From Flower to Medicine: Green-Synthesized Silver Nanoparticles as Promising Antibacterial Agents
Abstract
1. Introduction
2. Results and Discussion
2.1. HRS Flower Extract-Mediated Synthesis of AgNPs
2.2. UV–Vis-NIR Spectra of AgNPs
2.3. Morphological Characterization of HRS-AgNPs via Transmission Electron Microscopy (TEM)
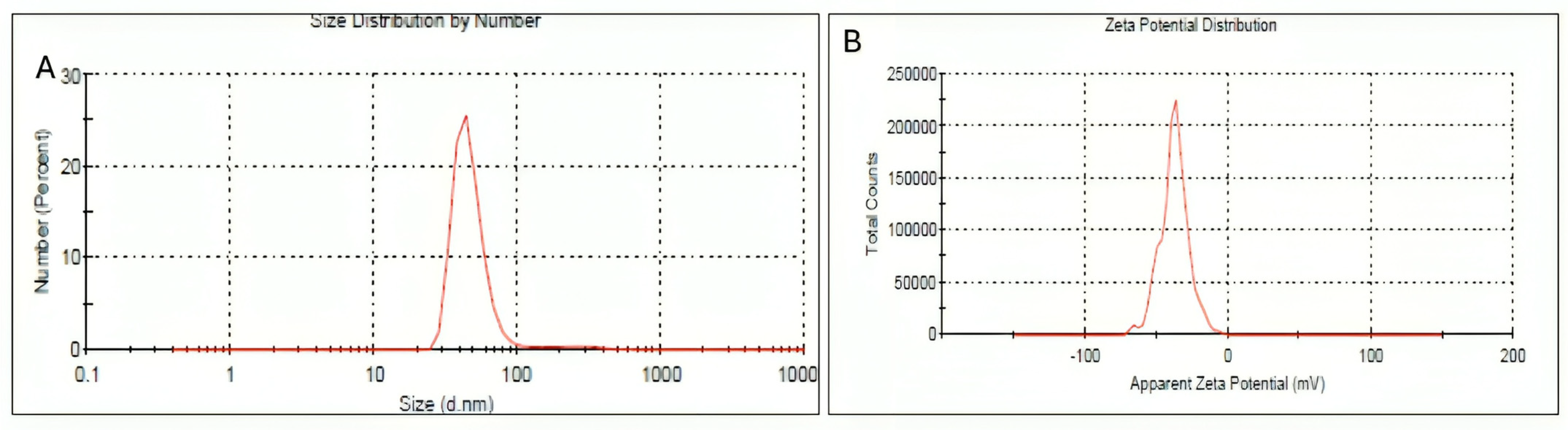
2.4. Dynamic Light Scattering and Zeta Potential
2.5. FTIR Analysis
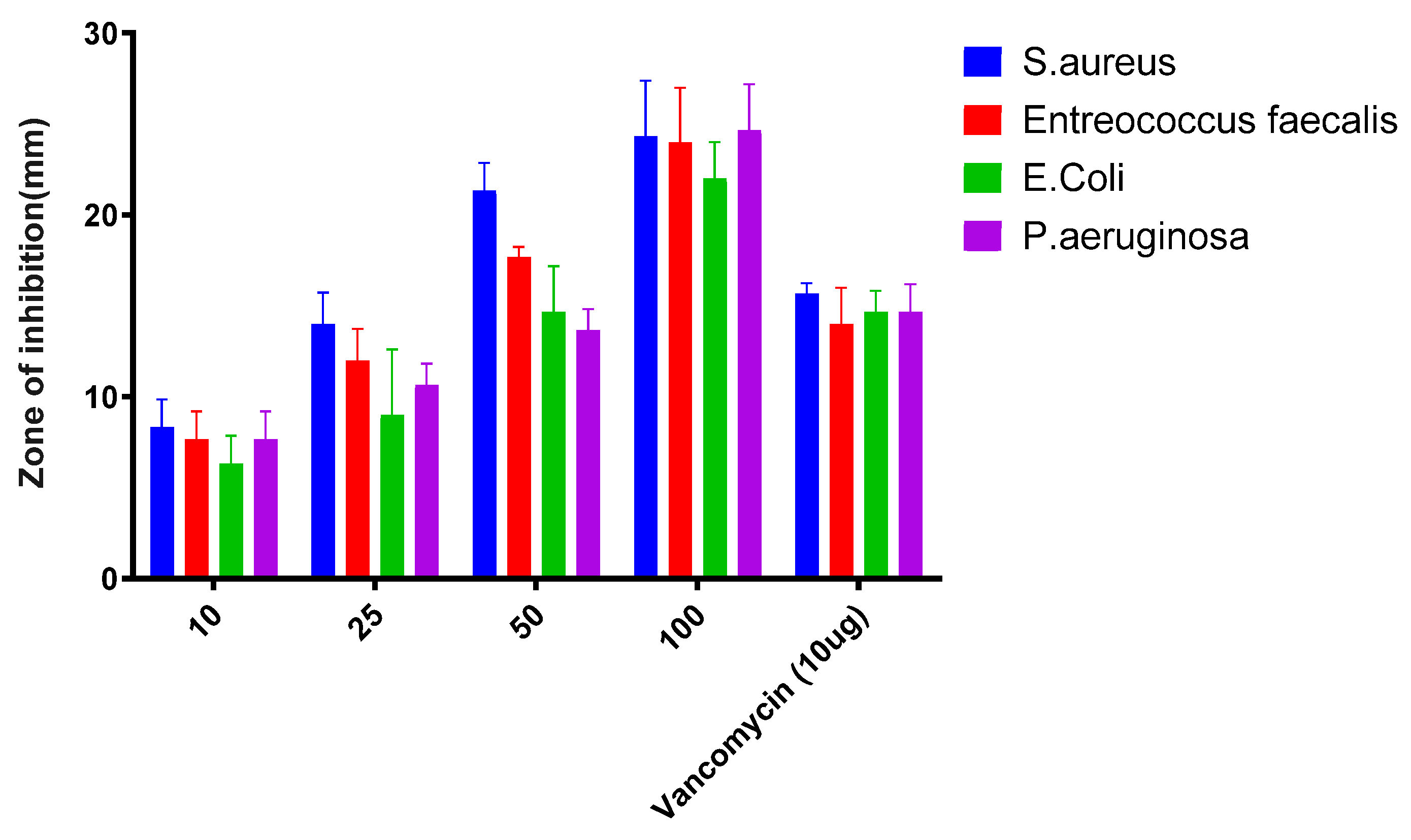
2.6. Antibacterial Activity
2.7. Cytotoxic Potential
3. Materials and Methods
3.1. Hibiscus Rosa Sinensis Flower Extract Preparation and Synthesis of Silver Nanoparticles
3.2. Absorption Spectra of Silver Nanoparticles (AgNPs)
3.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Measurements
3.4. Transmission Electron Microscopy (TEM) Measurements
3.5. In Vitro Antibacterial Activity
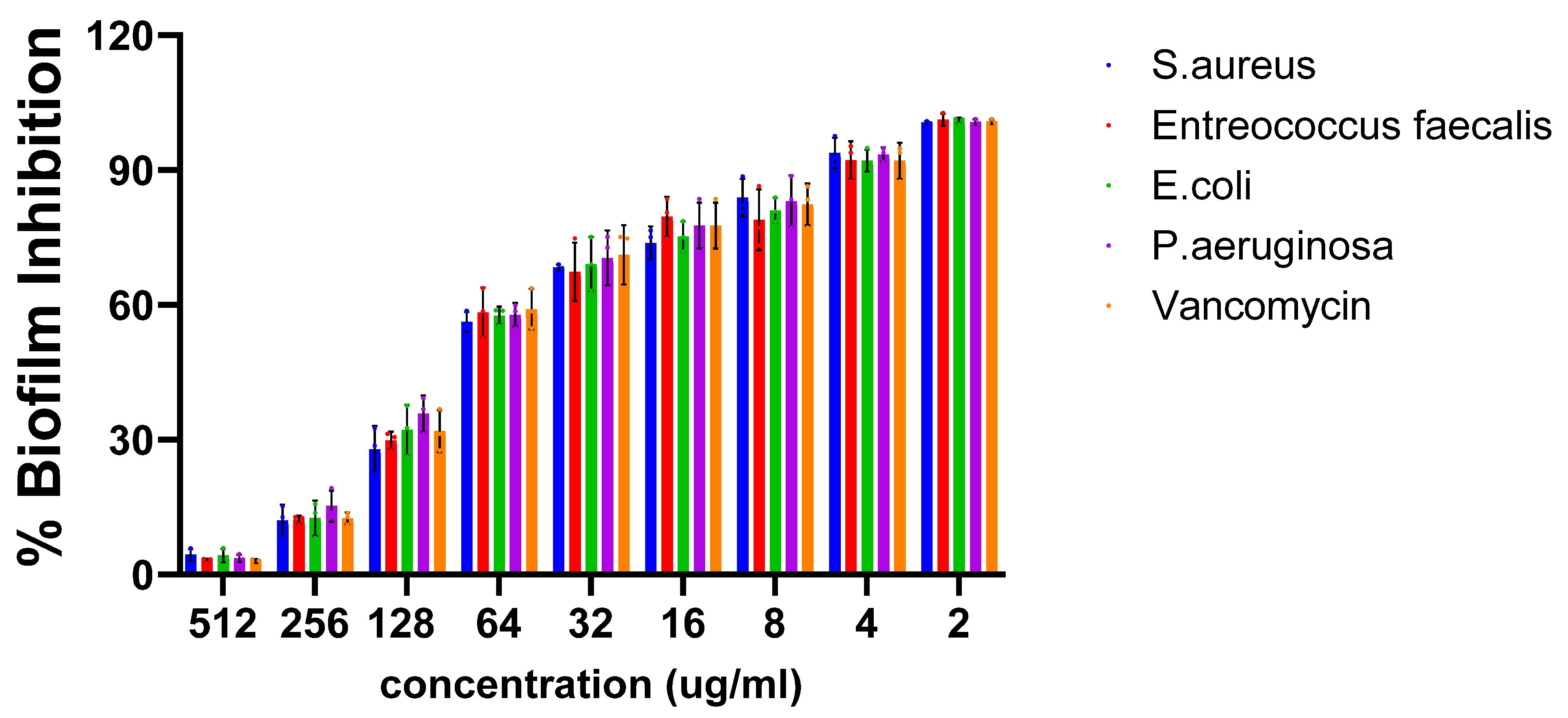
3.6. Evaluation of the Antibiofilm Activity of Silver Nanoparticles Through XTT Reduction Assay
3.7. Assessment of Cytotoxicity Utilizing the 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
3.8. Quantification of Intracellular Reactive Oxygen Species (ROS)
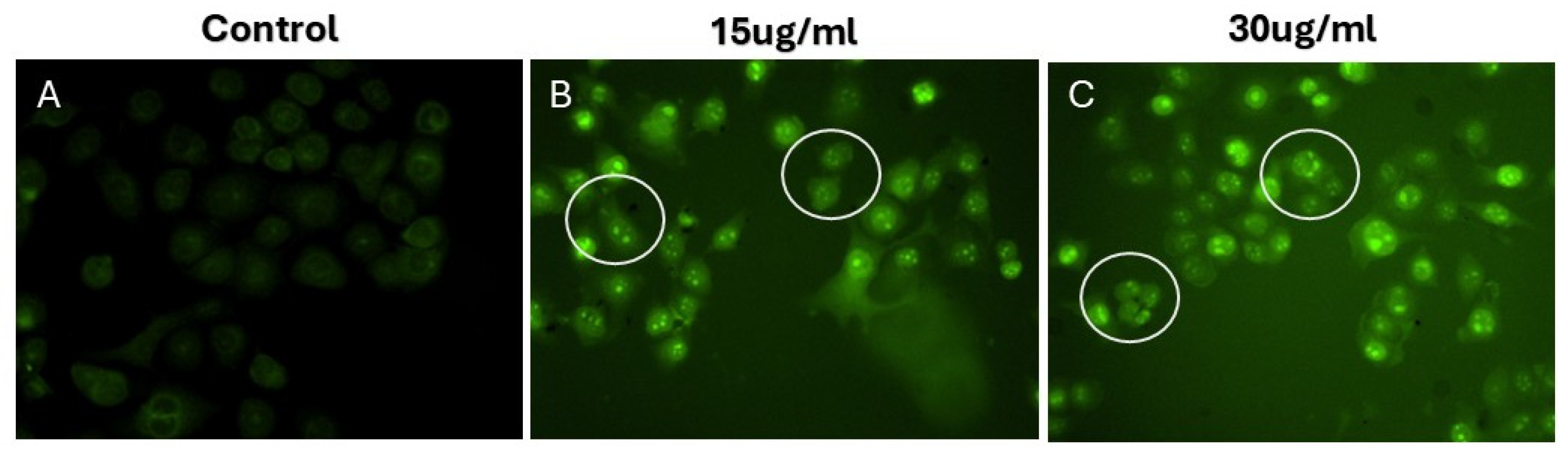
3.9. Evaluation of Morphological Changes Utilizing 4′,6-Diamidino-2-Phenylindole (DAPI) Staining
3.10. The Evaluation of Mitochondrial Membrane Potential (ΔΨm)
3.11. The Evaluation of Cleaved Caspase 3 Through Fluorescence Microscopy
4. Conclusions, Limitations, and Future Directions
Limitations and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef]
- Brieland, J.; Essig, D.; Jackson, C.; Frank, D.; Loebenberg, D.; Menzel, F.; Arnold, B.; DiDomenico, B.; Hare, R. Comparison of pathogenesis and host immune responses to Candida glabrata and Candida albicans in systemically infected immunocompetent mice. Infect. Immun. 2001, 69, 5046–5055. [Google Scholar] [CrossRef]
- Morris-Jones, R. ABC of Dermatology; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, L.; Ghosh, N.; Sharma, B. Pneumococcal biofilms and their intervention strategies. Int. J. Pharm. Pharm. Sci. 2018, 10, 5–8. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Biofilms in disease. In Antibiofilm Agents: From Diagnosis to Treatment and Prevention; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–13. [Google Scholar]
- Alvarado-Gomez, E.; Perez-Diaz, M.; Valdez-Perez, D.; Ruiz-Garcia, J.; Magana-Aquino, M.; Martinez-Castanon, G.; Martinez-Gutierrez, F. Adhesion forces of biofilms developed in vitro from clinical strains of skin wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 336–344. [Google Scholar] [CrossRef]
- Szczotka-Flynn, L.B.; Imamura, Y.; Chandra, J.; Yu, C.; Mukherjee, P.K.; Pearlman, E.; Ghannoum, M.A. Increased resistance of contact lens-related bacterial biofilms to antimicrobial activity of soft contact lens care solutions. Cornea 2009, 28, 918–926. [Google Scholar] [CrossRef]
- Wu, X.; Yang, H.; Yu, X.; Qin, J.J. Drug-resistant HER2-positive breast cancer: Molecular mechanisms and overcoming strategies. Front. Pharmacol. 2022, 13, 1012552. [Google Scholar] [CrossRef]
- Tatiparti, K.; Rauf, M.A.; Sau, S.; Iyer, A.K. Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor. Molecules 2020, 25, 2362. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef]
- Chauhan, G.; Madou, M.J.; Kalra, S.; Chopra, V.; Ghosh, D.; Martinez-Chapa, S.O. Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 2020, 14, 7760–7782. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.M.; Cheriyan, V.T.; Polin, L.A.; Vaishampayan, U.; Rishi, A.K.; Iyer, A.K. Tumor hypoxia directed multimodal nanotherapy for overcoming drug resistance in renal cell carcinoma and reprogramming macrophages. Biomaterials 2018, 183, 280–294. [Google Scholar] [CrossRef]
- Ramos-Martín, F.; D’amelio, N. Drug resistance: An incessant fight against evolutionary strategies of survival. Microbiol. Res. 2023, 14, 507–542. [Google Scholar] [CrossRef]
- Hernandez-Sierra, J.F.; Ruiz, F.; Pena, D.C.; Martinez-Gutierrez, F.; Martinez, A.E.; Guillen Ade, J.; Tapia-Perez, H.; Castanon, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Teli, M.K.; Mutalik, S.; Rajanikant, G.K. Nanotechnology and nanomedicine: Going small means aiming big. Curr. Pharm. Des. 2010, 16, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Espitia, P.J.P.; Soares, N.d.F.F.; Coimbra, J.S.d.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Zinc oxide nanoparticles: Synthesis, antimicrobial activity and food packaging applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef]
- Ahmad, J.; Akhter, S.; Rizwanullah, M.; Khan, M.A.; Pigeon, L.; Addo, R.T.; Greig, N.H.; Midoux, P.; Pichon, C.; Kamal, M.A. Nanotechnology Based Theranostic Approaches in Alzheimer’s Disease Management: Current Status and Future Perspective. Curr. Alzheimer Res. 2017, 14, 1164–1181. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramirez, J.T.; Yacaman, M.J. The bactericidal effect of silver nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Diez, I.; Eronen, P.; Osterberg, M.; Linder, M.B.; Ikkala, O.; Ras, R.H. Functionalization of nanofibrillated cellulose with silver nanoclusters: Fluorescence and antibacterial activity. Macromol. Biosci. 2011, 11, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Rauf, M.A.; Qari, H.A. Therapeutic Applications of Biogenic Silver Nanomaterial Synthesized from the Paper Flower of Bougainvillea glabra (Miami, Pink). Nanomaterials 2023, 13, 615. [Google Scholar] [CrossRef]
- Oves, M.; Ahmar Rauf, M.; Aslam, M.; Qari, H.A.; Sonbol, H.; Ahmad, I.; Sarwar Zaman, G.; Saeed, M. Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J. Biol. Sci. 2022, 29, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- dos Santos, F.K.; dos Santos, E.O.; Veiga-Junior, V.F.; Teixeira-Costa, B.E. Hibiscus rosa-sinensis. In Edible Flowers; Elsevier: Amsterdam, The Netherlands, 2024; pp. 127–156. [Google Scholar]
- Missoum, A. An update review on Hibiscus rosa sinensis phytochemistry and medicinal uses. J. Ayurvedic Herb. Med. 2018, 4, 135–146. [Google Scholar] [CrossRef]
- Khan, Z.A.; Naqvi, S.A.; Mukhtar, A.; Hussain, Z.; Shahzad, S.A.; Mansha, A.; Ahmad, M.; Zahoor, A.F.; Bukhari, I.H.; Ashraf-Janjua, M.R.; et al. Antioxidant and antibacterial activities of Hibiscus Rosa-sinensis Linn flower extracts. Pak. J. Pharm. Sci. 2014, 27, 469–474. [Google Scholar]
- Devaraji, M.; Thanikachalam, P.V. Phytoconstituents as emerging therapeutics for breast cancer: Mechanistic insights and clinical implications. Cancer Pathog. Ther. 2025, 3, E01–E19. [Google Scholar] [CrossRef]
- Ahirwar, B.; Ahirwar, D. In vivo and in vitro investigation of cytotoxic and antitumor activities of polyphenolic leaf extract of Hibiscus sabdariffa against breast cancer cell lines. Res. J. Pharm. Technol. 2020, 13, 615–620. [Google Scholar] [CrossRef]
- Thuwaini, M.M. Natural sources as promising future anticancer therapies—A review. GSC Biol. Pharm. Sci. 2022, 19, 084–113. [Google Scholar] [CrossRef]
- Nguyen, C.; Baskaran, K.; Pupulin, A.; Ruvinov, I.; Zaitoon, O.; Grewal, S.; Scaria, B.; Mehaidli, A.; Vegh, C.; Pandey, S. Hibiscus flower extract selectively induces apoptosis in breast cancer cells and positively interacts with common chemotherapeutics. BMC Complement. Altern. Med. 2019, 19, 98. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Dhaliwal, A.S.; Malik, C. Biogenic synthesis of silver nanoparticles, using Bougainvillea spectabilis willd. Bract extract. Natl. Acad. Sci. Lett. 2012, 35, 383–388. [Google Scholar] [CrossRef]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]
- Rauf, A.; Ahmad, Z.; Zhang, H.; Muhammad, N.; Akram, Z.; Ud Din, I. Green synthesis, characterization, and in vitro and in vivo biological screening of iron oxide nanoparticles (Fe3O4) generated with hydroalcoholic extract of aerial parts of Euphorbia milii. Green Process. Synth. 2024, 13, 20240155. [Google Scholar] [CrossRef]
- Abd-Elkhalek, H.F.; Badawy, A.A.; Al-Askar, A.A.; Abd Elgawad, H.; Hashem, A.H.; Salem, S.S. Biosynthesis and characterization of selenium and silver nanoparticles using Trichoderma viride filtrate and their impact on Culex pipiens. Green Process. Synth. 2024, 13, 20240025. [Google Scholar] [CrossRef]
- Ito, T.; Sun, L.; Bevan, M.A.; Crooks, R.M. Comparison of nanoparticle size and electrophoretic mobility measurements using a carbon-nanotube-based coulter counter, dynamic light scattering, transmission electron microscopy, and phase analysis light scattering. Langmuir 2004, 20, 6940–6945. [Google Scholar] [CrossRef]
- Ferro, A.; Mestre, T.; Carneiro, P.; Sahumbaiev, I.; Seruca, R.; Sanches, J.M. Blue intensity matters for cell cycle profiling in fluorescence DAPI-stained images. Lab. Investig. 2017, 97, 615–625. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Mosallam, F.M.; El-Sayyad, G.S. Synthesis of metallic silver nanoparticles by fluconazole drug and gamma rays to inhibit the growth of multidrug-resistant microbes. J. Clust. Sci. 2018, 29, 1003–1015. [Google Scholar] [CrossRef]
- Heng, M.H.; Win, Y.F.; Cheah, E.S.G.; Chan, Y.B.; Rahman, M.K.; Sultana, S.; Tey, L.-H.; Wong, L.S.; Djearamane, S.; Akhtaruzzaman, M. Microwave-assisted green synthesis, characterization, and in vitro antibacterial activity of NiO nanoparticles obtained from lemon peel extract. Green Process. Synth. 2024, 13, 20240071. [Google Scholar] [CrossRef]
- Rehman, F.U.; Bao, J.; Muhammad, P.; He, W.; Hanif, S.; Rauf, M.A. Blood-brain barrier amenable gold nanoparticles biofabrication in aged cell culture medium. Mater. Today Bio 2020, 8, 100072. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Wahab, R.; Khan, F.; Mishra, Y.K.; Musarrat, J.; Al-Khedhairy, A.A. Reactive oxygen species mediated bacterial biofilm inhibition via zinc oxide nanoparticles and their statistical determination. PLoS ONE 2014, 9, e111289. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.S.; Soukas, A.A. Identity, structure, and function of the mitochondrial permeability transition pore: Controversies, consensus, recent advances, and future directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Baev, A.Y.; Vinokurov, A.Y.; Potapova, E.V.; Dunaev, A.V.; Angelova, P.R.; Abramov, A.Y. Mitochondrial Permeability Transition, Cell Death and Neurodegeneration. Cells 2024, 13, 648. [Google Scholar] [CrossRef]
- Schultz, D.R.; Harringto, W.J., Jr. Apoptosis: Programmed cell death at a molecular level. Semin. Arthritis Rheum. 2003, 32, 345–369. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef]
- Creagh, E.M.; Conroy, H.; Martin, S.J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 2003, 193, 10–21. [Google Scholar] [CrossRef]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- McIlwain, D.; Berger, T.; Mak, T. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Kikuchi, M.; Kuroki, S.; Kayama, M.; Sakaguchi, S.; Lee, K.K.; Yonehara, S. Protease activity of procaspase-8 is essential for cell survival by inhibiting both apoptotic and nonapoptotic cell death dependent on receptor-interacting protein kinase 1 (RIP1) and RIP3. J. Biol. Chem. 2012, 287, 41165–41173. [Google Scholar] [CrossRef] [PubMed]
- Arland, S.E.; Kumar, J. Green and chemical syntheses of silver nanoparticles: Comparative and comprehensive study on characterization, therapeutic potential, and cytotoxicity. Eur. J. Med. Chem. Rep. 2024, 11, 100168. [Google Scholar]
- Ahmed, S.; Ikram, S. Silver nanoparticles: One pot green synthesis using Terminalia arjuna extract for biological application. J. Nanomed. Nanotechnol. 2015, 6, 1–6. [Google Scholar]
- Balderstone, L.A.; Dawson, J.C.; Welman, A.; Serrels, A.; Wedge, S.R.; Brunton, V.G. Development of a fluorescence-based cellular apoptosis reporter. Methods Appl. Fluoresc. 2018, 7, 015001. [Google Scholar] [CrossRef]
- Alenzi, F.Q.; Lotfy, M.; Wyse, R. Swords of cell death: Caspase activation and regulation. Asian Pac. J. Cancer Prev. 2010, 11, 271–280. [Google Scholar]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef]
- Reveendran, A.; Varghese, S.; Viswanathan, K. Green synthesis of silver nano particle using Hibiscus Rosa Sinensis. J. Apllied Phys. 2016, 8, 35–38. [Google Scholar]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnol. 2015, 9, 288–293. [Google Scholar] [CrossRef]
- Bilal, B.; Niazi, R.; Nadeem, S.; Farid, M.A.; Nazir, M.S.; Akhter, T.; Javed, M.; Mohyuddin, A.; Rauf, A.; Ali, Z.; et al. Fabrication of Guided Tissue Regeneration Membrane Using Lignin-Mediated ZnO Nanoparticles in Biopolymer Matrix for Antimicrobial Activity. Front. Chem. 2022, 10, 837858. [Google Scholar] [CrossRef]
- Alam, M.T.; Rauf, M.A.; Siddiqui, G.A.; Owais, M.; Naeem, A. Green synthesis of silver nanoparticles, its characterization, and chaperone-like activity in the aggregation inhibition of alpha-chymotrypsinogen A. Int. J. Biol. Macromol. 2018, 120, 2381–2389. [Google Scholar] [CrossRef]
- Ul Hassan, S.; Bilal, B.; Nazir, M.S.; Naqvi, S.A.R.; Ali, Z.; Nadeem, S.; Muhammad, N.; Palvasha, B.A.; Mohyuddin, A. Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration. Chem. Biol. Drug Des. 2021, 98, 1007–1024. [Google Scholar] [CrossRef]
- Allaker, R.; Vargas-Reus, M.; Ren, G. Nanometals as antimicrobials. Antimicrob. Polym. 2012, 2011, 327–350. [Google Scholar]
- Ramalingam, V.; Rajaram, R.; PremKumar, C.; Santhanam, P.; Dhinesh, P.; Vinothkumar, S.; Kaleshkumar, K. Biosynthesis of silver nanoparticles from deep sea bacterium Pseudomonas aeruginosa JQ989348 for antimicrobial, antibiofilm, and cytotoxic activity. J. Basic Microbiol. 2014, 54, 928–936. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. Am. Soc. Microbiol. 2009, 15, 1–23. [Google Scholar]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Supino, R. MTT assays. In In Vitro Toxicity Testing Protocols; Humana: Totowa, NJ, USA, 1995; pp. 137–149. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of cell viability by the MTT assay. Cold Spring Harb. Protoc. 2018, 2018, pdb.prot095505. [Google Scholar] [CrossRef]
- Eruslanov, E.; Kusmartsev, S. Advanced protocols in oxidative stress II. Methods Mol. Biol. 2010, 594, 57–72. [Google Scholar]
- Sherwani, M.A.; Tufail, S.; Khan, A.A.; Owais, M. Dendrimer-PLGA based multifunctional immuno-nanocomposite mediated synchronous and tumor selective delivery of siRNA and cisplatin: Potential in treatment of hepatocellular carcinoma. RSC Adv. 2015, 5, 39512–39531. [Google Scholar] [CrossRef]
- Ahmar Rauf, M.; Oves, M.; Ur Rehman, F.; Rauf Khan, A.; Husain, N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed. Pharmacother. 2019, 116, 108983. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.; Binsuwaidan, R.; Alshammari, N.; Alharbi, A.M.; Alabdallahd, N.M.; Alotaibi, N.A.; Siddiqui, S.; Obaidur, S. From Flower to Medicine: Green-Synthesized Silver Nanoparticles as Promising Antibacterial Agents. Pharmaceuticals 2025, 18, 691. https://doi.org/10.3390/ph18050691
Saeed M, Binsuwaidan R, Alshammari N, Alharbi AM, Alabdallahd NM, Alotaibi NA, Siddiqui S, Obaidur S. From Flower to Medicine: Green-Synthesized Silver Nanoparticles as Promising Antibacterial Agents. Pharmaceuticals. 2025; 18(5):691. https://doi.org/10.3390/ph18050691
Chicago/Turabian StyleSaeed, Mohd, Reem Binsuwaidan, Nawaf Alshammari, Ahmed M. Alharbi, Nadiyah M. Alabdallahd, Nawaf A. Alotaibi, Samra Siddiqui, and Safia Obaidur. 2025. "From Flower to Medicine: Green-Synthesized Silver Nanoparticles as Promising Antibacterial Agents" Pharmaceuticals 18, no. 5: 691. https://doi.org/10.3390/ph18050691
APA StyleSaeed, M., Binsuwaidan, R., Alshammari, N., Alharbi, A. M., Alabdallahd, N. M., Alotaibi, N. A., Siddiqui, S., & Obaidur, S. (2025). From Flower to Medicine: Green-Synthesized Silver Nanoparticles as Promising Antibacterial Agents. Pharmaceuticals, 18(5), 691. https://doi.org/10.3390/ph18050691






