Innovative Application of Medicinal Insects: Employing UHPLC-MS, Bioinformatics, In Silico Studies and In Vitro Experiments to Elucidate the Multi-Target Hemostatic Mechanism of Glenea cantor (Coleoptera: Cerambycidae) Charcoal-Based Medicine
Abstract
1. Introduction
2. Results
2.1. In Vitro Coagulation Assay Results of GC-CM
2.2. UHPLC-MS Analysis
2.3. Active Components of GC-CM and Its Potential Targets Related to Hemostasis
2.4. The “Active Ingredient–Intersecting Target–Hemostasis” Network and Key Components of GC-CM
2.5. PPI Network
2.6. GO and KEGG Pathway Analysis of GC-CM with Hemostatic Intersection Targets
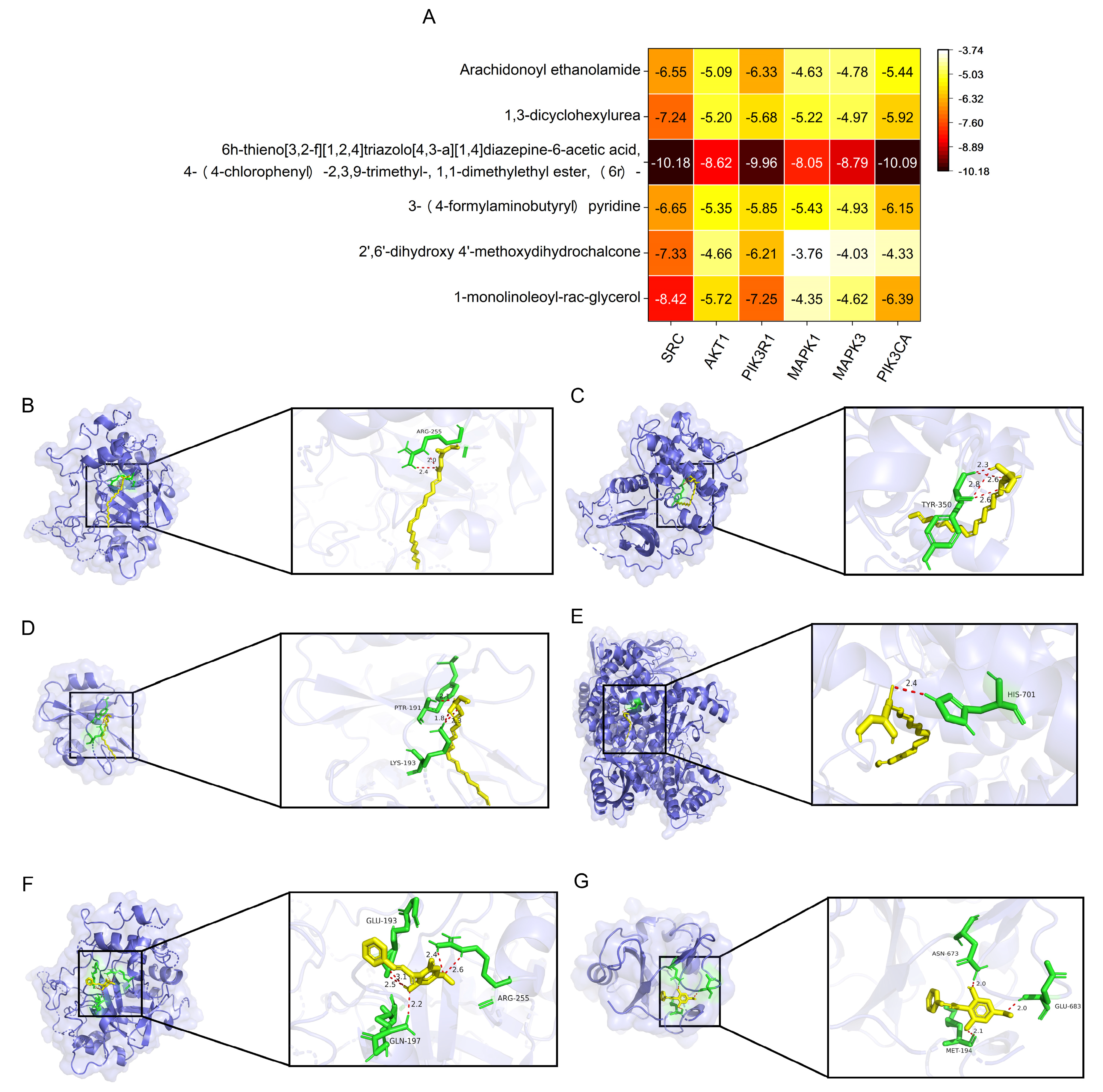
2.7. Verification with Molecular Docking
2.8. Verification with Molecular Dynamics Simulation
3. Discussion
4. Materials and Methods
4.1. Source of Glenea cantor
4.2. Reagents and Instruments
4.3. Experimental Animals
4.4. Preparation of GC-CM
4.5. In Vitro Coagulation Test
4.6. UHPLC-MS Analysis Conditions
4.6.1. UHPLC Conditions
4.6.2. Orbitrap Explorisrm™ 480 Mass Spectrometry Conditions
4.6.3. Data Analysis Process
4.7. Collection of Bioactive Components and Gene Targets of GC-CM
4.8. Collection of Potential Targets for Hemostasis
4.9. Construction of the “GC-CM Active Components–Intersection Targets–Hemostasis” Network
4.10. Construction of Protein–Protein Interaction (PPI) Network
4.11. Enrichment Analysis of GO and KEGG
4.12. Molecular Docking
4.13. Molecular Dynamics Simulation
4.14. Statistical Analysis Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| GC-CM | Glenea cantor charcoal medicine |
| APTT | activated partial thromboplastin time |
| PT | prothrombin time |
| DC | Degree Centrality |
| BC | Betweenness Centrality |
| CC | Closeness Centrality |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Spicer, P.P.; Mikos, A.G. Fibrin glue as a drug delivery system. J. Control. Release 2010, 148, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Cheng, B.; McCarthy, S.J.; Jung, M.; Whitelock, J.M. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials 2011, 32, 6655–6662. [Google Scholar] [PubMed]
- Zhang, S.; Li, J.; Chen, S.; Zhang, X.; Ma, J.; He, J. Oxidized cellulose-based hemostatic materials. Carbohydr. Polym. 2020, 230, 115585. [Google Scholar] [PubMed]
- Okata, S.; Hoshina, K.; Hanada, K.; Kamata, H.; Fujisawa, A.; Yoshikawa, Y.; Sakai, T. Hemostatic Capability of a Novel Tetra-Polyethylene Glycol Hydrogel. Ann. Vasc. Surg. 2022, 84, 398–404. [Google Scholar]
- Feng, Y.; Zhao, M.; He, Z.; Chen, Z.; Sun, L. Research and utilization of medicinal insects in China. Entomol. Res. 2009, 39, 313–316. [Google Scholar]
- Siddiqui, S.A.; Li, C.; Aidoo, O.F.; Fernando, I.; Haddad, M.A.; Pereira, J.A.M.; Blinov, A.; Golik, A.; Câmara, J.S. Unravelling the potential of insects for medicinal purposes—A comprehensive review. Heliyon 2023, 9, e15938. [Google Scholar]
- Wang, Q. Cerambycidae of the World: Biology and Pest Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Namba, T.; Ma, Y.H.; Inagaki, K. Insect-derived crude drugs in the Chinese Song dynasty. J. Ethnopharmacol. 1988, 24, 247–285. [Google Scholar]
- Deyrup, S.T.; Stagnitti, N.C.; Perpetua, M.J.; Wong-Deyrup, S.W. Drug Discovery Insights from Medicinal Beetles in Traditional Chinese Medicine. Biomol. Ther. 2021, 29, 105–126. [Google Scholar]
- Li, Y.; Hong, Y.; Han, Y.; Wang, Y.; Xia, L. Chemical characterization and antioxidant activities comparison in fresh, dried, stir-frying and carbonized ginger. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1011, 223–232. [Google Scholar]
- Chen, Z.; Ye, S.Y.; Yang, Y.; Li, Z.Y. A review on charred traditional Chinese herbs: Carbonization to yield a haemostatic effect. Pharm. Biol. 2019, 57, 498–506. [Google Scholar]
- Tomaiuolo, M.; Matzko, C.N.; Poventud-Fuentes, I.; Weisel, J.W.; Brass, L.F.; Stalker, T.J. Interrelationships between structure and function during the hemostatic response to injury. Proc. Natl. Acad. Sci. USA 2019, 116, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Su, L.; Pang, L.; Li, J.; Li, H.; Li, J.; Liu, Y.; Zhang, J. Natural exosome-like nanoparticles derived from ancient medicinal insect Periplaneta americana L. as a novel diabetic wound healing accelerator. J. Nanobiotechnol. 2023, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, N.A.; Mello, C.B.; Garcia, E.S.; Butt, T.M.; Azambuja, P. Insect natural products and processes: New treatments for human disease. Insect Biochem. Mol. Biol. 2011, 41, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Liao, J.; Chen, Q.; Lu, X.; Fan, X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin. J. Nat. Med. 2023, 21, 323–332. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Yuan, T.; Wang, H.; Zeng, Z.; Tian, L.; Cui, L.; Guo, J.; Chen, Y. Network pharmacology provides new insights into the mechanism of traditional Chinese medicine and natural products used to treat pulmonary hypertension. Phytomed. Int. J. Phytother. Phytopharm. 2024, 135, 156062. [Google Scholar] [CrossRef]
- Panagiotou, G.; Taboureau, O. The impact of network biology in pharmacology and toxicology. SAR QSAR Environ. Res. 2012, 23, 221–235. [Google Scholar] [CrossRef]
- Li, T.; Zhang, W.; Hu, E.; Sun, Z.; Li, P.; Yu, Z.; Zhu, X.; Zheng, F.; Xing, Z.; Xia, Z.; et al. Integrated metabolomics and network pharmacology to reveal the mechanisms of hydroxysafflor yellow A against acute traumatic brain injury. Comput. Struct. Biotechnol. J. 2021, 19, 1002–1013. [Google Scholar] [CrossRef]
- Li, X.; Wei, S.; Niu, S.; Ma, X.; Li, H.; Jing, M.; Zhao, Y. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput. Biol. Med. 2022, 144, 105389. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, Y.; Wang, W.; He, Y.; Zhong, H.; Zhou, X.; Chen, Y.; Cai, X.J.; Liu, L.Q. Mechanisms underlying the therapeutic effects of Qingfeiyin in treating acute lung injury based on GEO datasets, network pharmacology and molecular docking. Comput. Biol. Med. 2022, 145, 105454. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, W.; Wang, Y.; Jin, X.; Guo, H.; Wang, Y.; Tang, Y.; Yao, X. Explore the mechanism and substance basis of Mahuang FuziXixin Decoction for the treatment of lung cancer based on network pharmacology and molecular docking. Comput. Biol. Med. 2022, 151 Pt A, 106293. [Google Scholar] [CrossRef]
- Salmaso, V.; Moro, S. Bridging Molecular Docking to Molecular Dynamics in Exploring Ligand-Protein Recognition Process: An Overview. Front. Pharmacol. 2018, 9, 923. [Google Scholar]
- Liang, L.; He, C.; Han, X.; Liu, J.; Yang, L.; Chang, F.; Zhang, Y.; Lin, J. Zuojin Pill Alleviates Precancerous Lesions of Gastric Cancer by Modulating the MEK/ERK/c-Myc Pathway: An Integrated Approach of Network Pharmacology, Molecular Dynamics Simulation, and Experimental Validation. Drug Des. Dev. Ther. 2024, 18, 5905–5929. [Google Scholar]
- Chen, Z.; Wang, X.; Huang, W. Exploring the mechanism of Radix Bupleuri in the treatment of depression combined with SARS-CoV-2 infection through bioinformatics, network pharmacology, molecular docking, and molecular dynamic simulation. Metab. Brain Dis. 2025, 40, 105. [Google Scholar] [CrossRef]
- Lu, W.; Wang, Q.; Tian, M.Y.; Xu, J.; Qin, A.Z. Phenology and laboratory rearing procedures of an Asian longicorn beetle, Glenea cantor (Coleoptera: Cerambycidae: Lamiinae). J. Econ. Entomol. 2011, 104, 509–516. [Google Scholar] [PubMed]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar]
- Zhang, E.; Ji, X.; Ouyang, F.; Lei, Y.; Deng, S.; Rong, H.; Deng, X.; Shen, H. A minireview of the medicinal and edible insects from the traditional Chinese medicine (TCM). Front. Pharmacol. 2023, 14, 1125600. [Google Scholar]
- Kamal, A.H.; Tefferi, A.; Pruthi, R.K. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin. Proc. 2007, 82, 864–873. [Google Scholar]
- Li, S.; Xue, X.; Yang, X.; Zhou, S.; Wang, S.; Meng, J. A Network Pharmacology Approach Used to Estimate the Active Ingredients of Moutan Cortex Charcoal and the Potential Targets in Hemorrhagic Diseases. Biol. Pharm. Bull. 2019, 42, 432–441. [Google Scholar] [CrossRef]
- Hoffman, M.M.; Monroe, D.M. Rethinking the coagulation cascade. Curr. Hematol. Rep. 2005, 4, 391–396. [Google Scholar]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar]
- Xu, X.R.; Zhang, D.; Oswald, B.E.; Carrim, N.; Wang, X.; Hou, Y.; Zhang, Q.; Lavalle, C.; McKeown, T.; Marshall, A.H.; et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit. Rev. Clin. Lab. Sci. 2016, 53, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C.; Moreno, M.; García-Ruiz, J.C. The physiology of hemostasis. Blood Coagul. Fibrinolysis 2022, 33, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Monroe, D.M.; Hoffman, M.; Roberts, H.R. Platelets and thrombin generation. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.R.; Hoffman, M.; Monroe, D.M. A cell-based model of thrombin generation. Semin. Thromb. Hemost. 2006, 32 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Wang, H.; Bang, K.W.; Blanchette, V.S.; Nurden, A.T.; Rand, M.L. Phosphatidylserine exposure, microparticle formation and mitochondrial depolarisation in Glanzmann thrombasthenia platelets. Thromb. Haemost. 2014, 111, 1184–1186. [Google Scholar] [CrossRef]
- Wang, L.; Liang, Q.; Zhang, Y.; Liu, F.; Sun, Y.; Wang, S.; Cao, H.; Meng, J. iTRAQ-based quantitative proteomics and network pharmacology revealing hemostatic mechanism mediated by Zingiberis Rhizome Carbonisata in deficiency-cold and Hemorrhagic Syndrome rat models. Chem. Biol. Interact. 2021, 343, 109465. [Google Scholar]
- Du, X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr. Opin. Hematol. 2007, 14, 262–269. [Google Scholar] [CrossRef]
- De Kock, L.; Freson, K. The (Patho)Biology of SRC Kinase in Platelets and Megakaryocytes. Medicina 2020, 56, 633. [Google Scholar] [CrossRef]
- Yin, H.; Stojanovic, A.; Hay, N.; Du, X. The role of Akt in the signaling pathway of the glycoprotein Ib-IX induced platelet activation. Blood 2008, 111, 658–665. [Google Scholar] [CrossRef]
- Petrosino, M.; Novak, L.; Pasquo, A.; Turina, P.; Capriotti, E.; Minicozzi, V.; Consalvi, V.; Chiaraluce, R. The complex impact of cancer-related missense mutations on the stability and on the biophysical and biochemical properties of MAPK1 and MAPK3 somatic variants. Hum. Genom. 2023, 17, 95. [Google Scholar] [CrossRef]
- Kramer, R.M.; Roberts, E.F.; Um, S.L.; Börsch-Haubold, A.G.; Watson, S.P.; Fisher, M.J.; Jakubowski, J.A. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996, 271, 27723–27729. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Feys, H.B.; De Meyer, S.F.; Vanhoorelbeke, K.; Deckmyn, H. Platelets at work in primary hemostasis. Blood Rev. 2011, 25, 155–167. [Google Scholar] [PubMed]
- Kim, A.Y.; Yang, H.; David, T.; Tien, J.; Coughlin, S.R.; Jan, Y.N.; Jan, L. TMEM16F Ion Channel Regulates Calcium-Dependent PS Exposure, Hemostasis, and Thrombosis. Blood 2012, 120, 1111. [Google Scholar]
- Varga-Szabo, D.; Braun, A.; Nieswandt, B. Calcium signaling in platelets. J. Thromb. Haemost. JTH 2009, 7, 1057–1066. [Google Scholar]
- Safaroghli-Azar, A.; Sanaei, M.J.; Pourbagheri-Sigaroodi, A.; Bashash, D. Phosphoinositide 3-kinase (PI3K) classes: From cell signaling to endocytic recycling and autophagy. Eur. J. Pharmacol. 2023, 953, 175827. [Google Scholar]
- Tsay, A.; Wang, J.-C. The Role of PIK3R1 in Metabolic Function and Insulin Sensitivity. Int. J. Mol. Sci. 2023, 24, 12665. [Google Scholar] [CrossRef]
- Guidetti, G.F.; Canobbio, I.; Torti, M. PI3K/Akt in platelet integrin signaling and implications in thrombosis. Adv. Biol. Regul. 2015, 59, 36–52. [Google Scholar]
- Chen, Z.; Li, T.; Kareem, K.; Tran, D.; Griffith, B.P.; Wu, Z.J. The role of PI3K/Akt signaling pathway in non-physiological shear stress-induced platelet activation. Artif. Organs 2019, 43, 897–908. [Google Scholar]
- Khezri, M.R.; Varzandeh, R.; Ghasemnejad-Berenji, M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell. Mol. Biol. Lett. 2022, 27, 6. [Google Scholar]
- Hao, X.; Jin, Y.; Zhang, Y.; Li, S.; Cui, J.; He, H.; Guo, L.; Yang, F.; Liu, H. Inhibition of Oncogenic Src Ameliorates Silica-Induced Pulmonary Fibrosis via PI3K/AKT Pathway. Int. J. Mol. Sci. 2023, 24, 774. [Google Scholar] [CrossRef]
- Li, W.; Lv, L.; Ruan, M.; Xu, J.; Zhu, W.; Li, Q.; Jiang, X.; Zheng, L.; Zhu, W. Qin Huang formula enhances the effect of Adriamycin in B-cell lymphoma via increasing tumor infiltrating lymphocytes by targeting toll-like receptor signaling pathway. BMC Complement. Med. Ther. 2022, 22, 185. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Xia, M.; Song, Y.; Gao, Y.; Zhang, L.; Zhang, C. Investigation of the hemostatic mechanism of Gardeniae fructus Praeparatus based on pharmacological evaluation and network pharmacology. Ann. Transl. Med. 2022, 10, 1093. [Google Scholar] [CrossRef]
- Zhang, G.; Xiang, B.; Dong, A.; Skoda, R.C.; Daugherty, A.; Smyth, S.S.; Du, X.; Li, Z. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood 2011, 118, 3670–3679. [Google Scholar] [CrossRef] [PubMed]
- Balmes, A.; Rodríguez, J.G.; Seifert, J.; Pinto-Quintero, D.; Khawaja, A.A.; Boffito, M.; Frye, M.; Friebe, A.; Emerson, M.; Seta, F.; et al. Role of the NO-GC/cGMP signaling pathway in platelet biomechanics. Platelets 2024, 35, 2313359. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; Wiersinga, W.J.; Levi, M.; van der Poll, T. Inflammation, endothelium, and coagulation in sepsis. J. Leukoc. Biol. 2008, 83, 536–545. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Menichelli, A.; Del Principe, D.; Agrò, A.F. Anandamide activates human platelets through a pathway independent of the arachidonate cascade. FEBS Lett. 1999, 447, 277–282. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Battista, N.; Finazzi-Agrò, A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood 2002, 100, 4040–4048. [Google Scholar] [CrossRef]
- Brantl, S.A.; Khandoga, A.L.; Siess, W. Mechanism of platelet activation induced by endocannabinoids in blood and plasma. Platelets 2014, 25, 151–161. [Google Scholar] [CrossRef]
- Schmidt, G.J.; Reumiller, C.M.; Ercan, H.; Resch, U.; Butt, E.; Heber, S.; Liutkevičiūte, Z.; Basílio, J.; Schmid, J.A.; Assinger, A.; et al. Comparative proteomics reveals unexpected quantitative phosphorylation differences linked to platelet activation state. Sci. Rep. 2019, 9, 19009. [Google Scholar] [CrossRef]
- Stagaard, R.; Øvlisen, G.O.; Klæbel, J.H.; Danielsen, D.; Lund, A.; Elm, T.; Ley, C.D. In vivo effect of rFVIII and rFVIIa in hemophilia A rats evaluated by the Tail Vein Transection Bleeding Model. J. Thromb. Haemost. JTH 2023, 21, 1189–1199. [Google Scholar] [CrossRef]
- Ali-Mohamad, N.; Cau, M.F.; Zenova, V.; Baylis, J.R.; Beckett, A.; McFadden, A.; Donnellan, F.; Kastrup, C.J. Self-propelling thrombin powder enables hemostasis with no observable recurrent bleeding or thrombosis over 3 days in a porcine model of upper GI bleeding. Gastrointest. Endosc. 2023, 98, 245–248. [Google Scholar] [PubMed]
- Dong, Z.; Yang, Y.; Dou, F.; Zhang, Y.; Huang, H.; Zheng, X.; Wang, X.; Lu, W. Observations on the Ultrastructure of Antennal Sensilla of Adult Glenea cantor (Cerambycidae: Lamiinae). J. Insect Sci. 2020, 20, 7. [Google Scholar] [PubMed]
- Yang, B.; Xu, Z.Q.; Zhang, H.; Xu, F.Y.; Shi, X.Y.; Zou, Z.; Ling, C.Q.; Tang, L. The efficacy of Yunnan Baiyao on haemostasis and antiulcer: A systematic review and meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2014, 7, 461–482. [Google Scholar]
- Ignjatovic, V. Activated partial thromboplastin time. Methods Mol. Biol. 2013, 992, 111–120. [Google Scholar]
- Ignjatovic, V. Prothrombin time/international normalized ratio. Methods Mol. Biol. 2013, 992, 121–129. [Google Scholar]
- Xu, X.X.; Bi, J.P.; Ping, L.; Li, P.; Li, F. A network pharmacology approach to determine the synergetic mechanisms of herb couple for treating rheumatic arthritis. Drug Des. Dev. Ther. 2018, 12, 967–979. [Google Scholar]
- Yao, Y.; Zhang, X.; Wang, Z.; Zheng, C.; Li, P.; Huang, C.; Tao, W.; Xiao, W.; Wang, Y.; Huang, L.; et al. Deciphering the combination principles of Traditional Chinese Medicine from a systems pharmacology perspective based on Ma-huang Decoction. J. Ethnopharmacol. 2013, 150, 619–638. [Google Scholar]
- Yue, S.J.; Liu, J.; Feng, W.W.; Zhang, F.L.; Chen, J.X.; Xin, L.T.; Peng, C.; Guan, H.S.; Wang, C.Y.; Yan, D. System Pharmacology-Based Dissection of the Synergistic Mechanism of Huangqi and Huanglian for Diabetes Mellitus. Front. Pharmacol. 2017, 8, 694. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar]
- Shi, S.; Zhao, S.; Tian, X.; Liu, F.; Lu, X.; Zang, H.; Li, F.; Xiang, L.; Li, L.; Jiang, S. Molecular and metabolic mechanisms of bufalin against lung adenocarcinoma: New and comprehensive evidences from network pharmacology, metabolomics and molecular biology experiment. Comput. Biol. Med. 2023, 157, 20. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.M.; Shakil, S.; Haneef, M. A simple click by click protocol to perform docking: AutoDock 4.2 made easy for non-bioinformaticians. EXCLI J. 2013, 12, 831–857. [Google Scholar] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Hornak, V.; Abel, R.; Okur, A.; Strockbine, B.; Roitberg, A.; Simmerling, C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 2006, 65, 712–725. [Google Scholar] [CrossRef]
- Mark, P. and Lennart Nilsson, Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Jiang, T.; Li, C.; Li, Y.; Hu, W.; Guo, J.; Du, X.; Meng, Q.; Zhu, X.; Song, W.; Guo, J.; et al. Multi-omics and bioinformatics for the investigation of therapeutic mechanism of roucongrong pill against postmenopausal osteoporosis. J. Ethnopharmacol. 2025, 337 Pt 2, 118873. [Google Scholar] [CrossRef]
- Hassan, M.A.; Abd El-Aziz, S.; Nabil-Adam, A.; Tamer, T.M. Formulation of novel bioactive gelatin inspired by cinnamaldehyde for combating multi-drug resistant bacteria: Characterization, molecular docking, pharmacokinetic analyses, and in vitro assessments. Int. J. Pharm. 2024, 652, 123827. [Google Scholar]
- Helmy, M.W.; Youssef, M.H.; Yamari, I.; Amr, A.; Moussa, F.I.; El Wakil, A.; Chtita, S.; El-Samad, L.M.; Hassan, M.A. Repurposing of sericin combined with dactolisib or vitamin D to combat non-small lung cancer cells through computational and biological investigations. Sci. Rep. 2024, 14, 27034. [Google Scholar]







| Groups | APTT (s) | PT (s) |
|---|---|---|
| blank control group | 31.97 ± 1.90 | 12.77 ± 0.73 |
| GC-CM group | 24.93 ± 0.85 ** | 10.04 ± 0.70 ** |
| positive control group | 22.86 ± 1.42 ** | 9.68 ± 0.83 ** |
| No. | Name | Degree | BC | CC |
|---|---|---|---|---|
| 1 | 1-monolinoleoyl-rac-glycerol | 23 | 0.15123 | 0.38356 |
| 2 | 2′,6′-dihydroxy 4′-methoxydihydrochalcone | 20 | 0.13134 | 0.38043 |
| 3 | 3-(4-formylaminobutyryl)pyridine | 13 | 0.08402 | 0.37333 |
| 4 | 6h-thieno [3,2-f][1,2,4]triazolo [4,3-a][1,4]diazepine-6-acetic acid, 4-(4-chlorophenyl)-2,3,9-trimethyl-, 1,1-dimethylethyl ester, (6r)- | 9 | 0.05643 | 0.36939 |
| 5 | 1,3-dicyclohexylurea | 7 | 0.04247 | 0.36745 |
| 6 | Arachidonoyl ethanolamide | 7 | 0.04247 | 0.36745 |
| 7 | Galiellalactone | 7 | 0.04247 | 0.36745 |
| 8 | Neoabietic acid | 7 | 0.04247 | 0.36745 |
| 9 | 1-(4-piperidinyl)-1,3-dihydro-2h-indol-2-one | 6 | 0.03546 | 0.36649 |
| 10 | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol | 6 | 0.03546 | 0.36649 |
| 11 | 4-acetamidoantipyrin | 6 | 0.03546 | 0.36649 |
| 12 | alpha-ionone | 5 | 0.02842 | 0.36554 |
| 13 | (1r,5s)-8-methyl-8-azabicyclo [3.2.1]octan-3-amine | 5 | 0.02842 | 0.36554 |
| 14 | 10-hydroxydecanoate | 5 | 0.02842 | 0.36554 |
| 15 | Dibutyl adipate | 5 | 0.02842 | 0.36554 |
| 16 | Dodecanoic acid, 12-[[(cyclohexylamino)carbonyl]amino]- | 5 | 0.02842 | 0.36554 |
| 17 | L-Fucose | 5 | 0.02842 | 0.36554 |
| 18 | N-acetyl-5-hydroxytryptamine | 5 | 0.02842 | 0.36554 |
| 19 | Primaquine | 5 | 0.02842 | 0.36554 |
| 20 | Pro-leu | 5 | 0.02842 | 0.36554 |
| Target | Description | UniProt | DC | BC | CC |
|---|---|---|---|---|---|
| SRC | Proto-oncogene tyrosine-protein kinase Src | P12931 | 26 | 275.73 | 0.01176 |
| AKT1 | RAC-alpha serine/threonine-protein kinase | P31749 | 26 | 337.79 | 0.0119 |
| PIK3R1 | Phosphatidylinositol 3-kinase regulatory subunit alpha | P27986 | 22 | 87.27 | 0.01087 |
| MAPK1 | Mitogen-activated protein kinase 1 | P28482 | 22 | 138.64 | 0.01099 |
| MAPK3 | Mitogen-activated protein kinase 3 | P27361 | 21 | 154.09 | 0.01124 |
| PIK3CA | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | P42336 | 21 | 72.93 | 0.01075 |
| TP53 | Cellular tumor antigen p53 | P04637 | 21 | 218.78 | 0.01087 |
| PTK2 | Focal adhesion kinase 1 | Q05397 | 20 | 103.93 | 0.01042 |
| STAT3 | Signal transducer and activator of transcription 3 | P40763 | 19 | 190.94 | 0.01087 |
| PIK3CD | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform | O00329 | 18 | 41.64 | 0.0101 |
| PIK3CB | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform | P42338 | 18 | 41.64 | 0.0101 |
| JUN | Transcription factor Jun | P05412 | 18 | 204.33 | 0.01075 |
| EGFR | Epidermal growth factor receptor | P00533 | 17 | 97.91 | 0.0101 |
| RAF1 | RAF proto-oncogene serine/threonine-protein kinase | P04049 | 15 | 43.79 | 0.00935 |
| GRB2 | Growth factor receptor-bound protein 2 | P62993 | 14 | 83.64 | 0.00962 |
| ESR1 | Estrogen receptor | P03372 | 14 | 34.25 | 0.0101 |
| FYN | Tyrosine-protein kinase Fyn | P06241 | 14 | 81.66 | 0.00909 |
| LYN | Tyrosine-protein kinase Lyn | P07948 | 14 | 35.55 | 0.00935 |
| TNF | Tumor necrosis factor | P01375 | 14 | 285.73 | 0.01031 |
| CASP3 | Caspase-3 | P42574 | 12 | 90.93 | 0.0098 |
| HIF1A | Hypoxia-inducible factor 1-alpha | Q16665 | 12 | 101.36 | 0.00962 |
| MTOR | Serine/threonine-protein kinase mTOR | P42345 | 11 | 43.51 | 0.00909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, B.; Zhang, W.; Ming, L.; Fan, Q.; Zhang, L.; Lai, H.; Huang, G.; Liu, H.; Dong, Z. Innovative Application of Medicinal Insects: Employing UHPLC-MS, Bioinformatics, In Silico Studies and In Vitro Experiments to Elucidate the Multi-Target Hemostatic Mechanism of Glenea cantor (Coleoptera: Cerambycidae) Charcoal-Based Medicine. Pharmaceuticals 2025, 18, 479. https://doi.org/10.3390/ph18040479
Zhong B, Zhang W, Ming L, Fan Q, Zhang L, Lai H, Huang G, Liu H, Dong Z. Innovative Application of Medicinal Insects: Employing UHPLC-MS, Bioinformatics, In Silico Studies and In Vitro Experiments to Elucidate the Multi-Target Hemostatic Mechanism of Glenea cantor (Coleoptera: Cerambycidae) Charcoal-Based Medicine. Pharmaceuticals. 2025; 18(4):479. https://doi.org/10.3390/ph18040479
Chicago/Turabian StyleZhong, Bangyu, Wen Zhang, Liangshan Ming, Qimeng Fan, Lei Zhang, Hongyu Lai, Genwang Huang, Hongning Liu, and Zishu Dong. 2025. "Innovative Application of Medicinal Insects: Employing UHPLC-MS, Bioinformatics, In Silico Studies and In Vitro Experiments to Elucidate the Multi-Target Hemostatic Mechanism of Glenea cantor (Coleoptera: Cerambycidae) Charcoal-Based Medicine" Pharmaceuticals 18, no. 4: 479. https://doi.org/10.3390/ph18040479
APA StyleZhong, B., Zhang, W., Ming, L., Fan, Q., Zhang, L., Lai, H., Huang, G., Liu, H., & Dong, Z. (2025). Innovative Application of Medicinal Insects: Employing UHPLC-MS, Bioinformatics, In Silico Studies and In Vitro Experiments to Elucidate the Multi-Target Hemostatic Mechanism of Glenea cantor (Coleoptera: Cerambycidae) Charcoal-Based Medicine. Pharmaceuticals, 18(4), 479. https://doi.org/10.3390/ph18040479







