Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines
Abstract
1. Introduction
2. Results and Discussion
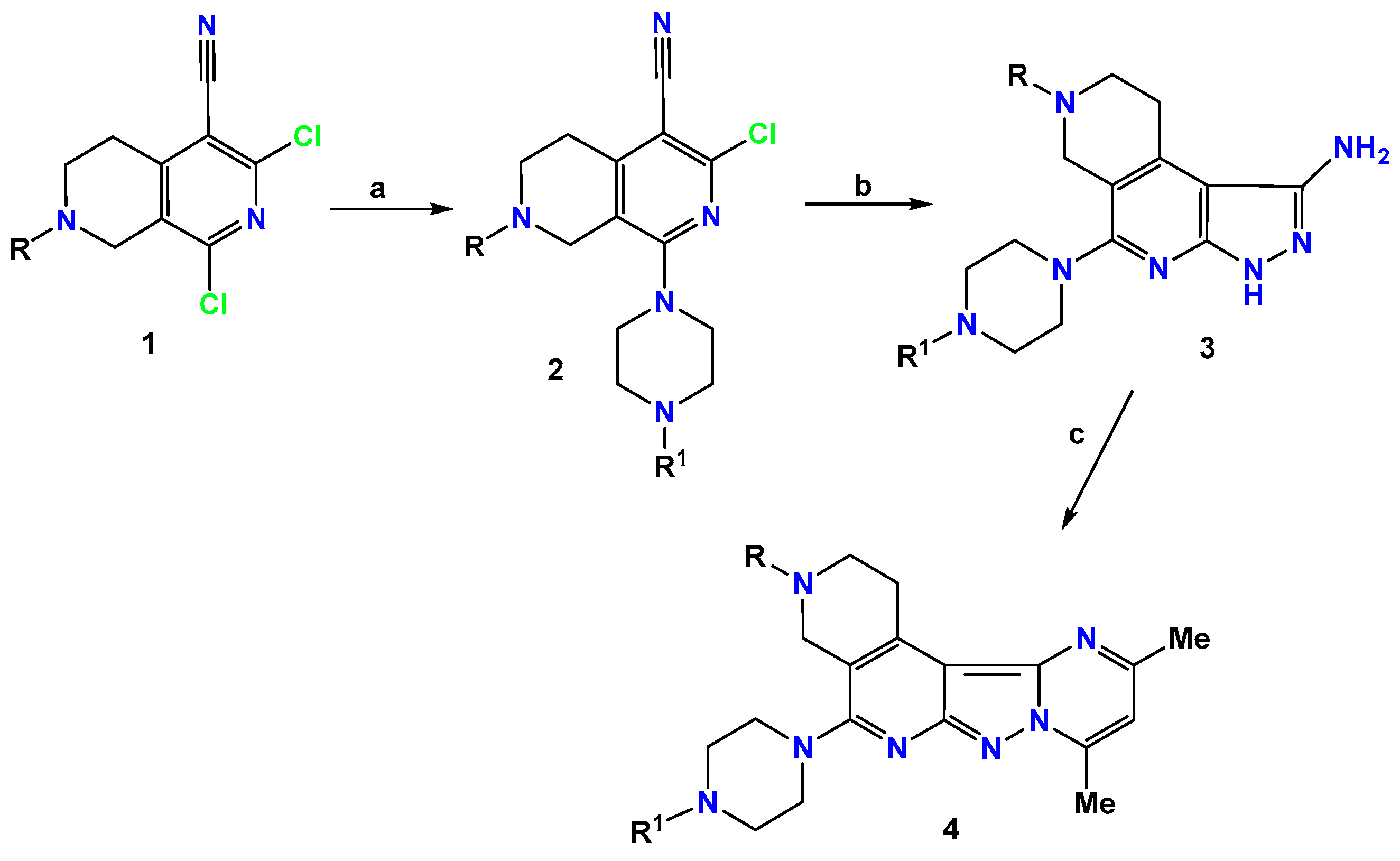
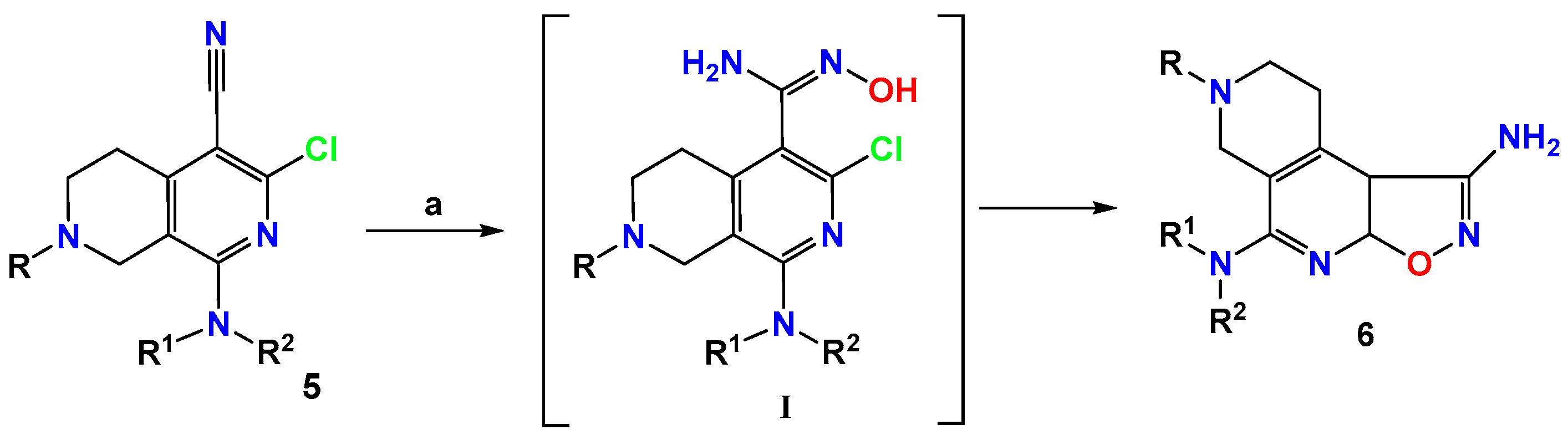
2.1. Chemistry
2.2. Biological Evaluation
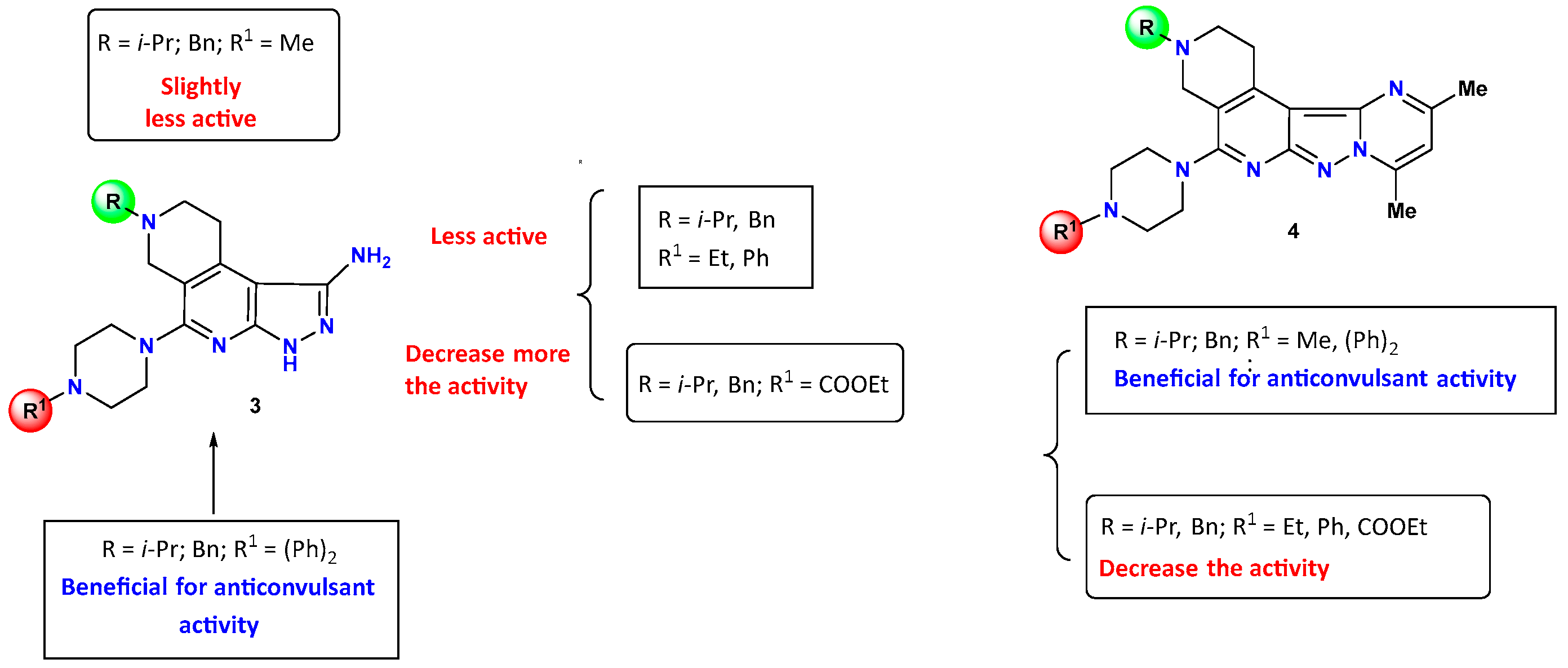
2.2.1. Evaluation of Anticonvulsant Activity
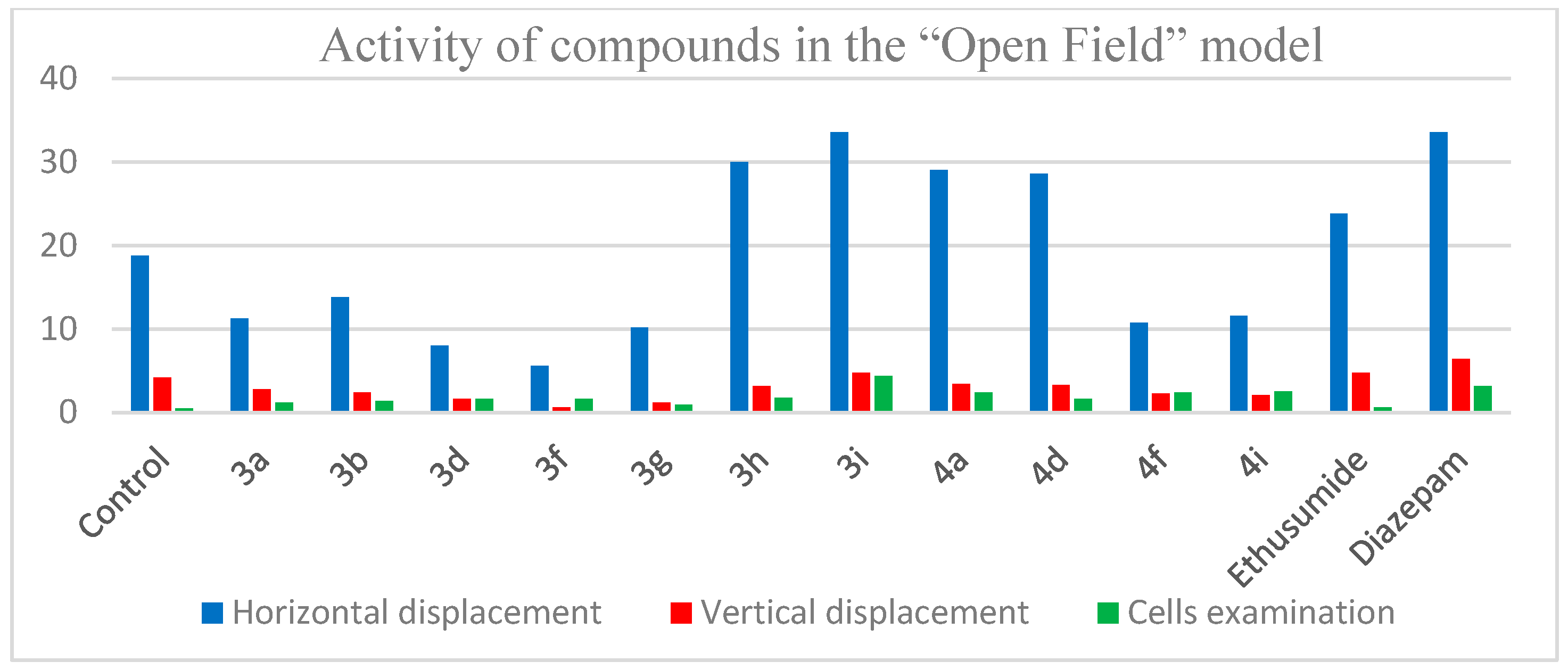
2.2.2. Evaluation of Psychotropic Effects
2.3. Molecular Docking
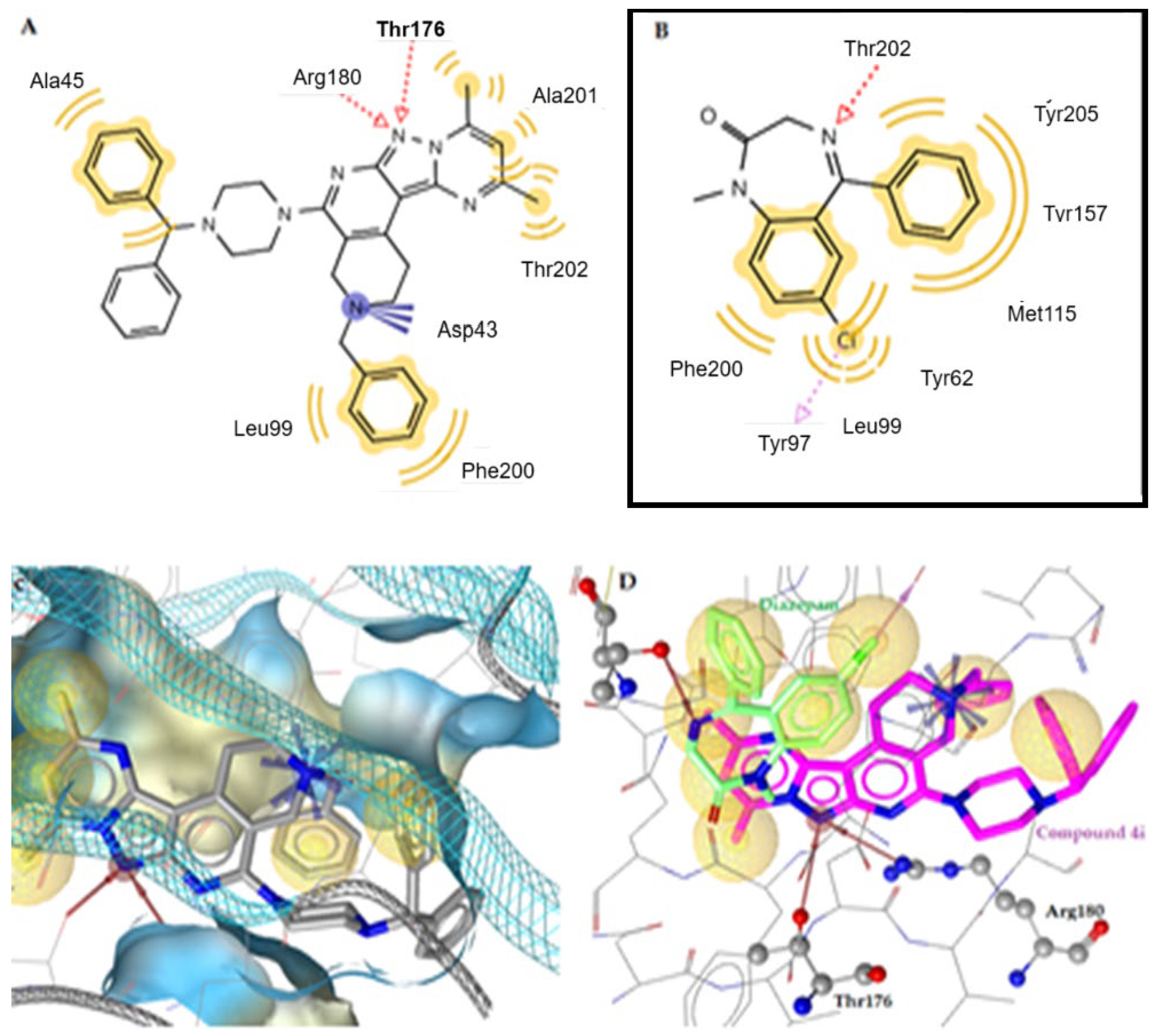
2.3.1. Docking to GABAA Receptor—Prediction of Mechanism of Anticonvulsant and Anxiolytic Activity
2.3.2. Docking to SERT Transporter and 5-HT1A Receptor
3. Materials and Methods
3.1. Chemistry: Experimental Part
3.1.1. General Procedure for Synthesis of Compounds 2a–d,f–j
3.1.2. General Procedure for Synthesis of Compounds 3a–j
3.1.3. General Procedure for Synthesis of Compounds 4a–j
3.1.4. General Procedure for Synthesis of Compounds 6a–d
3.2. Biological Evaluation: Experimental Part
3.2.1. Evaluation of Anticonvulsant Potency
3.2.2. PTZ-Induced Convulsions
3.2.3. Thiosemicarbazide-Induced Convulsions
3.2.4. MES Test
3.2.5. Examination of Psychotropic Effects of Synthesized Compounds
3.2.6. Open Field Test
3.2.7. EPM Test
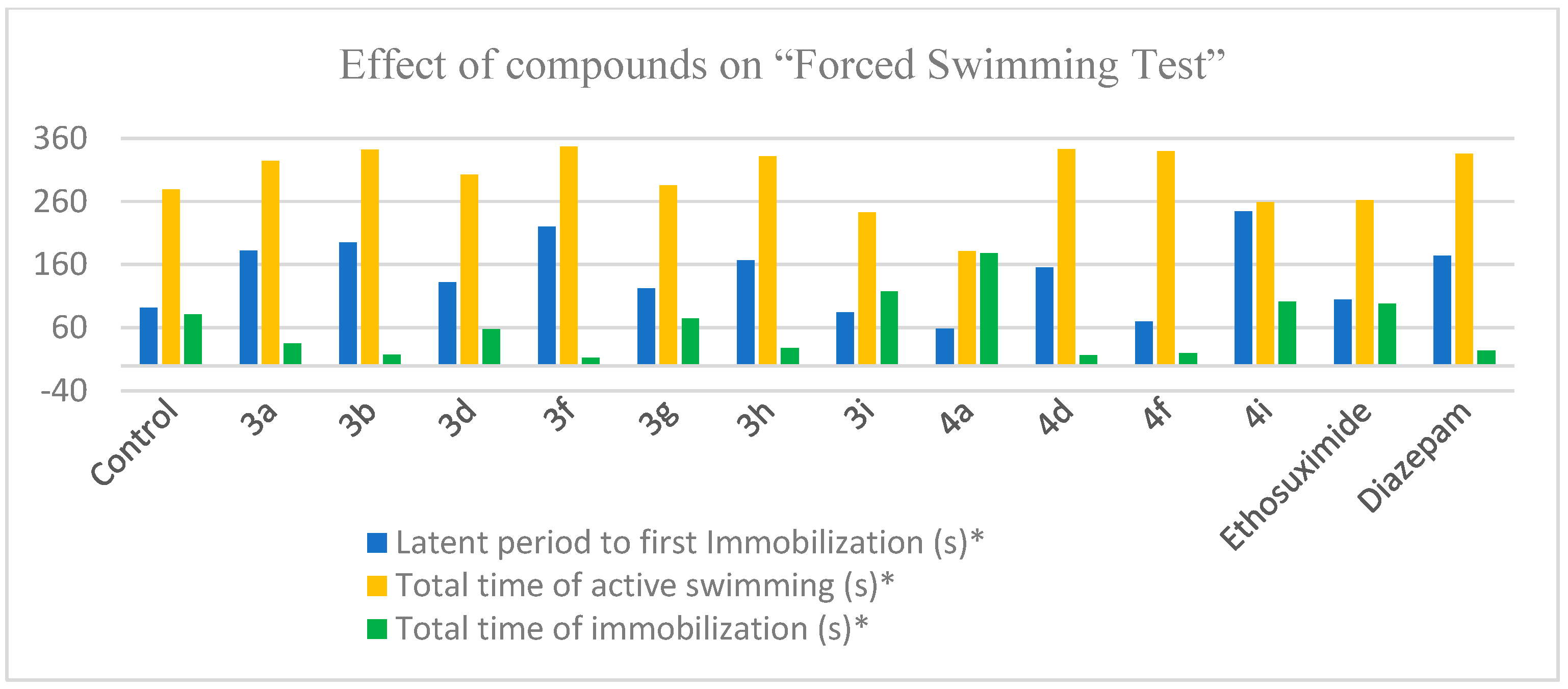
3.2.8. Forced Swimming Test
3.2.9. Evaluation of Coordination of Movement (Muscle Relaxant)
3.3. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Epilepsy: A Public Health Imperative. Summary; (WHO/MSD/MER/19.2). License: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Riney, K.; Bogacz, A.; Somerville, E.; Hirsch, E.; Nabbout, R.; Scheffer, I.E.; Zuberi, S.M.; Alsaadi, T.; Jain, S.; French, J.; et al. International League Against Epilepsy classification and definition of epilepsy syndromes with onset at a variable age: Position statement by the ILAE Task Force on Nosology and Definitions. Epilepsia 2022, 63, 1443–1474. [Google Scholar] [CrossRef] [PubMed]
- Wirrell, E.C.; Tinuper, P.; Perucca, E.; Moshe, S.L. Introduction to the epilepsy syndrome papers. Epilepsia 2022, 63, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Saadi, A.; Patenaude, B.; Mateen, F.J. Quality of life in epilepsy—31 inventory (QOLIE-31) scores: A global comparison. Epilepsy Behav. 2016, 65, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Basir, N.H.; Ramle, A.Q.; Ng, M.P.; Tan, C.H.; Tiekink, E.R.T.; Sim, K.S.; Basirun, W.J.; Khairuddean, M. Discovery of indoleninylpyrazolo[3,4-b]pyridines as potent chemotherapeutic agents against colorectal cancer cells. Bioorg. Chem. 2024, 146, 107256. [Google Scholar] [CrossRef]
- Salem, M.S.; Ali, M.A.M. Novel Pyrazolo[3,4-b]pyridine Derivatives: Synthesis, Characterization, Antimicrobial and Antiproliferative Profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef]
- El-Borai, M.A.; Rizk, H.F.; Abd-Aal, M.F.; El-Deeb, I.Y. Synthesis of pyrazolo[3,4-b]pyridines under microwave irradiation in multi-component reactions and their antitumor and antimicrobial activities—Part 1. Eur. J. Med. Chem. 2012, 48, 92–96. [Google Scholar] [CrossRef]
- Variya, H.H.; Panchal, V.; Patel, G.R. Synthesis and characterization of 4-((5-bromo-1H-pyrazolo[3,4-b]pyridin-3-yl)amino)-N-(substituted)benzenesulfonamide as antibacterial, and antioxidant candidates. Curr. Chem. Lett. 2019, 8, 177–186. Available online: https://www.growingscience.com/ccl/Vol8/ccl_2019_16.pdf (accessed on 15 January 2025). [CrossRef]
- Elsherif, M.A. Antibacterial evaluation and molecular properties of pyrazolo[3,4-b]pyridines and thieno[2,3-b]pyridines. J. Appl. Pharm. Sci. 2021, 11, 118–124. [Google Scholar] [CrossRef]
- Abeed, A.A.; El-Emary, T.I.; Youssef, M.S.; Hefzy, I.; Ibrahim, A.M. Synthesis and reactions of fused pyrazolo[3,4-b]pyridine derivatives: Insecticidal activity and digestive dysfunction against Mosquito Larvae. Curr. Org. Chem. 2023, 27, 852–859. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, H.; Wang, X.; Feng, F.; Fan, T.; Zhao, D.; Xiong, B.; Xie, H.; Liu, T. Identification of 1H-pyrazolo[3,4-b]pyridine derivatives as novel and potent TBK1 inhibitors: Design, synthesis, biological evaluation, and molecular docking study. J. Enzyme Inhib. Med. Chem. 2022, 37, 1411–1425. [Google Scholar] [CrossRef]
- Bharate, S.B.; Mahajan, T.R.; Gole, Y.R.; Nambiar, M.; Matan, T.T.; Kulkarni-Almeida, A.; Balachandran, S.; Junjappa, H.; Balakrishnan, A.; Vishwakarma, R.A. Synthesis and evaluation of pyrazolo[3,4-b]pyridines and its structural analogues as TNF-α and IL-6 inhibitors. Bioorg. Med. Chem. 2008, 16, 7167–7176. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, X.; Fu, Q.; Qin, Q.; Wu, T.; Lv, R.; Zhao, D.; Cheng, M. Design, synthesis and biological evaluation of pyrazolo[3,4-b]pyridine derivatives as TRK inhibitors. RSC Med. Chem. 2023, 14, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Donaire-Arias, A.; Montagut, A.M.; Bellacasa, R.P.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo[3,4-b]pyridines: Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef] [PubMed]
- Dodiya, D.K.; Trivedi, A.R.; Kataria, V.B.; Shah, V.H. Advances in the synthesis of pyrazolo[3,4-b]pyridines. Curr. Org. Chem. 2012, 16, 400–417. [Google Scholar] [CrossRef]
- Smolobochkin, A.V.; Gazizov, A.S.; Garifzyanov, A.R.; Burilov, A.R.; Pudovik, M.A. Methods for the synthesis of 1H-pyrazolo[3,4-b]pyridine derivatives. Russ. Chem. Bull. 2022, 71, 878–884. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hovakimyan, A.A. New methods for the synthesis of 3-amino-6,7-dihydro-5H-cyclopenta[c]pyridine-4-carbonitriles and cyclopenta[d]pyrazolo[3,4-b]pyridines via a Smiles-type rearrangement. J. Heterocycl. Chem. 2017, 54, 1199–1209. [Google Scholar] [CrossRef]
- Becerra, D.; Abonia, R.; Juan-Carlos, C. Recent Applications of the Multicomponent Synthesis for Bioactive Pyrazole Derivatives. Molecules 2022, 27, 4723. [Google Scholar] [CrossRef]
- Becerra, D.; Juan-Carlos, C. Recent advances in the synthesis of anticancer pyrazole derivatives using microwave, ultrasound, and mechanochemical techniques. RSC Adv. 2025, 15, 7018–7038. [Google Scholar] [CrossRef]
- Wójcicka, A. Synthesis and Biological Activity of 2,7-Naphthyridine Derivatives: An Overview. Curr. Org. Chem. 2021, 22, 2740–2764. [Google Scholar] [CrossRef]
- Guo, C.; Guzzo, P.R.; Hadden, M.; Sargent, B.J.; Yet, L.; Kan, Y.; Palyha, O.; Kelly, T.M.; Guan, X.; Rosko, K.; et al. Synthesis of 7-benzyl-5-(piperidin-1-yl)-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c][3,8]-naphthyridin-1-ylamine and its analogs as Bombesin receptor subtype-3 agonists. Bioorg. Med. Chem. Lett. 2010, 20, 2785–2789. [Google Scholar] [CrossRef]
- Gabos, B.; Lundkvist, M.; Af Rosenschold, M.M.; Shamovsky, I.; Zlatoidsky, P. Novel Hydantoin Derivatives as Metalloproteinase Inhibitors. U.S. Patent 12/697,625, 7 October 2010. [Google Scholar]
- Ukita, T.; Nakamura, Y.; Kubo, A.; Yamamoto, Y.; Moritani, Y.; Saruta, K.; Higashijima, T.; Kotera, J.; Fujishige, K.; Takagi, M.; et al. 1,7- and 2,7-naphthyridine derivatives as potent and highly specific PDE5 inhibitors. Bioorganic Med. Chem. Lett. 2003, 13, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Tonoyants, N.A.; Noravyan, A.S.; Dzhagatspanyan, I.A.; Nazaryan, I.M.; Akopyan, A.G.; Paronikyan, R.G.; Minasyan, N.S. Synthesis and neurotropic activity of triazolo[3,4-a]- and triazolo[5,1-a]-2,7-naphthyridines. Pharm. Chem. J. 2014, 48, 231–234. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Hakobyan, E.K.; Nikoghosyan, A.G.; Paronikyan, R.G.; Dzhagatspanyan, I.A.; Nazaryan, I.M.; Akopyan, A.G.; Hovakimyan, A.A. Synthesis and neurotropic activity of new 7-cyclohexyl-6,7,8,9-tetrahydro-3H-pyrazolo[3,4-c]-2,7-naphthyridine-1,5-diamines. Pharm. Chem. J. 2018, 52, 108–111. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Dotsenko, V.V. Synthesis of isoxazolo[5,4-b]pyridine derivatives (microreview). Chem. Heterocycl. Compd. 2021, 57, 627–629. [Google Scholar] [CrossRef]
- Poreba, K.; Wietrzyk, J.; Opolski, A. Synthesis and antiproliferative activity in vitro of new 3-substituted aminoisoxazolo[5,4-b]pyridines. Acta Pol. Pharm. Drug Res. 2003, 60, 293–301. Available online: https://pubmed.ncbi.nlm.nih.gov/14714858 (accessed on 15 January 2025). [PubMed]
- Astolfi, A.; Kudolo, M.; Brea, J.; Manni, G.; Manfroni, G.; Palazzotti, D.; Sabatini, S.; Cecchetti, F.; Felicetti, T.; Cannalire, R.; et al. Discovery of potent p38a MAPK inhibitors through a funnel like workflow combining in silico screening and in vitro validation. Eur. J. Med. Chem. 2019, 182, 111624. [Google Scholar] [CrossRef]
- Chikkula, K.V.; Raja, S. Isoxazole—A Potent Pharmacophore. Int. J. Pharm. Pharm. Sci. 2017, 9, 13–24. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hakobyan, E.K.; Kartsev, V.G.; Yegoryan, H.A.; Paronikyan, E.G.; Ghazaryan, S.G.; Hovakimyan, A.A. Synthesis of new pentaheterocyclic systems based on the triazolo[3,4-a]-2,7-naphthyridine core. Arkivoc 2022, part ii, 236–245. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Hakobyan, E.K.; Spinelli, D.; Geronikaki, A.; Kartsev, V.G.; Yegoryan, H.A.; Hovakimyan, A.A. Synthesis of new heterocyclic systems fused at pyrazolo[3,4-c]-2,7-naphthyridine core. Mendeleev Commun. 2022, 32, 393–394. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hovakimyan, A.A.; Noravyan, A.S. New heterocyclic systems derived from pyridine: New substrates for the investigation of the azide/tetrazole equilibrium. Tetrahedron 2014, 70, 8648–8656. [Google Scholar] [CrossRef]
- Sirakanyan, S.N.; Spinelli, D.; Mattioli, E.J.; Calvaresi, M.; Geronikaki, A.; Kartsev, V.G.; Hakobyan, E.K.; Yegoryan, H.A.; Jughetsyan, H.V.; Manukyan, M.E.; et al. Synthesis and Rearrangement of New 1,3-Diamino-2,7-naphthyridines and 1-Amino-3-oxo-2,7-naphthyridines. Int. J. Mol. Sci. 2024, 25, 11977. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, Y.; Zhang, C.; Yang, X.-J.; Wu, B. Isolation of the half-condensed pyrazoline intermediates en route to N-(1,10-phenanthroline)-pyrazole derivatives from the Knorr reaction. Synth. Commun. 2009, 39, 907–916. [Google Scholar] [CrossRef]
- El-Enany, M.M.; El-Meligie, S.E.M.; Abdou, N.A.; El-Nassan, H.B. Synthesis of pyrazolo[3,4-b]pyridine and pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine derivatives. J. Chem. Res. 2010, 34, 470–474. [Google Scholar] [CrossRef]
- Vogel, H.G.; Vogel, V.H. Psychotropic and Neurotropic Activity. In Drug Discovery and Evaluation: Pharmacological Assays; Vogel, H.E., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 58, p. 569874. [Google Scholar]
- Swinyard, E.A. Experimental Models of Epilepsy; Purpura, D.P., Penry, J.K., Tower, D., Woodbury, D.M., Walter, R., Eds.; Raven Press: New York, NY, USA, 1992; pp. 433–458. [Google Scholar]
- Katzung, B.G. Drugs Used in Generalized Seizures. In Basic and Clinical Pharmacology, 9th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Yuen, E.S.M.; Troconiz, I.F. Can pentylenetetrazole and maximal electroshock rodent seizure models quantitatively predict antiepileptic efficacy in humans? Seizure 2015, 24, 21–27. [Google Scholar] [CrossRef]
- Mironov, A.H. The 1th Part. In Manual for Preclinical Studies of Drugs; Medicine: Moscow, Russia, 2012; pp. 235–250. [Google Scholar]
- Löscher, W.; Klein, P. The Pharmacology and Clinical Efficacy of Antiseizure Medications: From Bromide Salts to Cenobamate and Beyond. CNS Drugs 2021, 35, 935–963. [Google Scholar] [CrossRef]
- Zhang, M.; Kou, L.; Qin, Y.; Chen, J.; Bai, D.; Zhao, L.; Lin, H.; Jiang, G. A bibliometric analysis of the recent advances in diazepam from 2012 to 2021. Front. Pharmacol. 2022, 13, 1042594. [Google Scholar] [CrossRef]
- Lubrich, C.; Giesler, P.; Kipp, M. Motor behavioral deficits in the cuprizone model: Validity of the rotarod test paradigm. Int. J. Mol. Sci. 2022, 23, 11342. [Google Scholar] [CrossRef]
- File, S.E. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001, 125, 151–157. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open: Closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Graeff, F.G.; Netto, C.F.; Zangrossi, H., Jr. The elevated T-maze as an experimental model of anxiety. Neurosci. Biobehav. Rev. 1998, 23, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A.; Löscher, W. The neurobiology of antiepileptic drugs. Nat. Rev. Neurosci. 2004, 5, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, M.; Rassner, M.P.; Freiman, T.M.; Feuerstein, T.J. Effects of antiepileptic drugs on GABA release from rat and human neocortical synaptosomes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 384, 47–57. [Google Scholar] [CrossRef]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef]
- Saier, M.H., Jr. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 2000, 64, 354–411. [Google Scholar] [CrossRef]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Lacivita, E.; Larizza, C.; Leopoldo, M.; Tortorella, V. Design and synthesis of long-chain arylpiperazines with mixed affinity for serotonin transporter (SERT) and 5-HT(1A) receptor. J. Pharm. Pharmacol. 2005, 57, 1319–1327. [Google Scholar] [CrossRef]
- Blier, P. Pharmacology of rapid-onset antidepressant treatment strategies. J. Clin. Psychiatry 2001, 62, 12–17. [Google Scholar] [PubMed]
- Singh, S.K.; Piscitelli, C.L.; Yamashita, A.; Gouaux, E. A competitive inhibitor traps LeuT in an opento-out conformation. Science 2008, 322, 1655–1661. [Google Scholar] [CrossRef]
- Kuipers, W.; Link, R.; Standaar, P.J.; Stoit, A.R.; Van Wijngaarden, I.; Leurs, R.; Ijzerman, A.P. Study of the interaction between aryloxypropanolamines and Asn386 in helix VII of the human 5hydroxytryptamine1A receptor. Mol. Pharmacol. 1997, 51, 889–896. [Google Scholar] [CrossRef]
- Wacker, D.; Fenalti, G.; Brown, M.A.; Katritch, V.; Abagyan, R.; Cherezov, V.; Stevens, R.C. Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J. Am. Chem. Soc. 2010, 132, 11443–11445. [Google Scholar] [CrossRef]
- Litchfield, J.T., Jr.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sirakanyan, S.N.; Spinelli, D.; Geronikaki, A.; Hakobyan, E.K.; Petrou, A.; Kartsev, V.; Yegoryan, H.A.; Paronikyan, E.G.; Zuppiroli, L.; Jughetsyan, H.V.; et al. New triazole-based hybrids as neurotropic agents. RSC Adv. 2024, 14, 32922–32943. [Google Scholar] [CrossRef] [PubMed]
- Bandaru, S.; Tiwari, G.; Akka, J.; Marri, V.K.; Alvala, M.; Gutlapalli, V.R.; Nayarisseri, A.; Mundluru, H.P. Identification of high affinity bioactive Salbutamol conformer directed against mutated (Thr164Ile) beta 2 adrenergic receptor. Curr. Top. Med. Chem. 2015, 15, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Accelrys Discovery Studio® Visualizer, Version 3.5.0.12158; Accelrys Software Inc.: San Diego, CA, USA, 2005.




| Compound | R | R1 | Compound | R | R1 |
|---|---|---|---|---|---|
| 1a | i-Pr | – | 2e/3e/4e | i-Pr | COOEt |
| 1b | Bn | – | 2f/3f/4f | Bn | Me |
| 1c | i-Bu | – | 2g/3g/4g | Bn | Et |
| 2a/3a/4a | i-Pr | Me | 2h/3h/4h | Bn | Ph |
| 2b/3b/4b | i-Pr | Et | 2i/3i/4i | Bn | CH(Ph)2 |
| 2c/3c/4c | i-Pr | Ph | 2j/3j/4j | Bn | COOEt |
| 2d/3d/4d | i-Pr | CH(Ph)2 |
| Compound | R | NR1R2 | Yield *, % (For Compounds 6) |
|---|---|---|---|
| 5a/6a | i-Pr | 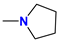 | 63 |
| 5b/6b | i-Pr | 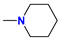 | 67 |
| 5c/6c | i-Pr | 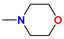 | 62 |
| 5d/6d | i-Bu | 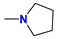 | 65 |
| Compound | ED50 * mg/kg (by PTZ Antagonism) | TD50 * mg/kg | LD50 * mg/kg | TI | Latency of Convulsions Induced by TSC, min | |
|---|---|---|---|---|---|---|
| M ± m | I** | |||||
| Control | – | – | – | – | 54.0 ± 2.5 | 1.0 |
| 3a | 34.0 (29.6 ÷ 39.1) | >200 | >500 | >14.7 | 66.9 ± 7.1 | 1.24 |
| 3b | 32.5 (27.1 ÷ 39.0) | >200 | >500 | >15.4 | 61.0 ± 3.5 | 1.13 |
| 3d | 41.2 (35.2 ÷ 48.2) | >200 | >700 | >17.0 | 94.6 ± 9.2 | 1.75 |
| 3f | 37.5 (28.8 ÷ 43.1) | >200 | >600 | >16.0 | 64.4 ± 6.9 | 1.20 |
| 3g | 38.5 (32.1 ÷ 46.2) | >200 | >700 | >18.2 | 73.4 ± 7.2 | 1.40 |
| 3h | 28.5 (23.9 ÷ 33.9) | >200 | >600 | >21.0 | 67.6 ± 8.7 | 1.25 |
| 3i | 25.8 (21.5 ÷ 31.0) | >200 | >600 | >23.3 | 66.2 ± 7.9 | 1.23 |
| 4a | 33.0 (27.5 ÷ 39.6) | >200 | >600 | >18.2 | 58.8 ± 5.8 | 1.10 |
| 4d | 40.0 (32.0 ÷ 50.0) | >200 | >600 | >15.0 | 110.0 ± 11.5 | 2.00 |
| 4f | 44.2 (36.8 ÷ 53.0) | >200 | >700 | >15.8 | 60.2 ± 5.2 | 1.15 |
| 4i | 23.8 (19.8 ÷ 28.6) | >200 | >800 | >33.6 | 68.0 ± 6.1 | 1.26 |
| Ethosuximide (200 mg/kg) | 155 (117.5 ÷ 204.5) | 520 (413 ÷ 655) | 1325 (1200 ÷ 1462) | 8.50 | 101.0 ± 14.0 | 1.87 |
| Diazepam (2 mg/kg) | 0.5 (0.4 ÷ 0.7) | 2.7 (1.4 ÷ 5.5) | 180 (128.5 ÷ 252.0) | 360 | 55.0 ± 3.5 | 1.0 |
| Compound | Dose mg/kg | Amount (Absolute Data During 5 min) * | ||
|---|---|---|---|---|
| Horizontal Displacement | Vertical Displacement | Cells | ||
| Control | – | 18.8 ± 3.2 | 4.2 ± 0.7 | 0.5 ± 0.2 |
| 3a | 50 | 11.3 ± 2.8 ** | 2.8 ± 0.3 ** | 1.2 ± 0.2 ** |
| 3b | 50 | 12.8 ± 1.6 ** | 2.4 ± 0.7 ** | 1.4 ± 0.3 ** |
| 3d | 50 | 8.0 ± 1.1 ** | 1.6 ± 0.4 ** | 1.6 ± 0.5 ** |
| 3f | 50 | 5.6 ± 1.4 ** | 0.6 ± 0.4 ** | 2.6 ± 0.2 ** |
| 3g | 50 | 10.2 ± 2.2 ** | 1.2 ± 0.3 ** | 0.9 ± 0.1 ** |
| 3h | 50 | 30.0 ± 4.1 ** | 3.2 ± 0.8 | 1.8 ± 0.1 ** |
| 3i | 50 | 33.5 ± 4.2 ** | 6.4 ± 0.7 ** | 4.4 ± 1.1 ** |
| 4a | 50 | 29.0 ± 3.1 ** | 3.4 ± 0.6 | 2.5 ± 0.5 ** |
| 4d | 50 | 28.6 ± 2.8 ** | 3.1 ± 0.1 ** | 1.6 ± 0.3 ** |
| 4f | 50 | 10.8 ± 3.3 ** | 2.3 ± 0.8 ** | 2.4 ± 0.6 ** |
| 4i | 50 | 11.6 ± 1.3 ** | 2.1 ± 0.9 ** | 2.7 ± 0.5 ** |
| Ethosuximide | 200 | 23.8 ± 3.8 | 4.8 ± 0.9 | 0.6 ± 0.08 |
| Diazepam | 2 | 33.6 ± 4.2 ** | 6.4 ± 1.0 ** | 3.2 ± 0.9 ** |
| Compound | Dose mg/kg | Time Spent in Closed Arms; /s/ * | Number of Entries into Closed Arms * | Time Spent in the Center; /s/ * | Time Spent in Open Arms; /s/ * |
|---|---|---|---|---|---|
| Control | − | 278.2 ± 20.0 | 7.0 ± 1.2 | 21.8 ± 4.4 | – |
| 3a | 50 | 243.0 ± 16.9 ** | 4.8 ± 1.3 ** | 15.7 ± 4.2 | 41.3 ± 13.0 ** |
| 3b | 50 | 228.0 ± 15.9 ** | 3.8 ± 1.7 ** | 34.0 ± 7.2 ** | 38.0 ± 8.7 ** |
| 3d | 50 | 241.0 ± 16.9 ** | 3.2 ± 0.7 ** | 30.0 ± 3.1 | 29.0 ± 12.0 ** |
| 3f | 50 | 184.0 ± 21.6 ** | 2.6 ± 0.7 ** | 8.0 ± 1.2 ** | 108.0 ± 22.7 ** |
| 3g | 50 | 230.0 ± 17.0 ** | 3.8 ± 0.9 ** | 26.0 ± 5.6 | 44.0 ± 8.1 ** |
| 3h | 50 | 240.0 ± 11.9 ** | 3.9 ± 0.9 ** | 24.0 ± 5.1 | 36.0 ± 10.2 ** |
| 3i | 50 | 225.0 ± 23.7 ** | 4.6 ± 0.8 ** | 40.0 ± 5.2 ** | 35.0 ± 6.6 ** |
| 4a | 50 | 105.0 ± 15.0 ** | 4.8 ± 0.9 ** | 33.0 ± 5.7 ** | 162.0 ± 12.6 ** |
| 4d | 50 | 248.0 ± 16.1 ** | 4.2 ± 1.0 ** | 20.0 ± 3.5 ** | 32.0 ± 7.7 ** |
| 4f | 50 | 233.0 ± 21.0 ** | 4.7 ± 1.0 ** | 31.0 ± 5.7 ** | 36.0 ± 5.4 ** |
| 4i | 50 | 238.2 ± 17.8 ** | 3.9 ± 1.1 ** | 38.0 ± 5.5 ** | 24.0 ± 5.5 ** |
| Ethosuximide | 200 | 245.2 ± 15.0 | 8.1 ± 2.5 | 54.8 ± 4.7 ** | – |
| Diazepam | 2 | 200.5 ± 15.2 ** | 5.5 ± 1.2 | 42.5 ± 3.9 ** | 57.0 ± 4.2 ** |
| Compound | Dose mg/kg | Latent Period to I Immobilization (s) * | Total Time of Immobilization (s) * | Total Time of Active Swimming (s) * |
|---|---|---|---|---|
| Control | – | 92.0 ± 7.8 | 81.0 ± 8.8 | 279.0 ± 23.3 |
| 3a | 50 | 182.0 ± 15.3 ** | 35.5 ± 8.1 ** | 324.5 ± 20.1 ** |
| 3b | 50 | 195.0 ± 15.2 ** | 17.5 ± 2.7 | 342.0 ± 25.3 ** |
| 3d | 50 | 132.5± 9.7 ** | 57.5 ± 9.7 ** | 302.5 ± 20.1 ** |
| 3f | 50 | 220.0 ± 18.6 ** | 12.5 ± 0.6 ** | 347.5 ± 21.0 ** |
| 3g | 50 | 122.0 ± 15.3 ** | 74.5 ± 21.0 ** | 285.5 ± 23.0 ** |
| 3h | 50 | 167.0 ± 11.8 ** | 28.3 ± 7.8 ** | 331.7 ± 21.0 ** |
| 3i | 50 | 85.0 ± 12.0 | 117.5 ± 4.8 ** | 242.5 ± 18.8 ** |
| 4a | 50 | 59.0 ± 10.6 ** | 178.0 ± 15.5 ** | 181.8 ± 19.9 ** |
| 4d | 50 | 156.0 ± 15.8 ** | 16.7 ± 2.7 ** | 343.3 ± 29.7 ** |
| 4f | 50 | 70.0 ± 8.6 | 20.0 ± 5.7 | 340.0 ± 27.7 ** |
| 4i | 50 | 245.0 ± 26.4 ** | 101.0 ± 11.1 ** | 259.0 ± 28.0 ** |
| Ethosuximide | 200 | 105.0 ± 9.6 | 98.0 ± 9.9 | 262.0 ± 14.4 |
| Diazepam | 2 | 174.0 ± 18.1 ** | 24.0 ± 6.6 ** | 336.0 ± 18.9 ** |
| No. | Est. Binding Energy (kcal/mol) | I-H | Residues Involved in Hydrogen Bond Formation | Hydrophobic Interactions | Positive Ionizable Interactions |
|---|---|---|---|---|---|
| 3a | −7.20 | 1 | Thr202 (N···H, 3.18 Å), | Tyr62, Leu99, Phe200 | Asp43 |
| 3b | −6.96 | 1 | Tyr97 (N···H, 3.23 Å) | Thr176, Phe200, Tyr205 | – |
| 3d | −6.32 | 1 | Thr202 (N···H, 3.75 Å) | Thr176, Phe200, Ala201 | – |
| 3f | −6.40 | – | – | Thr176, Phe200 | – |
| 3g | −6.72 | 1 | Tyr205 (N···H, 2.76 Å) | Tyr62, Thr176, Phe200 | - |
| 3h | −7.72 | 1 | Thr202 (N···H, 3.54 Å) | Tyr62, Thr176, Ala201, Phe200 | - |
| 3i | −8.12 | 1 | Thr176 (N···H, 3.20 Å) | Ala45, Tyr62, Ala201, Thr202 | - |
| 4a | −6.93 | – | – | Tyr62, Thr176, Ala201, Tyr205 | - |
| 4d | −6.22 | – | – | Ty157, Phe200, Tyr205 | - |
| 4f | −6.56 | – | – | Ala201, Tyr205 | - |
| 4i | −8.81 | 2 | Thr176 (N···H, 3.78 Å), Arg180 (N···H, 3.26 Å) | Ala45, Leu99, Val198, Phe200, Ala201, Thr202 | Asp43 |
| Diazepam | −8.90 | 1 | Thr202 (N···H, 2.67 Å) | Tyr62, Leu99, Met115, Tyr157, Phe200, Tyr205 | - |
| No. | Est. Binding Energy (kcal/mol) | I-H | Residues Involved in Hydrogen Bond Formation | Residues Involved in Hydrophobic Interactions | Residues Involved in Aromatic Interactions |
|---|---|---|---|---|---|
| 3a | −4.86 | - | - | - | - |
| 3b | −7.16 | 1 | Arg11 (N···H, 2.57 Å) | Asp267, Gly433 | - |
| 3d | −6.40 | - | - | His7, Arg431 | - |
| 3f | −5.67 | - | - | His7, Ile441 | - |
| 3g | −6.92 | - | - | Asp267, Gly433 | - |
| 3h | −6.45 | - | - | His7, Arg431 | - |
| 3i | −7.48 | 1 | His7 (N···H, 3.25 Å) | Asp267, Gly433 | Lys264 |
| 4a | −5.58 | - | - | Asp267, Arg431 | - |
| 4d | −5.29 | - | - | Asp267, Gly432 | - |
| 4f | −6.26 | - | - | Gly433 | - |
| 4i | −7.28 | 1 | Gly439 (N···H, 2.54 Å) | Ile441 | Arg11 |
| No. | Est. Binding Energy (kcal/mol) | Residues Involved in Hydrogen Bond Formation | Residues Involved in Hydrophobic Interactions | Residues Involved in Positive Ionizable Interactions |
|---|---|---|---|---|
| 3a | −5.80 | - | Thr195, Phe193, Val114, The290 | - |
| 3b | −8.82 | Tyr316 (N···H, 3.76 Å) | Val114, Phe193, Ala200, Phe289, Tyr316, Phe290 | N-Asp113 |
| 3d | −6.92 | - | Ala200, Ile309, Asn312 | - |
| 3f | −5.08 | - | Trp286, Asn312 | - |
| 3g | −5.27 | - | Phe193, Trp313, Phe290 | - |
| 3h | −6.17 | - | Trp109, Val117, Phe193, Thr195, Trp286 | - |
| 3i | −7.08 | Tyr316 (N···H, 3.28 Å) | Trp109, Tyr308, Thr195, Ala200, Asn312 | - |
| 4a | −8.25 | Tyr118 (N···H, 2.74 Å), Tyr316 (N···H, 3.59 Å) | Trp109, Tyr308, Phe193, Val114, Tyr110, Ile201, Ala200 | - |
| 4d | −4.30 | - | Ile309, Thr195, Phe193, Trp313 | - |
| 4f | −7.12 | Tyr316 (N···H, 2.58 Å) | Tyr316, Trp286, Asn312, Ala200 | - |
| 4i | −9.10 | Tyr316 (N···H, 3.04 Å), Tyr316 (N···H, 2.57 Å) | Trp109, Trp313, Ile309, Thr195, Phe193, Val114, The290, Tyr308, Val117, Tyr199, Ala200 | N-Asp113 |
| Alprenolol | −13.19 | Asp113, Asn312, Tyr316 | Val117, Val114, Thr195, Phe193, Tyr199, Phe290 | N-Asp113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirakanyan, S.N.; Hakobyan, E.K.; Geronikaki, A.; Spinelli, D.; Petrou, A.; Kartsev, V.G.; Yegoryan, H.A.; Jughetsyan, H.V.; Manukyan, M.E.; Paronikyan, R.G.; et al. Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines. Pharmaceuticals 2025, 18, 597. https://doi.org/10.3390/ph18040597
Sirakanyan SN, Hakobyan EK, Geronikaki A, Spinelli D, Petrou A, Kartsev VG, Yegoryan HA, Jughetsyan HV, Manukyan ME, Paronikyan RG, et al. Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines. Pharmaceuticals. 2025; 18(4):597. https://doi.org/10.3390/ph18040597
Chicago/Turabian StyleSirakanyan, Samvel N., Elmira K. Hakobyan, Athina Geronikaki, Domenico Spinelli, Anthi Petrou, Victor G. Kartsev, Hasmik A. Yegoryan, Hasmik V. Jughetsyan, Mariam E. Manukyan, Ruzanna G. Paronikyan, and et al. 2025. "Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines" Pharmaceuticals 18, no. 4: 597. https://doi.org/10.3390/ph18040597
APA StyleSirakanyan, S. N., Hakobyan, E. K., Geronikaki, A., Spinelli, D., Petrou, A., Kartsev, V. G., Yegoryan, H. A., Jughetsyan, H. V., Manukyan, M. E., Paronikyan, R. G., Araqelyan, T. A., & Hovakimyan, A. A. (2025). Synthesis and Neurotropic Activity of New 5-Piperazinopyrazolo[3,4-c]-2,7-naphthyridines and Isoxazolo[5,4-c]-2,7-naphthyridines. Pharmaceuticals, 18(4), 597. https://doi.org/10.3390/ph18040597









