Using Physiologically Based Pharmacokinetic Models for Assessing Pharmacokinetic Drug–Drug Interactions in Patients with Chronic Heart Failure Taking Narrow Therapeutic Window Drugs
Abstract
1. Introduction
2. Results
2.1. Evaluation of Drugs with a Narrow Therapeutic Window for pDDIs in Patients with CHF
2.2. Simulation of CYP3A4 Interactions with Simcyp® Software
2.3. Simulation of CYP2C9 Interactions with Simcyp® Software
2.4. Simulation of P-gp Interactions with Simcyp® Software
3. Discussion
4. Materials and Methods
4.1. Data and Software
4.2. Data Analysis
5. Limitations and Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cmax | Maximum plasma concentration |
| CmaxR | Maximum plasma concentration ratio |
| AUC | Area under the curve |
| AUCR | Area under the curve ratio |
| P-gp | P-glycoprotein |
| CYP450 | Cytochrome P450 enzymes |
| PBPK | Physiology-based pharmacokinetic |
| HF | Heart failure |
| pPKDDIs | Potential pharmacokinetic drug–drug interactions |
| pDDIs | Potential drug–drug interactions |
| OAT | Organic anion transporter |
| PD | Pharmacodynamics |
| PPB | Plasma protein binding; |
| PPI | Proton pump inhibitors; |
| 1,4-DHP-CCB | 1,4-dihydropyridine calcium antagonist; |
| SMZ/TMP | Sulfamethoxazole/trimethoprim; |
| NSAIDs | Non-steroidal anti-inflammatory drugs. |
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858, Erratum in Lancet 2019, 393, e44. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Sperrin, M.; Watson, M.C.; Coutts, A.; Wilde, K.; Burton, C.; Kadam, U.T.; Kwok, C.S.; Clark, A.B.; Murchie, P.; et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur. J. Heart Fail. 2017, 19, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef]
- Beezer, J.; Al Hatrushi, M.; Husband, A.; Kurdi, A.; Forsyth, P. Polypharmacy definition and prevalence in heart failure: A systematic review. Heart Fail. Rev. 2022, 27, 465–492. [Google Scholar] [CrossRef]
- Buda, V.; Prelipcean, A.; Cozma, D.; Man, D.E.; Negres, S.; Scurtu, A.; Suciu, M.; Andor, M.; Danciu, C.; Crisan, S.; et al. An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure. J. Clin. Med. 2022, 11, 2020. [Google Scholar] [CrossRef]
- Sager, J.E.; Yu, J.; Ragueneau-Majlessi, I.; Isoherranen, N. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification. Drug Metab. Dispos. 2015, 43, 1823–1837. [Google Scholar] [CrossRef]
- Jones, H.; Chen, Y.; Gibson, C.; Heimbach, T.; Parrott, N.; Peters, S.; Snoeys, J.; Upreti, V.; Zheng, M.; Hall, S. Physiologically Based Pharmacokinetic Modeling in Drug Discovery and Development: A Pharmaceutical Industry Perspective. Clin. Pharmacol. Ther. 2015, 97, 247–262. [Google Scholar] [CrossRef]
- Shebley, M.; Sandhu, P.; Riedmaier, A.E.; Jamei, M.; Narayanan, R.; Patel, A.; Peters, S.A.; Reddy, V.P.; Zheng, M.; de Zwart, L.; et al. Physiologically Based Pharmacokinetic Model Qualification and Reporting Procedures for Regulatory Submissions: A Consortium Perspective. Clin. Pharmacol. Ther. 2018, 104, 88–110. [Google Scholar] [CrossRef]
- Perry, C.; Davis, G.; Conner, T.M.; Zhang, T. Utilization of Physiologically Based Pharmacokinetic Modeling in Clinical Pharmacology and Therapeutics: An Overview. Curr. Pharmacol. Rep. 2020, 6, 71–84. [Google Scholar] [CrossRef]
- Cheng, J.W. Current Perspectives on the Role of the Pharmacist in Heart Failure Management. Integr. Pharm. Res. Pract. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jasińska-Stroschein, M.; Waszyk-Nowaczyk, M. Multidimensional Interventions on Supporting Disease Management for Hospitalized Patients with Heart Failure: The Role of Clinical and Community Pharmacists. J. Clin. Med. 2023, 12, 3037. [Google Scholar] [CrossRef] [PubMed]
- Schachter, M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: An update. Fundam. Clin. Pharmacol. 2005, 19, 117–125. [Google Scholar] [CrossRef]
- Ufer, M. Comparative pharmacokinetics of vitamin K antagonists: Warfarin, phenprocoumon, and acenocoumarol. Clin. Pharmacokinet. 2005, 44, 1227–1246. [Google Scholar] [CrossRef]
- Lee, C.R.; Goldstein, J.A.; Pieper, J.A. Cytochrome P450 2C9 polymorphisms: A comprehensive review of the in-vitro and human data. Pharmacogenetics 2002, 12, 251–263. [Google Scholar] [CrossRef]
- Yang, J.; He, M.M.; Niu, W.; Wrighton, S.A.; Li, L.; Liu, Y.; Li, C. Metabolic capabilities of cytochrome P450 enzymes in Chinese liver microsomes compared with those in Caucasian liver microsomes. Br. J. Clin. Pharmacol. 2012, 73, 268–284. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; von Richter, O.; Burk, O.; Ziegler, A.; Mettang, T.; Eichelbaum, M.; Fromm, M.F. Characterization of the major metabolites of verapamil as substrates and inhibitors of P-glycoprotein. J. Pharmacol. Exp. Ther. 2000, 293, 376–382. [Google Scholar] [CrossRef]
- Georgiev, K.D.; Hvarchanova, N.; Stoychev, E.; Kanazirev, B. Prevalence of polypharmacy and risk of potential drug-drug interactions among hospitalized patients with emphasis on the pharmacokinetics. Sci. Prog. 2022, 105, 368504211070183. [Google Scholar] [CrossRef]
- Stone, S.M.; Rai, N.; Nei, J. Problems and pitfalls in cardiac drug therapy. Rev. Cardiovasc. Med. 2001, 2, 126–142. [Google Scholar]
- Itakura, H.; Vaughn, D.; Haller, D.G.; O’Dwyer, P.J. Rhabdomyolysis from cytochrome P-450 interaction of ketoconazole and simvastatin in prostate cancer. J. Urol. 2003, 169, 613. [Google Scholar] [CrossRef]
- Vlahakos, D.V.; Manginas, A.; Chilidou, D.; Zamanika, C.; Alivizatos, P.A. Itraconazole-induced rhabdomyolysis and acute renal failure in a heart transplant recipient treated with simvastatin and cyclosporine. Transplantation 2002, 73, 1962–1964. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, P.J.; Kantola, T.; Kivistö, K.T. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin. Pharmacol. Ther. 1998, 63, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.A.; Hoehns, J.D.; Purcell, J.L.; Friedman, R.L.; Elhawi, Y. Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin, amiodarone, and atazanavir. J. Am. Board Fam. Med. 2007, 20, 411–416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hsyu, P.H.; Schultz-Smith, M.D.; Lillibridge, J.H.; Lewis, R.H.; Kerr, B.M. Pharmacokinetic interactions between nelfinavir and 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors atorvastatin and simvastatin. Antimicrob. Agents Chemother. 2001, 45, 3445–3450. [Google Scholar] [CrossRef]
- Patel, A.M.; Shariff, S.; Bailey, D.G.; Juurlink, D.N.; Gandhi, S.; Mamdani, M.; Gomes, T.; Fleet, J.; Hwang, Y.J.; Garg, A.X. Statin toxicity from macrolide antibiotic coprescription: A population-based cohort study. Ann. Intern. Med. 2013, 158, 869–876. [Google Scholar] [CrossRef]
- Kaleem, Z.; Khan, J.A.; Mushtaq, Z.; Altaf, S.; Javed, I. Assessment of potential interaction between simvastatin and clarithromycin in healthy adult male subjects. Pak. J. Pharm. Sci. 2018, 31, 801–806. [Google Scholar]
- Xie, H.G.; Prasad, H.C.; Kim, R.B.; Stein, C.M. CYP2C9 allelic variants: Ethnic distribution and functional significance. Adv. Drug Deliv. Rev. 2002, 54, 1257–1270. [Google Scholar] [CrossRef]
- Neal, J.M.; Kunze, K.L.; Levy, R.H.; O’Reilly, R.A.; Trager, W.F. Kiiv, an in vivo parameter for predicting the magnitude of a drug interaction arising from competitive enzyme inhibition. Drug Metab. Dispos. 2003, 31, 1043–1048. [Google Scholar] [CrossRef]
- Klein, H.O.; Lang, R.; Weiss, E.; Di Segni, E.; Libhaber, C.; Guerrero, J.; Kaplinsky, E. The influence of verapamil on serum digoxin concentration. Circulation 1982, 65, 998–1003. [Google Scholar] [CrossRef]
- Härtter, S.; Sennewald, R.; Nehmiz, G.; Reilly, P. Oral bioavailability of dabigatran etexilate (Pradaxa®) after co-medication with verapamil in healthy subjects. Br. J. Clin. Pharmacol. 2013, 75, 1053–1062. [Google Scholar] [CrossRef]
- Georgiev, K.D.; Hvarchanova, N.; Georgieva, M.; Kanazirev, B. The role of the clinical pharmacist in the prevention of potential drug interactions in geriatric heart failure patients. Int. J. Clin. Pharm. 2019, 41, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, K.; Hvarchanova, N.; Georgieva, M.; Kanazirev, B. Potential drug-drug interactions in heart failure patients. Int. J. Pharm. Pharm. Sci. 2019, 11, 37–41. [Google Scholar] [CrossRef][Green Version]
- Georgiev, K.; Hvarchanova, N.; Georgieva, M.; Kanazirev, B. Potential drug interactions in heart failure patients involving cardiac glycosides. Int. J. Pharm. Res. 2019, 11, 524–529. [Google Scholar] [CrossRef]
- Marsousi, N.; Desmeules, J.A.; Rudaz, S.; Daali, Y. Prediction of drug-drug interactions using physiologically-based pharmacokinetic models of CYP450 modulators included in Simcyp software. Biopharm. Drug Dispos. 2018, 39, 3–17. [Google Scholar] [CrossRef]
- Certara. Simcyp PBPK. Available online: https://www.certara.com/software/simcyp-pbpk/ (accessed on 6 March 2025).
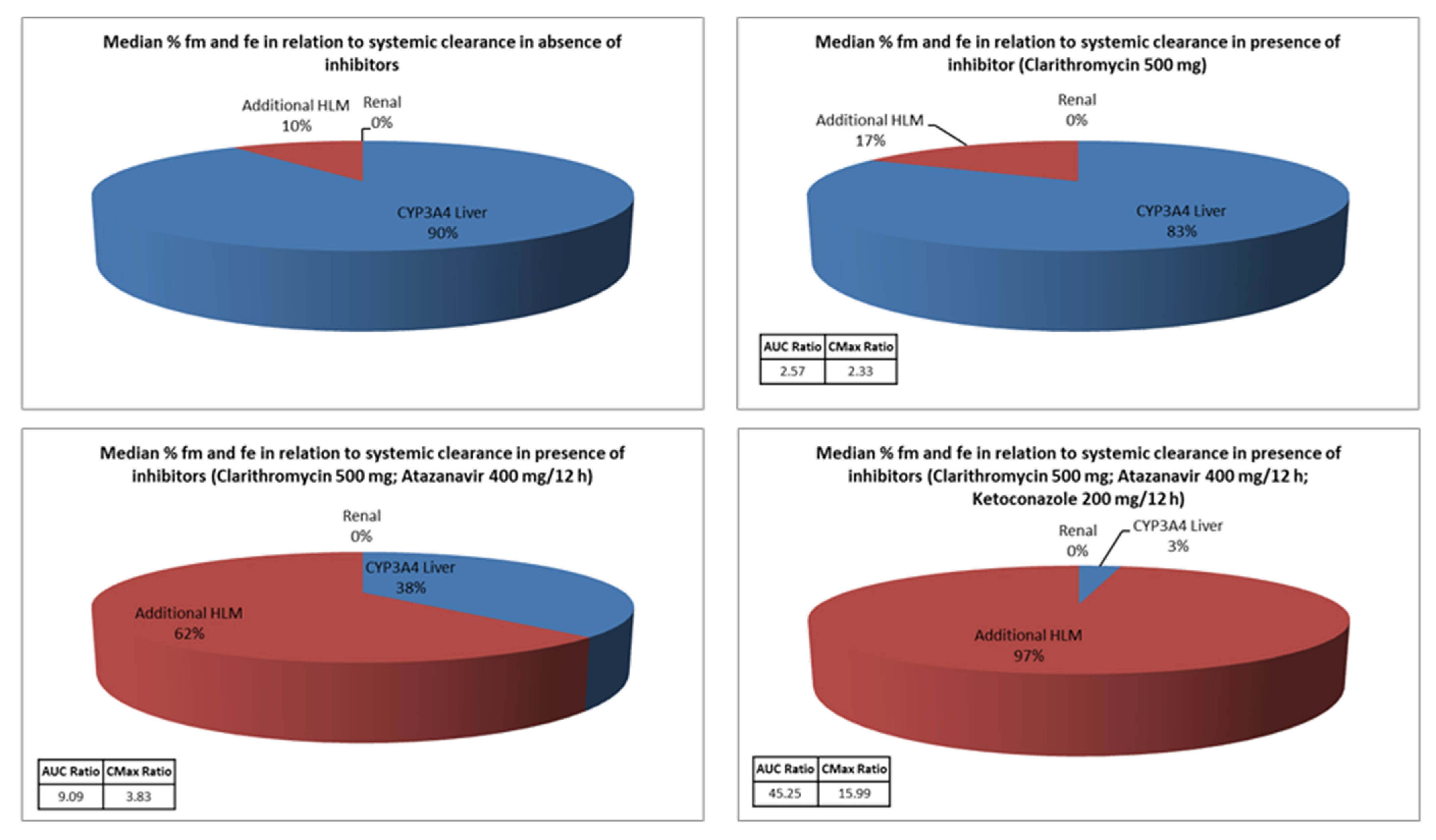
| Potential Drug–Drug Interactions | Severity/Risk Category | Frequency for 2014 | Frequency for 2015 | Mechanism of Interaction |
|---|---|---|---|---|
| HMG-CoA reductase inhibitors (statins) | ||||
| Simvastatin + 1,4-DHP-CCB | Major/D | 14 (5.9%) | 6 (2.4%) | CYP3A4 |
| Statin + Colchicine | Major/D | 1 (0.4%) | - | CYP3A4/OAT/PD |
| Statin + Fenofibrate | Major/C | 12 (5%) | 9 (3.6%) | PD |
| Rosuvastin + Amiodarone | Major/B | - | 3 (1.2%) | CYP2C9 |
| Statin + Verapamil | Major/D | - | 3 (1.2%) | CYP3A4 |
| Anticoagulants (coumarins) | ||||
| Acenocoumarol + Allopurinol | Moderate/D | 4 (1.7%) | 2 (0.8%) | CYP2C9 |
| Acenocoumarol + SMZ/TMP | Major/D | 1 (0.4%) | - | CYP2C9, PPB/PD |
| Acenocoumarol + Fenofibrate | Major/D | 1 (0.4%) | 4 (1.6%) | CYP2C9 |
| Acenocoumarol + Amiodarone | Major/D | 2 (0.8%) | 4 (1.6%) | CYP2C9 |
| Acenocoumarol + Thyreostatic | Moderate/D | 2 (0.8%) | 2 (0.8%) | PD |
| Acenocoumarol + NSAIDs | Moderate/D | - | 3 (1.2%) | N/A |
| New oral anticoagulants (NOACs) | ||||
| Apixaban + Aspirin | Major/D | - | 2 (0.8%) | PD |
| Apixaban + Clopidogrel | Major/D | - | 2 (0.8%) | PD? |
| Dabigatran + Amiodarone | Major/D | 1 (0.4%) | 2 (0.8%) | P-gp |
| Dabigatran + Verapamil | Major/D | 1 (0.4%) | - | P-gp |
| Dabigatran + Carvedilol | Major/D | 1 (0.4%) | 4 (1.6%) | P-gp |
| Dabigatran + Aspirin | Major/D | - | 2 (0.8%) | PD |
| Dabigatran + Fluconazole | Major/C | - | 1 (0.4%) | CYP3A4?, P-gp? |
| Rivaroxaban + Verapamil | Major/D | 1 (0.4%) | - | CYP3A4, P-gp |
| Antithrombotic drugs | ||||
| Clopidogrel + PPI | Moderate/D | 17 (7.1%) | 24 (9.7%) | CYP2C19 |
| Clopidogrel + Aspirin | Moderate/C | 12 (5%) | 10 (4%) | PD? |
| Ticagrelor + Aspirin | Major/D | - | 1 (0.4%) | PD? |
| Cardiac glycosides | ||||
| Digoxin + Amiodarone | Major/D | 1 (0.4%) | 1 (0.4%) | P-gp |
| Digoxin + Telmisartan | Moderate/C | 4 (1.7%) | 3 (1.2%) | P-gp |
| Digoxin + Colchicine | Moderate/C | 1 (0.4%) | - | P-gp |
| Inhibitors of CYP3A4 | AUCR ± SD | CmaxR ± SD |
|---|---|---|
| Clarithromycin 500 mg/24 h | 2.57 ± 0.51 | 2.33 ± 0.41 |
| Ketoconazole 200 mg/12 h | 28.42 ± 11.85 | 14.02 ± 5.90 |
| Ketoconazole 400 mg/24 h | 34.10 ± 15.75 | 15.77 ± 7.08 |
| Itraconazole 200 mg/24 h | 20.63 ± 8.79 | 11.56 ± 4.47 |
| Atazanavir 400 mg/12 h | 5.49 ± 3.75 | 2.28 ± 1.29 |
| Ritonavir 100 mg/12 h | 5.85 ± 3.59 | 2.67 ± 1.13 |
| Physicochemical and Pharmacokinetic Parameters | Warfarin | Acenocoumarol |
|---|---|---|
| Molecular weight | 308.3 | 353.3 |
| pKa/LogP | 5.0/2.9 | 5.0/1.98 |
| Vd (L/kg) | 0.08–0.12 | 0.22–0.52 |
| Plasma protein binding (PPB) | >99% | >98% |
| Plasma concentration (µM/L) | 1.5–8 | 0.03–0.3 |
| Terminal half-life (h) | S-War: 24–33 R-War: 35–58 | S-Ac: 1.8 R-Ac: 6.6 |
| Main metabolic pathway | CYP2C9 | CYP2C9 |
| Plasma clearance (L/h) | S-War: 0.1–1.0 R-War: 0.07–0.35 | S-Ac: 28.5 R-Ac: 1.9 |
| CYP2C9 Genotype | European Caucasian | Chinese | Japanese |
|---|---|---|---|
| *1/*1 | 0.672 | 0.924 | 0.96 |
| *1/*2 | 0.186 | 0.0024 | 0 |
| *1/*3 | 0.111 | 0.0712 | 0.0396 |
| *2/*2 | 0.011 | 0 | 0 |
| *2/*3 | 0.017 | 0 | 0 |
| *3/*3 | 0.003 | 0.0024 | 0.0004 |
| Population | Cmax (mg/L) | AUC (mg·h/L) |
|---|---|---|
| European Caucasian | 0.987 | 17.21 |
| Chinese | 1.267 | 23.58 |
| Japanese | 1.205 | 21.53 |
| Inhibitor of CYP2C9 | European Caucasian | Chinese | ||
|---|---|---|---|---|
| AUCR ± SD | CmaxR ± SD | AUCR ± SD | CmaxR ± SD | |
| Fluconazole 100 mg | 1.49 ± 0.16 | 1.31 ± 0.09 | 1.06 ± 0.06 | 1.02 ± 0.01 |
| Fluconazole 200 mg | 1.89 ± 0.33 | 1.56 ± 0.16 | 1.10 ± 0.11 | 1.03 ± 0.02 |
| Fluconazole 400 mg | 2.51 ± 0.65 | 1.94 ± 0.29 | 1.16 ± 0.17 | 1.04 ± 0.03 |
| Digoxin + Verapamil/Norverapamil | CmaxR ± SD | AUCR ± SD |
|---|---|---|
| Digoxin + Verapamil/Norverapamil 240 mg p.o. (80 mg/8 h) | 1.63 ± 0.28 | 1.32 ± 0.23 |
| Digoxin + Verapamil/Norverapamil 240 mg i.v. bolus (80 mg/8 h) | 1.09 ± 0.05 | 1.06 ± 0.04 |
| Digoxin + Verapamil (deactivation of P-gp in liver and GIT)/Norverapamil 240 mg | 1.25 ± 0.20 | 1.45 ± 0.24 |
| Digoxin + Verapamil/Norverapamil (deactivation of P-gp in liver and GIT) 240 mg | 1.27 ± 0.20 | 1.51 ± 0.25 |
| Digoxin + Verapamil (deactivation of P-gp in liver)/Norverapamil (deactivation of P-gp in liver) 240 mg | 1.25 ± 0.24 | 1.45 ± 0.20 |
| Digoxin + Verapamil (deactivation of P-gp in GIT)/Norverapamil (deactivation of P-gp in GIT) 240 mg | 1.14 ± 0.07 | 1.07 ± 0.05 |
| AUCR ± SD | CmaxR ± SD | |
|---|---|---|
| Simultaneous use | ||
| DE + verapamil/norverapamil | 2.61 ± 0.75 | 2.58 ± 0.80 |
| Dabigatran + verapamil/norverapamil | 2.34 ± 0.71 | 2.39 ± 0.75 |
| Application of the inhibitor after 2 h | ||
| DE + verapamil/norverapamil | 1.55 ± 0.28 | 1.26 ± 0.32 |
| Dabigatran + verapamil/norverapamil | 1.47 ± 0.26 | 1.42 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hvarchanova, N.; Radeva-Ilieva, M.; Georgiev, K.D. Using Physiologically Based Pharmacokinetic Models for Assessing Pharmacokinetic Drug–Drug Interactions in Patients with Chronic Heart Failure Taking Narrow Therapeutic Window Drugs. Pharmaceuticals 2025, 18, 477. https://doi.org/10.3390/ph18040477
Hvarchanova N, Radeva-Ilieva M, Georgiev KD. Using Physiologically Based Pharmacokinetic Models for Assessing Pharmacokinetic Drug–Drug Interactions in Patients with Chronic Heart Failure Taking Narrow Therapeutic Window Drugs. Pharmaceuticals. 2025; 18(4):477. https://doi.org/10.3390/ph18040477
Chicago/Turabian StyleHvarchanova, Nadezhda, Maya Radeva-Ilieva, and Kaloyan D. Georgiev. 2025. "Using Physiologically Based Pharmacokinetic Models for Assessing Pharmacokinetic Drug–Drug Interactions in Patients with Chronic Heart Failure Taking Narrow Therapeutic Window Drugs" Pharmaceuticals 18, no. 4: 477. https://doi.org/10.3390/ph18040477
APA StyleHvarchanova, N., Radeva-Ilieva, M., & Georgiev, K. D. (2025). Using Physiologically Based Pharmacokinetic Models for Assessing Pharmacokinetic Drug–Drug Interactions in Patients with Chronic Heart Failure Taking Narrow Therapeutic Window Drugs. Pharmaceuticals, 18(4), 477. https://doi.org/10.3390/ph18040477








