Structure-Activity Relationships of closo- and nido-Carborane Erlotinib Analogs: Lipophilicity as a Key Modulator of Anti-Glioma Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Lipophilicity Descriptors
2.1.1. Determination of RM0 and flogP
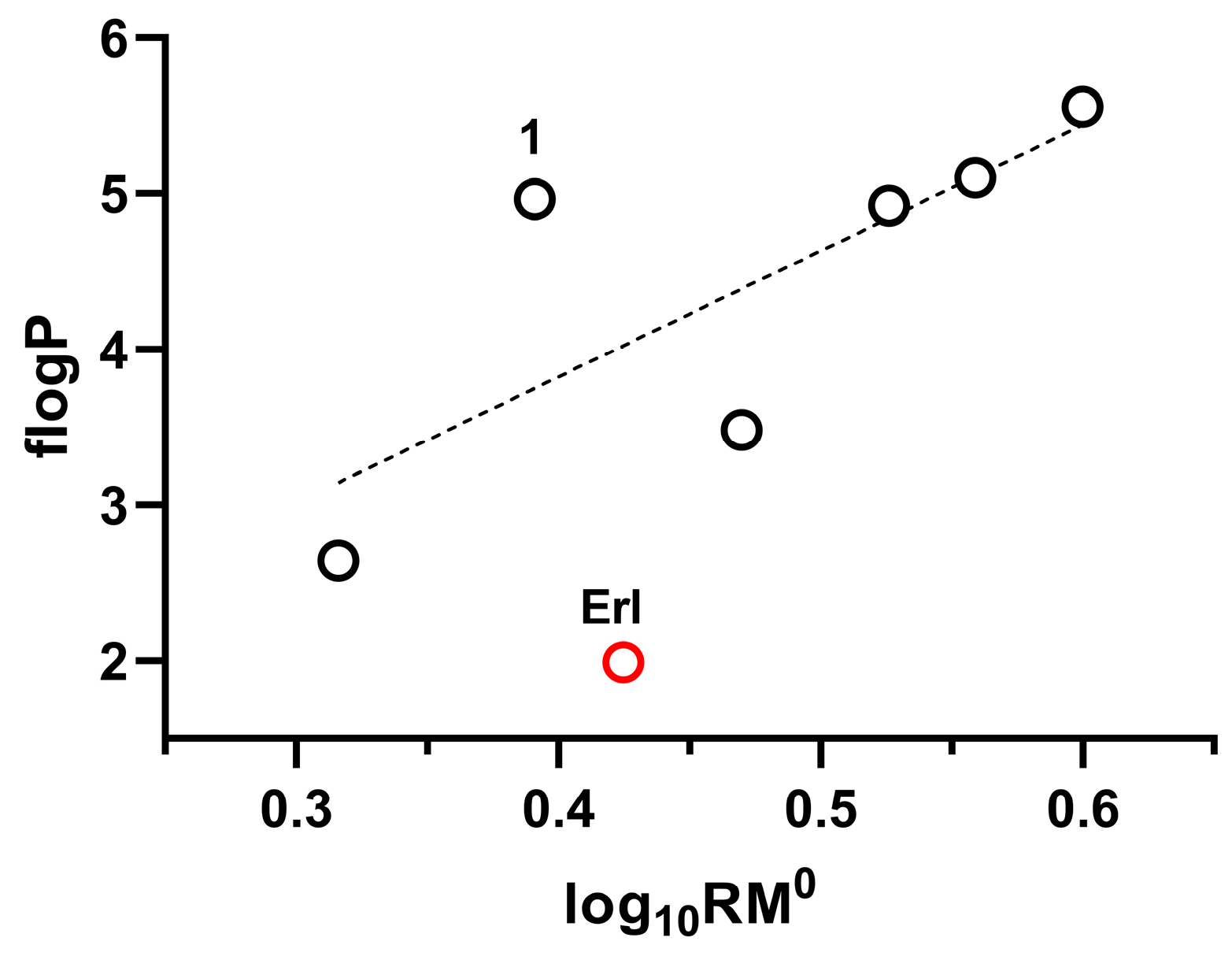
2.1.2. Correlation Between RM0 and flogP
2.1.3. Approaching the Hansch–Fujita Hydrophobic Parameter π for the nido-Carboranyl Substituent
2.2. Structure–Activity Relationships
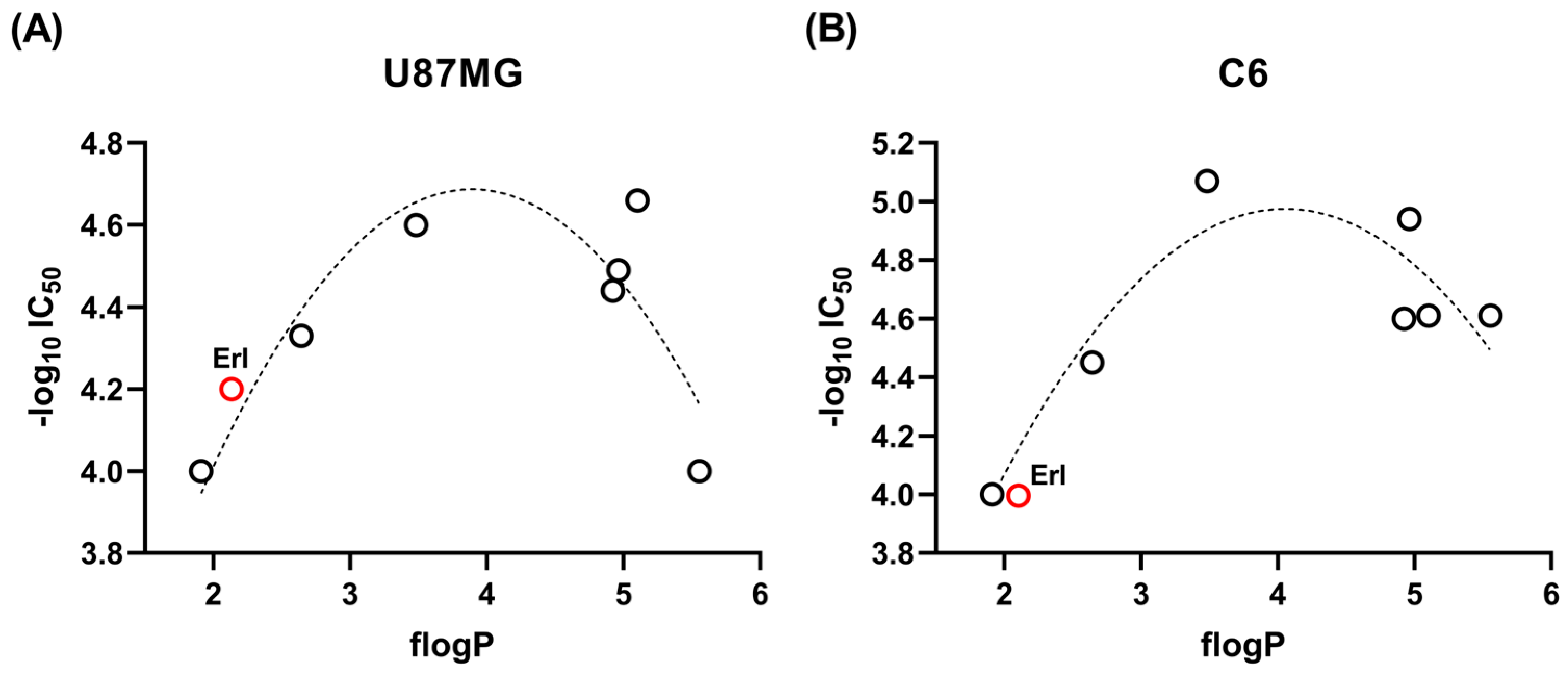
2.2.1. Correlations Between Lipophilicity and Cellular Cytotoxicity
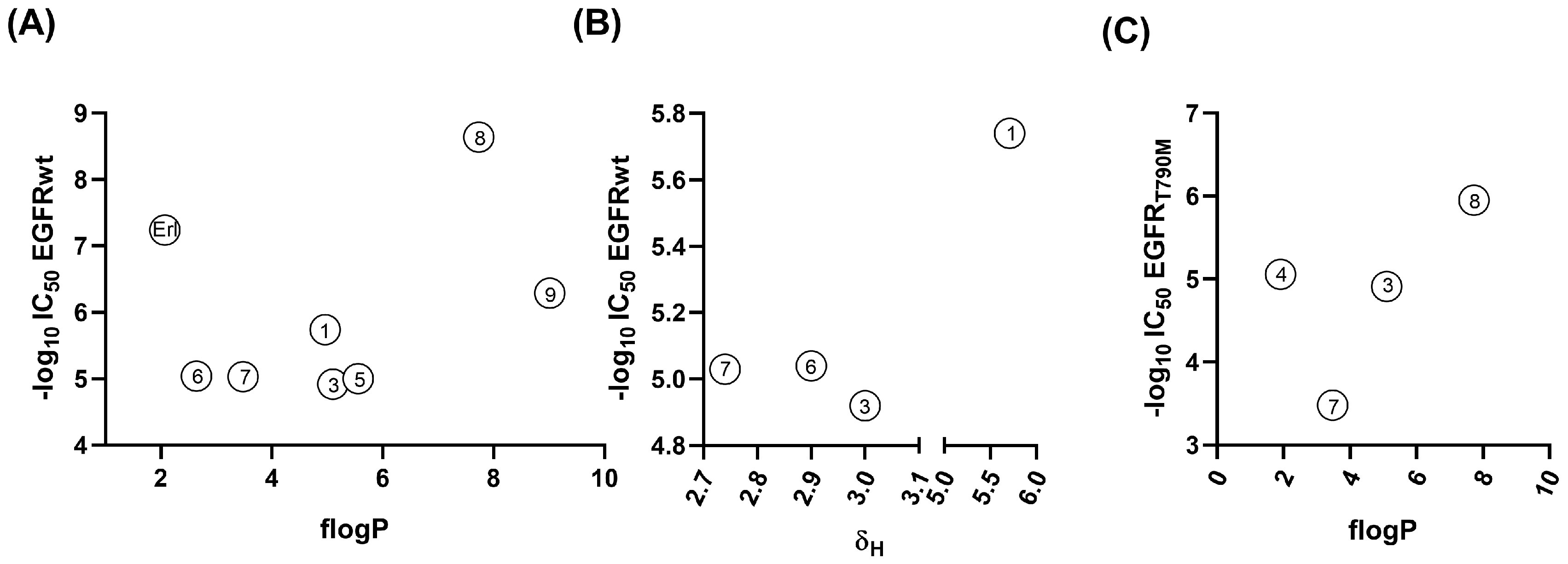
2.2.2. Correlations Between Lipophilicity and EGFR Inhibitions
2.2.3. Towards the Inclusion of the Electronic Effects of Boron Clusters in the Correlations
r = 0.9453, radj = 0.8567, RSS = 0.0298, F = 5.60
r = 0.8101, radj = 0.3751, RSS = 0.1208, F = 1.27
r = 0.9580, radj = 0.8913, RSS = 0.0591, F = 7.44
r = 0.9949, radj = 0.9872, RSS = 0.0006, F = 64.97
3. Materials and Methods
3.1. Chemical Compounds and Cell Lines
3.2. RM0 Determination [22]
3.3. 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline logD7.4 Determination [36]
3.4. Quantitative Structure–Activity Relationship Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TKR | Tyrosine Kinase Receptor |
| EGFR | Epidermal Growth Factor Receptor |
| PDGF | Platelet-Derived Growth Factors |
| HER2 | Human Epidermal Growth Factor 2 |
| Erl | Erlotinib |
| Sun | Sunitinib |
| Lap | Lapatinib |
| BNCT | Boron Neutron Capture Therapy |
| NDR | No Dose-Response |
| RPTLC | Reverse Phase Thin-Layer Chromatography |
| RPHPLC | Reverse Phase High Performance Liquid Chromatography |
References
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Singh, S.; Dey, D.; Barik, D.; Mohapatra, I.; Kim, S.; Sharma, M.; Prasad, S.; Wang, P.; Singh, A.; Singh, G. Glioblastoma at the Crossroads: Current Understanding and Future Therapeutic Horizons. Signal Transduct. Target. Ther. 2025, 10, 213. [Google Scholar] [CrossRef]
- Tang, J.; Karbhari, N.; Campian, J.L. Therapeutic Targets in Glioblastoma: Molecular Pathways, Emerging Strategies, and Future Directions. Cells 2025, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, Y. Receptor Tyrosine Kinases: Biological Functions and Anticancer Targeted Therapy. MedComm 2023, 4, e446. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Schmidt, M.H.H. EGFR and EGFRvIII promote angiogenesis and cell invasion in glioblastoma: Combination therapies for an effective treatment. Int. J. Mol. Sci. 2017, 18, 1295. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Joushi, S.; Bashiri, H.; Saso, L.; Sheibani, V. Characterization of Prevalent Tyrosine Kinase Inhibitors and Their Challenges in Glioblastoma Treatment. Front. Chem. 2023, 11, 1325214. [Google Scholar] [CrossRef]
- Brar, H.K.; Jose, J.; Wu, Z.; Sharma, M. Tyrosine Kinase Inhibitors for Glioblastoma Multiforme: Challenges and Opportunities for Drug Delivery. Pharmaceutics 2022, 15, 59. [Google Scholar] [CrossRef]
- Marfavi, A.; Kavianpour, P.; Rendina, L.M. Carboranes in Drug Discovery, Chemical Biology and Molecular Imaging. Nat. Rev. Chem. 2022, 6, 486–504. [Google Scholar] [CrossRef]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New Keys for Old Locks: Carborane-Containing Drugs as Platforms for Mechanism-Based Therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; Cho, C.H.; et al. Carboranes as Unique Pharmacophores in Antitumor Medicinal Chemistry. Mol. Ther. Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Soriano-Ursúa, M.A.; Das, B.C.; Trujillo-Ferrara, J.G. Boron-Containing Compounds: Chemico-Biological Properties and Expanding Medicinal Potential in Prevention, Diagnosis and Therapy. Expert Opin. Ther. Pat. 2014, 24, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Leśnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef] [PubMed]
- Couto, M.; Mastandrea, I.; Cabrera, M.; Cabral, P.; Teixidor, F.; Cerecetto, H.; Viñas, C. Small-molecule kinase-inhibitors-loaded boron cluster as hybrid agents for glioma-cell-targeting therapy. Chem. Eur. J. 2017, 23, 9233–9238. [Google Scholar] [CrossRef]
- Couto, M.; García, M.F.; Alamón, C.; Cabrera, M.; Cabral, P.; Merlino, A.; Teixidor, F.; Cerecetto, H.; Viñas, C. Discovery of potent EGFR inhibitors through the incorporation of a 3D-aromatic-boron-rich-cluster into the 4-anilinoquinazoline scaffold: Potential drugs for glioma treatment. Chem. Eur. J. 2018, 24, 3122–3126. [Google Scholar] [CrossRef]
- Couto, M.; Alamón, C.; Sánchez, C.; Dávila, B.; Fernández, M.; Lecot, N.; Cabral, P.; Teixidor, F.; Viñas, C.; Cerecetto, H. Carboranylanilinoquinazoline EGFR-inhibitors: Toward “lead-to-candidate” stage in the drug-development pipeline. Fut. Med. Chem. 2019, 11, 2273–2285. [Google Scholar] [CrossRef]
- Alamón, C.; Dávila, B.; García, M.F.; Sánchez, C.; Kovacs, M.; Trias, E.; Barbeito, L.; Gabay, M.; Zeineh, N.; Gavish, M.; et al. Sunitinib-containing carborane pharmacophore with the ability to inhibit tyrosine kinases receptors FLT3, KIT and PDGFR-β, exhibits powerful in vivo anti-glioblastoma activity. Cancers 2020, 12, 3423. [Google Scholar] [CrossRef]
- Alamón, C.; Dávila, B.; Cerecetto, H.; Couto, M. Exploring the cell death mechanisms of cytotoxic [1,2,3]triazolylcarborane lead compounds against U87 MG human glioblastoma cells. Chem. Biol. Drug Des. 2023, 101, 1435–1445. [Google Scholar] [CrossRef]
- Alamón, C.; Dávila, B.; García, M.F.; Nievas, S.; Dagrosa, M.A.; Thorp, S.; Kovacs, M.; Trias, E.; Faccio, R.; Gabay, M.; et al. A potential boron neutron capture therapy agent selectively suppresses high-grade glioma: In vitro and in vivo exploration. Mol. Pharm. 2023, 20, 2702–2713. [Google Scholar] [CrossRef]
- Dávila, B.; Vignolo, P.; González, I.; Lecot, N.; Bonanata, J.; García, M.F.; Echeverría, G.; Piro, O.; Cerecetto, H.; Couto, M. Escaping from Flatland: 3D Carborane-Based Bioisosteres of Erlotinib as a Potential Anticancer Agents. ACS Omega 2025, 10, 50468–50487. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. ρ-σ-π Analysis. A method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hansch, C. Quantitative approach to biochemical structure-activity relationships. Acc. Chem. Res. 1969, 8, 232–239. [Google Scholar] [CrossRef]
- Fernández, M.; Becco, L.; Correia, I.; Benítez, J.; Piro, O.E.; Echeverria, G.A.; Medeiros, A.; Comini, M.; Lavaggi, M.L.; González, M.; et al. Oxidovanadium(IV) and dioxidovanadium(V) complexes of tridentate salicylaldehyde semicarbazones: Searching for prospective antitrypanosomal agents. J. Inorg. Biochem. 2013, 127, 150–160. [Google Scholar] [CrossRef]
- Trifunović, J.; Borčić, V.; Vukmirović, S.; Goločorbin Kon, S.; Mikov, M. Retention data of bile acids and their oxo derivatives in characterization of pharmacokinetic properties and in silico ADME modeling. Eur. J. Pharm. Sci. 2016, 92, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, M.; Starek, M.; Komsta, L.; Szafrański, P.; Stasiewicz-Urban, A.; Opoka, W. Assessment of the chromatographic lipophilicity of eight cephalosporins on different stationary phases. Eur. J. Pharm. Sci. 2017, 101, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Hoekman, D. Exporing QSAR: Hydrophobic, Elecgronic and Steric Constants; American Chemical Society: Washington, DC, USA, 1995. [Google Scholar]
- Yamamoto, K.; Endo, Y. Utility of boron clusters for drug design. Hansch–Fujita hydrophobic parameters π of dicarba-closo-dodecaboranyl groups. Bioorg. Med. Chem. Lett. 2001, 11, 2389–2392. [Google Scholar] [CrossRef]
- Endo, Y.; Yamamoto, K.; Kagechika, H. Utility of boron clusters for drug design. Relation between estrogen receptor binding affinity and hydrophobicity of phenols bearing various types of carboranyl groups. Bioorg. Med. Chem. Lett. 2003, 13, 4089–4092. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in Drug Discovery: Carboranes as Unique Pharmacophores in Biologically Active Compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Couto, M.; Cerecetto, H. Advancements in the synthesis and biological properties of carboranes and high-boron related compounds: A comprehensive exploration with emphasis on BNCT applications. J. Braz. Chem. Soc. 2024, 35, e20240109. [Google Scholar] [CrossRef]
- Godfrey, P.D.; Grigsby, W.J.; Nichols, P.J.; Raston, C.L. Aza-crown ether: 1,2-Dicarbadodecaborane(12) supramolecular assemblies. J. Am. Chem. Soc. 1997, 119, 9283–9284. [Google Scholar] [CrossRef]
- Donati, A.; Ristori, S.; Bonechi, C.; Panza, L.; Martini, G.; Rossi, C. Evidences of strong C-H⋯·O bond in an o-carboranyl β-lactoside in solution. J. Am. Chem. Soc. 2002, 124, 8778–8779. [Google Scholar] [CrossRef]
- Rivas, F.; Medeiros, A.; Quiroga, C.; Benítez, D.; Comini, M.; Rodríguez-Arce, E.; Machado, I.; Cerecetto, H.; Gambino, D. New Pd-Fe ferrocenyl antiparasitic compounds with bioactive 8-hydroxyquinoline ligands: A comparative study with their Pt-Fe analogues. Dalton Trans. 2021, 50, 1651–1665. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H. 13C NMR chemical shift prediction of diverse chemical compounds. Environ. Res. 2019, 30, 477–490. [Google Scholar]
- Verma, R.P.; Hansch, C. Use of 13C NMR Chemical Shift as QSAR/QSPR Descriptor. Chem. Rev. 2011, 111, 2865–2899. [Google Scholar] [CrossRef] [PubMed]
- Sparatore, F.; Caliendo, G.; La Rotonda, M.I.; Novellino, E.; Silipo, C.; Vittoria, A. 13C-NMR and 1H-NMR Chemical Shifts in a Correlation Analysis of Benzotriazole Derivatives Active as Plant Growth Regulators. Quant. Struct.-Act. Relatsh. 1988, 7, 178–183. [Google Scholar] [CrossRef]
- Rodríguez, G.; Nargoli, J.; López, A.; Moyna, G.; Álvarez, G.; Fernández, M.; Osorio-Martínez, C.A.; González, M.; Cerecetto, H. Synthesis and in vivo proof of concept of a BODIPY-based fluorescent probe as a tracer for biodistribution studies of a new anti-Chagas agent. RSC Adv. 2017, 7, 7983–7989. [Google Scholar] [CrossRef]
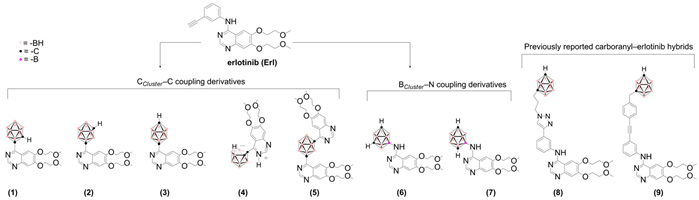 | ||||||
|---|---|---|---|---|---|---|
| Compd. | IC50 (µM) 1 | |||||
| U87 MG | F98 | C6 | Glial Cell 2 | EGFRwt | EGFRT790M | |
| 1 | 32.5 | 54.8 | 11.6 | 60.4 | 1.81 | NDR 3 |
| 2 | 36.0 | 24.6 | 24.9 | 51.0 | NDR | NDR |
| 3 | 22.0 | 24.2 | 24.3 | 50.0 | 12.13 | 12.31 |
| 4 | >100 | 50.1 | >100 | >100 | NDR | 8.73 |
| 5 | >100 | 24.2 | 24.8 | >100 | >5.0 | NDR |
| 6 | 47.0 | 75.5 | 35.4 | 71.2 | 9.19 | NDR |
| 7 | 25.4 | 15.6 | 8.50 | 58.5 | 9.23 | 7.19 |
| 8 | 70 | ND | 30 | >100 | 0.002 | 1.13 |
| 9 | ND | ND | >100 | 517.2 | NDR | |
| Erl | 63.0 | >100 | >100 | >100 | 0.057 | NDR |
| Compd. | Descriptor | ||||
|---|---|---|---|---|---|
| RM0 | log10RM0 | flogP 1 | π substituent 2 | δH (ppm) 3 | |
| 1 | 2.46 | 0.391 | 4.96 | o-carboran-1-yl, +4.33 | 5.71 |
| 2 | 3.36 | 0.526 | 4.92 | m-carboran-1-yl, +4.29 | 3.33 |
| 3 | 3.62 | 0.559 | 5.10 | p-carboran-1-yl, +4.47 | 3.00 |
| 4 | 1.46 | 0.164 | - | - | 2.41 |
| 5 | 3.94 | 0.600 | 5.56 | m-carboran-1-yl, +4.29 | - |
| 6 | 2.07 | 0.316 | 2.64 | m-carboran-9-yl, +3.20 | 2.90 |
| 7 | 2.95 | 0.470 | 3.48 | p-carboran-2-yl, +4.04 | 2.74 |
| 8 | - | - | 7.73 | - | - |
| 9 | - | - | 9.01 | - | - |
| Erl | 2.67 | 0.427 | 2.07 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dávila, B.; Vignolo, P.; Silvarrey, M.; Benítez, A.; González Schmidt, J.; de Arteaga Guidotti, C.; García, M.F.; Cerecetto, H.; Couto, M. Structure-Activity Relationships of closo- and nido-Carborane Erlotinib Analogs: Lipophilicity as a Key Modulator of Anti-Glioma Activity. Pharmaceuticals 2025, 18, 1753. https://doi.org/10.3390/ph18111753
Dávila B, Vignolo P, Silvarrey M, Benítez A, González Schmidt J, de Arteaga Guidotti C, García MF, Cerecetto H, Couto M. Structure-Activity Relationships of closo- and nido-Carborane Erlotinib Analogs: Lipophilicity as a Key Modulator of Anti-Glioma Activity. Pharmaceuticals. 2025; 18(11):1753. https://doi.org/10.3390/ph18111753
Chicago/Turabian StyleDávila, Belén, Pablo Vignolo, Martina Silvarrey, Andrés Benítez, Juliana González Schmidt, Carmela de Arteaga Guidotti, María Fernanda García, Hugo Cerecetto, and Marcos Couto. 2025. "Structure-Activity Relationships of closo- and nido-Carborane Erlotinib Analogs: Lipophilicity as a Key Modulator of Anti-Glioma Activity" Pharmaceuticals 18, no. 11: 1753. https://doi.org/10.3390/ph18111753
APA StyleDávila, B., Vignolo, P., Silvarrey, M., Benítez, A., González Schmidt, J., de Arteaga Guidotti, C., García, M. F., Cerecetto, H., & Couto, M. (2025). Structure-Activity Relationships of closo- and nido-Carborane Erlotinib Analogs: Lipophilicity as a Key Modulator of Anti-Glioma Activity. Pharmaceuticals, 18(11), 1753. https://doi.org/10.3390/ph18111753








