Synergistic Catalysis of Gold–Platinum Alloy Nanozymes: A Novel Colorimetric Sensor for ALP Detection in Complex Biological Matrices
Abstract
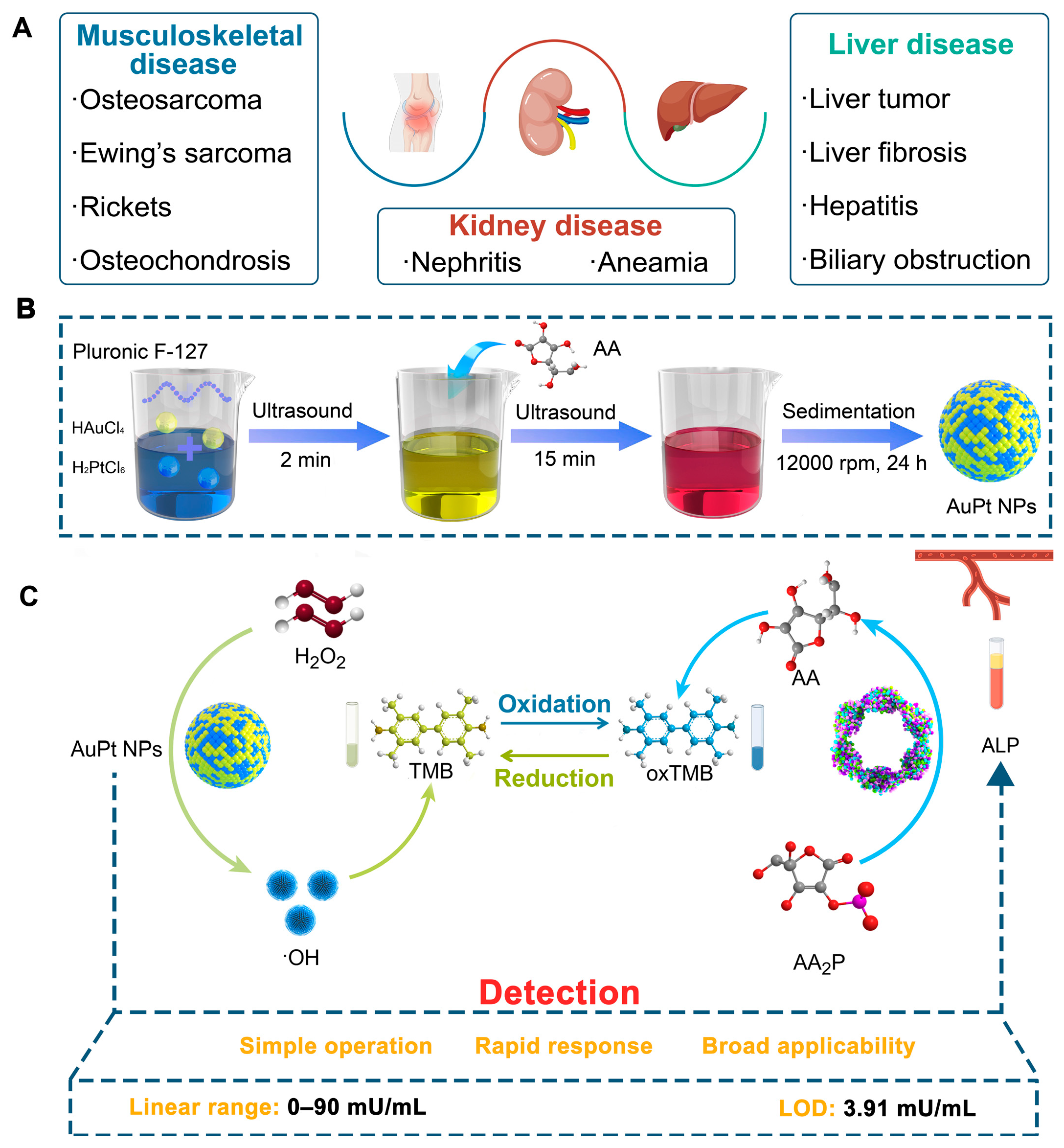
1. Introduction
2. Results
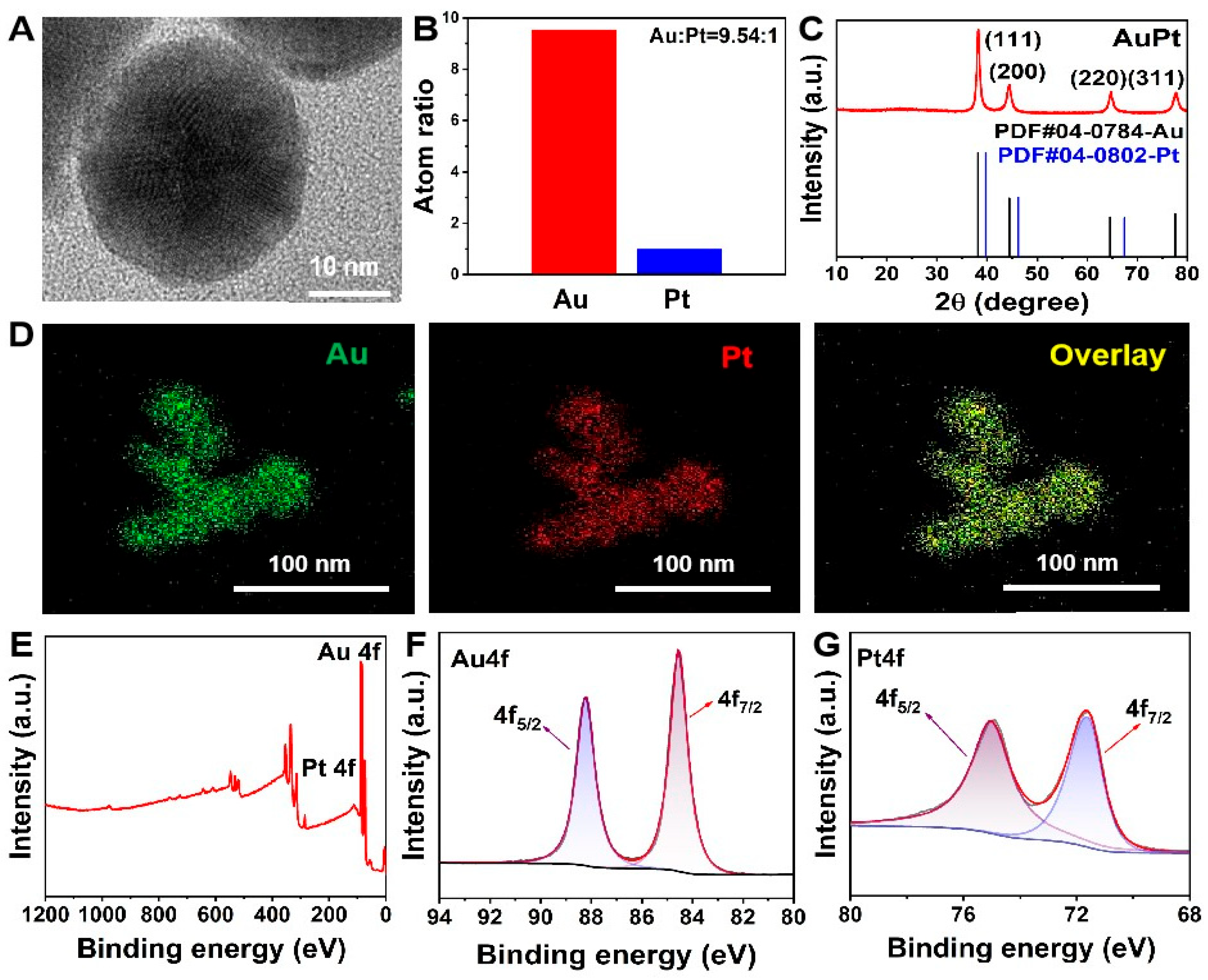
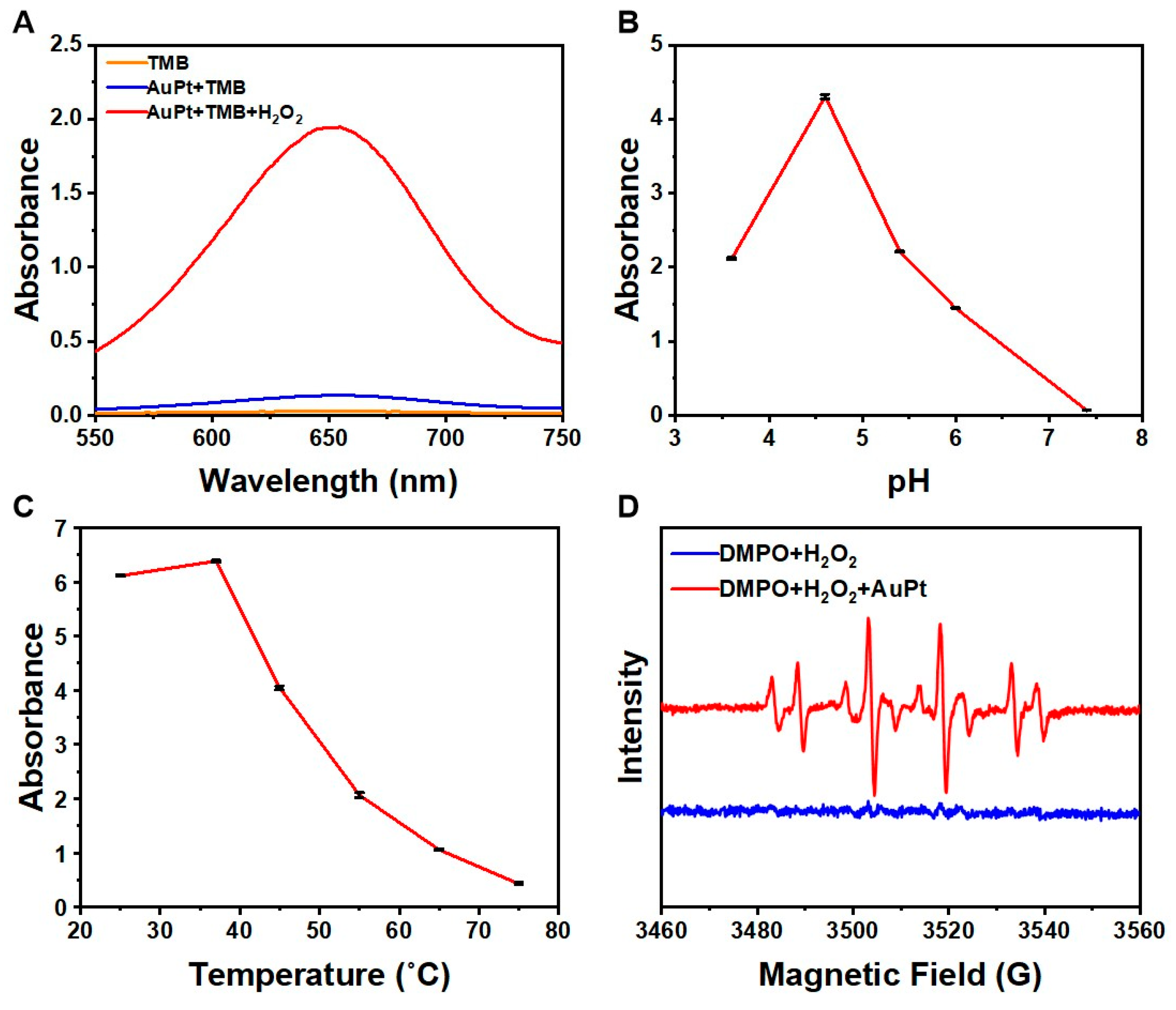
2.1. Characterization of AuPt NPs Catalyzing the Color Reaction of Chromogenic Agents
2.2. The Colorimetric Detection of ALP Based on AuPt NPs
2.3. Calculation of the Steady-State Kinetics
2.4. Inhibiting Effect of AA on POD-like Activity of AuPt NPs
2.5. ALP Quantification in Clinical Serum Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of AuPt NPs
4.3. Construction and Optimization of the Colorimetric System
4.4. Steady-State Kinetic Analysis of AuPt NPs in Colorimetric Systems
4.5. Detection of the Reducing Property and Selectivity of ALP Catalytic Products
4.6. Anti-Interference Detection of ALP Based on AuPt NPs
4.7. Assay of ALP in Serum Samples
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Khusbu, F.Y.; Wu, K.F.; Chen, H.C.; Chen, F.Z.; Ma, C.B. A label-free ThT-assisted fluorescence detection strategy of alkaline phosphatase activity based on MnO2 nanosheets. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 293, 122487. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, R.; Chen, W.; Wang, C.; Xing, D. The development of biosensors for alkaline phosphatase activity detection based on a phosphorylated DNA probe. Talanta 2024, 270, 125622. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, H.; Zhao, Y.; Wang, Y.; Ge, L.; Huang, Y.; Li, F. Immobilization-free dual-aptamer-based photoelectrochemical platform for ultrasensitive exosome assay. Talanta 2024, 266, 125001. [Google Scholar] [CrossRef] [PubMed]
- Daryaei, I.; Mohammadebrahim Ghaffari, M.; Jones, K.M.; Pagel, M.D. Detection of alkaline phosphatase enzyme activity with a catalyCEST MRI biosensor. ACS Sens. 2016, 1, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Qin, M.; Zhao, Y.; Li, H.; Luo, L.; Ding, C.; Cheng, P.; Su, M.; Li, H.; Song, Y.; et al. Supramolecular photonic hydrogel for high-sensitivity alkaline phosphatase detection via synergistic driving force. Small 2023, 19, e2206461. [Google Scholar] [CrossRef]
- Huang, J.M.; Zhao, H.; Chen, X.L.; Lin, T.R.; Hou, L.; Zhao, S.L. Pt nanoparticles functionalized hydrogen-bonded organic frameworks: A three-in-one nanozyme for colorimetric detection of alkaline phosphatase. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2025, 333, 125894. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Li, Z.; Yang, H.; Li, W.; Liu, Y.; Miao, M. Electrochemical detection of alkaline phosphatase activity via atom transfer radical polymerization. Bioelectrochemistry 2022, 144, 107998. [Google Scholar] [CrossRef]
- Guo, J.; Yu, H.; Cui, T. Applications of fluorescent materials in the detection of alkaline phosphatase activity. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 214–226. [Google Scholar] [CrossRef]
- Shang, X.; Yan, Y.; Li, J.; Zhou, X.; Xiang, X.; Huang, R.; Li, X.; Ma, C.; Nie, X. A turn-on fluorescent strategy for alkaline phosphatase detection based on enzyme-assisted signal amplification. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2023, 286, 121939. [Google Scholar] [CrossRef]
- Mintz Hemed, N.; Convertino, A.; Shacham-Diamand, Y. Alkaline phosphatase detection using electrochemical impedance of anti-alkaline phosphatase antibody (Ab354) functionalized silicon-nanowire-forest in phosphate buffer solution. Sens. Actuators B 2018, 259, 809–815. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, K.; Bartling, B.; Liu, C.C. The detection of alkaline phosphatase using an electrochemical biosensor in a single-step approach. Sensors 2009, 9, 8709–8721. [Google Scholar] [CrossRef]
- Iha, K.; Kyosei, Y.; Namba, M.; Makioka, D.; Yamura, S.; Watabe, S.; Yoshimura, T.; Ito, E. Zeptomole detection of an enzyme by a simple colorimetric method. Anal. Sci. 2021, 37, 1469–1472. [Google Scholar] [CrossRef]
- Jilma-Stohlawetz, P.; Lysy, K.; Belik, S.; Jilma, B.; Quehenberger, P. Interference in specialized coagulation assays affecting the protein C pathway: Effects of marked haemolysis, hyperbilirubinaemia and lipaemia on chromogenic and clotting tests on two coagulation platforms. Int. J. Lab. Hematol. 2019, 41, 404–411. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.D. Recent advances in the bioactive structure and application of single-atom nanozymes. Nano Biomed. Eng. 2024, 16, 1–27. [Google Scholar] [CrossRef]
- Ji, T.K.; Li, J.J.; Jiao, C.N.; Wang, J.; Cai, X.W. A unique core–shell nanoreactor sSiO2@CeO2/Pt@mSiO2 as artificial nanozyme for ultra-sensitive detection of ascorbic acid and alkaline phosphatase activity. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2025, 341, 126459. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.H.; Yeh, C.Y.; Wang, C.M.; Liao, W.S.; Chen, C.Y. Specializing carbon nanozyme active sites for sensitive alkaline phosphatase activity metal-free detection. Chem. Asian J. 2024, 19, e202300878. [Google Scholar] [CrossRef]
- Song, Y.F.; Huang, C.Z.; Li, Y.F. Nanozyme rich in oxygen vacancies derived from Mn-based metal–organic gel for the determination of alkaline phosphatase. Inorg. Chem. 2023, 62, 12697–12707. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Ding, S.; He, L.; Bian, X.-W.; Tian, G. Triple-synergistic hollow AuAg@CeO2 plasmonic nanozymes enable rapid alkaline phosphatase detection via smartphone-integrated dual-mode biosensing. J. Colloid Interface Sci. 2025, 699, 138272. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, Z.; Zhang, T.; Lin, H.; Li, Z.; Chen, J.; Deng, S.; Liu, F. Inorganic boron-based nanostructures: Synthesis, optoelectronic properties, and prospective applications. Nanomaterials 2019, 9, 538. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Zhou, X.; Chen, J.; Wang, M.; Su, X. Fe-N-C single-atom nanozymes with peroxidase-like activity for the detection of alkaline phosphatase. Analyst 2021, 146, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dong, Z.; Geng, F.; Wang, J.; Song, X.; Ding, C. Electrochemiluminescence Biosensor Based on an Efficient Antifouling Cell Membrane Hydrogel for the Direct Detection of Alkaline Phosphatase in Human Serum and Cell Lysate. Anal. Chem. 2025, 97, 11090–11098. [Google Scholar] [CrossRef]
- Wu, H.; Bu, T.; Sun, B.; Xi, J.; Cao, Y.; Wang, Y.; Xuan, C.; Feng, Q.; Yan, H.; Wang, L. “Three-in-One” multifunctional hollow nanocages with colorimetric photothermal catalytic activity for enhancing sensitivity in biosensing. Anal. Chem. 2024, 96, 4825–4834. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Wang, X. Alkaline phosphatase-responsive Zn(2+) double-triggered nucleotide capped gold nanoclusters/alginate hydrogel with recyclable nanozyme capability. Biosens. Bioelectron. 2021, 173, 112786. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, L.; Hou, H.; Jia, W.; Zhang, A.; Zhang, B.; Bu, Y.; Gong, Y.; Yan, L.; Du, B. Construct a magnetic Pt/Ru alloy peroxidase mimic as a reusable and cost-effective “signal-off” sensing platform for sensitive and wide-linear-range assay. Anal. Chem. 2024, 96, 10467–10475. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, J.; Wang, J.; Zheng, Z.; Liu, X.; Wang, J.; Zhang, C.; He, S.; Wei, H.; Yu, C.Y. A programmable microfluidic paper-based analytical device for simultaneous colorimetric and photothermal visual sensing of multiple enzyme activities. Anal. Chem. 2024, 96, 12181–12188. [Google Scholar] [CrossRef]
- Wang, H.; Su, P.; Wei, W.; Song, J.; Yang, Y. Hollow Cu/CoS(2) Nanozyme with defect-induced enzymatic catalytic sites and binding pockets for highly sensitive fluorescence detection of alkaline phosphatase. Small 2024, 20, e2401416. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, Y.; Cao, Z.; Lin, W.; Lu, N. DNA-programmed tuning of the growth and enzyme-like activity of a bimetallic nanozyme and its biosensing applications. ACS Appl. Mater. Interfaces 2023, 15, 18620–18629. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Gong, W.; Zhang, M.; Lu, N. Modulating the biomimetic and fluorescence quenching activities of metal-organic framework/platinum nanoparticle composites and their applications in molecular biosensing. ACS Appl. Mater. Interfaces 2022, 14, 21677–21686. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Z.; Zhao, X.; Miao, Y.; Yuan, Z.; Jiang, Y.; Lu, Y. Self-deposited ultrasmall Ru nanoparticles on carbon nitride with high peroxidase-mimicking activity for the colorimetric detection of alkaline phosphatase. J. Colloid Interface Sci. 2023, 631, 86–95. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, D.Y.; Choung, J.W.; Kim, C.H.; Ham, H.C.; Lee, K.Y. Roles of noble metals (M = Ag, Au, Pd, Pt and Rh) on CeO(2) in enhancing activity toward soot oxidation: Active oxygen species and DFT calculations. J. Hazard. Mater. 2021, 403, 124085. [Google Scholar] [CrossRef]
- Gao, Y.C.; Wang, C.; Zhang, C.X.; Li, H.W.; Wu, Y. Controlled preparation and application of glutathione capped gold and platinum alloy nanoclusters with high peroxidase-like activity. J. Mater. Sci. Technol. 2022, 109, 140–146. [Google Scholar] [CrossRef]
- Pearce, A.K.; Wilks, T.R.; Arno, M.C.; O’Reilly, R.K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 2021, 5, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, Z.; Xie, P.; Huang, Z.; Li, T.; Morris, D.; Finfrock, Z.; Zhou, J.; Jiao, M.; Gao, J.; et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 2020, 6, eaaz0510. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Wang, H.; Uemura, Y.; Nakamura, T.; Yu, L.; Sekizawa, O.; Uruga, T.; Tada, M.; Samjeské, G.; Iwasawa, Y.; et al. In situ study of oxidation states of platinum nanoparticles on a polymer electrolyte fuel cell electrode by near ambient pressure hard X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 6013–6021. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
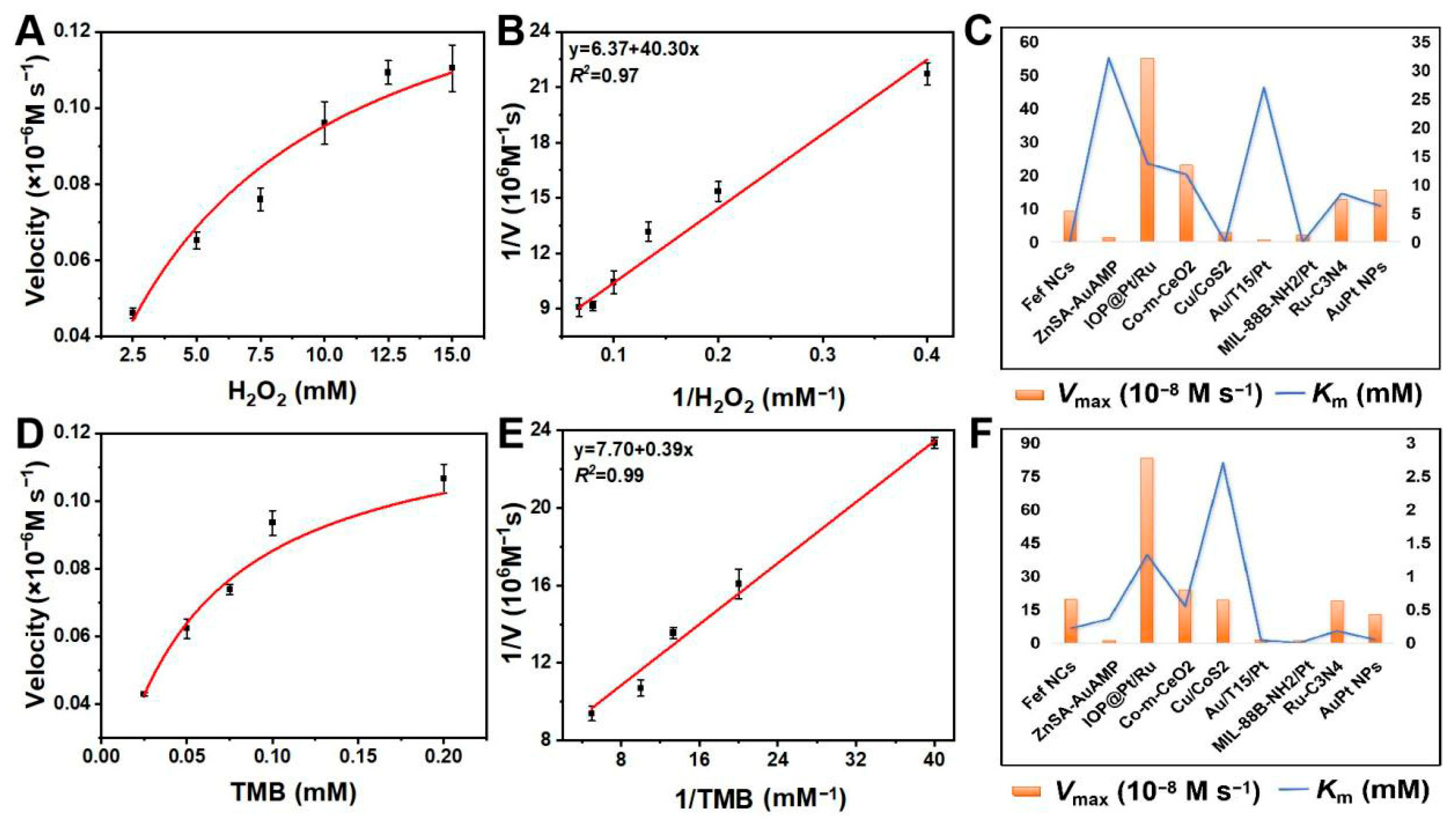
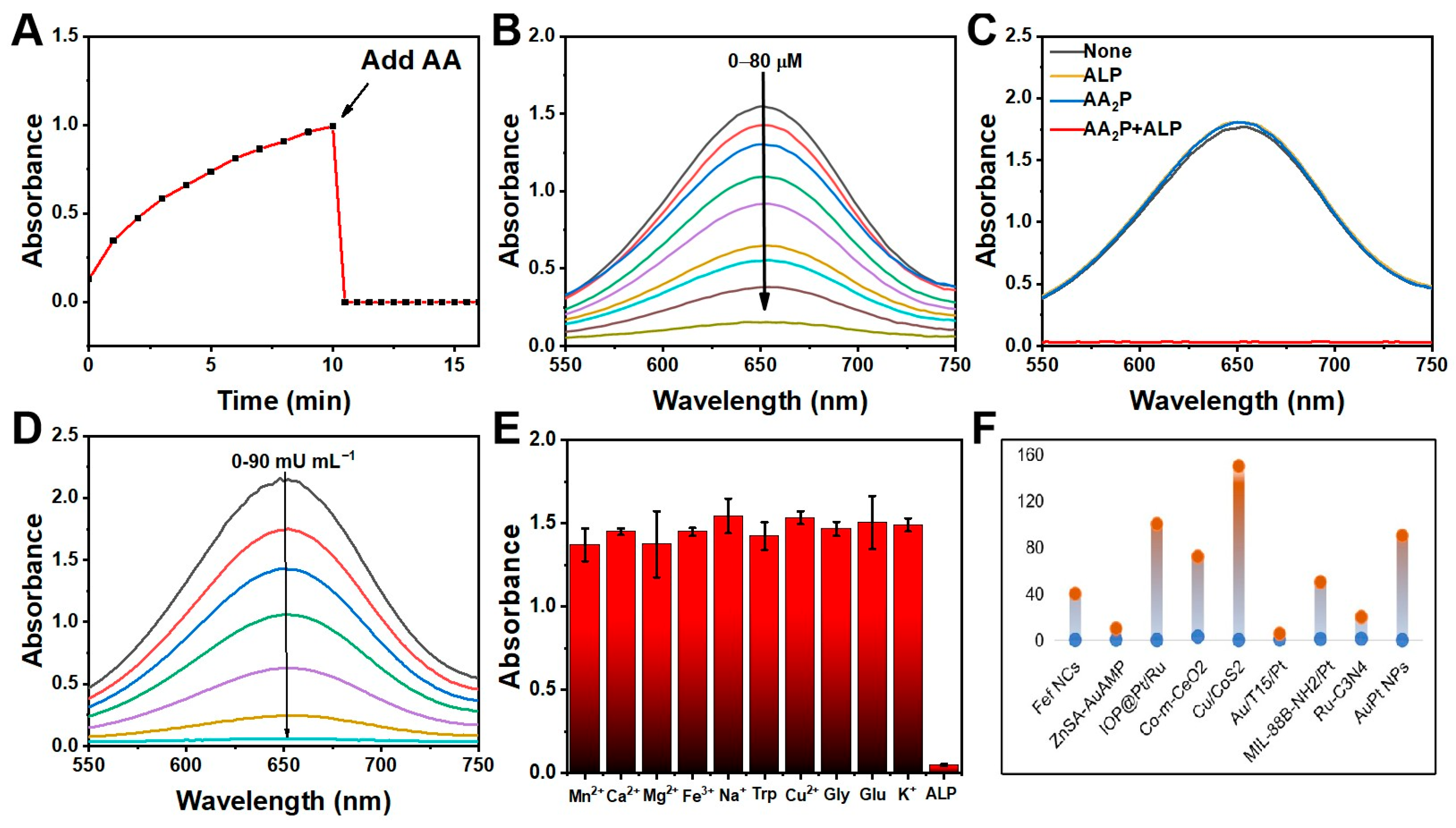
| Materials | Liner Range (mU mL−1) | LODs (mU mL−1) | Ref. |
|---|---|---|---|
| Fef NCs | 0.2–40 | 0.19 | [22] |
| ZnSA-AuAMP | 0.47–10.03 | 0.09 | [23] |
| IOP@Pt/Ru | 0.1–100 | 0.05 | [24] |
| Co-m-CeO2 | 3–72 | 0.021 | [25] |
| Cu/CoS2 | 0.05–150 | 0.035 | [26] |
| Au/T15/Pt | 0.625–5.625 | 0.35 | [27] |
| MIL-88B-NH2/Pt | 1–50 | 1.89 | [28] |
| Ru-C3N4 | 1.25–20 | 0.75 | [29] |
| AuPt NPs | 0–90 | 3.91 | This work |
| Sample | Added (mU mL−1) | Measured (mU mL−1) | RSD (100%) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0 | 0.80 ± 0.09 | 11.37 | / |
| 10 | 9.69 ± 0.46 | 4.73 | 96.94 ± 4.59 | |
| 30 | 28.63 ± 1.75 | 6.10 | 95.45 ± 5.82 | |
| 60 | 60.31 ± 2.57 | 4.26 | 100.51 ± 4.28 | |
| 2 | 0 | 0.60 ± 0.10 | 16.79 | / |
| 10 | 10.78 ± 2.91 | 27.04 | 107.78 ± 29.14 | |
| 30 | 33.59 ± 3.55 | 10.57 | 111.97 ± 11.83 | |
| 60 | 60.78 ± 1.46 | 2.40 | 101.29 ± 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, B.; Zhang, B.; Ren, X.; Yang, J.; Yang, F.; Yan, C.; Li, L.; Zhang, R. Synergistic Catalysis of Gold–Platinum Alloy Nanozymes: A Novel Colorimetric Sensor for ALP Detection in Complex Biological Matrices. Pharmaceuticals 2025, 18, 1795. https://doi.org/10.3390/ph18121795
Du B, Zhang B, Ren X, Yang J, Yang F, Yan C, Li L, Zhang R. Synergistic Catalysis of Gold–Platinum Alloy Nanozymes: A Novel Colorimetric Sensor for ALP Detection in Complex Biological Matrices. Pharmaceuticals. 2025; 18(12):1795. https://doi.org/10.3390/ph18121795
Chicago/Turabian StyleDu, Baojie, Bingqing Zhang, Xiaofeng Ren, Jie Yang, Fan Yang, Chunyu Yan, Liping Li, and Ruiping Zhang. 2025. "Synergistic Catalysis of Gold–Platinum Alloy Nanozymes: A Novel Colorimetric Sensor for ALP Detection in Complex Biological Matrices" Pharmaceuticals 18, no. 12: 1795. https://doi.org/10.3390/ph18121795
APA StyleDu, B., Zhang, B., Ren, X., Yang, J., Yang, F., Yan, C., Li, L., & Zhang, R. (2025). Synergistic Catalysis of Gold–Platinum Alloy Nanozymes: A Novel Colorimetric Sensor for ALP Detection in Complex Biological Matrices. Pharmaceuticals, 18(12), 1795. https://doi.org/10.3390/ph18121795








