Norfloxacin Derivative with Carbazole at C-7 FQB-1 Induces Cytotoxic, Antiproliferative, and Antitumor Effects in an Experimental Lung Carcinoma Model
Abstract
1. Introduction
2. Results
2.1. Synthesis and Molecular Docking of FQB-1 Compound with IIα Human Topoisomerase
2.2. Cytotoxicity and Anti-Proliferative Effect of the FQB-1 Compound in Lewis Lung Carcinoma Cells
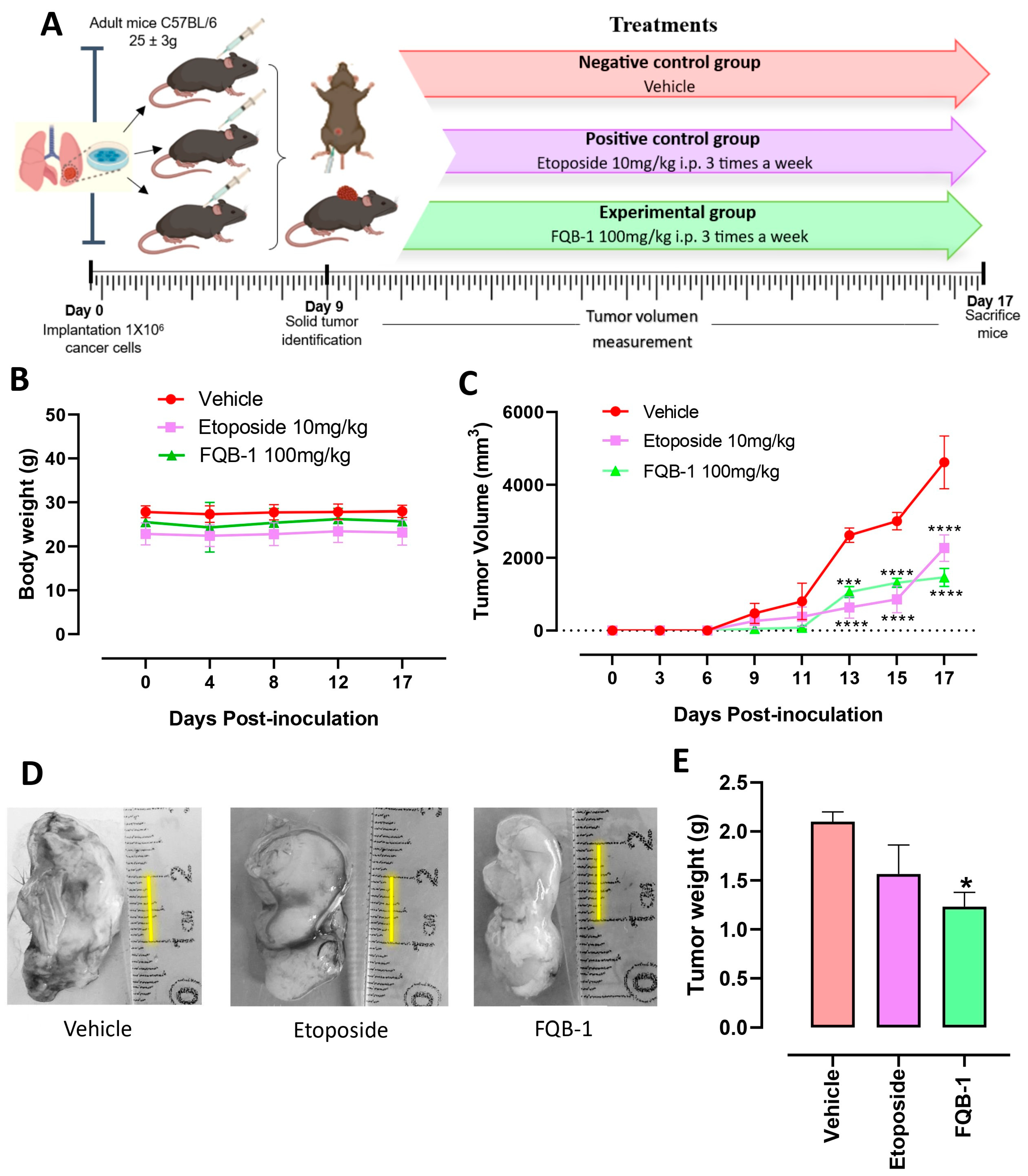
2.3. FQB-1 Decreases Tumor Growth in Lung Carcinoma of C57BL/6 Strain Mice
2.4. FQB-1 Decreases Mitosis and Increases Necrosis in Lewis Lung Carcinoma Tumors
3. Discussion
4. Materials and Methods
4.1. Synthesis of Difluoroboroyl 1-Ethyl-7-(1,2,3,4-tetrahydro-9H-carbazol-9-yl)-6-fluoro-4-oxo-1,4-dihydroquinolin-3-carboxylate (FQB-1)
4.2. Molecular Docking
4.3. Cytotoxicity Evaluation
4.4. Cell Proliferation Assay
4.5. Syngeneic Lung Carcinoma Model
4.6. Histopathological Analysis
4.7. Quantification of Mitotic Cells and Analysis of Necrotic Areas
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FQ | Fluoroquinolones |
| DMSO | Dimethylsulfoxide |
| LLC | Lewis lung cancer |
| PDB | Protein data bank |
References
- Brau-Figueroa, H.; Palafox-Parrilla, E.A.; Mohar-Betancourt, A. The National Cancer Registry in Mexico, a reality. Gac. Mex. Oncol. 2020, 19, 107–111. [Google Scholar]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo. Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Cucchiara, F.; Petrini, I.; Romei, C.; Crucitta, S.; Lucchesi, M.; Valleggi, S.; Scavone, C.; Capuano, A.; De Liperi, A.; Chella, A.; et al. Combining liquid biopsy and radiomics for personalized treatment of lung cancer patients. State of the art and new perspectives. Pharmacol. Res. 2021, 169, 105643. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Kalemkerian, G.P. Cisplatin, Etoposide, and Irinotecan for Relapsed Small-Cell Lung Cancer. Transl. Cancer Res 2016, 5 (Suppl. S6), S1142–S1144. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Paz-Ares, L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J. Clin. Oncol. 2022, 40, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef]
- Cabel, L.; Carton, M.; Cheaib, B.; Pierga, J.Y.; Dalenc, F.; Mailliez, A.; Levy, C.; Jacot, W.; Debled, M.; Leheurteur, M.; et al. Oral etoposide in heavily pre-treated metastatic breast cancer: Results from the ESME cohort and comparison with other chemotherapy regimens. Breast Cancer Res. Treat. 2019, 173, 397–406. [Google Scholar] [CrossRef]
- Yadav, V.; Talwar, P. Repositioning of fluoroquinolones from antibiotic to anti-cancer agents: An underestimated truth. Biomed. Pharmacother. 2019, 111, 934–946. [Google Scholar] [CrossRef]
- Kloskowski, T.; Frackowiak, S.; Adamowicz, J.; Szeliski, K.; Rasmus, M.; Drewa, T.; Pokrywczynska, M. Quinolones as a Potential Drug in Genitourinary Cancer Treatment-A Literature Review. Front. Oncol. 2022, 12, 890337. [Google Scholar] [CrossRef]
- Leyva-Ramos, S.; Hernandez-Lopez, H. Fluoroquinolones: Non-antibacterial properties. Rev. Esp. Quimioter. 2017, 30, 1–8. [Google Scholar]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Chen, S.F.; Wu, C.C.; Liao, Y.W.; Lin, T.S.; Liu, K.T.; Chen, Y.S.; Li, T.K.; Chien, T.C.; Chan, N.L. Producing irreversible topoisomerase II-mediated DNA breaks by site-specific Pt(II)-methionine coordination chemistry. Nucleic Acids Res. 2017, 45, 10861–10871. [Google Scholar] [CrossRef]
- Hernández-López, H.; Sánchez-Miranda, G.; Araujo-Huitrado, J.G.; Granados-López, A.J.; López, J.A.; Leyva-Ramos, S.; Chacón-García, L. Synthesis of Hybrid Fluoroquinolone-Boron Complexes and Their Evaluation in Cervical Cancer Cell Lines. J. Chem. 2019, 2019, 5608652. [Google Scholar] [CrossRef]
- Asche, C.; Demeunynck, M. Antitumor carbazoles. Anticancer. Agents Med. Chem. 2007, 7, 247–267. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tripathi, N.M.; Bandyopadhyay, A. The modern role of boron as a ‘magic element’ in biomedical science: Chemistry perspective. Chem. Commun. 2021, 57, 13629–13640. [Google Scholar] [CrossRef] [PubMed]
- Khaliq, H.; Juming, Z.; Ke-Mei, P. The Physiological Role of Boron on Health. Biol. Trace Elem. Res. 2018, 186, 31–51. [Google Scholar] [CrossRef]
- Abonia, D.D.A.R. Synthetic approaches for BF2-containing adducts of outstanding biological potential. A review. Arab. J. Chem. 2022, 15, 103528. [Google Scholar]
- Han, S.; Mei, L.; Quach, T.; Porter, C.; Trevaskis, N. Lipophilic Conjugates of Drugs: A Tool to Improve Drug Pharmacokinetic and Therapeutic Profiles. Pharm. Res. 2021, 38, 1497–1518. [Google Scholar] [CrossRef]
- Hande, K.R. Etoposide: Four decades of development of a topoisomerase II inhibitor. Eur. J. Cancer 1998, 34, 1514–1521. [Google Scholar] [CrossRef]
- Reyhanoglu, G.; Tadi, P. Etoposide. In StatPearls; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Veyna-Hurtado, L.A.; Hernández-López, H.; Reyes-Escobedo, F.; Medellín-Luna, M.; García-Cruz, S.; Troncoso-Vázquez, L.; González-Curiel, I.E.; Galván-Valencia, M.; Castañeda-Delgado, J.E.; Cervantes-Villagrana, A.R. The difluoroboranyl-norfloxacin complex “7a” induces an antimicrobial effect against K. pneumoniae strain in acute pneumonia murine model. Med. Drug Discov. 2023, 19, 100160. [Google Scholar] [CrossRef]
- Jadhav, A.K.; Karuppayil, S.M. Molecular docking studies on thirteen fluoroquinolines with human topoisomerase II a and b. Silico Pharmacol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Chen, S.H.; Chan, N.L.; Hsieh, T.S. New mechanistic and functional insights into DNA topoisomerases. Annu. Rev. Biochem. 2013, 82, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, T.; Rilak, A.; Bugarcic, Z.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Saberkari, M.; Abiri, R.; Motlagh, H.M.; Saberkari, H. In vitro evaluation of Zn-norfloxacin complex as a potent cytotoxic and antibacterial agent, proposed model for DNA binding. Appl. Biochem. Biotechnol. 2013, 170, 988–1009. [Google Scholar] [CrossRef] [PubMed]
- Abumansour, H.; Abusara, O.H.; Khalil, W.; Abul-Futouh, H.; Ibrahim, A.I.M.; Harb, M.K.; Abulebdah, D.H.; Ismail, W.H. Biological evaluation of levofloxacin and its thionated derivatives: Antioxidant activity, aldehyde dehydrogenase enzyme inhibition, and cytotoxicity on A549 cell line. Naunyn. Schmiedebergs Arch. Pharmacol. 2024, 397, 6963–6973. [Google Scholar] [CrossRef]
- Mahmoud, Z.; Ismail, M.M.; Kamel, M.; Youssef, A. Levofloxacin reposition-based design: Synthesis, biological evaluation of new levofloxacin derivatives targeting topoisomerase II beta polymerase as promising anticancer agents, molecular docking, and physicochemical characterization. RSC Adv. 2024, 14, 28098–28119. [Google Scholar] [CrossRef]
- Elanany, M.A.; Osman, E.E.A.; Gedawy, E.M.; Abou-Seri, S.M. Design and synthesis of novel cytotoxic fluoroquinolone analogs through topoisomerase inhibition, cell cycle arrest, and apoptosis. Sci. Rep. 2023, 13, 4144. [Google Scholar] [CrossRef]
- Kumar, N.; Dhamija, I.; Raj, P.V.; Jayashree, B.S.; Parihar, V.; Manjula, S.N.; Thomas, S.; Kutty, N.G.; Rao, C.M. Preliminary investigation of cytotoxic potential of 2-quinolone derivatives using in vitro and in vivo (solid tumor and liquid tumor) models of cancer. Arab. J. Chem. 2014, 7, 409–417. [Google Scholar] [CrossRef]
- Joseph, B.; Darro, F.; Behard, A.; Lesur, B.; Collignon, F.; Decaestecker, C.; Frydman, A.; Guillaumet, G.; Kiss, R. 3-Aryl-2-quinolone derivatives: Synthesis and characterization of in vitro and in vivo antitumor effects with emphasis on a new therapeutical target connected with cell migration. J. Med. Chem. 2002, 45, 2543–2555. [Google Scholar] [CrossRef]
- Gonzalez, A.S.C.; Valencia, M.G.; Cervantes-Villagrana, R.D.; Zapata, A.B.; Cervantes-Villagrana, A.R. Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells. Molecules 2023, 28, 7055. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Qashou, E.; Al-Hiari, Y.; Kasabri, V.; AlBashiti, R.; AlAlawi, S.; Telfah, A.; AlHadid, A. Antiproliferative Activities of Lipophililic Fluoroquinolones- Based Scaffold Against a Panel of Solid and Liquid Cancer Cell Lines. Asian. Pac. J. Cancer Prev. 2022, 23, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Jimenez, I.; Cervantes-Villagrana, R.D.; Del-Rio-Robles, J.E.; Castillo-Kauil, A.; Beltran-Navarro, Y.M.; Garcia-Roman, J.; Reyes-Cruz, G.; Vazquez-Prado, J. Gbetagamma mediates activation of Rho guanine nucleotide exchange factor ARHGEF17 that promotes metastatic lung cancer progression. J. Biol. Chem. 2022, 298, 101440. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Villagrana, R.D.; Color-Aparicio, V.M.; Reyes-Cruz, G.; Vazquez-Prado, J. Protumoral bone marrow-derived cells migrate via Gbetagamma-dependent signaling pathways and exhibit a complex repertoire of RhoGEFs. J. Cell Commun. Signal. 2019, 13, 179–191. [Google Scholar] [CrossRef]
- Cervantes-Villagrana, R.D.; Mendoza, V.; Hinck, C.S.; de la Fuente-Leon, R.L.; Hinck, A.P.; Reyes-Cruz, G.; Vazquez-Prado, J.; Lopez-Casillas, F. Betaglycan sustains HGF/Met signaling in lung cancer and endothelial cells promoting cell migration and tumor growth. Heliyon 2024, 10, e30520. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocanegra-Zapata, A.; Hernández-López, H.; Leyva-Ramos, S.; Cervantes-Villagrana, R.D.; Galván-Valencia, M.; Veyna-Hurtado, L.A.; Tovar, N.G.R.; Albores-García, D.; de la Torre, J.A.F.; Cervantes-Villagrana, A.R. Norfloxacin Derivative with Carbazole at C-7 FQB-1 Induces Cytotoxic, Antiproliferative, and Antitumor Effects in an Experimental Lung Carcinoma Model. Pharmaceuticals 2025, 18, 664. https://doi.org/10.3390/ph18050664
Bocanegra-Zapata A, Hernández-López H, Leyva-Ramos S, Cervantes-Villagrana RD, Galván-Valencia M, Veyna-Hurtado LA, Tovar NGR, Albores-García D, de la Torre JAF, Cervantes-Villagrana AR. Norfloxacin Derivative with Carbazole at C-7 FQB-1 Induces Cytotoxic, Antiproliferative, and Antitumor Effects in an Experimental Lung Carcinoma Model. Pharmaceuticals. 2025; 18(5):664. https://doi.org/10.3390/ph18050664
Chicago/Turabian StyleBocanegra-Zapata, Alondra, Hiram Hernández-López, Socorro Leyva-Ramos, Rodolfo Daniel Cervantes-Villagrana, Marisol Galván-Valencia, L. Angel Veyna-Hurtado, Norma Guadalupe Ramírez Tovar, Damaris Albores-García, Juan Armando Flores de la Torre, and Alberto Rafael Cervantes-Villagrana. 2025. "Norfloxacin Derivative with Carbazole at C-7 FQB-1 Induces Cytotoxic, Antiproliferative, and Antitumor Effects in an Experimental Lung Carcinoma Model" Pharmaceuticals 18, no. 5: 664. https://doi.org/10.3390/ph18050664
APA StyleBocanegra-Zapata, A., Hernández-López, H., Leyva-Ramos, S., Cervantes-Villagrana, R. D., Galván-Valencia, M., Veyna-Hurtado, L. A., Tovar, N. G. R., Albores-García, D., de la Torre, J. A. F., & Cervantes-Villagrana, A. R. (2025). Norfloxacin Derivative with Carbazole at C-7 FQB-1 Induces Cytotoxic, Antiproliferative, and Antitumor Effects in an Experimental Lung Carcinoma Model. Pharmaceuticals, 18(5), 664. https://doi.org/10.3390/ph18050664