In Vitro Percutaneous Absorption of Permeation-Enhancing Estrogen Formulations
Abstract
1. Introduction
2. Results
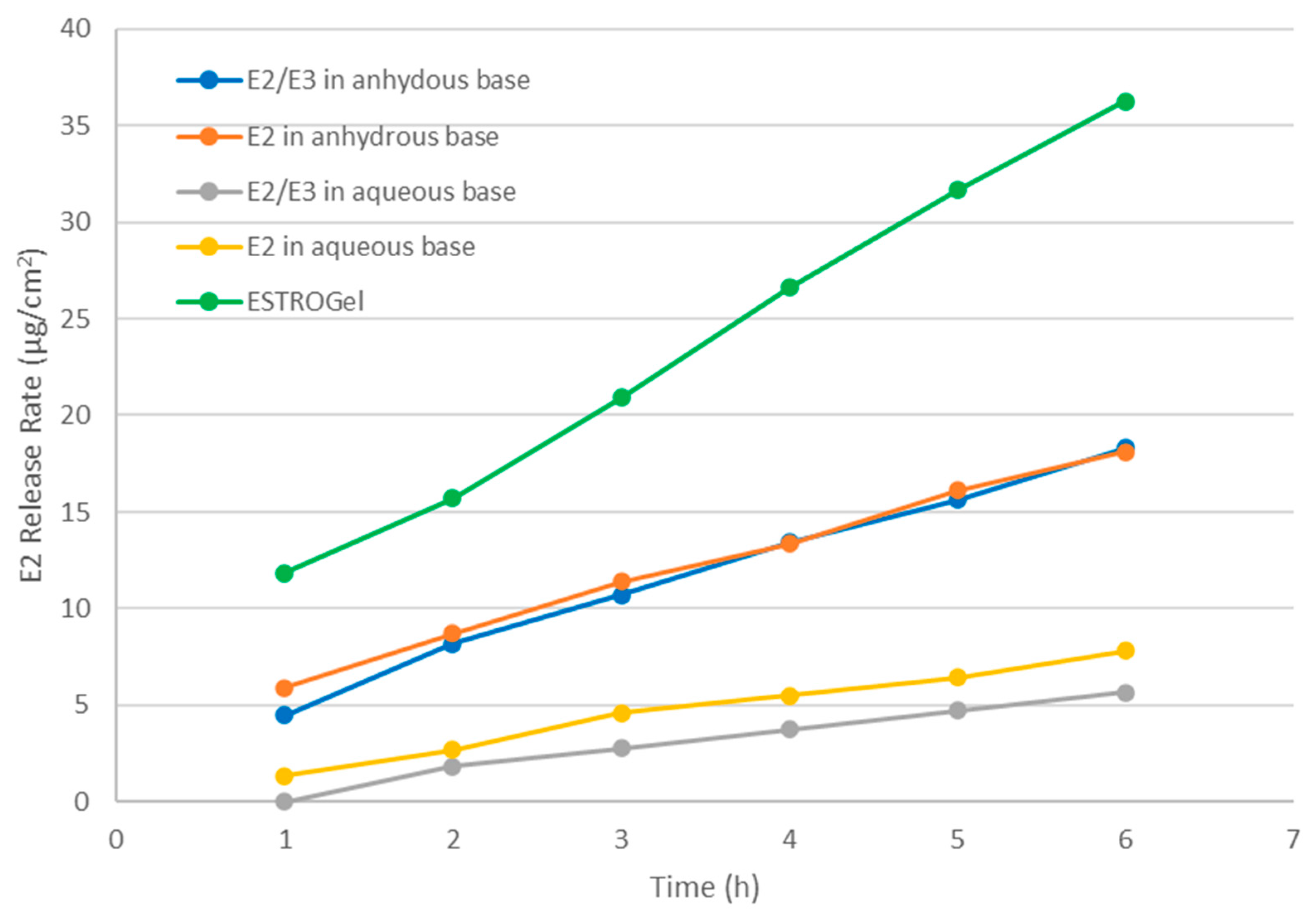
2.1. In Vitro Drug Release
2.2. In Vitro Permeation Test (IVPT) Validation
2.2.1. IVPT Apparatus Qualification
2.2.2. Solubility and Stability of Estradiol in the Receptor Medium
2.2.3. Dilution Integrity
2.2.4. Sensitivity
2.2.5. Selectivity
2.2.6. Robustness
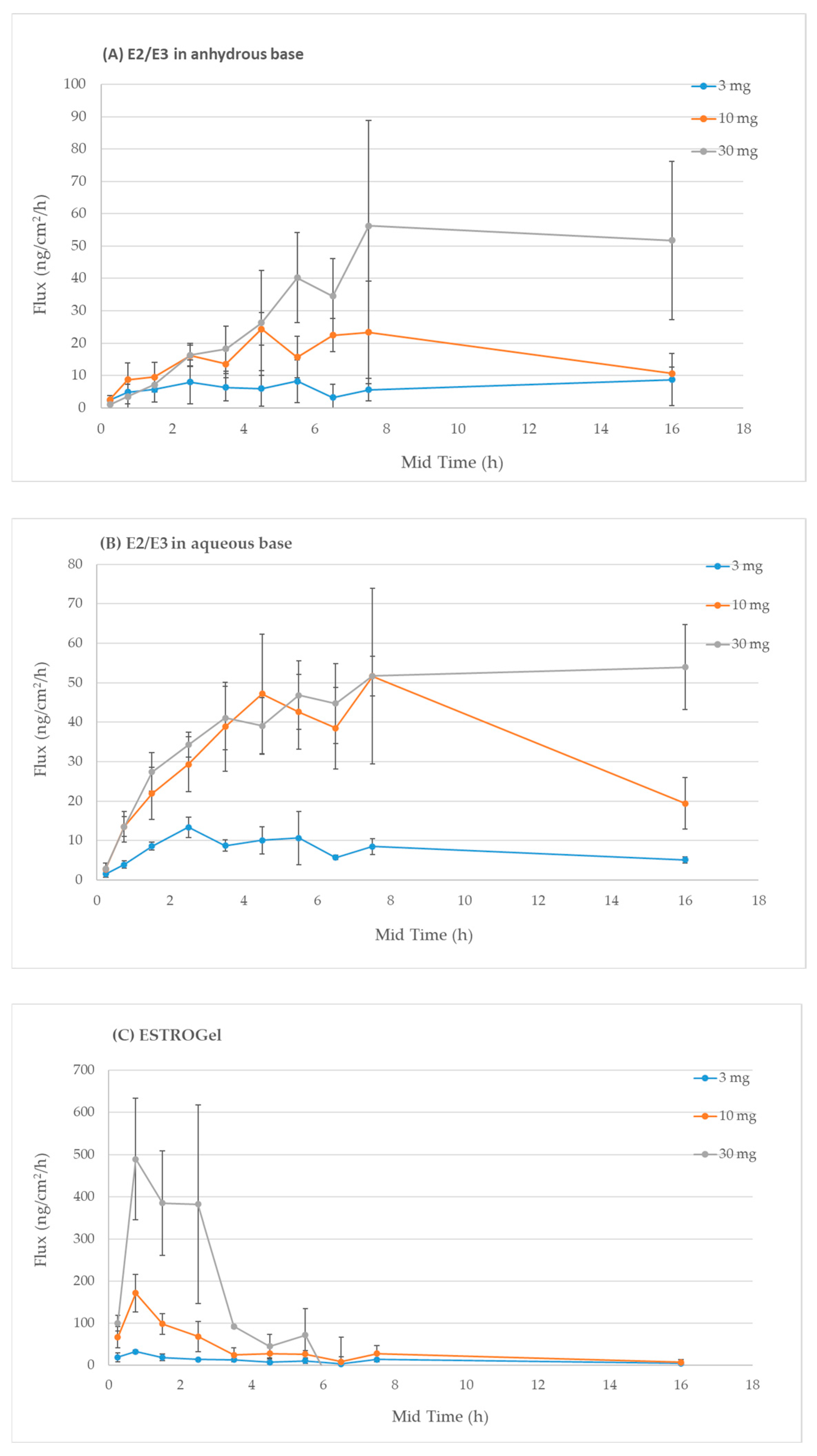
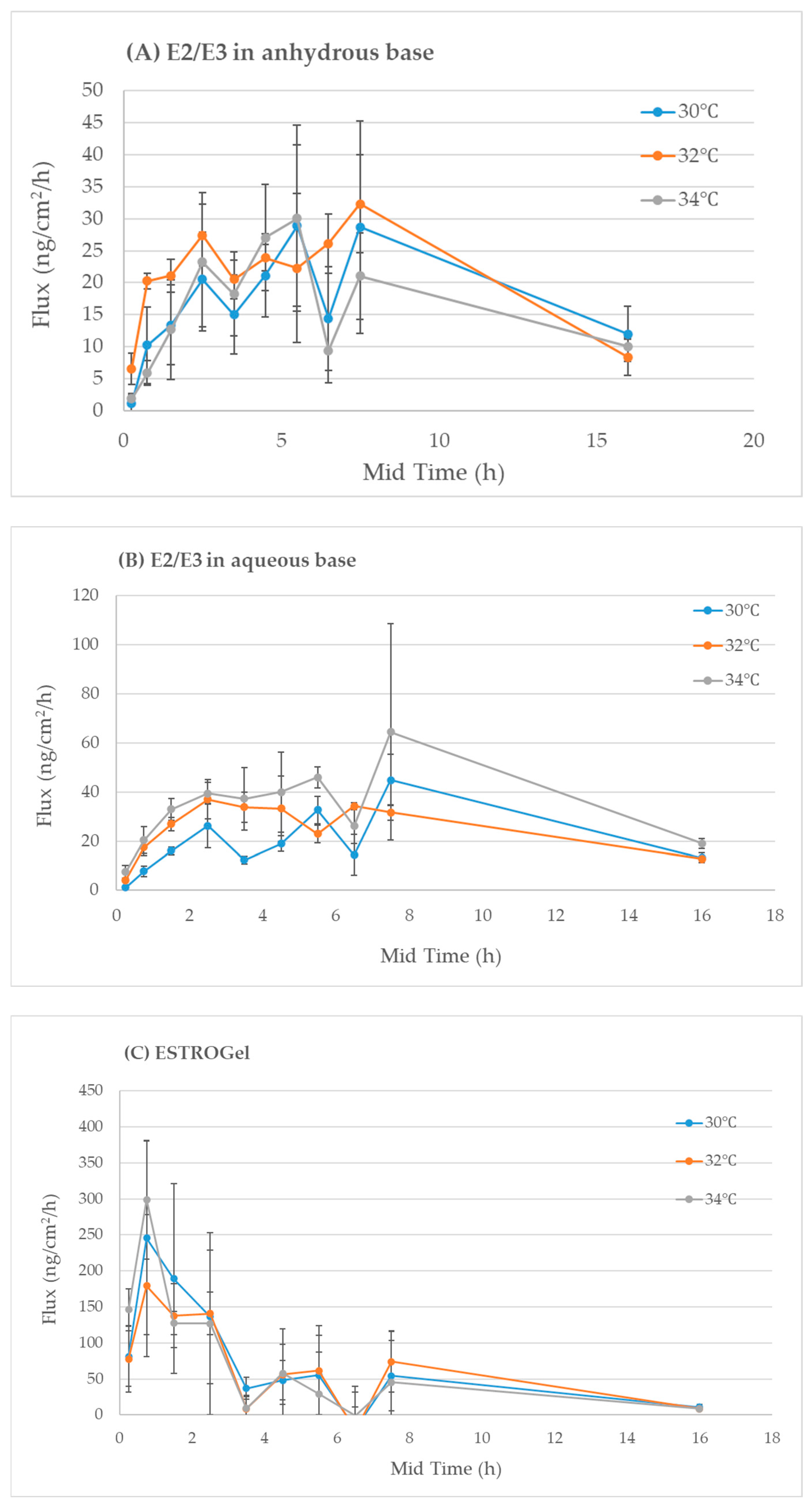
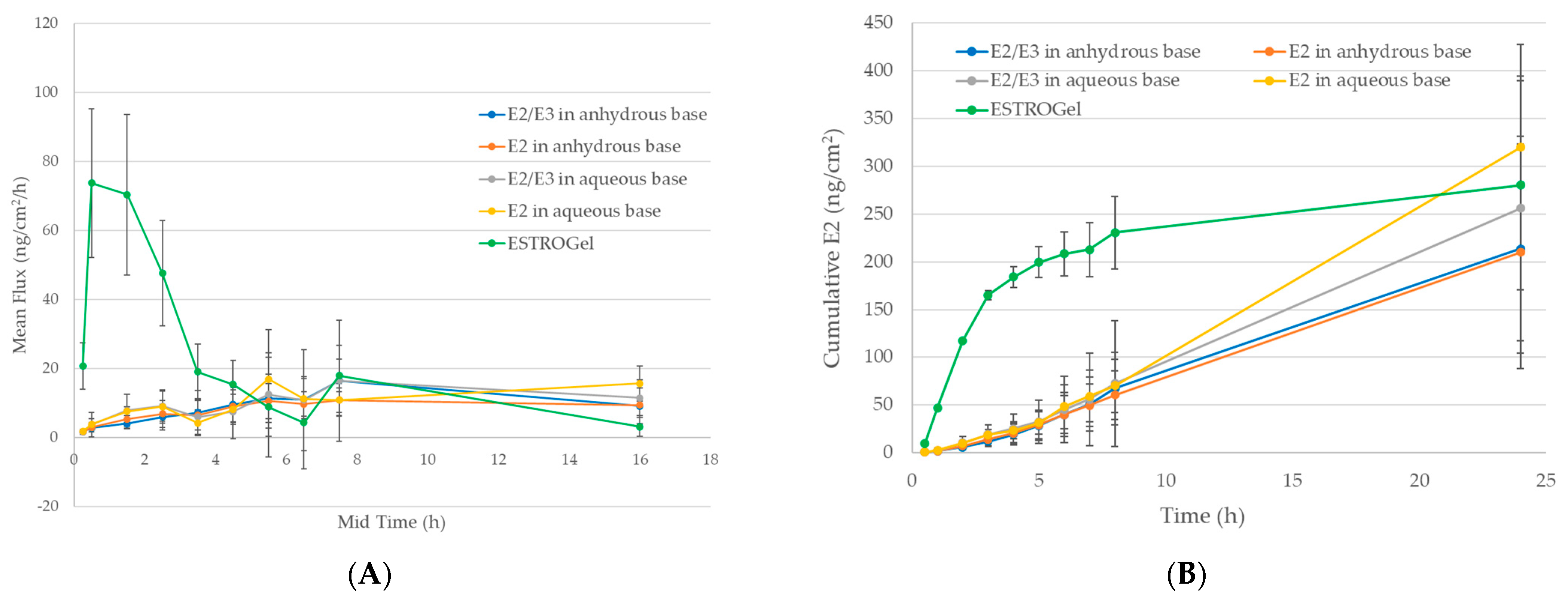
2.3. In Vitro Percutaneous Absorption
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Test Formulations
- (1)
- ESTROGel containing 0.06% estradiol (E2)
- (2)
- Estradiol 0.06% (w/w) (E2) in anhydrous base
- (3)
- Estradiol 0.06% (w/w) (E2) in aqueous base
- (4)
- Estradiol 0.06%/Estriol 0.1% (w/w) (E2/E3) in anhydrous base
- (5)
- Estradiol 0.06%/Estriol 0.1% (w/w) (E2/E3) in aqueous base
4.3. In Vitro Drug Release
4.4. IVPT Validation
4.4.1. IVPT Apparatus Qualification
4.4.2. Estradiol Solubility in the Receptor Medium
4.4.3. Bench-Top Stability and Long-Term Stability of Estradiol in the Receptor Medium
4.4.4. Skin Qualification
4.4.5. Dilution Integrity
4.4.6. IVPT Sensitivity and Selectivity
4.4.7. IVPT Robustness
4.5. Assessment of Differences Between Commercial and Compounded Formulations
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, P.W. What You Must Know About Women’s Hormones: Your Guide to Natural Hormone Treatments for PMS, Menopause, Osteoporosis, PCOS, and More, 2nd ed.; Square One Publishers: Garden City Park, NY, USA, 2022. [Google Scholar]
- FDA. Drugs@FDA: FDA-Approved Drugs: ESTROGEL® 0.06% (Estradiol Gel); Label ORIG-1 500123 1E Rev 2/2004; FDA: Silver Spring, MD, USA, 2004. [Google Scholar]
- USP. USP General Chapter 〈1724〉 SEMISOLID Drug Products—Performance Tests; USP: Rockville, MD, USA, 2023. [Google Scholar]
- Glaser, R.L.; Zava, D.T.; Wurtzbacher, D. Pilot study: Absorption and efficacy of multiple hormones delivered in a single cream applied to the mucous membranes of the labia and vagina. Gynecol. Obstet. Investig. 2008, 66, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Bassani, A.S.; Banov, D.; Carvalho, M. Evaluation of the in vitro human skin percutaneous absorption of progesterone in VersaBase® using the Franz skin finite dose model. J. Women’s Health Care 2017, 6, 1000384. [Google Scholar] [CrossRef]
- Allen, L.V., Jr. Progesterone 50 mg/g in VersaBase Cream. US Pharm. 2017, 42, 47–48. [Google Scholar]
- Needham, S.; Needham, S. Case study: Absorption of testosterone cream via scrotal delivery. Int. J. Pharm. Compd. 2018, 22, 466–468. [Google Scholar] [PubMed]
- Song, G.; Banov, D.; Song, H.; Liu, Y.; Ip, K.; Bassani, A.S.; Valdez, B.C. Evaluation of an anhydrous permeation-enhancing vehicle for percutaneous absorption of hormones. AAPS PharmSciTech 2022, 23, 198. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Shim, J.; Han, S.; Chang, I. The skin-permeation-enhancing effect of phosphatidylcholine: Caffeine as a model active ingredient. J. Cosmet. Sci. 2002, 53, 363–374. [Google Scholar] [PubMed]
- Wang, L.H.; Wang, C.C.; Kuo, S.C. Vehicle and enhancer effects on human skin penetration of aminophylline from cream formulations: Evaluation in vivo. J. Cosmet. Sci. 2007, 58, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. Estrogel Side Effects. 2024. Available online: https://www.drugs.com/sfx/estrogel-side-effects.html (accessed on 11 November 2024).
- Bartosova, L.; Bajgar, J. Transdermal drug delivery in vitro using diffusion cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- PCCA. ANHYDROUS VERSABASE® HRT (30-5056). 2024. Available online: https://www.pccarx.com/products/PCCA-VERSABASE%C2%AE-ANHYDROUS-HRT/30-5056/PROPRIETARYBASES (accessed on 22 December 2024).
- PCCA. VERSABASE® CREAM (30-3641). 2024. Available online: https://www.pccarx.com/products/VERSABASE%C2%AECREAM/30-3641/PROPRIETARYBASES (accessed on 22 December 2024).
- Song, G.; Ip, K.; Shan, S.; Banov, D.; Song, H.; Bassani, A.S.; Carvalho, M.; Day, A.J. Evaluation of the in vitro percutaneous absorption of progesterone, testosterone, estriol and estradiol topical compounded formulations. J. Investig. Dermatol. 2021, 141, S82. [Google Scholar] [CrossRef]

| Parameter | Acceptance Criteria | Results | Range of Variation V | Pass |
|---|---|---|---|---|
| Mean | Mean | |||
| Capacity of the cells | 12 ± 0.6 mL | 11.93 mL | 0.4 mL | Pass |
| Diameter of the orifice | 15 ± 0.75 mm | 14.7 mm | 0.1 mm | Pass |
| Temperature of the skin surface | 32 ± 1 °C | 31.7 °C | 0.6 °C | Pass |
| Speed of the magnetic stirrer | 600 ± 60 rpm | 600 ± 10 rpm | NA | Pass |
| Dispensed sampling volume | 1000 ± 30 µL | 1002 µL | 6.4 µL | Pass |
| Parameter | Acceptance Criteria | Results | ||||
|---|---|---|---|---|---|---|
| Bench-top | The accuracy (nominal) at each level should be ±15% | Formulation | Concentration (µg/mL) | 0 h % | 24 h % | Pass |
| ESTROGel | 1.64 | 100.00 | 101.57 | Pass | ||
| E2/E3 in anhydrous base | 1.01 | 100.00 | 99.67 | Pass | ||
| E2/E3 in aqueous base | 1.01 | 100.00 | 99.28 | Pass | ||
| Long term-stability | The accuracy (nominal) at each level should be ±15% | Formulation | Concentration (µg/mL) | 0 day % | 14 days % | Pass |
| ESTROGel | 1.64 | 100.00 | 99.98 | Pass | ||
| E2/E3 in anhydrous base | 1.01 | 100.00 | 95.45 | Pass | ||
| E2/E3 in aqueous base | 1.01 | 100.00 | 99.04 | Pass | ||
| Acceptance Criteria | Results | |||
|---|---|---|---|---|
| Dilution Factor | Accuracy (%) | Precision (%) | Pass | |
| 5 replicates per dilution factor Accuracy: ±20% nominal concentrations Precision: ±20% nominal concentrations | 1:5 | 103.50 | 7.24 | Pass |
| 1:50 | 91.15 | 5.59 | Pass | |
| 1:100 | 87.52 | 4.99 | Pass | |
| 1:200 | 82.76 | 9.54 | Pass | |
| 1:300 | 70.52 | 5.43 | Reject | |
| Source of Variation | ESTROGEL Versus | ||||
|---|---|---|---|---|---|
| E2/E3 Anhydrous | E2 Anhydrous | E2/E3 Aqueous | E2 Aqueous | ||
| Flux | Formulation | 2.7 × 10−9 | 5.7 × 10−10 | 8.5 × 10−8 | 1.1 × 10−8 |
| Time | 1.2 × 10−6 | 2.9 × 10−7 | 6 × 10−6 | 9.5 × 10−7 | |
| Interaction | 5.7 × 10−9 | 5.7 × 10−9 | 2.5 × 10−7 | 9 × 10−9 | |
| Cumulative | Formulation | 6 × 10−11 | 7.5 × 10−11 | 4.4 × 10−9 | 1.1 × 10−9 |
| Time | 9.6 × 10−8 | 1.8 × 10−7 | 2 × 10−7 | 5.5 × 10−10 | |
| Interaction | 0.079 | 0.088 | 0.12 | 0.0068 | |
| Donor ID | Age, Years | Race | Sex | Integrity Test Results (kΩ) (Mean ± SD) |
|---|---|---|---|---|
| FHU-S-040822A | 55 | African American | Female | 16.71 ± 6.56 |
| FHU-S-042722B | 59 | Caucasian | Female | 23.72 ± 6.41 |
| FHU-S-011323 | 54 | Caucasian | Female | 21.86 ± 5.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Ip, K.; Biundo, B.; Carvalho, M.; Day, A.J.; Bassani, A.S.; Song, H.; Valdez, B.C.; Banov, D. In Vitro Percutaneous Absorption of Permeation-Enhancing Estrogen Formulations. Pharmaceuticals 2025, 18, 596. https://doi.org/10.3390/ph18040596
Song G, Ip K, Biundo B, Carvalho M, Day AJ, Bassani AS, Song H, Valdez BC, Banov D. In Vitro Percutaneous Absorption of Permeation-Enhancing Estrogen Formulations. Pharmaceuticals. 2025; 18(4):596. https://doi.org/10.3390/ph18040596
Chicago/Turabian StyleSong, Guiyun, Kendice Ip, Bruce Biundo, Maria Carvalho, A. J. Day, August S. Bassani, Hui Song, Benigno C. Valdez, and Daniel Banov. 2025. "In Vitro Percutaneous Absorption of Permeation-Enhancing Estrogen Formulations" Pharmaceuticals 18, no. 4: 596. https://doi.org/10.3390/ph18040596
APA StyleSong, G., Ip, K., Biundo, B., Carvalho, M., Day, A. J., Bassani, A. S., Song, H., Valdez, B. C., & Banov, D. (2025). In Vitro Percutaneous Absorption of Permeation-Enhancing Estrogen Formulations. Pharmaceuticals, 18(4), 596. https://doi.org/10.3390/ph18040596








