Abstract
Background: Inflammatory bowel disease (IBD) is a chronic inflammatory disorder marked by persistent gastrointestinal inflammation and a spectrum of systemic effects, including extraintestinal manifestations (EIMs) that impact the joints, skin, liver, and eyes. Conventional therapies primarily target intestinal inflammation, yet they frequently fail to ameliorate these systemic complications. Recent investigations have highlighted the complex interplay among the immune system, gut, and nervous system in IBD pathogenesis, thereby underscoring the need for innovative therapeutic approaches. Methods: We conducted a comprehensive literature search using databases such as PubMed, Scopus, Web of Science, Science Direct, and Google Scholar. Keywords including “cannabinoids”, “endocannabinoid system”, “endocannabinoidome”, “inflammatory bowel disease”, and “extraintestinal manifestations” were used to identify peer-reviewed original research and review articles that explore the role of the endocannabinoidome (eCBome) in IBD. Results: Emerging evidence suggests that eCBome—a network comprising lipid mediators, receptors (e.g., CB1, CB2, GPR55, GPR35, PPARα, TRPV1), and metabolic enzymes—plays a critical role in modulating immune responses, maintaining gut barrier integrity, and regulating systemic inflammation. Targeting eCBome not only improves intestinal inflammation but also appears to mitigate metabolic, neurological, and extraintestinal complications such as arthritis, liver dysfunction, and dermatological disorders. Conclusions: Modulation of eCBome represents a promising strategy for comprehensive IBD management by addressing both local and systemic disease components. These findings advocate for further mechanistic studies to develop targeted interventions that leverage eCBome as a novel therapeutic avenue in IBD.
1. Introduction
Inflammatory bowel disease (IBD), including ulcerative colitis and Crohn’s disease, affects millions of people with rising prevalence globally [1]. While the exact etiology of IBD is unclear, it is reported to be driven by intricate crosstalk between genetic predisposition, environmental triggers, gut microbial imbalances, and immune dysfunction [2,3,4,5,6]. Despite advancements in IBD therapy, significant gaps remain, including the management of extraintestinal manifestations (EIMs), metabolic dysfunction, and quality of life concerns. Although the primary symptoms are localized in the gut, IBD often exerts systemic effects, manifesting in a variety of extraintestinal complications such as arthritis, uveitis, osteoporosis, liver disorders, skin disorders, anxiety, and depression [7,8]. These EIMs are conditions occurring outside the gastrointestinal tract yet are closely linked to IBD, significantly adding to the disease burden and impacting patients’ quality of life. They may emerge alongside active intestinal symptoms or develop independently, and in some cases, precede the onset of IBD diagnosis. The presence of EIMs not only complicates disease management but also leads to increased healthcare demands [9,10]. As novel therapeutic strategies continue to be investigated, the endocannabinoidome (eCBome) emerges as a multifaceted therapeutic target for addressing both intestinal symptoms and extraintestinal manifestations of IBD [11,12,13].
The endocannabinoidome is an expanded network of bioactive lipids, receptors, and metabolic enzymes that regulate diverse physiological functions [13]. Core components of eCBome include the canonical cannabinoid receptors CB1 and CB2, key endocannabinoids (eCBs) such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG), and their metabolizing enzymes, fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) [14,15]. CB1 receptors are primarily expressed in the central nervous system and gut epithelium, while CB2 receptors are predominant in immune cells [16,17,18,19]. Endocannabinoids are bioactive lipids that are synthesized on demand by the host in response to physiological stimuli such as neuronal activity, stress, or immune signaling. Factors such as stress, infections, inflammation, and dietary intake can influence endocannabinoid tone and signaling, thereby affecting physiological and immunological functions. For example, acute stress can transiently elevate endocannabinoid levels to restore homeostasis, while chronic stress may lead to dysregulation, contributing to pathological conditions [20]. Beyond the classical endocannabinoids, eCBome includes lipid mediators such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA), which play significant roles in modulating immune responses, maintaining gut barrier integrity, influencing gastrointestinal motility, and regulating energy homeostasis. For instance, OEA was shown to reduce intestinal motility and enhance gut barrier function [21], while PEA exhibits anti-inflammatory properties in gastrointestinal disorders [22]. In addition to endocannabinoids and lipid mediators, certain plant-derived cannabinoids and dietary lipids can influence eCBome. For instance, fatty acids from foods such as fish, nuts, and seeds serve as precursors for endocannabinoid synthesis, while phytocannabinoids (active constituents of cannabis plant) can modulate eCBome through different receptors. We have reported the use of cannabis and different phytocannabinoids such as tetrahydrocannabinol (THC), cannabidiol (CBD), tetrahydrocannabivarin (THCV), and cannabigerol (CBG) in modulation of IBD in our previous papers [23,24,25].
eCBome is highly dynamic and responds to various external and internal stimuli. Factors such as stress, infections, inflammation, and dietary intake can influence endocannabinoid tone and signaling, thereby affecting physiological and immunological functions. For example, acute stress can transiently elevate endocannabinoid levels to restore homeostasis, while chronic stress may lead to dysregulation, contributing to pathological conditions. Disruptions in eCBome signaling have been increasingly implicated in the pathogenesis of IBD, highlighting its potential as a novel therapeutic target [26,27]. This review explores the role of eCBome in managing both intestinal and EIMs of IBD.
2. Extraintestinal Complications of IBD: A Role for eCBome
Current therapies for IBD mainly target intestinal symptoms but often fail to manage its EIMs. Extraintestinal manifestations of IBD such as arthritis, uveitis, hepatobiliary disorders, and central nervous system disorders such as anxiety and depressions significantly impact morbidity. Extraintestinal manifestations often present alongside gastrointestinal symptoms but can also arise independently, adding complexity to disease management. Figure 1 provides a summary of the intestinal and extraintestinal manifestations of IBD. While current therapies mainly target GI-related disorders, eCBome could offer a novel therapeutic target for managing intestinal as well as EIMs. Unlike conventional therapies that predominantly focus on intestinal inflammation, eCBome modulation offers a more comprehensive approach by addressing both local and systemic manifestations of the disease. Therefore, targeting eCBome has the potential to not only reduce intestinal inflammation but also mitigate EIMs such as arthritis, metabolic dysfunction, and neurological complications associated with IBD. This broader therapeutic impact underscores translational potential. Table 1 outlines the various types of EIMs associated with IBD and highlights the potential benefits of eCBome modulation in their management.
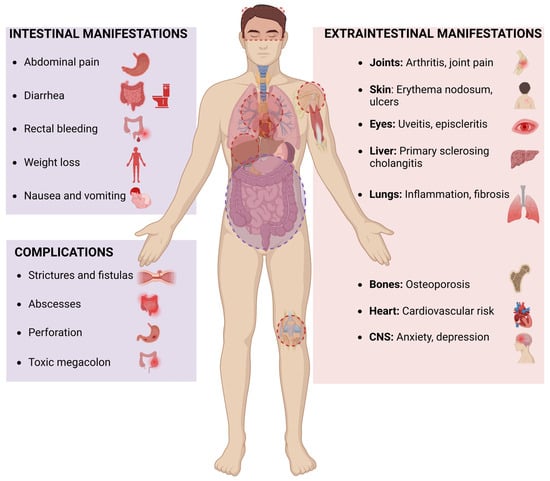
Figure 1.
Intestinal and extraintestinal manifestations of inflammatory bowel disease.

Table 1.
Benefits role of endocannabinoidome modulation in extraintestinal manifestations of IBD.
3. Current Therapies in IBD: Challenges and Limitations
Despite advancements in IBD treatment, current therapies have several limitations, including incomplete response rates, loss of efficacy over time, significant adverse effects, and a failure to address EIMs of IBD, as discussed below.
3.1. Limited Long-Term Efficacy and Loss of Response
Many patients initially respond to conventional treatments such as aminosalicylates (e.g., mesalamine, sulfasalazine), corticosteroids (e.g., prednisone, budesonide), immunomodulators (e.g., azathioprine, methotrexate, cyclosporine), and biologics (e.g., TNF inhibitors like infliximab and adalimumab). However, up to 40% of patients experience primary non-response, and an additional 30–50% lose response over time, requiring dose escalation or switching to alternative therapies [114,115].
3.2. Adverse Effects and Safety Concerns
Corticosteroids, though effective for short-term symptom control, are associated with serious long-term complications, including osteoporosis, hyperglycemia, hypertension, and increased infection risk [103,104]. Immunosuppressants such as azathioprine, 6-mercaptopurine, methotrexate, cyclosporine carry risks of hepatotoxicity, myelosuppression, pancreatitis, and opportunistic infections [116,117,118]. Small-molecule inhibitors (tofacitinib) and biologic therapies such as anti-TNF, anti-integrin, and anti-IL-12/23 can increase susceptibility to infections (e.g., tuberculosis, opportunistic fungal infections) and, in rare cases, lead to the development of lymphomas or demyelinating disorders [114,119].
3.3. High Treatment Costs and Accessibility Issues
Biologic therapies and small-molecule inhibitors are expensive, limiting access in many regions of the world. The high cost burden can lead to treatment discontinuation, inadequate disease control, and increased hospitalization rates [114,119].
3.4. Surgical Interventions
Due to limitations with medical treatments and disease complications, surgical interventions remain a common intervention for 30–50% of Crohn’s disease patients, while 20–30% of ulcerative colitis patients undergo colectomy. However, infection and post-surgical recurrence in Crohn’s disease remains a significant challenge, often requiring lifelong medical therapy [120,121,122].
3.5. Extraintestinal Manifestations and Unmet Needs
Up to 47% of IBD patients experience extraintestinal manifestations (EIMs) affecting the joints, skin, liver, and eyes, along with symptoms such as fatigue, mental health disorders (including anxiety and depression), and dysbiosis—all of which significantly impair quality of life. Notably, EIMs can develop in up to 24% of patients before the onset of intestinal symptoms, underscoring the importance of early recognition for timely diagnosis and intervention [10,123,124]. These multifaceted complications highlight the need for novel, holistic therapeutic strategies that extend beyond conventional treatments. Current pharmacological interventions, including aminosalicylates, corticosteroids, immunosuppressants, and biologics, predominantly target inflammation within the gastrointestinal tract but often fail to address the EIMs, including systemic immune and metabolic dysregulation, associated with IBD. In contrast, eCBome modulation has demonstrated promise in regulating systemic inflammation, improving metabolic homeostasis, and restoring gut barrier integrity, making it a unique and promising therapeutic avenue. An overview of the current therapeutic landscape in IBD management, their mechanism of action, and the complexities associated with each treatment modality are highlighted below (Table 2).

Table 2.
Current IBD therapies and challenges.
In summary, current pharmacological interventions, including aminosalicylates, corticosteroids, immunosuppressants, and biologics, primarily target inflammation within the gastrointestinal tract but fail to comprehensively manage the systemic immune and metabolic dysregulation observed in IBD [135]. For example, biologics such as TNF-α inhibitors have shown limited effectiveness in addressing metabolic alterations, including insulin resistance and lipid dysregulation, which are frequently observed in IBD patients. Furthermore, these treatments do not significantly impact the gut–brain axis, which is increasingly recognized as a key player in IBD pathology. In contrast, eCBome modulation has demonstrated promise in regulating systemic inflammation, improving metabolic homeostasis, and restoring gut barrier integrity, making it a unique and promising therapeutic avenue [13,26].
4. The Endocannabinoidome: Components and Functions
The discovery of tetrahydrocannabinol (THC), the primary psychoactive compound in cannabis, led to the identification of the endocannabinoid system (ECS) in the early 1990s. Initially, the ECS was defined as an endogenous lipid signaling system composed of three primary components: (1) cannabinoid receptors (CB1 and CB2), (2) endogenous ligands, AEA and 2-AG, and (3) metabolic enzymes involved in their synthesis (NAPE-PLD, DAGL) and degradation (FAAH, MAGL) [14].
The ECS was originally associated with neurotransmission, pain modulation, appetite regulation, and immune responses. However, as research advanced, it became evident that additional lipid mediators, receptors, and metabolic enzymes interact with these classical components. This led to the recognition of a broader signaling network, termed the endocannabinoidome [136]. eCBome includes lipid-derived mediators synthesized from membrane phospholipids through enzymatic pathways similar to those producing classical endocannabinoids like AEA and 2-AG. To be classified within this system, these molecules must interact with cannabinoid receptors (CB1 and CB2) or functionally related receptors such as PPARs (peroxisome proliferator-activated receptors), TRPV1 (transient receptor potential vanilloid 1), and G-protein coupled receptors (GPR35, GPR55, GPR119). They must also undergo metabolic processes regulated by enzymes such as NAPE-PLD, DAGL, FAAH, and MAGL and influence physiological processes, including inflammation, metabolism, pain modulation, and gut barrier integrity [13,23,27]
For example, although compounds like oleoylethanolamide and palmitoylethanolamide do not bind to CB1 or CB2 receptors, they activate PPARα to regulate lipid metabolism, inflammation, and energy homeostasis [137]. Similarly, TRPV1 is activated by AEA and other bioactive lipids, regulating nociception and inflammatory pathways [138]. Figure 2 provides a visual summary of the inclusion criteria and boundaries of eCBome, illustrating how its components interconnect to modulate key physiological responses.
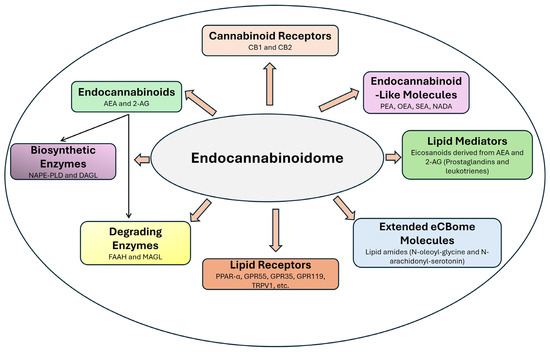
Figure 2.
Components of endocannabinoidome. CB1—cannabinoid 1; CB2—cannabinoid 2; AEA—anandamide; 2-AG—2 arachidonylglycerol; NAPE-PLD—N-Acyl-phosphatidylethanolamine phospholipase D; DAGL—diacylglycerol lipase; FAAH—fatty acid amide hydrolase; MAGL—monoacylglycerol lipase; PPAR-α—peroxisome proliferator-activated receptor-alpha; GPR55—G protein coupled receptor 55; GPR35—G protein coupled receptor 55; GPR119—G protein coupled receptor 55; TRPV1—transient receptor potential vanilloid 1; PEA—palmitoylethanolamide; OEA—oleoylethanolamide; SEA—Stearoylethanolamide; NADA—N-arachidonoyl dopamine.
This expanded conceptualization of the ECS—eCBome—offers new insights into therapeutic targets for various pathological conditions, including inflammatory bowel disease. Dysregulation of eCBome was implicated in IBD pathogenesis, affecting epithelial barrier integrity, cytokine production, and immune cell activation. A comprehensive evaluation of eCBome alterations in IBD is performed through biochemical, molecular, and functional assays such as lipidomic profiling (using LC-MS/MS), qPCR, Western blotting, immunohistochemistry, and receptor binding studies [16]. One key approach is lipidomic profiling, which quantifies endocannabinoids (e.g., AEA, 2-AG) and related lipid mediators (e.g., OEA, PEA) in tissues, blood, or stool samples from IBD patients using high-resolution liquid chromatography–tandem mass spectrometry (LC-MS/MS). Additionally, qPCR, Western blotting, and immunohistochemistry can evaluate gene and protein expression of key eCBome components, including CB1, CB2, PPARα, and TRPV1, and metabolic enzymes (FAAH, MAGL, DAGL, NAPE-PLD). Functional assays, such as receptor binding studies and downstream signaling assessments (e.g., ERK or AKT phosphorylation), provide further insight into the functional consequences of eCBome dysregulation. Integrating these approaches with clinical markers, histopathological findings, and disease severity assessments enhances our understanding of eCBome alterations in IBD [14,20]. Studies have reported elevated levels of AEA and 2-AG, reduced CB1/CB2 expression, and altered activity of metabolic enzymes in inflamed colonic tissues, supporting the role of eCBome dysregulation in IBD [16,139,140]. Additionally, lower levels of PEA and OEA in IBD further highlight the importance of these non-canonical mediators and underscore the therapeutic potential of targeting eCBome [141]. Dietary interventions enriched with PEA and OEA have shown therapeutic benefits in IBD [21,22]. Overall, this historical background and definition establish clear boundaries for eCBome, providing a framework for understanding the inclusion of molecules such as PPARα and TRPV1, and underscoring their relevance as potential therapeutic targets in IBD.
A range of ethnomedicinal, preclinical, and clinical studies suggest that targeting eCBome with phytocannabinoids (e.g., cannabidiol, THC), synthetic cannabinoids (e.g., HU308), or enzyme inhibitors (e.g., JZL195) may reduce colonic inflammation, alleviate symptoms, and promote mucosal healing [23,24,25,139,142]. However, despite promising findings, further clinical trials are needed to optimize dosing, delivery methods, and long-term safety, positioning eCBome as a novel therapeutic target for IBD.
5. Metabolic Dysregulation in IBD
Metabolic disorders are among the most commonly reported EIMs of IBD, significantly impacting morbidity and overall patient health [143]. These disturbances often arise from chronic systemic inflammation, nutritional deficiencies, altered gut microbiota, and the side effects of medications. Below, we detail the key metabolic complications in IBD along with their underlying mechanisms.
5.1. Dyslipidemia
IBD patients frequently exhibit altered lipid profiles, characterized by decreased high-density lipoprotein (HDL) and increased triglycerides and low-density lipoproteins (LDL) [144]. Chronic inflammation in IBD elevates pro-inflammatory cytokines (e.g., IL-6, TNF-α) that disrupt normal lipid metabolism. These cytokines promote lipid peroxidation and alter hepatic lipid clearance. The imbalance in lipid processing contributes to the development of a pro-atherogenic profile, thus increasing the risk of cardiovascular diseases [145,146,147].
5.2. Insulin Resistance and Diabetes
Systemic inflammation and corticosteroid use in IBD can impair glucose metabolism, leading to insulin resistance and an increased risk of diabetes [148]. Pro-inflammatory cytokines such as TNF-α interfere with insulin receptor signaling by promoting serine phosphorylation of insulin receptor substrates, thereby reducing insulin sensitivity. Additionally, corticosteroids enhance gluconeogenesis, further exacerbating insulin resistance. This creates a vicious cycle where impaired insulin signaling contributes to sustained inflammation and metabolic dysregulation [149].
5.3. Bone Metabolism Abnormalities
Osteoporosis and osteopenia are common in IBD, largely due to malabsorption of calcium and vitamin D, chronic inflammation, and prolonged corticosteroid use [150]. Inflammatory cytokines, notably IL-6 and TNF-α, stimulate osteoclast differentiation and activation, leading to increased bone resorption and reduced bone mineral density. Corticosteroids further impair osteoblast function and contribute to calcium loss, collectively increasing the risk of fractures and compromising skeletal integrity [151].
5.4. Body Composition Changes
Conditions such as cachexia and sarcopenia are frequently observed in IBD patients. Additionally, fat redistribution—especially central obesity—can negatively impact functional capacity and disease prognosis [73,152]. Systemic inflammation, driven by cytokines like TNF-α, IL-1, and IL-6, accelerates muscle protein degradation while inhibiting muscle protein synthesis, leading to sarcopenia and cachexia. Hormonal imbalances and adipose tissue inflammation further contribute to the redistribution of fat mass, often resulting in central obesity despite overall weight loss.
5.5. Gut Microbiota and Metabolic Alterations
Gut microbiota and metabolomic alterations play a crucial role in IBD-related metabolic disturbances [153]. Dysbiosis, characterized by reduced microbial diversity and altered metabolomic profiles, is a hallmark of IBD and plays a crucial role in metabolic disturbances [154]. Alterations in the gut microbiota led to decreased production of beneficial short-chain fatty acids (SCFAs), such as butyrate, which normally have anti-inflammatory effects and support energy homeostasis. Disruption in bile acid metabolism also contributes to impaired lipid absorption and energy regulation, further exacerbating systemic metabolic imbalances [21,155].
6. Modulatory Role of eCBome in Metabolic Dysregulation
eCBome plays a pivotal role in regulating energy balance, lipid metabolism, insulin sensitivity, and gut–brain signaling. Its dysregulation has been linked to obesity, type 2 diabetes, and metabolic syndrome—conditions that frequently co-exist with IBD and worsen disease outcomes [15,27,153,156]. Endocannabinoids such as anandamide and 2-arachidonoylglycerol signal through cannabinoid receptors (CB1 and CB2), as well as TRP channels and PPARs. In IBD, these molecules are dysregulated in both the intestinal mucosa and systemic circulation [11,112,157,158]. Activation of CB2 receptors on immune by endocannabinoids reduces cytokine production and inhibits macrophage activation, thereby mitigating inflammation [159,160]. CB1 receptor activity in adipose tissue and liver regulates lipid storage and oxidation. Targeting peripheral CB1 receptors may help ameliorate dyslipidemia while avoiding central side effects [161,162,163]. Through interactions with CB1 and PPARγ pathways, endocannabinoids regulate insulin sensitivity and glucose uptake. Inhibition of CB1 receptors has been shown to improve insulin resistance in IBD [153,164,165]. CB2 receptor activation is reported to inhibit osteoclastogenesis, promoting bone preservation. This pathway offers therapeutic potential for IBD-associated osteoporosis [166]. Altered gut microbiota is shown to affect eCBome tone, affecting metabolite production and intestinal barrier integrity. Restoring balance in this axis may help mitigate metabolic dysregulation [153]. Endocannabinoid-like molecules such as OEA and PEA further support metabolic regulation. OEA promotes GLP-1 release, enhances fat oxidation, and reduces food intake—primarily through GPR119—while PEA exerts anti-inflammatory and gut-protective effects [21,22,79,141,167]. In high-fat diet-induced obese mice, treatment with synthetic GPR119 agonists such as ps297 and ps3188, in combination with sitagliptin—a DPP-IV inhibitor—has been shown to retard body weight gain. This combination therapy also improved glycaemic control and enhanced hepatic health, as evidenced by improved GLP-1 regulation, better liver histopathological scores, and modulation of metabolic hormones [79,80]. In addition to endogenously produced endocannabinoids and endocannabinoid-like molecules, several exogenous compounds including diet, synthetic and phytocannabinoids play a pivotal role in regulation of metabolic processes and pathogenesis of metabolic dysregulation as discussed previously [13,168,169,170]
Together, these insights underscore the critical role of metabolic dysregulation in IBD pathogenesis and highlight the potential of eCBome-targeted therapies to address these complex systemic disturbances.
7. Role of GLP-1 and eCBome in Metabolic Dysregulation in IBD
GLP-1 is an incretin hormone secreted by intestinal L-cells in response to food intake and plays a pivotal role in glucose homeostasis, lipid metabolism, and energy regulation [171,172,173]. Despite its influence on metabolism and inflammation, GLP-1 is not considered part of eCBome because eCBome is defined as a network of lipid-derived mediators, receptors, and metabolic enzymes that interact with cannabinoid receptors (CB1, CB2) or related receptors (e.g., PPARs, TRPV1, GPR55) [136,174]. Instead, GLP-1 is a peptide hormone derived from proglucagon and acts on GLP-1 receptor (GLP-1R), a GPCR unrelated to ECS signaling [172]. Furthermore, the biosynthesis and degradation pathways differ markedly between these systems. Endocannabinoidome molecules are synthesized from membrane phospholipids and are metabolized by enzymes such as FAAH and MAGL, whereas GLP-1 is produced by intestinal L-cells and rapidly degraded by DPP-IV [175]. Although GLP-1 signaling modulates ECS activity, such as reducing CB1 overactivation, enhancing CB2-mediated anti-inflammatory effects, and modulating gut microbiota, it only exhibits crosstalk with the ECS rather than being a component of eCBome [176,177].
Growing evidence suggests a complex interplay between GLP-1 and eCBome in regulating metabolism, gut homeostasis, and inflammation, making them promising therapeutic targets for IBD-associated metabolic disorders [173,178,179,180]. Dual modulation of GLP-1 and ECS, for example, combining GLP-1 receptor agonists (such as liraglutide or semaglutide) with CB1 antagonists may enhance insulin sensitivity, reduce fat accumulation, and control inflammation. Additionally, GLP-1 analogs help restore gut barrier integrity, complementing the ECS’s function in regulating intestinal permeability and inflammation. Additionally, CB2 receptor activation, using selective agonists like HU308, could complement GLP-1 therapy by reducing systemic inflammation, metabolic dysregulation and maintaining gut homeostasis [24]. Furthermore, endocannabinoid-like molecules such as PEA and OEA are shown to enhance GLP-1 potency, providing further metabolic benefits and improving overall therapeutic outcomes [181]. Table 3 summarizes key studies on the interaction between GLP-1 and eCBome in metabolic dysregulation in IBD.

Table 3.
Interaction between glucagonlike peptide-1 and the endocannabinoidome in metabolic dysregulation in inflammatory bowel disease.
In summary, the interplay between GLP-1 and eCBome plays a crucial role in regulating metabolic dysregulation in IBD. Targeting this axis may not only alleviate metabolic complications but also mitigate inflammation and promote gut health, offering a promising therapeutic approach for IBD management. Further clinical studies are needed to explore the synergistic effects of GLP-1 and ECS-targeted therapies in treating metabolic disorders associated with IBD.
8. Beyond the Gut: Future Perspectives on eCBome Modulation in IBD
The complexity of IBD extends far beyond gut inflammation, with extraintestinal manifestations significantly contributing to disease burden and reducing patients’ quality of life. Conventional therapies primarily focus on intestinal inflammation, often overlooking the systemic effects of IBD. The endocannabinoidome, an intricate network of lipid mediators, receptors, and enzymes, has emerged as a promising target for modulating both intestinal and extraintestinal complications of IBD. Recent evidence suggests that eCBome regulation influences key physiological processes, including immune responses, metabolism, and neuroinflammation, positioning it as a potential therapeutic avenue for holistic disease management. CB1 and CB2 receptors, two key components of eCBome, are shown to be highly dysregulated in IBD. Use of selective/peripheral CB1 antagonists and CB2 agonists could be employed as a promising drug candidate for addressing specific metabolic disturbances without central side effects. Our previous studies using DSS-induced acute and chronic colitis murine models demonstrated that activation of the CB2 receptor with the selective agonist HU308, and activation of the CB1 receptor using a sub-therapeutic dose of THC in combination with the CB1 allosteric modulator ZCZ011 significantly improved both colonic and systemic markers of IBD [24,25]. These included reductions in diarrhea, rectal bleeding, body weight loss, colon histopathological scores, organ dysfunction (liver, kidney, and spleen), and pain-related behaviors.
Most importantly, both treatments restored systemic GLP-1 and ammonia levels to those observed in healthy controls, underscoring their potential role in mitigating systemic complications of IBD. Elevated systemic ammonia levels have been linked to central nervous system (CNS) disorders, including Alzheimer’s disease. Notably, IBD patients frequently experience CNS-related symptoms such as anxiety and depression, which may be influenced by ammonia dysregulation [190,191]. Investigating the role of ammonia regulation in IBD patients could provide valuable insights into the gut–brain axis and its implications for disease management.
Diet and nutrition also play a significant role in etiopathogenesis of IBD. While triggers may vary from person to person, a Western-style diet rich in processed foods, unhealthy fats (trans fats), sugars, and additives generally worsens IBD [192]. Moreover, diet is pivotal in modulating both the gut microbiome and gut–brain axis. Unhealthy dietary patterns often linked to microbial dysbiosis; a condition characterized by an altered balance in the gut microbiota. This dysbiosis is well-documented in IBD and is associated with increased intestinal permeability, immune dysregulation, and chronic dietary interventions rich in fiber, polyphenols, and omega-3 fatty acids have shown promise in modulating the gut microbiota and reducing inflammation [22,193]. For example, beta-caryophyllene, a terpene abundant in cannabis and also considered a dietary cannabinoid, has been demonstrated to reduce DSS-induced colitis in mice through CB2 and PPAR-α activation [194,195] and has also shown beneficial effects in diabetes [185,186].
Probiotic supplementation has gained attention as an adjunct therapy for colitis due to its ability to restore microbial balance and enhance intestinal barrier integrity. Clinical studies suggest that probiotics such as Escherichia coli Nissle 1917 and multi-strain formulations like VSL#3 can induce remission and maintain mucosal healing in IBD patients [196,197]. Mechanistically, probiotics modulate the immune response by increasing short-chain fatty acid production, promoting regulatory T cell responses, and suppressing pro-inflammatory cytokine secretion [198,199]. Additionally, through modulation of microbiome, probiotics can also positively influence eCBome tone in IBD [200]. Integrating cannabinoid-targeted therapies with existing dietary, anti-inflammatory, and metabolic interventions could enhance therapeutic outcomes.
However, while promising, the efficacy of probiotic remains strain-dependent, and further clinical trials are needed to establish optimal formulations and long-term benefits. A personalized probiotic regimen, tailored to individual patient profiles and combined with standard pharmacotherapy, may offer significant benefits in managing both the intestinal and EIMs of IBD. Furthermore, emerging evidence suggests that other components of eCBome, such as GPR35 and GPR55, are dysregulated in IBD. The activation of GPR35 by olsalazine, an aminosalicylate used in IBD therapy, appears to play a role in preventing colitis [201,202].
While eCBome represents a promising therapeutic avenue for IBD, Table 4 outlines key challenges in its clinical translation and proposes potential solutions for future research.

Table 4.
Challenges and potential solutions for modulating endocannabinoidome in inflammatory bowel disease.
9. Conclusions
Extraintestinal manifestations of IBD pose a significant and diverse array of clinical challenges, significantly impacting patients’ lives and healthcare utilization. While conventional therapies primarily target gut inflammation, the endocannabinoidome emerges as a promising and versatile target for managing inflammatory, metabolic, and extraintestinal complications of IBD. Preliminary evidence highlights its therapeutic potential, but further research is essential to optimize clinical applications and ensure safety.
A multidisciplinary approach is crucial to delivering holistic care, addressing not only gut inflammation but also improving overall quality of life and reducing the burden of EIMs. Mechanistic investigations of eCBome signaling at the cellular level, particularly its downstream pathways, such as MAPK/ERK and PI3K/AKT, could provide deeper insights for designing effective targeted therapies. By addressing these unmet needs, eCBome modulation has the potential to transform IBD management and significantly enhance patient outcomes.
Author Contributions
Conceptualization, D.T.; methodology, D.T. and A.G.; validation, D.T., L.N.W. and R.C.; investigation, D.T. and A.G.; writing—original draft preparation, D.T. and A.G.; writing—review and editing, D.T., L.N.W. and R.C.; supervision, R.C. and L.N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude for the infrastructure and support provided by the Curtin Medical Research Institute and Curtin Medical School at Curtin University.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 2-AG | 2-Arachidonoylglycerol |
| AEA | Anandamide |
| ASA | Aminosalicylates |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CBD | Cannabidiol |
| DAGL | Diacylglycerol lipase |
| ECS | Endocannabinoid system |
| eCBome | Endocannabinoidome |
| EIMs | Extraintestinal manifestations |
| FAAH | Fatty acid amide hydrolase |
| GLP-1 | Glucagon-Like Peptide-1 |
| GLP-1R | Glucagon-Like Peptide-1 Receptor |
| GPR35 | G Protein-Coupled Receptor 35 |
| GPR55 | G Protein-Coupled Receptor 55 |
| GPR119 | G Protein-Coupled Receptor 119 |
| HDL | High-density lipoprotein |
| IBD | Inflammatory bowel disease |
| IL-6 | Interleukin-6 |
| IL-12 | Interleukin-12 |
| IL-23 | Interleukin-23 |
| JAK | Janus kinase |
| LDL | Low-density lipoprotein |
| MAGL | Monoacylglycerol lipase |
| NADA | N-arachidonoyl dopamine |
| NAPE-PLD | N-Acyl-phosphatidylethanolamine phospholipase D |
| OEA | Oleoylethanolamide |
| PEA | Palmitoylethanolamide |
| PPAR-α | Peroxisome proliferator-activated receptor alpha |
| SCFAs | Short-chain fatty acids |
| SEA | Stearoylethanolamide |
| THC | Tetrahydrocannabinol |
| TNF-α | Tumor Necrosis Factor-Alpha |
| TRPV1 | Transient receptor potential vanilloid 1 |
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The IBD Interactome: An Integrated View of Aetiology, Pathogenesis and Therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental Triggers in IBD: A Review of Progress and Evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut Microbiota and IBD: Causation or Correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef]
- Goethel, A.; Croitoru, K.; Philpott, D.J. The Interplay between Microbes and the Immune Response in Inflammatory Bowel Disease. J. Physiol. 2018, 596, 3869–3882. [Google Scholar] [CrossRef]
- Argollo, M.; Gilardi, D.; Peyrin-Biroulet, C.; Chabot, J.-F.; Peyrin-Biroulet, L.; Danese, S. Comorbidities in Inflammatory Bowel Disease: A Call for Action. Lancet Gastroenterol. Hepatol. 2019, 4, 643–654. [Google Scholar] [CrossRef]
- Goyal, M.K.; Kalra, S.; Rao, A.; Khubber, M.; Gupta, A.; Vuthaluru, A.R. Beyond the Gut: Exploring Neurological Manifestations in Inflammatory Bowel Disease. Brain Heart 2024, 2, 3486. [Google Scholar] [CrossRef]
- Barreiro-de Acosta, M.; Molero, A.; Artime, E.; Díaz-Cerezo, S.; Lizán, L.; De Paz, H.D.; Martín-Arranz, M.D. Epidemiological, Clinical, Patient-Reported and Economic Burden of Inflammatory Bowel Disease (Ulcerative Colitis and Crohn’s Disease) in Spain: A Systematic Review. Adv. Ther. 2023, 40, 1975–2014. [Google Scholar] [CrossRef]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Alhouayek, M.; Muccioli, G.G. The Endocannabinoid System in Inflammatory Bowel Diseases: From Pathophysiology to Therapeutic Opportunity. Trends Mol. Med. 2012, 18, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ambwani, S.R.; Singh, S. Endocannabinoid system: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Casari, I.; Falasca, M. Modulatory Role of the Endocannabinoidome in the Pathophysiology of the Gastrointestinal Tract. Pharmacol. Res. 2022, 175, 106025. [Google Scholar] [CrossRef] [PubMed]
- Marzo, V.D.; Bifulco, M.; Petrocellis, L.D. The Endocannabinoid System and Its Therapeutic Exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Grill, M.; Högenauer, C.; Blesl, A.; Haybaeck, J.; Golob-Schwarzl, N.; Ferreirós, N.; Thomas, D.; Gurke, R.; Trötzmüller, M.; Köfeler, H.C.; et al. Members of the Endocannabinoid System Are Distinctly Regulated in Inflammatory Bowel Disease and Colorectal Cancer. Sci. Rep. 2019, 9, 2358. [Google Scholar] [CrossRef]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid Receptors as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef]
- Di Marzo, V.; Petrosino, S. Endocannabinoids and the Regulation of Their Levels in Health and Disease. Curr. Opin. Lipidol. 2007, 18, 129–140. [Google Scholar] [CrossRef]
- De Filippo, C.; Costa, A.; Becagli, M.V.; Monroy, M.M.; Provensi, G.; Passani, M.B. Gut Microbiota and Oleoylethanolamide in the Regulation of Intestinal Homeostasis. Front. Endocrinol. 2023, 14, 1135157. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a Naturally Occurring Lipid, Is an Orally Effective Intestinal Anti-Inflammatory Agent: Palmitoylethanolamide and Colitis. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Warne, L.N.; Falasca, M. Pharmacohistory of Cannabis Use-A New Possibility in Future Drug Development for Gastrointestinal Diseases. Int. J. Mol. Sci. 2023, 24, 14677. [Google Scholar] [CrossRef]
- Thapa, D.; Patil, M.; Warne, L.N.; Carlessi, R.; Falasca, M. Comprehensive Assessment of Cannabidiol and HU308 in Acute and Chronic Colitis Models: Efficacy, Safety, and Mechanistic Innovations. Cells 2024, 13, 2013. [Google Scholar] [CrossRef]
- Thapa, D.; Patil, M.; Warne, L.N.; Carlessi, R.; Falasca, M. Enhancing Tetrahydrocannabinol’s Therapeutic Efficacy in Inflammatory Bowel Disease: The Roles of Cannabidiol and the Cannabinoid 1 Receptor Allosteric Modulator ZCZ011. Pharmaceuticals 2025, 18, 148. [Google Scholar] [CrossRef]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Veilleux, A.; Di Marzo, V.; Silvestri, C. The Expanded Endocannabinoid System/Endocannabinoidome as a Potential Target for Treating Diabetes Mellitus. Curr. Diabetes Rep. 2019, 19, 117. [Google Scholar] [CrossRef]
- Ali, T.; Lam, D.; Bronze, M.S.; Humphrey, M.B. Osteoporosis in Inflammatory Bowel Disease. Am. J. Med. 2009, 122, 599–604. [Google Scholar] [CrossRef]
- Bourikas, L.A.; Papadakis, K.A. Musculoskeletal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2009, 15, 1915–1924. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Bakshi, A.K.; Borum, M.L. The Frequency of Osteoporosis Screening in Men With Inflammatory Bowel Disease. Am. J. Men’s Health 2010, 4, 71–74. [Google Scholar] [CrossRef]
- Targownik, L.E.; Bernstein, C.N.; Nugent, Z.; Leslie, W.D. Inflammatory Bowel Disease Has a Small Effect on Bone Mineral Density and Risk for Osteoporosis. Clin. Gastroenterol. Hepatol. 2013, 11, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.T.; McDougall, J.J. Combatting Joint Pain and Inflammation by Dual Inhibition of Monoacylglycerol Lipase and Cyclooxygenase-2 in a Rat Model of Osteoarthritis. Arthritis Res. Ther. 2020, 22, 9. [Google Scholar] [CrossRef]
- O’Brien, M.; McDougall, J.J. Cannabis and Joints: Scientific Evidence for the Alleviation of Osteoarthritis Pain by Cannabinoids. Curr. Opin. Pharmacol. 2018, 40, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Frane, N.; Stapleton, E.; Iturriaga, C.; Ganz, M.; Rasquinha, V.; Duarte, R. Cannabidiol as a Treatment for Arthritis and Joint Pain: An Exploratory Cross-Sectional Study. J. Cannabis Res. 2022, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Paland, N.; Hamza, H.; Pechkovsky, A.; Aswad, M.; Shagidov, D.; Louria-Hayon, I. Cannabis and Rheumatoid Arthritis: A Scoping Review Evaluating the Benefits, Risks, and Future Research Directions. Rambam Maimonides Med. J. 2023, 14, e0022. [Google Scholar] [CrossRef]
- Hu, S.; Cheng, G.; Chen, G.; Zhou, H.; Zhang, Q.; Zhao, Q.; Lian, C.; Zhao, Z.; Zhang, Q.; Han, T.; et al. Cannabinoid Receptors Type 2: Function and Development in Agonist Discovery from Synthetic and Natural Sources with Applications for the Therapy of Osteoporosis. Arab. J. Chem. 2024, 17, 105536. [Google Scholar] [CrossRef]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; Van’T Hof, R.J.; Ralston, S.H. Cannabinoid Receptor Type 1 Protects against Age- Related Osteoporosis by Regulating Osteoblast and Adipocyte Differentiation in Marrow Stromal Cells. Cell Metab. 2009, 10, 139–147. [Google Scholar] [CrossRef]
- Saponaro, F.; Ferrisi, R.; Gado, F.; Polini, B.; Saba, A.; Manera, C.; Chiellini, G. The Role of Cannabinoids in Bone Metabolism: A New Perspective for Bone Disorders. Int. J. Mol. Sci. 2021, 22, 12374. [Google Scholar] [CrossRef]
- Antonelli, E.; Bassotti, G.; Tramontana, M.; Hansel, K.; Stingeni, L.; Ardizzone, S.; Genovese, G.; Marzano, A.V.; Maconi, G. Dermatological Manifestations in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 364. [Google Scholar] [CrossRef]
- He, R.; Zhao, S.; Cui, M.; Chen, Y.; Ma, J.; Li, J.; Wang, X. Cutaneous Manifestations of Inflammatory Bowel Disease: Basic Characteristics, Therapy, and Potential Pathophysiological Associations. Front. Immunol. 2023, 14, 1234535. [Google Scholar] [CrossRef]
- Huang, B.L.; Chandra, S.; Shih, D.Q. Skin Manifestations of Inflammatory Bowel Disease. Front. Physiol. 2012, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Kuzumi, A.; Yamashita, T.; Fukasawa, T.; Yoshizaki-Ogawa, A.; Sato, S.; Yoshizaki, A. Cannabinoids for the Treatment of Autoimmune and Inflammatory Skin Diseases: A Systematic Review. Exp. Dermatol. 2024, 33, e15064. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Mihai, L.-G.; Scheau, A.-E.; Costache, D.O.; Constantin, C.; Calina, D.; Caruntu, C.; Costache, R.S.; Caruntu, A. Cannabinoids in the Pathophysiology of Skin Inflammation. Molecules 2020, 25, 652. [Google Scholar] [CrossRef] [PubMed]
- Sivesind, T.E.; Maghfour, J.; Rietcheck, H.; Kamel, K.; Malik, A.S.; Dellavalle, R.P. Cannabinoids for the Treatment of Dermatologic Conditions. JID Innov. 2022, 2, 100095. [Google Scholar] [CrossRef]
- Yoo, E.H.; Lee, J.H. Cannabinoids and Their Receptors in Skin Diseases. Int. J. Mol. Sci. 2023, 24, 16523. [Google Scholar] [CrossRef]
- Maida, V.; Shi, R.B.; Fazzari, F.G.T.; Zomparelli, L. Topical Cannabis-based Medicines—A Novel Adjuvant Treatment for Venous Leg Ulcers: An Open-label Trial. Exp. Dermatol. 2021, 30, 1258–1267. [Google Scholar] [CrossRef]
- Shah, P.; Holmes, K.; Chibane, F.; Wang, P.; Chagas, P.; Salles, E.; Jones, M.; Palines, P.; Masoumy, M.; Baban, B.; et al. Cutaneous Wound Healing and the Effects of Cannabidiol. Int. J. Mol. Sci. 2024, 25, 7137. [Google Scholar] [CrossRef]
- Wang, L.-L.; Zhao, R.; Li, J.-Y.; Li, S.-S.; Liu, M.; Wang, M.; Zhang, M.-Z.; Dong, W.-W.; Jiang, S.-K.; Zhang, M.; et al. Pharmacological Activation of Cannabinoid 2 Receptor Attenuates Inflammation, Fibrogenesis, and Promotes Re-Epithelialization during Skin Wound Healing. Eur. J. Pharmacol. 2016, 786, 128–136. [Google Scholar] [CrossRef]
- Licona Vera, E.; Betancur Vasquez, C.; Peinado Acevedo, J.S.; Rivera Bustamante, T.; Martinez Redondo, J.M. Ocular Manifestations of Inflammatory Bowel Disease. Cureus 2023, 15, e40299. [Google Scholar] [CrossRef]
- Migliorisi, G.; Vella, G.; Dal Buono, A.; Gabbiadini, R.; Busacca, A.; Loy, L.; Bezzio, C.; Vinciguerra, P.; Armuzzi, A. Ophthalmological Manifestations in Inflammatory Bowel Diseases: Keep an Eye on It. Cells 2024, 13, 142. [Google Scholar] [CrossRef]
- Richardson, H.; Yoon, G.; Moussa, G.; Kumar, A.; Harvey, P. Ocular Manifestations of IBD: Pathophysiology, Epidemiology, and Iatrogenic Associations of Emerging Treatment Strategies. Biomedicines 2024, 12, 2856. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Duran, M.; O’Keefe, G.A.D. Ocular Extraintestinal Manifestations and Treatments in Patients with Inflammatory Bowel Disease. Front. Ophthalmol. 2024, 3, 1257068. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.; Shah, A.; Hassman, L.; Gutierrez, A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Cairns, E.A.; Toguri, J.T.; Porter, R.F.; Szczesniak, A.M.; Kelly, M.E. Seeing over the Horizon—Targeting the Endocannabinoid System for the Treatment of Ocular Disease. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 253–265. [Google Scholar] [CrossRef]
- Szczesniak, A.-M.; Porter, R.F.; Toguri, J.T.; Borowska-Fielding, J.; Gebremeskel, S.; Siwakoti, A.; Johnston, B.; Lehmann, C.; Kelly, M.E.M. Cannabinoid 2 Receptor Is a Novel Anti-Inflammatory Target in Experimental Proliferative Vitreoretinopathy. Neuropharmacology 2017, 113 Pt B, 627–638. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.-M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25, 417. [Google Scholar] [CrossRef]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Toguri, J.T.; Caldwell, M.D.; Kelly, M.E.M. The Cannabinoids Delta(8)THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation. Cannabis Cannabinoid Res. 2018, 3, 11–20. [Google Scholar] [CrossRef]
- Toguri, J.T.; Caldwell, M.; Kelly, M.E. Turning Down the Thermostat: Modulating the Endocannabinoid System in Ocular Inflammation and Pain. Front. Pharmacol. 2016, 7, 304. [Google Scholar] [CrossRef]
- Toguri, J.T.; Moxsom, R.; Szczesniak, A.M.; Zhou, J.; Kelly, M.E.; Lehmann, C. Cannabinoid 2 Receptor Activation Reduces Leukocyte Adhesion and Improves Capillary Perfusion in the Iridial Microvasculature during Systemic Inflammation. Clin. Hemorheol. Microcirc. 2015, 61, 237–249. [Google Scholar] [CrossRef]
- Toguri, J.T.; Lehmann, C.; Laprairie, R.B.; Szczesniak, A.M.; Zhou, J.; Denovan-Wright, E.M.; Kelly, M.E. Anti-Inflammatory Effects of Cannabinoid CB(2) Receptor Activation in Endotoxin-Induced Uveitis. Br. J. Pharmacol. 2014, 171, 1448–1461. [Google Scholar] [CrossRef]
- Barberio, B.; Massimi, D.; Cazzagon, N.; Zingone, F.; Ford, A.C.; Savarino, E.V. Prevalence of Primary Sclerosing Cholangitis in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 161, 1865–1877. [Google Scholar] [CrossRef] [PubMed]
- Mertz, A.; Nguyen, N.A.; Katsanos, K.H.; Kwok, R.M. Primary Sclerosing Cholangitis and Inflammatory Bowel Disease Comorbidity: An Update of the Evidence. Ann. Gastroenterol. 2019, 32, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Palmela, C.; Peerani, F.; Castaneda, D.; Torres, J.; Itzkowitz, S.H. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver 2018, 12, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef]
- Berk, K.; Bzdega, W.; Konstantynowicz-Nowicka, K.; Charytoniuk, T.; Zywno, H.; Chabowski, A. Phytocannabinoids—A Green Approach toward Non-Alcoholic Fatty Liver Disease Treatment. J. Clin. Med. 2021, 10, 393. [Google Scholar] [CrossRef]
- Dibba, P.; Li, A.; Cholankeril, G.; Iqbal, U.; Gadiparthi, C.; Khan, M.A.; Kim, D.; Ahmed, A. Mechanistic Potential and Therapeutic Implications of Cannabinoids in Nonalcoholic Fatty Liver Disease. Medicines 2018, 5, 47. [Google Scholar] [CrossRef]
- Louvet, A.; Teixeira-Clerc, F.; Chobert, M.-N.; Deveaux, V.; Pavoine, C.; Zimmer, A.; Pecker, F.; Mallat, A.; Lotersztajn, S. Cannabinoid CB2 Receptors Protect against Alcoholic Liver Disease by Regulating Kupffer Cell Polarization in Mice. Hepatology 2011, 54, 1217–1226. [Google Scholar] [CrossRef]
- Mallat, A.; Teixeira-Clerc, F.; Lotersztajn, S. Cannabinoid Signaling and Liver Therapeutics. J. Hepatol. 2013, 59, 891–896. [Google Scholar] [CrossRef]
- Mohamed Ali, A.; Samir El-Tawil, O.; Samir Abd El-Rahman, S. Inhibited TLR-4/NF- κB Pathway Mediated by Cannabinoid Receptor 2 Activation Curbs Ongoing Liver Fibrosis in Bile Duct Ligated Rats. Adv. Anim. Vet. Sci. 2020, 9, 253–264. [Google Scholar] [CrossRef]
- Adolph, T.E.; Meyer, M.; Jukic, A.; Tilg, H. Heavy Arch: From Inflammatory Bowel Diseases to Metabolic Disorders. Gut 2024, 73, 1376–1387. [Google Scholar] [CrossRef]
- Dragasevic, S.; Stankovic, B.; Kotur, N.; Sokic-Milutinovic, A.; Milovanovic, T.; Lukic, S.; Milosavljevic, T.; Srzentic Drazilov, S.; Klaassen, K.; Pavlovic, S.; et al. Metabolic Syndrome in Inflammatory Bowel Disease: Association with Genetic Markers of Obesity and Inflammation. Metab. Syndr. Relat. Disord. 2020, 18, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C.-K. Molecular and Pathophysiological Links between Metabolic Disorders and Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 9139. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A. Relationship(s) between Obesity and Inflammatory Bowel Diseases: Possible Intertwined Pathogenic Mechanisms. Clin. J. Gastroenterol. 2020, 13, 139–152. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review Article: Vitamin D and Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Olczyk, M.; Czkwianianc, E.; Socha-Banasiak, A. Metabolic Bone Disorders in Children with Inflammatory Bowel Diseases. Life 2022, 12, 423. [Google Scholar] [CrossRef]
- Bielawiec, P.; Harasim-Symbor, E.; Chabowski, A. Phytocannabinoids: Useful Drugs for the Treatment of Obesity? Special Focus on Cannabidiol. Front. Endocrinol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Gruden, G.; Barutta, F.; Kunos, G.; Pacher, P. Role of the Endocannabinoid System in Diabetes and Diabetic Complications: Role of Endocannabinoid System in Diabetes. Br. J. Pharmacol. 2016, 173, 1116–1127. [Google Scholar] [CrossRef]
- Patil, M.; Casari, I.; Thapa, D.; Warne, L.N.; Dallerba, E.; Massi, M.; Carlessi, R.; Falasca, M. Preclinical Pharmacokinetics, Pharmacodynamics, and Toxicity of Novel Small-Molecule GPR119 Agonists to Treat Type-2 Diabetes and Obesity. Biomed. Pharmacother. 2024, 177, 117077. [Google Scholar] [CrossRef]
- Patil, M.; Thapa, D.; Warne, L.N.; Lareu, R.R.; Dallerba, E.; Lian, J.; Massi, M.; Carlessi, R.; Falasca, M. Chronic Metabolic Effects of Novel Gut-Oriented Small-Molecule GPR119 Agonists in Diet-Induced Obese Mice. Biomed. Pharmacother. 2024, 181, 117675. [Google Scholar] [CrossRef]
- Rakotoarivelo, V.; Sihag, J.; Flamand, N. Role of the Endocannabinoid System in the Adipose Tissue with Focus on Energy Metabolism. Cells 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Ambruzs, J.M.; Larsen, C.P. Renal Manifestations of Inflammatory Bowel Disease. Rheum. Dis. Clin. N. Am. 2018, 44, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Braysh, K.; Geagea, A.G.; Matar, C.; Rizzo, M.; Eid, A.; Massaad-Massade, L.; Mallat, S.; Jurjus, A. Kidney Manifestations of Inflammatory Bowel Diseases. Open J. Gastroenterol. 2018, 08, 172–191. [Google Scholar] [CrossRef][Green Version]
- Dincer, M.T.; Dincer, Z.T.; Bakkaloglu, O.K.; Yalin, S.F.; Trabulus, S.; Celik, A.F.; Seyahi, N.; Altiparmak, M.R. Renal Manifestations in Inflammatory Bowel Disease: A Cohort Study During the Biologic Era. Med. Sci. Monit. 2022, 28, e936497-1–e936497-10. [Google Scholar] [CrossRef]
- Kim, Y.N.; Jung, Y. Renal and Urinary Manifestations of Inflammatory Bowel Disease. Korean J. Gastroenterol. 2019, 73, 260. [Google Scholar] [CrossRef]
- Singh, A.; Khanna, T.; Mahendru, D.; Kahlon, J.; Kumar, V.; Sohal, A.; Yang, J. Insights into Renal and Urological Complications of Inflammatory Bowel Disease. World J. Nephrol. 2024, 13, 96574. [Google Scholar] [CrossRef]
- Fernandes, C.D.A.L.; Paulo, D.G.; Alves, L.F. Exploring the Role of the Endocannabinoid System in Chronic Kidney Disease: Implications for Therapeutic Interventions. J. Adv. Med. Med. Res. 2023, 35, 14–22. [Google Scholar] [CrossRef]
- Tam, J. The Emerging Role of the Endocannabinoid System in the Pathogenesis and Treatment of Kidney Diseases. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 267–276. [Google Scholar] [CrossRef]
- Bergamaschi, G.; Di Sabatino, A.; Albertini, R.; Ardizzone, S.; Biancheri, P.; Bonetti, E.; Cassinotti, A.; Cazzola, P.; Markopoulos, K.; Massari, A.; et al. Prevalence and Pathogenesis of Anemia in Inflammatory Bowel Disease. Influence of Anti-Tumor Necrosis Factor-Alpha Treatment. Haematologica 2010, 95, 199–205. [Google Scholar] [CrossRef]
- Resál, T.; Farkas, K.; Molnár, T. Iron Deficiency Anemia in Inflammatory Bowel Disease: What Do We Know? Front. Med. 2021, 8, 686778. [Google Scholar] [CrossRef]
- Giaginis, C.; Lakiotaki, E.; Korkolopoulou, P.; Konstantopoulos, K.; Patsouris, E.; Theocharis, S. Endocannabinoid System: A Promising Therapeutic Target for the Treatment of Haematological Malignancies? Curr. Med. Chem. 2016, 23, 2350–2362. [Google Scholar] [CrossRef]
- Sharma, D.S.; Paddibhatla, I.; Raghuwanshi, S.; Malleswarapu, M.; Sangeeth, A.; Kovuru, N.; Dahariya, S.; Gautam, D.K.; Pallepati, A.; Gutti, R.K. Endocannabinoid System: Role in Blood Cell Development, Neuroimmune Interactions and Associated Disorders. J. Neuroimmunol. 2021, 353, 577501. [Google Scholar] [CrossRef] [PubMed]
- Alkhawajah, M.M.; Caminero, A.B.; Freeman, H.J.; Oger, J.J. Multiple Sclerosis and Inflammatory Bowel Diseases: What We Know and What We Would Need to Know! Mult. Scler. 2013, 19, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Nemati, R.; Mehdizadeh, S.; Salimipour, H.; Yaghoubi, E.; Alipour, Z.; Tabib, S.M.; Assadi, M. Neurological Manifestations Related to Crohn’s Disease: A Boon for the Workforce. Gastroenterol. Rep. 2019, 7, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Bagüés, A.; Martín, M.I.; Sánchez-Robles, E.M. Involvement of Central and Peripheral Cannabinoid Receptors on Antinociceptive Effect of Tetrahydrocannabinol in Muscle Pain. Eur. J. Pharmacol. 2014, 745, 69–75. [Google Scholar] [CrossRef]
- Castillo-Arellano, J.; Canseco-Alba, A.; Cutler, S.J.; León, F. The Polypharmacological Effects of Cannabidiol. Molecules 2023, 28, 3271. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid System: Role in Depression, Reward and Pain Control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar] [CrossRef]
- Liu, C.; Walker, J.M. Effects of a Cannabinoid Agonist on Spinal Nociceptive Neurons in a Rodent Model of Neuropathic Pain. J. Neurophysiol. 2006, 96, 2984–2994. [Google Scholar] [CrossRef]
- Russo, M.; Naro, A.; Leo, A.; Sessa, E.; D’Aleo, G.; Bramanti, P.; Calabro, R.S. Evaluating Sativex(R) in Neuropathic Pain Management: A Clinical and Neurophysiological Assessment in Multiple Sclerosis. Pain Med. 2016, 17, 1145–1154. [Google Scholar] [CrossRef]
- Wu, S.; Yi, J.; Wu, B. Casual Associations of Thyroid Function with Inflammatory Bowel Disease and the Mediating Role of Cytokines. Front. Endocrinol. 2024, 15, 1376139. [Google Scholar] [CrossRef]
- Xian, W.; Wu, D.; Liu, B.; Hong, S.; Huo, Z.; Xiao, H.; Li, Y. Graves Disease and Inflammatory Bowel Disease: A Bidirectional Mendelian Randomization. J. Clin. Endocrinol. Metab. 2023, 108, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Farraj, K.L.; Pellegrini, J.R.; Munshi, R.F.; Russe-Russe, J.; Kaliounji, A.; Tiwana, M.S.; Srivastava, P.; Subramani, K. Chronic Steroid Use: An Overlooked Impact on Patients with Inflammatory Bowel Disease. JGH Open 2022, 6, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Dahlqvist, P.; Olsson, T.; Lundgren, D.; Werner, M.; Suhr, O.B.; Karling, P. The Clinical Course after Glucocorticoid Treatment in Patients with Inflammatory Bowel Disease Is Linked to Suppression of the Hypothalamic-Pituitary-Adrenal Axis: A Retrospective Observational Study. Ther. Adv. Gastroenterol. 2017, 10, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Nachawi, N.; Li, D.; Lansang, M.C. Glucocorticoid-Induced Adrenal Insufficiency and Glucocorticoid Withdrawal Syndrome: Two Sides of the Same Coin. Clevel. Clin. J. Med. 2024, 91, 245–255. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Mondal, S.; Jana, S. Cannabidiol Improves Thyroid Function via Modulating Vitamin D3 Receptor in Vitamin D3 Deficiency Diet-Induced Rat Model. J. Food Sci. Technol. 2022, 59, 3237–3244. [Google Scholar] [CrossRef]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Woliński, K.; Płazińska, M.T.; Mikołajczak, P.; Ruchała, M. The Effects of Cannabinoids on the Endocrine System. Endokrynol. Pol. 2018, 69, 705–719. [Google Scholar] [CrossRef]
- Meah, F.; Lundholm, M.; Emanuele, N.; Amjed, H.; Poku, C.; Agrawal, L.; Emanuele, M.A. The Effects of Cannabis and Cannabinoids on the Endocrine System. Rev. Endocr. Metab. Disord. 2022, 23, 401–420. [Google Scholar] [CrossRef]
- Bigeh, A.; Sanchez, A.; Maestas, C.; Gulati, M. Inflammatory Bowel Disease and the Risk for Cardiovascular Disease: Does All Inflammation Lead to Heart Disease? Trends Cardiovasc. Med. 2020, 30, 463–469. [Google Scholar] [CrossRef]
- Biondi, R.B.; Salmazo, P.S.; Bazan, S.G.Z.; Hueb, J.C.; de Paiva, S.A.R.; Sassaki, L.Y. Cardiovascular Risk in Individuals with Inflammatory Bowel Disease. Clin. Exp. Gastroenterol. 2020, 13, 107–113. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; Van Der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters Van Lennep, J.E.; De Vries, A.C. Cardiovascular Risk Profiles in Patients with Inflammatory Bowel Disease Differ from Matched Controls from the General Population. Eur. J. Prev. Cardiol. 2023, 30, 1615–1622. [Google Scholar] [CrossRef]
- Ho, W.S.V.; Kelly, M.E.M. Cannabinoids in the Cardiovascular System. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 80, pp. 329–366. ISBN 978-0-12-811232-8. [Google Scholar]
- O’Sullivan, S.E. Endocannabinoids and the Cardiovascular System in Health and Disease. In Endocannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; Volume 231, pp. 393–422. ISBN 978-3-319-20824-4. [Google Scholar]
- Sierra, S.; Luquin, N.; Navarro-Otano, J. The Endocannabinoid System in Cardiovascular Function: Novel Insights and Clinical Implications. Clin. Auton. Res. 2018, 28, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Na, S.-Y.; Moon, W. Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.-F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef]
- Cohen, B.L.; Torres, J.; Colombel, J.-F. Immunosuppression in Inflammatory Bowel Disease: How Much Is Too Much? Curr. Opin. Gastroenterol. 2012, 28, 341–348. [Google Scholar] [CrossRef]
- Vukovic, J.; Jukic, I.; Tonkic, A. The Challenges in Treating Inflammatory Bowel Diseases During the COVID-19 Pandemic: An Opinion. J. Clin. Med. 2024, 13, 7128. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting Inflammatory Bowel Disease: Pathology, Treatments, Challenges and Emerging Therapeutics Including Drug Leads from Natural Products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- D’Haens, G. Risks and Benefits of Biologic Therapy for Inflammatory Bowel Diseases. Gut 2007, 56, 725–732. [Google Scholar] [CrossRef]
- Bakes, D.; Kiran, R.P. Overview of Common Complications in Inflammatory Bowel Disease Surgery. Gastrointest. Endosc. Clin. 2022, 32, 761–776. [Google Scholar] [CrossRef]
- Nickerson, T.P.; Merchea, A. Perioperative Considerations in Crohn Disease and Ulcerative Colitis. Clin. Colon. Rectal Surg. 2016, 29, 80–84. [Google Scholar] [CrossRef]
- Parray, F.Q.; Wani, M.L.; Malik, A.A.; Wani, S.N.; Bijli, A.H.; Irshad, I.; Ul-Hassan, N. Ulcerative Colitis: A Challenge to Surgeons. Int. J. Prev. Med. 2012, 3, 749–763. [Google Scholar]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Busacca, A.; Ingrassia Strano, G.; Giuffrida, E.; Guida, L.; Scrivo, B.; Carrozza, L.; Calvaruso, V.; Cappello, M. P114 A Screening Tool for the Early Diagnosis of Extraintestinal Manifestations in Inflammatory Bowel Disease: The EMAIL Questionnaire. J. Crohn’s Colitis 2020, 14, S192–S193. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Zingone, F. Editorial: Challenges in Inflammatory Bowel Disease: Current, Future and Unmet Needs. Front. Med. 2022, 9, 979535. [Google Scholar] [CrossRef] [PubMed]
- Jefremow, A.; Neurath, M.F. Novel Small Molecules in IBD: Current State and Future Perspectives. Cells 2023, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Martín-Acosta, P.; Xiao, X. PROTACs to Address the Challenges Facing Small Molecule Inhibitors. Eur. J. Med. Chem. 2021, 210, 112993. [Google Scholar] [CrossRef]
- Faye, A.S.; Allin, K.H.; Iversen, A.T.; Agrawal, M.; Faith, J.; Colombel, J.-F.; Jess, T. Antibiotic Use as a Risk Factor for Inflammatory Bowel Disease across the Ages: A Population-Based Cohort Study. Gut 2023, 72, 663–670. [Google Scholar] [CrossRef]
- Lo, B.; Biederman, L.; Rogler, G.; Dora, B.; Kreienbühl, A.; Vind, I.; Bendtsen, F.; Burisch, J. Specific Antibiotics Increase the Risk of Flare-Ups in Patients with Inflammatory Bowel Disease: Results from a Danish Nationwide Population-Based Nested Case-Control Study. J. Crohn’s Colitis 2024, 18, 1232–1240. [Google Scholar] [CrossRef]
- Culligan, E.P.; Hill, C.; Sleator, R.D. Probiotics and Gastrointestinal Disease: Successes, Problems and Future Prospects. Gut Pathog. 2009, 1, 19. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Yuan, Y.; Rodríguez-Lago, I.; Sousa-Pimenta, M.; Dias, C.C.; Barreiro-de Acosta, M.; Jairath, V.; Magro, F. Efficacy and Safety of Probiotics in IBD: An Overview of Systematic Reviews and Updated Meta-analysis of Randomized Controlled Trials. UEG J. 2024, 12, 960–981. [Google Scholar] [CrossRef]
- Almeida, C.; Oliveira, R.; Baylina, P.; Fernandes, R.; Teixeira, F.G.; Barata, P. Current Trends and Challenges of Fecal Microbiota Transplantation-An Easy Method That Works for All? Biomedicines 2022, 10, 2742. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Anderco, P.; Birsan, S. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Biomedicines 2023, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Yadegar, A.; Bar-Yoseph, H.; Monaghan, T.M.; Pakpour, S.; Severino, A.; Kuijper, E.J.; Smits, W.K.; Terveer, E.M.; Neupane, S.; Nabavi-Rad, A.; et al. Fecal Microbiota Transplantation: Current Challenges and Future Landscapes. Clin. Microbiol. Rev. 2024, 37, e00060-22. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Arturo, I.F.; Fabiana, P. Endocannabinoidome. In Encyclopedia of Life Sciences; Wiley: New York, NY, USA, 2018; pp. 1–10. ISBN 978-0-470-01617-6. [Google Scholar]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Louis-Gray, K.; Tupal, S.; Premkumar, L.S. TRPV1: A Common Denominator Mediating Antinociceptive and Antiemetic Effects of Cannabinoids. Int. J. Mol. Sci. 2022, 23, 10016. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.-L.; Sibaev, A.; Storr, M.; Lutz, B. The Endogenous Cannabinoid System Protects against Colonic Inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef]
- Wolyniak, M.; Wlodarczyk, M.; Piscitelli, F.; Verde, R.; Di Marzo, V.; Mokrowiecka, A.; Malecka-Wojciesko, E.; Fabisiak, A. Modulation of CB1 and CB2 Receptors and Endocannabinoid Activity in Inflammatory Bowel Diseases. J. Physiol. Pharmacol. 2024, 75, 547–555. [Google Scholar] [CrossRef]
- Branković, M.; Gmizić, T.; Dukić, M.; Zdravković, M.; Daskalović, B.; Mrda, D.; Nikolić, N.; Brajković, M.; Gojgić, M.; Lalatović, J.; et al. Therapeutic Potential of Palmitoylethanolamide in Gastrointestinal Disorders. Antioxidants 2024, 13, 600. [Google Scholar] [CrossRef]
- Anderson, W.B.; Gould, M.J.; Torres, R.D.; Mitchell, V.A.; Vaughan, C.W. Actions of the Dual FAAH/MAGL Inhibitor JZL195 in a Murine Inflammatory Pain Model. Neuropharmacology 2014, 81, 224–230. [Google Scholar] [CrossRef]
- Michalak, A.; Mosińska, P.; Fichna, J. Common Links between Metabolic Syndrome and Inflammatory Bowel Disease: Current Overview and Future Perspectives. Pharmacol. Rep. 2016, 68, 837–846. [Google Scholar] [CrossRef]
- Sappati Biyyani, R.S.R.; Putka, B.S.; Mullen, K.D. Dyslipidemia and Lipoprotein Profiles in Patients with Inflammatory Bowel Disease. J. Clin. Lipidol. 2010, 4, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and Cardiovascular Disease: From Mechanisms to Therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Fan, J.; Su, Q.; Yang, Z. Cytokines and Abnormal Glucose and Lipid Metabolism. Front. Endocrinol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An Update on Lipid Oxidation and Inflammation in Cardiovascular Diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Eder, A.E.; Karlstad, M.D.; Burk, D.H.; Noland, R.C.; Floyd, Z.E.; Collier, J.J. Oral Corticosterone Administration Reduces Insulitis but Promotes Insulin Resistance and Hyperglycemia in Male Nonobese Diabetic Mice. Am. J. Pathol. 2017, 187, 614–626. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The Crucial Role and Mechanism of Insulin Resistance in Metabolic Disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef]
- Andreassen, H.; Rungby, J.; Dahlerup, J.F.; Mosekilde, L. Inflammatory Bowel Disease and Osteoporosis. Scand. J. Gastroenterol. 1997, 32, 1247–1255. [Google Scholar] [CrossRef]
- Van Bodegraven, A.A.; Bravenboer, N. Perspective on Skeletal Health in Inflammatory Bowel Disease. Osteoporos. Int. 2020, 31, 637–646. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Quinlan, J.I.; Overthrow, K.; Greig, C.; Lord, J.M.; Armstrong, M.J.; Cooper, S.C. Sarcopenia in Inflammatory Bowel Disease: A Narrative Overview. Nutrients 2021, 13, 656. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Di Marzo, V. The Gut Microbiome, Endocannabinoids and Metabolic Disorders. J. Endocrinol. 2021, 248, R83–R97. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lin, J.; Zhang, C.; Gao, H.; Lu, H.; Gao, X.; Zhu, R.; Li, Z.; Li, M.; Liu, Z. Microbiota Metabolite Butyrate Constrains Neutrophil Functions and Ameliorates Mucosal Inflammation in Inflammatory Bowel Disease. Gut Microbes 2021, 13, 1968257. [Google Scholar] [CrossRef] [PubMed]
- Marzo, V.D. Endocannabinoid Signaling in the Brain: Biosynthetic Mechanisms in the Limelight. Nat. Neurosci. 2011, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- DiPatrizio, N.V. Endocannabinoids in the Gut. Cannabis Cannabinoid Res. 2016, 1, 67–77. [Google Scholar] [CrossRef]
- Alhamoruni, A.; Wright, K.; Larvin, M.; O’Sullivan, S. Cannabinoids Mediate Opposing Effects on Inflammation-induced Intestinal Permeability. Br. J. Pharmacol. 2012, 165, 2598–2610. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Battistini, L.; Maccarrone, M. Endocannabinoid Signalling in Innate and Adaptive Immunity. Immunology 2015, 144, 352–364. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- O’Sullivan, S.E.; Yates, A.S.; Porter, R.K. The Peripheral Cannabinoid Receptor Type 1 (CB1) as a Molecular Target for Modulating Body Weight in Man. Molecules 2021, 26, 6178. [Google Scholar] [CrossRef]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte Cannabinoid Receptor CB1 Regulates Energy Homeostasis and Alternatively Activated Macrophages. J. Clin. Investig. 2017, 127, 4148–4162. [Google Scholar] [CrossRef]
- Tam, J.; Hinden, L.; Drori, A.; Udi, S.; Azar, S.; Baraghithy, S. The Therapeutic Potential of Targeting the Peripheral Endocannabinoid/CB 1 Receptor System. Eur. J. Intern. Med. 2018, 49, 23–29. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lee, T.-Y.; Kwok, C.-F.; Hsu, Y.-P.; Shih, K.-C.; Lin, Y.-J.; Ho, L.-T. Cannabinoid Receptor Type 1 Mediates High-Fat Diet-Induced Insulin Resistance by Increasing Forkhead Box O1 Activity in a Mouse Model of Obesity. Int. J. Mol. Med. 2016, 37, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.H.; Lee, M.H.; Kim, J.E.; Song, H.K.; Kang, Y.S.; Lee, J.E.; Kim, H.W.; Cha, J.J.; Hyun, Y.Y.; Kim, S.H.; et al. Blockade of Cannabinoid Receptor 1 Improves Insulin Resistance, Lipid Metabolism, and Diabetic Nephropathy in Db/Db Mice. Endocrinology 2012, 153, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral Cannabinoid Receptor, CB2, Regulates Bone Mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Couch, D.G.; Tasker, C.; Theophilidou, E.; Lund, J.N.; O’Sullivan, S.E. Cannabidiol and Palmitoylethanolamide Are Anti-Inflammatory in the Acutely Inflamed Human Colon. Clin. Sci. 2017, 131, 2611–2626. [Google Scholar] [CrossRef]
- Nagappan, A.; Shin, J.; Jung, M.H. Role of Cannabinoid Receptor Type 1 in Insulin Resistance and Its Biological Implications. Int. J. Mol. Sci. 2019, 20, 2109. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, S.; Niu, J.; Li, P.; Deng, J.; Xu, S.; Wang, Z.; Wang, W.; Kong, D.; Li, C. Cannabinoid 2 Receptor Agonist Improves Systemic Sensitivity to Insulin in High-Fat Diet/Streptozotocin-Induced Diabetic Mice. Cell. Physiol. Biochem. 2016, 40, 1175–1185. [Google Scholar] [CrossRef]
- Dörnyei, G.; Vass, Z.; Juhász, C.B.; Nádasy, G.L.; Hunyady, L.; Szekeres, M. Role of the Endocannabinoid System in Metabolic Control Processes and in the Pathogenesis of Metabolic Syndrome: An Update. Biomedicines 2023, 11, 306. [Google Scholar] [CrossRef]
- Holst, J.J. Glucagon and Other Proglucagon-Derived Peptides in the Pathogenesis of Obesity. Front. Nutr. 2022, 9, 964406. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Hunt, J.E.; Holst, J.J.; Jeppesen, P.B.; Kissow, H. GLP-1 and Intestinal Diseases. Biomedicines 2021, 9, 383. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Biro, T.; Cabral, G.A.; Dey, S.K.; Marzo, V.D.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid Signaling at the Periphery: 50 Years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- González-Mariscal, I.; Krzysik-Walker, S.M.; Kim, W.; Rouse, M.; Egan, J.M. Blockade of Cannabinoid 1 Receptor Improves GLP-1R Mediated Insulin Secretion in Mice. Mol. Cell. Endocrinol. 2016, 423, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zizzari, P.; He, R.; Falk, S.; Bellocchio, L.; Allard, C.; Clark, S.; Lesté-Lasserre, T.; Marsicano, G.; Clemmensen, C.; Perez-Tilve, D.; et al. CB1 and GLP-1 Receptors Cross Talk Provides New Therapies for Obesity. Diabetes 2021, 70, 415–422. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; Zhang, H.; Li, L.; Fan, T.; Jin, Z. The Alleviating Effect and Mechanism of GLP-1 on Ulcerative Colitis. Aging 2023, 15, 8044–8060. [Google Scholar] [CrossRef]
- Lebrun, L.J.; Lenaerts, K.; Kiers, D.; Pais De Barros, J.-P.; Le Guern, N.; Plesnik, J.; Thomas, C.; Bourgeois, T.; Dejong, C.H.C.; Kox, M.; et al. Enteroendocrine L Cells Sense LPS after Gut Barrier Injury to Enhance GLP-1 Secretion. Cell Rep. 2017, 21, 1160–1168. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Jun, H.-S. Anti-Inflammatory Effects of GLP-1-Based Therapies beyond Glucose Control. Mediat. Inflamm. 2016, 2016, 3094642. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Ho, M.-S.; Huang, W.-T.; Chou, Y.-T.; King, K. Modulation of Glucagon-like Peptide-1 (GLP-1) Potency by Endocannabinoid-like Lipids Represents a Novel Mode of Regulating GLP-1 Receptor Signaling. J. Biol. Chem. 2015, 290, 14302–14313. [Google Scholar] [CrossRef]
- Villumsen, M.; Schelde, A.B.; Jimenez-Solem, E.; Jess, T.; Allin, K.H. GLP-1 Based Therapies and Disease Course of Inflammatory Bowel Disease. eClinicalMedicine 2021, 37, 100979. [Google Scholar] [CrossRef]
- Vasincu, A.; Rusu, R.-N.; Ababei, D.-C.; Neamțu, M.; Arcan, O.D.; Macadan, I.; Beșchea Chiriac, S.; Bild, W.; Bild, V. Exploring the Therapeutic Potential of Cannabinoid Receptor Antagonists in Inflammation, Diabetes Mellitus, and Obesity. Biomedicines 2023, 11, 1667. [Google Scholar] [CrossRef]
- Matias, I.; Lehmann, E.W.; Zizzari, P.; Byberg, S.; Cota, D.; Torekov, S.S.; Quarta, C. Endocannabinoid-Related Molecules Predict the Metabolic Efficacy of GLP-1 Receptor Agonism in Humans with Obesity. J. Endocrinol. Investig. 2023, 47, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Azimullah, S.; Nagoor Meeran, M.F.; Saraswathiamma, D.; Arunachalam, S.; Jha, N.K.; Sadek, B.; Adeghate, E.; Sethi, G.; Albawardi, A.; et al. Cannabinoid 2 Receptor Activation Protects against Diabetic Cardiomyopathy through Inhibition of AGE/RAGE-Induced Oxidative Stress, Fibrosis, and Inflammasome Activation. J. Pharmacol. Exp. Ther. 2024, 391, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Hashiesh, H.M.; Meeran, M.F.N.; Sharma, C.; Sadek, B.; Kaabi, J.A.; Ojha, S.K. Therapeutic Potential of β-Caryophyllene: A Dietary Cannabinoid in Diabetes and Associated Complications. Nutrients 2020, 12, 2963. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-Jahani, P.; Yan, S.; Kazemi, M.H.; Li, X.; Akbari, A.; Sakano, K.; Sakano, Y.; Hurrell, B.P.; Akbari, O. CB2 Stimulation of Adipose Resident ILC2s Orchestrates Immune Balance and Ameliorates Type 2 Diabetes Mellitus. Cell Rep. 2024, 43, 114434. [Google Scholar] [CrossRef] [PubMed]
- Pegah, A.; Abbasi-Oshaghi, E.; Khodadadi, I.; Mirzaei, F.; Tayebinai, H. Probiotic and Resveratrol Normalize GLP-1 Levels and Oxidative Stress in the Intestine of Diabetic Rats. Metabol. Open 2021, 10, 100093. [Google Scholar] [CrossRef]
- Wei, S.-H.; Chen, Y.-P.; Chen, M.-J. Selecting Probiotics with the Abilities of Enhancing GLP-1 to Mitigate the Progression of Type 1 Diabetes in Vitro and in Vivo. J. Funct. Foods 2015, 18, 473–486. [Google Scholar] [CrossRef]
- Chen, T.; Pan, F.; Huang, Q.; Xie, G.; Chao, X.; Wu, L.; Wang, J.; Cui, L.; Sun, T.; Li, M.; et al. Metabolic Phenotyping Reveals an Emerging Role of Ammonia Abnormality in Alzheimer’s Disease. Nat. Commun. 2024, 15, 3796. [Google Scholar] [CrossRef]
- Duan, Y.; Wu, X.; Liang, S.; Jin, F. Elevated Blood Ammonia Level Is a Potential Biological Risk Factor of Behavioral Disorders in Prisoners. Behav. Neurol. 2015, 2015, 797862. [Google Scholar] [CrossRef]
- Knight-Sepulveda, K.; Kais, S.; Santaolalla, R.; Abreu, M.T. Diet and Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2015, 11, 511–520. [Google Scholar]
- Pagano, E.; Capasso, R.; Piscitelli, F.; Romano, B.; Parisi, O.A.; Finizio, S.; Lauritano, A.; Marzo, V.D.; Izzo, A.A.; Borrelli, F. An Orally Active Cannabis Extract with High Content in Cannabidiol Attenuates Chemically-Induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Pereira Leite, D.F.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.-Z.; Xie, X.-Q.; Altmann, K.-H.; Karsak, M.; Zimmer, A. Beta-Caryophyllene Is a Dietary Cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed]
- Kruis, W. Maintaining Remission of Ulcerative Colitis with the Probiotic Escherichia Coli Nissle 1917 Is as Effective as with Standard Mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Pascarella, F.; Giannetti, E.; Quaglietta, L.; Baldassano, R.N.; Staiano, A. Effect of a Probiotic Preparation (VSL#3) on Induction and Maintenance of Remission in Children With Ulcerative Colitis. Am. J. Gastroenterol. 2009, 104, 437–443. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Lutz, B.; Ruiz de Azua, I. The Microbiome and Gut Endocannabinoid System in the Regulation of Stress Responses and Metabolism. Front. Cell. Neurosci. 2022, 16, 867267. [Google Scholar] [CrossRef]
- Otkur, W.; Wang, J.; Hou, T.; Liu, F.; Yang, R.; Li, Y.; Xiang, K.; Pei, S.; Qi, H.; Lin, H.; et al. Aminosalicylates Target GPR35, Partly Contributing to the Prevention of DSS-Induced Colitis. Eur. J. Pharmacol. 2023, 949, 175719. [Google Scholar] [CrossRef]
- Farooq, S.M.; Hou, Y.; Li, H.; O’Meara, M.; Wang, Y.; Li, C.; Wang, J.-M. Disruption of GPR35 Exacerbates Dextran Sulfate Sodium-Induced Colitis in Mice. Dig. Dis. Sci. 2018, 63, 2910–2922. [Google Scholar] [CrossRef]
- Gonzalez, S.; Cebeira, M.; Fernandez-Ruiz, J. Cannabinoid Tolerance and Dependence: A Review of Studies in Laboratory Animals. Pharmacol. Biochem. Behav. 2005, 81, 300–318. [Google Scholar] [CrossRef]
- Compton, D.R.; Dewey, W.L.; Martin, B.R. Cannabis Dependence and Tolerance Production. Adv. Alcohol Subst. Abus. 1990, 9, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Burggren, A.C.; Shirazi, A.; Ginder, N.; London, E.D. Cannabis Effects on Brain Structure, Function, and Cognition: Considerations for Medical Uses of Cannabis and Its Derivatives. Am. J. Drug Alcohol Abus. 2019, 45, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Griffin-Thomas, L. Emerging Role of the Cannabinoid Receptor CB2 in Immune Regulation: Therapeutic Prospects for Neuroinflammation. Expert Rev. Mol. Med. 2009, 11, e3. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).