Fluoroquinolones for Dermatologists: A Practical Guide to Clinical Use and Risk Management
Abstract
1. Introduction
2. Overview of Fluoroquinolone Antibiotics
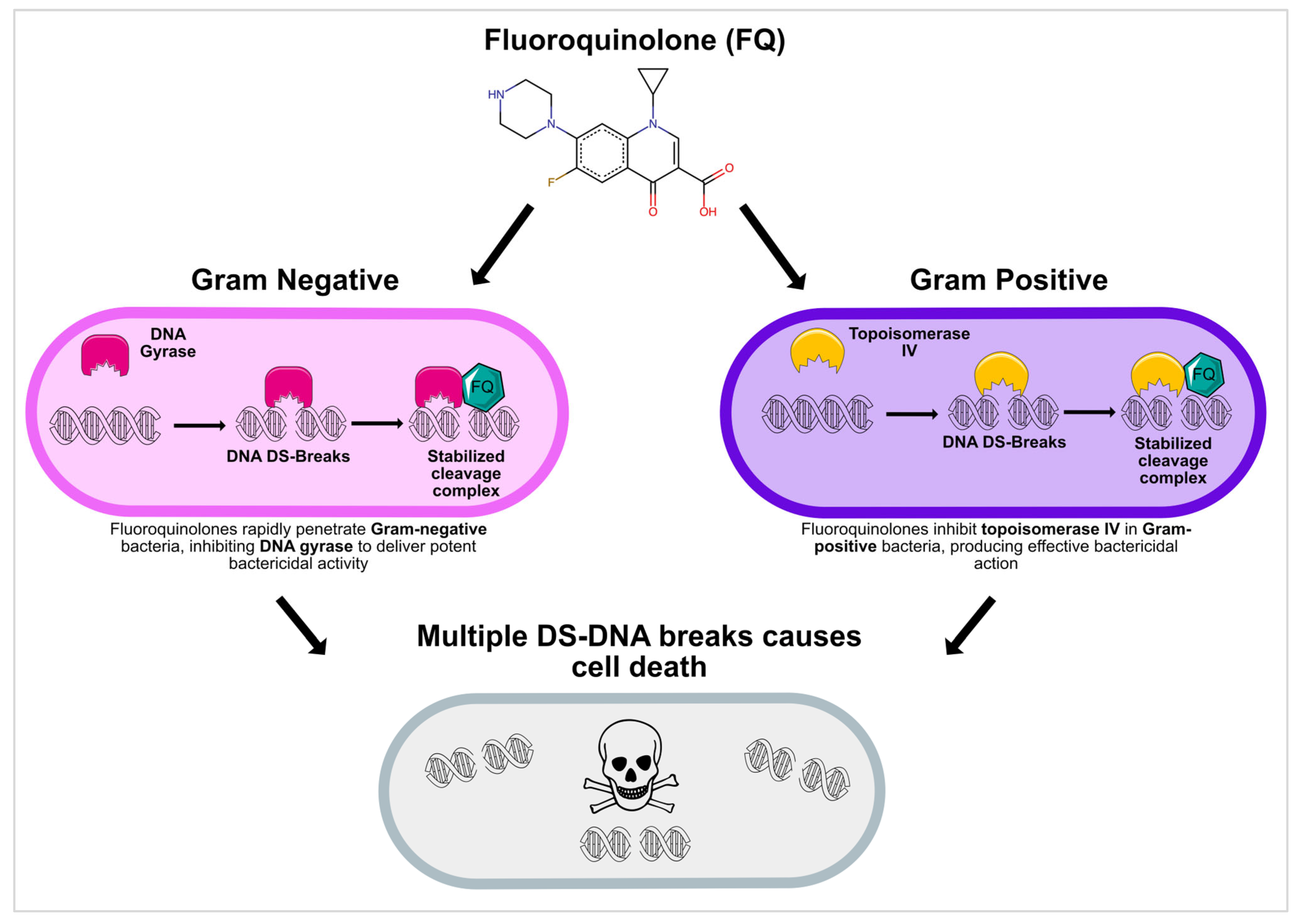
2.1. Mechanism of Action
2.2. Fluoroquinolone Resistance
2.3. Pharmacokinetics
2.4. Anti-Inflammatory Properties
3. Clinical Indications in Dermatology
3.1. Common Skin and Soft Tissue Infections
3.1.1. Cellulitis
3.1.2. Impetigo
3.1.3. Pseudomonas Infections
3.1.4. Necrotizing Fasciitis
3.1.5. Diabetic Foot Infections
3.2. Atypical and Opportunistic Infections
3.2.1. Atypical Mycobacterial Infections
3.2.2. Gram-Negative Bacilli in Immunocompromised Hosts
3.3. Special Dermatological Conditions
3.3.1. Burn Wound Infections
3.3.2. Acne Vulgaris
3.3.3. Surgical Prophylaxis
3.3.4. Hidradenitis Suppurativa (HS)
3.3.5. Zoonotic and Vector-Borne Infections
4. Safety Profile and Risk Management
4.1. Adverse Effects (Table 5)
4.1.1. Tendinopathy and Tendon Rupture
4.1.2. QT Interval Prolongation
4.1.3. Photosensitivity
4.1.4. Gastrointestinal and Neurological Effects
4.1.5. Hypo- and Hyperglycemia
4.1.6. Aortic Aneurysm and Aortic Dissection
4.2. Box Warnings and Regulatory Considerations
4.3. Risk Mitigation Strategies
5. Antibiotic Stewardship in Dermatology
6. Special Patient Populations and Considerations
6.1. Geriatric Patients
6.1.1. Dosing Adjustments
6.1.2. Medication Adherence
6.2. Patients with Rheumatologic–Dermatologic Diseases
6.3. Immunocompromised Patients
6.4. Pediatric Patients
6.5. Pregnant Populations
6.6. Patients with Seizure Disorders
7. Discussion
7.1. Clinical Decision-Making
7.2. Clinical Pearls
- Screen for Risk Factors: Identify patients at higher risk of FQ-related complications, such as the elderly, those with tendon disorders, aortic aneurysm risk, or those on corticosteroids, and consider alternative therapies if possible;
- Shorten Treatment Duration: Whenever clinically feasible, opt for the minimum effective course to limit adverse events and reduce the likelihood of resistance;
- Monitor for Toxicity: Advise patients to watch for early signs of tendon pain, neuropathy, or cardiac symptoms and to stop therapy immediately if they develop these symptoms;
- Leverage Topical Formulations: In localized infections amenable to topical therapy (e.g., chronic otitis externa or mild wound infections), a topical FQ can achieve high local concentrations while minimizing systemic effects [7];
- Engage in Stewardship: Confirm bacterial pathogens with cultures and tailor therapy to sensitivity results, collaborating with infectious disease specialists or stewardship teams as needed.
7.3. Controversies and Debates
7.4. Limitations of Current Evidence
8. Key Takeaways for Dermatologists
9. Future Directions
9.1. Research Gaps and Innovation Priorities
9.2. Addressing Resistance and Personalization in Antibiotic Therapy
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABSSSI | Acute Bacterial Skin and Skin Structure Infections |
| DNA | Deoxyribonucleic Acid |
| ECG | Electrocardiogram |
| FDA | Food and Drug Administration |
| FQ | Fluoroquinolone |
| FQAD | Fluoroquinolone-Associated Disability |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| IV | Intravenous |
| MSSA | Methicillin-Susceptible Staphylococcus aureus |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells |
| PO | By Mouth (Per Os) |
| QT | QT Interval (on Electrocardiogram) |
| QTc | Corrected QT Interval |
| SSTI | Skin and Soft Tissue Infection |
| SSSIs | Skin and Skin Structure Infections |
| TNF-α | Tumor Necrosis Factor Alpha |
| TLR4 | Toll-Like Receptor 4 |
References
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The Current Case of Quinolones: Synthetic Approaches and Antibacterial Activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.E. Pharmacokinetics and Pharmacodynamics of Newer Fluoroquinolones. Clin. Infect. Dis. 1996, 23 (Suppl. 1), S19–S24. [Google Scholar] [CrossRef]
- Martin, S.J.; Zeigler, D.G. The Use of Fluoroquinolones in the Treatment of Skin Infections. Expert Opin. Pharmacother. 2004, 5, 237–246. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H.M. Risks Associated with the Therapeutic Use of Fluoroquinolones. Expert Opin. Drug Saf. 2013, 12, 497–505. [Google Scholar] [CrossRef]
- Appelbaum, P.C.; Hunter, P.A. The Fluoroquinolone Antibacterials: Past, Present and Future Perspectives. Int. J. Antimicrob. Agents 2000, 16, 5–15. [Google Scholar] [CrossRef]
- Blondeau, J.M. Expanded Activity and Utility of the New Fluoroquinolones: A Review. Clin. Ther. 1999, 21, 3–40, discussion 1–2. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, J.M. The Role of Fluoroquinolones in Skin and Skin Structure Infections. Am. J. Clin. Dermatol. 2002, 3, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Karchmer, A.W. Fluoroquinolone Treatment of Skin and Skin Structure Infections. Drugs 1999, 58 (Suppl. 2), 82–84. [Google Scholar] [CrossRef]
- Tulkens, P.M.; Van Bambeke, F.; Zinner, S.H. Profile of a Novel Anionic Fluoroquinolone-Delafloxacin. Clin. Infect. Dis. 2019, 68, S213–S222. [Google Scholar] [CrossRef]
- McCurdy, S.; Lawrence, L.; Quintas, M.; Woosley, L.; Flamm, R.; Tseng, C.; Cammarata, S. In Vitro Activity of Delafloxacin and Microbiological Response against Fluoroquinolone-Susceptible and Nonsusceptible Staphylococcus aureus Isolates from Two Phase 3 Studies of Acute Bacterial Skin and Skin Structure Infections. Antimicrob. Agents Chemother. 2017, 61, e00772-17. [Google Scholar] [CrossRef]
- Bassetti, M.; Della Siega, P.; Pecori, D.; Scarparo, C.; Righi, E. Delafloxacin for the Treatment of Respiratory and Skin Infections. Expert Opin. Investig. Drugs 2015, 24, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Delafloxacin: A Review in Acute Bacterial Skin and Skin Structure Infections. Drugs 2020, 80, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Mogle, B.T.; Steele, J.M.; Thomas, S.J.; Bohan, K.H.; Kufel, W.D. Clinical Review of Delafloxacin: A Novel Anionic Fluoroquinolone. J. Antimicrob. Chemother. 2018, 73, 1439–1451. [Google Scholar] [CrossRef]
- Hooper, D.C. Mode of Action of Fluoroquinolones. Drugs 1999, 58 (Suppl. 2), 6–10. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31 (Suppl. 2), S24–S28. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones. Clin. Infect. Dis. 2001, 32 (Suppl. 1), S9–S15. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Ferrándiz, M.J.; Martín-Galiano, A.J.; Arnanz, C.; Zimmerman, T.; de la Campa, A.G. Reactive Oxygen Species Contribute to the Bactericidal Effects of the Fluoroquinolone Moxifloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 2016, 60, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, Q.; Gao, Q.; Xie, J.; Huang, H.; Drlica, K.; Zhao, X. Reactive Oxygen Species Play a Dominant Role in All Pathways of Rapid Quinolone-Mediated Killing. J. Antimicrob. Chemother. 2020, 75, 576–585. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J.V. Fluoroquinolone Resistance: Mechanisms, Impact on Bacteria, and Role in Evolutionary Success. Trends Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Turnidge, J. Pharmacokinetics and Pharmacodynamics of Fluoroquinolones. Drugs 1999, 58 (Suppl. 2), 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rodvold, K.A.; Neuhauser, M. Pharmacokinetics and Pharmacodynamics of Fluoroquinolones. Pharmacotherapy 2001, 21, 233S–252S. [Google Scholar] [CrossRef]
- Aminimanizani, A.; Beringer, P.; Jelliffe, R. Comparative Pharmacokinetics and Pharmacodynamics of the Newer Fluoroquinolone Antibacterials. Clin. Pharmacokinet. 2001, 40, 169–187. [Google Scholar] [CrossRef]
- Roland, P.S.; Wall, M. Ciprofloxacin 0.3%/Dexamethasone 0.1% Topical Drops for the Management of Otic Infections. Expert Opin. Pharmacother. 2008, 9, 3129–3135. [Google Scholar] [CrossRef]
- Tsivkovskii, R.; Sabet, M.; Tarazi, Z.; Griffith, D.C.; Lomovskaya, O.; Dudley, M.N. Levofloxacin Reduces Inflammatory Cytokine Levels in Human Bronchial Epithelia Cells: Implications for Aerosol MP-376 (Levofloxacin Solutionfor Inhalation) Treatment of Chronic Pulmonary Infections. FEMS Immunol. Med. Microbiol. 2011, 61, 141–146. [Google Scholar] [CrossRef]
- Zusso, M.; Lunardi, V.; Franceschini, D.; Pagetta, A.; Lo, R.; Stifani, S.; Frigo, A.C.; Giusti, P.; Moro, S. Ciprofloxacin and Levofloxacin Attenuate Microglia Inflammatory Response via TLR4/NF-kB Pathway. J. Neuroinflamm. 2019, 16, 148. [Google Scholar] [CrossRef]
- Dalhoff, A.; Shalit, I. Immunomodulatory Effects of Quinolones. Lancet Infect. Dis. 2003, 3, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.A.A.; Almotawa, A.A.; Almutairi, M.A.M.; Baabbad, R.S.; Abdullah, S.A.A.; Alagedi, H.S.K.; Aljaizani, E.A.H.; Alahmar, M.A.; Shabi, F.A.A.; Alharbi, S.A.; et al. Ciprofloxacin: An Overview of Uses, Mechanism of Action, and Adverse Effects. J. Ecohumanism 2024, 3, 9328–9336. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice Guidelines for the Diagnosis and Management of Skin and Soft Tissue Infections: 2014 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, 79, ciae403. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.D.; Hood Watson, K.L. Cellulitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Giordano, P.; Weber, K.; Gesin, G.; Kubert, J. Skin and Skin Structure Infections: Treatment with Newer Generation Fluoroquinolones. Ther. Clin. Risk Manag. 2007, 3, 309–317. [Google Scholar] [CrossRef]
- Nardi, N.M.; Schaefer, T.J. Impetigo. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bessa, G.R.; Quinto, V.P.; Machado, D.C.; Lipnharski, C.; Weber, M.B.; Bonamigo, R.R.; D’Azevedo, P.A. Staphylococcus aureus Resistance to Topical Antimicrobials in Atopic Dermatitis. An. Bras. Dermatol. 2016, 91, 604–610. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. XEPI™ (Ozenoxacin) Cream, for Topical Use. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208945lbl.pdf (accessed on 12 April 2025).
- Schachner, L.; Andriessen, A.; Bhatia, N.; Grada, A.; Patele, D. Topical Ozenoxacin Cream 1% for Impetigo: A Review. J. Drugs Dermatol. 2019, 18, 655–661. [Google Scholar]
- Aste, N.; Atzori, L.; Zucca, M.; Pau, M.; Biggio, P. Gram-Negative Bacterial Toe Web Infection: A Survey of 123 Cases from the District of Cagliari, Italy. J. Am. Acad. Dermatol. 2001, 45, 537–541. [Google Scholar] [CrossRef]
- Lee, H.; Mun, J.-H.; Cho, S.; Park, H. Clinical Analysis of Pseudomonas aeruginosa-Positive and -Negative Green Nail Syndrome Cases: A Single Center Retrospective Analysis. J. Dermatol. 2021, 48, 1073–1076. [Google Scholar] [CrossRef]
- Müller, S.; Ebnöther, M.; Itin, P. Green Nail Syndrome (Pseudomonas aeruginosa Nail Infection): Two Cases Successfully Treated with Topical Nadifloxacin, an Acne Medication. Case Rep. Dermatol. 2014, 6, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Huang, J.; Kassamali Escobar, Z. Antipseudomonal Antibiotics in Diabetic Foot Infections: A Practical Perspective From a Community Hospital. Open Forum Infect. Dis. 2024, 11, ofae258. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Everett, E.D.; Dellinger, P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, E.L.; Montoya, J.G.; et al. Practice Guidelines for the Diagnosis and Management of Skin and Soft-Tissue Infections. Clin. Infect. Dis. 2005, 41, 1373–1406. [Google Scholar] [CrossRef]
- Bader, M.S. Diabetic Foot Infection. Am. Fam. Physician Afp 2008, 78, 71–79. [Google Scholar]
- Aubry, A.; Jarlier, V.; Escolano, S.; Truffot-Pernot, C.; Cambau, E. Antibiotic Susceptibility Pattern of Mycobacterium marinum. Antimicrob. Agents Chemother. 2000, 44, 3133–3136. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Wan, Q. Pseudomonas aeruginosa Bacteremia among Liver Transplant Recipients. Infect. Drug Resist. 2018, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.L.; Champigneulle, O.; Bennett, A.; Witherspoon, B.; Bove, C. Fluoroquinolone-Associated Disability and Other Fluoroquinolone-Associated Serious Adverse Events: Unexpected Toxicities Have Emerged in Recent Years. In Cancer Drug Safety and Public Health Policy; Cancer Treatment and Research; Springer: Cham, Switzerland, 2022; Volume 184, pp. 1–39. [Google Scholar] [CrossRef]
- Grada, A.; Armstrong, A.; Bunick, C.; Salem, R.; Feldman, S. Trends in Oral Antibiotic Use for Acne Treatment: A Retrospective, Population-Based Study in the United States, 2014 to 2016. J. Drugs Dermatol. 2023, 22, 265–270. [Google Scholar] [CrossRef]
- Bettoli, V.; Join-Lambert, O.; Nassif, A. Antibiotic Treatment of Hidradenitis Suppurativa. Dermatol. Clin. 2016, 34, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, J.; Villani, A.P.; Guillem, P.; Tristan, A.; Boibieux, A.; Jullien, D. Oral Ofloxacin and Clindamycin as an Alternative to the Classic Rifampicin-Clindamycin in Hidradenitis Suppurativa: Retrospective Analysis of 65 Patients. Br. J. Dermatol. 2018, 178, e15–e16. [Google Scholar] [CrossRef]
- Khaliq, Y.; Zhanel, G.G. Fluoroquinolone-Associated Tendinopathy: A Critical Review of the Literature. Clin. Infect. Dis. 2003, 36, 1404–1410. [Google Scholar] [CrossRef] [PubMed]
- Wise, B.; Peloquin, C.; Choi, H.; Lane, N.; Zhang, Y. Impact of Age, Sex, Obesity, and Steroid Use on Quinolone-Associated Tendon Disorders. Am. J. Med. 2012, 125, 1228.e23–1228.e28. [Google Scholar] [CrossRef]
- Sendzik, J.; Shakibaei, M.; Schäfer-Korting, M.; Stahlmann, R. Fluoroquinolones Cause Changes in Extracellular Matrix, Signalling Proteins, Metalloproteinases and Caspase-3 in Cultured Human Tendon Cells. Toxicology 2005, 212, 24–36. [Google Scholar] [CrossRef]
- Yu, C.; Giuffre, B. Achilles Tendinopathy after Treatment with Fluoroquinolone. Australas. Radiol. 2005, 49, 407–410. [Google Scholar] [CrossRef]
- Damuth, E.; Heidelbaugh, J.; Malani, P.N.; Cinti, S.K. An Elderly Patient with Fluoroquinolone-Associated Achilles Tendinitis. Am. J. Geriatr. Pharmacother. 2008, 6, 264–268. [Google Scholar] [CrossRef]
- Huston, K.A. Achilles Tendinitis and Tendon Rupture Due to Fluoroquinolone Antibiotics. N. Engl. J. Med. 1994, 331, 748. [Google Scholar] [CrossRef] [PubMed]
- Sanguinetti, M.C.; Tristani-Firouzi, M. hERG Potassium Channels and Cardiac Arrhythmia. Nature 2006, 440, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.J.; Ackerman, M.J.; Funk, M.; Gibler, W.B.; Kligfield, P.; Menon, V.; Philippides, G.J.; Roden, D.M.; Zareba, W.; American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology; et al. Prevention of Torsade de Pointes in Hospital Settings: A Scientific Statement from the American Heart Association and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2010, 55, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Post, W.S.; Blasco-Colmenares, E.; Dalal, D.; Tomaselli, G.F.; Guallar, E. Electrocardiographic QT Interval and Mortality: A Meta-Analysis. Epidemiology 2011, 22, 660–670. [Google Scholar] [CrossRef]
- Beach, S.R.; Celano, C.M.; Noseworthy, P.A.; Januzzi, J.L.; Huffman, J.C. QTc Prolongation, Torsades de Pointes, and Psychotropic Medications. Psychosomatics 2013, 54, 1–13. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; LaPointe, N.M.A.; Kramer, J.M.; Califf, R.M. What Clinicians Should Know about the QT Interval. JAMA 2003, 289, 2120–2127. [Google Scholar] [CrossRef]
- Martínez, L.J.; Sik, R.H.; Chignell, C.F. Fluoroquinolone Antimicrobials: Singlet Oxygen, Superoxide and Phototoxicity. Photochem. Photobiol. 1998, 67, 399–403. [Google Scholar] [CrossRef]
- Tokura, Y.; Seo, N.; Yagi, H.; Furukawa, F.; Takigawa, M. Cross-Reactivity in Murine Fluoroquinolone Photoallergy: Exclusive Usage of TCR Vβ13 by Immune T Cells That Recognize Fluoroquinolone-Photomodified Cells. J. Immunol. 1998, 160, 3719–3728. [Google Scholar] [CrossRef]
- Dawe, R.S.; Ibbotson, S.H.; Sanderson, J.B.; Thomson, E.M.; Ferguson, J. A Randomized Controlled Trial (Volunteer Study) of Sitafloxacin, Enoxacin, Levofloxacin and Sparfloxacin Phototoxicity. Br. J. Dermatol. 2003, 149, 1232–1241. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Toxicity of Quinolones. Drugs 1999, 58 (Suppl. 2), 37–42. [Google Scholar] [CrossRef]
- Kowalska, J.; Banach, K.; Rok, J.; Beberok, A.; Rzepka, Z.; Wrześniok, D. Molecular and Biochemical Basis of Fluoroquinolones-Induced Phototoxicity—The Study of Antioxidant System in Human Melanocytes Exposed to UV-A Radiation. Int. J. Mol. Sci. 2020, 21, 9714. [Google Scholar] [CrossRef]
- Mehlhorn, A.J.; Brown, D.A. Safety Concerns with Fluoroquinolones. Ann. Pharmacother. 2007, 41, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Blakely, K.M.; Drucker, A.M.; Rosen, C.F. Drug-Induced Photosensitivity-An Update: Culprit Drugs, Prevention and Management. Drug Saf. 2019, 42, 827–847. [Google Scholar] [CrossRef]
- Xie, W.-L.; Ge, M.-L.; Chen, D.; Chen, G.-Q.; Mei, Y.-X.; Lai, Y.-J. Psychiatric Disorders Associated with Fluoroquinolones: A Pharmacovigilance Analysis of the FDA Adverse Event Reporting System Database. Front. Pharmacol. 2024, 15, 1435923. [Google Scholar] [CrossRef]
- Freeman, M.Z.; Cannizzaro, D.N.; Naughton, L.F.; Bove, C. Fluoroquinolones-Associated Disability: It Is Not All in Your Head. NeuroSci 2021, 2, 235–253. [Google Scholar] [CrossRef]
- Aspinall, S.L.; Good, C.B.; Jiang, R.; McCarren, M.; Dong, D.; Cunningham, F.E. Severe Dysglycemia with the Fluoroquinolones: A Class Effect? Clin. Infect. Dis. 2009, 49, 402–408. [Google Scholar] [CrossRef]
- Saraya, A.; Yokokura, M.; Gonoi, T.; Seino, S. Effects of Fluoroquinolones on Insulin Secretion and β-Cell ATP-Sensitive K+ Channels. Eur. J. Pharmacol. 2004, 497, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Tamagawa, T.; Niki, I.; Miura, H.; Ozawa, K.; Watanabe, G.; Nonogaki, K.; Uemura, K.; Iguchi, A. Increase in Insulin Release from Rat Pancreatic Islets by Quinolone Antibiotics. Br. J. Pharmacol. 1996, 117, 372–376. [Google Scholar] [CrossRef]
- Ambrose, P.G.; Bhavnani, S.M.; Cirincione, B.B.; Piedmonte, M.; Grasela, T.H. Gatifloxacin and the Elderly: Pharmacokinetic-Pharmacodynamic Rationale for a Potential Age-Related Dose Reduction. J. Antimicrob. Chemother. 2003, 52, 435–440. [Google Scholar] [CrossRef]
- Newton, E.R.; Akerman, A.W.; Strassle, P.D.; Kibbe, M.R. Association of Fluoroquinolone Use with Short-Term Risk of Development of Aortic Aneurysm. JAMA Surg. 2021, 156, 264–272. [Google Scholar] [CrossRef]
- FDA Center for Drug Evaluation and Research. FDA Warns about Increased Risk of Ruptures or Tears in the Aorta Blood Vessel with Fluoroquinolone Antibiotics in Certain Patients. 2025. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics (accessed on 11 April 2025).
- FDA Center for Drug Evaluation and Research. Fluoroquinolone Antimicrobial Drugs Information. 2019. Available online: https://www.fda.gov/drugs/information-drug-class/fluoroquinolone-antimicrobial-drugs-information (accessed on 1 March 2025).
- Corrao, G.; Zambon, A.; Bertù, L.; Mauri, A.; Paleari, V.; Rossi, C.; Venegoni, M. Evidence of Tendinitis Provoked by Fluoroquinolone Treatment: A Case-Control Study. Drug Saf. 2006, 29, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Pacurariu, A.; Slattery, J.; Pinheiro, L.; McGettigan, P.; Kurz, X. Association Between Peripheral Neuropathy and Exposure to Oral Fluoroquinolone or Amoxicillin-Clavulanate Therapy. JAMA Neurol. 2019, 76, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Guo, I.J.; Maximos, M.; Gamble, J.-M. Examining Hypoglycemia Risk with Systemic Fluoroquinolone Use: A Systematic Review and Meta-Analysis. CMI Commun. 2024, 1, 105038. [Google Scholar] [CrossRef]
- Kelesidis, T.; Canseco, E. Quinolone-Induced Hypoglycemia: A Life-Threatening but Potentially Reversible Side Effect. Am. J. Med. 2010, 123, e5–e6. [Google Scholar] [CrossRef]
- Li, C.; Mercuro, N.J.; Chapin, R.W.; Gold, H.S.; McCoy, C. Fluoroquinolone Prescribing for Diabetic Foot Infections Following an FDA Drug Safety Communication for Aortic Aneurysm Risk. Antimicrob. Agents Chemother. 2021, 65, e0070821. [Google Scholar] [CrossRef]
- Wildermuth, A.; Holmes, M. A Preventable, Life-Altering Case of Fluoroquinolone-Associated Tendonitis. JAAPA 2022, 35, 33–36. [Google Scholar] [CrossRef]
- Farzam, K.; Tivakaran, V.S. QT Prolonging Drugs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shu, Y.; Zhang, Q.; He, X.; Liu, Y.; Wu, P.; Chen, L. Fluoroquinolone-Associated Suspected Tendonitis and Tendon Rupture: A Pharmacovigilance Analysis from 2016 to 2021 Based on the FAERS Database. Front. Pharmacol. 2022, 13, 990241. [Google Scholar] [CrossRef]
- Landers, Z.D.; Mazhar, A. Fluoroquinolone-Induced Multisystem Toxicity: A Case Report. Cureus 2024, 16, e61174. [Google Scholar] [CrossRef]
- Michalak, K.; Sobolewska-Włodarczyk, A.; Włodarczyk, M.; Sobolewska, J.; Woźniak, P.; Sobolewski, B. Treatment of the Fluoroquinolone-Associated Disability: The Pathobiochemical Implications. Oxid. Med. Cell. Longev. 2017, 2017, 8023935. [Google Scholar] [CrossRef]
- Outpatient Antibiotic Prescriptions—United States, 2022|Antibiotic Use|CDC. Available online: https://archive.cdc.gov/www_cdc_gov/antibiotic-use/data/report-2022.html (accessed on 1 March 2025).
- Barbieri, J.S.; Bhate, K.; Hartnett, K.P.; Fleming-Dutra, K.E.; Margolis, D.J. Trends in Oral Antibiotic Prescription in Dermatology, 2008 to 2016. JAMA Dermatol. 2019, 155, 290–297. [Google Scholar] [CrossRef]
- MacGibeny, M.A.; Jo, J.-H.; Kong, H.H. Antibiotic Stewardship in Dermatology: Reducing the Risk of Prolonged Antimicrobial Resistance in Skin. JAMA Dermatol. 2022, 158, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Rengarajan, S.; MacGibeny, M.A.; Kong, H.H. Comment on: Antibiotic Resistance in Dermatology: The Scope of the Problem and Strategies to Address It. J. Am. Acad. Dermatol. 2022, 87, e195–e196. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.; Conlon, A.; Chopra, V.; Malani, A.N.; Flanders, S.A. The Association of Antibiotic Stewardship with Fluoroquinolone Prescribing in Michigan Hospitals: A Multi-Hospital Cohort Study. Clin. Infect. Dis. 2019, 69, 1269–1277. [Google Scholar] [CrossRef]
- Hecker, M.T.; Son, A.H.; Murphy, N.N.; Sethi, A.K.; Wilson, B.M.; Watkins, R.R.; Donskey, C.J. Impact of Syndrome-Specific Antimicrobial Stewardship Interventions on Use of and Resistance to Fluoroquinolones: An Interrupted Time Series Analysis. Am. J. Infect. Control 2019, 47, 869–875. [Google Scholar] [CrossRef]
- Yarrington, M.E.; Anderson, D.J.; Dodds Ashley, E.; Jones, T.; Davis, A.; Johnson, M.; Lokhnygina, Y.; Sexton, D.J.; Moehring, R.W. Impact of FDA Black Box Warning on Fluoroquinolone and Alternative Antibiotic Use in Southeastern US Hospitals. Infect. Control Hosp. Epidemiol. 2019, 40, 1297–1300. [Google Scholar] [CrossRef]
- Grada, A.; Ghannoum, M.A.; Bunick, C.G. Sarecycline Demonstrates Clinical Effectiveness against Staphylococcal Infections and Inflammatory Dermatoses: Evidence for Improving Antibiotic Stewardship in Dermatology. Antibiotics 2022, 11, 722. [Google Scholar] [CrossRef]
- Stahlmann, R.; Lode, H. Safety Considerations of Fluoroquinolones in the Elderly: An Update. Drugs Aging 2010, 27, 193–209. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Munteanu, A.-C.; Arbănași, E.-M.; Uivarosi, V. Overview of Side-Effects of Antibacterial Fluoroquinolones: New Drugs versus Old Drugs, a Step Forward in the Safety Profile? Pharmaceutics 2023, 15, 804. [Google Scholar] [CrossRef]
- Werth, B.J. Fluoroquinolones—Infectious Diseases. Available online: https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-medications/fluoroquinolones (accessed on 1 March 2025).
- Committee on Infectious Diseases, American Academy of Pediatrics. Fluoroquinolones. In Red Book: 2021–2024 Report of the Committee on Infectious Diseases; Kimberlin, D.W., Barnett, E.D., Lynfield, R., Sawyer, M.H., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2021; ISBN 978-1-61002-578-2. [Google Scholar]
- Kim, Y.; Park, G.W.; Kim, S.; Moon, H.J.; Won, S.; Chung, W.; Yang, H.-J. Fluoroquinolone and No Risk of Achilles-Tendinopathy in Childhood Pneumonia under Eight Years of Age—A Nationwide Retrospective Cohort. J. Thorac. Dis. 2021, 13, 3399–3408. [Google Scholar] [CrossRef]
- Schluter, G. Ciprofloxacin: Toxicologic Evaluation of Additional Safety Data. Am. J. Med. 1989, 87, 37S–39S. [Google Scholar] [CrossRef]
- Corrado, M.L.; Struble, W.E.; Peter, C.; Hoagland, V.; Sabbaj, J. Norfloxacin: Review of Safety Studies. Am. J. Med. 1987, 82, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Loebstein, R.; Addis, A.; Ho, E.; Andreou, R.; Sage, S.; Donnenfeld, A.E.; Schick, B.; Bonati, M.; Moretti, M.; Lalkin, A.; et al. Pregnancy Outcome Following Gestational Exposure to Fluoroquinolones: A Multicenter Prospective Controlled Study. Antimicrob. Agents Chemother. 1998, 42, 1336–1339. [Google Scholar] [CrossRef]
- Padberg, S.; Wacker, E.; Meister, R.; Panse, M.; Weber-Schoendorfer, C.; Oppermann, M.; Schaefer, C. Observational Cohort Study of Pregnancy Outcome after First-Trimester Exposure to Fluoroquinolones. Antimicrob. Agents Chemother. 2014, 58, 4392–4398. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, R.F.; Davey, P.G.; Lambert, J.J. Antagonism of GABAA Receptors by 4-Quinolones. J. Antimicrob. Chemother. 1993, 31, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Famularo, G.; Pizzicannella, M.; Gasbarrone, L. Levofloxacin and Seizures: What Risk for Elderly Adults? J. Am. Geriatr. Soc. 2014, 62, 2018–2019. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. CIPRO (Ciprofloxacin Hydrochloride). 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/Label/2016/019537s086lbl.pdf (accessed on 12 April 2025).
- United States Food and Drug Administration. AVELOX (Moxifloxacin Hydrochloride). 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021085s063lbl.pdf (accessed on 12 April 2025).
- Pulia, M.S.; Schwei, R.J.; Hesse, S.P.; Werner, N.E. Characterizing Barriers to Antibiotic Stewardship for Skin and Soft-Tissue Infections in the Emergency Department Using a Systems Engineering Framework. Antimicrob. Steward. Healthc. Epidemiol. 2022, 2, e180. [Google Scholar] [CrossRef]
- Eckmann, C.; Tulkens, P.M. Current and Future Options for Treating Complicated Skin and Soft Tissue Infections: Focus on Fluoroquinolones and Long-Acting Lipoglycopeptide Antibiotics. J. Antimicrob. Chemother. 2021, 76, iv9–iv22. [Google Scholar] [CrossRef]
- Geremia, N.; Giovagnorio, F.; Colpani, A.; De Vito, A.; Botan, A.; Stroffolini, G.; Toc, D.-A.; Zerbato, V.; Principe, L.; Madeddu, G.; et al. Fluoroquinolones and Biofilm: A Narrative Review. Pharmaceuticals 2024, 17, 1673. [Google Scholar] [CrossRef]
- Pollack, L.A.; Srinivasan, A. Core Elements of Hospital Antibiotic Stewardship Programs From the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59, S97–S100. [Google Scholar] [CrossRef]
- Stocco, G.; Lucafò, M.; Decorti, G. Pharmacogenomics of Antibiotics. Int. J. Mol. Sci. 2020, 21, 5975. [Google Scholar] [CrossRef]
| Generation | Agents | Half Life | Comments |
|---|---|---|---|
| 1st | Nalidixic acid | 4–6 h | Prototypical quinolone; limited use in dermatology |
| 2nd | Ciprofloxacin, Norfloxacin, Ofloxacin, Nadifloxacin (topical) | 6–8 h | Enhanced Gram-negative coverage; common in SSTIs; Used off-label topically for skin infections like Pseudomonas nail (nadifloxacin) |
| 3rd | Levofloxacin, Moxifloxacin | 8–10 h | Expanded Gram-positive coverage; once-daily dosing |
| 4th | Delafloxacin, Trovafloxacin | 10–12 h | Broad-spectrum, including MRSA and anaerobes; acidic pH activity (delafloxacin) |
| Indication | Regimen(s) | Target(s) | Evidence | Notes |
|---|---|---|---|---|
| Incisional surgical site infections following operations on the axilla, perineum, or female genital tract | Combination therapy
| Gram-negative bacteria and anaerobes | Strong, low | |
| Treatment of necrotizing infections of the skin | Antimicrobial Agent for Patients with Severe Penicillin Hypersensitivity
| Mixed Infections | ||
| Treatment of necrotizing infections of the skin |
| Aeromonas hydrophila | Not recommended for children, but may need to use in life-threatening situations. |
| Indication | Regimen(s) | Target(s) | Evidence | Notes |
|---|---|---|---|---|
| Patients with SSTIs during the initial episode of fever and neutropenia |
| If fluoroquinolones are used for prophylaxis, broad-spectrum β-lactam antibiotics should be used for empiric therapy | ||
| Cutaneous Nocardia |
| Nocardia farcinica, Nocardia brasiliensis, and other Nocardia species | Combine with other agents for patients with severe infections or profound/lasting immunodeficiency |
| Indication | Regimen(s) | Target(s) | Evidence | Notes |
|---|---|---|---|---|
| Infected animal bite-related wounds |
| Good activity against Pasturella multocida; lacks activity against MRSA and some anaerobes | Strong, moderate |
|
| Cutaneous anthrax | Ciprofloxacin 500 mg PO bid or levofloxacin 500 mg IV/PO every 24 h × 60 days is recommended for bioterrorism cases because of presumed aerosol exposure | Bacillus anthracis | Strong, low |
|
| Erysipeloid | Fluoroquinolones | Erysipelothrix rhusiopathiae |
| |
| Glanders | Ciprofloxacin 400 mg IV every 8 h or 750 mg PO every 12 h | Burkholderia mallei | Strong, low |
|
| Bubonic plague | Ciprofloxacin for 10–14 days Other fluoroquinolones may be effective | Yersinia pestis |
| |
| Tularemia | Levofloxacin 500 mg PO daily or ciprofloxacin 750 mg PO bid for at least 14 days | Francisella tularensis |
|
| Adverse Effect | Clinical Features/Risk Factors | Management Recommendations |
|---|---|---|
| Tendinopathy and Tendon Rupture | Achilles tendon is most affected; risk factors: age > 60, corticosteroids, renal failure, diabetes, musculoskeletal disorders | Immediately discontinue FQ; orthopedic referral, imaging, physical therapy; avoid physical activity |
| QT Interval Prolongation | Risk factors: hypokalemia, bradycardia, increased age, concurrent QT-prolonging drugs (macrolides, antipsychotics) | Baseline and follow-up ECG; discontinue FQ if QTc > 500 ms |
| Photosensitivity | Severe sunburn, erythema, blistering, edema (notably ciprofloxacin, lomefloxacin) | Avoid sun exposure; use protective clothing and broad-spectrum sunscreen; topical/systemic corticosteroids; discontinue FQ |
| Gastrointestinal and Neurological | Nausea, vomiting, dizziness, headaches; rare severe effects: hallucinations, confusion, seizures (especially elderly); chronic FQAD symptoms | Symptomatic management; discontinue FQ if severe neuropsychiatric symptoms occur |
| Hypo- and Hyperglycemia | Glucose homeostasis disruption; hypoglycemia (insulin-dependent diabetics), hyperglycemia (non-diabetics) | Monitor blood glucose closely; adjust diabetic medications accordingly |
| Aortic Aneurysm and Aortic Dissection | Chest, abdominal, or back pain; risk factors: connective tissue disorders (e.g., pseudoxanthoma elasticum, Ehlers-Danlos syndrome, Marfan syndrome), history of obstructions or aneurysms of the aorta or other blood vessels, hypertension, genetic disorders that involve blood vessel changes, and advanced age | Monitor for chest, abdominal, or back pain occurring within two months of starting an FQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahood, S.; Alani, O.; Draw, I.; Shqair, L.; Wang, D.; Bunick, C.G.; Damiani, G.; Ho, J.D.; Obagi, S.; Akbarialiabad, H.; et al. Fluoroquinolones for Dermatologists: A Practical Guide to Clinical Use and Risk Management. Pharmaceuticals 2025, 18, 800. https://doi.org/10.3390/ph18060800
Wahood S, Alani O, Draw I, Shqair L, Wang D, Bunick CG, Damiani G, Ho JD, Obagi S, Akbarialiabad H, et al. Fluoroquinolones for Dermatologists: A Practical Guide to Clinical Use and Risk Management. Pharmaceuticals. 2025; 18(6):800. https://doi.org/10.3390/ph18060800
Chicago/Turabian StyleWahood, Samer, Omar Alani, Iyla Draw, Lara Shqair, David Wang, Christopher G. Bunick, Giovanni Damiani, Jonathan D. Ho, Sabine Obagi, Hossein Akbarialiabad, and et al. 2025. "Fluoroquinolones for Dermatologists: A Practical Guide to Clinical Use and Risk Management" Pharmaceuticals 18, no. 6: 800. https://doi.org/10.3390/ph18060800
APA StyleWahood, S., Alani, O., Draw, I., Shqair, L., Wang, D., Bunick, C. G., Damiani, G., Ho, J. D., Obagi, S., Akbarialiabad, H., Galimberti, F., Ghannoum, M., & Grada, A. (2025). Fluoroquinolones for Dermatologists: A Practical Guide to Clinical Use and Risk Management. Pharmaceuticals, 18(6), 800. https://doi.org/10.3390/ph18060800











