Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence
Abstract
1. Introduction
2. Results
3. Discussion
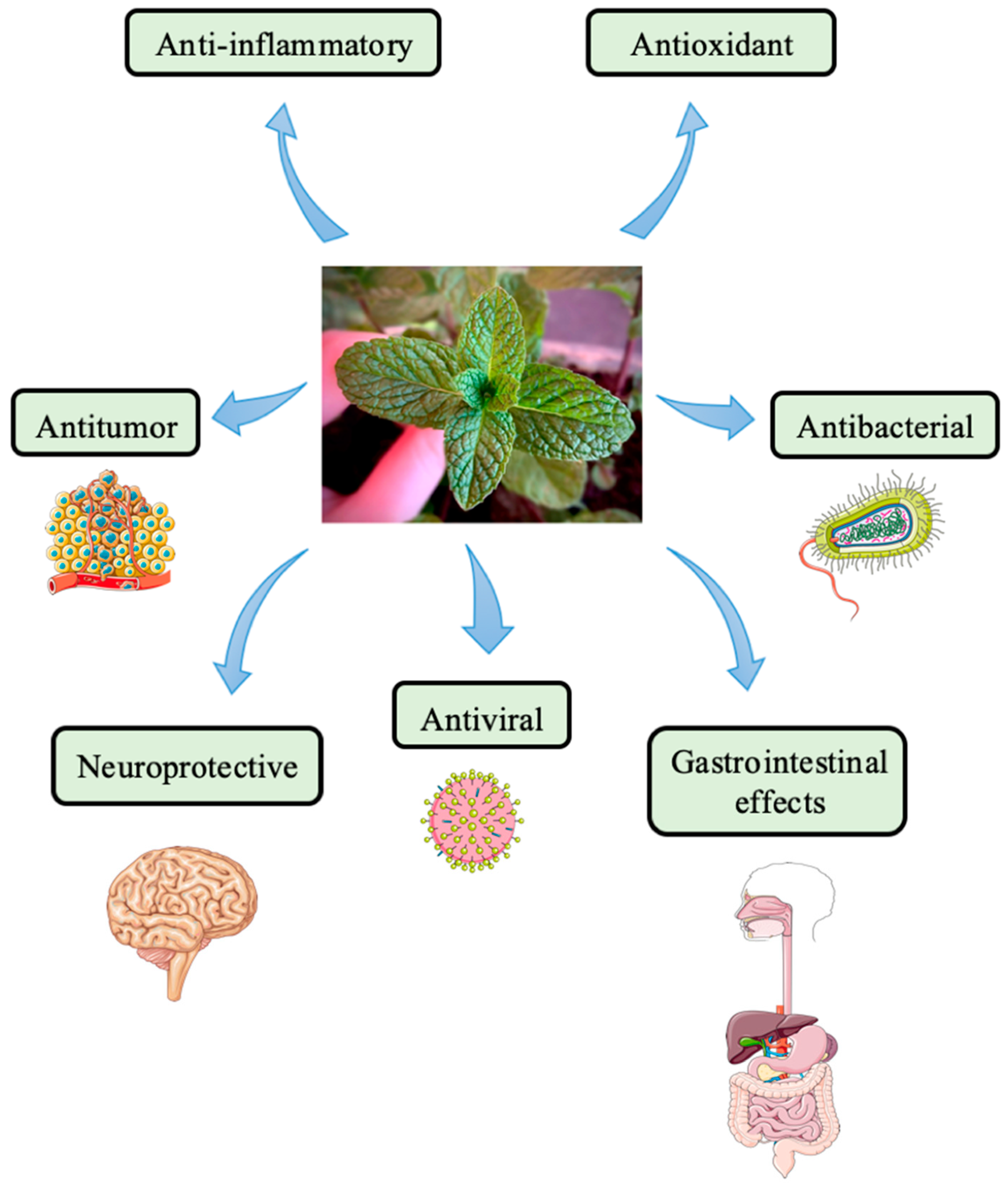
3.1. Mentha Species
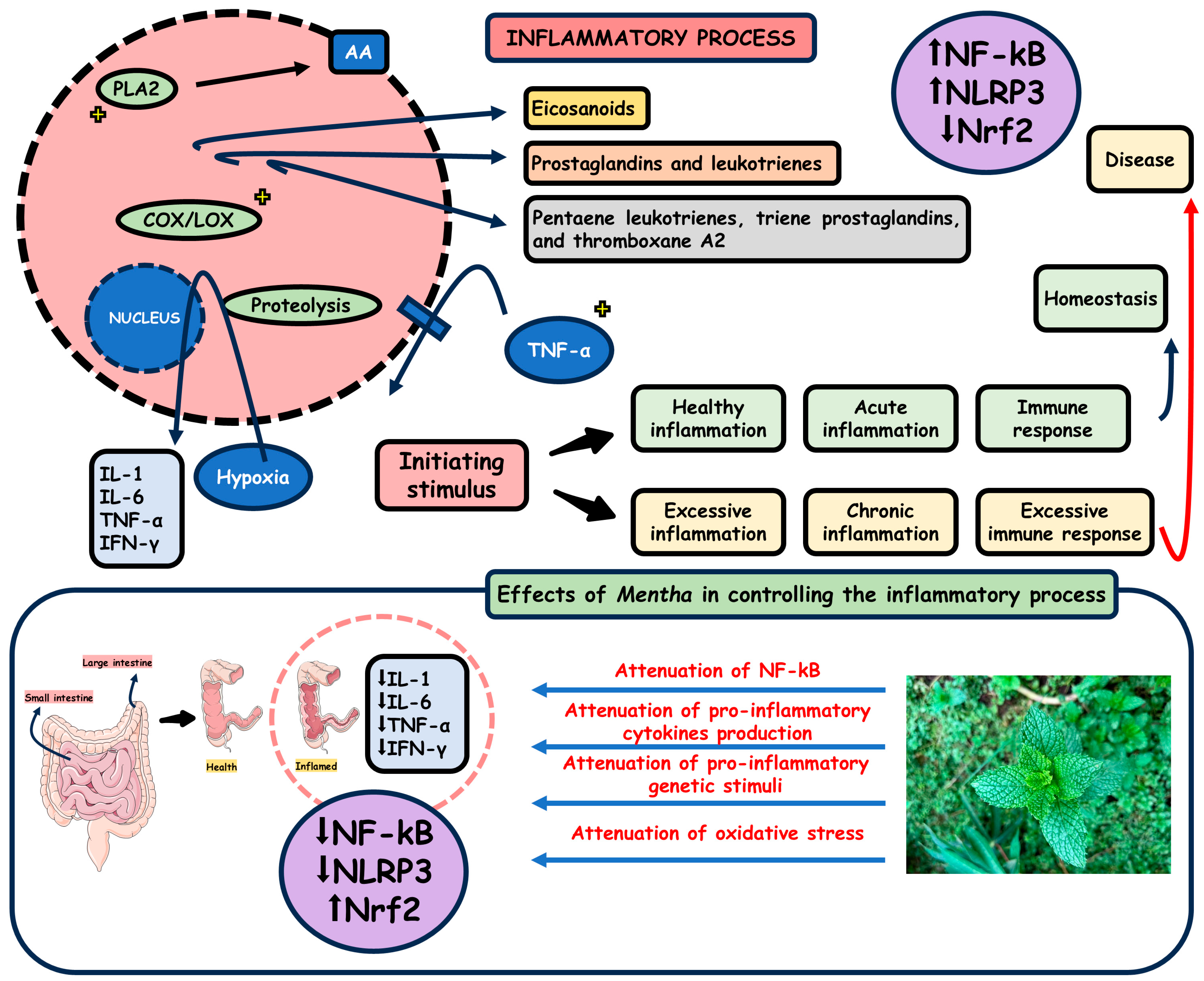
3.2. Mentha and Inflammation
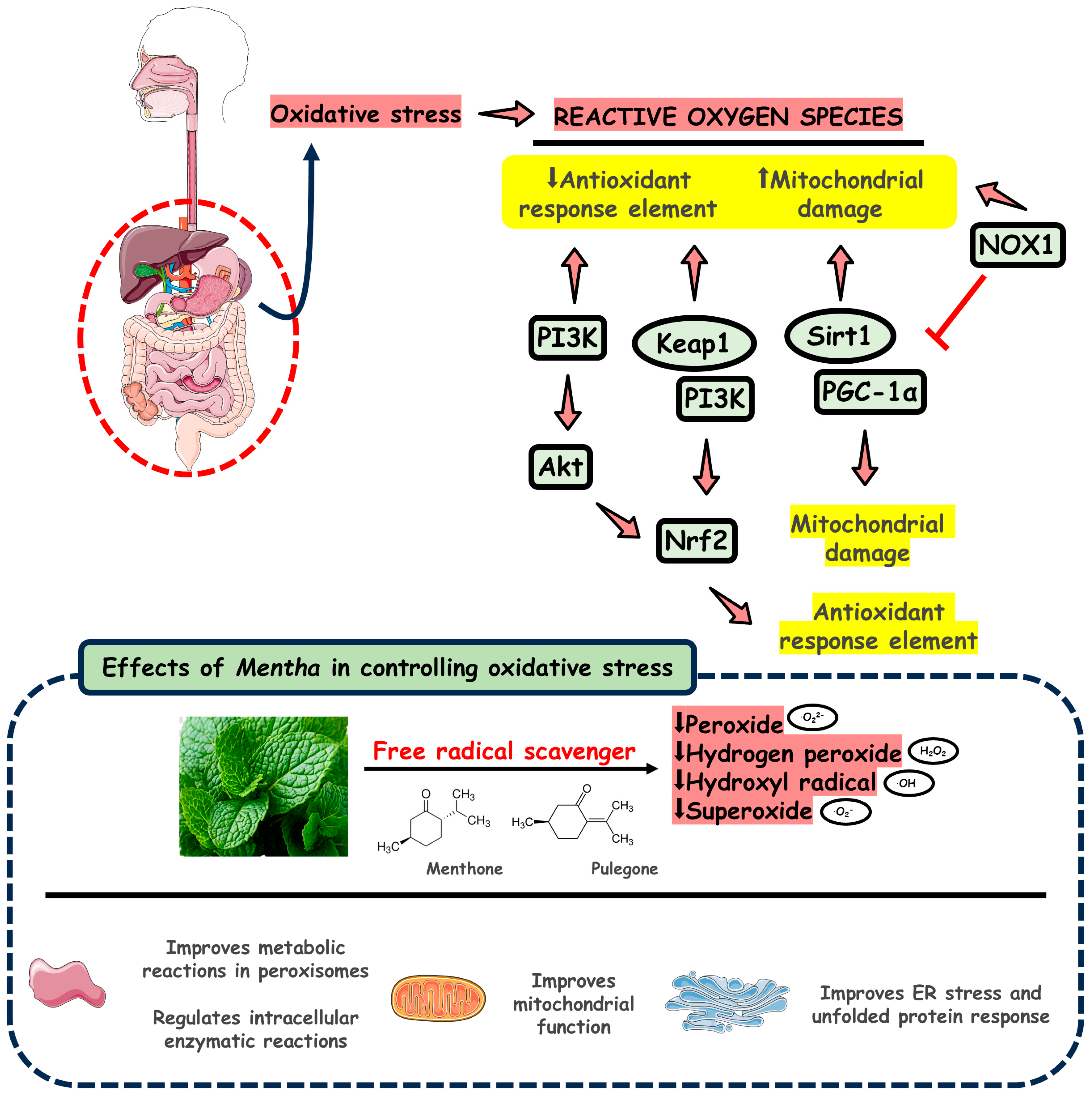
3.3. Mentha Species and Oxidative Stress
3.4. Mentha, Abdominal Pain, and Dyspepsia: The Results of Clinical Trials
4. Materials and Methods
4.1. Focal Question
4.2. Language
4.3. Databases
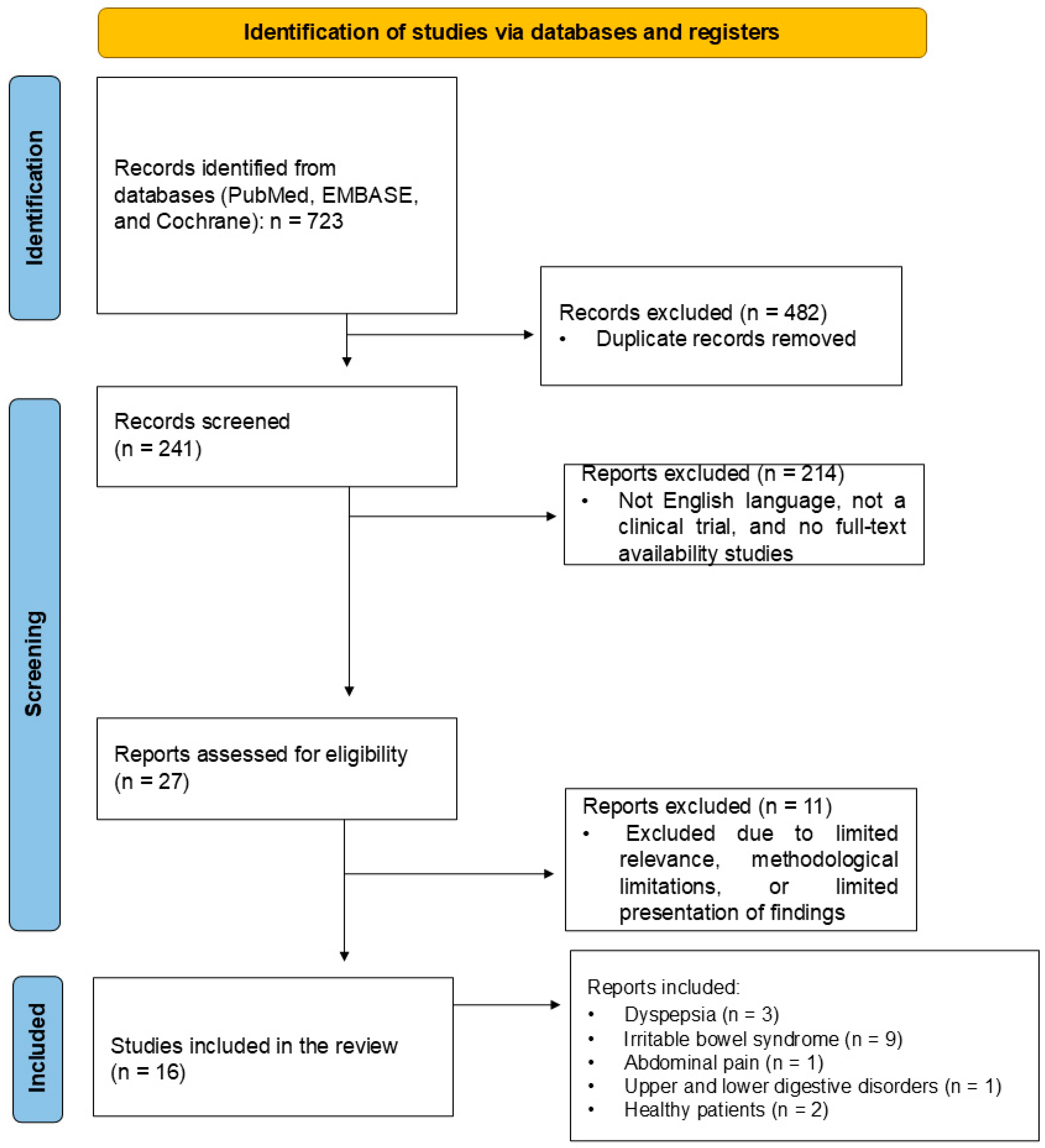
4.4. Study Selection
4.5. Data Extraction
4.6. Quality Assessment
5. Conclusions and Future Research Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Black, C.J.; Drossman, D.A.; Talley, N.J.; Ruddy, J.; Ford, A.C. Functional gastrointestinal disorders: Advances in understanding and management. Lancet 2020, 396, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Häuser, W.; Andresen, V. Functional gastrointestinal disorders. Dtsch. Med. Wochenschr. 2022, 147, 595–604. [Google Scholar] [CrossRef]
- Ranjbar, R.; Ghasemian, M.; Maniati, M.; Khatami, S.H.; Jamali, N.; Taheri-Anganeh, M. Gastrointestinal disorder biomarkers. Clin. Chim. Acta 2022, 530, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Vanuytsel, T.; Bercik, P.; Boeckxstaens, G. Understanding neuroimmune interactions in disorders of gut-brain interaction: From functional to immune-mediated disorders. Gut 2023, 72, 787–798. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Xie, Y.; Zeng, F.; Chen, S.; Chen, R.; Zhang, X.; Huang, S.; Li, D.; Bai, F. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A prospective follow-up cohort study. BMC Infect. Dis. 2023, 23, 422. [Google Scholar] [CrossRef]
- Zhao, J.; Li, X.; Zheng, H.; Ye, K.; Wang, X.; Wang, X.; Sun, R.; Li, Z. Effects of acupuncture on functional gastrointestinal disorders: Special effects, coeffects, synergistic effects in terms of single or compatible acupoints. J. Tradit. Chin. Med. 2023, 43, 397–408. [Google Scholar] [CrossRef]
- Govender, I.; Rangiah, S.; Bongongo, T.; Mahuma, P. A Primary Care Approach to Abdominal Pain in Adults. S. Afr. Fam. Pract. 2021, 63, e1–e5. [Google Scholar] [CrossRef]
- Sabo, C.M.; Grad, S.; Dumitrascu, D.L. Chronic Abdominal Pain in General Practice. Dig. Dis. 2021, 39, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lukic, S.; Mijac, D.; Filipovic, B.; Sokic-Milutinovic, A.; Tomasevic, R.; Krstic, M.; Milosavljevic, T. Chronic Abdominal Pain: Gastroenterologist Approach. Dig. Dis. 2022, 40, 181–186. [Google Scholar] [CrossRef]
- Wolfe, C.; Halsey-Nichols, M.; Ritter, K.; McCoin, N. Abdominal Pain in the Emergency Department: How to Select the Correct Imaging for Diagnosis. Open Access Emerg. Med. 2022, 14, 335–345. [Google Scholar] [CrossRef]
- Mounsey, A.; Barzin, A.; Rietz, A. Functional Dyspepsia: Evaluation and Management. Am. Fam. Physician 2020, 101, 84–88. [Google Scholar]
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, P. Dyspepsia in Primary Care Medicine: A European Prospective. Dig. Dis. 2022, 40, 266–269. [Google Scholar] [CrossRef]
- Tziatzios, G.; Gkolfakis, P.; Leite, G.; Mathur, R.; Damoraki, G.; Giamarellos-Bourboulis, E.J.; Triantafyllou, K. Probiotics in Functional Dyspepsia. Microorganisms 2023, 11, 351. [Google Scholar] [CrossRef] [PubMed]
- Syam, A.F.; Miftahussurur, M.; Makmun, D.; Abdullah, M.; Rani, A.A.; Siregar, G.A.; Simadibrata, M.; Zubir, N.; Dewa Nyoman Wibawa, I.; Purnomo, H.D.; et al. Management of dyspepsia and Helicobacter pylori infection: The 2022 Indonesian Consensus Report. Gut Pathog. 2023, 15, 25. [Google Scholar] [CrossRef]
- Oshima, T. Functional Dyspepsia: Current Understanding and Future Perspective. Digestion 2024, 105, 26–33. [Google Scholar] [CrossRef]
- Amerikanou, C.; Kleftaki, S.A.; Valsamidou, E.; Chroni, E.; Biagki, T.; Sigala, D.; Koutoulogenis, K.; Anapliotis, P.; Gioxari, A.; Kaliora, A.C. Food, Dietary Patterns, or Is Eating Behavior to Blame? Analyzing the Nutritional Aspects of Functional Dyspepsia. Nutrients 2023, 15, 1544. [Google Scholar] [CrossRef]
- Ashagrie, G.; Abebe, A.; Umer, S. Analgesic and Anti-Inflammatory Activities of 80% Methanol Extract and Solvent Fractions of Ehretia cymosa Thonn (Boraginaceae) Leaves in Rodents. J. Exp. Pharmacol. 2023, 15, 63–79. [Google Scholar] [CrossRef]
- Sayre, C.L.; Yellepeddi, V.K.; Job, K.M.; Krepkova, L.V.; Sherwin, C.M.T.; Enioutina, E.Y. Current use of complementary and conventional medicine for treatment of pediatric patients with gastrointestinal disorders. Front. Pharmacol. 2023, 14, 1051442. [Google Scholar] [CrossRef]
- Thapa, S.; Luna, R.A.; Chumpitazi, B.P.; Oezguen, N.; Abdel-Rahman, S.M.; Garg, U.; Musaad, S.; Versalovic, J.; Kearns, G.L.; Shulman, R.J. Peppermint oil effects on the gut microbiome in children with functional abdominal pain. Clin. Transl. Sci. 2022, 15, 1036–1049. [Google Scholar] [CrossRef]
- Weerts, Z.; Masclee, A.A.M.; Witteman, B.J.M.; Clemens, C.H.M.; Winkens, B.; Brouwers, J.; Frijlink, H.W.; Muris, J.W.M.; De Wit, N.J.; Essers, B.A.B.; et al. Efficacy and Safety of Peppermint Oil in a Randomized, Double-Blind Trial of Patients With Irritable Bowel Syndrome. Gastroenterology 2020, 158, 123–136. [Google Scholar] [CrossRef]
- Silva, H. A Descriptive Overview of the Medical Uses Given to Mentha Aromatic Herbs throughout History. Biology 2020, 9, 484. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Sharma, R.; Atanassova, M.; Atanassov, L.; Valere Tsouh Fokou, P.; et al. The Wonderful Activities of the Genus Mentha: Not Only Antioxidant Properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef]
- Hamad Al-Mijalli, S.; ER, E.L.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid. Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Adel, M.; Sakhaie, F.; Hosseini Shekarabi, S.P.; Gholamhosseini, A.; Impellitteri, F.; Faggio, C. Dietary Mentha piperita essential oil loaded in chitosan nanoparticles mediated the growth performance and humoral immune responses in Siberian sturgeon (Acipenserbaerii). Fish Shellfish Immunol. 2024, 145, 109321. [Google Scholar] [CrossRef]
- Amirzade-Iranaq, M.H.; Tajik, M.; Takzaree, A.; Amirmohammadi, M.; Rezaei Yazdi, F.; Takzaree, N. Topical Mentha piperita Effects on Cutaneous Wound Healing: A Study on TGF-β Expression and Clinical Outcomes. World J. Plast. Surg. 2022, 11, 86–96. [Google Scholar] [CrossRef]
- Yousefian, S.; Esmaeili, F.; Lohrasebi, T. A Comprehensive Review of the Key Characteristics of the Genus Mentha, Natural Compounds and Biotechnological Approaches for the Production of Secondary Metabolites. Iran. J. Biotechnol. 2023, 21, e3605. [Google Scholar] [CrossRef]
- Mahendran, G.; Rahman, L.U. Ethnomedicinal, phytochemical and pharmacological updates on Peppermint (Mentha × piperita L.)—A review. Phytother. Res. 2020, 34, 2088–2139. [Google Scholar] [CrossRef] [PubMed]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential Oil and Extracts, Their Biological Activities, and Perspectives on the Development of New Medicinal and Cosmetic Products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef]
- Prasad, P.; Gupta, A.; Singh, V.; Kumar, B. Impact of induced mutation-derived genetic variability, genotype and varieties for quantitative and qualitative traits in Mentha species. Int. J. Radiat. Biol. 2024, 100, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Floare, A.D.; Dumitrescu, R.; Alexa, V.T.; Balean, O.; Szuhanek, C.; Obistioiu, D.; Cocan, I.; Neacsu, A.G.; Popescu, I.; Fratila, A.D.; et al. Enhancing the Antimicrobial Effect of Ozone with Mentha piperita Essential Oil. Molecules 2023, 28, 2032. [Google Scholar] [CrossRef]
- Łyczko, J.; Piotrowski, K.; Kolasa, K.; Galek, R.; Szumny, A. Mentha piperita L. Micropropagation and the Potential Influence of Plant Growth Regulators on Volatile Organic Compound Composition. Molecules 2020, 25, 2652. [Google Scholar] [CrossRef]
- Ferrati, M.; Spinozzi, E.; Baldassarri, C.; Maggi, F.; Pavela, R.; Canale, A.; Petrelli, R.; Cappellacci, L. Efficacy of Mentha aquatica L. Essential Oil (Linalool/Linalool Acetate Chemotype) against Insect Vectors and Agricultural Pests. Pharmaceuticals 2023, 16, 633. [Google Scholar] [CrossRef]
- Sobti, B.; Kamal-Eldin, A.; Rasul, S.; Alnuaimi, M.S.K.; Alnuaimi, K.J.J.; Alhassani, A.A.K.; Almheiri, M.M.A.; Nazir, A. Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method. Foods 2023, 12, 1838. [Google Scholar] [CrossRef]
- Ahmad, N.; Ali, S.; Abbas, M.; Fazal, H.; Saqib, S.; Ali, A.; Ullah, Z.; Zaman, S.; Sawati, L.; Zada, A.; et al. Antimicrobial efficacy of Mentha piperata-derived biogenic zinc oxide nanoparticles against UTI-resistant pathogens. Sci. Rep. 2023, 13, 14972. [Google Scholar] [CrossRef]
- Wu, C.; Yan, J.; Li, W. Acacetin as a Potential Protective Compound against Cardiovascular Diseases. Evid. Based Complement. Altern. Med. 2022, 2022, 6265198. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Mu, J.; Chen, H.; Ye, M.; Zhang, X.; Ma, H. Acacetin resists UVA photoaging by mediating the SIRT3/ROS/MAPKs pathway. J. Cell. Mol. Med. 2022, 26, 4624–4628. [Google Scholar] [CrossRef]
- Wu, Y.; Song, F.; Li, Y.; Li, J.; Cui, Y.; Hong, Y.; Han, W.; Wu, W.; Lakhani, I.; Li, G.; et al. Acacetin exerts antioxidant potential against atherosclerosis through Nrf2 pathway in apoE(−) Mice. J. Cell. Mol. Med. 2021, 25, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Ak, G.; Ceylan, R.; Uysal, S.; Llorent-Martínez, E.; Di Simone, S.C.; Rapino, M.; Acquaviva, A.; Libero, M.L.; Chiavaroli, A.; et al. Novel Perceptions on Chemical Profile and Biopharmaceutical Properties of Mentha spicata Extracts: Adding Missing Pieces to the Scientific Puzzle. Plants 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- de Groot, A.; Schmidt, E. Essential Oils, Part V: Peppermint Oil, Lavender Oil, and Lemongrass Oil. Dermatitis 2016, 27, 325–332. [Google Scholar] [CrossRef]
- Iorio, R.; Celenza, G.; Petricca, S. Multi-Target Effects of ß-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants 2022, 11, 1199. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases-Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Sun, R.; Wu, T.; Xing, S.; Wei, S.; Bielicki, J.K.; Pan, X.; Zhou, M.; Chen, J. Caffeic acid protects against atherosclerotic lesions and cognitive decline in ApoE(−) mice. J. Pharmacol. Sci. 2023, 151, 110–118. [Google Scholar] [CrossRef]
- El Menyiy, N.; Mrabti, H.N.; El Omari, N.; Bakili, A.E.; Bakrim, S.; Mekkaoui, M.; Balahbib, A.; Amiri-Ardekani, E.; Ullah, R.; Alqahtani, A.S.; et al. Medicinal Uses, Phytochemistry, Pharmacology, and Toxicology of Mentha spicata. Evid. Based Complement. Altern. Med. 2022, 2022, 7990508. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; Omari, N.E. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Bonilla-Carvajal, K.; Stashenko, E.E.; Moreno-Castellanos, N. Essential Oil of Carvone Chemotype Lippia alba (Verbenaceae) Regulates Lipid Mobilization and Adipogenesis in Adipocytes. Curr. Issues Mol. Biol. 2022, 44, 5741–5755. [Google Scholar] [CrossRef] [PubMed]
- Bernatova, I. Biological activities of (−)-epicatechin and (−)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 2018, 36, 666–681. [Google Scholar] [CrossRef]
- Ling, J.; Wu, Y.; Zou, X.; Chang, Y.; Li, G.; Fang, M. (-)-Epicatechin Reduces Neuroinflammation, Protects Mitochondria Function, and Prevents Cognitive Impairment in Sepsis-Associated Encephalopathy. Oxid. Med. Cell. Longev. 2022, 2022, 2657713. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Tariq, M.H.; Ullah, R.; Zafar, S.; Rizwan, M.; Bibi, N.; Khattak, A.; Amir, N.; Abdullah. Exploring the antibacterial, antidiabetic, and anticancer potential of Mentha arvensis extract through in-silico and in-vitro analysis. BMC Complement. Med. Ther. 2023, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Eddin, L.B.; Jha, N.K.; Meeran, M.F.N.; Kesari, K.K.; Beiram, R.; Ojha, S. Neuroprotective Potential of Limonene and Limonene Containing Natural Products. Molecules 2021, 26, 4535. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer activity of limonene: A systematic review of target signaling pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Du, P.; Du, Y.; Zhao, D.; Cai, Y.; Yang, Q.; Guo, Z. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021, 269, 119008. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.S.; Nunes, P.H.M.; Lima, G.M.; Brito, A.; Pacheco, J.F.R.; Medina, H.D.C.; Benigno, M.I.M.; de Sousa, D.P.; de Moura-Filho, O.F.; Cunha, F.V.M.; et al. Hypokinetic Activity of Menthofuran on the Gastrointestinal Tract in Rodents. Evid. Based Complement. Altern. Med. 2023, 2023, 2726794. [Google Scholar] [CrossRef] [PubMed]
- Alves, N.M.; Nunes, P.H.M.; Mendes Garcez, A.; Lima de Freitas, M.C.; Oliveira, I.S.; da Silva, F.V.; Fernandes, H.B.; de Sousa, D.P.; Oliveira, R.C.M.; Arcanjo, D.D.R.; et al. Antioxidant Mechanisms Underlying the Gastroprotective Effect of Menthofuran on Experimentally Induced Gastric Lesions in Rodents. Evid. Based Complement. Altern. Med. 2023, 2023, 9192494. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Wang, Y.; Li, Y.; Li, Q.; Zhang, L. The distinctive role of menthol in pain and analgesia: Mechanisms, practices, and advances. Front. Mol. Neurosci. 2022, 15, 1006908. [Google Scholar] [CrossRef]
- Cheng, H.; An, X. Cold stimuli, hot topic: An updated review on the biological activity of menthol in relation to inflammation. Front. Immunol. 2022, 13, 1023746. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, R.; Mazumder, A.; Salahuddin; Yadav, R.K.; Chauhan, B.; Abdulah, M.M. Camphor and Menthol as Anticancer Agents: Synthesis, Structure-Activity Relationship and Interaction with Cancer Cell Lines. Anticancer Agents Med. Chem. 2023, 23, 614–623. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M.; Lawrence, B.M. Menthol: A simple monoterpene with remarkable biological properties. Phytochemistry 2013, 96, 15–25. [Google Scholar] [CrossRef]
- Huang, M.; Duan, W.; Chen, N.; Lin, G.; Wang, X. Synthesis and Antitumor Evaluation of Menthone-Derived Pyrimidine-Urea Compounds as Potential PI3K/Akt/mTOR Signaling Pathway Inhibitor. Front. Chem. 2021, 9, 815531. [Google Scholar] [CrossRef]
- Zaia, M.G.; Cagnazzo, T.; Feitosa, K.A.; Soares, E.G.; Faccioli, L.H.; Allegretti, S.M.; Afonso, A.; Anibal Fde, F. Anti-Inflammatory Properties of Menthol and Menthone in Schistosoma mansoni Infection. Front. Pharmacol. 2016, 7, 170. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Xie, Q.; Jin, W.; Zeng, R.; Hong, Z.; Zhang, Y.; Zhang, Y. Preparation and Embedding Characterization of Hydroxypropyl-β-cyclodextrin/Menthyl Acetate Microcapsules with Enhanced Stability. Pharmaceutics 2023, 15, 1979. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Gruľová, D.; Elshafie, H.S. Chemical Composition and Antimicrobial Properties of Mentha × piperita cv. ‘Kristinka’ Essential Oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Marwa, C.; Fikri-Benbrahim, K.; Ou-Yahia, D.; Farah, A. African peppermint (Mentha piperita) from Morocco: Chemical composition and antimicrobial properties of essential oil. J. Adv. Pharm. Technol. Res. 2017, 8, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Fatima, K.; Masood, N.; Ahmad Wani, Z.; Meena, A.; Luqman, S. Neomenthol prevents the proliferation of skin cancer cells by restraining tubulin polymerization and hyaluronidase activity. J. Adv. Res. 2021, 34, 93–107. [Google Scholar] [CrossRef]
- Dhingra, A.K.; Chopra, B. Pulegone: An Emerging Oxygenated Cyclic Monoterpene Ketone Scaffold Delineating Synthesis, Chemical Reactivity, and Biological potential. Recent Adv. Antiinfect. Drug Discov. 2023, 18, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Silva, C.M.; Faustino-Rocha, A.I.; Gil da Costa, R.M.; Medeiros, R.; Pires, M.J.; Gaivão, I.; Gama, A.; Neuparth, M.J.; Barbosa, J.V.; Peixoto, F.; et al. Pulegone and Eugenol Oral Supplementation in Laboratory Animals: Results from Acute and Chronic Studies. Biomedicines 2022, 10, 2595. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic Acid-Human Pharmacokinetics and Health Benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Noor, S.; Mohammad, T.; Rub, M.A.; Raza, A.; Azum, N.; Yadav, D.K.; Hassan, M.I.; Asiri, A.M. Biomedical features and therapeutic potential of rosmarinic acid. Arch. Pharm. Res. 2022, 45, 205–228. [Google Scholar] [CrossRef]
- Kernou, O.N.; Azzouz, Z.; Madani, K.; Rijo, P. Application of Rosmarinic Acid with Its Derivatives in the Treatment of Microbial Pathogens. Molecules 2023, 28, 4243. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Rui, Y.; Han, X.; Jiang, A.; Hu, J.; Li, M.; Liu, B.; Qian, F.; Huang, L. Eucalyptol prevents bleomycin-induced pulmonary fibrosis and M2 macrophage polarization. Eur. J. Pharmacol. 2022, 931, 175184. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Górny, A.; Grabarczyk, M.; Wińska, K. Can Eucalyptol Replace Antibiotics? Molecules 2021, 26, 4933. [Google Scholar] [CrossRef] [PubMed]
- Kennedy-Feitosa, E.; Cattani-Cavalieri, I.; Barroso, M.V.; Romana-Souza, B.; Brito-Gitirana, L.; Valenca, S.S. Eucalyptol promotes lung repair in mice following cigarette smoke-induced emphysema. Phytomedicine 2019, 55, 70–79. [Google Scholar] [CrossRef]
- Rafiyan, M.; Sadeghmousavi, S.; Akbarzadeh, M.; Rezaei, N. Experimental animal models of chronic inflammation. Curr. Res. Immunol. 2023, 4, 100063. [Google Scholar] [CrossRef]
- Wen, J.H.; Li, D.Y.; Liang, S.; Yang, C.; Tang, J.X.; Liu, H.F. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front. Immunol. 2022, 13, 946832. [Google Scholar] [CrossRef]
- Soliman, A.M.; Barreda, D.R. Acute Inflammation in Tissue Healing. Int. J. Mol. Sci. 2022, 24, 641. [Google Scholar] [CrossRef]
- Duarte, L.R.F.; Pinho, V.; Rezende, B.M.; Teixeira, M.M. Resolution of Inflammation in Acute Graft-Versus-Host-Disease: Advances and Perspectives. Biomolecules 2022, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Cenni, S.; Sesenna, V.; Boiardi, G.; Casertano, M.; Russo, G.; Reginelli, A.; Esposito, S.; Strisciuglio, C. The Role of Gluten in Gastrointestinal Disorders: A Review. Nutrients 2023, 15, 1615. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, S.D.; Kim, M.; Mony, T.J.; Lee, E.S.; Kim, K.M.; Choi, S.H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Sapkota, A.; Bae, Y.J.; Choi, S.-H.; Bae, H.J.; Kim, H.-J.; Cho, Y.E.; Choi, Y.-Y.; An, J.-Y.; Cho, S.-Y.; et al. The Anti-Atopic Dermatitis Effects of Mentha arvensis Essential Oil Are Involved in the Inhibition of the NLRP3 Inflammasome in DNCB-Challenged Atopic Dermatitis BALB/c Mice. Int. J. Mol. Sci. 2023, 24, 7720. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fang, X. Inflammation and Overlap of Irritable Bowel Syndrome and Functional Dyspepsia. J. Neurogastroenterol. Motil. 2021, 27, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bernstein, C.N. Environmental risk factors for inflammatory bowel disease. United Eur. Gastroenterol. J. 2022, 10, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Allin, K.H.; Petralia, F.; Colombel, J.F.; Jess, T. Multiomics to elucidate inflammatory bowel disease risk factors and pathways. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 399–409. [Google Scholar] [CrossRef]
- Bisgaard, T.H.; Allin, K.H.; Keefer, L.; Ananthakrishnan, A.N.; Jess, T. Depression and anxiety in inflammatory bowel disease: Epidemiology, mechanisms and treatment. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 717–726. [Google Scholar] [CrossRef]
- Choi, E.L.; Taheri, N.; Chandra, A.; Hayashi, Y. Cellular Senescence, Inflammation, and Cancer in the Gastrointestinal Tract. Int. J. Mol. Sci. 2023, 24, 9810. [Google Scholar] [CrossRef]
- Direito, R.; Rocha, J.; Lima, A.; Gonçalves, M.M.; Duarte, M.P.; Mateus, V.; Sousa, C.; Fernandes, A.; Pinto, R.; Boavida Ferreira, R.; et al. Reduction of Inflammation and Colon Injury by a Spearmint Phenolic Extract in Experimental Bowel Disease in Mice. Medicines 2019, 6, 65. [Google Scholar] [CrossRef]
- Mohammad Hosein, F.; Roodabeh, B.; Ali, G.; Fatemeh, F.; Fariba, N. Pharmacological activity of Mentha longifolia and its phytoconstituents. J. Tradit. Chin. Med. 2017, 37, 710–720. [Google Scholar]
- Murad, H.A.; Abdallah, H.M.; Ali, S.S. Mentha longifolia protects against acetic-acid induced colitis in rats. J. Ethnopharmacol. 2016, 190, 354–361. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Kearns, G.L.; Shulman, R.J. The physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment. Pharmacol. Ther. 2018, 47, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Doolaanea, A.A.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118, 109351. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Ihsan, M.; Nisar, M.; Zahoor, M.; Rehman, K.U.; Kudratovich, K.K. Antioxidant properties of aromatic and medicinal plants. In Plants as Medicine and Aromatics; CRC Press: Boca Raton, FL, USA, 2024; pp. 394–412. [Google Scholar]
- Mendonça, J.D.S.; Guimarães, R.C.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.P.; Marcelino, G.; Bogo, D.; Freitas, K.C.; Hiane, P.A.; de Pádua Melo, E.S.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Aldoghachi, F.E.H.; Noor Al-Mousawi, U.M.; Shari, F.H. Antioxidant Activity of Rosmarinic Acid Extracted and Purified from Mentha piperita. Arch. Razi Inst. 2021, 76, 1279–1287. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Wang, R.; Ren, F.; Wang, X. Oxidative Stress and Antioxidant Nanotherapeutic Approaches for Inflammatory Bowel Disease. Biomedicines 2021, 10, 85. [Google Scholar] [CrossRef]
- Arabshomali, A.; Bazzazzadehgan, S.; Mahdi, F.; Shariat-Madar, Z. Potential Benefits of Antioxidant Phytochemicals in Type 2 Diabetes. Molecules 2023, 28, 7209. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Ferenc, K.; Filip, R. Antioxidants as Protection against Reactive Oxidative Stress in Inflammatory Bowel Disease. Metabolites 2023, 13, 573. [Google Scholar] [CrossRef]
- Stavely, R.; Ott, L.C.; Rashidi, N.; Sakkal, S.; Nurgali, K. The Oxidative Stress and Nervous Distress Connection in Gastrointestinal Disorders. Biomolecules 2023, 13, 1586. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative stress, hormones, and effects of natural antioxidants on intestinal inflammation in inflammatory bowel disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.; Costa, K.S.D.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid. Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef] [PubMed]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory bowel diseases and gut microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- Wolfe, W.; Xiang, Z.; Yu, X.; Li, P.; Chen, H.; Yao, M.; Fei, Y.; Huang, Y.; Yin, Y.; Xiao, H.; et al. The challenge of applications of probiotics in gastrointestinal diseases. Adv. Gut Microbiome Res. 2023, 2023, 1984200. [Google Scholar] [CrossRef]
- Lahlou, R.A.; Gonçalves, A.C.; Bounechada, M.; Nunes, A.R.; Soeiro, P.; Alves, G.; Moreno, D.A.; Garcia-Viguera, C.; Raposo, C.; Silvestre, S.; et al. Antioxidant, Phytochemical, and Pharmacological Properties of Algerian Mentha aquatica Extracts. Antioxidants 2024, 13, 1512. [Google Scholar] [CrossRef]
- Krstić, S.; Milanović, I.; Stilinović, N.; Vukmirović, S.; Pavlović, N.; Berežni, S.; Rašeta, M. Health Benefits of Traditional Sage and Peppermint Juices: Simple Solutions for Antioxidant and Antidiabetic Support. Foods 2025, 14, 1182. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, A. Exploring the Role of Mentha in Gut Microbiota: A Modern Perspective of an Ancient Herb. Recent Adv. Food Nutr. Agric. 2023, 14, 94–106. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, G.H.; Yeh, H.Z.; Huang, C.K.; Poon, S.K. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: A prospective, randomized trial. J. Gastroenterol. 1997, 32, 765–768. [Google Scholar] [CrossRef]
- Kline, R.M.; Kline, J.J.; Di Palma, J.; Barbero, G.J. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J. Pediatr. 2001, 138, 125–128. [Google Scholar] [CrossRef]
- May, B.; Köhler, S.; Schneider, B. Efficacy and tolerability of a fixed combination of peppermint oil and caraway oil in patients suffering from functional dyspepsia. Aliment. Pharmacol. Ther. 2000, 14, 1671–1677. [Google Scholar] [CrossRef]
- Goerg, K.J.; Spilker, T. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: A pharmacodynamic study using simultaneous determination of gastric and gall-bladder emptying and orocaecal transit time. Aliment. Pharmacol. Ther. 2003, 17, 445–451. [Google Scholar] [CrossRef]
- Micklefield, G.; Jung, O.; Greving, I.; May, B. Effects of intraduodenal application of peppermint oil (WS(R) 1340) and caraway oil (WS(R) 1520) on gastroduodenal motility in healthy volunteers. Phytother. Res. 2003, 17, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Vejdani, R.; Shalmani, H.R.; Mir-Fattahi, M.; Sajed-Nia, F.; Abdollahi, M.; Zali, M.R.; Mohammad Alizadeh, A.H.; Bahari, A.; Amin, G. The efficacy of an herbal medicine, Carmint, on the relief of abdominal pain and bloating in patients with irritable bowel syndrome: A pilot study. Dig. Dis. Sci. 2006, 51, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Cappello, G.; Spezzaferro, M.; Grossi, L.; Manzoli, L.; Marzio, L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: A prospective double blind placebo-controlled randomized trial. Dig. Liver Dis. 2007, 39, 530–536. [Google Scholar] [CrossRef]
- Merat, S.; Khalili, S.; Mostajabi, P.; Ghorbani, A.; Ansari, R.; Malekzadeh, R. The effect of enteric-coated, delayed-release peppermint oil on irritable bowel syndrome. Dig. Dis. Sci. 2010, 55, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Cash, B.D.; Epstein, M.S.; Shah, S.M. A Novel Delivery System of Peppermint Oil Is an Effective Therapy for Irritable Bowel Syndrome Symptoms. Dig. Dis. Sci. 2016, 61, 560–571. [Google Scholar] [CrossRef]
- Rich, G.; Shah, A.; Koloski, N.; Funk, P.; Stracke, B.; Köhler, S.; Holtmann, G. A randomized placebo-controlled trial on the effects of Menthacarin, a proprietary peppermint- and caraway-oil-preparation, on symptoms and quality of life in patients with functional dyspepsia. Neurogastroenterol. Motil. 2017, 29, e13132. [Google Scholar] [CrossRef]
- Khonche, A.; Fallah Huseini, H.; Abdi, H.; Mohtashami, R.; Nabati, F.; Kianbakht, S. Efficacy of Mentha pulegium extract in the treatment of functional dyspepsia: A randomized double-blind placebo-controlled clinical trial. J. Ethnopharmacol. 2017, 206, 267–273. [Google Scholar] [CrossRef]
- Ried, K.; Travica, N.; Dorairaj, R.; Sali, A. Herbal formula improves upper and lower gastrointestinal symptoms and gut health in Australian adults with digestive disorders. Nutr. Res. 2020, 76, 37–51. [Google Scholar] [CrossRef]
- Nee, J.; Ballou, S.; Kelley, J.M.; Kaptchuk, T.J.; Hirsch, W.; Katon, J.; Cheng, V.; Rangan, V.; Lembo, A.; Iturrino, J. Peppermint Oil Treatment for Irritable Bowel Syndrome: A Randomized Placebo-Controlled Trial. Am. J. Gastroenterol. 2021, 116, 2279–2285. [Google Scholar] [CrossRef] [PubMed]
- Weerts, Z.; Essers, B.A.B.; Jonkers, D.; Willems, J.I.A.; Janssen, D.; Witteman, B.J.M.; Clemens, C.H.M.; Westendorp, A.; Masclee, A.A.M.; Keszthelyi, D. A trial-based economic evaluation of peppermint oil for the treatment of irritable bowel syndrome. United Eur. Gastroenterol. J. 2021, 9, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Shulman, R.J.; Chumpitazi, B.P.; Abdel-Rahman, S.M.; Garg, U.; Musaad, S.; Kearns, G.L. Randomised trial: Peppermint oil (menthol) pharmacokinetics in children and effects on gut motility in children with functional abdominal pain. Br. J. Clin. Pharmacol. 2022, 88, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
| Bioactive Compounds | Molecular Structures | Plant Parts | Health Effects | References |
|---|---|---|---|---|
| Acacetin | 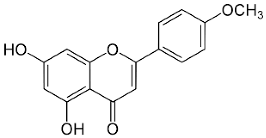 | Flowering aerial parts and leaves | Anti-inflammatory, anticancer, anti-obesity, anti-diabetic, antioxidant, neuroprotective, and cardioprotective | [38,39,40,41,42] |
| β-caryophyllene | 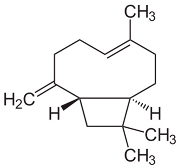 | Flowering aerial parts and leaves | Antioxidant, immunomodulatory, anti-inflammatory, anti-diabetic, antitumor, and gastroprotective | [43,44] |
| Caffeic acid | 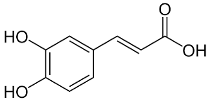 | Flowering aerial parts and leaves | Antioxidant, anticancer, antibacterial, antiviral, anti-inflammatory, anti-diabetic, and anti-dyslipidemia | [45,46,47,48,49] |
| Carvone | 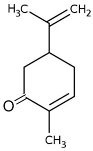 | Flowering aerial parts and leaves | Antibacterial, antifungal, anti-parasitic, antioxidant, anti-inflammatory, anticancer, and anti-diabetic | [49,50,51] |
| Epicatechin | 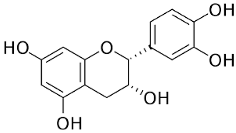 | Flowering aerial parts and leaves | Antihypertensive, vasorelaxant, antioxidant, anti-inflammatory, anti-diabetic, anti-dyslipidemia, and anti-atherosclerotic, hepatoprotective, and neuroprotective | [49,52,53,54] |
| Isomenthol | 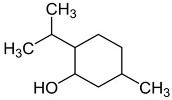 | Flowering aerial parts and leaves | Thermogenic, anti-spasmodic, antioxidant, hepatoprotective, anti-inflammatory, and antibacterial | [43,55] |
| Limonene | 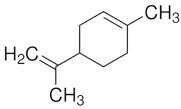 | Flowering aerial parts and leaves | Antioxidant, anti-diabetic, anticancer, anti-inflammatory, cardioprotective, gastroprotective, hepatoprotective, immunomodulatory, and anti-fibrotic | [43,56,57,58] |
| Luteolin | 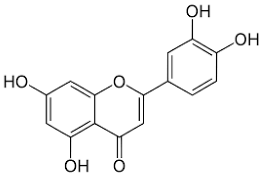 | Flowering aerial parts and leaves | Antioxidant, anti-inflammatory, anticancer, and antiviral | [49,59,60,61,62] |
| Menthofuran | 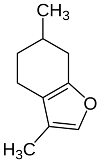 | Flowering aerial parts and leaves | Antidiarrheal and gastroprotective | [43,63,64] |
| Menthol | 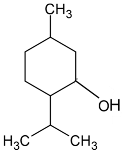 | Flowering aerial parts and leaves | Analgesic, antibacterial, antifungal, anesthetic, immunomodulatory effects, anti-inflammatory, and anticancer | [43,65,66,67,68] |
| Menthone | 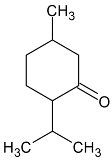 | Flowering aerial parts and leaves | Antiviral, anti-inflammatory, antioxidant, antidepressant, antifungal, anticancer, and antibacterial | [43,69,70] |
| Menthyl acetate | 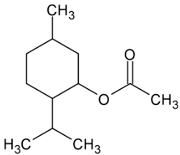 | Flowering aerial parts and leaves | Anti-inflammatory, analgesic, antifungal, antiviral, and antimicrobial | [71,72,73] |
| Neomenthol | 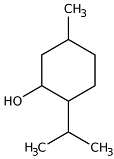 | Flowering aerial parts and leaves | Cooling–soothing effects, treatment of sore throat and mouth irritations | [43,74] |
| Pulegone | 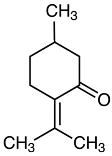 | Flowering aerial parts and leaves | Antioxidant, antimicrobial, antifungal, antiviral, antibacterial, antihistaminic, and anti-inflammatory | [43,75,76] |
| Rosmarinic acid | 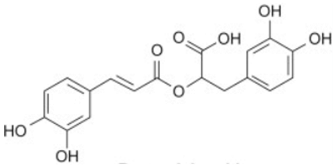 | Flowering aerial parts and leaves | Antioxidant, antibacterial, antiviral, anti-inflammatory, anti-hyperglycemic, analgesic, hepatoprotective, immunomodulatory, anticancer, cardioprotective, and neuroprotective | [49,77,78,79,80] |
| 1,8-cineole (eucalyptol) | 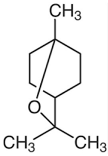 | Flowering aerial parts and leaves | Antimicrobial, anti-inflammatory, antioxidant, and anti-fibrotic | [43,81,82,83] |
| Ref | Model/Country | Population | Intervention/ Comparison | Outcomes | Side Effects |
|---|---|---|---|---|---|
| [120] | Randomized, prospective, double-blind, placebo-controlled clinical study, single-center/Taiwan. | 110 patients, 66 ♂, 44 ♀, 18–70 y, with active symptoms of IBS. | Participants received enteric-coated capsules containing 187 mg of PO (n = 52) or a placebo (n = 49), 3 to 4 times daily, 15–30 min before meals/4 weeks. | PO controlled most symptoms of IBS (abdominal pain, distension, stool frequency and consistency, borborygmi, and flatulence) in about 78% of patients (p < 0.05). | Heartburn was present at the beginning of treatment, and there was a mild skin rash over both forearms (PO group). |
| [121] | Randomized, double-blind, controlled trial, multicenter/USA. | 50 patients, 40% ♂, 60% ♀, 8–17 y, previously diagnosed with IBS. | Participants received enteric-coated capsules containing 187 mg of PO or a placebo. >45 kg = 2 capsules 3 times daily or 30–45 kg = 1 capsule 3 times daily/2 weeks. | PO group: 76% of the patients reported changes in the severity of symptom scale (p < 0.001), and the severity of pain symptoms was significantly lower (p < 0.03). There are no changes in abdominal rumbling, distention, belching, gas, or heartburn. | No side effects were reported. |
| [122] | Randomized, double-blind, parallel-group, multicenter/Germany. | 96 patients, 32 ♂, 64 ♀, at least 18 y with a secured diagnosis of FD. | Participants received enteric-coated capsules containing 90 mg PO and 50 mg caraway oil (n = 48) or a placebo (n = 48), two capsules daily (in the morning and at lunchtime) for 28 days. | The average pain intensity decreased by 40% (p = 0.0003), and the sensations of pressure, heaviness, and fullness decreased by 43.5% (p = 0.0005) in the treated group. | Neck and shoulder pain, hemorrhoids, bronchitis, influenza-like symptoms, mild eructation, nausea, and vomiting. |
| [123] | Clinical trial, single-center/Germany. | 12 patients, 6 ♂, 6 ♀, 24–51 y, healthy volunteers. | On day 1, a placebo was given; for the next 4 days, the substances 90 mg PO, 50 mg caraway oil, 10 mg cisapride, and 10 mg n-butyl scopolamine were studied in a randomized sequence, with a washout phase of 2 days after each investigation. | Orocaecal transit time was prolonged by PO (p = 0.004), and caraway oil was not significant. No effects on gastric emptying, inhibition of gallbladder emptying. | Eructation with a peppermint taste. One case was associated with mild heartburn that rapidly disappeared. |
| [124] | Randomized, prospective, controlled, double-blind, two-period crossover trial, single-center/Germany. | 24 patients, 12 ♂, 12 ♀, 21–42 y, healthy volunteers. | Participants received the lipophilic carrier/control + 90 mg PO or a placebo, control + 50 mg caraway oil, or control + 50 mg hydrophobic phase in 1 day. | Intraduodenal application of 90 mg PO and 50 mg caraway oil affects duodenal motility and reduces motility in the gastric antrum and corpus. | No adverse events were observed during the study. |
| [125] | Randomized, double-blind, placebo-controlled, multicenter/Iran. | 32 patients, 18 ♂, 14 ♀, 18–65 y with IBS diagnosed using the Rome II criteria. | Participants received 30 drops of Carmint (combination of Melissa officinalis, Mentha spicata, and Coriandrum sativum) (n = 14) or a placebo (n = 18) 3 times a day after each meal. They also received loperamide (2 mg/2x/d) (n = 10) or one spoonful of psyllium powder 1x/d (n = 22) for 8 weeks. | Carmint group: the average abdominal pain/discomfort severity and frequency were significantly lower (p = 0.016), and the average bloating severity score (p = 0.02) at the end of the treatment. | Anal pain before defecation, malaise, anxiety/chest pain/sweating. |
| [126] | Randomized, prospective, double-blind, placebo-controlled clinical trial, single-center/Italy. | According to the Rome II criteria, 50 patients, 12 ♂, 38 ♀, 20–60 y with IBS. | Participants received capsules with 225 mg PO + 45 mg Natrasorb (n = 24) or a placebo (n = 26). Two enteric-coated capsules twice a day, before meals, for 4 weeks. | 75% of patients in the PO group showed a > 50% reduction in basal TISS (p < 0.009), and a beneficial effect persisted after the other 4 weeks (p < 0.05). | Intense heartburn and a minty taste in his mouth. |
| [127] | Randomized, double-blind, placebo-controlled clinical trial, single-center/Iran. | 60 patients, 15 ♂, 45 ♀, 22–48 y with diagnosed IBS by Rome II criteria | Participants took one enteric-coated capsule of 187 mg PO or a placebo, 3 times daily, 30 min before each meal for 8 weeks. | 42.5% of PO group patients were free from abdominal pain or discomfort (p < 0.001), and the intensity of the pain was also significantly reduced (p < 0.001). | Heartburn, headache, and dizziness. |
| [128] | Randomized, double-blind, placebo-controlled clinical trial, multicenter/USA. | 72 patients, 18 ♂, 54 ♀, 18–60 y, who met Rome III criteria for IBS-M or IBS-D. | Patients were instructed to take two capsules of 180 mg PO or a placebo 3 times daily between 30 and 90 min before meals for 4 weeks. | The PO group showed a 40% reduction in the TISS from baseline (p = 0.0246) and a 21% decrease in abdominal pain and discomfort (p = 0.0138). | Flatulence, dyspepsia, and gastroesophageal reflux. |
| [129] | Randomized, prospective, double-blind, parallel-group, placebo-controlled clinical trial, multicenter/Australia and Germany. | 114 patients, 41 ♂, 73 ♀, 32–62 y with FD symptoms consistent with EPS and PDS. | Participants received 90 mg PO + 50 mg caraway oil or a placebo, two capsules per day to be taken with liquids in the morning and at noon, before meals for 4 weeks. | 88% of the patients from the treated group showed any improvement (p = 0.0001), and an overall therapeutic effect for 51.7% of patients was assessed to be very good (p < 0.0001). | Eleven patients from the treated group experienced adverse events, which were not reported in the study. |
| [130] | Randomized, 2-arm, double-blind, placebo-controlled parallel-group, single-center/Iran. | One hundred patients, 48 ♂, 52 ♀, aged 25–56 y, fulfilled Rome III criteria for FD. | Participants received 330 mg of Mentha pulegium extract powder or a placebo three times a day and one famotidine 40 mg tablet daily for 8 weeks. | Mentha pulegium significantly reduced the total dyspepsia score (Hong Kong dyspepsia index) (p = 0.011) and decreased stomach pain, upper abdominal bloating, dull ache, and belching. | No adverse events were reported. |
| [131] | Single-arm, pre-post study, single-center/Australia. | Forty-three patients, 24% ♂, 76% ♀, mean age of 50 y, experiencing one or multiple symptoms at least once a week for 3 months, such as heartburn, reflux, nausea, regurgitation, abdominal pain, bloating, diarrhea, constipation, and IBS. | After a 4-week control phase (0 g/d), patients received 5 g/d of the Nutrition Care Gut Relief Formula powder (a combination of curcumin, Aloe vera, guar gum, slippery elm, glutamine, and PO) for 4 weeks, followed by 10 g/d for another 4 weeks and more 4 weeks of the patient’s preferred dose (0/5/10 g/d). The powder is to be taken mixed with water or with food. | The formula improved the GI symptoms’ severity (56% to 62%) and frequency (64%) as indigestion, heartburn, regurgitation, and nausea (p < 0.001) and also the frequency and severity abdominal pain (62%) and troublesome flatulence (58%) (p < 0.0001). | No bothersome adverse events were reported. |
| [21] | Randomized, double-blind, placebo-controlled trial, multicenter/The Netherlands. | 189 patients, 42 ♂, 147 ♀, 18–70 y, fulfilling the Rome IV criteria for IBS. | Participants received 182 mg of small-intestinal release PO or 182 mg of ileocolonic-release PO or a placebo, three capsules daily, 30 min before breakfast, lunch, and dinner, for 8 weeks. | The proportion of patients who reported abdominal pain did not differ drastically between groups: 46.8% in the small-intestinal release group (p = 0.170) and 41.3% in the ileocolonic-release peppermint oil group (p = 0.385), compared with 34.4% in the placebo. | Heartburn, belching, headache, nausea, abdominal cramps, and altered anal sensation or sensitive urethra. |
| [132] | Randomized, double-blind, placebo-controlled trial, single-center/USA. | 133 patients, 35 ♂, 98 ♀, mean age in the early 40s, with IBS diagnosed by the Rome IV criteria. | Patients received 180 mg enteric-coated PO capsules or a placebo group 3 times a day for 6 weeks. | A substantial mean improvement in the IBS-SSS score for both groups showed no significant difference in scores between the two groups (p = 0.97). Both groups had equal benefits for most patients. | Belching and heartburn. |
| [133] | Randomized, double-blind, placebo-controlled trial, multicenter/The Netherlands. | 126 patients, 26 ♂, 100 ♀, 18–75 y, fulfilling the Rome IV criteria for IBS. | Patients received a placebo or 182 mg small-intestinal release PO for 8 weeks. | The small-intestinal release PO is more effective at a lower cost in 46% of the simulations, but at a higher cost in 31%, while it is inferior in 18% (less effective and higher costs). | No adverse events were reported. |
| [134] | Randomized trial, single center/USA. | 30 patients, 9 ♂, 21 ♀, 7–12 y, with functional abdominal pain (pediatric Rome III criteria). | At visit 1, patients received a WMC. After 1 week, they received 180 mg, 360 mg, or 540 mg of enteric-coated PO for 1 week, during which time the WMC test was repeated. | Small-intestinal transit time was slower (p = 0.066). Stomach (p = 0.02) and duodenal (p = 0.039) contraction frequency increased with increasing peppermint oil dose, as well as ileum (p = 0.028) and colonic (p = 0.075) mean contraction amplitude. | No adverse events were reported. |
| Study | Question Focus | Appropriate Randomization | Allocation Blinding | Double- Blind | Losses (>20%) | Prognostic or Demographic Characteristics | Outcomes | Intention to Treat Analysis | Sample Calculation | Adequate Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| [120] | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes |
| [121] | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes |
| [122] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| [123] | Yes | No | No | No | No | No | Yes | No | No | Yes |
| [124] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes |
| [125] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| [126] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| [127] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| [128] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| [129] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| [130] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| [131] | Yes | No | No | No | No | No | Yes | No | Yes | Yes |
| [21] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes |
| [132] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes |
| [133] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes |
| [134] | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, M.; Fornari Laurindo, L.; Dogani Rodrigues, V.; Cristina Castilho Caracio, F.; Valenti, V.E.; Pereira, E.d.S.B.M.; Haber Mellem, R.; Penteado Detregiachi, C.R.; dos Santos Bueno, M.; Guissoni Campos, L.M.; et al. Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence. Pharmaceuticals 2025, 18, 693. https://doi.org/10.3390/ph18050693
Hirata M, Fornari Laurindo L, Dogani Rodrigues V, Cristina Castilho Caracio F, Valenti VE, Pereira EdSBM, Haber Mellem R, Penteado Detregiachi CR, dos Santos Bueno M, Guissoni Campos LM, et al. Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence. Pharmaceuticals. 2025; 18(5):693. https://doi.org/10.3390/ph18050693
Chicago/Turabian StyleHirata, Mariana, Lucas Fornari Laurindo, Victória Dogani Rodrigues, Flávia Cristina Castilho Caracio, Vitor Engrácia Valenti, Eliana de Souza Bastos Mazuqueli Pereira, Rodrigo Haber Mellem, Cláudia Rucco Penteado Detregiachi, Manuela dos Santos Bueno, Leila Maria Guissoni Campos, and et al. 2025. "Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence" Pharmaceuticals 18, no. 5: 693. https://doi.org/10.3390/ph18050693
APA StyleHirata, M., Fornari Laurindo, L., Dogani Rodrigues, V., Cristina Castilho Caracio, F., Valenti, V. E., Pereira, E. d. S. B. M., Haber Mellem, R., Penteado Detregiachi, C. R., dos Santos Bueno, M., Guissoni Campos, L. M., Spilla, C. S. G., & Barbalho, S. M. (2025). Investigating the Health Potential of Mentha Species Against Gastrointestinal Disorders—A Systematic Review of Clinical Evidence. Pharmaceuticals, 18(5), 693. https://doi.org/10.3390/ph18050693








