Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review
Abstract
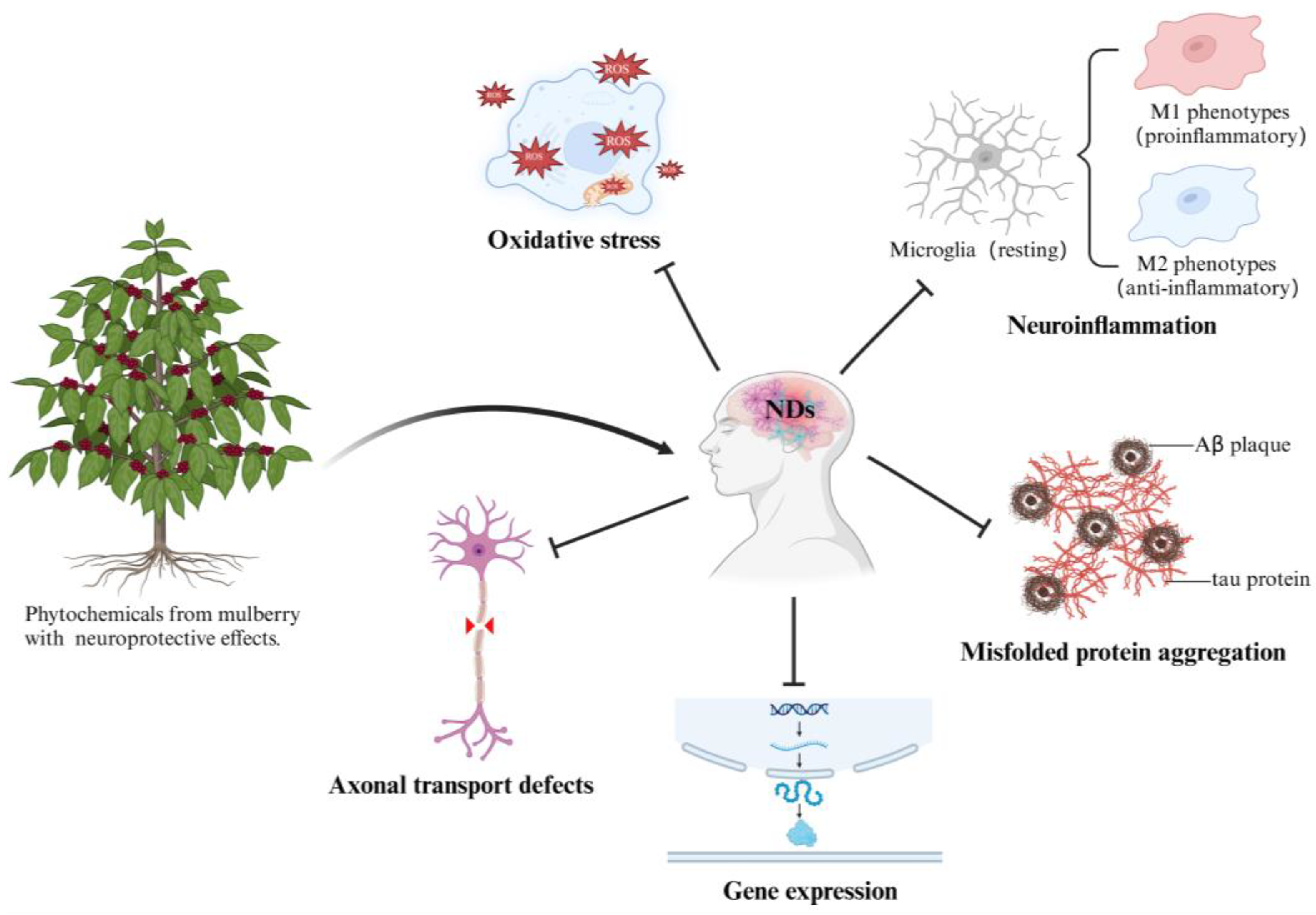
:1. Introduction
2. Mulberry Extracts with Neuroprotective Effects
2.1. Differences Between Mulberry Leaf and Fruit Extracts
2.2. Extract of Mulberry Fruit
2.3. Extract of Mulberry Leaf
3. Chemical Components of Mulberry with Neuroprotective Effects
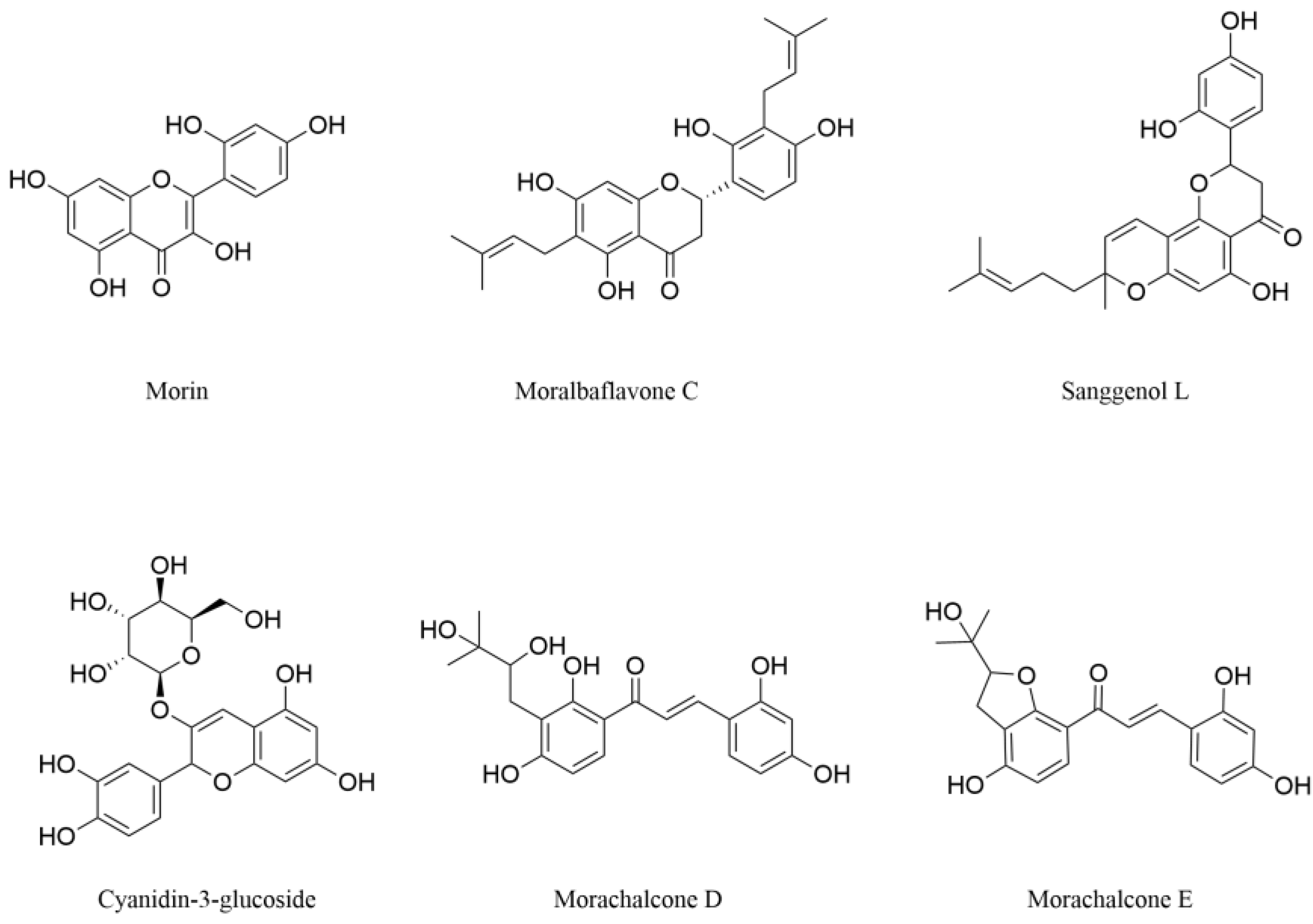
3.1. Flavonoids
3.1.1. Flavonols
3.1.2. Dihydroflavones
3.1.3. Anthocyanins
3.1.4. Chalcones
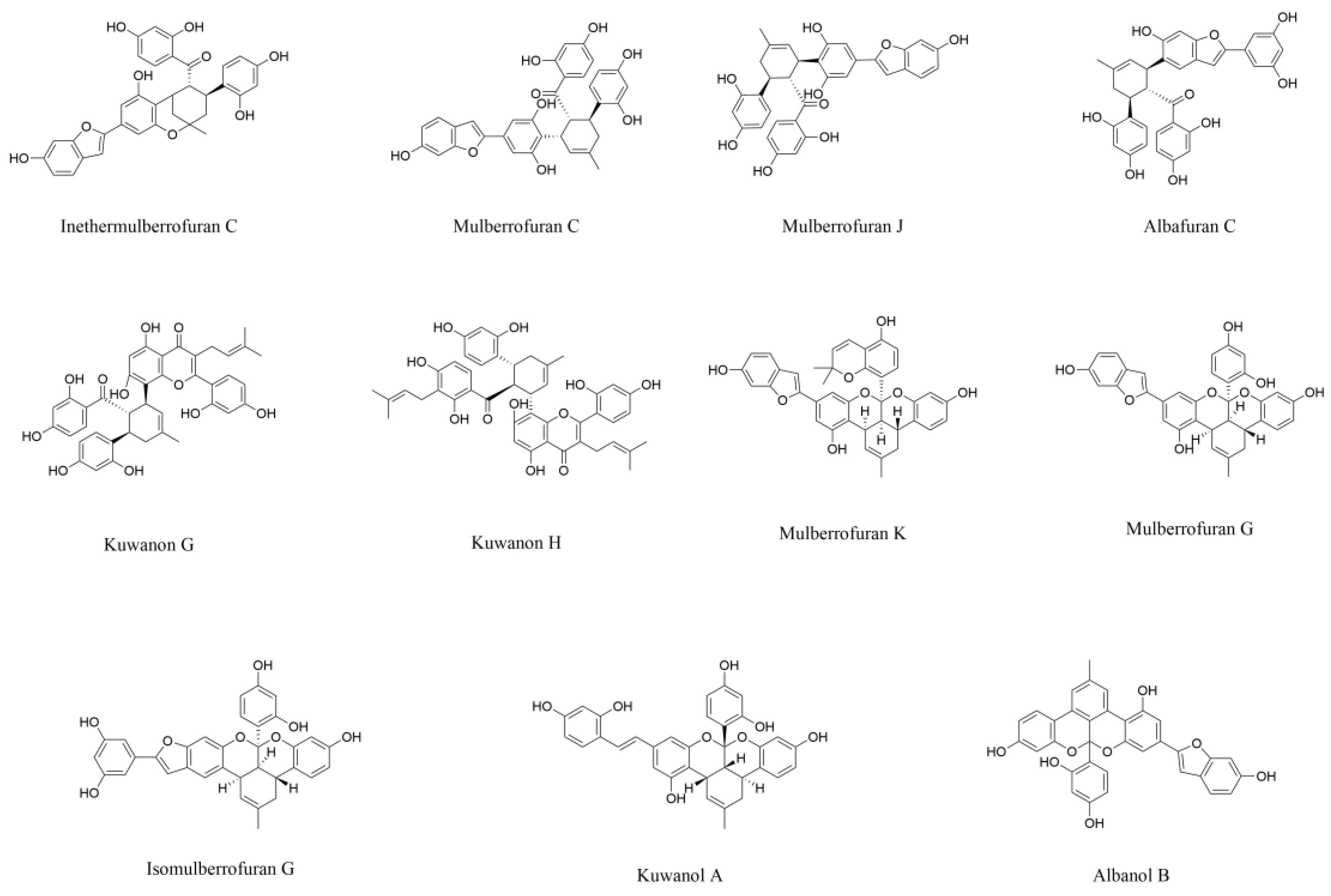
3.2. Diels-Alder-Type Adducts
3.3. Benzofurans
3.4. Quinones
3.5. Stilbenes
3.6. Alkaloids
4. Limitations and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Polyphenols Targeting NF-κB Pathway in Neurological Disorders: What We Know So Far? Int. J. Biol. Sci. 2024, 20, 1332–1355. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, Regional, and National Burden and Attributable Risk Factors of Neurological Disorders: The Global Burden of Disease Study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.D.; Seeher, K.M.; Schiess, N.; Nichols, E.; Cao, B.; Servili, C.; Cavallera, V.; Cousin, E.; Hagins, H.; Moberg, M.E.; et al. Global, Regional, and National Burden of Disorders Affecting the Nervous System, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Pan, H.; Han, L. Global, Regional, and National Burden of Neurological Disorders in 204 Countries and Territories Worldwide. J. Glob. Health 2023, 13, 04160. [Google Scholar] [CrossRef]
- Nunomura, A.; Perry, G.; Aliev, G.; Hirai, K.; Takeda, A.; Balraj, E.K.; Jones, P.K.; Ghanbari, H.; Wataya, T.; Shimohama, S.; et al. Oxidative Damage Is the Earliest Event in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2001, 60, 759–767. [Google Scholar] [CrossRef]
- Ansari, M.A.; Scheff, S.W. Oxidative Stress in the Progression of Alzheimer Disease in the Frontal Cortex. J. Neuropathol. Exp. Neurol. 2010, 69, 155–167. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Von Bernhardi, R.; Eugenín-von Bernhardi, L.; Eugenín, J. Microglial Cell Dysregulation in Brain Aging and Neurodegeneration. Front. Aging Neurosci. 2015, 7, 124. [Google Scholar] [CrossRef]
- Giacomelli, C.; Daniele, S.; Martini, C. Potential Biomarkers and Novel Pharmacological Targets in Protein Aggregation-Related Neurodegenerative Diseases. Biochem. Pharmacol. 2017, 131, 1–15. [Google Scholar] [CrossRef]
- Bignante, E.A.; Heredia, F.; Morfini, G.; Lorenzo, A. Amyloid β Precursor Protein as a Molecular Target for Amyloid β–Induced Neuronal Degeneration in Alzheimer’s Disease. Neurobiol. Aging 2013, 34, 2525–2537. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Z.; Lian, P.; Xu, Y.; Cao, X. Common Mechanisms Underlying Axonal Transport Deficits in Neurodegenerative Diseases: A Mini Review. Front. Mol. Neurosci. 2023, 16, 1172197. [Google Scholar] [CrossRef] [PubMed]
- Beers, D.R.; Appel, S.H. Immune Dysregulation in Amyotrophic Lateral Sclerosis: Mechanisms and Emerging Therapies. Lancet Neurol. 2019, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, S.; Li, X.-J. Genetically Modified Large Animal Models for Investigating Neurodegenerative Diseases. Cell Biosci. 2021, 11, 218. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Deng, H.; Wang, P.; Jankovic, J. The Genetics of Parkinson Disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Das, S.; Basu, S. Multi-Targeting Strategies for Alzheimer’s Disease Therapeutics: Pros and Cons. Curr. Top. Med. Chem. 2017, 17, 3017–3061. [Google Scholar] [CrossRef]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An Update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural Compounds as Medical Strategies in the Prevention and Treatment of Psychiatric Disorders Seen in Neurological Diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef]
- Raghunathan, T.; Srinivasan, S.; Jamuna, S. Neuroprotective Effect of Ethanolic Extract of Scoparia Dulcis on Acrylamide-Induced Neurotoxicity in Zebrafish Model. Appl. Biochem. Biotechnol. 2024, 196, 3992–4007. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.-W.; Shaw, P.-C. Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells 2022, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Cascao, R.; Fonseca, J.E.; Moita, L.F. Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 2017, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Jantas, D.; Lenda, T.; Domin, H.; Czarnecka, A.; Kuter, K.; Śmiałowska, M.; Lasoń, W.; Lorenc-Koci, E. Lack of Neuroprotective Effect of Celastrol Under Conditions of Proteasome Inhibition by Lactacystin in In Vitro and In Vivo Studies: Implications for Parkinson’s Disease. Neurotox. Res. 2014, 26, 255–273. [Google Scholar] [CrossRef]
- Echeverry, C.; Pazos, M.; Torres-Pérez, M.; Prunell, G. Plant-Derived Compounds and Neurodegenerative Diseases: Different Mechanisms of Action with Therapeutic Potential. Neuroscience 2025, 566, 149–160. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) Fruit—A Review of Characteristic Components and Health Benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Tang, C.; Bao, T.; Zhang, Q.; Qi, H.; Huang, Y.; Zhang, B.; Zhao, L.; Tong, X. Clinical Potential and Mechanistic Insights of Mulberry (Morus alba L.) Leaves in Managing Type 2 Diabetes Mellitus: Focusing on Gut Microbiota, Inflammation, and Metabolism. J. Ethnopharmacol. 2023, 306, 116143. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.-G.; Linhardt, R.J.; Liao, S.-T.; Wu, H.; Zou, Y.-X. Mulberry: A Review of Bioactive Compounds and Advanced Processing Technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Zhong, Y.; Song, B.; Zheng, C.; Zhang, S.; Yan, Z.; Tang, Z.; Kong, X.; Duan, Y.; Li, F. Flavonoids from Mulberry Leaves Alleviate Lipid Dysmetabolism in High Fat Diet-Fed Mice: Involvement of Gut Microbiota. Microorganisms 2020, 8, 860. [Google Scholar] [CrossRef]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of Mulberry Leaves’ Hypoglycemic Properties and Hypoglycemic Mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef]
- Zhao, L.; Ouyang, D.; Cheng, X.; Zhou, X.; Lin, L.; Wang, J.; Wu, Q.; Jia, J. Multi-Frequency Ultrasound-Assisted Cellulase Extraction of Protein from Mulberry Leaf: Kinetic, Thermodynamic, and Structural Properties. Ultrason. Sonochem. 2023, 99, 106554. [Google Scholar] [CrossRef] [PubMed]
- Martín-García, B.; Aznar-Ramos, M.J.; Verardo, V.; Gómez-Caravaca, A.M. The Establishment of Ultrasonic-Assisted Extraction for the Recovery of Phenolic Compounds and Evaluation of Their Antioxidant Activity from Morus alba Leaves. Foods 2022, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Bang, N.; Srichana, T. The Properties and Stability of Anthocyanins in Mulberry Fruits. Food Res. Int. 2010, 43, 1093–1097. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Jiang, L.; Zhang, J.-G.; Deng, W.; Wang, H.-L.; Wei, Z.-J. Quantitative Determination of 1-Deoxynojirimycin in Mulberry Leaves from 132 Varieties. Ind. Crops Prod. 2013, 49, 782–784. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y. Antioxidant Properties and Electrochemical Activity of Anthocyanins and Anthocyanidins in Mulberries. J. Food Meas. Charact. 2024, 18, 3569–3576. [Google Scholar] [CrossRef]
- Han, W.; Chen, X.; Yu, H.; Chen, L.; Shen, M. Seasonal Variations of Iminosugars in Mulberry Leaves Detected by Hydrophilic Interaction Chromatography Coupled with Tandem Mass Spectrometry. Food Chem. 2018, 251, 110–114. [Google Scholar] [CrossRef]
- Lin, M.; Li, Y.; Gao, Q.; Shi, L.; He, W.; Li, W.; Liang, Y.; Zhang, Z. Dynamic Changes in Physicochemical and Transcriptional Expression Profiles of Mulberry (Morus alba L.) Fruit during Ripening. Food Biosci. 2024, 57, 103606. [Google Scholar] [CrossRef]
- Chen, N.-C.; Chyau, C.-C.; Lee, Y.-J.; Tseng, H.-C.; Chou, F.-P. Promotion of Mitotic Catastrophe via Activation of PTEN by Paclitaxel with Supplement of Mulberry Water Extract in Bladder Cancer Cells. Sci. Rep. 2016, 6, 20417. [Google Scholar] [CrossRef]
- Kaewkaen, P.; Tong-un, T.; Wattanathorn, J.; Muchimapura, S.; Kaewrueng, W.; Wongcharoenwanakit, S. Mulberry Fruit Extract Protects against Memory Impairment and Hippocampal Damage in Animal Model of Vascular Dementia. Evid. Based Complement. Alternat. Med. 2012, 2012, 263520. [Google Scholar] [CrossRef]
- Liu, D.; Du, D. Mulberry Fruit Extract Alleviates Cognitive Impairment by Promoting the Clearance of Amyloid-β and Inhibiting Neuroinflammation in Alzheimer’s Disease Mice. Neurochem. Res. 2020, 45, 2009–2019. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Zhang, X.; Jin, Q.; Li, J. Phenolic Profiles, Antioxidant Activities, and Neuroprotective Properties of Mulberry (Morus Atropurpurea Roxb.) Fruit Extracts from Different Ripening Stages. J. Food Sci. 2016, 81, C2439–C2446. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Yang, H.; Pang, W.; Qie, Z.; Lu, H.; Tan, L.; Li, H.; Sun, S.; Lian, F.; Qin, C.; et al. Mulberry Extracts Alleviate Aβ 25–35-Induced Injury and Change the Gene Expression Profile in PC12 Cells. Evid. Based Complement. Alternat. Med. 2014, 2014, 150617. [Google Scholar] [CrossRef] [PubMed]
- Ochiishi, T.; Kaku, M.; Kajsongkram, T.; Thisayakorn, K. Mulberry Fruit Extract Alleviates the Intracellular Amyloid-β Oligomer-Induced Cognitive Disturbance and Oxidative Stress in Alzheimer’s Disease Model Mice. Genes Cells 2021, 26, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Ju, M.S.; Shim, J.S.; Kim, M.C.; Lee, S.-H.; Huh, Y.; Kim, S.Y.; Oh, M.S. Mulberry Fruit Protects Dopaminergic Neurons in Toxin-Induced Parkinson’s Disease Models. Br. J. Nutr. 2010, 104, 8–16. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Fattouch, S.; Amri, M. Phytochemicals from Mulberry Extract (Morus Sp.): Antioxidant and Neuroprotective Potentials. J. Appl. Pharm. Sci. 2017, 7, 217–222. [Google Scholar] [CrossRef]
- Hamdan, D.I.; Salah, S.; Hassan, W.H.B.; Morsi, M.; Khalil, H.M.A.; Ahmed-Farid, O.A.; El-Shiekh, R.A.; Nael, M.A.; Elissawy, A.M. Anticancer and Neuroprotective Activities of Ethyl Acetate Fractions from Morus macroura Miq. Plant Organs with Ultraperformance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry Profiling. ACS Omega 2022, 7, 16013–16027. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Boujelben, A.; Fattouch, S.; Amri, M. Morus alba Leaf Extract Mediates Neuroprotection against Glyphosate-Induced Toxicity and Biochemical Alterations in the Brain. Environ. Sci. Pollut. Res. 2017, 24, 9605–9613. [Google Scholar] [CrossRef]
- Kang, T.H.; Oh, H.R.; Jung, S.M.; Ryu, J.H.; Park, M.W.; Park, Y.K.; Kim, S.Y. Enhancement of Neuroprotection of Mulberry Leaves (Morus alba L.) Prepared by the Anaerobic Treatment against Ischemic Damage. Biol. Pharm. Bull. 2006, 29, 270–274. [Google Scholar] [CrossRef]
- Niidome, T.; Takahashi, K.; Goto, Y.; Goh, S.; Tanaka, N.; Kamei, K.; Ichida, M.; Hara, S.; Akaike, A.; Kihara, T.; et al. Mulberry Leaf Extract Prevents Amyloid Beta-Peptide Fibril Formation and Neurotoxicity. Neuroreport 2007, 18, 813–816. [Google Scholar] [CrossRef]
- Hynd, M.R.; Scott, H.L.; Dodd, P.R. Glutamate-Mediated Excitotoxicity and Neurodegeneration in Alzheimer’s Disease. Neurochem. Int. 2004, 45, 583–595. [Google Scholar] [CrossRef]
- Shih, A.Y.; Erb, H.; Sun, X.; Toda, S.; Kalivas, P.W.; Murphy, T.H. Cystine/Glutamate Exchange Modulates Glutathione Supply for Neuroprotection from Oxidative Stress and Cell Proliferation. J. Neurosci. 2006, 26, 10514–10523. [Google Scholar] [CrossRef] [PubMed]
- Dalmagro, A.P.; Camargo, A.; Bertarello Zeni, A.L. Morus Nigra and Its Major Phenolic, Syringic Acid, Have Antidepressant-like and Neuroprotective Effects in Mice. Metab. Brain Dis. 2017, 32, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A Flavonoid with Wider Biological Activities and Its Applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Olonode, E.T.; Aderibigbe, A.O.; Adeoluwa, O.A.; Eduviere, A.T.; Ben-Azu, B. Morin Hydrate Mitigates Rapid Eye Movement Sleep Deprivation-Induced Neurobehavioural Impairments and Loss of Viable Neurons in the Hippocampus of Mice. Behav. Brain Res. 2019, 356, 518–525. [Google Scholar] [CrossRef]
- Lee, K.M.; Lee, Y.; Chun, H.J.; Kim, A.H.; Kim, J.Y.; Lee, J.Y.; Ishigami, A.; Lee, J. Neuroprotective and Anti-inflammatory Effects of Morin in a Murine Model of Parkinson’s Disease. J. Neurosci. Res. 2016, 94, 865–878. [Google Scholar] [CrossRef]
- Wang, Z.; Cui, J.; Li, D.; Ran, S.; Huang, J.; Chen, G. Morin Exhibits a Neuroprotective Effect in MPTP-Induced Parkinson’s Disease Model via TFEB/AMPK-Mediated Mitophagy. Phytomedicine 2023, 116, 154866. [Google Scholar] [CrossRef]
- Berezhnov, A.V.; Soutar, M.P.M.; Fedotova, E.I.; Frolova, M.S.; Plun-Favreau, H.; Zinchenko, V.P.; Abramov, A.Y. Intracellular pH Modulates Autophagy and Mitophagy. J. Biol. Chem. 2016, 291, 8701–8708. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Weng, H.-Z.; Tang, D.-K.; Long, J.-C.; Tang, Z.-Y.; Chen, Y.; Yin, S.; Tang, G.-H. Prenylated Dihydroflavones from the Root Barks of Morus alba. Nat. Prod. Res. 2024, 38, 2569–2576. [Google Scholar] [CrossRef]
- Nam, M.S.; Jung, D.-B.; Seo, K.-H.; Kim, B.-I.; Kim, J.-H.; Kim, J.H.; Kim, B.; Baek, N.-I.; Kim, S.-H. Apoptotic Effect of Sanggenol L via Caspase Activation and Inhibition of NF-B Signaling in Ovarian Cancer Cells. Phytother. Res. 2016, 30, 90–96. [Google Scholar] [CrossRef]
- Wang, H.; Jin, T.; Mao, Y.; Wei, Y. Sanggenol L Reduces LPS-Induced Myocardial Injury and Inflammation by Activating PI3K/AKT/mTOR and Inhibiting NF-κB in Rats. Pharmacogn. Mag. 2024, 21, 09731296241264617. [Google Scholar] [CrossRef]
- Jung, J.-W.; Ko, W.-M.; Park, J.-H.; Seo, K.-H.; Oh, E.-J.; Lee, D.-Y.; Lee, D.-S.; Kim, Y.-C.; Lim, D.-W.; Han, D.; et al. Isoprenylated Flavonoids from the Root Bark of Morus alba and Their Hepatoprotective and Neuroprotective Activities. Arch. Pharm. Res. 2015, 38, 2066–2075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Wu, M.; Velu, P.; Annamalai, V.; Zhang, J. Sanggenol L Alleviates Rotenone-Induced Parkinson’s Disease and Inhibits Mitochondrial Complex I by Apoptosis Via P13K/AKT/mTOR Signalling. Comb. Chem. High Throughput Screen. 2024. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective Effects of Berry Fruits on Neurodegenerative Diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Nazir, A.; Sultan, M.T.; Schroen, K. Morus alba L. Nature’s Functional Tonic. Trends Food Sci. Technol. 2008, 19, 505–512. [Google Scholar] [CrossRef]
- Kang, T.H.; Hur, J.Y.; Kim, H.B.; Ryu, J.H.; Kim, S.Y. Neuroprotective Effects of the Cyanidin-3-O-β-D-Glucopyranoside Isolated from Mulberry Fruit against Cerebral Ischemia. Neurosci. Lett. 2006, 391, 122–126. [Google Scholar] [CrossRef]
- Bhuiyan, M.I.H.; Kim, H.-B.; Kim, S.Y.; Cho, K.-O. The Neuroprotective Potential of Cyanidin-3-Glucoside Fraction Extracted from Mulberry Following Oxygen-Glucose Deprivation. Korean J. Physiol. Pharmacol. 2011, 15, 353–361. [Google Scholar] [CrossRef]
- Samota, M.K.; Yadav, D.K.; Koli, P.; Kaur, M.; Kaur, M.; Rani, H.; Selvan, S.S.; Mahala, P.; Tripathi, K.; Kumar, S. Exploring Natural Chalcones: Innovative Extraction Techniques, Bioactivities, and Health Potential. Sustain. Food Technol. 2024, 2, 1456–1468. [Google Scholar] [CrossRef]
- Hofmann, E.; Webster, J.; Do, T.; Kline, R.; Snider, L.; Hauser, Q.; Higginbottom, G.; Campbell, A.; Ma, L.; Paula, S. Hydroxylated Chalcones with Dual Properties: Xanthine Oxidase Inhibitors and Radical Scavengers. Bioorg. Med. Chem. 2016, 24, 578–587. [Google Scholar] [CrossRef]
- Nowakowska, Z. A Review of Anti-Infective and Anti-Inflammatory Chalcones. Eur. J. Med. Chem. 2007, 42, 125–137. [Google Scholar] [CrossRef]
- Wen, L.; Shi, D.; Zhou, T.; Tu, J.; He, M.; Jiang, Y.; Yang, B. Identification of Two Novel Prenylated Flavonoids in Mulberry Leaf and Their Bioactivities. Food Chem. 2020, 315, 126236. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Hano, Y. Isoprenoid-Substituted Phenolic-Compounds of Moraceous Plants. Nat. Prod. Rep. 1994, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, Y.-X.; Chen, R.-Y.; Kang, J. The Latest Review on the Polyphenols and Their Bioactivities of Chinese Morus Plants. J. Asian Nat. Prod. Res. 2014, 16, 690–702. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.-L.; Tang, G.-H.; Guo, Y.-Q.; Xu, Y.-K.; Huang, Z.-S.; Yin, S. Mulberry Diels-Alder-Type Adducts from Morus alba as Multi-Targeted Agents for Alzheimer’s Disease. Phytochemistry 2019, 157, 82–91. [Google Scholar] [CrossRef]
- Chun, W. The Role of Tau Phosphorylation and Cleavage in Neuronal Cell Death. Front. Biosci. 2007, 12, 733. [Google Scholar] [CrossRef]
- Cario, A.; Berger, C.L. Tau, Microtubule Dynamics, and Axonal Transport: New Paradigms for Neurodegenerative Disease. BioEssays 2023, 45, 2200138. [Google Scholar] [CrossRef]
- Scarpini, E.; Schelterns, P.; Feldman, H. Treatment of Alzheimer’s Disease; Current Status and New Perspectives. Lancet Neurol. 2003, 2, 539–547. [Google Scholar] [CrossRef]
- Kuk, E.B.; Jo, A.R.; Oh, S.I.; Sohn, H.S.; Seong, S.H.; Roy, A.; Choi, J.S.; Jung, H.A. Anti-Alzheimer’s Disease Activity of Compounds from the Root Bark of Morus alba L. Arch. Pharm. Res. 2017, 40, 338–349. [Google Scholar] [CrossRef]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative Diseases and Effective Drug Delivery: A Review of Challenges and Novel Therapeutics. J. Controlled Release 2021, 330, 1152–1167. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRX 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Miao, Y.; Hu, Y.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Natural Source, Bioactivity and Synthesis of Benzofuran Derivatives. RSC Adv. 2019, 9, 27510–27540. [Google Scholar] [CrossRef] [PubMed]
- Qiao, A.; Wang, Y.; Zhang, W.; He, X. Neuroprotection of Brain-Targeted Bioactive Dietary Artoindonesianin O (AIO) from Mulberry on Rat Neurons as a Novel Intervention for Alzheimer’s Disease. J. Agric. Food Chem. 2015, 63, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhou, T.; Jiang, Y.; Gong, L.; Yang, B. Identification of Prenylated Phenolics in Mulberry Leaf and Their Neuroprotective Activity. Phytomedicine 2021, 90, 153641. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Lee, C.; Lee, D.; Kim, S.; Bang, S.; Shin, M.-S.; Lee, J.; Kang, K.S.; Shim, S.H. Neuroprotective Compound from an Endophytic Fungus, Colletotrichum Sp. JS-0367. J. Nat. Prod. 2018, 81, 1411–1416. [Google Scholar] [CrossRef]
- Fukui, M.; Song, J.-H.; Choi, J.; Choi, H.J.; Zhu, B.T. Mechanism of Glutamate-Induced Neurotoxicity in HT22 Mouse Hippocampal Cells. Eur. J. Pharmacol. 2009, 617, 1–11. [Google Scholar] [CrossRef]
- Stanciu, M.; Wang, Y.; Kentor, R.; Burke, N.; Watkins, S.; Kress, G.; Reynolds, I.; Klann, E.; Angiolieri, M.R.; Johnson, J.W.; et al. Persistent Activation of ERK Contributes to Glutamate-Induced Oxidative Toxicity in a Neuronal Cell Line and Primary Cortical Neuron Cultures. J. Biol. Chem. 2000, 275, 12200–12206. [Google Scholar] [CrossRef]
- Shen, T.; Xie, C.-F.; Wang, X.-N.; Lou, H.-X. Stilbenoids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1901–1949. ISBN 978-3-642-22144-6. [Google Scholar]
- Navarro-Orcajada, S.; Conesa, I.; Vidal-Sánchez, F.J.; Matencio, A.; Albaladejo-Maricó, L.; García-Carmona, F.; López-Nicolás, J.M. Stilbenes: Characterization, Bioactivity, Encapsulation and Structural Modifications. A Review of Their Current Limitations and Promising Approaches. Crit. Rev. Food Sci. Nutr. 2023, 63, 7269–7287. [Google Scholar] [CrossRef]
- Kim, J.-K.; Kim, M.; Cho, S.-G.; Kim, M.-K.; Kim, S.W.; Lim, Y.-H. Biotransformation of Mulberroside A from Morus alba Results in Enhancement of Tyrosinase Inhibition. J. Ind. Microbiol. Biotechnol. 2010, 37, 631–637. [Google Scholar] [CrossRef]
- Wang, C.-P.; Zhang, L.-Z.; Li, G.-C.; Shi, Y.; Li, J.-L.; Zhang, X.-C.; Wang, Z.-W.; Ding, F.; Liang, X.-M. Mulberroside a Protects against Ischemic Impairment in Primary Culture of Rat Cortical Neurons after Oxygen-Glucose Deprivation Followed by Reperfusion: Neuroprotective Effects of Mulberroside A Against Ischemic Injury. J. Neurosci. Res. 2014, 92, 944–954. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight Junctions in Inflammatory Bowel Diseases and Inflammatory Bowel Disease Associated Colorectal Cancer. World J. Gastroenterol. 2016, 22, 3117. [Google Scholar] [CrossRef]
- Yu, R.; Wen, S.; Wang, Q.; Wang, C.; Zhang, L.; Wu, X.; Li, J.; Kong, L. Mulberroside A Repairs High Fructose Diet-induced Damage of Intestinal Epithelial and Blood–Brain Barriers in Mice: A Potential for Preventing Hippocampal Neuroinflammatory Injury. J. Neurochem. 2021, 157, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Artime, M.C.; O’Brian, C.A. Resveratrol: A Candidate Nutritional Substance for Prostate Cancer Prevention. J. Nutr. 2003, 133, 2440S–2443S. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, P.; Khan, M.M.U.; Singh, H.; Verma, A. Resveratrol: A Potential Therapeutic Natural Polyphenol for Neurodegenerative Diseases Associated with Mitochondrial Dysfunction. Front. Pharmacol. 2022, 13, 922232. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Chen, S.-D.; Hsu, C.-Y.; Chen, S.-F.; Chen, N.-C.; Jou, S.-B. Resveratrol Promotes Mitochondrial Biogenesis and Protects against Seizure-Induced Neuronal Cell Damage in the Hippocampus Following Status Epilepticus by Activation of the PGC-1 Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 998. [Google Scholar] [CrossRef]
- Lorenz, P.; Roychowdhury, S.; Engelmann, M.; Wolf, G.; Horn, T.F.W. Oxyresveratrol and Resveratrol Are Potent Antioxidants and Free Radical Scavengers: Effect on Nitrosative and Oxidative Stress Derived from Microglial Cells. Nitric Oxide 2003, 9, 64–76. [Google Scholar] [CrossRef]
- Chao, J.; Yu, M.-S.; Ho, Y.-S.; Wang, M.; Chang, R.C.-C. Dietary Oxyresveratrol Prevents Parkinsonian Mimetic 6-Hydroxydopamine Neurotoxicity. Free Radic. Biol. Med. 2008, 45, 1019–1026. [Google Scholar] [CrossRef]
- Weber, J.T.; Lamont, M.; Chibrikova, L.; Fekkes, D.; Vlug, A.S.; Lorenz, P.; Kreutzmann, P.; Slemmer, J.E. Potential Neuroprotective Effects of Oxyresveratrol against Traumatic Injury. Eur. J. Pharmacol. 2012, 680, 55–62. [Google Scholar] [CrossRef]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of High 1-Deoxynojirimycin (DNJ) Content Mulberry Tea and Use of Response Surface Methodology to Optimize Tea-Making Conditions for Highest DNJ Extraction. LWT Food Sci. Technol. 2012, 45, 226–232. [Google Scholar] [CrossRef]
- Chen, W.; Liang, T.; Zuo, W.; Wu, X.; Shen, Z.; Wang, F.; Li, C.; Zheng, Y.; Peng, G. Neuroprotective Effect of 1-Deoxynojirimycin on Cognitive Impairment, β-Amyloid Deposition, and Neuroinflammation in the SAMP8 Mice. Biomed. Pharmacother. 2018, 106, 92–97. [Google Scholar] [CrossRef]
- Parida, I.S.; Takasu, S.; Ito, J.; Eitsuka, T.; Nakagawa, K. 1-Deoxynojirimycin Attenuates Pathological Markers of Alzheimer’s Disease in the in Vitro Model of Neuronal Insulin Resistance. FASEB J. 2024, 38, e23800. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Wang, H.; Capuano, A.W.; Khan, A.; Taïb, B.; Anokye-Danso, F.; Schneider, J.A.; Bennett, D.A.; Ahima, R.S.; Arnold, S.E. Brain Insulin Signaling, Alzheimer Disease Pathology, and Cognitive Function. Ann. Neurol. 2020, 88, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Shen, X.; Xu, F.; Liang, X. Research Progress of Nano-Delivery Systems for the Active Ingredients from Traditional Chinese Medicine. Phytochem. Anal. 2024. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Ruan, J.-Q.; Wu, W.-J.; Zhou, R.-N.; Lei, J.P.-C.; Zhao, H.-Y.; Yan, R.; Wang, Y.-T. In Vitro Pharmacokinetic Characterization of Mulberroside A, the Main Polyhydroxylated Stilbene in Mulberry (Morus alba L.), and Its Bacterial Metabolite Oxyresveratrol in Traditional Oral Use. J. Agric. Food Chem. 2012, 60, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, G.; Lu, Z.; Zhang, J.; Guo, D. Identification of Seven Metabolites of Oxyresveratrol in Rat Urine and Bile Using Liquid Chromatography/Tandem Mass Spectrometry. Biomed. Chromatogr. 2010, 24, 426–432. [Google Scholar] [CrossRef]
- Zhou, J.; Li, S.; Wang, W.; Guo, X.; Lu, X.-Y.; Yan, X.; Huang, D.; Wei, B.; Cao, L. Variations in the Levels of Mulberroside A, Oxyresveratrol, and Resveratrol in Mulberries in Different Seasons and during Growth. Sci. World J. 2013, 2013, 380692. [Google Scholar] [CrossRef]
- Jeandet, P.; Vasserot, Y.; Chastang, T.; Courot, E. Engineering Microbial Cells for the Biosynthesis of Natural Compounds of Pharmaceutical Significance. BioMed Res. Int. 2013, 2013, 780145. [Google Scholar] [CrossRef]
- Chen, C.; Mohamad Razali, U.H.; Saikim, F.H.; Mahyudin, A.; Mohd Noor, N.Q.I. Morus alba L. Plant: Bioactive Compounds and Potential as a Functional Food Ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in Nanoparticle and Microparticle Delivery Systems for Increasing the Dispersibility, Stability, and Bioactivity of Phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef]
- Gao, Q.; Feng, J.; Liu, W.; Wen, C.; Wu, Y.; Liao, Q.; Zou, L.; Sui, X.; Xie, T.; Zhang, J.; et al. Opportunities and Challenges for Co-Delivery Nanomedicines Based on Combination of Phytochemicals with Chemotherapeutic Drugs in Cancer Treatment. Adv. Drug Deliv. Rev. 2022, 188, 114445. [Google Scholar] [CrossRef]
- Sangsen, Y.; Wiwattanawongsa, K.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanapatapee, R. Modification of Oral Absorption of Oxyresveratrol Using Lipid Based Nanoparticles. Colloids Surf. B Biointerfaces 2015, 131, 182–190. [Google Scholar] [CrossRef]
- Nie, W.; Liu, Y.; Lan, J.; Li, T.; He, Y.; Li, Z.; Zhang, T.; Ding, Y. Self-Assembled Nanoparticles from Xie-Bai-San Decoction: Isolation, Characterization and Enhancing Oral Bioavailability. Int. J. Nanomed. 2024, 19, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Khanam, N.; Nath, D. Solid Lipid Nanoparticle: A Potent Vehicle of the Kaempferol for Brain Delivery through the Blood-Brain Barrier in the Focal Cerebral Ischemic Rat. Chem. Biol. Interact. 2024, 397, 111084. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Sobarzo-Sánchez, E.; Clément, C.; Nabavi, S.F.; Habtemariam, S.; Nabavi, S.M.; Cordelier, S. Engineering Stilbene Metabolic Pathways in Microbial Cells. Biotechnol. Adv. 2018, 36, 2264–2283. [Google Scholar] [CrossRef]
- Shrikanta, A.; Kumar, A.; Govindaswamy, V. Resveratrol Content and Antioxidant Properties of Underutilized Fruits. J. Food Sci. Technol. 2015, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Marienhagen, J.; Bott, M. Metabolic Engineering of Microorganisms for the Synthesis of Plant Natural Products. J. Biotechnol. 2013, 163, 166–178. [Google Scholar] [CrossRef]

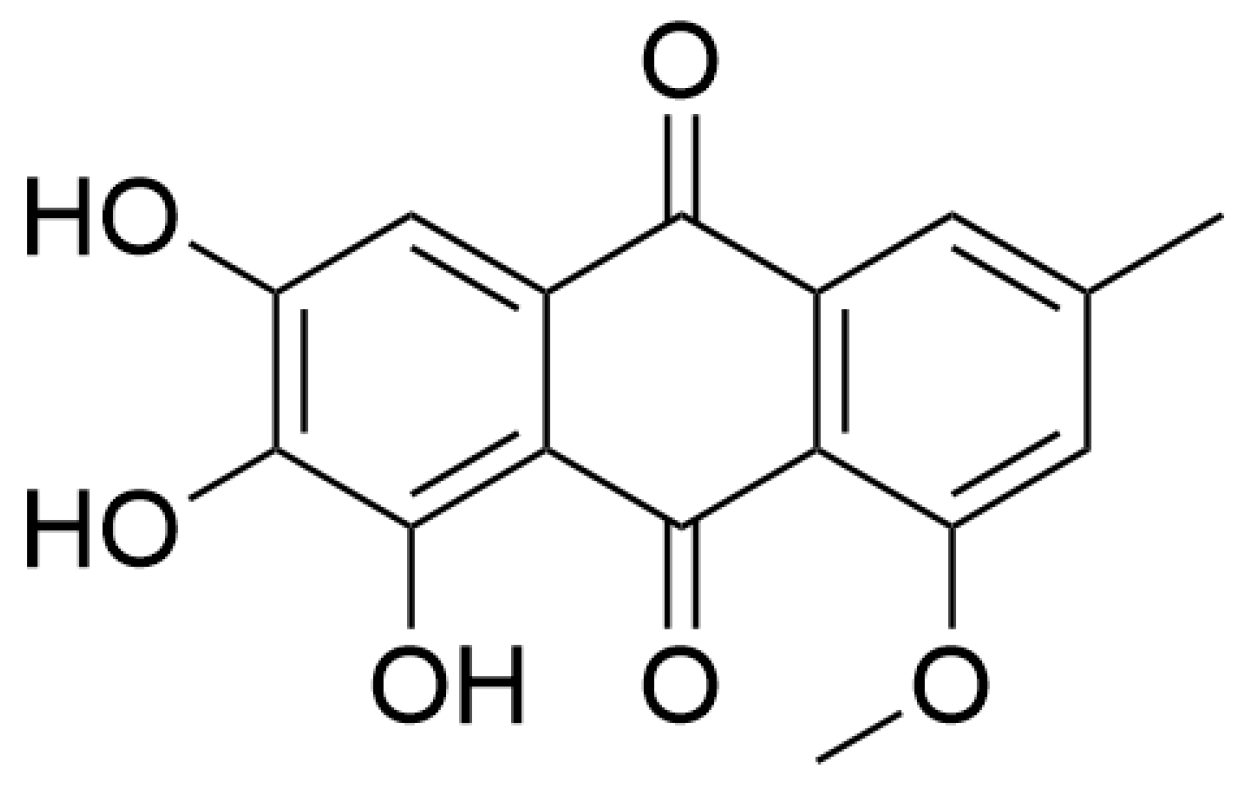

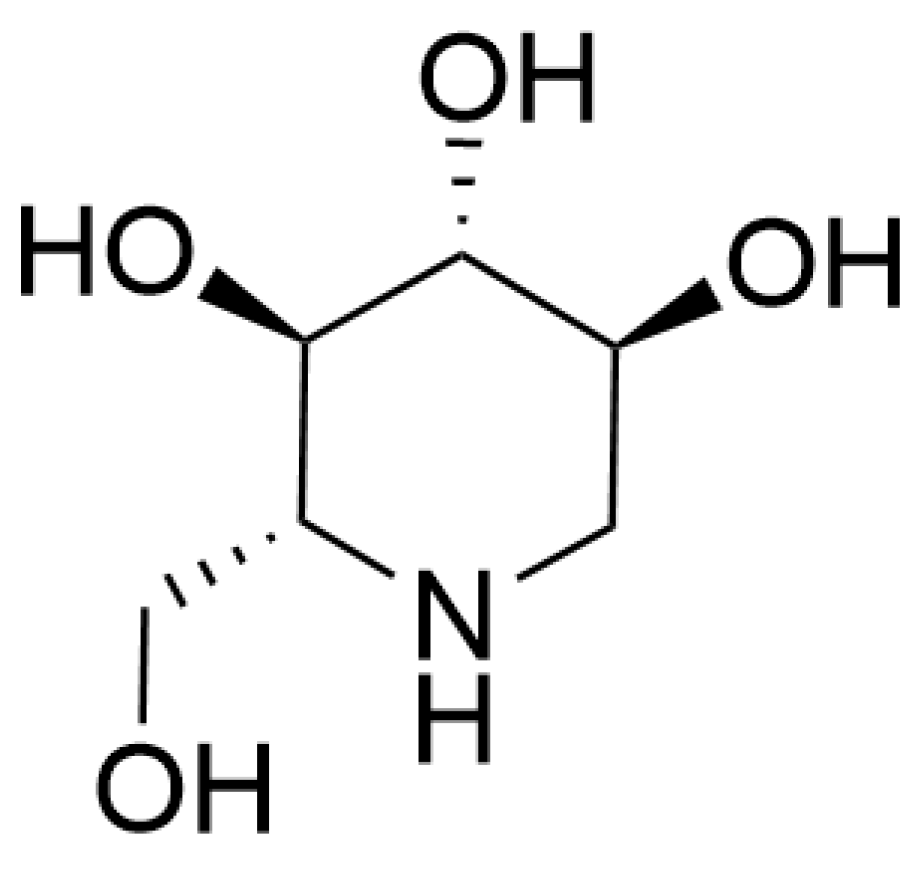
| Comparison Items | Mulberry Leaf | Mulberry Fruit | Refs. |
|---|---|---|---|
| Bioactivity focus | Anti-diabetic, hypolipidemic | Antioxidant, anti-inflammatory | [26,28,29] |
| Characteristic components | Alkaloids (1-deoxynojirimycin, DNJ), flavonoids | Anthocyanins (cyanidin-3-glucoside, C3G), phenolic acid derivatives | [26,30] |
| Extraction key points | Enzymatic hydrolysis/ultrasound-assisted cell wall disruption | Light avoidance and temperature control | [31,32,33] |
| Preferred cultivars | Morus australis (higher DNJ content) | Morus nigra (higher anthocyanins content) | [34,35] |
| Optimal harvest period | June–July (DNJ peak) | Full maturity (anthocyanins peak) | [36,37] |
| Structural Classes | Compound Name | Mechanisms of Action | Disease Models | Plant Parts | Refs. |
|---|---|---|---|---|---|
| Flavonoids | Morin | Alleviate oxidative stress | Neuronal injury in vivo model | Branch | [54,56] |
| Inhibit inflammation-mediated neuronal apoptosis | PD in vivo model | ||||
| Moralbaflavone C | Alleviate oxidative stress | PD in vitro model | Root bark | [58] | |
| Flavonoids | Sanggenol L | Alleviate oxidative stress and cell apoptosis | PD in vitro model | Root bark | [62] |
| Cyanidin-3-glucoside | Maintain mitochondria normal function | Cerebral ischemia in vitro and vivo models | Fruit | [65,66] | |
| Morachalcone D | Alleviate oxidative stress and inhibit ferroptosis | Neuronal injury in vitro models | Leaf | [70] | |
| Typical Diels-Alder-type adducts | Mulberrofuran C | Alleviate oxidative stress, inhibit aggregation of abnormal protein and activity of cholinesterases | AD in vitro models | Root bark | [74,78] |
| Mulberrofuran K | |||||
| Benzofurans | Artoindonesianin O | Inhibit aggregation of abnormal protein and promote dendritic spine regeneration | AD in vitro models | Fruit | [82] |
| Moracin N | Inhibit ferroptosis | Neuronal injury in vitro model | Leaf | [83] | |
| Quinones | Evariquinone | Alleviate oxidative stress and regulate intracellular Ca2+ homeostasis | Neuronal injury in vitro model | Endophytic fungi in leaf | [84] |
| Stilbenes | Mulberroside A | Alleviate oxidative stress, inhibit neuroinflammation, and repair internal barriers | Metabolic syndrome-associated neuronal injury in vivo model | Root and branch | [92] |
| Resveratrol | Promote mitochondrial biogenesis and functional repair, and inhibit neuronal apoptosis | Epileptic neuronal injury in vivo model | Fruit | [94,95] | |
| Oxyresveratrol | Alleviate oxidative stress, inhibit inflammation and apoptosis, and regulate glia functions | Neuronal injury in vitro models | Branch and fruit | [97,98] | |
| Alkaloids | 1-Deoxynojirimycin | Inhibit aggregation of abnormal protein and inflammation | AD in vitro and vivo models | Branch and leaf | [99,100,101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Gou, Z.; Huang, Y.; Yu, Q.; Kim, A.N.; Shi, W.; Zhou, Y. Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review. Pharmaceuticals 2025, 18, 695. https://doi.org/10.3390/ph18050695
Chen J, Gou Z, Huang Y, Yu Q, Kim AN, Shi W, Zhou Y. Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review. Pharmaceuticals. 2025; 18(5):695. https://doi.org/10.3390/ph18050695
Chicago/Turabian StyleChen, Junwei, Zhonglang Gou, Yufei Huang, Qianhui Yu, An Na Kim, Wenchao Shi, and You Zhou. 2025. "Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review" Pharmaceuticals 18, no. 5: 695. https://doi.org/10.3390/ph18050695
APA StyleChen, J., Gou, Z., Huang, Y., Yu, Q., Kim, A. N., Shi, W., & Zhou, Y. (2025). Research Progress on Phytochemicals from Mulberry with Neuroprotective Effects: A Review. Pharmaceuticals, 18(5), 695. https://doi.org/10.3390/ph18050695







