Recent Advancements in the Diversification and Applications of Boron-Containing Compounds in Medicinal Chemistry †
Abstract
1. Introduction
2. Significance of This Work
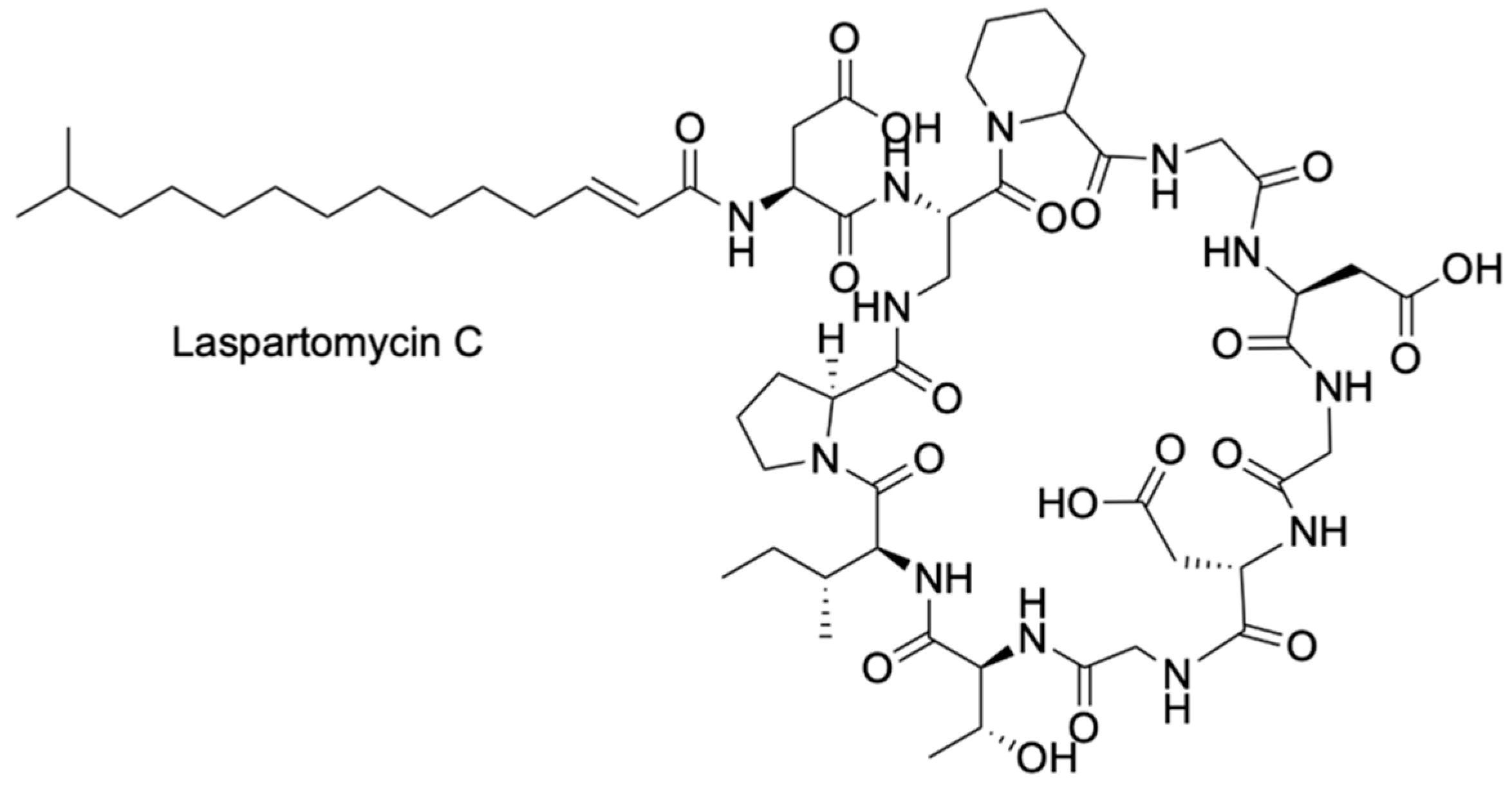
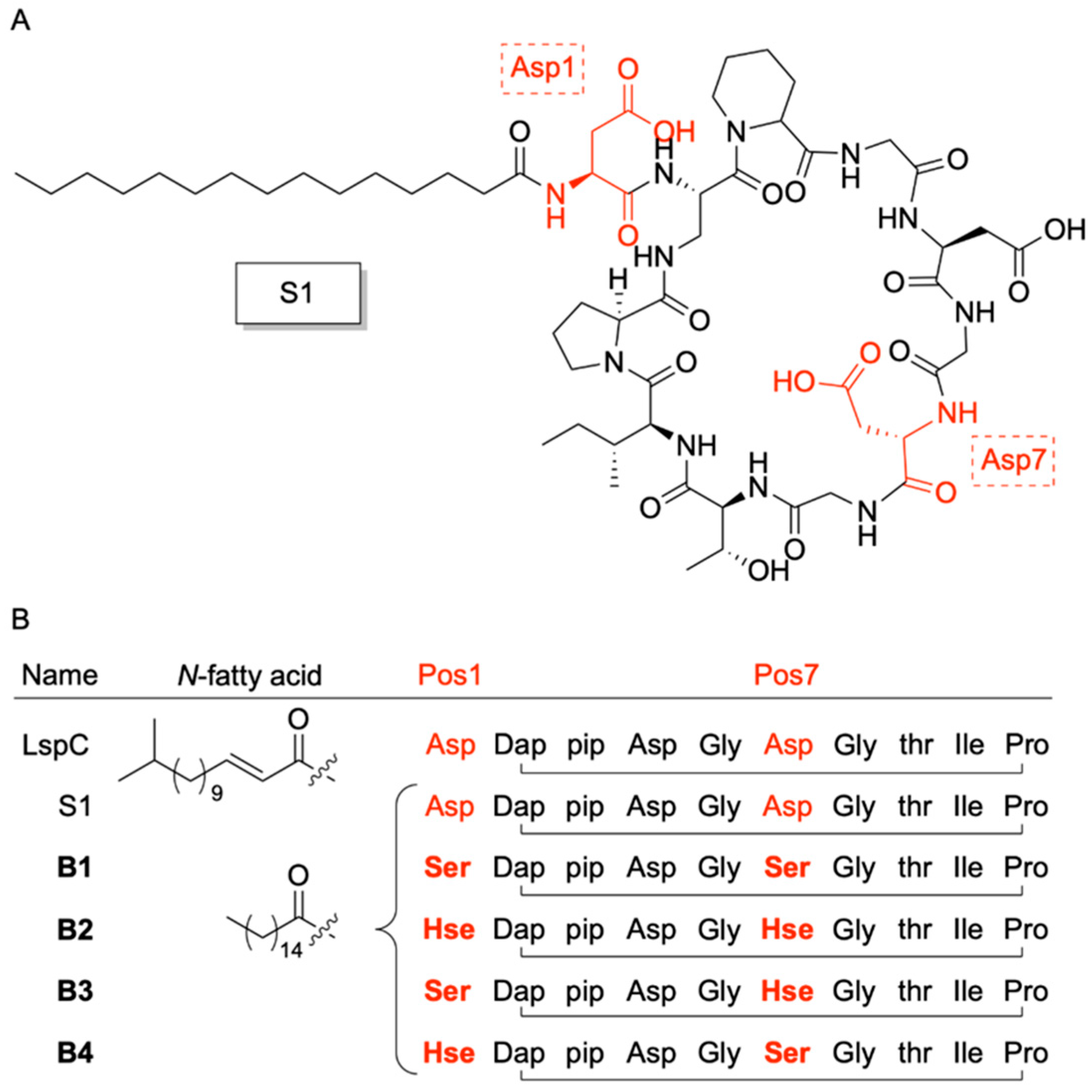
3. A Boron-Dependent Antibiotic
| Name | Pathogen | Supplement | MIC µg/mL | Reference |
|---|---|---|---|---|
| Laspartomycin C | Staphylococcus aureus | 5 mM Ca2+ | 4 | [39,44] |
| S1 | Bacillus subtillis | 5 mM Ca2+ | 4 | [36] |
| B1 | Bacillus subtillis | 0.82 mM PBA | 3.3 | [36] |
| B2 | Bacillus subtillis | 0.82 mM PBA | > | [36] |
| B3 | Bacillus subtillis | 0.82 mM PBA | 6.5 | [36] |
| B4 | Bacillus subtillis | 0.82 mM PBA | 6.5 | [36] |
| Daptomycin | Staphylococcus aureus | 5 mM Ca2+ | 0.25 | [39,44] |
| Friulimicin B | Staphylococcus aureus | 5 mM Ca2+ | 2 | [44] |
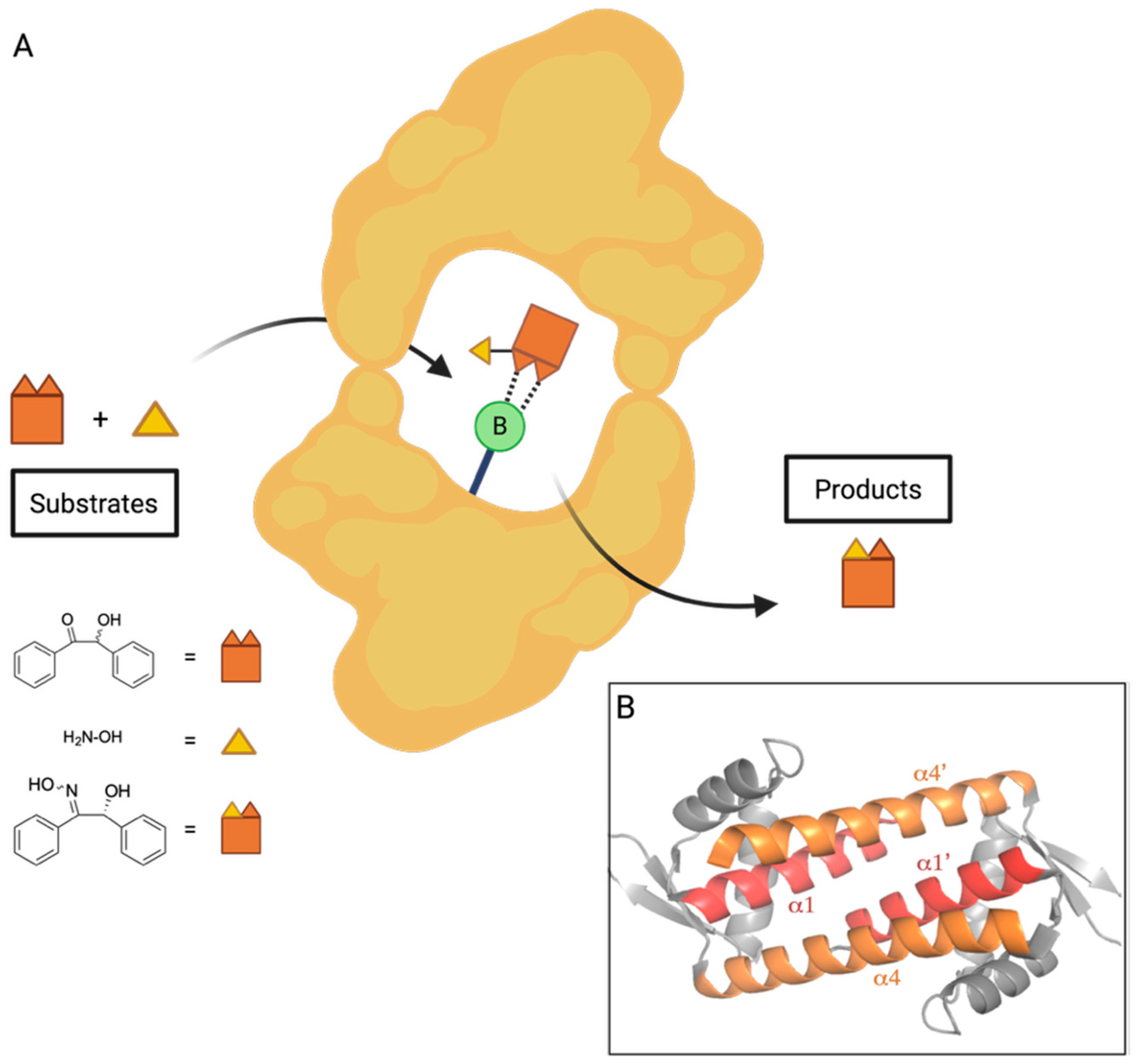
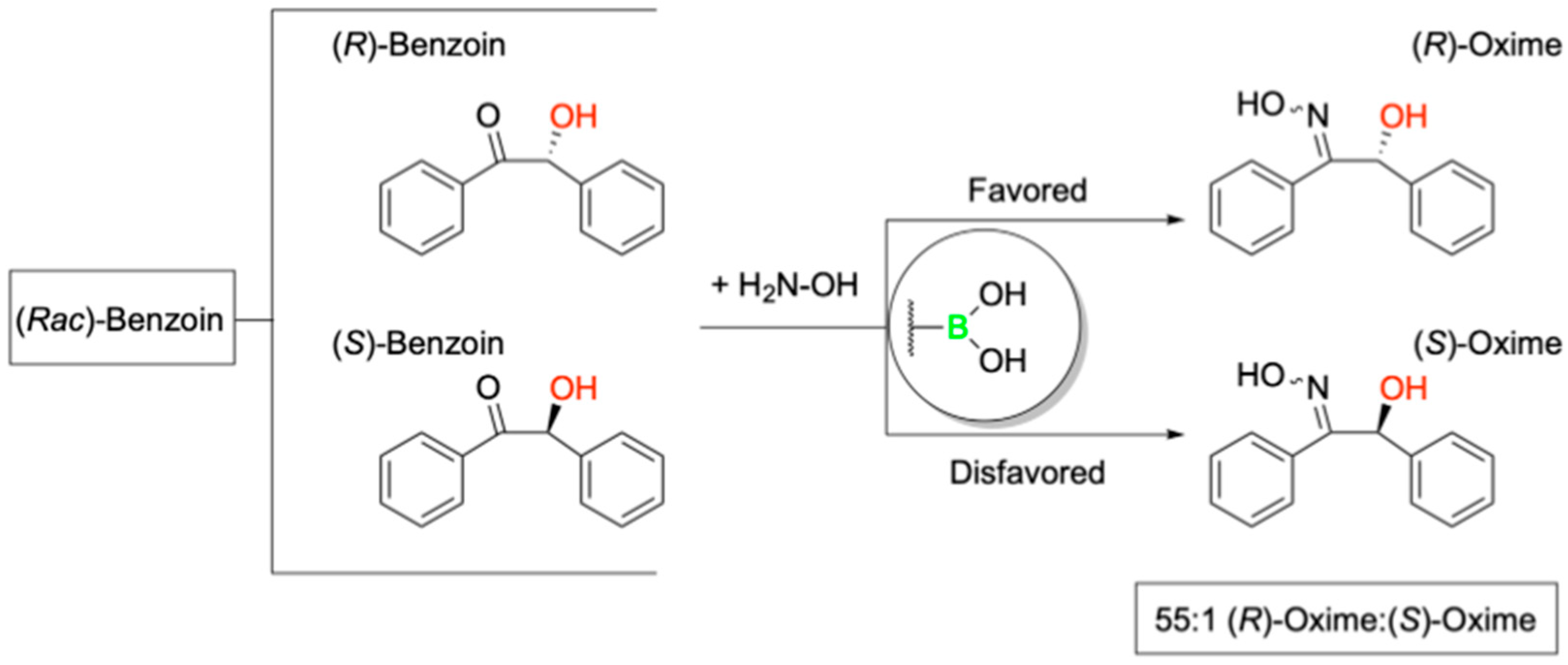
4. Boron Catalysis in a Designer Enzyme
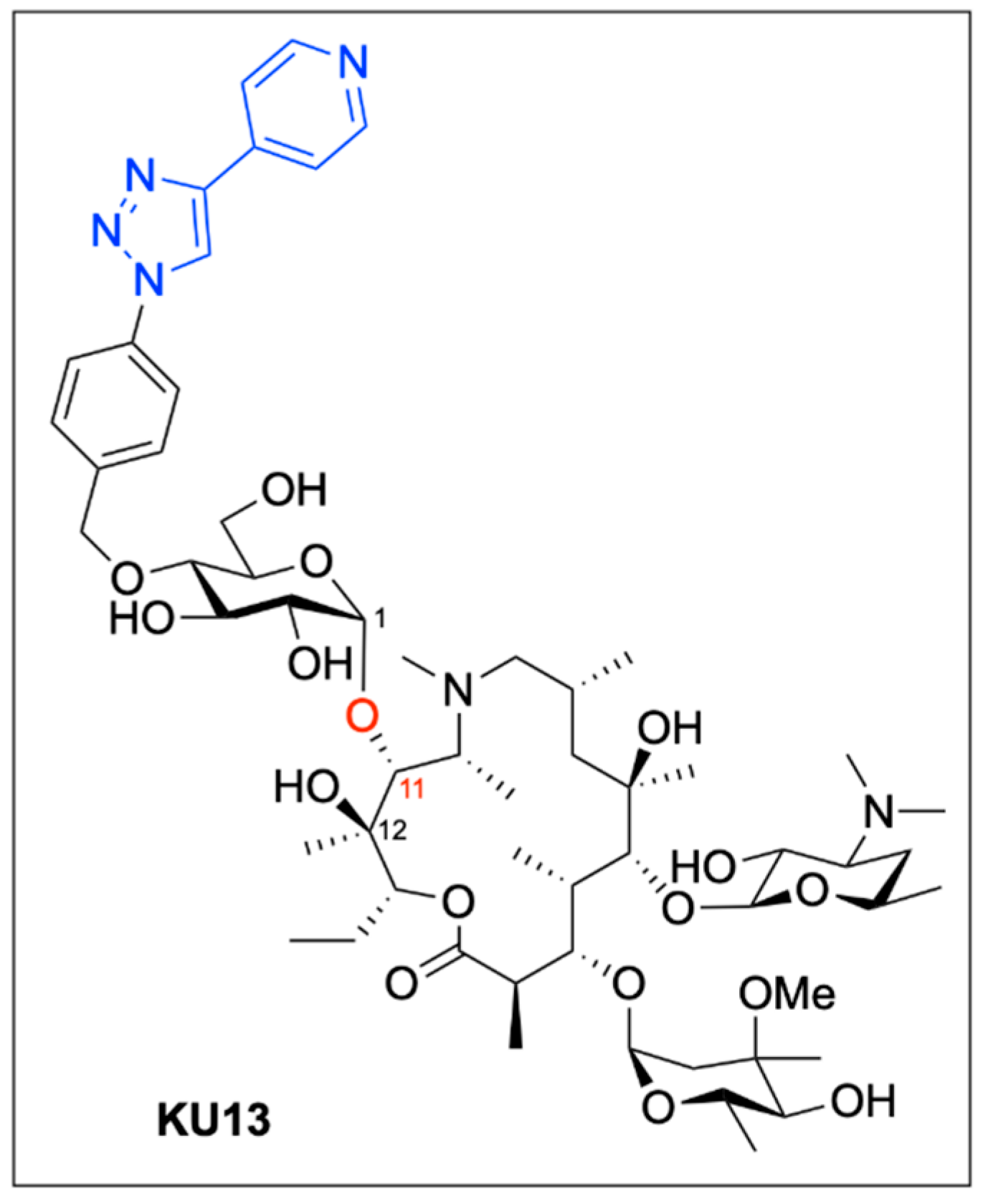
5. Boron-Mediated Aglycon Delivery
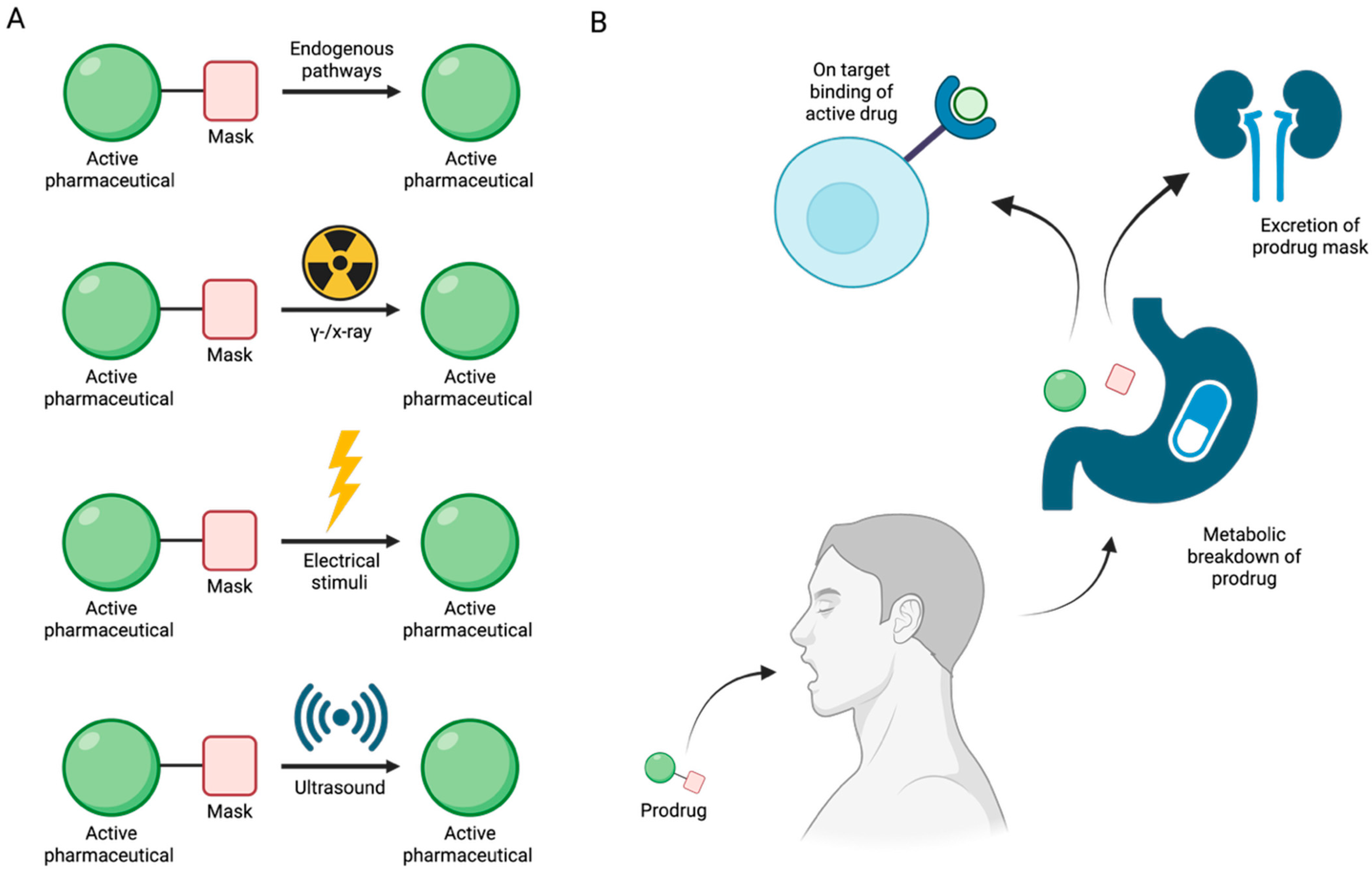
6. Enzyme-Independent Prodrug Activation Mechanism by Boron-Based Compounds
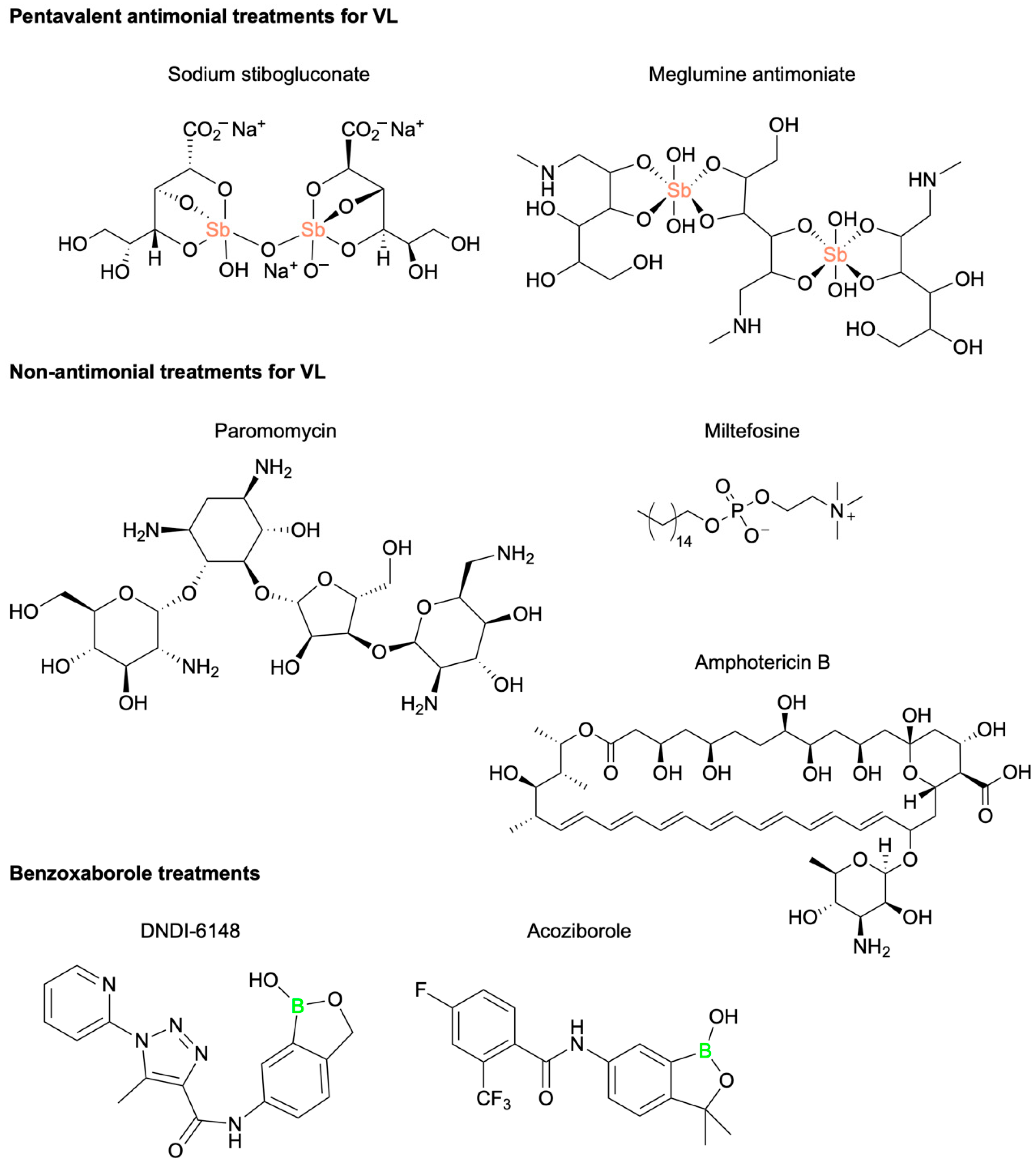
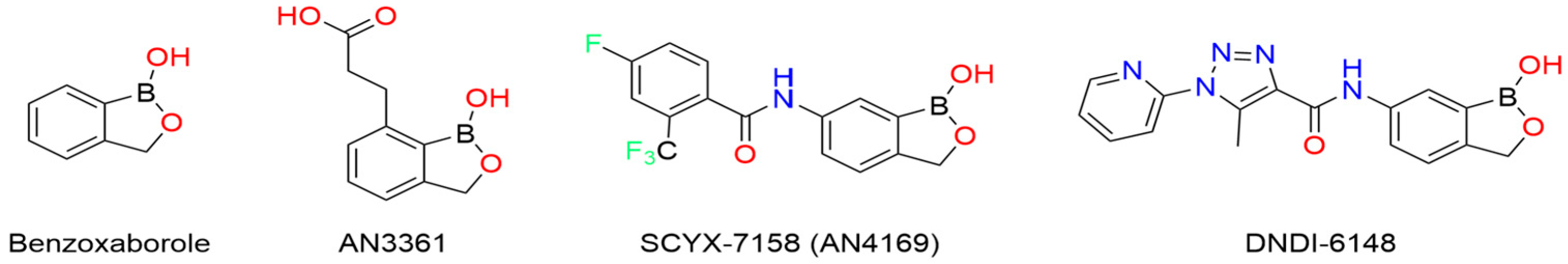
7. Novel Benzoxaborole for the Treatment of Visceral Leishmaniasis
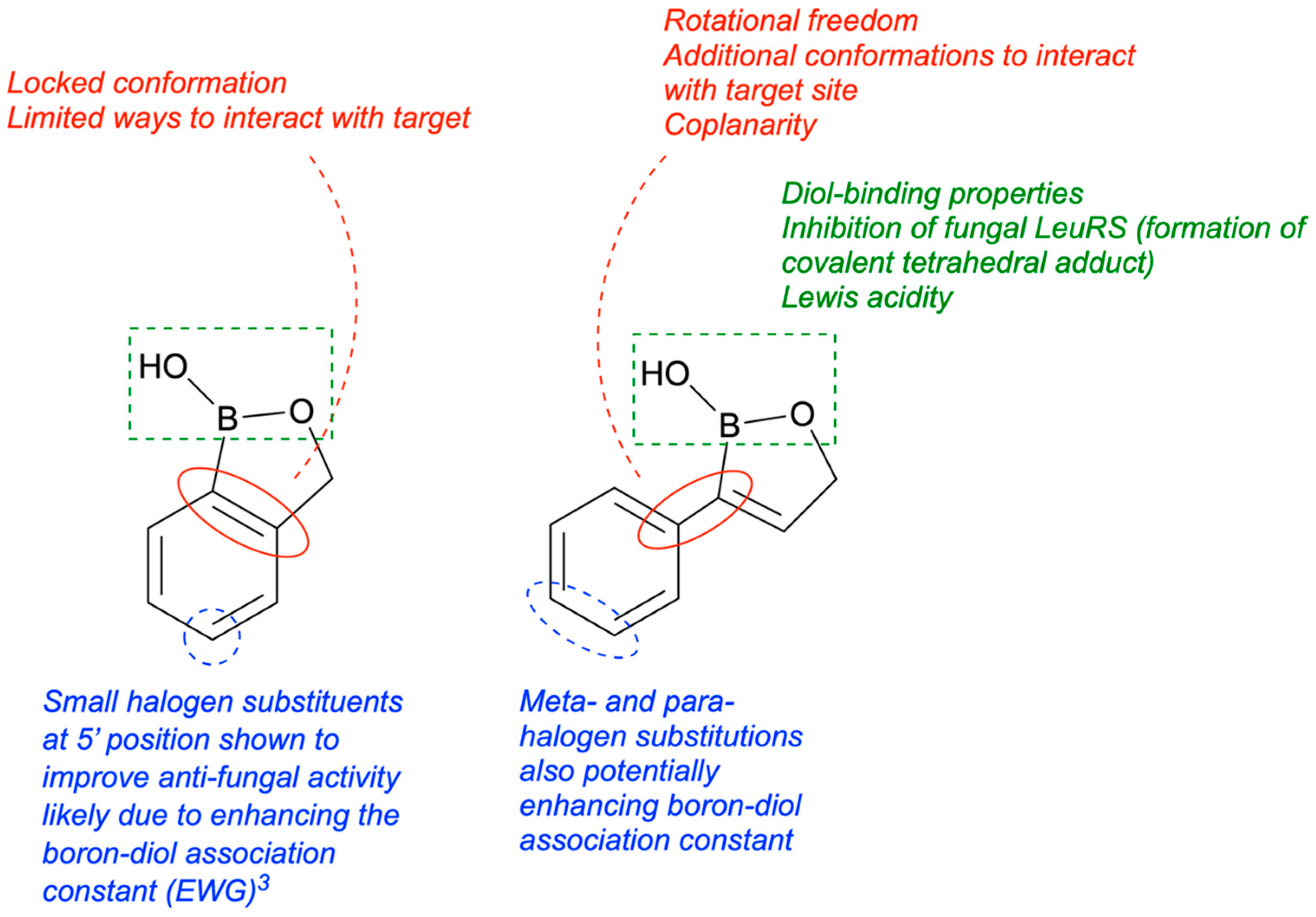
8. Antifungal Activity of 3-Substituted-2(5H)-Oxaboroles
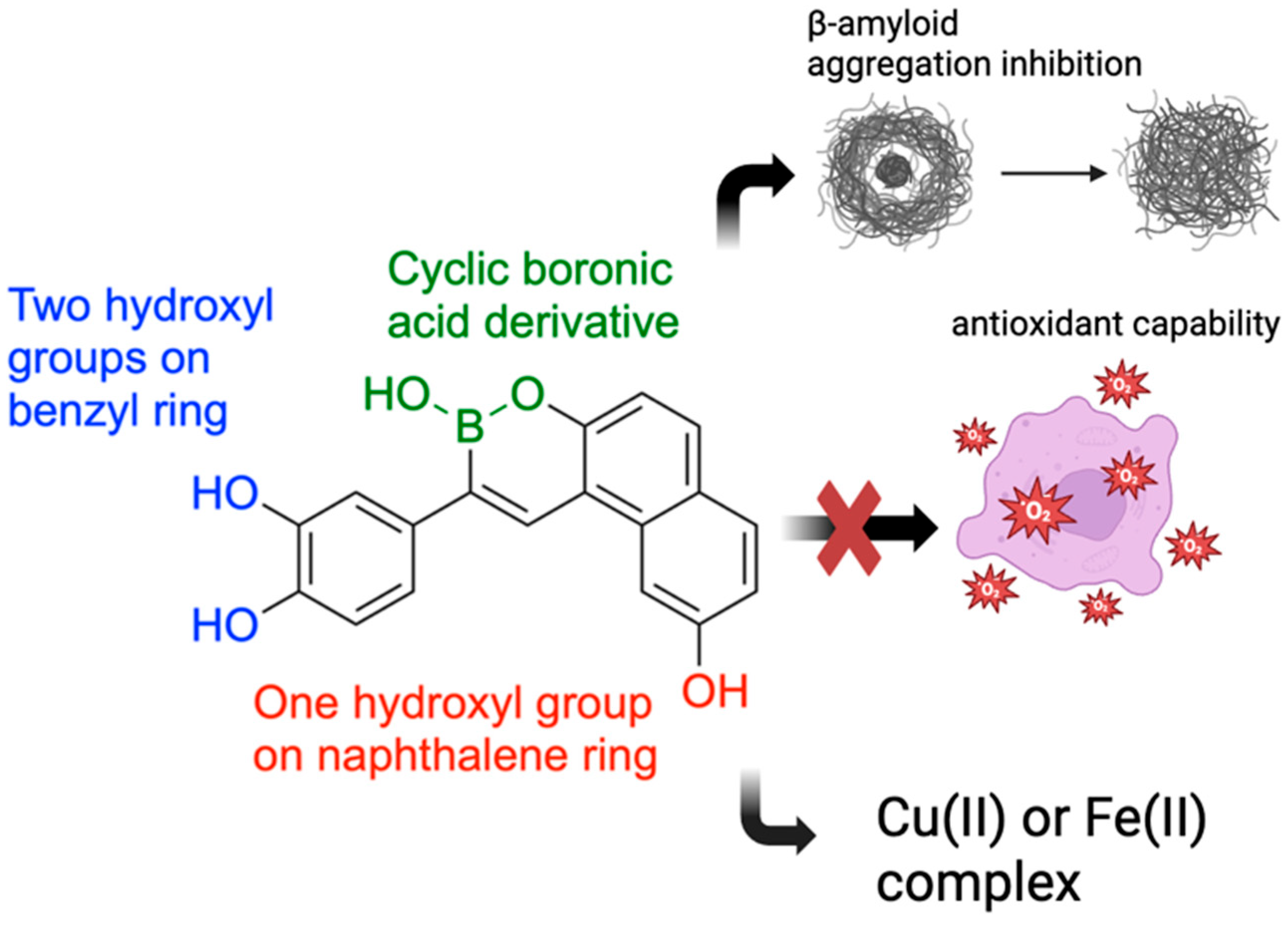
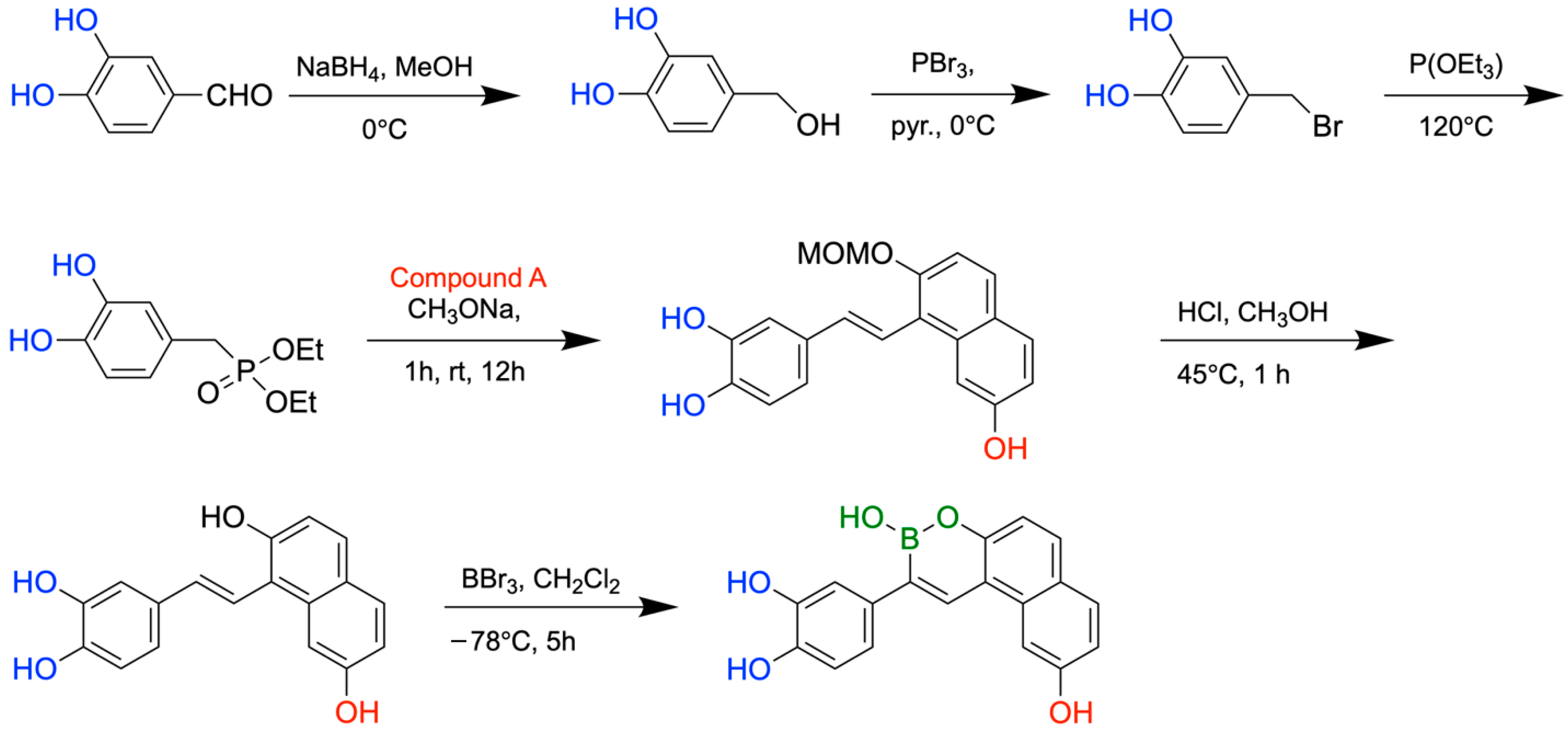
9. Boron-Containing Compounds for the Treatment of Alzheimer’s Disease (AD)
10. Boron in Biological Target Engagement
11. Boron in New Drug Chemotypes: Pharmaceutical Potential of Hemiboronic Naphthoids
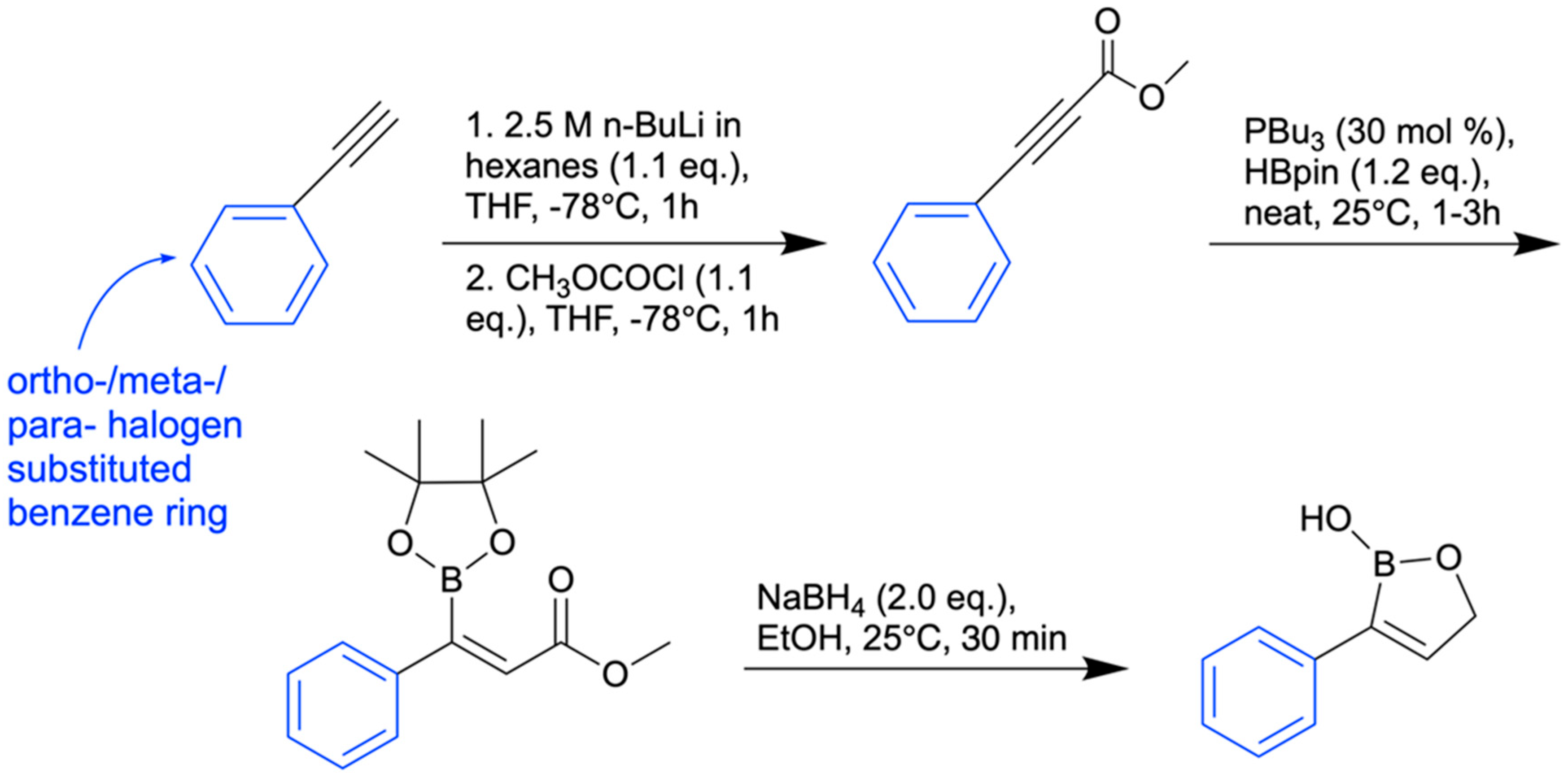
12. Phenyl Boronic Acid (PBA)-Based Non-β-Lactam β-Lactamase Inhibitors Against KPC-2-Producers
13. Discovery and Optimization of a Novel Boronic-Acid (BA) Arginase Inhibitor
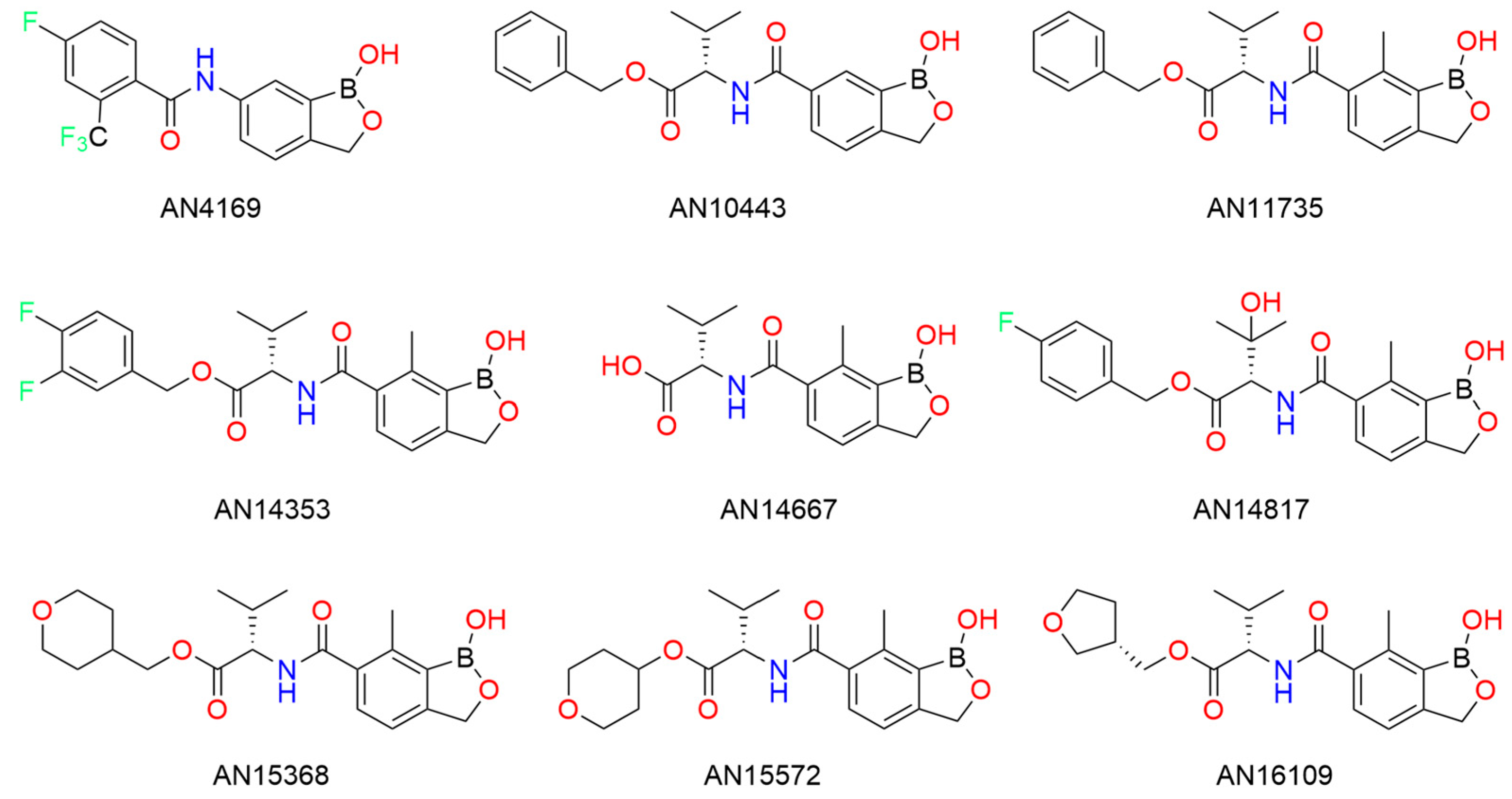
14. Boron in the Treatment of Chagas Disease
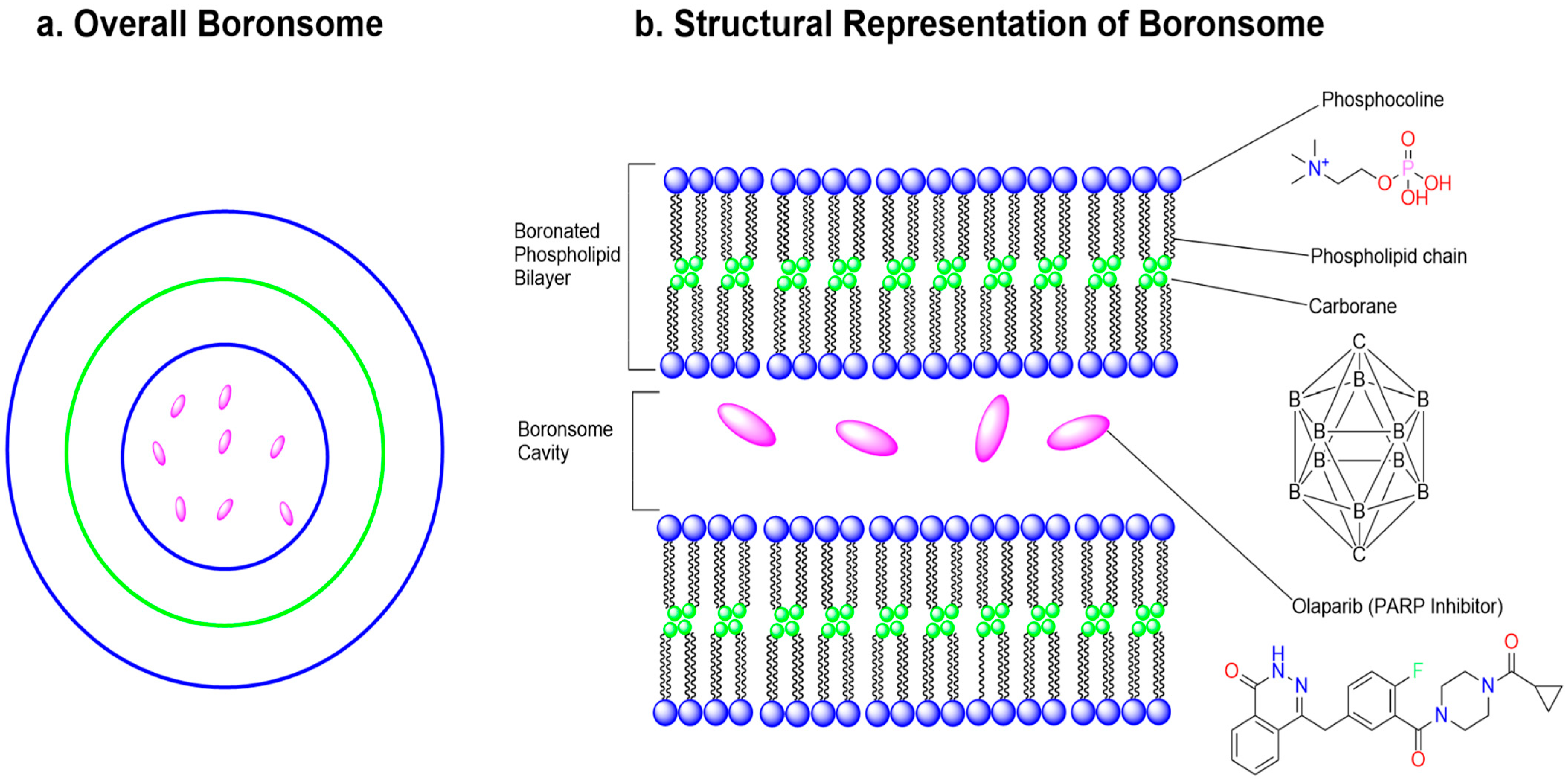
15. Boron Encapsulated in a Liposome for Neutron Capture Therapy
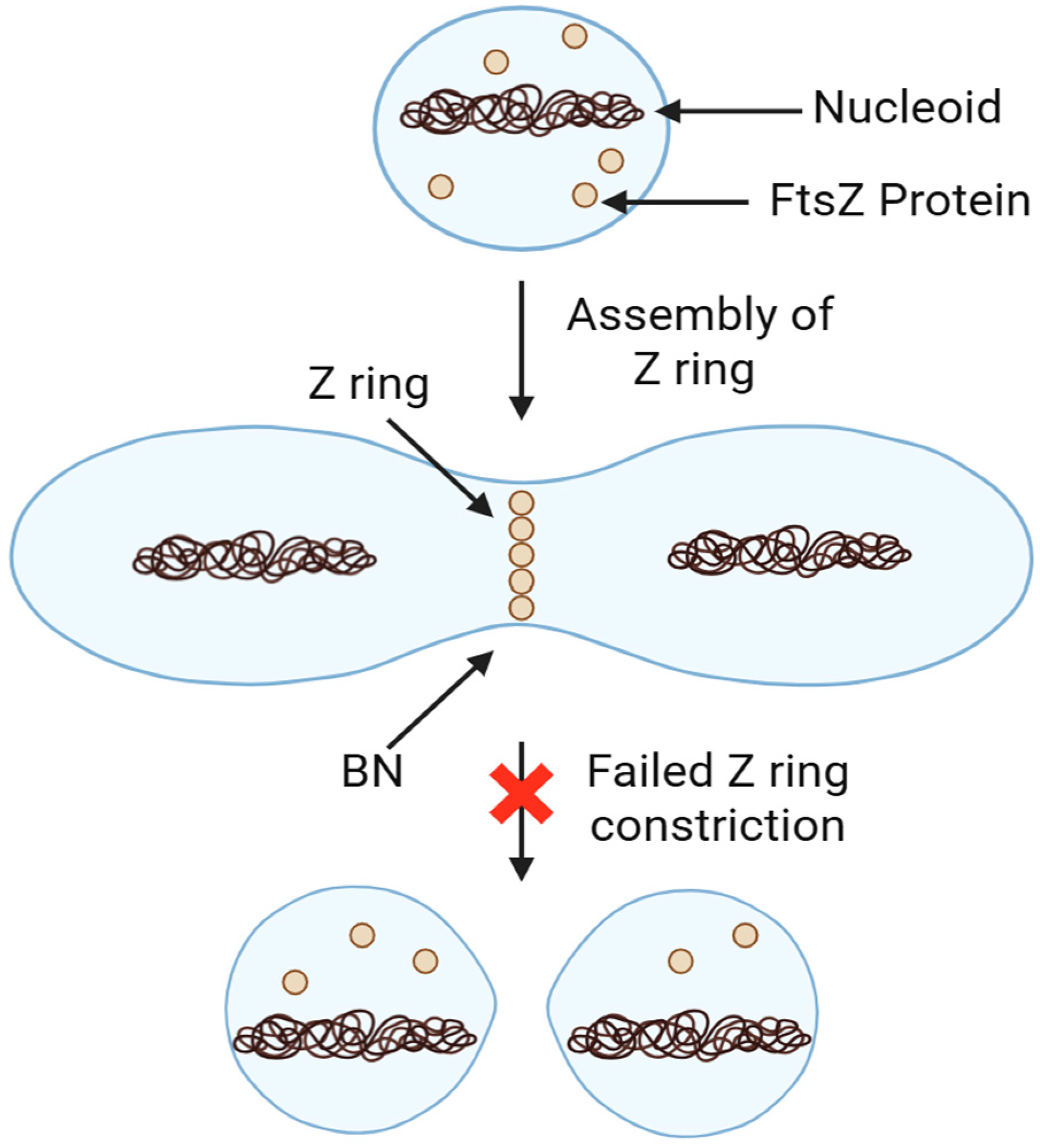
16. Boron Nitride for Combating Resistant Bacteria
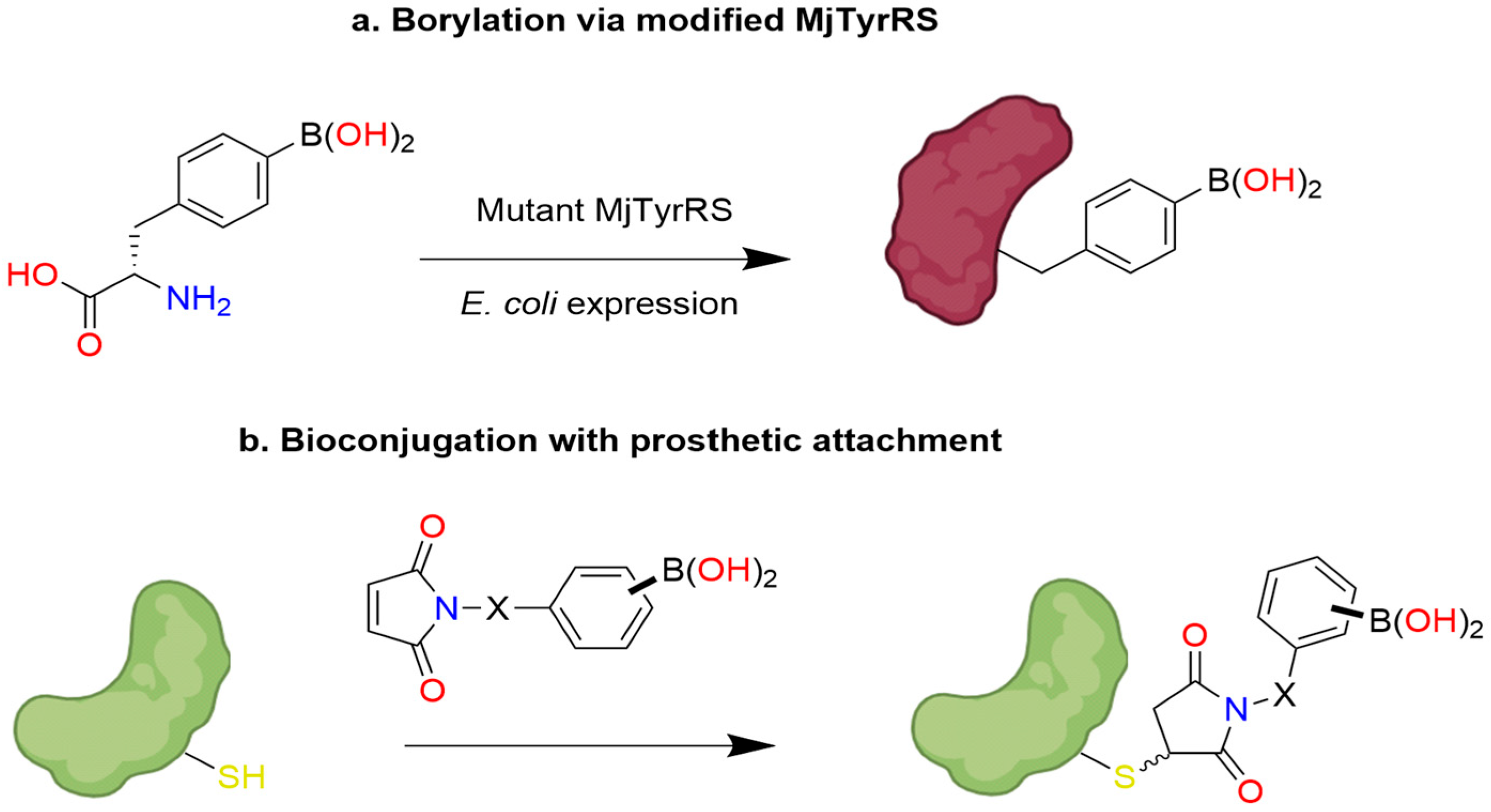
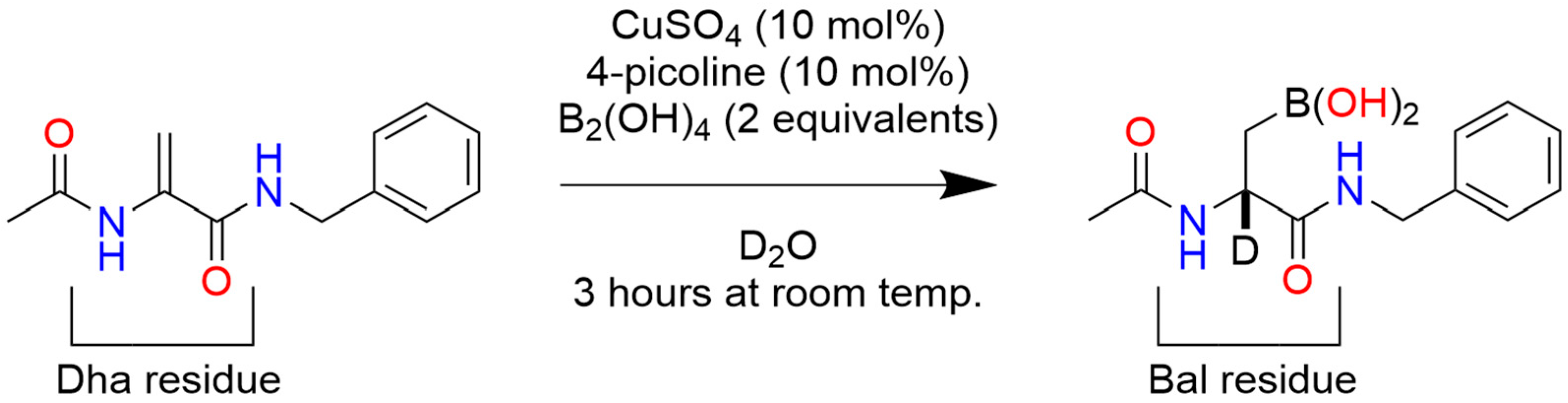
17. Post-Translational Insertion of Boron in Proteins
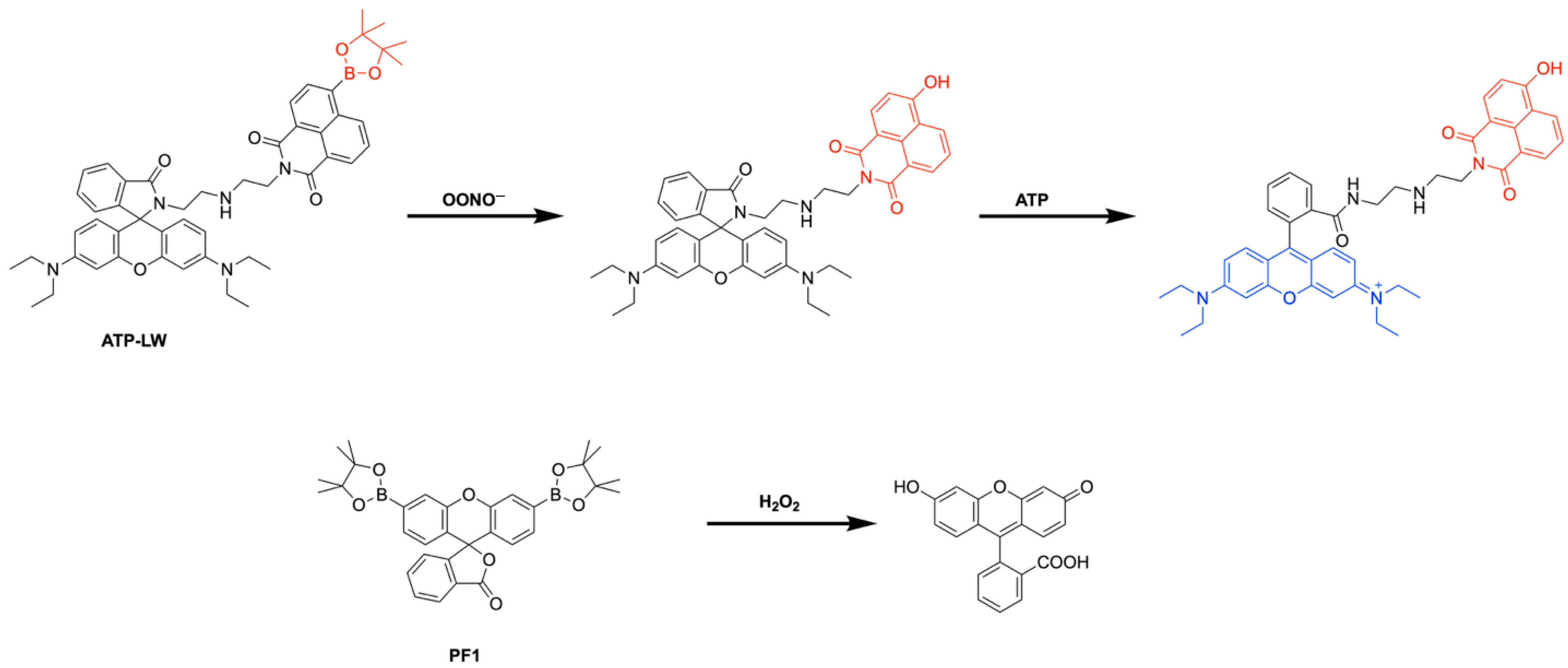
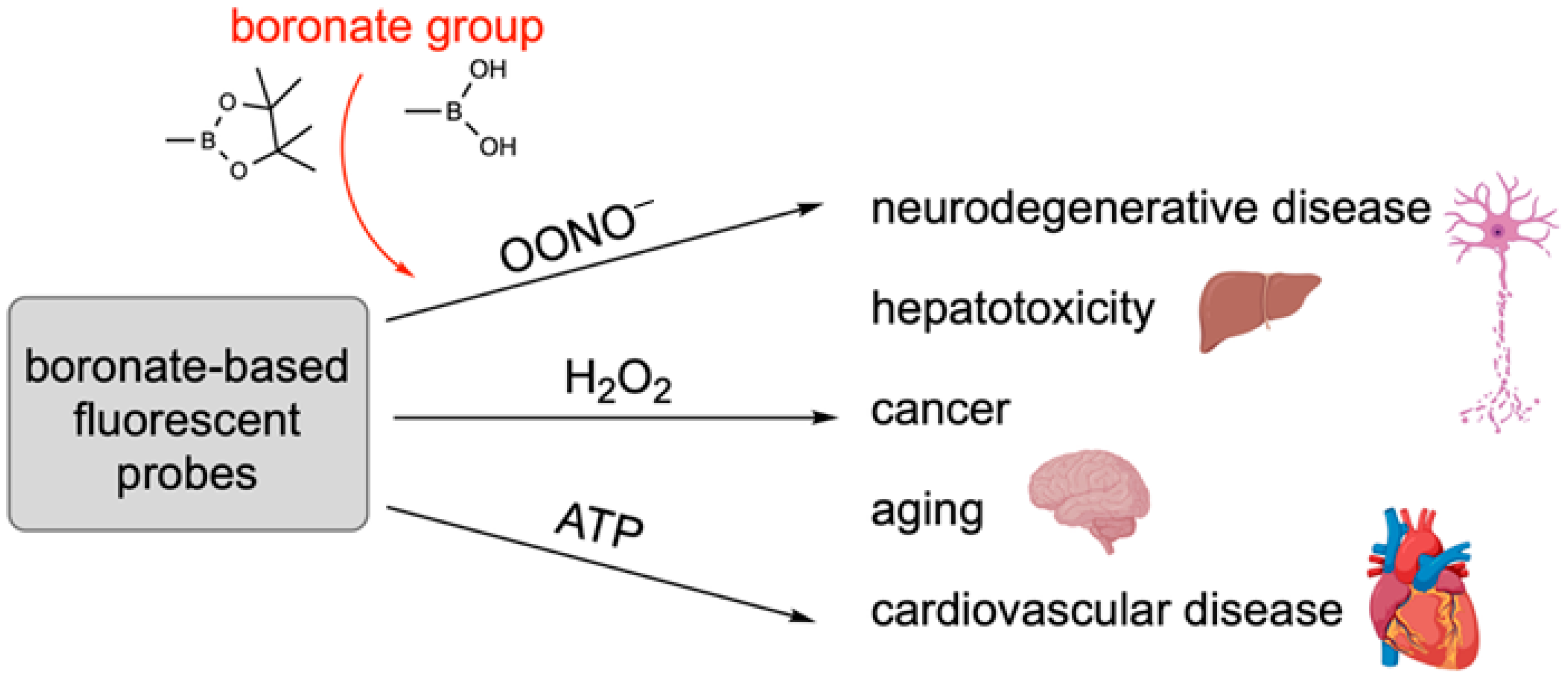
18. Boron-Based Probes Used in Biological Imaging
19. Toxicity of Boron-Containing Compounds
20. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gay-Lussac, J.L.; Thénard, L.J. Sur la décomposition et la recomposition de l’acide boracique. Ann. Chim. Phys. 1808, 68, 169–174. [Google Scholar]
- Brown, H.C.; Malhotra, S.V.; Ramachandran, P.V. Organoboranes for synthesis 17. Generality of hydroboration-amination for the conversion of terpenes into enantiomerically pure terpenylamines. Their utility for gas chromatographic analysis of chiral carboxylic acids. Tetrahedron Asymmetry 1996, 7, 3527–3534. [Google Scholar] [CrossRef]
- Yang, W.; Gao, X.; Wang, B. Boronic acid compounds as potential pharmaceutical agents. Med. Res. Rev. 2003, 23, 346–368. [Google Scholar] [CrossRef] [PubMed]
- DeFrancesco, H.; Dudley, J.; Coca, A. Boron Chemistry: An Overview. In Boron Reagents in Synthesis; American Chemical Society: Washington, DC, USA, 2016; pp. 1–25. [Google Scholar]
- Yang, F.; Zhu, M.; Zhang, J.; Zhou, H. Synthesis of biologically active boron-containing compounds. Medchemcomm 2018, 9, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Ban, H.S.; Nakamura, H. Boron-Based Drug Design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef]
- Grams, R.J.; Santos, W.L.; Scorei, I.R.; Abad-García, A.; Rosenblum, C.A.; Bita, A.; Cerecetto, H.; Viñas, C.; Soriano-Ursúa, M.A. The Rise of Boron-Containing Compounds: Advancements in Synthesis, Medicinal Chemistry, and Emerging Pharmacology. Chem. Rev. 2024, 124, 2441–2511. [Google Scholar] [CrossRef]
- Loomis, W.D.; Durst, R.W. Chemistry and biology of boron. Biofactors 1992, 3, 229–239. [Google Scholar]
- Kan, F.; Kucukkurt, I. The effects of boron on some biochemical parameters: A review. J. Trace Elem. Med. Biol. 2023, 79, 127249. [Google Scholar] [CrossRef]
- Das, B.C.; Chokkalingam, P.; Masilamani, P.; Shukla, S.; Das, S. Stimuli-Responsive Boron-Based Materials in Drug Delivery. Int. J. Mol. Sci. 2023, 24, 2757. [Google Scholar] [CrossRef]
- Das, B.C.; Nandwana, N.K.; Das, S.; Nandwana, V.; Shareef, M.A.; Das, Y.; Saito, M.; Weiss, L.M.; Almaguel, F.; Hosmane, N.S.; et al. Boron Chemicals in Drug Discovery and Development: Synthesis and Medicinal Perspective. Molecules 2022, 27, 2615. [Google Scholar] [CrossRef]
- Martin, R.; Buchwald, S.L. Palladium-catalyzed Suzuki− Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 2008, 41, 1461–1473. [Google Scholar] [CrossRef]
- Buskes, M.J.; Blanco, M.-J. Impact of cross-coupling reactions in drug discovery and development. Molecules 2020, 25, 3493. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.B.; Yudin, A.K. The versatility of boron in biological target engagement. Nat. Chem. 2017, 9, 731–742. [Google Scholar] [CrossRef]
- Fernandes, G.F.S.; Denny, W.A.; Dos Santos, J.L. Boron in drug design: Recent advances in the development of new therapeutic agents. Eur. J. Med. Chem. 2019, 179, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Lorand, J.P.; Edwards, J.O. Polyol complexes and structure of the benzeneboronate ion. J. Org. Chem. 1959, 24, 769–774. [Google Scholar] [CrossRef]
- Springsteen, G.; Ballard, C.E.; Gao, S.; Wang, W.; Wang, B. The development of photometric sensors for boronic acids. Bioorg. Chem. 2001, 29, 259–270. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Crépy, K.V. Synthesis of chiral binaphthalenes using the asymmetric Suzuki reaction. Tetrahedron 2004, 60, 4377–4386. [Google Scholar] [CrossRef]
- Lamandé, L.; Boyer, D.; Munoz, A. Structure et acidite de composes a atome de bore et de phosphore hypercoordonnes. J. Organomet. Chem. 1980, 329, 1–29. [Google Scholar] [CrossRef]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef]
- Jin, S.; Cheng, Y.; Reid, S.; Li, M.; Wang, B. Carbohydrate recognition by boronolectins, small molecules, and lectins. Med. Res. Rev. 2010, 30, 171–257. [Google Scholar] [CrossRef]
- Yang, W.; Fan, H.; Gao, X.; Gao, S.; Karnati, V.V.R.; Ni, W.; Hooks, W.B.; Carson, J.; Weston, B.; Wang, B. The first fluorescent diboronic acid sensor specific for hepatocellular carcinoma cells expressing sialyl Lewis X. Chem. Biol. 2004, 11, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, S.; Gao, X.; Karnati, V.V.R.; Ni, W.; Wang, B.; Hooks, W.B.; Carson, J.; Weston, B. Diboronic acids as fluorescent probes for cells expressing sialyl Lewis X. Bioorg. Med. Chem. Lett. 2002, 12, 2175–2177. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Zhu, D. A new saccharide sensor based on a tetrathiafulvalene-anthracene dyad with a boronic acid group. J. Org. Chem. 2005, 70, 5729–5732. [Google Scholar] [CrossRef]
- Manimala, J.C.; Wiskur, S.L.; Ellington, A.D.; Anslyn, E.V. Tuning the specificity of a synthetic receptor using a selected nucleic acid receptor. J. Am. Chem. Soc. 2004, 126, 16515–16519. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.J.; Zhang, Y.-K.; Akama, T.; Lau, A.; Zhou, H.; Hernandez, V.; Mao, W.; Alley, M.; Sanders, V.; Plattner, J.J. Discovery of a new boron-containing antifungal agent, 5-fluoro-1, 3-dihydro-1-hydroxy-2, 1-benzoxaborole (AN2690), for the potential treatment of onychomycosis. J. Med. Chem. 2006, 49, 4447–4450. [Google Scholar] [CrossRef]
- Windsor, I.W.; Palte, M.J.; Lukesh III, J.C.; Gold, B.; Forest, K.T.; Raines, R.T. Sub-picomolar inhibition of HIV-1 protease with a boronic acid. J. Am. Chem. Soc. 2018, 140, 14015–14018. [Google Scholar] [CrossRef]
- Chong, P.Y.; Shotwell, J.B.; Miller, J.; Price, D.J.; Maynard, A.; Voitenleitner, C.; Mathis, A.; Williams, S.; Pouliot, J.J.; Creech, K. Design of N-benzoxaborole benzofuran GSK8175—Optimization of human pharmacokinetics inspired by metabolites of a failed clinical HCV inhibitor. J. Med. Chem. 2019, 62, 3254–3267. [Google Scholar] [CrossRef]
- Malouff, T.D.; Seneviratne, D.S.; Ebner, D.K.; Stross, W.C.; Waddle, M.R.; Trifiletti, D.M.; Krishnan, S. Boron neutron capture therapy: A review of clinical applications. Front. Oncol. 2021, 11, 351. [Google Scholar] [CrossRef]
- Génin, E.; Reboud-Ravaux, M.; Vidal, J. Proteasome inhibitors: Recent advances and new perspectives in medicinal chemistry. Curr. Top. Med. Chem. 2010, 10, 232–256. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Versteeg, S.G. Tavaborole–a treatment for onychomycosis of the toenails. Expert Rev. Clin. Pharmacol. 2016, 9, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Zweegman, S.; O’Donnell, E.K.; Laubach, J.P.; Raje, N.; Voorhees, P.; Ferrari, R.H.; Skacel, T.; Kumar, S.K.; Lonial, S. Ixazomib for the treatment of multiple myeloma. Expert Opin. Pharmacother. 2018, 19, 1949–1968. [Google Scholar] [CrossRef]
- Jarnagin, K.; Chanda, S.; Coronado, D.; Ciaravino, V.; Zane, L.T.; Guttman-Yassky, E.; Lebwohl, M.G. Crisaborole Topical Ointment, 2%: A Nonsteroidal, Topical, Anti-Inflammatory Phosphodiesterase 4 Inhibitor in Clinical Development for the Treatment of Atopic Dermatitis. J. Drugs Dermatol. 2016, 15, 390–396. [Google Scholar]
- Burgos, R.M.; Biagi, M.J.; Rodvold, K.A.; Danziger, L.H. Pharmacokinetic evaluation of meropenem and vaborbactam for the treatment of urinary tract infection. Expert Opin. Drug Metab. Toxicol. 2018, 14, 1007–1021. [Google Scholar] [CrossRef]
- Fazal, T.; Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Compounds for Catalytic Applications. In Advances in Catalysis; Elsevier: Amsterdam, The Netherlands, 2022; Volume 71, pp. 169–199. [Google Scholar]
- Chiou, S.L.; Chen, Y.J.; Lee, C.T.; Ho, M.N.; Miao, J.; Kuo, P.C.; Hsu, C.C.; Lin, Y.S.; Chu, J. A Boron-Dependent Antibiotic Derived from a Calcium-Dependent Antibiotic. Angew. Chem. 2024, 136, e202317522. [Google Scholar] [CrossRef]
- Naganawa, H.; Hamada, M.; Maeda, K.; Okami, Y.; Takeushi, T. Laspartomycin, a new anti-staphylococcal peptide. J. Antibiot. 1968, 21, 55–62. [Google Scholar] [CrossRef]
- Kleijn, L.H.J.; Vlieg, H.C.; Wood, T.M.; Sastre Toraño, J.; Janssen, B.J.C.; Martin, N.I. A High-Resolution Crystal Structure that Reveals Molecular Details of Target Recognition by the Calcium-Dependent Lipopeptide Antibiotic Laspartomycin C. Angew. Chem. Int. Ed. Engl. 2017, 56, 16546–16549. [Google Scholar] [CrossRef] [PubMed]
- Kleijn, L.H.; Oppedijk, S.F.; Hart, P.T.; van Harten, R.M.; Martin-Visscher, L.A.; Kemmink, J.; Breukink, E.; Martin, N.I. Total Synthesis of Laspartomycin C and Characterization of Its Antibacterial Mechanism of Action. J. Med. Chem. 2016, 59, 3569–3574. [Google Scholar] [CrossRef]
- Atchison, D.K.; Beierwaltes, W.H. The influence of extracellular and intracellular calcium on the secretion of renin. Pflug. Arch. 2013, 465, 59–69. [Google Scholar] [CrossRef]
- Hover, B.M.; Kim, S.H.; Katz, M.; Charlop-Powers, Z.; Owen, J.G.; Ternei, M.A.; Maniko, J.; Estrela, A.B.; Molina, H.; Park, S.; et al. Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat. Microbiol. 2018, 3, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.M. Synthetic and Mechanistic Studies with Biologically Active Macrocyclic Peptides. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2021. [Google Scholar]
- Jung, D.; Rozek, A.; Okon, M.; Hancock, R.E. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 2004, 11, 949–957. [Google Scholar] [CrossRef]
- Wood, T.M.; Zeronian, M.R.; Buijs, N.; Bertheussen, K.; Abedian, H.K.; Johnson, A.V.; Pearce, N.M.; Lutz, M.; Kemmink, J.; Seirsma, T.; et al. Mechanistic insights into the C(55)-P targeting lipopeptide antibiotics revealed by structure-activity studies and high-resolution crystal structures. Chem. Sci. 2022, 13, 2985–2991. [Google Scholar] [CrossRef]
- Hall, D.G. Boronic acid catalysis. Chem. Soc. Rev. 2019, 48, 3475–3496. [Google Scholar] [CrossRef]
- Mo, X.; Hall, D.G. Dual Catalysis Using Boronic Acid and Chiral Amine: Acyclic Quaternary Carbons via Enantioselective Alkylation of Branched Aldehydes with Allylic Alcohols. J. Am. Chem. Soc. 2016, 138, 10762–10765. [Google Scholar] [CrossRef]
- Hayama, N.; Kobayashi, Y.; Takemoto, Y. Asymmetric hetero-Michael addition to α, β-unsaturated carboxylic acids using thiourea–boronic acid hybrid catalysts. Tetrahedron 2021, 89, 132089. [Google Scholar] [CrossRef]
- Longwitz, L.; Leveson-Gower, R.B.; Rozeboom, H.J.; Thunnissen, A.W.H.; Roelfes, G. Boron catalysis in a designer enzyme. Nature 2024, 629, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Madoori, P.K.; Agustiandari, H.; Driessen, A.J.; Thunnissen, A.M. Structure of the transcriptional regulator LmrR and its mechanism of multidrug recognition. Embo J. 2009, 28, 156–166. [Google Scholar] [CrossRef]
- Simionatto, E.L.; Yunes, P.R.; Yunes, R.A. The effect of boric acid on the dehydration step in the formation of oxime from salicylaldehyde. J. Chem. Soc. Perkin Trans. 2 1993, 1291–1294. [Google Scholar] [CrossRef]
- Ettinger, D.S. Amrubicin for the treatment of small cell lung cancer: Does effectiveness cross the Pacific? J. Thorac. Oncol. 2007, 2, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Booser, D.J.; Perez-Soler, R.; Cossum, P.; Esparza-Guerra, L.; Wu, Q.P.; Zou, Y.; Priebe, W.; Hortobagyi, G.N. Phase I study of liposomal annamycin. Cancer Chemother. Pharmacol. 2000, 46, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S. Development of arbekacin and synthesis of new derivatives stable to enzymatic modifications by methicillin-resistant Staphylococcus aureus. Jpn. J. Antibiot. 1994, 47, 561–574. [Google Scholar]
- Kawaguchi, H. Discovery, chemistry, and activity of amikacin. J. Infect. Dis. 1976, 134 (Suppl. S2), S242–S248. [Google Scholar] [CrossRef]
- Dutcher, J.D. The discovery and development of amphotericin B. Dis. Chest 1968, 54 (Suppl. S1), 296–298. [Google Scholar] [CrossRef]
- Dindere, M.E. New FDA-approved SGLT2 inhibitor bexagliflozin for type 2 diabetes therapy. Discov. Rep. 2023, 6, e41. [Google Scholar]
- Meng, W.; Ellsworth, B.A.; Nirschl, A.A.; McCann, P.J.; Patel, M.; Girotra, R.N.; Wu, G.; Sher, P.M.; Morrison, E.P.; Biller, S.A. Discovery of dapagliflozin: A potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2008, 51, 1145–1149. [Google Scholar] [CrossRef]
- Gold, H.; Bellet, S. Acetyldigitoxin in the treatment of heart failure. N. Engl. J. Med. 1957, 256, 536–540. [Google Scholar] [CrossRef]
- Eskwith, I.S.; Fogarty, T.F. The treatment of auricular arrhythmias with deslanoside. Am. J. Cardiol. 1958, 2, 579–585. [Google Scholar] [CrossRef]
- Hanessian, S.; Banoub, J. Chemistry of the glycosidic linkage. An efficient synthesis of 1, 2-trans-di-saccharides. Carbohydr. Res. 1977, 53, C13–C16. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, N.; Xiao, H.; Li, Y. Efficient syntheses of a series of glycosphingolipids with 1, 2-trans-glycosidic linkages. J. Carbohydr. Chem. 2006, 25, 471–489. [Google Scholar] [CrossRef]
- Hahm, H.S.; Hurevich, M.; Seeberger, P.H. Automated assembly of oligosaccharides containing multiple cis-glycosidic linkages. Nat. Commun. 2016, 7, 12482. [Google Scholar] [CrossRef]
- Jeanneret, R.A.; Johnson, S.E.; Galan, M.C. Conformationally Constrained Glycosyl Donors as Tools to Control Glycosylation Outcomes. J. Org. Chem. 2020, 85, 15801–15826. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.S.; Lowary, T.L. A Siloxane-Bridged Glycosyl Donor Enables Highly Stereoselective β-Xylulofuranosylation. J. Org. Chem. 2020, 85, 15895–15907. [Google Scholar] [CrossRef]
- Jia, X.G.; Demchenko, A.V. Intramolecular glycosylation. Beilstein J. Org. Chem. 2017, 13, 2028–2048. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, Y.; Toshima, K.; Takahashi, D. Boron-mediated aglycon delivery for late-stage modifications: Applications in chemical biology and pharmaceutical lead development. Chem. Lett. 2025, 54, upaf088. [Google Scholar] [CrossRef]
- Fahr, G.; LaDue, J. A preliminary investigation of the therapeutic value of lanatoside C (digilanid C). Am. Heart J. 1941, 21, 133–150. [Google Scholar] [CrossRef]
- Kang, M.A.; Kim, M.S.; Kim, W.; Um, J.H.; Shin, Y.J.; Song, J.Y.; Jeong, J.H. Lanatoside C suppressed colorectal cancer cell growth by inducing mitochondrial dysfunction and increased radiation sensitivity by impairing DNA damage repair. Oncotarget 2016, 7, 6074–6087. [Google Scholar] [CrossRef]
- Schneider, N.F.Z.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef]
- Denis, A.; Agouridas, C.; Auger, J.M.; Benedetti, Y.; Bonnefoy, A.; Bretin, F.; Chantot, J.F.; Dussarat, A.; Fromentin, C.; D’Ambrières, S.G.; et al. Synthesis and antibacterial activity of HMR 3647 a new ketolide highly potent against erythromycin-resistant and susceptible pathogens. Bioorg Med. Chem. Lett. 1999, 9, 3075–3080. [Google Scholar] [CrossRef]
- Albert, A. Chemical aspects of selective toxicity. Nature 1958, 182, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Liu, L.H.; Jia, H.Z.; Qiu, W.X.; Rong, L.; Cheng, H.; Zhang, X.Z. A pH-responsive prodrug for real-time drug release monitoring and targeted cancer therapy. Chem. Commun. 2014, 50, 11852–11855. [Google Scholar] [CrossRef]
- Peng, X.; Gandhi, V. ROS-activated anticancer prodrugs: A new strategy for tumor-specific damage. Ther. Deliv. 2012, 3, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Zhang, Y.; Gao, Q.; Neumann, K.; Dong, H.; Porter, H.; Potter, M.; Ren, H.; Argyle, D.; Bradley, M. Switching on prodrugs using radiotherapy. Nat. Chem. 2021, 13, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Norman, D.J.; González-Fernández, E.; Clavadetscher, J.; Tucker, L.; Staderini, M.; Mount, A.R.; Murray, A.F.; Bradley, M. Electrodrugs: An electrochemical prodrug activation strategy. Chem. Commun. 2018, 54, 9242–9245. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of Beta-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- Ganapathy, U.S.; Gengenbacher, M.; Dick, T. Epetraborole Is Active against Mycobacterium abscessus. Antimicrob. Agents Chemother. 2021, 65, e0115621. [Google Scholar] [CrossRef]
- Jacobs, R.T.; Nare, B.; Wring, S.A.; Orr, M.D.; Chen, D.; Sligar, J.M.; Jenks, M.X.; Noe, R.A.; Bowling, T.S.; Mercer, L.T.; et al. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Negl. Trop. Dis. 2011, 5, e1151. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, D. An upcoming drug for onychomycosis: Tavaborole. J. Pharmacol. Pharmacother. 2015, 6, 236–239. [Google Scholar] [CrossRef]
- Paller, A.S.; Tom, W.L.; Lebwohl, M.G.; Blumenthal, R.L.; Boguniewicz, M.; Call, R.S.; Eichenfield, L.F.; Forsha, D.W.; Rees, W.C.; Simpson, E.L.; et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J. Am. Acad. Dermatol. 2016, 75, 494–503.e496. [Google Scholar] [CrossRef]
- Hoffmann, G.; Le Gorrec, M.; Mestdach, E.; Cusack, S.; Salmon, L.; Jensen, M.R.; Palencia, A. Adenosine-Dependent Activation Mechanism of Prodrugs Targeting an Aminoacyl-tRNA Synthetase. J. Am. Chem. Soc. 2023, 145, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, U.S.; Del Rio, R.G.; Cacho-Izquierdo, M.; Ortega, F.; Lelièvre, J.; Barros-Aguirre, D.; Lindman, M.; Dartois, V.; Gengenbacher, M.; Dick, T. A Leucyl-tRNA Synthetase Inhibitor with Broad-Spectrum Anti-Mycobacterial Activity. Antimicrob. Agents Chemother. 2023, 95, 10–1128. [Google Scholar] [CrossRef]
- Palencia, A.; Li, X.; Bu, W.; Choi, W.; Ding, C.Z.; Easom, E.E.; Feng, L.; Hernandez, V.; Houston, P.; Liu, L.; et al. Discovery of Novel Oral Protein Synthesis Inhibitors of Mycobacterium tuberculosis That Target Leucyl-tRNA Synthetase. Antimicrob. Agents Chemother. 2016, 60, 6271–6280. [Google Scholar] [CrossRef]
- Scarpini, S.; Dondi, A.; Totaro, C.; Biagi, C.; Melchionda, F.; Zama, D.; Pierantoni, L.; Gennari, M.; Campagna, C.; Prete, A. Visceral leishmaniasis: Epidemiology, diagnosis, and treatment regimens in different geographical areas with a focus on pediatrics. Microorganisms 2022, 10, 1887. [Google Scholar] [CrossRef] [PubMed]
- Uliana, S.R.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of leishmaniasis: Present challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.K.; Sen, P.; Roy, S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Mol. Biol. Int. 2011, 2011, 571242. [Google Scholar] [CrossRef]
- Sundar, S. Drug resistance in Indian visceral leishmaniasis. Trop. Med. Int. Health 2001, 6, 849–854. [Google Scholar] [CrossRef]
- Sundar, S.; More, D.K.; Singh, M.K.; Singh, V.P.; Sharma, S.; Makharia, A.; Kumar, P.C.; Murray, H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: Report from the center of the Indian epidemic. Clin. Infect. Dis. 2000, 31, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Thakur, C.; Dedet, J.; Narain, S.; Pratlong, F. Leishmania species, drug unresponsiveness and visceral leishmaniasis in Bihar, India. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 187–189. [Google Scholar] [CrossRef]
- Lira, R.; Sundar, S.; Makharia, A.; Kenney, R.; Gam, A.; Saraiva, E.; Sacks, D. Evidence that the high incidence of treatment failures in Indian kala-azar is due to the emergence of antimony-resistant strains of Leishmania donovani. J. Infect. Dis. 1999, 180, 564–567. [Google Scholar] [CrossRef]
- Sundar, S.; Sinha, P.R.; Agrawal, N.K.; Srivastava, R.; Rainey, P.M.; Berman, J.D.; Murray, H.W.; Singh, V.P. A cluster of cases of severe cardiotoxicity among kala-azar patients treated with a high-osmolarity lot of sodium antimony gluconate. Am. J. Trop. Med. Hyg. 1998, 59, 139–143. [Google Scholar] [CrossRef]
- Alves, F.; Bilbe, G.; Blesson, S.; Goyal, V.; Monnerat, S.; Mowbray, C.; Muthoni Ouattara, G.; Pécoul, B.; Rijal, S.; Rode, J. Recent development of visceral leishmaniasis treatments: Successes, pitfalls, and perspectives. Clin. Microbiol. Rev. 2018, 31, 10–1128. [Google Scholar] [CrossRef]
- Wyllie, S.; Brand, S.; Thomas, M.; De Rycker, M.; Chung, C.-w.; Pena, I.; Bingham, R.P.; Bueren-Calabuig, J.A.; Cantizani, J.; Cebrian, D. Preclinical candidate for the treatment of visceral leishmaniasis that acts through proteasome inhibition. Proc. Natl. Acad. Sci. USA 2019, 116, 9318–9323. [Google Scholar]
- Mowbray, C.E.; Braillard, S.; Glossop, P.A.; Whitlock, G.A.; Jacobs, R.T.; Speake, J.; Pandi, B.; Nare, B.; Maes, L.; Yardley, V.; et al. DNDI-6148: A novel benzoxaborole preclinical candidate for the treatment of visceral leishmaniasis. J. Med. Chem. 2021, 64, 16159–16176. [Google Scholar] [CrossRef]
- Kumeso, V.K.B.; Kalonji, W.M.; Rembry, S.; Mordt, O.V.; Tete, D.N.; Prêtre, A.; Delhomme, S.; Kyhi, M.I.W.; Camara, M.; Catusse, J. Efficacy and safety of acoziborole in patients with human African trypanosomiasis caused by Trypanosoma brucei gambiense: A multicentre, open-label, single-arm, phase 2/3 trial. Lancet Infect. Dis. 2023, 23, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Begolo, D.; Vincent, I.M.; Giordani, F.; Poehner, I.; Witty, M.J.; Rowan, T.G.; Bengaly, Z.; Gillingwater, K.; Freund, Y.; Wade, R.C.; et al. The trypanocidal benzoxaborole AN7973 inhibits trypanosome mRNA processing. PLoS Pathog. 2018, 14, e1007315. [Google Scholar] [CrossRef] [PubMed]
- Sonoiki, E.; Ng, C.L.; Lee, M.C.; Guo, D.; Zhang, Y.-K.; Zhou, Y.; Alley, M.; Ahyong, V.; Sanz, L.M.; Lafuente-Monasterio, M.J.; et al. A potent antimalarial benzoxaborole targets a Plasmodium falciparum cleavage and polyadenylation specificity factor homologue. Nat. Commun. 2017, 8, 14574. [Google Scholar] [CrossRef]
- Bellini, V.; Swale, C.; Brenier-Pinchart, M.P.; Pezier, T.; Georgeault, S.; Laurent, F.; Hakimi, M.A.; Bougdour, A. Target Identification of an Antimalarial Oxaborole Identifies AN13762 as an Alternative Chemotype for Targeting CPSF3 in Apicomplexan Parasites. iScience 2020, 23, 101871. [Google Scholar] [CrossRef]
- Campbell, R.; Buchbinder, N.W.; Szwetkowski, C.; Zhu, Y.; Piedl, K.; Truong, M.; Matson, J.B.; Santos, W.L.; Mevers, E. Design, Synthesis, and Antifungal Activity of 3-Substituted-2(5H)-Oxaboroles. ACS Med. Chem. Lett. 2024, 15, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T.; Winum, J.Y. Benzoxaborole compounds for therapeutic uses: A patent review (2010–2018). Expert Opin. Ther. Pat. 2018, 28, 493–504. [Google Scholar] [CrossRef]
- Lu, C.J.; Hu, J.; Wang, Z.; Xie, S.; Pan, T.; Huang, L.; Li, X. Discovery of boron-containing compounds as Aβ aggregation inhibitors and antioxidants for the treatment of Alzheimer’s disease. Medchemcomm 2018, 9, 1862–1870. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. A century of Alzheimer’s disease. Science 2006, 314, 777–781. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Bonda, D.J.; Wang, X.; Perry, G.; Nunomura, A.; Tabaton, M.; Zhu, X.; Smith, M.A. Oxidative stress in Alzheimer disease: A possibility for prevention. Neuropharmacology 2010, 59, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress, antioxidants, and Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2000, 14 (Suppl. S1), S62–S66. [Google Scholar] [CrossRef]
- Usher, K.C.; Blaszczak, L.C.; Weston, G.S.; Shoichet, B.K.; Remington, S.J. Three-dimensional structure of AmpC beta-lactamase from Escherichia coli bound to a transition-state analogue: Possible implications for the oxyanion hypothesis and for inhibitor design. Biochemistry 1998, 37, 16082–16092. [Google Scholar] [CrossRef]
- Katz, B.A.; Finer-Moore, J.; Mortezaei, R.; Rich, D.H.; Stroud, R.M. Episelection: Novel Ki approximately nanomolar inhibitors of serine proteases selected by binding or chemistry on an enzyme surface. Biochemistry 1995, 34, 8264–8280. [Google Scholar] [CrossRef]
- Diaz, D.B.; Scully, C.C.; Liew, S.K.; Adachi, S.; Trinchera, P.; St Denis, J.D.; Yudin, A.K. Synthesis of Aminoboronic Acid Derivatives from Amines and Amphoteric Boryl Carbonyl Compounds. Angew. Chem. Int. Ed. Engl. 2016, 55, 12659–12663. [Google Scholar] [CrossRef] [PubMed]
- Llona-Minguez, S.; Höglund, A.; Jacques, S.A.; Johansson, L.; Calderón-Montaño, J.M.; Claesson, M.; Loseva, O.; Valerie, N.C.K.; Lundbäck, T.; Piedrafita, J.; et al. Discovery of the First Potent and Selective Inhibitors of Human dCTP Pyrophosphatase 1. J. Med. Chem. 2016, 59, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Zajdlik, A.; Wang, Z.; Hickey, J.L.; Aman, A.; Schimmer, A.D.; Yudin, A.K. α-Boryl isocyanides enable facile preparation of bioactive boropeptides. Angew. Chem. Int. Ed. Engl. 2013, 52, 8411–8415. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a Cyclic Boronic Acid β-Lactamase Inhibitor (RPX7009) with Utility vs Class A Serine Carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.K.; Liu, Y.; Ding, C.Z.; Zhou, Y.; Li, Q.; Plattner, J.J.; Baker, S.J.; Zhang, S.; Kazmierski, W.M.; et al. Novel macrocyclic HCV NS3 protease inhibitors derived from α-amino cyclic boronates. Bioorg Med. Chem. Lett. 2010, 20, 5695–5700. [Google Scholar] [CrossRef]
- Stoll, V.S.; Eger, B.T.; Hynes, R.C.; Martichonok, V.; Jones, J.B.; Pai, E.F. Differences in binding modes of enantiomers of 1-acetamido boronic acid based protease inhibitors: Crystal structures of gamma-chymotrypsin and subtilisin Carlsberg complexes. Biochemistry 1998, 37, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Zervosen, A.; Herman, R.; Kerff, F.; Herman, A.; Bouillez, A.; Prati, F.; Pratt, R.F.; Frère, J.M.; Joris, B.; Luxen, A.; et al. Unexpected tricovalent binding mode of boronic acids within the active site of a penicillin-binding protein. J. Am. Chem. Soc. 2011, 133, 10839–10848. [Google Scholar] [CrossRef] [PubMed]
- Martichonok, V.; Jones, J.B. Cysteine proteases such as papain are not inhibited by substrate analogue peptidyl boronic acids. Bioorg Med. Chem. 1997, 5, 679–684. [Google Scholar] [CrossRef]
- Bone, R.; Frank, D.; Kettner, C.A.; Agard, D.A. Structural analysis of specificity: Alpha-lytic protease complexes with analogues of reaction intermediates. Biochemistry 1989, 28, 7600–7609. [Google Scholar] [CrossRef]
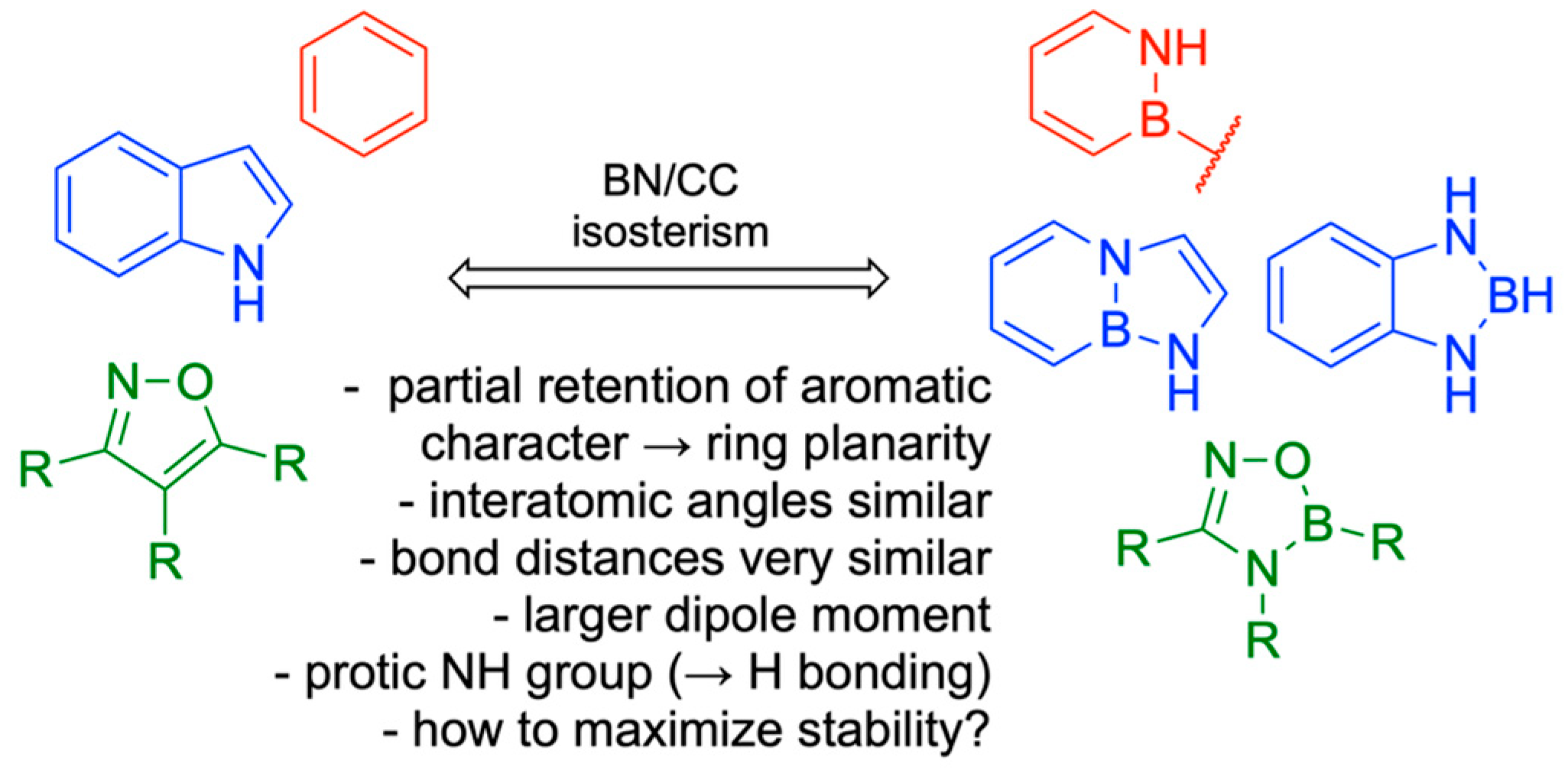
- Zhao, P.; Nettleton, D.O.; Karki, R.G.; Zécri, F.J.; Liu, S.Y. Medicinal Chemistry Profiling of Monocyclic 1,2-Azaborines. ChemMedChem 2017, 12, 358–361. [Google Scholar] [CrossRef]
- Boghi, M.; Hall, D.G. Valdecoxib vs. borazavaldecoxib: Isoxazole BN/CC isosterism as a case study in designing and stabilizing boron heterocycles. Org. Biomol. Chem. 2018, 16, 4849–4856. [Google Scholar] [CrossRef] [PubMed]
- Akama, T.; Dong, C.; Virtucio, C.; Sullivan, D.; Zhou, Y.; Zhang, Y.K.; Rock, F.; Freund, Y.; Liu, L.; Bu, W.; et al. Linking phenotype to kinase: Identification of a novel benzoxaborole hinge-binding motif for kinase inhibition and development of high-potency rho kinase inhibitors. J. Pharmacol. Exp. Ther. 2013, 347, 615–625. [Google Scholar] [CrossRef]
- Kazmi, M.Z.H.; Schneider, O.M.; Hall, D.G. Expanding the Role of Boron in New Drug Chemotypes: Properties, Chemistry, Pharmaceutical Potential of Hemiboronic Naphthoids. J. Med. Chem. 2023, 66, 13768–13787. [Google Scholar] [CrossRef]
- Tattersall, A.; Ryan, N.; Wiggans, A.J.; Rogozińska, E.; Morrison, J. Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst. Rev. 2022, 2, Cd007929. [Google Scholar] [CrossRef]
- Kazmi, M.Z.H.; Rygus, J.P.G.; Ang, H.T.; Paladino, M.; Johnson, M.A.; Ferguson, M.J.; Hall, D.G. Lewis or Brønsted? A Rectification of the Acidic and Aromatic Nature of Boranol-Containing Naphthoid Heterocycles. J. Am. Chem. Soc. 2021, 143, 10143–10156. [Google Scholar] [CrossRef]
- Freund, Y.R.; Akama, T.; Alley, M.R.; Antunes, J.; Dong, C.; Jarnagin, K.; Kimura, R.; Nieman, J.A.; Maples, K.R.; Plattner, J.J.; et al. Boron-based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012, 586, 3410–3414. [Google Scholar] [CrossRef]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crépin, T.; Zhou, H.; Zhang, Y.K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An antifungal agent inhibits an aminoacyl-tRNA synthetase by trapping tRNA in the editing site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Papp-Wallace, K.M.; Bethel, C.R.; Distler, A.M.; Kasuboski, C.; Taracila, M.; Bonomo, R.A. Inhibitor resistance in the KPC-2 beta-lactamase, a preeminent property of this class A beta-lactamase. Antimicrob. Agents Chemother. 2010, 54, 890–897. [Google Scholar] [CrossRef]
- Singh, M.; Boomgaren, M.; Bakht, P.; Ihle, P.; Leiros, H.S.; Bayer, A.; Pathania, R. Correction to “Design and SAR Analysis of Phenylboronic Acid-Based Inhibitors for Sensitizing KPC-2-Producing Klebsiella pneumoniae to β-Lactam Antibiotics”. J. Med. Chem. 2025, 68, 19791. [Google Scholar] [CrossRef] [PubMed]
- Celenza, G.; Vicario, M.; Bellio, P.; Linciano, P.; Perilli, M.; Oliver, A.; Blazquez, J.; Cendron, L.; Tondi, D. Phenylboronic Acid Derivatives as Validated Leads Active in Clinical Strains Overexpressing KPC-2: A Step against Bacterial Resistance. ChemMedChem 2018, 13, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Skagseth, S.; Akhter, S.; Paulsen, M.H.; Muhammad, Z.; Lauksund, S.; Samuelsen, Ø.; Leiros, H.S.; Bayer, A. Metallo-β-lactamase inhibitors by bioisosteric replacement: Preparation, activity and binding. Eur. J. Med. Chem. 2017, 135, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Mitcheltree, M.J.; Li, D.; Achab, A.; Beard, A.; Chakravarthy, K.; Cheng, M.; Cho, H.; Eangoor, P.; Fan, P.; Gathiaka, S.; et al. Discovery and Optimization of Rationally Designed Bicyclic Inhibitors of Human Arginase to Enhance Cancer Immunotherapy. ACS Med. Chem. Lett. 2020, 11, 582–588. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef]
- Bonney, K.M.; Engman, D.M. Chagas heart disease pathogenesis: One mechanism or many? Curr. Mol. Med. 2008, 8, 510–518. [Google Scholar] [CrossRef]
- Padilla, A.M.; Wang, W.; Akama, T.; Carter, D.S.; Easom, E.; Freund, Y.; Halladay, J.S.; Liu, Y.; Hamer, S.A.; Hodo, C.L.; et al. Discovery of an orally active benzoxaborole prodrug effective in the treatment of Chagas disease in non-human primates. Nat. Microbiol. 2022, 7, 1536–1546. [Google Scholar] [CrossRef]
- Ding, D.; Zhao, Y.; Meng, Q.; Xie, D.; Nare, B.; Chen, D.; Bacchi, C.J.; Yarlett, N.; Zhang, Y.K.; Hernandez, V.; et al. Discovery of novel benzoxaborole-based potent antitrypanosomal agents. ACS Med. Chem. Lett. 2010, 1, 165–169. [Google Scholar] [CrossRef]
- Maier, A.G.; Matuschewski, K.; Zhang, M.; Rug, M. Plasmodium falciparum. Trends Parasitol. 2019, 35, 481–482. [Google Scholar] [CrossRef] [PubMed]
- Molina, I.; Gómez i Prat, J.; Salvador, F.; Treviño, B.; Sulleiro, E.; Serre, N.; Pou, D.; Roure, S.; Cabezos, J.; Valerio, L.; et al. Randomized trial of posaconazole and benznidazole for chronic Chagas’ disease. N. Engl. J. Med. 2014, 370, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Torrico, F.; Gascon, J.; Ortiz, L.; Alonso-Vega, C.; Pinazo, M.J.; Schijman, A.; Almeida, I.C.; Alves, F.; Strub-Wourgaft, N.; Ribeiro, I. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018, 18, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.J.; Rico, E.; Lukac, I.; Zuccotto, F.; Elg, S.; Gilbert, I.H.; Freund, Y.; Alley, M.R.K.; Field, M.C.; Wyllie, S.; et al. Clinical and veterinary trypanocidal benzoxaboroles target CPSF3. Proc. Natl. Acad. Sci. USA 2018, 115, 9616–9621. [Google Scholar] [CrossRef]
- Giordani, F.; Paape, D.; Vincent, I.M.; Pountain, A.W.; Fernández-Cortés, F.; Rico, E.; Zhang, N.; Morrison, L.J.; Freund, Y.; Witty, M.J.; et al. Veterinary trypanocidal benzoxaboroles are peptidase-activated prodrugs. PLoS Pathog. 2020, 16, e1008932. [Google Scholar] [CrossRef]
- Palencia, A.; Bougdour, A.; Brenier-Pinchart, M.P.; Touquet, B.; Bertini, R.L.; Sensi, C.; Gay, G.; Vollaire, J.; Josserand, V.; Easom, E.; et al. Targeting Toxoplasma gondii CPSF3 as a new approach to control toxoplasmosis. EMBO Mol. Med. 2017, 9, 385–394. [Google Scholar] [CrossRef]
- Nedunchezhian, K.; Aswath, N.; Thiruppathy, M.; Thirugnanamurthy, S. Boron Neutron Capture Therapy—A Literature Review. J. Clin. Diagn. Res. 2016, 10, Ze01–Ze04. [Google Scholar] [CrossRef]
- Jin, W.H.; Seldon, C.; Butkus, M.; Sauerwein, W.; Giap, H.B. A Review of Boron Neutron Capture Therapy: Its History and Current Challenges. Int. J. Part. Ther. 2022, 9, 71–82. [Google Scholar] [CrossRef]
- Li, J.; Sun, Q.; Lu, C.; Xiao, H.; Guo, Z.; Duan, D.; Zhang, Z.; Liu, T.; Liu, Z. Boron encapsulated in a liposome can be used for combinational neutron capture therapy. Nat. Commun. 2022, 13, 2143. [Google Scholar] [CrossRef]
- Lee, K.; González-Montiel, G.A.; Eom, H.; Kim, T.H.; Noh, H.C.; Farah, A.O.; Wise, H.R.; Kim, D.; Cheong, P.H.; Lee, P.H. Site- and enantioselective B-H functionalization of carboranes. Nat. Commun. 2025, 16, 4182. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Wang, C.; Jankovic, K.E.; Dong, Y. Lipids and Lipid Derivatives for RNA Delivery. Chem. Rev. 2021, 121, 12181–12277. [Google Scholar] [CrossRef]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef]
- Mechetin, G.V.; Zharkov, D.O. DNA Damage Response and Repair in Boron Neutron Capture Therapy. Genes 2023, 14, 127. [Google Scholar] [CrossRef]
- Curtin, N.J.; Szabo, C. Poly(ADP-ribose) polymerase inhibition: Past, present and future. Nat. Rev. Drug Discov. 2020, 19, 711–736. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, H.; Li, G.; Li, Y.; Jiang, J.; Chen, J.; Xie, Q.; Wu, D.; Ma, R.; Liu, X.; et al. Antibiotic-Like Activity of Atomic Layer Boron Nitride for Combating Resistant Bacteria. ACS Nano 2022, 16, 7674–7688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, R.P.; Brown, J.M. Engineered nanomaterials and oxidative stress: Current understanding and future challenges. Curr. Opin. Toxicol. 2019, 13, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Goley, E.D. Redefining the roles of the FtsZ-ring in bacterial cytokinesis. Curr. Opin. Microbiol. 2016, 34, 90–96. [Google Scholar] [CrossRef]
- Monahan, L.G.; Hajduk, I.V.; Blaber, S.P.; Charles, I.G.; Harry, E.J. Coordinating bacterial cell division with nutrient availability: A role for glycolysis. mBio 2014, 5, e00935-14. [Google Scholar] [CrossRef]
- Männik, J.; Walker, B.E.; Männik, J. Cell cycle-dependent regulation of FtsZ in Escherichia coli in slow growth conditions. Mol. Microbiol. 2018, 110, 1030–1044. [Google Scholar] [CrossRef]
- Uluisik, I.; Karakaya, H.C.; Koc, A. The importance of boron in biological systems. J. Trace Elem. Med. Biol. 2018, 45, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Mollner, T.A.; Isenegger, P.G.; Josephson, B.; Buchanan, C.; Lercher, L.; Oehlrich, D.; Hansen, D.F.; Mohammed, S.; Baldwin, A.J.; Gouverneur, V.; et al. Post-translational insertion of boron in proteins to probe and modulate function. Nat. Chem. Biol. 2021, 17, 1245–1261. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Bains, W.; Seager, S. On the Potential of Silicon as a Building Block for Life. Life 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Ding, Z.; Li, X.; Dong, Y.; Fu, T.; Wu, Y. Colorimetric Sensor Array Based on Wulff-Type Boronate Functionalized AgNPs at Various pH for Bacteria Identification. Anal. Chem. 2019, 91, 12134–12137. [Google Scholar] [CrossRef]
- Brustad, E.; Bushey, M.L.; Lee, J.W.; Groff, D.; Liu, W.; Schultz, P.G. A genetically encoded boronate-containing amino acid. Angew. Chem. Int. Ed. Engl. 2008, 47, 8220–8223. [Google Scholar] [CrossRef] [PubMed]
- Akgun, B.; Hall, D.G. Fast and Tight Boronate Formation for Click Bioorthogonal Conjugation. Angew. Chem. Int. Ed. Engl. 2016, 55, 3909–3913. [Google Scholar] [CrossRef]
- Ramsay, W.J.; Bayley, H. Single-Molecule Determination of the Isomers of d-Glucose and d-Fructose that Bind to Boronic Acids. Angew. Chem. Int. Ed. Engl. 2018, 57, 2841–2845. [Google Scholar] [CrossRef]
- Dadová, J.; Galan, S.R.; Davis, B.G. Synthesis of modified proteins via functionalization of dehydroalanine. Curr. Opin. Chem. Biol. 2018, 46, 71–81. [Google Scholar] [CrossRef]
- Müller, S.; Hoege, C.; Pyrowolakis, G.; Jentsch, S. SUMO, ubiquitin’s mysterious cousin. Nat. Rev. Mol. Cell Biol. 2001, 2, 202–210. [Google Scholar] [CrossRef]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef]
- Han, H.-H.; Ge, P.-X.; Li, W.-J.; Hu, X.-L.; He, X.-P. Recent Advancement in Fluorescent Probes for Peroxynitrite (ONOO−). Sensors 2025, 25, 3018. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Tian, X.; Groleau, R.R.; Feng, B.; Yang, Y.; Sedgwick, A.C.; Han, H.-H.; Wang, Y.; Wang, H.-M. Dual-channel fluorescent probe for the simultaneous monitoring of peroxynitrite and adenosine-5′-triphosphate in cellular applications. J. Am. Chem. Soc. 2021, 144, 174–183. [Google Scholar] [CrossRef]
- Torreilles, F.; Salman-Tabcheh, S.; Guérin, M.C.; Torreilles, J. Neurodegenerative disorders: The role of peroxynitrite. Brain Res. Rev. 1999, 30, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, N.; Tranmer, G.K. Hydrogen peroxide and amyotrophic lateral sclerosis: From biochemistry to pathophysiology. Antioxidants 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil. Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Estevez-Fregoso, E.; Kilic, A.; Rodríguez-Vera, D.; Nicanor-Juárez, L.E.; Romero-Rizo, C.E.M.; Farfán-García, E.D.; Soriano-Ursúa, M.A. Effects of boron-containing compounds on liposoluble hormone functions. Inorganics 2023, 11, 84. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Peroni, G.; Infantino, V.; Gasparri, C.; Iannello, G.; Perna, S.; Riva, A.; Petrangolini, G.; Tartara, A. Pivotal role of boron supplementation on bone health: A narrative review. J. Trace Elem. Med. Biol. 2020, 62, 126577. [Google Scholar] [CrossRef]
- Jin, E.; Li, S.; Ren, M.; Hu, Q.; Gu, Y.; Li, K. Boron affects immune function through modulation of splenic T lymphocyte subsets, cytokine secretion, and lymphocyte proliferation and apoptosis in rats. Biol. Trace Elem. Res. 2017, 178, 261–275. [Google Scholar] [CrossRef]
- World Health Organization. Trace Elements in Human Nutrition and Health; World Health Organization: Geneva, Switzerland, 1996; pp. 105–122. [Google Scholar]
- Acid, B.; Salts, S.B. HED Chapter of the Tolerance Reassessment Eligibility Decision Document (TRED); US Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances, Health Effects Division: Washington, DC, USA, 2006. [Google Scholar]
- Lim, E.; Pon, A.; Djoumbou, Y.; Knox, C.; Shrivastava, S.; Guo, A.C.; Neveu, V.; Wishart, D.S. T3DB: A comprehensively annotated database of common toxins and their targets. Nucleic Acids Res. 2010, 38, D781–D786. [Google Scholar] [CrossRef]
- Hadrup, N.; Frederiksen, M.; Sharma, A.K. Toxicity of boric acid, borax and other boron containing compounds: A review. Regul. Toxicol. Pharmacol. 2021, 121, 104873. [Google Scholar] [CrossRef] [PubMed]

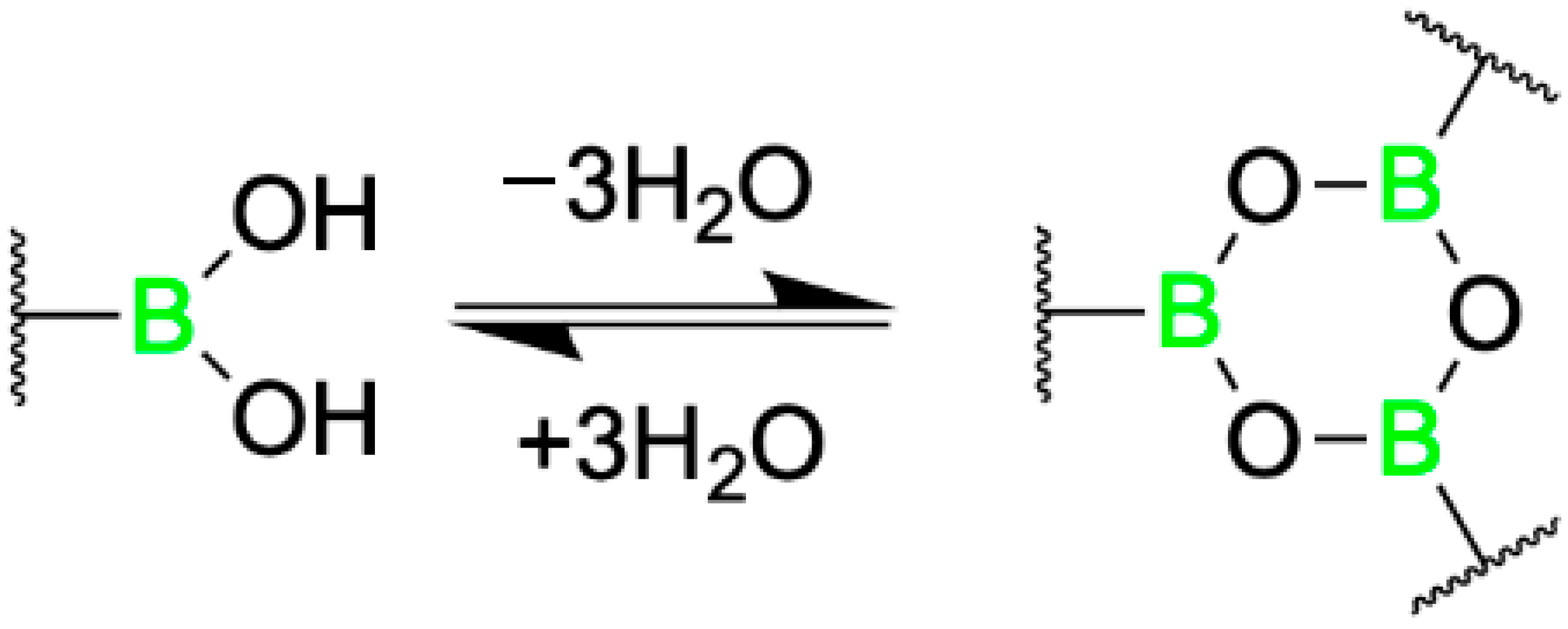
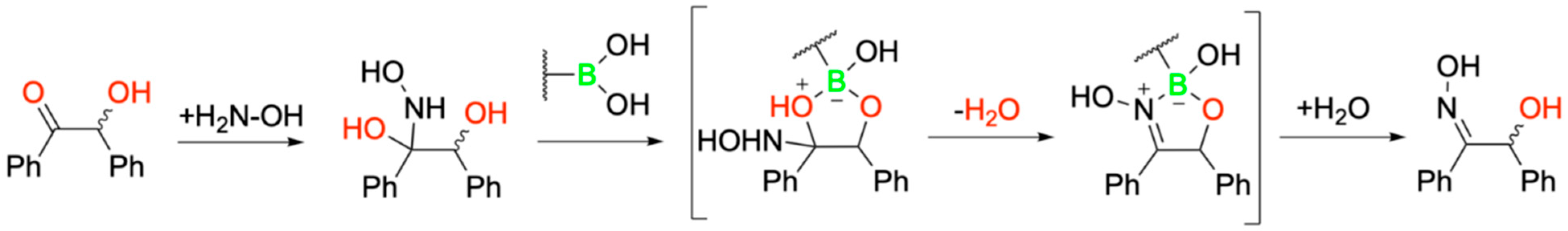

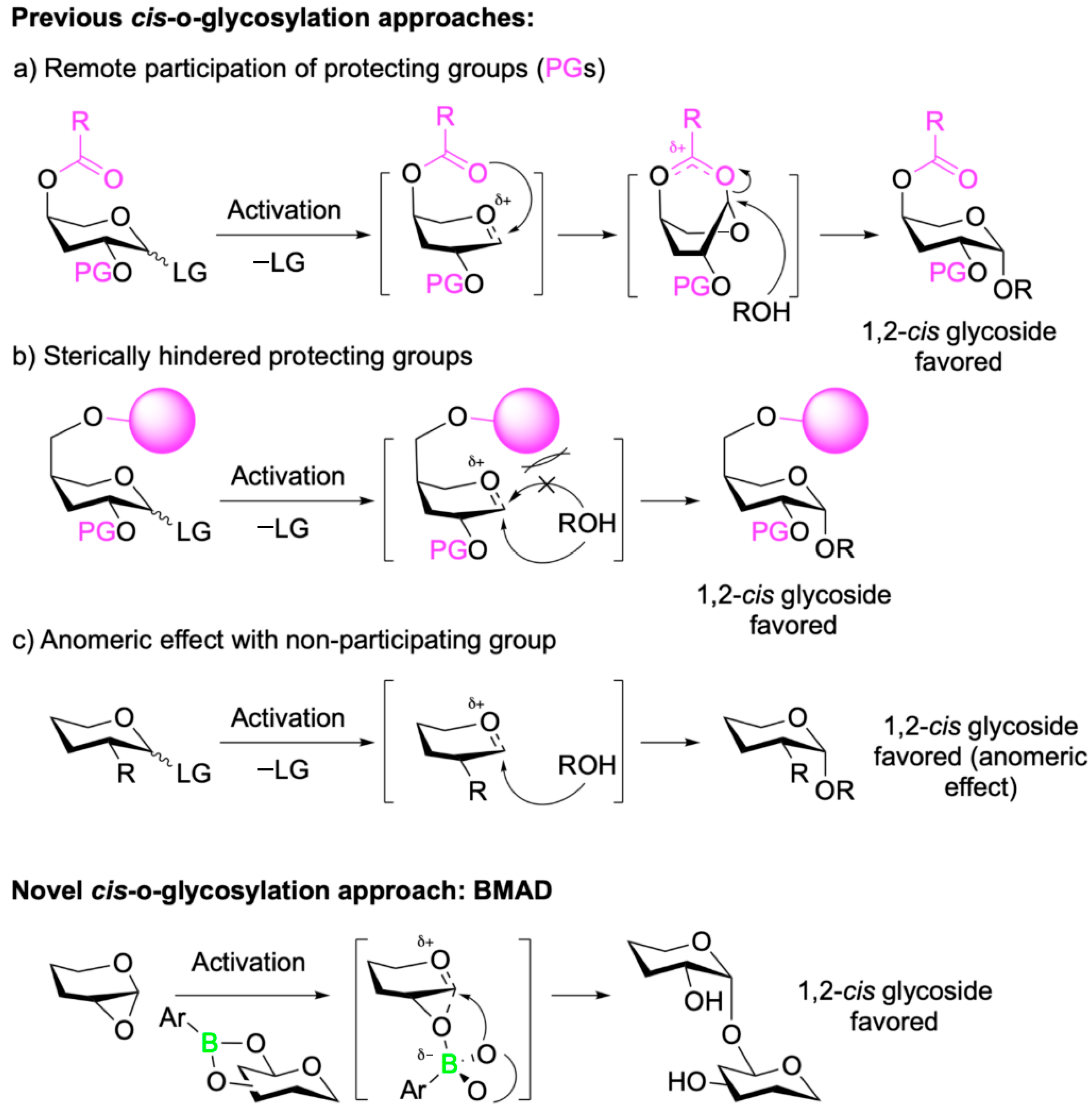
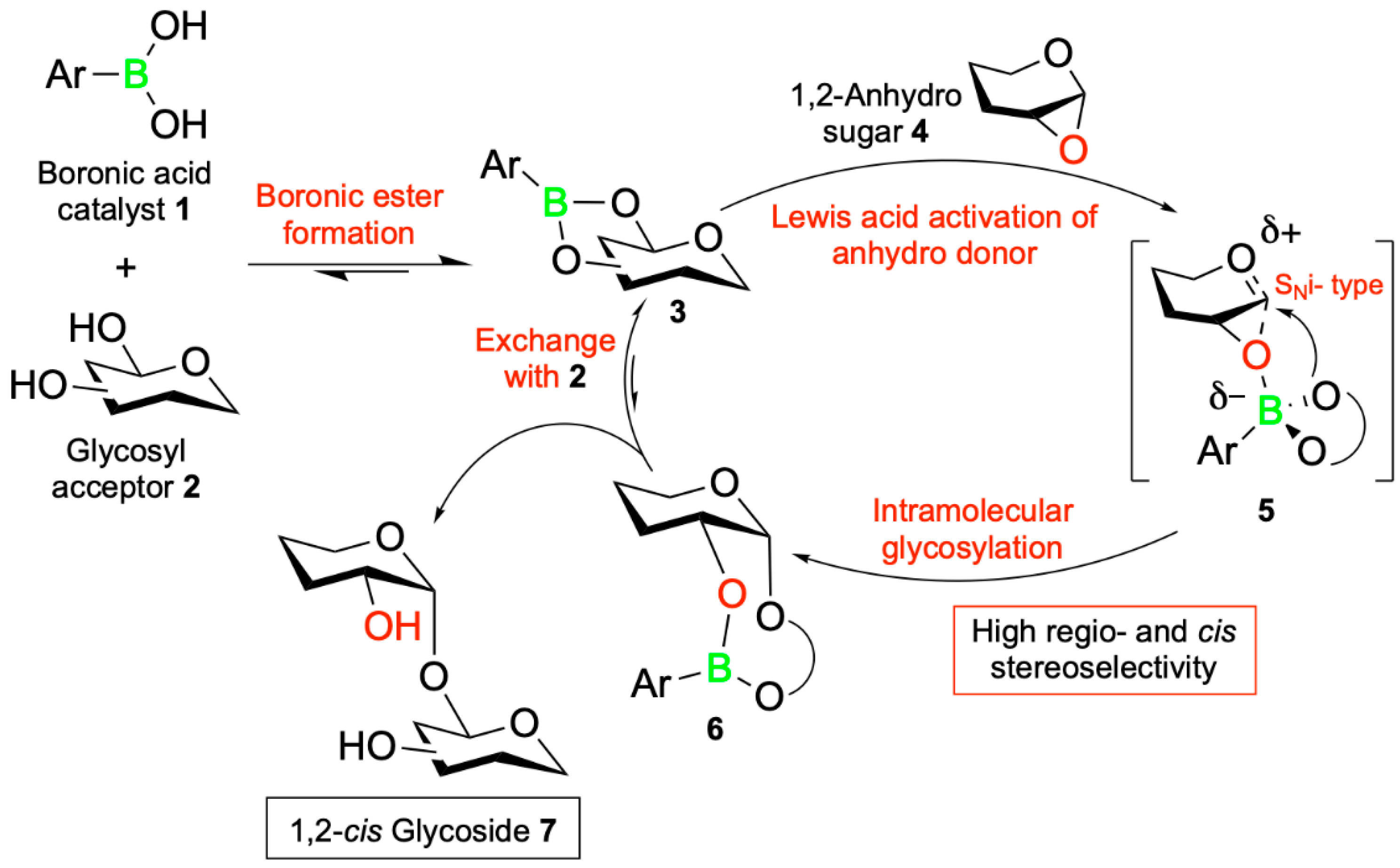











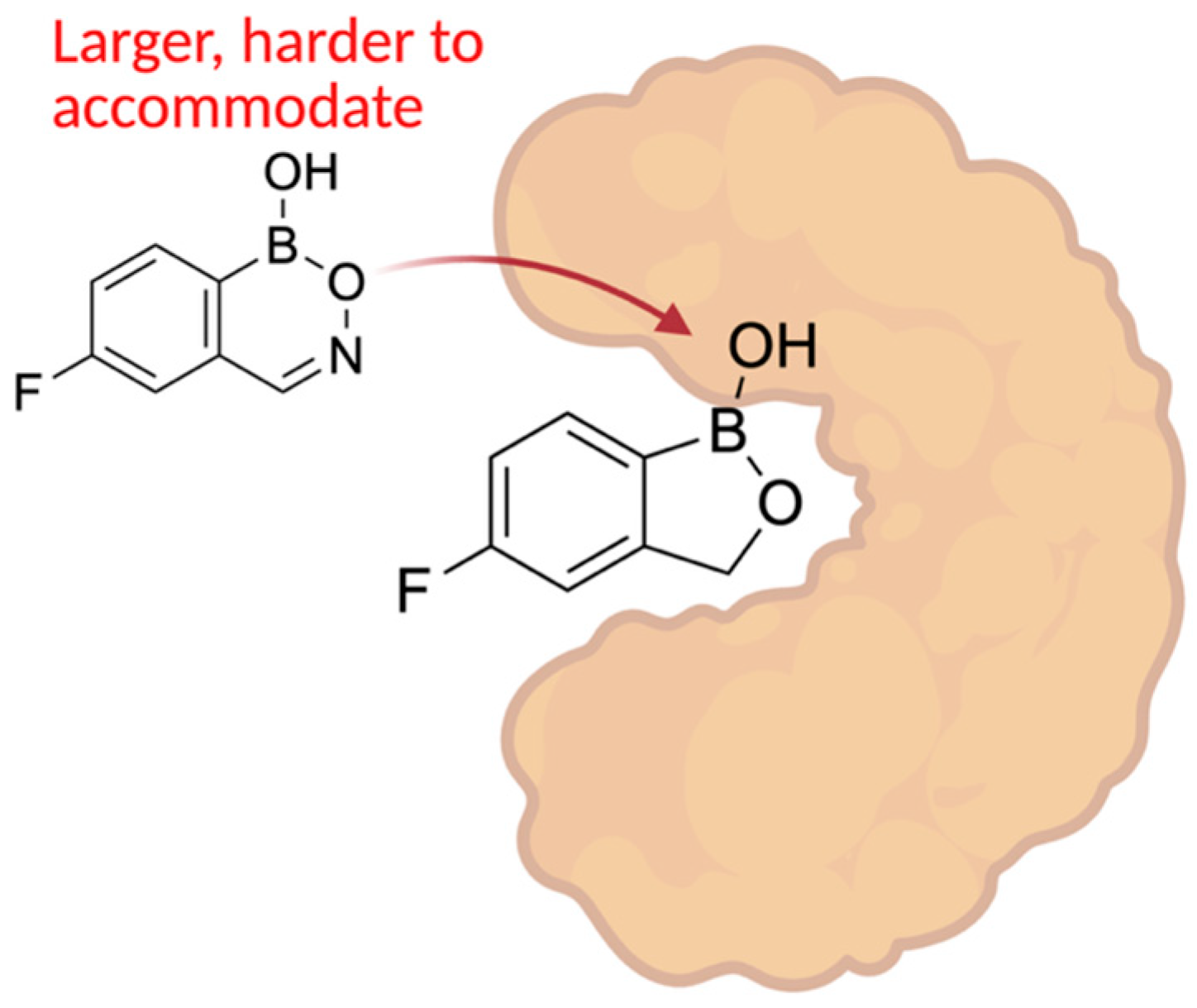

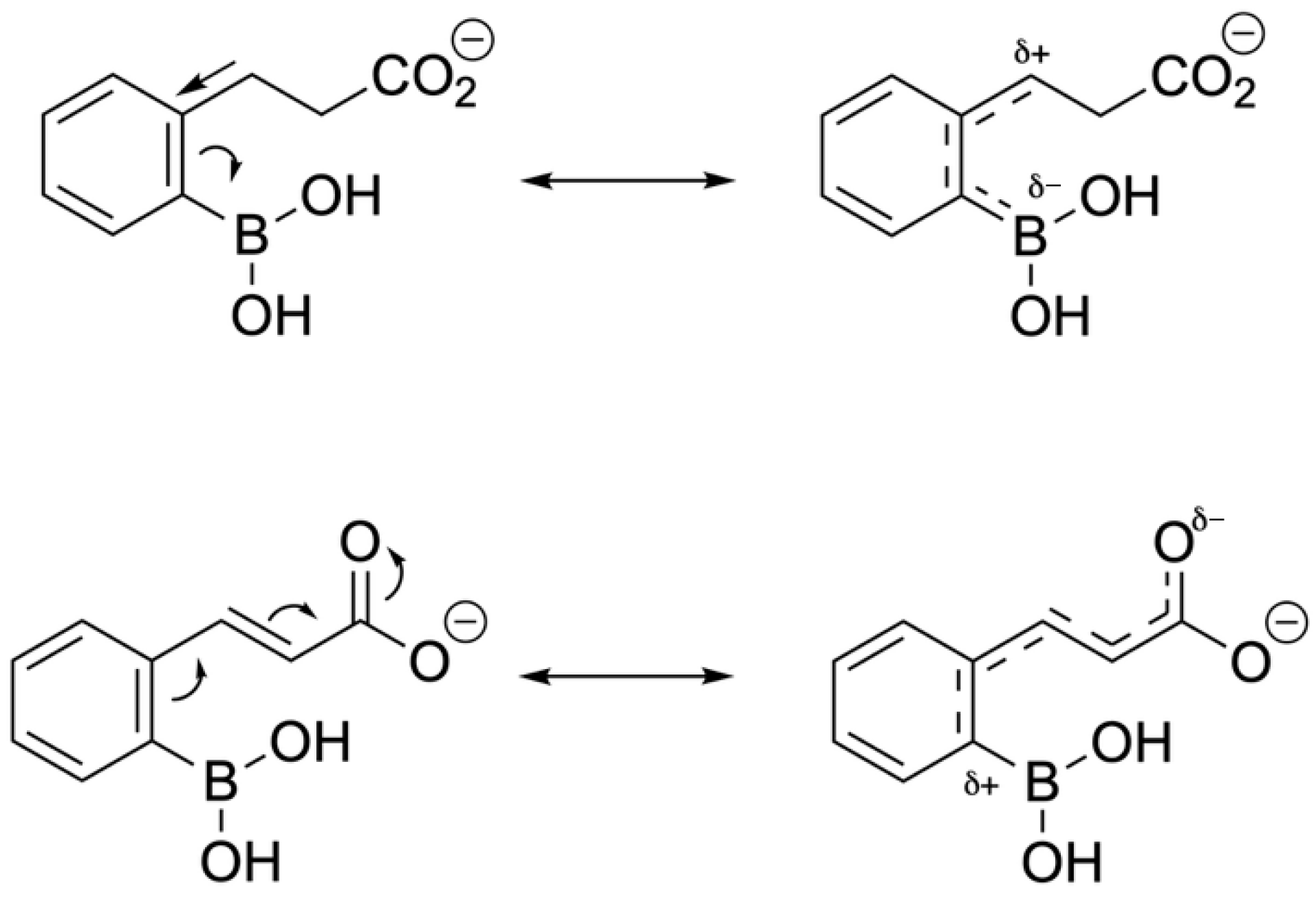
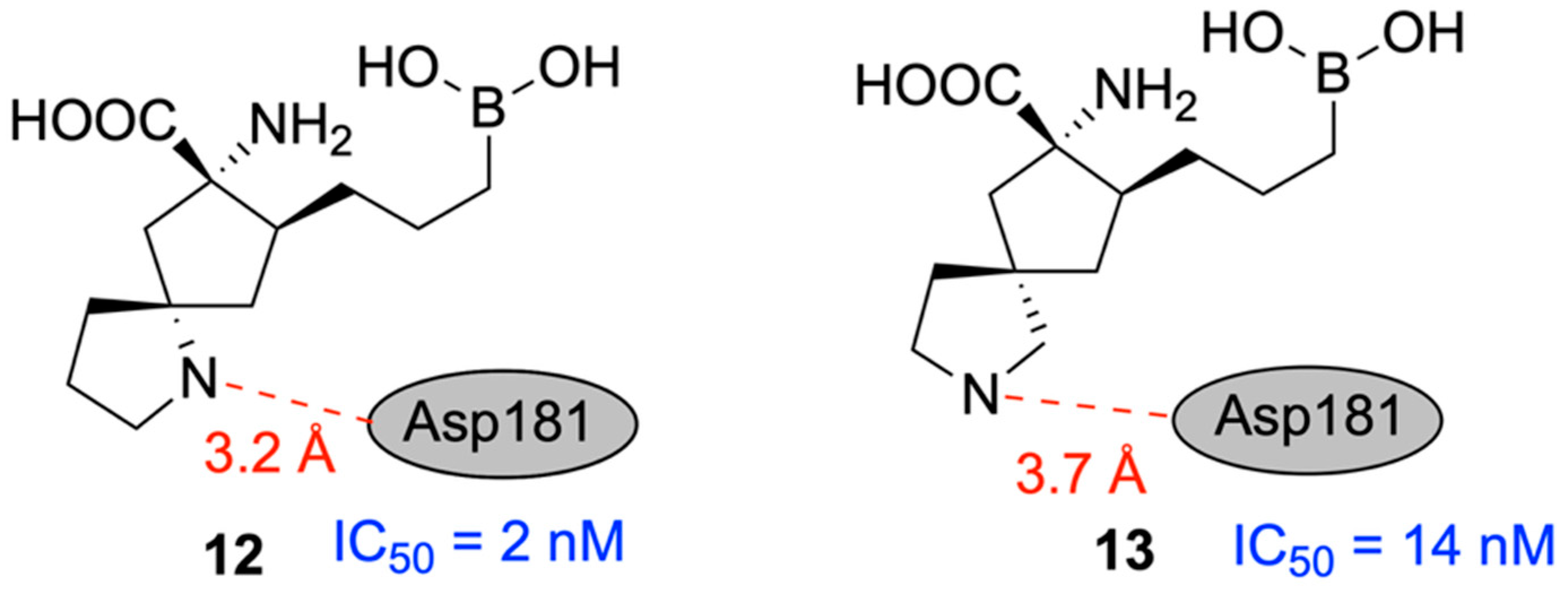
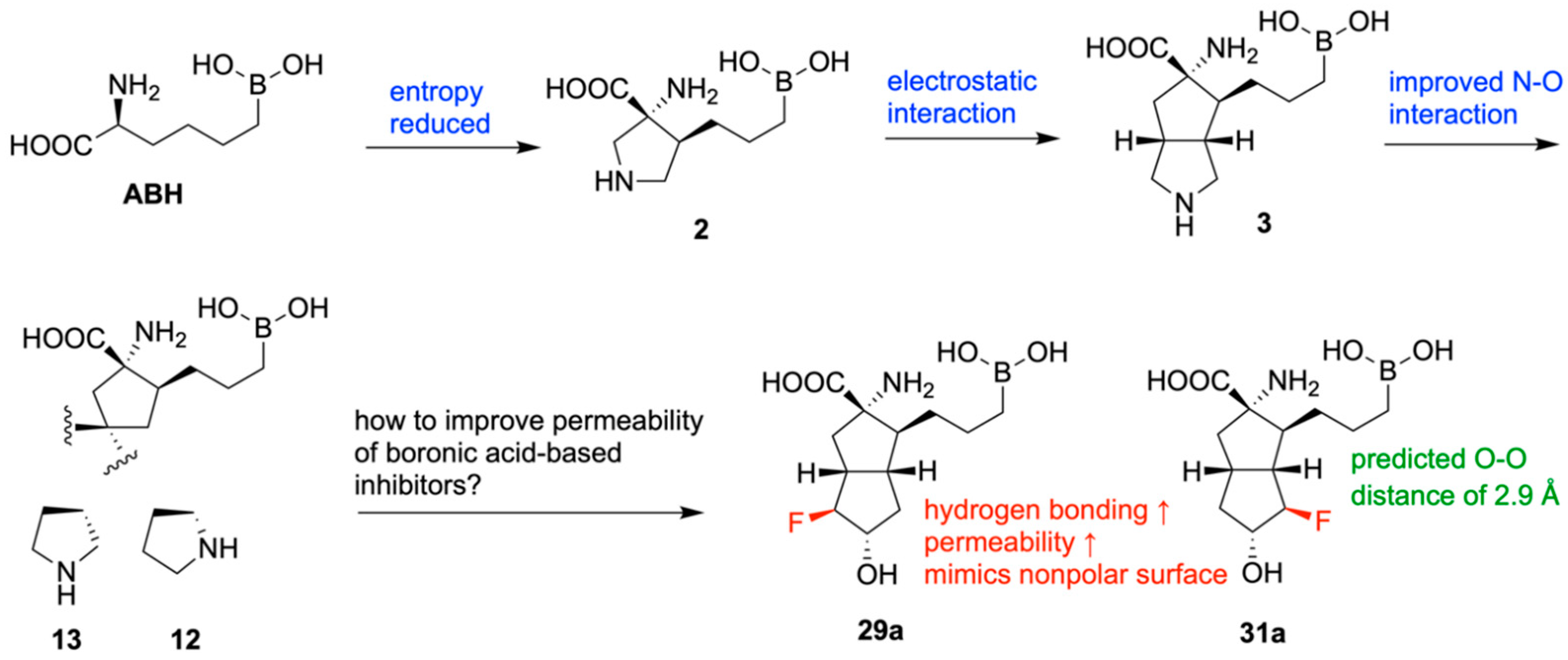

| Promising ENMs | MIC (µg/mL) | Less Effective ENMs | MIC (µg/mL) |
|---|---|---|---|
| BN | 125 | SnO2 | >500 |
| Ag | 125 | SiO2 | >500 |
| Ni2O3 | 250 | Mn2O3 | >500 |
| GO | 500 | In2O3 | >500 |
| CuO | 500 | In2O3 | >500 |
| WS2 | >500 | NiO | >500 |
| MoS2 | >500 | CNF | >500 |
| ZnO | >500 | CNT | >500 |
| Candidate | Type | Yield |
|---|---|---|
| 2,2′-bipyridine | Bidentate Ligand | 6–11% |
| 1,8-bis(dimethylamino)naphthalene | Bidentate Ligand | 6–11% |
| Pyridine | Monodentate Pyridine Additive | 96–99% |
| 4-picoline | Monodentate Pyridine Additive | 96–99% |
| Imidazole | Monodentate imidazole | 66–88% |
| 1-histidine | Monodentate imidazole | 66–88% |
| Guanidine hydrochloride | Protein Denaturant | 18% |
| Urea | Protein Denaturant | 0% |
| Protein | Geometry | Bal Conversion Yield |
|---|---|---|
| Histone H3 | Small, α-helical | 9: 73% 10: >90% |
| Histone H4 | Small, α-helical | 16: >95% |
| pre-SUMO1 | Small, globular with α-helices and β-sheets | 51: >95% |
| Npβ | β-helical repeats | 61: >95% |
| mCherry | β barrel | 131: 35% |
| Pantothenate Synthetase (PanC) | Dimer | 44: 71% 47: 60% |
| Modified phosphate-binding protein (PstS) | Globular domains with β-sheets and α-helices | 197: 79% |
| Annexin V | Globular α-helical | 316: >95% |
| Amyloid Precursor Protein (AcrA) | Contains a single helix with a short tail | 123: 77% |
| Protein | Scope of Analysis | ROS Concentration | Relative Accessibility |
|---|---|---|---|
| H3-Bal10 | 1.0–2.8 Å | 5 mM | 71–108% |
| H4-Bal16 | 2.8 Å | 20 mM | 60% |
| H3-Bal9 | 2.8 Å | 20 mM | 34% |
| Npβ-Bal61 | 2.8 Å | – | 25 |
| PstS-Bal197 | 2.8 Å | – | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frooman, M.B.; Deb, M.K.; Peters, J.; Leggett, S.; Sanghai, N.; Rizvi, N.Z.; Atukorallaya, D.; Tranmer, G.K. Recent Advancements in the Diversification and Applications of Boron-Containing Compounds in Medicinal Chemistry. Pharmaceuticals 2025, 18, 1798. https://doi.org/10.3390/ph18121798
Frooman MB, Deb MK, Peters J, Leggett S, Sanghai N, Rizvi NZ, Atukorallaya D, Tranmer GK. Recent Advancements in the Diversification and Applications of Boron-Containing Compounds in Medicinal Chemistry. Pharmaceuticals. 2025; 18(12):1798. https://doi.org/10.3390/ph18121798
Chicago/Turabian StyleFrooman, Marielle B., Moinak K. Deb, Jaxon Peters, Sasha Leggett, Nitesh Sanghai, Nafees Zahra Rizvi, Devi Atukorallaya, and Geoffrey K. Tranmer. 2025. "Recent Advancements in the Diversification and Applications of Boron-Containing Compounds in Medicinal Chemistry" Pharmaceuticals 18, no. 12: 1798. https://doi.org/10.3390/ph18121798
APA StyleFrooman, M. B., Deb, M. K., Peters, J., Leggett, S., Sanghai, N., Rizvi, N. Z., Atukorallaya, D., & Tranmer, G. K. (2025). Recent Advancements in the Diversification and Applications of Boron-Containing Compounds in Medicinal Chemistry. Pharmaceuticals, 18(12), 1798. https://doi.org/10.3390/ph18121798








