Involvement of CB1R and CB2R Ligands in Sleep Disorders and Addictive Behaviors in the Last 25 Years
Abstract
1. Introduction
2. Search Strategy
3. Phytocannabinoids, Endocannabinoids, and Synthetic Cannabinoids
4. Cannabinoid Receptors
CB1R/CB2R Ligands
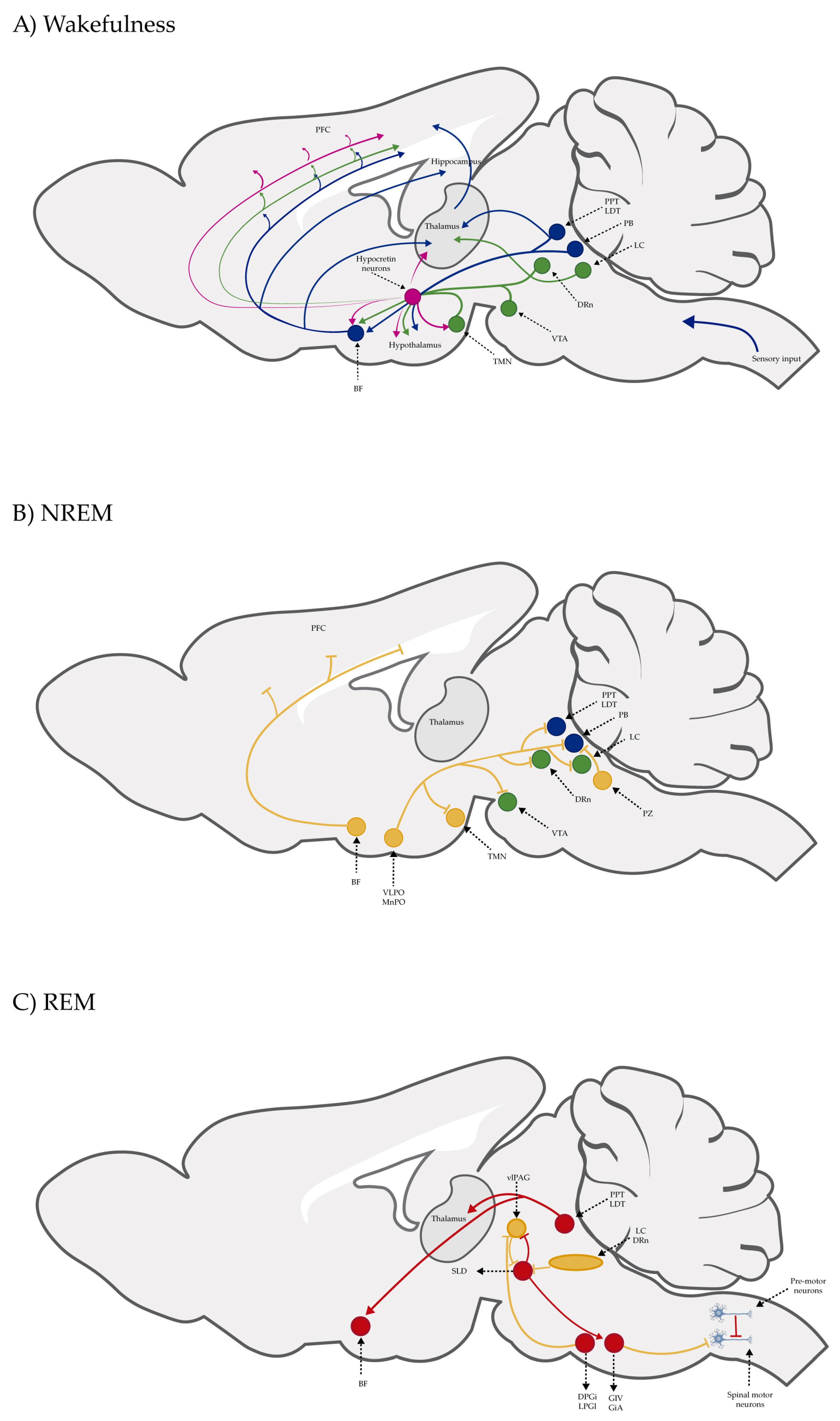
5. Sleep–Wake Cycle
Sleep Disorders
6. Addictive Behaviors
6.1. The Brain’s Reward System
6.2. CB1R/CB2R Ligands and the Brain’s Reward System
6.3. Psychostimulants and the Central Nervous System
6.4. Depressants of the Central Nervous System
7. Discussion
8. Conclusions
9. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bai, Y.; Jiang, M.; Xie, T.; Jiang, C.; Gu, M.; Zhou, X.; Yan, X.; Yuan, Y.; Huang, L. Archaeobotanical evidence of the use of medicinal cannabis in a secular context unearthed from south China. J. Ethnopharmacol. 2021, 275, 114114. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef]
- Charitos, I.A.; Gagliano-Candela, R.; Santacroce, L.; Bottalico, L. The cannabis spread throughout the continents and its therapeutic use in history. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 407–417. [Google Scholar]
- United Nations Office on Drugs and Crime. Available online: https://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1962-01-01_4_page005.html (accessed on 15 January 2025).
- Campbell, R.S.; Freeland, J.B. The hippie turns junkie: The emergence of a type. Int. J. Addict. 1974, 9, 719–730. [Google Scholar] [CrossRef]
- Kaufman, J.; Allen, J.R.; West, L.J. Runaways, hippies, and marijuana. Am. J. Psychiatry 1969, 126, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Kulick, M.A.V.; Espinoza-Kulick, A. Hippies. In Marijuana in America: Cultural, Political and Medical Controversies; Hawdon, J., Miller, B.L., Costello, M., Eds.; Bloomsbury Publishing: London, UK, 2022; pp. 435–440. [Google Scholar]
- Ball, D. Beat generation writers. In Marijuana in America: Cultural, political and medical controversies; Hawdon, J., Miller, B.L., Costello, M., Eds.; Bloomsbury Publishing: London, UK, 2022; pp. 101–106. [Google Scholar]
- Sandberg, S. Cannabis culture: A stable subculture in a changing world. Criminol. Crim. Justice 2013, 13, 63–79. [Google Scholar] [CrossRef]
- Dekeseredy, P.; Brownstein, H.; Haggerty, T.; Sedney, C.L. Using medical cannabis for chronic pain: A social-ecological framework. Cannabis Cannabinoid Res. 2024, 9, 1339–1348. [Google Scholar] [CrossRef]
- Mikuriya, T.H.; Aldrich, M.R. Cannabis 1988. Old drug, new dangers. The potency question. J. Psychoact. Drugs 1988, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Gertsch, J.; Pertwee, R.G.; Di Marzo, V. Phytocannabinoids beyond the Cannabis plant–Do they exist? Br. J. Pharmacol. 2010, 160, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J. Biochemistry and pharmacology of the endocannabinoids arachidonylethanolamide and 2-arachidonylglycerol. Prostaglandins Other Lipid Mediat. 2000, 61, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Zuurman, L.; Ippel, A.E.; Moin, E.; van Gerven, J.M. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br. J. Clin. Pharmacol. 2009, 67, 5–21. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and Endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef]
- McPartland, J.M.; Agraval, J.; Gleeson, D.; Heasman, K.; Glass, M. Cannabinoid receptors in invertebrates. J. Evol. Biol. 2006, 19, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; De Petrocellis, L. Why do cannabinoid receptors have more than one endogenous ligand? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3216–3228. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Schlicker, E. Effects of cannabinoids on neurotransmission. Handb. Exp. Pharmacol. 2005, 168, 327–365. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Kim, W.K.; Chavarría, I.; Hillard, C.J.; Mackie, K.; Tolón, R.M.; Williams, K.; Romero, J. A glial endogenous cannabinoid system is upregulated in the brains of macaques with simian immunodeficiency virus-induced encephalitis. J. Neurosci. 2005, 25, 2530–2536. [Google Scholar] [CrossRef]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef] [PubMed]
- Stella, N. THC and CBD: Similarities and differences between siblings. Neuron 2023, 111, 302–327. [Google Scholar] [CrossRef] [PubMed]
- Jakowiecki, J.; Abel, R.; Orzeł, U.; Pasznik, P.; Preissner, R.; Filipek, S. Allosteric modulation of the CB1 cannabinoid receptor by cannabidiol–A molecular modeling study of the N-Terminal domain and the allosteric-orthosteric coupling. Molecules 2021, 26, 2456. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoid pharmacology: The first 66 years. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S163–S171. [Google Scholar] [CrossRef]
- Spaderna, M.; Addy, P.H.; D’Souza, D.C. Spicing things up: Synthetic cannabinoids. Psychopharmacology 2013, 228, 525–540. [Google Scholar] [CrossRef]
- Hudson, S.; Ramsey, J. The emergence and analysis of synthetic cannabinoids. Drug Test. Anal. 2011, 3, 466–478. [Google Scholar] [CrossRef]
- United Nations Office on Drugs and Crime. Available online: https://www.unodc.org/LSS/SubstanceGroup/Details/ae45ce06-6d33-4f5f-916a-e873f07bde02 (accessed on 5 December 2024).
- Castaneto, M.S.; Gorelick, D.A.; Desrosiers, N.A.; Hartman, R.L.; Pirard, S.; Huestis, M.A. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol. Depend. 2014, 144, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.L.; Marusich, J.A.; Huffman, J.W. Moving around the molecule: Relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci. 2014, 97, 55–63. [Google Scholar] [CrossRef]
- Brents, L.K.; Prather, P.L. The K2/Spice phenomenon: Emergence, identification, legislation and metabolic characterization of synthetic cannabinoids in herbal incense products. Drug Metab. Rev. 2014, 46, 72–85. [Google Scholar] [CrossRef]
- Deng, H.; Verrico, C.D.; Kosten, T.R.; Nielsen, D.A. Psychosis and synthetic cannabinoids. Psychiatry Res. 2018, 268, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Tait, R.J.; Caldicott, D.; Mountain, D.; Hill, S.L.; Lenton, S. A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin. Toxicol. 2016, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, A.; Busardò, F.P.; Tittarelli, R.; Auwärter, V.; Giorgetti, R. Post-mortem toxicology: A systematic review of death cases involving synthetic cannabinoid receptor agonists. Front. Psychiatry 2020, 11, 464. [Google Scholar] [CrossRef]
- Sánchez-de la Torre, A.; Ezquerro-Herce, S.; Huerga-Gómez, A.; Sánchez-Martín, E.; Chara, J.C.; Matute, C.; Monory, K.; Mato, S.; Lutz, B.; Guzmán, M.; et al. CB1 receptors in NG2 cells mediate cannabinoid-evoked functional myelin regeneration. Prog. Neurobiol. 2024, 243, 102683. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, K.A.; Wiley, J.W. The Role of the Endocannabinoid System in the Brain-Gut Axis. Gastroenterology 2016, 151, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Burston, J.J.; Sim-Selley, L.J.; Harloe, J.P.; Mahadevan, A.; Razdan, R.K.; Selley, D.E.; Wiley, J.L. N-arachidonyl maleimide potentiates the pharmacological and biochemical effects of the endocannabinoid 2-arachidonylglycerol through inhibition of monoacylglycerol lipase. J. Pharmacol. Exp. Ther. 2008, 327, 546–553. [Google Scholar] [CrossRef]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Mock, E.D.; Gagestein, B.; van der Stelt, M. Anandamide and other N-acylethanolamines: A class of signaling lipids with therapeutic opportunities. Prog. Lipid Res. 2023, 89, 101194. [Google Scholar] [CrossRef]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid receptor-related orphan G Protein-coupled receptors. Adv. Pharmacol. 2017, 80, 223–247. [Google Scholar]
- Lutters, B.; Foley, P.; Koehler, P.J. The centennial lesson of encephalitis lethargica. Neurology 2018, 90, 563–567. [Google Scholar] [CrossRef]
- Neylan, T.C. Physiology of arousal: Moruzzi and Magoun’s ascending reticular activating system. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 250. [Google Scholar] [PubMed]
- Aserinksy, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar]
- Jones, B.E. Arousal and sleep circuits. Neuropsychopharmacology 2020, 45, 6–20. [Google Scholar] [CrossRef]
- Jones, B.E.; Hassani, O.K. The role of Hcrt/Orx and MCH neurons in sleep-wake state regulation. Sleep 2023, 36, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E.; Coronado-Álvarez, A.; López-Muciño, L.A.; Pastrana-Trejo, J.C.; Viana-Torre, G.; Barberena, J.J.; Soriano-Nava, D.M.; García-García, F. Neurobiology of Dream Activity and Effects of Stimulants on Dream. Curr. Top. Med. Chem. 2022, 22, 1280–1295. [Google Scholar] [CrossRef]
- Lavender, I.; McGregor, I.S.; Suraev, A.; Grunstein, R.R.; Hoyos, C.M. Cannabinoids, Insomnia, and Other Sleep Disorders. Chest 2022, 162, 452–465. [Google Scholar] [CrossRef]
- Carlini, E.A.; Cunha, J.M. Hypnotic and antiepileptic effects of cannabidiol. J. Clin. Pharmacol. 1981, 21 (Suppl. S1), 417S–427S. [Google Scholar] [CrossRef] [PubMed]
- Cousens, K.; DiMascio, A. (-) Delta 9 THC as an hypnotic. An experimental study of three dose levels. Psychopharmacologia 1973, 33, 355–364. [Google Scholar] [CrossRef]
- Hanlon, E.C. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 2020, 111, 104471. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; de Wit, H.; Hillard, C.J.; Van Cauter, E. Sleep Restriction Enhances the Daily Rhythm of Circulating Levels of Endocannabinoid 2-Arachidonoylglycerol. Sleep 2016, 39, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Monti, J. M Hypnoticlike effects of cannabidiol in the rat. Psychopharmacology 1977, 55, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Santucci, V.; Storme, J.J.; Soubrié, P.; Le Fur, G. Arousal-enhancing properties of the CB1 cannabinoid receptor antagonist SR 141716A in rats as assessed by electroencephalographic spectral and sleep-waking cycle analysis. Life Sci. 1996, 58, PL103–PL110. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Rodríguez, E.; Cabeza, R.; Méndez-Díaz, M.; Navarro, L.; Prospéro-García, O. Anandamide-induced sleep is blocked by SR141716A, a CB1 receptor antagonist and by U73122, a phospholipase C inhibitor. Neuroreport 2001, 12, 2131–2136. [Google Scholar] [CrossRef]
- Rueda-Orozco, P.E.; Soria-Gómez, E.; Montes-Rodríguez, C.J.; Pérez-Morales, M.; Prospéro-García, O. Intrahippocampal administration of anandamide increases REM sleep. Neurosci. Lett. 2010, 473, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Morales, M.; De La Herrán-Arita, A.K.; Méndez-Díaz, M.; Ruiz-Contreras, A.E.; Drucker-Colín, R.; Prospéro-García, O. 2-AG into the lateral hypothalamus increases REM sleep and cFos expression in melanin concentrating hormone neurons in rats. Pharmacol. Biochem. Behav. 2013, 108, 1–7. [Google Scholar] [CrossRef]
- Pérez-Morales, M.; Fajardo-Valdez, A.; Méndez-Díaz, M.; Ruiz-Contreras, A.E.; Prospéro-García, O. 2-Arachidonoylglycerol into the lateral hypothalamus improves reduced sleep in adult rats subjected to maternal separation. Neuroreport 2014, 25, 1437–1441. [Google Scholar] [CrossRef]
- Xue, J.; Xu, Z.; Zhang, J.; Hou, H.; Ge, L.; Yang, K. Systematic review/meta-analysis on the role of CB1R regulation in sleep-wake cycle in rats. J. Evid. Based Med. 2024; Advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Lavender, I.; Garden, G.; Grunstein, R.R.; Yee, B.J.; Hoyos, C.M. Using Cannabis and CBD to sleep: An updated review. Curr. Psychiatry Rep. 2024, 26, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Gates, P.J.; Albertella, L.; Copeland, J. The effects of cannabinoid administration on sleep: A systematic review of human studies. Sleep Med. Rev. 2014, 18, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Karacan, I.; Fernández-Salas, A.; Coggins, W.J.; Carter, W.E.; Williams, R.L.; Thornby, J.I.; Salis, P.J.; Okawa, M.; Villaume, J.P. Sleep electroencephalographic-electrooculographic characteristics of chronic marijuana users: Part I. Ann. N. Y. Acad. Sci. 1976, 282, 348–374. [Google Scholar] [CrossRef]
- Tassinari, C.A.; Ambrosetto, G.; Peraita-Adrado, M.R.; Gastaut, H. The neuropsychiatric syndrome of δ9-tetrahydrocannabinol and cannabis intoxication in naive subjects. In Marijuana and Medicine, 1st ed.; Nahas, G.G., Sutin, K.M., Harvey, D., Agurell, S., Pace, N., Cancro, R., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 649–664. [Google Scholar]
- Barratt, E.S.; Beaver, W.; White, R. The effects of marijuana on human sleep patterns. Biol. Psychiatry 1974, 8, 47–54. [Google Scholar] [PubMed]
- Nicholson, A.N.; Turner, C.; Stone, B.M.; Robson, P.J. Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J. Clin. Psychopharmacol. 2004, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pranikoff, K.; Karacan, I.; Larson, E.A.; Williams, R.L.; Thornby, J.I.; Hursch, C.J. Effects of marijuana smoking on the sleep EEG. JFMA 1973, 60, 28–31. [Google Scholar] [PubMed]
- Goonawardena, A.V.; Plano, A.; Robinson, L.; Ross, R.; Greig, I.; Pertwee, R.G.; Hampson, R.E.; Platt, B.; Riedel, G. Modulation of food consumption and sleep-wake cycle in mice by the neutral CB1 antagonist ABD459. Behav. Pharmacol. 2015, 26, 289–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murillo-Rodríguez, E.; Machado, S.; Rocha, N.B.; Budde, H.; Yuan, T.F.; Arias-Carrión, O. Revealing the role of the endocannabinoid system modulators, SR141716A, URB597 and VDM-11, in sleep homeostasis. Neuroscience 2016, 339, 433–449. [Google Scholar] [CrossRef]
- Macías-Triana, L.; Romero-Cordero, K.; Tatum-Kuri, A.; Vera-Barrón, A.; Millán-Aldaco, D.; Arankowsky-Sandoval, G.; Piomelli, D.; Murillo-Rodríguez, E. Exposure to the cannabinoid agonist WIN 55, 212–2 in adolescent rats causes sleep alterations that persist until adulthood. Eur. J. Pharmacol. 2020, 874, 172911. [Google Scholar] [CrossRef] [PubMed]
- Puskar, P.; Sengupta, T.; Sharma, B.; Nath, S.S.; Mallick, H.; Akhtar, N. Changes in sleep-wake cycle after microinjection of agonist and antagonist of endocannabinoid receptors at the medial septum of rats. Physiol. Behav. 2021, 237, 113448. [Google Scholar] [CrossRef]
- Xie, J.F.; Wang, L.X.; Ren, W.T.; Wang, C.; Gao, J.X.; Chen, H.L.; Zhao, X.Q.; Ren, Y.L.; Xie, Y.P.; Shao, Y.F.; et al. An α-hemoglobin-derived eptide (m)VD-Hemopressin (α) promotes NREM sleep via the CB1 cannabinoid receptor. Front. Pharmacol. 2023, 14, 1213215. [Google Scholar] [CrossRef]
- Belali, R.; Mard, S.A.; Khoshnam, S.E.; Bavarsad, K.; Sarkaki, A.; Farbood, Y. anandamide attenuates neurobehavioral deficits and EEG irregularities in the chronic sleep deprivation rats: The role of oxidative stress and neuroinflammation. Neurochem. Res. 2024, 49, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Belali, R.; Mard, S.A.; Khoshnam, S.E.; Bavarsad, K.; Sarkaki, A.; Farbood, Y. Anandamide improves food intake and orexinergic neuronal activity in the chronic sleep deprivation induction model in rats by modulating the expression of the CB1 receptor in the lateral hypothalamus. Neuropeptides 2023, 101, 102336. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.C.; Occelli Hanbury-Brown, C.V.; Anderson, L.L.; Bedoya-Pérez, M.A.; Udoh, M.; Sharman, L.A.; Raymond, J.S.; Doohan, P.T.; Ametovski, A.; McGregor, I.S. A sleepy cannabis constituent: Cannabinol and its active metabolite influence sleep architecture in rats. Neuropsychopharmacology 2024, 50, 586–595. [Google Scholar] [CrossRef]
- Prasad, B.; Radulovacki, M.G.; Carley, D.W. Proof of concept trial of dronabinol in obstructive sleep apnea. Front. Psychiatry 2013, 4, 1. [Google Scholar]
- Calik, M.W.; Carley, D.W. Effects of cannabinoid agonists and antagonists on sleep and breathing in Sprague-Dawley rats. Sleep 2017, 40, zsx112. [Google Scholar] [PubMed]
- Kisiolek, J.N.; Flores, V.A.; Ramani, A.; Butler, B.; Haughian, J.M.; Stewart, L.K. Eight weeks of daily cannabidiol supplementation improves sleep quality and immune cell cytotoxicity. Nutrients 2023, 15, 4173. [Google Scholar] [CrossRef]
- First, M.B. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J. Nerv. Ment. Dis. 2013, 201, 727–729. [Google Scholar] [PubMed]
- Grant, J.E.; Chamberlain, S.R. Expanding the definition of addiction: DSM-5 vs. ICD-11. CNS Spectr. 2016, 21, 300–303. [Google Scholar]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar]
- Zehra, A.; Burns, J.; Liu, C.K.; Manza, P.; Wiers, C.E.; Volkow, N.D.; Wang, G.J. Cannabis addiction and the brain: A review. J. Neuroimmune Pharmacol. 2018, 13, 438–452. [Google Scholar]
- Baumeister, A.A. Serendipity and the cerebral localization of pleasure. J. Hist. Neurosci. 2006, 15, 92–98. [Google Scholar]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar]
- Castro, D.C.; Cole, S.L.; Berridge, K.C. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: Interactions between homeostatic and reward circuitry. Front. Syst. Neurosci. 2015, 9, 90. [Google Scholar]
- Tindell, A.J.; Berridge, K.C.; Zhang, J.; Peciña, S.; Aldridge, J.W. Ventral pallidal neurons code incentive motivation: Amplification by mesolimbic sensitization and amphetamine. Eur. J. Neurosci. 2005, 22, 2617–2634. [Google Scholar] [PubMed]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [PubMed]
- Bloomfield, M.A.; Ashok, A.H.; Volkow, N.D.; Howes, O.D. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 2016, 539, 369–377. [Google Scholar] [PubMed]
- De Luca, M.A.; Solinas, M.; Bimpisidis, Z.; Goldberg, S.R.; Di Chiara, G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 2012, 63, 161–168. [Google Scholar] [PubMed]
- Pontieri, F.E.; Tanda, G.; Di Chiara, G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. USA 1995, 92, 12304–12308. [Google Scholar]
- Bloom, A.S.; Dewey, W.L. A comparison of some pharmacological actions of morphine and delta9-tetrahydrocannabinol in the mouse. Psychopharmacology 1978, 57, 243–248. [Google Scholar] [PubMed]
- Poddar, M.K.; Dewey, W.L. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J. Pharmacol. Exp. Ther. 1980, 214, 63–67. [Google Scholar]
- Seif, T.; Makriyannis, A.; Kunos, G.; Bonci, A.; Hopf, F.W. The endocannabinoid 2-arachidonoylglycerol mediates D1 and D2 receptor cooperative enhancement of rat nucleus accumbens core neuron firing. Neuroscience 2011, 193, 21–33. [Google Scholar]
- Volkow, N.D.; Wang, G.J.; Telang, F.; Fowler, J.S.; Alexoff, D.; Logan, J.; Jayne, M.; Wong, C.; Tomasi, D. Decreased dopamine brain reactivity in marijuana abusers is associated with negative emotionality and addiction severity. Proc. Natl. Acad. Sci. USA 2014, 111, E3149–E3156. [Google Scholar]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar]
- Robinson, T.E.; Berridge, K.C. The Incentive-Sensitization Theory of Addiction 30 Years On. Annu. Rev. Psychol. 2025, 76, 29–58. [Google Scholar] [CrossRef]
- Chomchai, C.; Chomchai, S. Global patterns of methamphetamine use. Curr. Opin. Psychiatry 2015, 28, 269–274. [Google Scholar] [CrossRef]
- Karila, L.; Petit, A.; Lowenstein, W.; Reynaud, M. Diagnosis and consequences of cocaine addiction. Curr. Med. Chem. 2012, 19, 5612–5618. [Google Scholar] [CrossRef]
- Morley, K.C.; Cornish, J.L.; Faingold, A.; Wood, K.; Haber, P.S. Pharmacotherapeutic agents in the treatment of methamphetamine dependence. Exp. Opin. Investig. Drugs 2017, 26, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Psychostimulants. In Neurobiology of Addiction, 1st ed.; Koob, G.F., Le Moal, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 69–109. [Google Scholar]
- Luján, M.Á.; Castro-Zavala, A.; Alegre-Zurano, L.; Valverde, O. Repeated Cannabidiol treatment reduces cocaine intake and modulates neural proliferation and CB1R expression in the mouse hippocampus. Neuropharmacology 2018, 143, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Luján, M.Á.; Alegre-Zurano, L.; Martín-Sánchez, A.; Cantacorps, L.; Valverde, O. CB1 receptor antagonist AM4113 reverts the effects of cannabidiol on cue and stress-induced reinstatement of cocaine-seeking behaviour in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110462. [Google Scholar] [CrossRef] [PubMed]
- Anooshe, M.; Nouri, K.; Karimi-Haghighi, S.; Mousavi, Z.; Haghparast, A. Cannabidiol efficiently suppressed the acquisition and expression of methamphetamine-induced conditioned place preference in the rat. Behav. Brain Res. 2021, 404, 113158. [Google Scholar] [CrossRef]
- Karimi-Haghighi, S.; Mahmoudi, M.; Sayehmiri, F.; Mozafari, R.; Haghparast, A. Endocannabinoid system as a therapeutic target for psychostimulants relapse: A systematic review of preclinical studies. Eur. J. Pharmacol. 2023, 951, 175669. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, P.; Miszkiel, J.; McCreary, A.C.; Filip, M.; Papp, M.; Przegaliński, E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012, 1444, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.J.; Feng, Z.W.; Galaj, E.; Bi, G.H.; Xue, Y.; Liang, Y.; McGuire, T.; Xie, X.Q.; Xi, Z.X. Xie2-64, a novel CB2 receptor inverse agonist, reduces cocaine abuse-related behaviors in rodents. Neuropharmacology 2020, 176, 108241. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.X.; Peng, X.Q.; Li, X.; Song, R.; Zhang, H.Y.; Liu, Q.R.; Yang, H.J.; Bi, G.H.; Li, J.; Gardner, E.L. Brain cannabinoid CB2 receptors modulate cocaine’s actions in mice. Nat. Neurosci. 2011, 14, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hiranita, T.; Nawata, Y.; Sakimura, K.; Yamamoto, T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology 2008, 55, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.B.; Bastos, J.R.; Costa, R.B.; Aguiar, D.C.; Moreira, F.A. The roles of cannabinoid CB1 and CB2 receptors in cocaine-induced behavioral sensitization and conditioned place preference in mice. Psychopharmacology 2020, 237, 385–394. [Google Scholar] [CrossRef]
- Canseco-Alba, A.; Schanz, N.; Sanabria, B.; Zhao, J.; Lin, Z.; Liu, Q.R.; Onaivi, E.S. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav. Brain Res. 2019, 360, 286–297. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Opioids. In Neurobiology of Addiction, 1st ed.; Koob, G.F., Le Moal, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 121–159. [Google Scholar]
- Lecca, D.; Scifo, A.; Pisanu, A.; Valentini, V.; Piras, G.; Sil, A.; Cadoni, C.; Di Chiara, G. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology 2020, 166, 107974. [Google Scholar] [CrossRef]
- De Vries, T.J.; Homberg, J.R.; Binnekade, R.; Raasø, H.; Schoffelmeer, A.N.M. Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology 2003, 168, 164–169. [Google Scholar] [CrossRef]
- Alvarez-Jaimes, L.; Polis, I.; Parsons, L.H. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology 2008, 33, 2483–2493. [Google Scholar] [CrossRef]
- Maguire, D.R.; France, C.P. Effects of daily delta-9-tetrahydrocannabinol treatment on heroin self-administration in rhesus monkeys. Behav. Pharmacol. 2016, 27, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Oliva, I.; Kazi, F.; Cantwell, L.N.; Thakur, G.A.; Crystal, J.D.; Hohmann, A.G. Negative allosteric modulation of CB1 cannabinoid receptor signaling decreases intravenous morphine self-administration and relapse in mice. Addict. Biol. 2024, 29, e13429. [Google Scholar] [CrossRef]
- Norwood, C.S.; Cornish, J.L.; Mallet, P.E.; McGregor, I.S. Pre-exposure to the cannabinoid receptor agonist CP 55940 enhances morphine behavioral sensitization and alters morphine self-administration in Lewis rats. Eur. J. Pharmacol. 2003, 465, 105–114. [Google Scholar] [CrossRef]
- Imtiaz, S.; Elton-Marshall, T.; Rehm, J. Cannabis liberalisation and the US opioid crisis. BMJ 2021, 372, n163. [Google Scholar] [CrossRef] [PubMed]
- Nurco, D.N.; Kinlock, T.W.; Hanlon, T.E.; Ball, J.C. Nonnarcotic drug use over an addiction career--a study of heroin addicts in Baltimore and New York City. Compr. Psychiatry 1988, 29, 450–459. [Google Scholar] [CrossRef]
- Pritchett, C.E.; Flynn, H.; Wang, Y.; Polston, J.E. Medical cannabis patients report improvements in health functioning and reductions in opiate use. Subst. Use Misuse 2022, 57, 1883–1892. [Google Scholar] [CrossRef]
- Scavone, J.L.; Sterling, R.C.; Van Bockstaele, E.J. Cannabinoid and opioid interactions: Implications for opiate dependence and withdrawal. Neuroscience 2013, 248, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M. Opiate withdrawal. Addiction 1994, 89, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, D.R. Opiate withdrawal syndrome: Acute and protracted aspects. Ann. N. Y. Acad. Sci. 1981, 362, 183–186. [Google Scholar] [CrossRef]
- Houser, S.J.; Eads, M.; Embrey, J.P.; Welch, S.P. Dynorphin B and spinal analgesia: Induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Res. 2000, 857, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Pugh, G., Jr.; Smith, P.B.; Dombrowski, D.S.; Welch, S.P. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J. Pharmacol. Exp. Ther. 1996, 279, 608–616. [Google Scholar] [CrossRef]
- Pugh, G., Jr.; Mason, D.J., Jr.; Combs, V.; Welch, S.P. Involvement of dynorphin B in the antinociceptive effects of the cannabinoid CP55,940 in the spinal cord. J. Pharmacol. Exp. Ther. 1997, 281, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, J.; Corchero, J.; Romero, J.; Fernández-Ruiz, J.J.; Ramos, J.A.; Fuentes, J.A. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol. Sci. 1999, 20, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Corchero, J.; Oliva, J.M.; García-Lecumberri, C.; Martin, S.; Ambrosio, E.; Manzanares, J. Repeated administration with Delta9-tetrahydrocannabinol regulates mu-opioid receptor density in the rat brain. J. Psychopharmacol. 2004, 18, 54–58. [Google Scholar] [PubMed]
- Gueye, A.B.; Pryslawsky, Y.; Trigo, J.M.; Poulia, N.; Delis, F.; Antoniou, K.; Loureiro, M.; Laviolette, S.R.; Vemuri, K.; Makriyannis, A.; et al. The CB1 neutral antagonist AM4113 retains the therapeutic efficacy of the inverse agonist rimonabant for nicotine dependence and weight loss with better psychiatric tolerability. Int. J. Neuropsychopharmacol. 2016, 19, pyw068. [Google Scholar]
- Myers, A.M.; Siegele, P.B.; Foss, J.D.; Tuma, R.F.; Ward, S.J. Single and combined effects of plant-derived and synthetic cannabinoids on cognition and cannabinoid-associated withdrawal signs in mice. Br. J. Pharmacol. 2019, 176, 1552–1567. [Google Scholar] [PubMed]
- Spiller, K.J.; Bi, G.-H.; He, Y.; Galaj, E.; Gardner, E.L.; Xi, Z.X. Cannabinoid CB 1 and CB 2 receptor mechanisms underlie cannabis reward and aversion in rats. Br. J. Pharmacol. 2019, 176, 1268–1281. [Google Scholar]
- Xi, Z.X.; Muldoon, P.; Wang, X.F.; Bi, G.H.; Damaj, M.I.; Lichtman, A.H.; Pertwee, R.G.; Gardner, E.L. Δ8-tetrahydrocannabivarin has potent anti-nicotine effects in several rodent models of nicotine dependence. Br. J. Pharmacol. 2019, 176, 4773–4784. [Google Scholar] [PubMed]
- Murray, C.H.; Glazer, J.E.; Lee, R.; Nusslock, R.; de Wit, H. Δ9-THC reduces reward-related brain activity in healthy adults. Psychopharmacology 2022, 239, 2829–2840. [Google Scholar]
- Galaj, E.; Hempel, B.; Moore, A.; Klein, B.; Bi, G.H.; Gardner, E.L.; Seltzman, H.H.; Xi, Z.X. Therapeutic potential of PIMSR, a novel CB1 receptor neutral antagonist, for cocaine use disorder: Evidence from preclinical research. Transl. Psychiatry 2022, 12, 286. [Google Scholar] [PubMed]
- Seltzman, H.H.; Maitra, R.; Bortoff, K.; Henson, J.; Reggio, P.H.; Wesley, D.; Tam, J. Metabolic Profiling of CB1 Neutral Antagonists. Methods Enzymol. 2017, 593, 199–215. [Google Scholar]
- McReynolds, J.R.; Wolf, C.P.; Starck, D.M.; Mathy, J.C.; Schaps, R.; Krause, L.A.; Hillard, C.J.; Mantsch, J.R. Role of mesolimbic cannabinoid receptor 1 in stress-driven increases in cocaine self-administration in male rats. Neuropsychopharmacology 2023, 48, 1121–1132. [Google Scholar] [PubMed]
- Han, S.; Thoresen, L.; Zhu, X.; Narayanan, S.; Jung, J.K.; Strah-Pleynet, S.; Decaire, M.; Choi, K.; Xiong, Y.; Yue, D.; et al. Discovery of 1a,2,5,5a-tetrahydro-1h-2,3-diaza-cyclopropa[a]pentalen-4-carboxamides as potent and selective CB2 receptor agonists. Bioorganic Med. Chem. Lett. 2015, 25, 322–326. [Google Scholar]
- Li, J.; Wang, H.; Liu, D.; Li, X.; He, L.; Pan, J.; Shen, Q.; Peng, Y. CB2R activation ameliorates late adolescent chronic alcohol exposure-induced anxiety-like behaviors during withdrawal by preventing morphological changes and suppressing NLRP3 inflammasome activation in prefrontal cortex microglia in mice. Brain Behav. Immun. 2023, 110, 60–79. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Xu, Z.; Rajic, G.; Makriyannis, A.; Romero, J.; Hillard, C.; Mackie, K.; Hohmann, A.G. Peripheral sensory neuron CB2 cannabinoid receptors are necessary for both CB2-mediated antinociceptive efficacy and sparing of morphine tolerance in a mouse model of anti-retroviral toxic neuropathy. Pharmacol. Res. 2023, 187, 106560. [Google Scholar] [CrossRef]
- Saleska, J.L.; Bryant, C.; Kolobaric, A.; D’Adamo, C.R.; Colwell, C.S.; Loewy, D.; Chen, J.; Pauli, E.K. The safety and comparative effectiveness of non-psychoactive cannabinoid formulations for the improvement of sleep: A double-blinded, randomized controlled trial. J. Am. Nutr. Assoc. 2024, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suraev, A.S.; Marshall, N.S.; Vandrey, R.; McCartney, D.; Benson, M.J.; McGregor, I.S.; Grunstein, R.R.; Hoyos, C.M. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med. Rev. 2020, 53, 101339. [Google Scholar] [CrossRef]
- Chye, Y.; Christensen, E.; Solowij, N.; Yücel, M. The endocannabinoid system and cannabidiol’s promise for the treatment of substance use disorder. Front. Psychiatry 2019, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- García-Blanco, A.; Ramírez-López, Á.; Navarrete, F.; García-Gutiérrez, M.S.; Manzanares, J.; Martín-García, E.; Maldonado, R. Role of CB2 cannabinoid receptor in the development of food addiction in male mice. Neurobiol. Dis. 2023, 179, 106034. [Google Scholar] [CrossRef] [PubMed]
- Onaivi, E.S.; Ishiguro, H.; Gong, J.P.; Patel, S.; Meozzi, P.A.; Myers, L.; Perchuk, A.; Mora, Z.; Tagliaferro, P.A.; Gardner, E.; et al. Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann. N. Y. Acad. Sci. 2008, 1139, 434–449. [Google Scholar] [CrossRef] [PubMed]
- Gobira, P.H.; Joca, S.R.; Moreira, F.A. Roles of cannabinoid CB1 and CB2 receptors in the modulation of psychostimulant responses. Acta Neuropsychiatr. 2024, 36, 67–77. [Google Scholar] [CrossRef] [PubMed]
- He, X.H.; Jordan, C.J.; Vemuri, K.; Bi, G.H.; Zhan, J.; Gardner, E.L.; Makriyannis, A.; Wang, Y.L.; Xi, Z.X. Cannabinoid CB1 receptor neutral antagonist AM4113 inhibits heroin self-administration without depressive side effects in rats. Acta Pharmacol. Sin. 2019, 40, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Schindler, C.W.; Redhi, G.H.; Vemuri, K.; Makriyannis, A.; Le Foll, B.; Bergman, J.; Goldberg, S.R.; Justinova, Z. Blockade of nicotine and cannabinoid reinforcement and relapse by a cannabinoid CB1-receptor neutral antagonist AM4113 and inverse agonist rimonabant in squirrel monkeys. Neuropsychopharmacology 2016, 41, 2283–2293. [Google Scholar] [CrossRef]
- Soler-Cedeño, O.; Alton, H.; Bi, G.H.; Linz, E.; Ji, L.; Makriyannis, A.; Xi, Z.X. AM6527, a neutral CB1 receptor antagonist, suppresses opioid taking and seeking, as well as cocaine seeking in rodents without aversive effects. Neuropsychopharmacology 2024, 49, 1678–1688. [Google Scholar] [CrossRef]
- Guenther, K.G.; Wirt, J.L.; Oliva, I.; Saberi, S.A.; Crystal, J.D.; Hohmann, A.G. The cannabinoid CB2 agonist LY2828360 suppresses neuropathic pain behavior and attenuates morphine tolerance and conditioned place preference in rats. Neuropharmacology 2025, 265, 110257. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Slivicki, R.A.; Thomaz, A.C.; Crystal, J.D.; Mackie, K.; Hohmann, A.G. The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur. J. Pharmacol. 2020, 886, 173544. [Google Scholar] [CrossRef] [PubMed]
- Spanagel, R. Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues Clin. Neurosci. 2020, 22, 241–250. [Google Scholar] [CrossRef]
- Hurd, Y.L.; Spriggs, S.; Alishayev, J.; Winkel, G.; Gurgov, K.; Kudrich, C.; Oprescu, A.M.; Salsitz, E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am. J. Psychiatry 2019, 176, 911–922. [Google Scholar] [CrossRef]
- Kaul, M.; Zee, P.C.; Sahni, A.S. Effects of cannabinoids on sleep and their therapeutic potential for sleep disorders. NeuroTherapeutics 2021, 18, 217–227. [Google Scholar] [PubMed]
- van den Brink, W.; van Ree, J.M. Pharmacological treatments for heroin and cocaine addiction. Eur. Neuropsychopharmacol. 2003, 13, 476–487. [Google Scholar] [CrossRef]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic potential of cannabis, cannabidiol, and cannabinoid-based pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Robson, P. Therapeutic aspects of cannabis and cannabinoids. Br. J. Psychiatry 2001, 178, 107–115. [Google Scholar]
- Wiskerke, J.; Pattij, T.; Schoffelmeer, A.N.; De Vries, T.J. The role of CB1 receptors in psychostimulant addiction. Addict. Biol. 2008, 13, 225–238. [Google Scholar] [PubMed]
- Cohen, Y.; Kolodziej, A.; Morningstar, M. Seventeen years since Rimonabant’s downfall: Reassessing its suicidality risk profile. Obesity 2024, 32, 1235–1244. [Google Scholar] [PubMed]
- Ettaro, R.; Laudermilk, L.; Clark, S.D.; Maitra, R. Behavioral assessment of Rimonabant under acute and chronic conditions. Behav. Brain Res. 2020, 390, 112697. [Google Scholar] [PubMed]
- Han, J.; Kesner, P.; Metna-Laurent, M.; Duan, T.; Xu, L.; Georges, F.; Koehl, M.; Abrous, D.N.; Mendizabal-Zubiaga, J.; Grandes, P.; et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 2012, 148, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Soler-Cedeno, O.; Xi, Z.X. Neutral CB1 Receptor Antagonists as Pharmacotherapies for Substance Use Disorders: Rationale, Evidence, and Challenge. Cells 2022, 11, 3262. [Google Scholar] [CrossRef] [PubMed]
- Angarita, G.A.; Emadi, N.; Hodges, S.; Morgan, P.T. Sleep abnormalities associated with alcohol, cannabis, cocaine, and opiate use: A comprehensive review. Addict. Sci. Clin. Pract. 2016, 11, 9. [Google Scholar] [PubMed]
| Classification | Chemical Structure | Animal Model/Clinical Trial | Route of Administration, Dose, and Period | Main Effects | Ref. |
|---|---|---|---|---|---|
| CB1R | |||||
| ABD459/Antagonist | 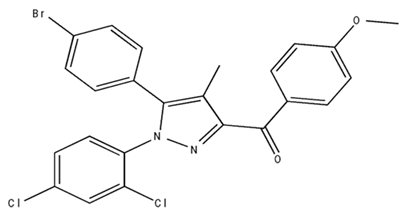 | C57Bl/6 mice | IP, 3, 10 and 20 mg/kg, 1 day | Reduced REM sleep, without alterations to NREM and total sleep. | [68] |
| SR141716A/Antagonist |  | Male Wistar rats | IP, 5, 10 or 20 mg/kg, 1 day | Prevention of the sleep rebound in total sleep-deprived rats in a dose-dependent fashion. | [69] |
| WIN 55, 212–2/Agonist | 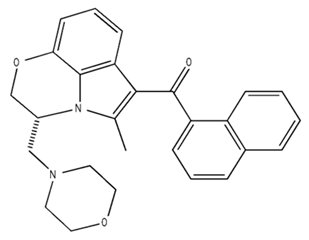 | Male Wistar rats | IP, 0.1, 0.3, or 1.0 mg/kg, 14 days | Alterations in sleep patterns; decreased wakefulness and enhanced REM sleep in adult rats. | [70] |
| WIN 55,212–2 mesylate salt/Agonist | 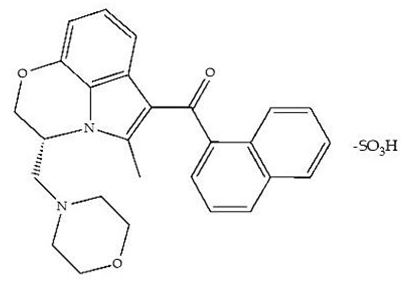 | Male Wistar rats | Medial septum MI, 200 nL, 6 h | MI of a CB1R agonist decreased NREM and increased total sleep time with a high percentage of REM sleep, while the MI of a CB1R antagonist decreased NREM and REM sleep. | [71] |
| LY 320,135/Antagonist | 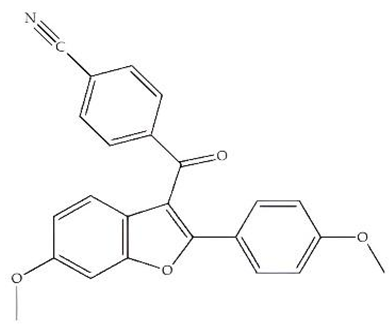 | ||||
| VD-hemopressin α/Agonist | Val-Asp-Pro-Val-Asn-Phe-Lys-Leu-Leu-Ser-His-OH | Male Sprague-Dawley rats | ICV 6.7, 13.4, and 20.1 nmol, 24 h | NREM sleep enhancement and stabilization. | [72] |
| Anandamide/Agonist |  | Male Wistar rats | Oral, 50 mg, 8 wk | Improved sleep quality and increased Natural Killer immune cell function. | [73] |
| Anandamide/Agonist |  | Male Wistar rats | Oral, 20 mg/kg, 21 days | Reduction in anxiety symptoms in sleep-deprived rats. | [74] |
| Cannabinol (CBN)/Partial agonist |  | Male Long–Evans rats | IP, 10, 30, and 100 mg/kg, 3 injections, 15 days | Increased total sleep time, also increased REM and NREM sleep. The impact on NREM was similar to the administration of zolpidem. | [75] |
| CB2R | |||||
| Dronabinol/Agonist (synthetic THC) |  | Clinical | Oral, 2.5, 5, and 10 mg, 21 days | Reduction in obstructive sleep apnea. | [76] |
| Dronabinol/Agonist (synthetic THC) |  | Male Sprague-Dawley rats | IP, 5 mg/kg, and combined 5/5 mg/kg, 6 h | Dronabinol decreased the percent of REM sleep and apnea during sleep, but when combined with the antagonist, the reduction in sleep apnea was reversed. | [77] |
| AM 251/Antagonist | 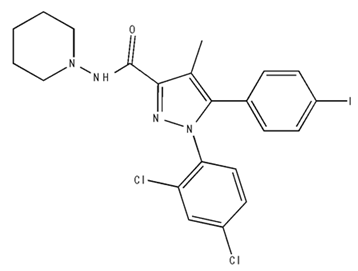 | ||||
| AM 630/Antagonist |  | ||||
| Cannabidiol/Negative allosteric modulator of CB1R | 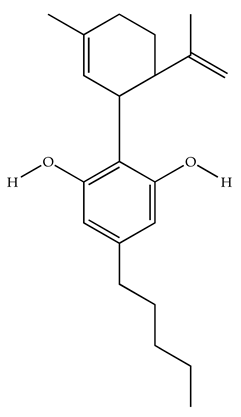 | Clinical | Oral, 50 mg, 8 wk | Improved sleep quality and increased Natural Killer immune cell function. | [78] |
| Classification | Chemical Structure | Animal Model/Clinical Trial | Route of Administration, Dose, and Period | Main Effects | Ref. |
|---|---|---|---|---|---|
| CB1R | |||||
| AM4113/Neutral antagonist |  | Male Long–Evans and Wistar rats | IP, 1,3, 10 mg/kg, 10 days | Reduced nicotine consumption, decreased motivation for nicotine, and diminished reinstatement of nicotine-seeking behavior. | [129] |
| SR141716/Antagonist/Inverse agonist | 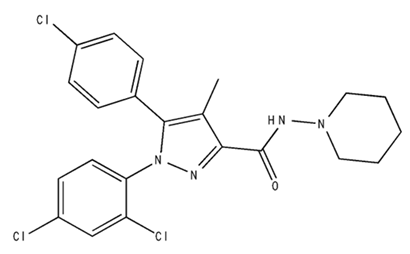 | Male C57Bl6 mice | IP, 10 mg/kg, 1 day | Precipitation of withdrawal signs in mice chronically treated with THC or WIN55,212. | [130] |
| Δ9-THC/CB1 and CB2 agonist |  | Adult male Long–Evans rats | IP, 3 mg/kg | Biphasic effects mildly enhance BSR at low doses, but inhibit it at higher doses. Pretreatment with AM251 attenuated low dose-enhanced BSR, while AM630 attenuated high dose-inhibited BSR. | [131] |
| WIN55,212-2 CB1 and CB2/Agonist | 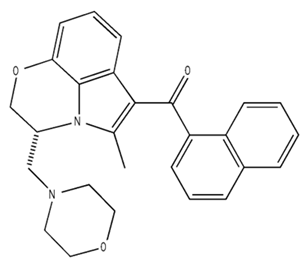 | ||||
| AM251 CB1/Antagonist |  | ||||
| AM630 CB2/Antagonist | 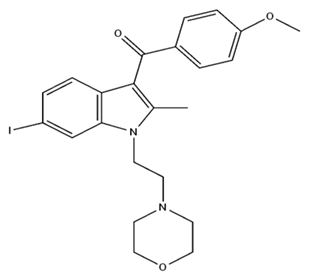 | ||||
| Δ8-THCV/CB1 antagonist and CB2 agonist | 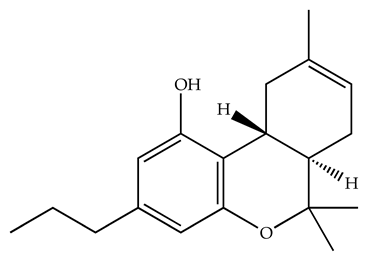 | Male alcohol-preferring Wistar rats and male drug-naïve ICR mice | IP, 3 or 10 mg/kg, and 10 or 20 mg/kg | Attenuated intravenous nicotine self-administration and both cue-induced and nicotine-induced relapse to nicotine-seeking behavior in rats. In mice it also significantly attenuated nicotine-induced conditioned place preference and nicotine withdrawal. | [132] |
| ∆9-THC/Agonist | 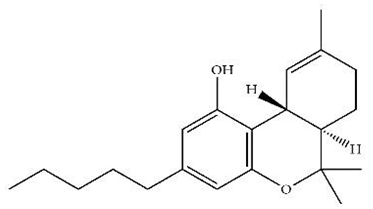 | Clinical | Oral, 7.5 and 15 mg | Dampens respond to reward and loss feedback, which may reflect an “amotivational” state. | [133] |
| PIMSR/Antagonist |  | Male Long–Evans rats and male and female wild-type (C57/BL6J) mice | IP, 3, 10 and 30 mg/kg 4 wk | Dose dependently inhibited cocaine self-administration, decreased motivation to seek cocaine under progressive ratio reinforcement, and reduced cue-induced reinstatement of cocaine-seeking behavior. | [134,135] |
| AM251/Antagonist/Inverse agonist | 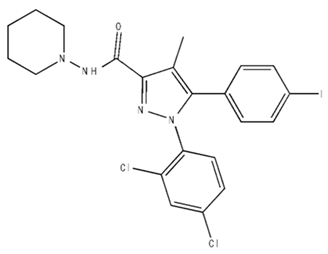 | Male Sprague-Dawley rats | IP, 1 mg/kg 2 wk | Attenuated cocaine intake only in rats with a history of stress. | [136] |
| CB2R | |||||
| APD 371/CB2 agonist | 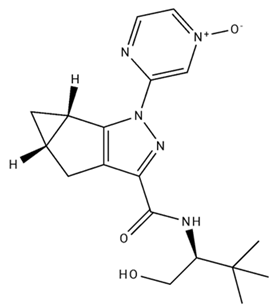 | Male Sprague-Dawley rats | Oral 30 mg/kg, time not specified | Generated hyperalgesia without tachyphylaxis in morphine-treated osteoarthritis rats. | [137] |
| AM1241/Agonist | 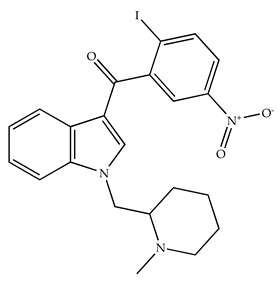 | Male C57B6/J mice (6 weeks) | IP 3 or 6 mg/kg, 4 wk | Reduction in anxiety-like behaviors in chronic alcohol-exposed adolescent mice. | [138] |
| AM1710/Agonist |  | Adult male and female C57Bl6/J mice | IP 25 mg/kg 3 wk | Reversal of morphine tolerance and dependence. | [139] |
| LY2828360/Agonist | 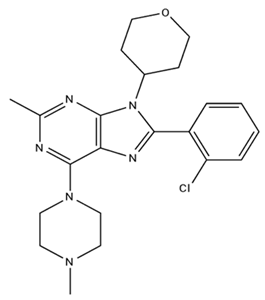 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Morales, M.; Espinoza-Abad, R.; García-García, F. Involvement of CB1R and CB2R Ligands in Sleep Disorders and Addictive Behaviors in the Last 25 Years. Pharmaceuticals 2025, 18, 266. https://doi.org/10.3390/ph18020266
Pérez-Morales M, Espinoza-Abad R, García-García F. Involvement of CB1R and CB2R Ligands in Sleep Disorders and Addictive Behaviors in the Last 25 Years. Pharmaceuticals. 2025; 18(2):266. https://doi.org/10.3390/ph18020266
Chicago/Turabian StylePérez-Morales, Marcel, Rodolfo Espinoza-Abad, and Fabio García-García. 2025. "Involvement of CB1R and CB2R Ligands in Sleep Disorders and Addictive Behaviors in the Last 25 Years" Pharmaceuticals 18, no. 2: 266. https://doi.org/10.3390/ph18020266
APA StylePérez-Morales, M., Espinoza-Abad, R., & García-García, F. (2025). Involvement of CB1R and CB2R Ligands in Sleep Disorders and Addictive Behaviors in the Last 25 Years. Pharmaceuticals, 18(2), 266. https://doi.org/10.3390/ph18020266









