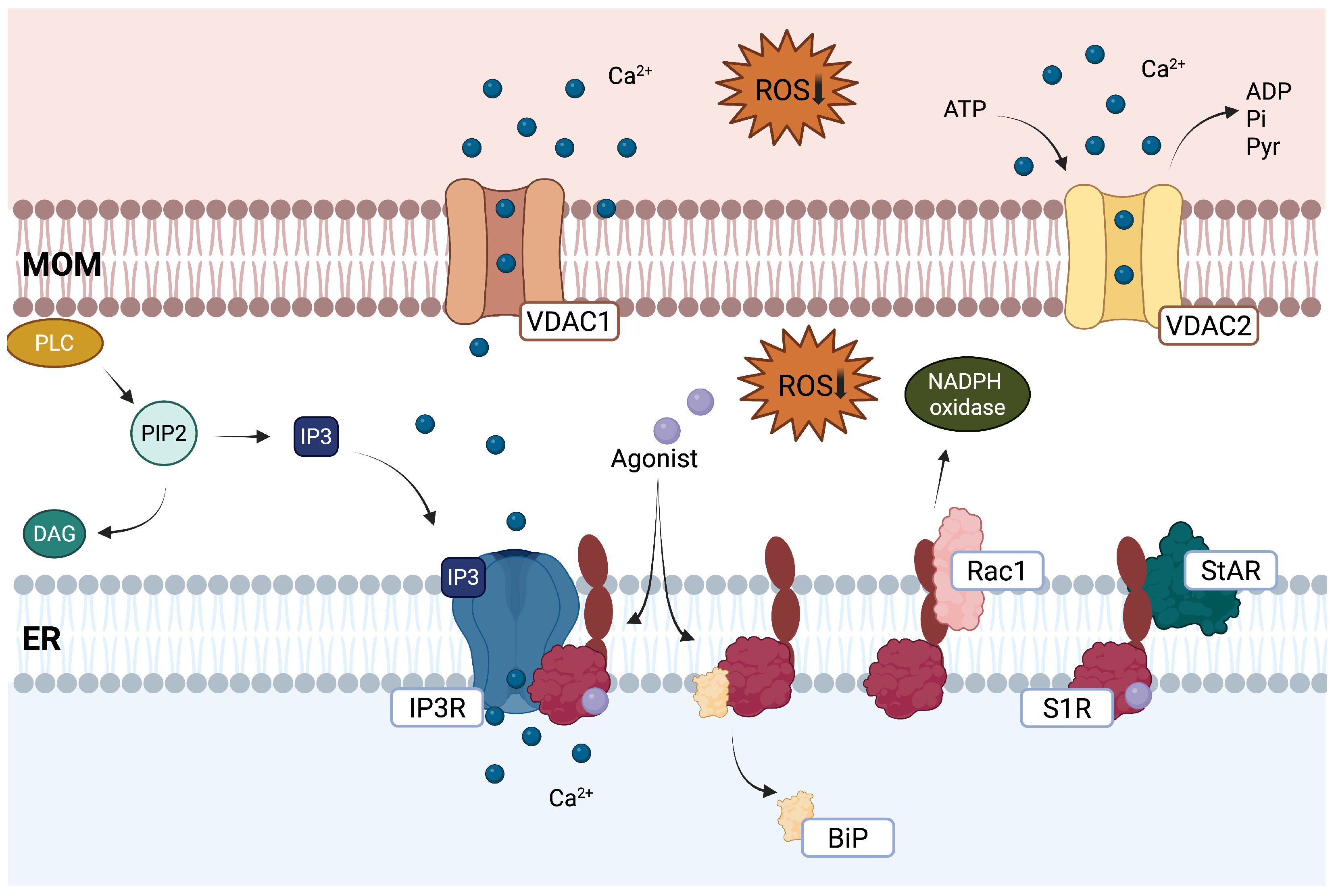
2.2. Mitochondrial Function
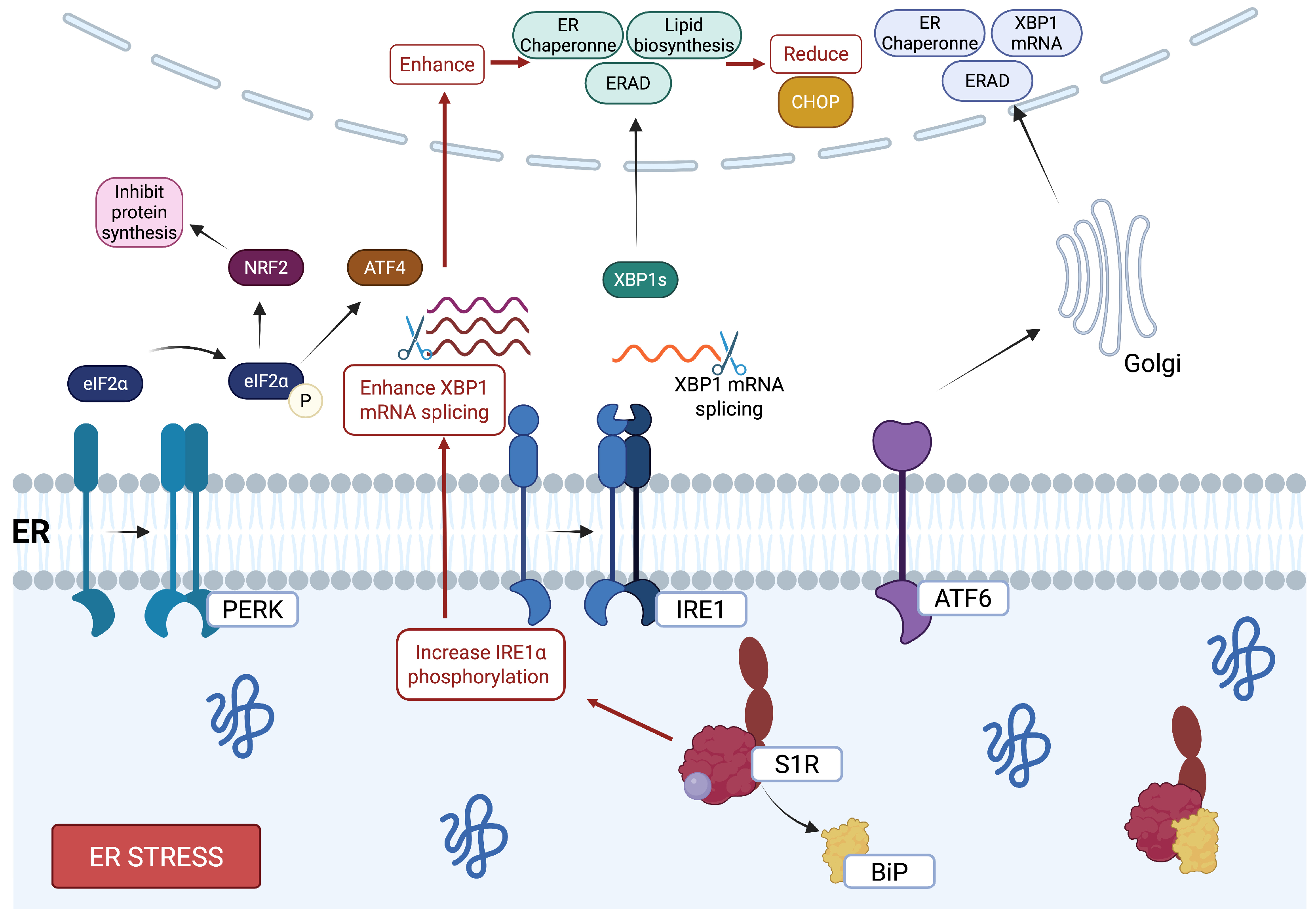
2.3. Endoplasmic Reticulum Stress
2.4. Autophagy
2.5. Neuroinflammation
2.6. Neurotrophic Factors
3. Repurposing Existing Drugs Targeting the S1R for Neurodegenerative Diseases
3.1. Fluvoxamine
3.2. Citalopram and Escitalopram
3.3. Fluoxetine
3.4. Memantine
3.5. Dextromethorphan
3.6. Amantadine
3.7. Donepezil
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| ALS | Amyotrophic lateral sclerosis |
| MS | Multiple sclerosis |
| Aβ | β-amyloid |
| TDP-43 | TAR DNA-binding protein 43 |
| S1R | Sigma-1 receptor |
| ER | Endoplasmic reticulum |
| MAM | Mitochondria-associated membrane |
| BiP | Binding immunoglobulin protein |
| IP3R | Inositol triphosphate receptor |
| PLC | Phospholipase C |
| TCA | Tricarboxylic acid cycle |
| ETC | Electron transport chain |
| ROS | Reactive oxygen species |
| Rac1 | Ras-related C3 botulinum toxin substrate 1 |
| VDAC2 | Voltage-dependent anion channel 2 |
| NQO1 | NAD(P)H quinone oxidoreductase 1 |
| SOD1 | Superoxide dismutase 1 |
| XBP1 | X-box-binding protein 1 |
| CHOP | C/EBP homologous protein |
| BCL-2 | B-cell lymphoma 2 |
| BAX | BCL-2-associated X protein |
| BAK | BCL-2 antagonist/killer |
| ULK | Unc-51-like autophagy-activating kinase |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| AMPK | AMP-activated protein kinase |
| TFEB | Transcription factor EB |
| BDNF | Brain-derived neurotrophic factor |
| NGF | Nerve growth factor |
| GDNF | Glial cell-derived neurotrophic factor |
| EGF | Epidermal growth factor |
| TrkB | Tropomyosin receptor kinase B |
| CaMKIV/II | Calcium/calmodulin-dependent protein kinases IV/II |
| CREB | cAMP response element-binding protein |
| NMDA | N-Methyl-D-aspartate |
| GluN2A/B | Glutamate receptor subunits 2A/B |
| JAK2 | Janus kinase 2 |
| STAT3 | Signal transducer and activator of transcription 3 |
| GFAP | Glial fibrillary acidic protein |
| ERK | Extracellular signal-regulated kinase |
| NF-κB | Nuclear factor kappa B |
| TLR4 | Toll-like receptor 4 |
| SSRI | Selective serotonin reuptake inhibitor |
| LTP | Long-term potentiation |
| CYP2D6 | Cytochrome P450 2D6 |
References
- Schulte, J.; Littleton, J.T. The biological function of the Huntingtin protein and its relevance to Huntington’s Disease pathology. Curr. Trends Neurol. 2011, 5, 65. [Google Scholar] [PubMed]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef]
- Sajjad, R.; Arif, R.; Shah, A.; Manzoor, I.; Mustafa, G. Pathogenesis of Alzheimer’s disease: Role of amyloid-beta and hyperphosphorylated tau protein. Indian J. Pharm. Sci. 2018, 80, 581–591. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef]
- Rowinska-Zyrek, M.; Salerno, M.; Kozlowski, H. Neurodegenerative diseases—Understanding their molecular bases and progress in the development of potential treatments. Coord. Chem. Rev. 2015, 284, 298–312. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef]
- Ashburn, T.T.; Thor, K.B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004, 3, 673–683. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Nosengo, N. Can you teach old drugs new tricks? Nature 2016, 534, 314–316. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F. New perspectives on how to discover drugs from herbal medicines: CAM′ S outstanding contribution to modern therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef] [PubMed]
- Kourrich, S.; Su, T.-P.; Fujimoto, M.; Bonci, A. The sigma-1 receptor: Roles in neuronal plasticity and disease. Trends Neurosci. 2012, 35, 762–771. [Google Scholar] [CrossRef]
- Lachance, V.; Bélanger, S.-M.; Hay, C.; Le Corvec, V.; Banouvong, V.; Lapalme, M.; Tarmoun, K.; Beaucaire, G.; Lussier, M.P.; Kourrich, S. Overview of sigma-1R subcellular specific biological functions and role in neuroprotection. Int. J. Mol. Sci. 2023, 24, 1971. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, I. Alzheimer Agent Blarcamesine Shows Significant Reduction of Amyloid-ß Biomarkers in Phase 2b/3 Trial. NeurologyLive, 8 September 2023. Available online: https://www.neurologylive.com/view/ad-agent-blarcamesine-significant-reduction-amyloid-beta-biomarkers-phase-2b3-trial (accessed on 28 December 2024).
- Hayashi, T.; Su, T.-P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human σ1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef]
- Munguia-Galaviz, F.J.; Miranda-Diaz, A.G.; Cardenas-Sosa, M.A.; Echavarria, R. Sigma-1 receptor signaling: In search of new therapeutic alternatives for cardiovascular and renal diseases. Int. J. Mol. Sci. 2023, 24, 1997. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.-P. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14949–14954. [Google Scholar] [CrossRef]
- Alonso, G.; Phan, V.-L.; Guillemain, I.; Saunier, M.; Legrand, A.; Anoal, M.; Maurice, T. Immunocytochemical localization of the sigma1 receptor in the adult rat central nervous system. Neuroscience 2000, 97, 155–170. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.-P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Pal, A.; Chu, U.B.; Ramachandran, S.; Grawoig, D.; Guo, L.-W.; Hajipour, A.R.; Ruoho, A.E. Juxtaposition of the steroid binding domain-like I and II regions constitutes a ligand binding site in the σ-1 receptor. J. Biol. Chem. 2008, 283, 19646–19656. [Google Scholar] [CrossRef]
- Su, T.-P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-Y.A.; Chuang, J.-Y.; Tsai, M.-S.; Wang, X.-f.; Xi, Z.-X.; Hung, J.-J.; Chang, W.-C.; Bonci, A.; Su, T.-P. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. USA 2015, 112, E6562–E6570. [Google Scholar] [CrossRef] [PubMed]
- Kourrich, S. Sigma-1 receptor and neuronal excitability. In Sigma Proteins: Evolution of the Concept of Sigma Receptors; Springer: Berlin/Heidelberg, Germany, 2017; pp. 109–130. [Google Scholar]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.E.; Starkov, A.; Blass, J.P.; Ratan, R.R.; Beal, M.F. Cause and consequence: Mitochondrial dysfunction initiates and propagates neuronal dysfunction, neuronal death and behavioral abnormalities in age-associated neurodegenerative diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 122–134. [Google Scholar] [CrossRef]
- Leuner, K.; Schütt, T.; Kurz, C.; Eckert, S.H.; Schiller, C.; Occhipinti, A.; Mai, S.; Jendrach, M.; Eckert, G.P.; Kruse, S.E. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxid. Redox Signal. 2012, 16, 1421–1433. [Google Scholar] [CrossRef]
- Ballard, P.A.; Tetrud, J.W.; Langston, J.W. Permanent human parkinsonism due to 1-methy 1–4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): Seven cases. Neurology 1985, 35, 949–956. [Google Scholar] [CrossRef]
- Morin-Surun, M.-P.; Collin, T.; Denavit-Saubié, M.; Baulieu, E.-E.; Monnet, F. Intracellular σ1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc. Natl. Acad. Sci. USA 1999, 96, 8196–8199. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol.-Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Goguadze, N.; Zhuravliova, E.; Morin, D.; Mikeladze, D.; Maurice, T. Sigma-1 receptor agonists induce oxidative stress in mitochondria and enhance complex I activity in physiological condition but protect against pathological oxidative stress. Neurotox. Res. 2019, 35, 1–18. [Google Scholar] [CrossRef]
- Chatterjee, S. Oxidative stress, inflammation, and disease. In Oxidative Stress and Biomaterials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–58. [Google Scholar]
- Madesh, M.; Hajnóczky, G.r. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 2001, 155, 1003–1016. [Google Scholar] [CrossRef]
- Natsvlishvili, N.; Goguadze, N.; Zhuravliova, E.; Mikeladze, D. Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria. BMC Biochem. 2015, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Fontanilla, D.; Gopalakrishnan, A.; Chae, Y.-K.; Markley, J.L.; Ruoho, A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012, 682, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegeneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Khair, M. Endoplasmic reticulum stress and unfolded protein response in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Zhang, D.; Hannink, M.; Arvisais, E.; Kaufman, R.J.; Diehl, J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 2003, 23, 7198–7209. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef]
- Le Goupil, S.; Laprade, H.; Aubry, M.; Chevet, E. Exploring the IRE1 interactome: From canonical signaling functions to unexpected roles. J. Biol. Chem. 2024, 300, 107169. [Google Scholar] [CrossRef]
- Park, S.M.; Kang, T.I.; So, J.S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 2021, 9, 791. [Google Scholar] [CrossRef]
- Haze, K.; Yoshida, H.; Yanagi, H.; Yura, T.; Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 1999, 10, 3787–3799. [Google Scholar] [CrossRef]
- Xiang, C.; Wang, Y.; Zhang, H.; Han, F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017, 22, 1–26. [Google Scholar]
- Marwarha, G.; Dasari, B.; Ghribi, O. Endoplasmic reticulum stress-induced CHOP activation mediates the down-regulation of leptin in human neuroblastoma SH-SY5Y cells treated with the oxysterol 27-hydroxycholesterol. Cell. Signal. 2012, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Li, H.; Zhang, Y.; Ron, D.; Walter, P. Divergent effects of PERK and IRE1 signaling on cell viability. PLoS ONE 2009, 4, e4170. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Abdullah, C.S.; Aishwarya, R.; Orr, A.W.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; Pattillo, C.B.; Bhuiyan, M.S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci. Rep. 2017, 37, BSR20170898. [Google Scholar] [CrossRef]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.-P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, L.; Liu, D.; Chi, T.; Ji, X.; Liu, P.; Yang, X.; Tian, X.; Zou, L. Sigma-1 receptor protects against endoplasmic reticulum stress-mediated apoptosis in mice with cerebral ischemia/reperfusion injury. Apoptosis 2019, 24, 157–167. [Google Scholar] [CrossRef]
- Mitsuda, T.; Omi, T.; Tanimukai, H.; Sakagami, Y.; Tagami, S.; Okochi, M.; Kudo, T.; Takeda, M. Sigma-1Rs are upregulated via PERK/eIF2α/ATF4 pathway and execute protective function in ER stress. Biochem. Biophys. Res. Commun. 2011, 415, 519–525. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Settembre, C.; Di Malta, C.; Polito, V.A.; Arencibia, M.G.; Vetrini, F.; Erdin, S.; Erdin, S.U.; Huynh, T.; Medina, D.; Colella, P. TFEB links autophagy to lysosomal biogenesis. Science 2011, 332, 1429–1433. [Google Scholar] [CrossRef]
- Paquette, M.; El-Houjeiri, L.; Zirden, L.C.; Puustinen, P.; Blanchette, P.; Jeong, H.; Dejgaard, K.; Siegel, P.M.; Pause, A. AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3. Autophagy 2021, 17, 3957–3975. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.D.; Flores-Morelos, D.S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.M.; Zacapala-Gómez, A.E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O.; et al. Autophagy: A Key Regulator of Homeostasis and Disease: An Overview of Molecular Mechanisms and Modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Yu, W.H.; Cuervo, A.M.; Kumar, A.; Peterhoff, C.M.; Schmidt, S.D.; Lee, J.-H.; Mohan, P.S.; Mercken, M.; Farmery, M.R.; Tjernberg, L.O. Macroautophagy—A novel β-amyloid peptide-generating pathway activated in Alzheimer’s disease. J. Cell Biol. 2005, 171, 87–98. [Google Scholar] [CrossRef]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [CrossRef]
- Lachance, V.; Wang, Q.; Sweet, E.; Choi, I.; Cai, C.-Z.; Zhuang, X.-X.; Zhang, Y.; Jiang, J.L.; Blitzer, R.D.; Bozdagi-Gunal, O. Autophagy protein NRBF2 has reduced expression in Alzheimer’s brains and modulates memory and amyloid-beta homeostasis in mice. Mol. Neurodegener. 2019, 14, 43. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2018, 28, 3–13. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Treis, A.; Skujat, D.; Weber, S.S.; Fiesel, F.C.; Kahle, P.J.; Springer, W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 2010, 6, 871–878. [Google Scholar] [CrossRef]
- Zhou, T.-Y.; Ma, R.-X.; Li, J.; Zou, B.; Yang, H.; Ma, R.-Y.; Wu, Z.-Q.; Li, J.; Yao, Y. Review of PINK1-Parkin-mediated mitochondrial autophagy in Alzheimer’s disease. Eur. J. Pharmacol. 2023, 959, 176057. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, W.; Zhang, S.; Iyaswamy, A.; Sun, J.; Wang, J.; Yang, C. Novel insight into functions of transcription factor EB (TFEB) in alzheimer’s disease and Parkinson’s disease. Aging Dis. 2023, 14, 652. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Guan, Y.; Wang, Q.; Zhou, F.; Jie, L.; Ju, J.; Pu, L.; Du, H.; Wang, X. The altered autophagy mediated by TFEB in animal and cell models of amyotrophic lateral sclerosis. Am. J. Transl. Res. 2015, 7, 1574. [Google Scholar] [PubMed]
- Dreser, A.; Vollrath, J.T.; Sechi, A.; Johann, S.; Roos, A.; Yamoah, A.; Katona, I.; Bohlega, S.; Wiemuth, D.; Tian, Y. The ALS-linked E102Q mutation in Sigma receptor-1 leads to ER stress-mediated defects in protein homeostasis and dysregulation of RNA-binding proteins. Cell Death Differ. 2017, 24, 1655–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wan, C.; He, T.; Han, C.; Zhu, K.; Waddington, J.L.; Zhen, X. Sigma-1 receptor regulates mitophagy in dopaminergic neurons and contributes to dopaminergic protection. Neuropharmacology 2021, 196, 108360. [Google Scholar] [CrossRef]
- Yang, H.; Shen, H.; Li, J.; Guo, L.-W. SIGMAR1/Sigma-1 receptor ablation impairs autophagosome clearance. Autophagy 2019, 15, 1539–1557. [Google Scholar] [CrossRef]
- Wang, S.-M.; Wu, H.-E.; Yasui, Y.; Geva, M.; Hayden, M.; Maurice, T.; Cozzolino, M.; Su, T.-P. Nucleoporin POM121 signals TFEB-mediated autophagy via activation of SIGMAR1/sigma-1 receptor chaperone by pridopidine. Autophagy 2023, 19, 126–151. [Google Scholar] [CrossRef] [PubMed]
- Deczkowska, A.; Keren-Shaul, H.; Weiner, A.; Colonna, M.; Schwartz, M.; Amit, I. Disease-associated microglia: A universal immune sensor of neurodegeneration. Cell 2018, 173, 1073–1081. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Bisht, K.; Tremblay, M.-È. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef]
- Colton, C.A.; Wilcock, D.M. Assessing activation states in microglia. CNS Neurol. Disord.-Drug Targets 2010, 9, 174–191. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Miao, H.; Li, R.; Han, C.; Lu, X.; Zhang, H. Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J. Neurophysiol. 2018, 120, 1307–1317. [Google Scholar] [CrossRef]
- Arroyo, D.S.; Soria, J.A.; Gaviglio, E.A.; Rodriguez-Galan, M.C.; Iribarren, P. Toll-like receptors are key players in neurodegeneration. Int. Immunopharmacol. 2011, 11, 1415–1421. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.; Benítez, J.A.; Razgado, L.F.; Vergara, P.; Segovia, J. Adenoviral astrocyte-specific expression of BDNF in the striata of mice transgenic for Huntington’s disease delays the onset of the motor phenotype. Cell. Mol. Neurobiol. 2011, 31, 1229–1243. [Google Scholar] [CrossRef]
- Wang, L.; Lin, F.; Wang, J.; Wu, J.; Han, R.; Zhu, L.; Zhang, G.; DiFiglia, M.; Qin, Z. Truncated N-terminal huntingtin fragment with expanded-polyglutamine (htt552-100Q) suppresses brain-derived neurotrophic factor transcription in astrocytes. Acta Biochim. Biophys. Sin. 2012, 44, 249–258. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [PubMed]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Cuevas, J.; Rodriguez, A.; Behensky, A.; Katnik, C. Afobazole modulates microglial function via activation of both σ-1 and σ-2 receptors. J. Pharmacol. Exp. Ther. 2011, 339, 161–172. [Google Scholar] [CrossRef]
- Jia, J.; Cheng, J.; Wang, C.; Zhen, X. Sigma-1 receptor-modulated neuroinflammation in neurological diseases. Front. Cell. Neurosci. 2018, 12, 314. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.A.; Herrera, Y.; Ajmo, C.T., Jr.; Cuevas, J.; Pennypacker, K.R. Sigma receptors suppress multiple aspects of microglial activation. Glia 2009, 57, 744–754. [Google Scholar] [CrossRef]
- Francardo, V.; Bez, F.; Wieloch, T.; Nissbrandt, H.; Ruscher, K.; Cenci, M.A. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain 2014, 137, 1998–2014. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Zheng, L.T.; Xu, Z.; Guo, L.; Zhen, X. Allosteric modulation of sigma-1 receptors by SKF 83959 inhibits microglia-mediated inflammation. J. Neurochem. 2015, 134, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Heiss, K.; Vanella, L.; Murabito, P.; Prezzavento, O.; Marrazzo, A.; Castracani, C.C.; Barbagallo, I.; Zappalà, A.; Arena, E.; Astuto, M. (+)-Pentazocine reduces oxidative stress and apoptosis in microglia following hypoxia/reoxygenation injury. Neurosci. Lett. 2016, 626, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ha, Y.; Liou, G.I.; Gonsalvez, G.B.; Smith, S.B.; Bollinger, K.E. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3375–3384. [Google Scholar] [CrossRef]
- Peviani, M.; Salvaneschi, E.; Bontempi, L.; Petese, A.; Manzo, A.; Rossi, D.; Salmona, M.; Collina, S.; Bigini, P.; Curti, D. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol. Dis. 2014, 62, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, T.; Gao, C.; Jia, X.; Ni, J.; Han, C.; Wang, Y. Stimulation of astrocytic sigma-1 receptor is sufficient to ameliorate inflammation- induced depression. Behav. Brain Res. 2021, 410, 113344. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, T.; Zhang, X.; Chao, J.; Hu, G.; Yao, H. Role of high-mobility group box 1 in methamphetamine-induced activation and migration of astrocytes. J. Neuroinflamm. 2015, 12, 156. [Google Scholar] [CrossRef]
- Moon, J.; Roh, D.; Yoon, S.; Choi, S.; Kwon, S.; Choi, H.; Kang, S.; Han, H.; Beitz, A.; Oh, S. σ1 receptors activate astrocytes via p38 MAPK phosphorylation leading to the development of mechanical allodynia in a mouse model of neuropathic pain. Br. J. Pharmacol. 2014, 171, 5881–5897. [Google Scholar] [CrossRef]
- Ajmo, C.T., Jr.; Vernon, D.O.; Collier, L.; Pennypacker, K.R.; Cuevas, J. Sigma receptor activation reduces infarct size at 24 hours after permanent middle cerebral artery occlusion in rats. Curr. Neurovasc. Res. 2006, 3, 89–98. [Google Scholar] [CrossRef]
- Robson, M.J.; Turner, R.C.; Naser, Z.J.; McCurdy, C.R.; O’Callaghan, J.P.; Huber, J.D.; Matsumoto, R.R. SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation. Exp. Neurol. 2014, 254, 180–189. [Google Scholar] [CrossRef]
- Dalwadi, D.A.; Kim, S.; Schetz, J.A. Activation of the sigma-1 receptor by haloperidol metabolites facilitates brain-derived neurotrophic factor secretion from human astroglia. Neurochem. Int. 2017, 105, 21–31. [Google Scholar] [CrossRef]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549–557. [Google Scholar] [PubMed]
- Levy, Y.S.; Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs 2005, 19, 97–127. [Google Scholar] [CrossRef]
- Samadi, P.; Boutet, A.; Rymar, V.; Rawal, K.; Maheux, J.; Kvann, J.C.; Tomaszewski, M.; Beaubien, F.; Cloutier, J.; Levesque, D. Relationship between BDNF expression in major striatal afferents, striatum morphology and motor behavior in the R6/2 mouse model of Huntington’s disease. Genes Brain Behav. 2013, 12, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB neurotrophic activity stimulates δ-secretase by upregulating C/EBPβ in Alzheimer’s disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef]
- Counts, S.E.; Mufson, E.J. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol. 2005, 64, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Cuello, A.C.; Bruno, M.A.; Allard, S.; Leon, W.; Iulita, M.F. Cholinergic involvement in Alzheimer’s disease. A link with NGF maturation and degradation. J. Mol. Neurosci. 2010, 40, 230–235. [Google Scholar] [CrossRef]
- Lin, L.-F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Araujo, D.M.; Hilt, D.C.; Sheng, J.; Jiao, S. Adenoviral vector-mediated GDNF gene therapy in a rodent lesion model of late stage Parkinson’s disease. Brain Res. 1997, 777, 153–160. [Google Scholar] [CrossRef]
- Sha, S.; Qu, W.J.; Li, L.; Lu, Z.H.; Chen, L.; Yu, W.F.; Chen, L. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors. CNS Neurosci. Ther. 2013, 19, 705–713. [Google Scholar] [CrossRef]
- Franchini, S.; Linciano, P.; Puja, G.; Tait, A.; Borsari, C.; Denora, N.; Iacobazzi, R.M.; Brasili, L.; Sorbi, C. Novel dithiolane-based ligands combining sigma and NMDA receptor interactions as potential neuroprotective agents. ACS Med. Chem. Lett. 2020, 11, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Turcotte, M.E.B.; Halman, S.; Bergeron, R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol. 2007, 578, 143–157. [Google Scholar] [CrossRef]
- Pabba, M.; Wong, A.Y.; Ahlskog, N.; Hristova, E.; Biscaro, D.; Nassrallah, W.; Ngsee, J.K.; Snyder, M.; Beique, J.-C.; Bergeron, R. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J. Neurosci. 2014, 34, 11325–11338. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, K.; Moriguchi, S. Stimulation of the sigma-1 receptor and the effects on neurogenesis and depressive behaviors in mice. In Sigma Receptors: Their Role in Disease and as Therapeutic Targets; Springer: Berlin/Heidelberg, Germany, 2017; pp. 201–211. [Google Scholar]
- Yagasaki, Y.; Numakawa, T.; Kumamaru, E.; Hayashi, T.; Su, T.-P.; Kunugi, H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006, 281, 12941–12949. [Google Scholar] [CrossRef]
- Saito, A.; Cai, L.; Matsuhisa, K.; Ohtake, Y.; Kaneko, M.; Kanemoto, S.; Asada, R.; Imaizumi, K. Neuronal activity-dependent local activation of dendritic unfolded protein response promotes expression of brain-derived neurotrophic factor in cell soma. J. Neurochem. 2018, 144, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Shinoda, Y.; Yamamoto, Y.; Sasaki, Y.; Miyajima, K.; Tagashira, H.; Fukunaga, K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS ONE 2013, 8, e60863. [Google Scholar] [CrossRef]
- Ruscher, K.; Shamloo, M.; Rickhag, M.; Ladunga, I.; Soriano, L.; Gisselsson, L.; Toresson, H.; Ruslim-Litrus, L.; Oksenberg, D.; Urfer, R. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain 2011, 134, 732–746. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hayashi, T.; Su, T.-P. Nerve growth factor-induced neurite sprouting in PC12 cells involves ς-1 receptors: Implications for antidepressants. J. Pharmacol. Exp. Ther. 2002, 303, 1227–1237. [Google Scholar] [CrossRef]
- Takebayashi, M.; Hayashi, T.; Su, T.P. σ-1 Receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: Potential relation to lipid raft reconstitution. Synapse 2004, 53, 90–103. [Google Scholar] [CrossRef]
- Penas, C.; Pascual-Font, A.; Mancuso, R.; Forés, J.; Casas, C.; Navarro, X. Sigma receptor agonist 2-(4-morpholinethyl) 1 phenylcyclohexanecarboxylate (Pre084) increases GDNF and BiP expression and promotes neuroprotection after root avulsion injury. J. Neurotrauma 2011, 28, 831–840. [Google Scholar] [CrossRef]
- Liu, D.; Yang, L.; Liu, P.; Ji, X.; Qi, X.; Wang, Z.; Chi, T.; Zou, L. Sigma-1 receptor activation alleviates blood-brain barrier disruption post cerebral ischemia stroke by stimulating the GDNF-GFRα1-RET pathway. Exp. Neurol. 2022, 347, 113867. [Google Scholar] [CrossRef] [PubMed]
- Narita, N.; Hashimoto, K.; Tomitaka, S.-i.; Minabe, Y. Interactions of selective serotonin reuptake inhibitors with subtypes of σ receptors in rat brain. Eur. J. Pharmacol. 1996, 307, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Romieu, P.; Maurice, T.; Su, T.P.; Maloteaux, J.M.; Hermans, E. Involvement of the sigma1 receptor in the modulation of dopaminergic transmission by amantadine. Eur. J. Neurosci. 2004, 19, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.-C.; Liao, J.-F.; Chang, W.-Y.; Lin, M.-F.; Chen, C.-F. Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan. Brain Res. 1999, 821, 516–519. [Google Scholar] [CrossRef]
- Kato, K.; Hayako, H.; Ishihara, Y.; Marui, S.; Iwane, M.; Miyamoto, M. TAK-147, an acetylcholinesterase inhibitor, increases choline acetyltransferase activity in cultured rat septal cholinergic neurons. Neurosci. Lett. 1999, 260, 5–8. [Google Scholar] [CrossRef]
- Laux, G. Serotonin reuptake inhibitors: Citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline. In NeuroPsychopharmacotherapy; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–13. [Google Scholar]
- Omi, T.; Kudo, T.; Tanimukai, H.; Kanayama, D.; Sakagami, Y.; Hara, H.; Takeda, M. P1–400: Fluvoxamine attenuates tau phosphorylation through an upregulation of sigma-1 receptors (Sig-1Rs). Alzheimer’s Dement. 2013, 9, P304. [Google Scholar] [CrossRef]
- Hindmarch, I.; Hashimoto, K. Cognition and depression: The effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Hum. Psychopharmacol. Clin. Exp. 2010, 25, 193–200. [Google Scholar] [CrossRef]
- McCann, D.J.; Weissman, A.D.; Su, T.P. Sigma-1 and Sigma-2 sites in rat brain: Comparison of regional, ontogenetic, and subcellular patterns. Synapse 1994, 17, 182–189. [Google Scholar] [CrossRef]
- Tagashira, H.; Bhuiyan, M.S.; Shioda, N.; Fukunaga, K. Fluvoxamine rescues mitochondrial Ca2+ transport and ATP production through σ1-receptor in hypertrophic cardiomyocytes. Life Sci. 2014, 95, 89–100. [Google Scholar] [CrossRef]
- Omi, T.; Tanimukai, H.; Kanayama, D.; Sakagami, Y.; Tagami, S.; Okochi, M.; Morihara, T.; Sato, M.; Yanagida, K.; Kitasyoji, A. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014, 5, e1332. [Google Scholar] [CrossRef]
- Ahsan, H.; Ayub, M.; Irfan, H.M.; Saleem, M.; Anjum, I.; Haider, I.; Asif, A.; Abbas, S.Q.; ul Hulassan, S.S. Tumor necrosis factor-alpha, prostaglandin-E2 and interleukin-1β targeted anti-arthritic potential of fluvoxamine: Drug repurposing. Environ. Sci. Pollut. Res. 2023, 30, 14580–14591. [Google Scholar] [CrossRef] [PubMed]
- Dallé, E.; Daniels, W.M.; Mabandla, M.V. Fluvoxamine maleate normalizes striatal neuronal inflammatory cytokine activity in a Parkinsonian rat model associated with depression. Behav. Brain Res. 2017, 316, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Zibara, K.; Sadeghi, H.; Dokoohaki, S.; Sadeghi, H.; Aryanpour, R.; Ghanbari, A. Fluvoxamine stimulates oligodendrogenesis of cultured neural stem cells and attenuates inflammation and demyelination in an animal model of multiple sclerosis. Sci. Rep. 2017, 7, 4923. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wu, H.-E.; Weng, E.F.-J.; Wu, H.-C.; Su, T.-P.; Wang, S.-M. Fluvoxamine Exerts Sigma-1R to Rescue Autophagy via Pom121-Mediated Nucleocytoplasmic Transport of TFEB. Mol. Neurobiol. 2024, 61, 5282–5294. [Google Scholar] [CrossRef]
- Terada, K.; Izumo, N.; Suzuki, B.; Karube, Y.; Morikawa, T.; Ishibashi, Y.; Kameyama, T.; Chiba, K.; Sasaki, N.; Iwata, K. Fluvoxamine moderates reduced voluntary activity following chronic dexamethasone infusion in mice via recovery of BDNF signal cascades. Neurochem. Int. 2014, 69, 9–13. [Google Scholar] [CrossRef]
- Matsushima, Y.; Terada, K.; Takata, J.; Karube, Y.; Kamei, C.; Sugimoto, Y. Effects of fluvoxamine on nerve growth factor-induced neurite outgrowth inhibition by dexamethasone in PC12 cells. Biosci. Biotechnol. Biochem. 2019, 83, 659–665. [Google Scholar] [CrossRef]
- Moriguchi, S.; Sakagami, H.; Yabuki, Y.; Sasaki, Y.; Izumi, H.; Zhang, C.; Han, F.; Fukunaga, K. Stimulation of sigma-1 receptor ameliorates depressive-like behaviors in CaMKIV null mice. Mol. Neurobiol. 2015, 52, 1210–1222. [Google Scholar] [CrossRef]
- Nishimura, T.; Ishima, T.; Iyo, M.; Hashimoto, K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: Role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PLoS ONE 2008, 3, e2558. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, K.; Sharma, A.; Sandha, K.K.; Ali, S.M.; Ahmed, R.; Ramajayan, P.; Singh, P.P.; Ahmed, Z.; Kumar, A. Fluvoxamine maleate alleviates amyloid-beta load and neuroinflammation in 5XFAD mice to ameliorate Alzheimer disease pathology. Front. Immunol. 2024, 15, 1418422. [Google Scholar] [CrossRef]
- Kim, W.S.; Fu, Y.; Dobson-Stone, C.; Hsiao, J.-H.T.; Shang, K.; Hallupp, M.; Schofield, P.R.; Garner, B.; Karl, T.; Kwok, J.B. Effect of fluvoxamine on amyloid-β peptide generation and memory. J. Alzheimer’s Dis. 2018, 62, 1777–1787. [Google Scholar] [CrossRef]
- Kurita, M.; Sato, T.; Nishino, S.; Ohtomo, K.; Shirakawa, H.; Mashiko, H.; SHIN-ICHI, N.; Nakahata, N. Effects of fluvoxamine on behavioral and psychological symptoms of dementia in Alzheimer’s disease: A report of three cases. Fukushima J. Med. Sci. 2006, 52, 143–148. [Google Scholar] [CrossRef]
- Teranishi, M.; Kurita, M.; Nishino, S.; Takeyoshi, K.; Numata, Y.; Sato, T.; Tateno, A.; Okubo, Y. Efficacy and tolerability of risperidone, yokukansan, and fluvoxamine for the treatment of behavioral and psychological symptoms of dementia: A blinded, randomized trial. J. Clin. Psychopharmacol. 2013, 33, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Fujita, Y.; Iyo, M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: Role of sigma-1 receptors. Neuropsychopharmacology 2007, 32, 514–521. [Google Scholar] [CrossRef]
- Ishima, T.; Fujita, Y.; Kohno, M.; Kunitachi, S.; Horio, M.; Takatsu, M.; Minase, T.; Tanibuchi, Y.; Hagiwara, H.; Iyo, M. Improvement of phencyclidine-induced cognitive deficits in mice by subsequent subchronic administration of fluvoxamine, but not sertraline. Open Clin. Chem. J. 2009, 2, 7–11. [Google Scholar] [CrossRef]
- Albayrak, Y.; Uğurlu, G.K.; Uğurlu, M.; Çayköylü, A. Beneficial effects of fluvoxamine for chorea in a patient with huntington’s disease: A case report. Prim. Care Companion CNS Disord. 2012, 14, 26696. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Zheng, L.; Wan, Y.; Chen, Z.; Li, P.; Wang, Y. Metoprolol, N-Acetylcysteine, and Escitalopram Prevents Chronic Unpredictable Mild Stress-Induced Depression by Inhibition of Endoplasmic Reticulum Stress. Front. Psychiatry 2018, 9, 696. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Li, J.; Ding, P.; Wang, Y. Effects of Escitalopram on Endoplasmic Reticulum Stress and Oxidative Stress Induced by Tunicamycin. Front. Neurosci. 2021, 15, 737509. [Google Scholar] [CrossRef]
- Reddy, A.P.; Yin, X.; Sawant, N.; Reddy, P.H. Protective effects of antidepressant citalopram against abnormal APP processing and amyloid beta-induced mitochondrial dynamics, biogenesis, mitophagy and synaptic toxicities in Alzheimer’s disease. Hum. Mol. Genet. 2021, 30, 847–864. [Google Scholar]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers–A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-inflammatory cytokines and treatment response to escitaloprsam in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 445–450. [Google Scholar]
- Su, F.; Yi, H.; Xu, L.; Zhang, Z. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 2015, 294, 60–68. [Google Scholar] [CrossRef]
- Tan, Y.; Duan, J.; Li, Y.; Cai, W. Effects of citalopram on serum deprivation induced PC12 cell apoptosis and BDNF expression. Pharmazie 2010, 65, 845–848. [Google Scholar]
- Ishima, T.; Fujita, Y.; Hashimoto, K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur. J. Pharmacol. 2014, 727, 167–173. [Google Scholar] [CrossRef]
- Cirrito, J.R.; Disabato, B.M.; Restivo, J.L.; Verges, D.K.; Goebel, W.D.; Sathyan, A.; Hayreh, D.; D’Angelo, G.; Benzinger, T.; Yoon, H. Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 14968–14973. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; West, T.; Yarasheski, K.; Swarm, R.; Jasielec, M.S.; Fisher, J.R.; Ficker, W.D.; Yan, P.; Xiong, C.; Frederiksen, C. An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 2014, 6, 236re4. [Google Scholar] [CrossRef] [PubMed]
- Cirrito, J.R.; Wallace, C.E.; Yan, P.; Davis, T.A.; Gardiner, W.D.; Doherty, B.M.; King, D.; Yuede, C.M.; Lee, J.-M.; Sheline, Y.I. Effect of escitalopram on Aβ levels and plaque load in an Alzheimer mouse model. Neurology 2020, 95, e2666–e2674. [Google Scholar] [CrossRef]
- Sheline, Y.I.; Snider, B.J.; Beer, J.C.; Seok, D.; Fagan, A.M.; Suckow, R.F.; Lee, J.-M.; Waligorska, T.; Korecka, M.; Aselcioglu, I. Effect of escitalopram dose and treatment duration on CSF Aβ levels in healthy older adults: A controlled clinical trial. Neurology 2020, 95, e2658–e2665. [Google Scholar] [CrossRef]
- Wu, C.; Gong, W.-G.; Wang, Y.-J.; Sun, J.-J.; Zhou, H.; Zhang, Z.-J.; Ren, Q.-G. Escitalopram alleviates stress-induced Alzheimer’s disease-like tau pathologies and cognitive deficits by reducing hypothalamic-pituitary-adrenal axis reactivity and insulin/GSK-3β signal pathway activity. Neurobiol. Aging 2018, 67, 137–147. [Google Scholar] [CrossRef]
- Sawant, N.; Kshirsagar, S.; Reddy, P.H.; Reddy, A.P. Protective effects of SSRI, Citalopram in mutant APP and mutant Tau expressed dorsal raphe neurons in Alzheimer’s disease. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2024, 1870, 166942. [Google Scholar] [CrossRef]
- Ovlyakulov, B.; Hu, B.-L.; Kan, H.-Y.; Guo, Q.; Li, X.-F.; Fan, H.-H.; Wu, H.-M.; Wang, J.-Y.; Zhang, X.; Zhu, J.-H. Escitalopram moderately outperforms citalopram towards anti-neuroinflammation and neuroprotection in 6-hydroxydopamine-induced mouse model of Parkinson’s disease. Int. Immunopharmacol. 2024, 139, 112715. [Google Scholar] [CrossRef]
- Rampello, L.; Chiechio, S.; Raffaele, R.; Vecchio, I.; Nicoletti, F. The SSRI, citalopram, improves bradykinesia in patients with Parkinson’s disease treated with L-dopa. Clin. Neuropharmacol. 2002, 25, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Safrany, S.; Brimson, J. Are fluoxetine’s effects due to sigma-1 receptor agonism? Pharmacol. Res. 2016, 113, 707–708. [Google Scholar] [CrossRef]
- Bougea, A.; Angelopoulou, E.; Vasilopoulos, E.; Gourzis, P.; Papageorgiou, S. Emerging Therapeutic Potential of Fluoxetine on Cognitive Decline in Alzheimer’s Disease: Systematic Review. Int. J. Mol. Sci. 2024, 25, 6542. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Bagini, L.; Gorini, A.; Brunello, N.; Tascedda, F. Mitochondrial energy metabolism of rat hippocampus after treatment with the antidepressants desipramine and fluoxetine. Neuropharmacology 2017, 121, 30–38. [Google Scholar] [CrossRef]
- Sonei, N.; Amiri, S.; Jafarian, I.; Anoush, M.; Rahimi-Balaei, M.; Bergen, H.; Haj-Mirzaian, A.; Hosseini, M.-J. Mitochondrial dysfunction bridges negative affective disorders and cardiomyopathy in socially isolated rats: Pros and cons of fluoxetine. World J. Biol. Psychiatry 2017, 18, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Filipović, D.; Costina, V.; Perić, I.; Stanisavljević, A.; Findeisen, P. Chronic fluoxetine treatment directs energy metabolism towards the citric acid cycle and oxidative phosphorylation in rat hippocampal nonsynaptic mitochondria. Brain Res. 2017, 1659, 41–54. [Google Scholar] [CrossRef]
- da Silva, A.I.; Braz, G.R.F.; Silva-Filho, R.; Pedroza, A.A.; Ferreira, D.S.; Manhães de Castro, R.; Lagranha, C. Effect of fluoxetine treatment on mitochondrial bioenergetics in central and peripheral rat tissues. Appl. Physiol. Nutr. Metab. 2015, 40, 565–574. [Google Scholar] [CrossRef]
- Peng, T.; Liu, X.; Wang, J.; Liu, Y.; Fu, Z.; Ma, X.; Li, J.; Sun, G.; Ji, Y.; Lu, J.; et al. Fluoxetine-mediated inhibition of endoplasmic reticulum stress is involved in the neuroprotective effects of Parkinson’s disease. Aging 2018, 10, 4188–4196. [Google Scholar] [CrossRef]
- Sierra-Fonseca, J.A.; Rodriguez, M.; Themann, A.; Lira, O.; Flores-Ramirez, F.J.; Vargas-Medrano, J.; Gadad, B.S.; Iñiguez, S.D. Autophagy Induction and Accumulation of Phosphorylated Tau in the Hippocampus and Prefrontal Cortex of Adult C57BL/6 Mice Subjected to Adolescent Fluoxetine Treatment. J. Alzheimer’s Dis. 2021, 83, 1691–1702. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.S.; Yang, H.J.; Song, G.J. Fluoxetine Potentiates Phagocytosis and Autophagy in Microglia. Front. Pharmacol. 2021, 12, 770610. [Google Scholar] [CrossRef]
- Tan, X.; Du, X.; Jiang, Y.; Botchway, B.O.A.; Hu, Z.; Fang, M. Inhibition of Autophagy in Microglia Alters Depressive-Like Behavior via BDNF Pathway in Postpartum Depression. Front. Psychiatry 2018, 9, 434. [Google Scholar] [CrossRef]
- García-García, M.L.; Tovilla-Zárate, C.A.; Villar-Soto, M.; Juárez-Rojop, I.E.; González-Castro, T.B.; Genis-Mendoza, A.D.; Ramos-Méndez, M.Á.; López-Nárvaez, M.L.; Saucedo-Osti, A.S.; Ruiz-Quiñones, J.A. Fluoxetine modulates the pro-inflammatory process of IL-6, IL-1β and TNF-α levels in individuals with depression: A systematic review and meta-analysis. Psychiatry Res. 2022, 307, 114317. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Reiersen, A.M.; Lenze, E.J.; Mennerick, S.J.; Zorumski, C.F. SSRIs differentially modulate the effects of pro-inflammatory stimulation on hippocampal plasticity and memory via sigma 1 receptors and neurosteroids. Transl. Psychiatry 2023, 13, 39. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, S.R.; Yune, T.Y. Fluoxetine prevents oligodendrocyte cell death by inhibiting microglia activation after spinal cord injury. J. Neurotrauma 2015, 32, 633–644. [Google Scholar] [CrossRef]
- Mostert, J.; Admiraal-Behloul, F.; Hoogduin, J.; Luyendijk, J.; Heersema, D.; Van Buchem, M.; De Keyser, J. Effects of fluoxetine on disease activity in relapsing multiple sclerosis: A double-blind, placebo-controlled, exploratory study. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Jing, P.; Liu, Z.; Li, Z.; Ma, H.; Tu, W.; Zhang, W.; Zhuo, C. Beneficial effect of fluoxetine treatment aganist psychological stress is mediated by increasing BDNF expression in selected brain areas. Oncotarget 2017, 8, 69527. [Google Scholar] [CrossRef]
- Hui, J.; Zhang, J.; Kim, H.; Tong, C.; Ying, Q.; Li, Z.; Mao, X.; Shi, G.; Yan, J.; Zhang, Z. Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3β/β-catenin signaling. Int. J. Neuropsychopharmacol. 2015, 18, pyu099. [Google Scholar] [CrossRef]
- Grote, H.E.; Bull, N.D.; Howard, M.L.; Van Dellen, A.; Blakemore, C.; Bartlett, P.F.; Hannan, A.J. Cognitive disorders and neurogenesis deficits in Huntington’s disease mice are rescued by fluoxetine. Eur. J. Neurosci. 2005, 22, 2081–2088. [Google Scholar] [CrossRef]
- Marotta, G.; Basagni, F.; Rosini, M.; Minarini, A. Memantine derivatives as multitarget agents in Alzheimer’s disease. Molecules 2020, 25, 4005. [Google Scholar] [CrossRef]
- Parsons, C.; Danysz, W.; Quack, G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist—A review of preclinical data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Esposito, Z.; Belli, L.; Toniolo, S.; Sancesario, G.; Bianconi, C.; Martorana, A. Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci. Ther. 2013, 19, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Wenk, G.L. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Rev. 2003, 9, 275–308. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Stöffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system-too little activation is bad, too much is even worse. Neuropharmacology 2007, 53, 699–723. [Google Scholar] [CrossRef]
- Transm, J.N. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm. 1994, 43, 91–104. [Google Scholar]
- Keshavarz, M.; Farrokhi, M.R.; Fard, E.A.; Mehdipour, M. Contribution of lysosome and sigma receptors to neuroprotective effects of memantine against beta-amyloid in the SH-SY5Y cells. Adv. Pharm. Bull. 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Hidau, M.K.; Rai, S. Memantine treatment exerts an antidepressant-like effect by preventing hippocampal mitochondrial dysfunction and memory impairment via upregulation of CREB/BDNF signaling in the rat model of chronic unpredictable stress-induced depression. Neurochem. Int. 2021, 142, 104932. [Google Scholar] [CrossRef]
- Bommaraju, S.; Dhokne, M.D.; Rakeshkumar, P.P.; Datusalia, A.K. Memantine Alleviates PTSD-like Symptoms and Improves Dendritic Arborization through Modulation of the HPA Axis and Neuroinflammation in Rats. Neurochem. Res. 2025, 50, 58. [Google Scholar] [CrossRef]
- Moreau, C.; Delval, A.; Tiffreau, V.; Defebvre, L.; Dujardin, K.; Duhamel, A.; Petyt, G.; Hossein-Foucher, C.; Blum, D.; Sablonnière, B.; et al. Memantine for axial signs in Parkinson’s disease: A randomised, double-blind, placebo-controlled pilot study. J. Neurol. Neurosurg Psychiatry 2013, 84, 552–555. [Google Scholar] [CrossRef]
- Leroi, I.; Overshott, R.; Byrne, E.J.; Daniel, E.; Burns, A. Randomized controlled trial of memantine in dementia associated with Parkinson’s disease. Mov. Disord. 2009, 24, 1217–1221. [Google Scholar] [CrossRef]
- Emre, M.; Tsolaki, M.; Bonuccelli, U.; Destée, A.; Tolosa, E.; Kutzelnigg, A.; Ceballos-Baumann, A.; Zdravkovic, S.; Bladström, A.; Jones, R. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010, 9, 969–977. [Google Scholar] [CrossRef]
- Ondo, W.G.; Mejia, N.I.; Hunter, C.B. A pilot study of the clinical efficacy and safety of memantine for Huntington’s disease. Park. Relat. Disord. 2007, 13, 453–454. [Google Scholar] [CrossRef]
- Dicpinigaitis, P. The current and emerging treatment landscape for chronic cough. Am. J. Manag. Care 2022, 28, S159–S165. [Google Scholar] [PubMed]
- Carpenter, C.L.; Marks, S.S.; Watson, D.L.; Greenberg, D.A. Dextromethorphan and dextrorphan as calcium channel antagonists. Brain Res. 1988, 439, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.P.; Traynelis, S.F.; Siffert, J.; Pope, L.E.; Matsumoto, R.R. Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta®) clinical use. Pharmacol. Ther. 2016, 164, 170–182. [Google Scholar] [CrossRef]
- Shin, E.-J.; Nah, S.-Y.; Chae, J.S.; Bing, G.; Shin, S.W.; Yen, T.P.H.; Baek, I.-H.; Kim, W.-K.; Maurice, T.; Nabeshima, T. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats. Neurochem. Int. 2007, 50, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.; Thomas, K.L.; Lucke-Wold, B.P.; Cavendish, J.Z.; Crowe, M.S.; Matsumoto, R.R. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol. Ther. 2016, 159, 1–22. [Google Scholar] [CrossRef]
- Metman, L.V.; Blanchet, P.J.; Van Den Munckhof, P.; Dotto, P.D.; Natté, R.; Chase, T.N. A trial of dextromethorphan in parkinsonian patients with motor response complications. Mov. Disord. 1998, 13, 414–417. [Google Scholar] [CrossRef]
- Paquette, M.A.; Brudney, E.G.; Putterman, D.B.; Meshul, C.K.; Johnson, S.W.; Berger, S.P. Sigma ligands, but not N-methyl-D-aspartate antagonists, reduce levodopa-induced dyskinesias. Neuroreport 2008, 19, 111–115. [Google Scholar] [CrossRef]
- Yang, J.-J.; Liu, Y.-X.; Wang, Y.-F.; Ge, B.-Y.; Wang, Y.; Wang, Q.-S.; Li, S.; Zhang, J.-J.; Jin, L.-L.; Hong, J.-S. Anti-epileptic and neuroprotective effects of ultra-low dose NADPH oxidase inhibitor dextromethorphan on kainic acid-induced chronic temporal lobe epilepsy in rats. Neurosci. Bull. 2024, 40, 577–593. [Google Scholar] [CrossRef]
- Yang, Y.-N.; Yang, Y.-C.S.; Wu, P.-L.; Yang, C.-H.; Kuo, K.-C.; Yang, S.-N. Dextromethorphan Suppresses Lipopolysaccharide-Induced Epigenetic Histone Regulation in the Tumor Necrosis Factor-α Expression in Primary Rat Microglia. Mediat. Inflamm. 2020, 2020, 9694012. [Google Scholar] [CrossRef]
- Li, G.; Cui, G.; Tzeng, N.-S.; Wei, S.-J.; Wang, T.; Block, M.L.; Hong, J.-S. Femtomolar concentrations of dextromethorphan protect mesencephalic dopaminergic neurons from inflammatory damage. FASEB J. 2005, 19, 489–496. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, L.; Li, G.; Zhang, W.; An, L.; Liu, B.; Hong, J.-S. Dextromethorphan protects dopaminergic neurons against inflammation-mediated degeneration through inhibition of microglial activation. J. Pharmacol. Exp. Ther. 2003, 305, 212–218. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, T.; Qin, L.; Gao, H.M.; Wilson, B.; Ali, S.F.; Zhang, W.; Hong, J.S.; Liu, B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: Role of NADPH oxidase. FASEB J. 2004, 18, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Pleasure, D.E.; Hong, J.-S.; Deng, W. Low dose dextromethorphan attenuates moderate experimental autoimmune encephalomyelitis by inhibiting NOX2 and reducing peripheral immune cells infiltration in the spinal cord. Neurobiol. Dis. 2011, 44, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Dextromethorphan/Bupropion: First approval. CNS Drugs 2022, 36, 1229–1238. [Google Scholar] [CrossRef]
- Parincu, Z.; Iosifescu, D.V. Combinations of dextromethorphan for the treatment of mood disorders—A review of the evidence. Expert Rev. Neurother. 2023, 23, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, W.M. Recognizing and treating pseudobulbar affect. CNS Spectr. 2016, 21, 34–44. [Google Scholar] [CrossRef]
- McClure, E.W.; Daniels, R.N. Classics in chemical neuroscience: Dextromethorphan (DXM). ACS Chem. Neurosci. 2023, 14, 2256–2270. [Google Scholar] [CrossRef]
- Smith, R.; Pioro, E.; Myers, K.; Sirdofsky, M.; Goslin, K.; Meekins, G.; Yu, H.; Wymer, J.; Cudkowicz, M.; Macklin, E.A. Enhanced bulbar function in amyotrophic lateral sclerosis: The nuedexta treatment trial. Neurotherapeutics 2017, 14, 762–772. [Google Scholar] [CrossRef]
- Green, J.R.; Allison, K.M.; Cordella, C.; Richburg, B.D.; Pattee, G.L.; Berry, J.D.; Macklin, E.A.; Pioro, E.P.; Smith, R.A. Additional evidence for a therapeutic effect of dextromethorphan/quinidine on bulbar motor function in patients with amyotrophic lateral sclerosis: A quantitative speech analysis. Br. J. Clin. Pharmacol. 2018, 84, 2849–2856. [Google Scholar] [CrossRef]
- Pioro, E.P.; Brooks, B.R.; Cummings, J.; Schiffer, R.; Thisted, R.A.; Wynn, D.; Hepner, A.; Kaye, R. Dextromethorphan plus ultra Low-Dose quinidine reduces pseudobulbar affect. Ann. Neurol. 2010, 68, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Metman, L.V.; Nutt, J.G.; Brodsky, M.; Factor, S.A.; Lang, A.E.; Pope, L.E.; Knowles, N.; Siffert, J. Trial of dextromethorphan/quinidine to treat levodopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2017, 32, 893–903. [Google Scholar] [CrossRef]
- Cummings, J.; Lyketsos, C.G.; Tariot, P.; Peskind, E.; Nguyen, U.; Knowles, N.; Shin, P.; Siffert, J. Dextromethorphan/quinidine (AVP-923) Efficacy and Safety for Treatment of Agitation in Persons with Alzheimer’s Disease: Results from a Phase 2 Study (NCT01584440). Am. J. Geriatr. Psychiatry 2015, 23, S164–S165. [Google Scholar] [CrossRef]
- Fralick, M.; Sacks, C.A.; Kesselheim, A.S. Assessment of use of combined dextromethorphan and quinidine in patients with dementia or Parkinson disease after US Food and Drug Administration approval for pseudobulbar affect. JAMA Intern. Med. 2019, 179, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Blanpied, T.A.; Clarke, R.J.; Johnson, J.W. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005, 25, 3312–3322. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef]
- Uitti, R.; Rajput, A.; Ahlskog, J.; Offord, K.; Schroeder, D.; Ho, M.; Prasad, M.; Rajput, A.; Basran, P. Amantadine treatment is an independent predictor of improved survival in Parkinson’s disease. Neurology 1996, 46, 1551–1556. [Google Scholar] [CrossRef]
- Gianutsos, G.; Chute, S.; Dunn, J.P. Pharmacological changes in dopaminergic systems induced by long-term administration of amantadine. Eur. J. Pharmacol. 1985, 110, 357–361. [Google Scholar] [CrossRef]
- Mata-Bermudez, A.; Ríos, C.; Burelo, M.; Pérez-González, C.; García-Martínez, B.A.; Jardon-Guadarrama, G.; Calderón-Estrella, F.; Manning-Balpuesta, N.; Diaz-Ruiz, A. Amantadine prevented hypersensitivity and decreased oxidative stress by NMDA receptor antagonism after spinal cord injury in rats. Eur. J. Pain 2021, 25, 1839–1851. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, H.-W.; Hwang, J.; Kim, J.; Lee, M.-J.; Han, H.-S.; Lee, W.-H.; Suk, K. Microglia-inhibiting activity of Parkinson’s disease drug amantadine. Neurobiol. Aging 2012, 33, 2145–2159. [Google Scholar] [CrossRef]
- Al-Salama, Z.T. Amantadine extended release capsules (GOCOVRI®) in Parkinson’s disease: A profile of its use in the USA. Drugs Ther. Perspect. 2022, 38, 203–214. [Google Scholar] [CrossRef]
- Hauser, R.A.; Mehta, S.H.; Kremens, D.; Chernick, D.; Formella, A.E. Effects of Gocovri (amantadine) extended-release capsules on motor aspects of experiences of daily living in people with Parkinson’s disease and dyskinesia. Neurol. Ther. 2021, 10, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Verhagen Metman, L.; Morris, M.; Farmer, C.; Gillespie, M.; Mosby, K.; Wuu, J.; Chase, T. Huntington’s disease: A randomized, controlled trial using the NMDA-antagonist amantadine. Neurology 2002, 59, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Geerts, H.; Guillaumat, P.-O.; Grantham, C.; Bode, W.; Anciaux, K.; Sachak, S. Brain levels and acetylcholinesterase inhibition with galantamine and donepezil in rats, mice, and rabbits. Brain Res. 2005, 1033, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kandiah, N.; Hsu, J.L.; Suthisisang, C.; Udommongkol, C.; Dash, A. Beyond symptomatic effects: Potential of donepezil as a neuroprotective agent and disease modifier in Alzheimer’s disease. Br. J. Pharmacol. 2017, 174, 4224–4232. [Google Scholar] [CrossRef]
- Li, Q.; He, S.; Chen, Y.; Feng, F.; Qu, W.; Sun, H. Donepezil-based multi-functional cholinesterase inhibitors for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 158, 463–477. [Google Scholar] [CrossRef]
- Tsuno, N. Donepezil in the treatment of patients with Alzheimer’s disease. Expert Rev. Neurother. 2009, 9, 591–598. [Google Scholar] [CrossRef]
- Ramakrishnan, N.K.; Visser, A.K.; Schepers, M.; Luurtsema, G.; Nyakas, C.J.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.; van Waarde, A. Dose-dependent sigma-1 receptor occupancy by donepezil in rat brain can be assessed with 11 C-SA4503 and microPET. Psychopharmacology 2014, 231, 3997–4006. [Google Scholar] [CrossRef]
- Kenche, H.; Singh, M.; Smith, J.; Shen, K. Neuronal mitochondrial dysfunction in a cellular model of circadian rhythm disruption is rescued by donepezil. Biochem. Biophys. Res. Commun. 2021, 567, 56–62. [Google Scholar] [CrossRef]
- Kim, E.; Park, M.; Jeong, J.; Kim, H.; Lee, S.K.; Lee, E.; Oh, B.H.; Namkoong, K. Cholinesterase inhibitor donepezil increases mitochondrial biogenesis through AMP-activated protein kinase in the hippocampus. Neuropsychobiology 2016, 73, 81–91. [Google Scholar] [CrossRef]
- Takahashi, K.; Tsuji, M.; Nakagawasai, O.; Katsuyama, S.; Hong, L.; Miyagawa, K.; Kurokawa, K.; Mochida-Saito, A.; Takeda, H.; Tadano, T. Donepezil prevents olfactory dysfunction and α-synuclein aggregation in the olfactory bulb by enhancing autophagy in zinc sulfate-treated mice. Behav. Brain Res. 2023, 438, 114175. [Google Scholar] [CrossRef]
- Meunier, J.; Ieni, J.; Maurice, T. The anti-amnesic and neuroprotective effects of donepezil against amyloid β25-35 peptide-induced toxicity in mice involve an interaction with the σ1 receptor. Br. J. Pharmacol. 2006, 149, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.Y.; Lei, Y.; Tang, X.C.; Zhang, H.Y. Donepezil attenuates Aβ-associated mitochondrial dysfunction and reduces mitochondrial Aβ accumulation in vivo and in vitro. Neuropharmacology 2015, 95, 29–36. [Google Scholar] [CrossRef]
- Solntseva, E.; Kapai, N.; Popova, O.; Rogozin, P.; Skrebitsky, V. The involvement of sigma1 receptors in donepezil-induced rescue of hippocampal LTP impaired by beta-amyloid peptide. Brain Res. Bull. 2014, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Kunitachi, S.; Fujita, Y.; Ishima, T.; Kohno, M.; Horio, M.; Tanibuchi, Y.; Shirayama, Y.; Iyo, M.; Hashimoto, K. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: Role of sigma-1 receptors. Brain Res. 2009, 1279, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Ishima, T.; Nishimura, T.; Iyo, M.; Hashimoto, K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: Role of sigma-1 receptors and IP3 receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1656–1659. [Google Scholar] [CrossRef]
- Valencia, M.E.; Herrera-Arozamena, C.; de Andrés, L.; Pérez, C.; Morales-García, J.A.; Pérez-Castillo, A.; Ramos, E.; Romero, A.; Viña, D.; Yáñez, M. Neurogenic and neuroprotective donepezil-flavonoid hybrids with sigma-1 affinity and inhibition of key enzymes in Alzheimer’s disease. Eur. J. Med. Chem. 2018, 156, 534–553. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, H.-Y.; Oh, J.-P.; Park, J.; Bae, S.-H.; Park, H.; Kim, E.J.; Lee, J.-H. Therapeutic effects of combination of Nebivolol and donepezil: Targeting multifactorial mechanisms in ALS. Neurotherapeutics 2023, 20, 1779–1795. [Google Scholar] [CrossRef]
- Chen, S.; Liang, T.; Xue, T.; Xue, S.; Xue, Q. Pridopidine for the improvement of motor function in patients with Huntington’s disease: A systematic review and Meta-analysis of randomized controlled trials. Front. Neurol. 2021, 12, 658123. [Google Scholar] [CrossRef]
- Matić, T.; Hendriks, M.; Vinke, R.S.; Sadikov, A.; Georgiev, D. The effect of serotonin reuptake and serotonin-noradrenaline reuptake inhibitors on motor symptoms in Parkinson’s disease: A PPMI-based matched-subject study. J. Park. Dis. 2024, 14, 1642–1651. [Google Scholar] [CrossRef]
- Richard, I.; McDermott, M.; Kurlan, R.; Lyness, J.; Como, P.; Pearson, N.; Factor, S.; Juncos, J.; Serrano Ramos, C.; Brodsky, M. A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012, 78, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tabu, H.; Ozaki, A.; Hamano, T.; Takeshima, T.; Group, R.S. Antidepressants for depression, apathy, and gait instability in Parkinson’s disease: A multicenter randomized study. Intern. Med. 2019, 58, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ceravolo, R.; Nuti, A.; Piccinni, A.; Agnello, G.D.; Bellini, G.; Gambaccini, G.; Osso, L.D.; Murri, L.; Bonuccelli, U. Paroxetine in Parkinson’s disease: Effects on motor and depressive symptoms. Neurology 2000, 55, 1216–1218. [Google Scholar] [CrossRef]
- Dell’Agnello, G.; Ceravolo, R.; Nuti, A.; Bellini, G.; Piccinni, A.; D’Avino, C.; Dell’Osso, L.; Bonuccelli, U. SSRIs do not worsen Parkinson’s disease: Evidence from an open-label, prospective study. Clin. Neuropharmacol. 2001, 24, 221–227. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Grimshaw, A.A.; Axson, S.A.; Choe, S.H.; Miller, J.E. Drug repurposing: A systematic review on root causes, barriers and facilitators. BMC Health Serv. Res. 2022, 22, 970. [Google Scholar] [CrossRef]


| Drug Name | 2D Structure | Primary Target MOA | Currently Approved | Radioligand S1R Ki | S1R MOA | Potential Repurposing Disease(s) |
|---|---|---|---|---|---|---|
| Fluvoxamine | 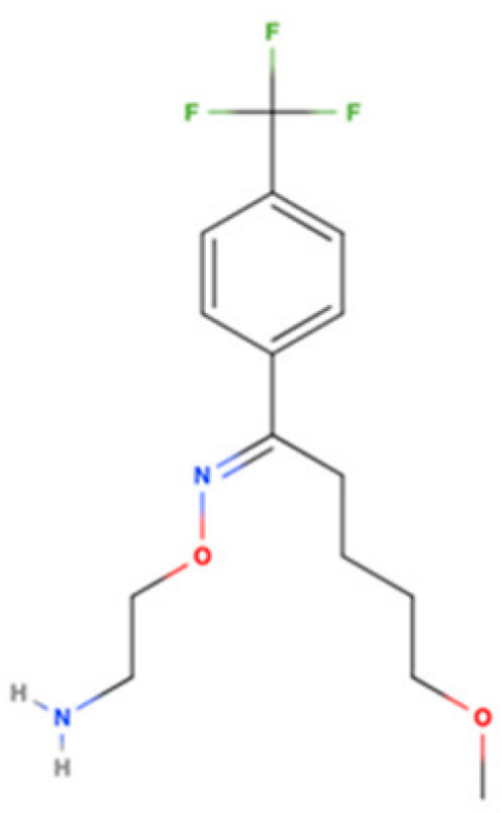 | SSRI | Psychiatric disorders (e.g., depression and obsessive-compulsive disorder) | [3H](+)-pentazocine 36 nM [116] | Agonist neuroprotection (↑calcium influx into mitochondria) ↓γ-secretase activity ↓ER stress ↑autophagy ↓neuroinflammation ↑neurotrophic factors like BDNF and NGF | Cognitive impairments, AD, MS, ALS, and HD |
| Citalopram | 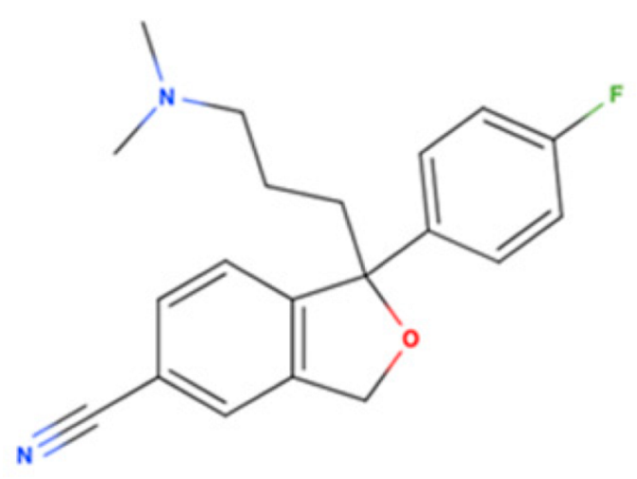 | SSRI | Psychiatric disorders (e.g., depression and obsessive-compulsive disorder) | [3H](+)-pentazocine 292 nM [116] | Agonist ↓pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β) ↑autophagy ↓mutant amyloid precursor protein (APP) and Aβ ↑neurotrophins like BDNF | Amyloid-related disorders (e.g., AD) and movement disorders (e.g., PD) |
| Escitalopram | 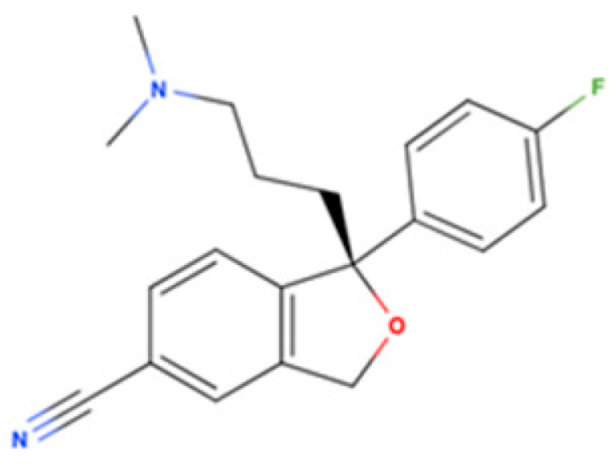 | SSRI | Psychiatric disorders (e.g., depression and obsessive-compulsive disorder) | [3H](+)-pentazocine 288.3 nM [116] | Agonist ↓ER stress ↑autophagy ↑M2 microglial activation ↓neuroinflammatory response ↓Aβ burden ↑NGF-induced neurite outgrowth | Amyloid-related disorders (e.g., AD) and movement disorders (e.g., PD) |
| Fluoxetine | 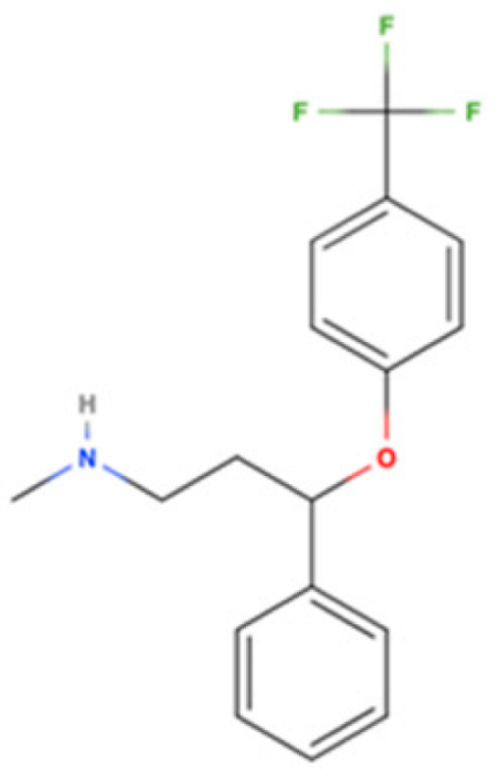 | SSRI | Psychiatric disorders (e.g., depression and obsessive-compulsive disorder) | [3H](+)-pentazocine 240 nM [116] | Agonist ↑ATP by increasing mitochondrial function ↓ER-stress ↓pro-inflammatory cytokines (TNF-α, IL-1β, and INF-γ) regulate microglial activation (↓M1 and ↑M2 activity) ↑autophagy ↑BDNF and NGF | AD, PD, and HD |
| Memantine | 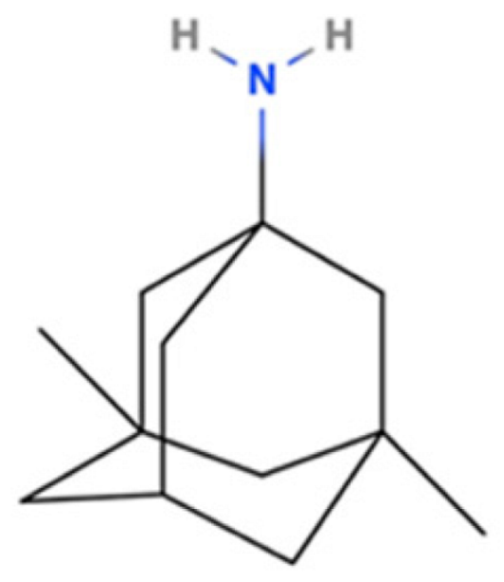 | NMDA Receptor Antagonist | AD | [3H]-(+)SKF-10,047 2.5 μM [117] | Agonist ↑mitochondrial function ↓oxidative stress ↓inflammatory cytokines (TNF-α and IL-6) ↓Aβ production ↑BDNF | Neurodegene-rative disorders (e.g., PD and HD) |
| DXM | 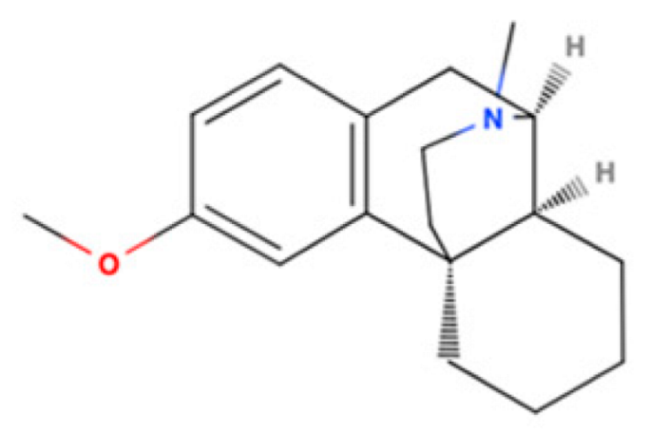 | NMDAR Antagonist | Cough suppressant and pseudobulbar effect in combination with quinidine | [3H]-(+)SKF-10,047 205 nM [118] | Agonist ↓neuroinflammation ↓ER stress ↓ROS generation ↑NGF | PD, MS, and ALS |
| Amantadine | 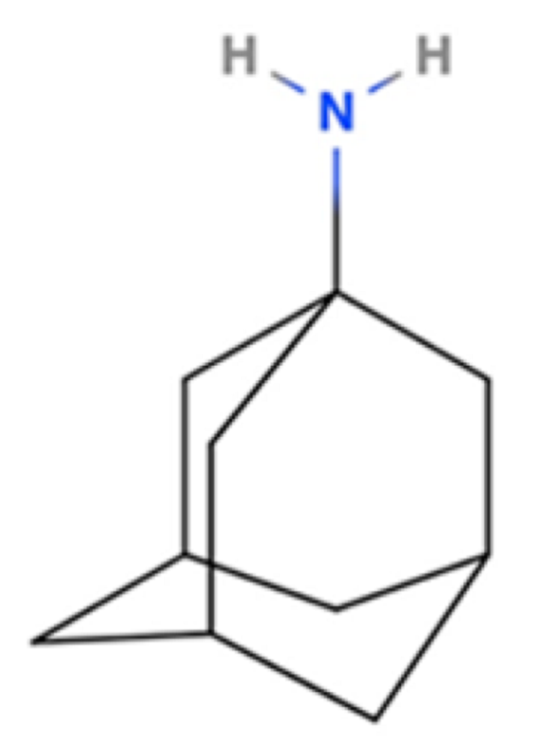 | NMDA Receptor Antagonist | PD | [3H]-(+)SKF-10,047 7 μM [117] | Agonist ↑dopaminergic transmission ↓oxidative stress ↓neuroinflammation | Movement disorders (e.g., HD) |
| Donepezil | 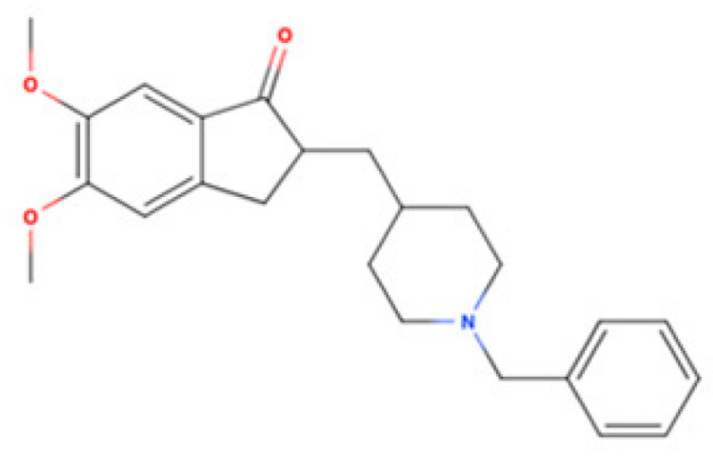 | AChE Inhibitor | AD | [3H]DTG 14.6 nM [119] | Agonist ↑mitochondrial function ↓Aβ accumulation ↓neuroinflammatory responses ↓excitotoxicity ↑NGF | Cognitive deficits, ALS, and other neurodegene- rative disorders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eskandari, K.; Bélanger, S.-M.; Lachance, V.; Kourrich, S. Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders. Pharmaceuticals 2025, 18, 700. https://doi.org/10.3390/ph18050700
Eskandari K, Bélanger S-M, Lachance V, Kourrich S. Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders. Pharmaceuticals. 2025; 18(5):700. https://doi.org/10.3390/ph18050700
Chicago/Turabian StyleEskandari, Kiarash, Sara-Maude Bélanger, Véronik Lachance, and Saïd Kourrich. 2025. "Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders" Pharmaceuticals 18, no. 5: 700. https://doi.org/10.3390/ph18050700
APA StyleEskandari, K., Bélanger, S.-M., Lachance, V., & Kourrich, S. (2025). Repurposing Sigma-1 Receptor-Targeting Drugs for Therapeutic Advances in Neurodegenerative Disorders. Pharmaceuticals, 18(5), 700. https://doi.org/10.3390/ph18050700









