Neuroprotective Effects of Fluoxetine Derivative 4-[3-Oxo-3-(2-trifluoromethyl-phenyl)-propyl]-morpholinium Chloride (OTPM) as a Potent Modulator of Motor Deficits and Neuroinflammatory Pathways in LPS-Induced BV-2 Microglial Cells and MPTP-Induced Parkinsonian Models
Abstract
1. Introduction
2. Results
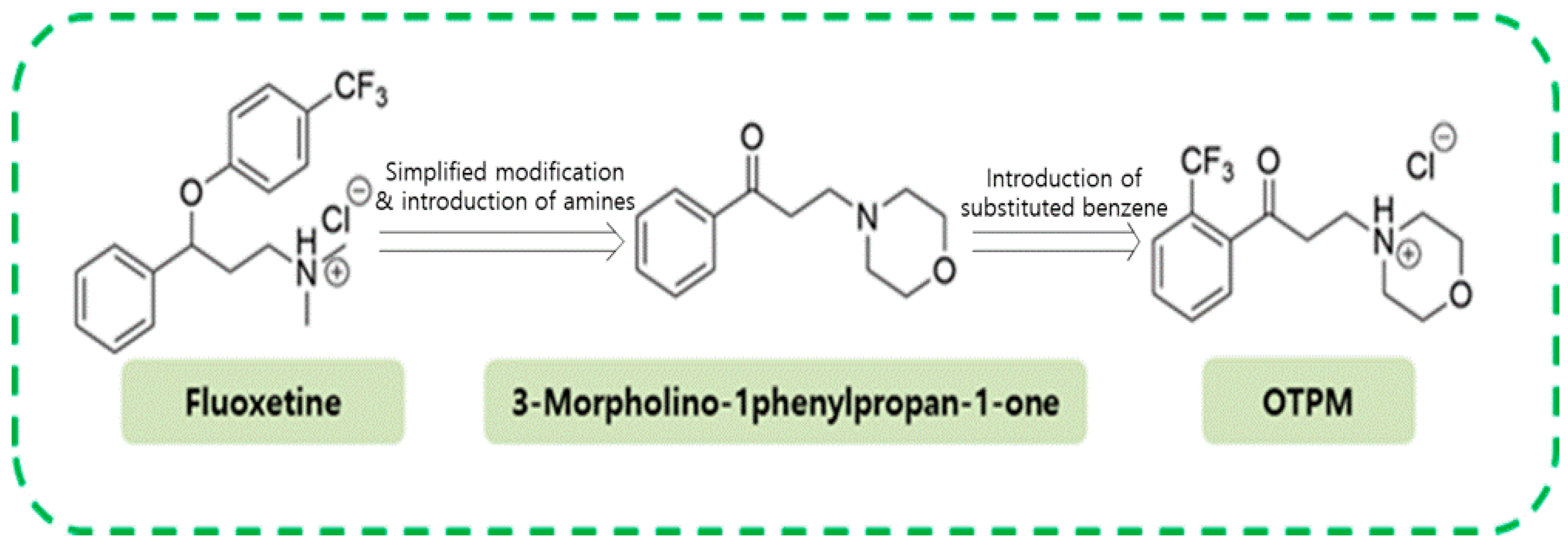
2.1. OTPM Synthesis
2.2. OTPM Inhibits NO Release Without Affecting the Viability of LPS-Treated BV-2 Microglial Cells
2.3. OTPM Attenuates iNOS and COX-2 Production in LPS-Stimulated BV-2 Microglial Cells
2.4. OTPM Attenuates the Production of Pro-Inflammatory Cytokines in LPS-Stimulated BV-2 Microglial Cells
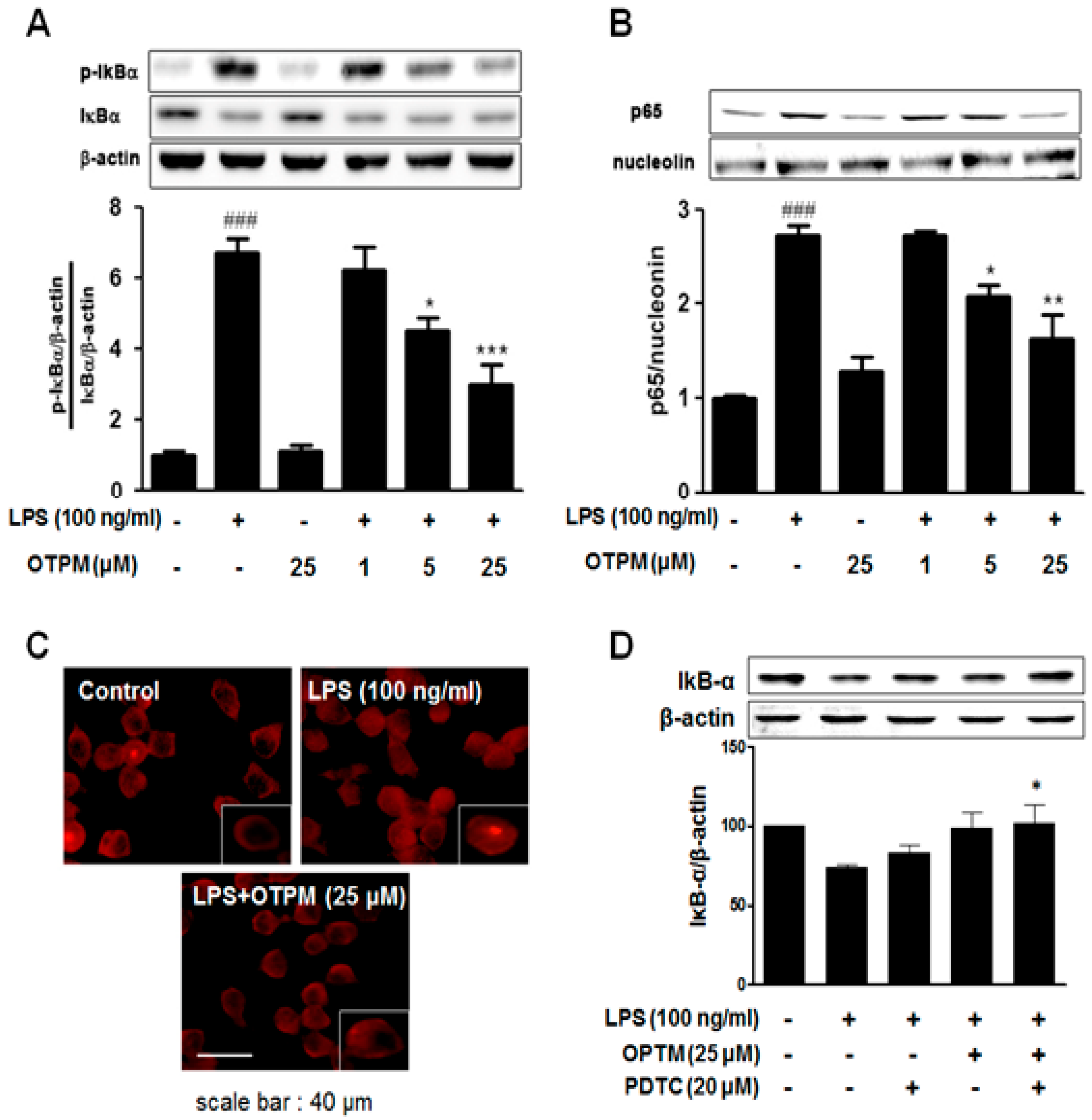
2.5. OTPM Inhibits LPS-Induced NF-κB Activation in BV-2 Microglial Cells
2.6. OTPM Attenuates Glial Activation in the MPTP-Induced PD Mouse Model
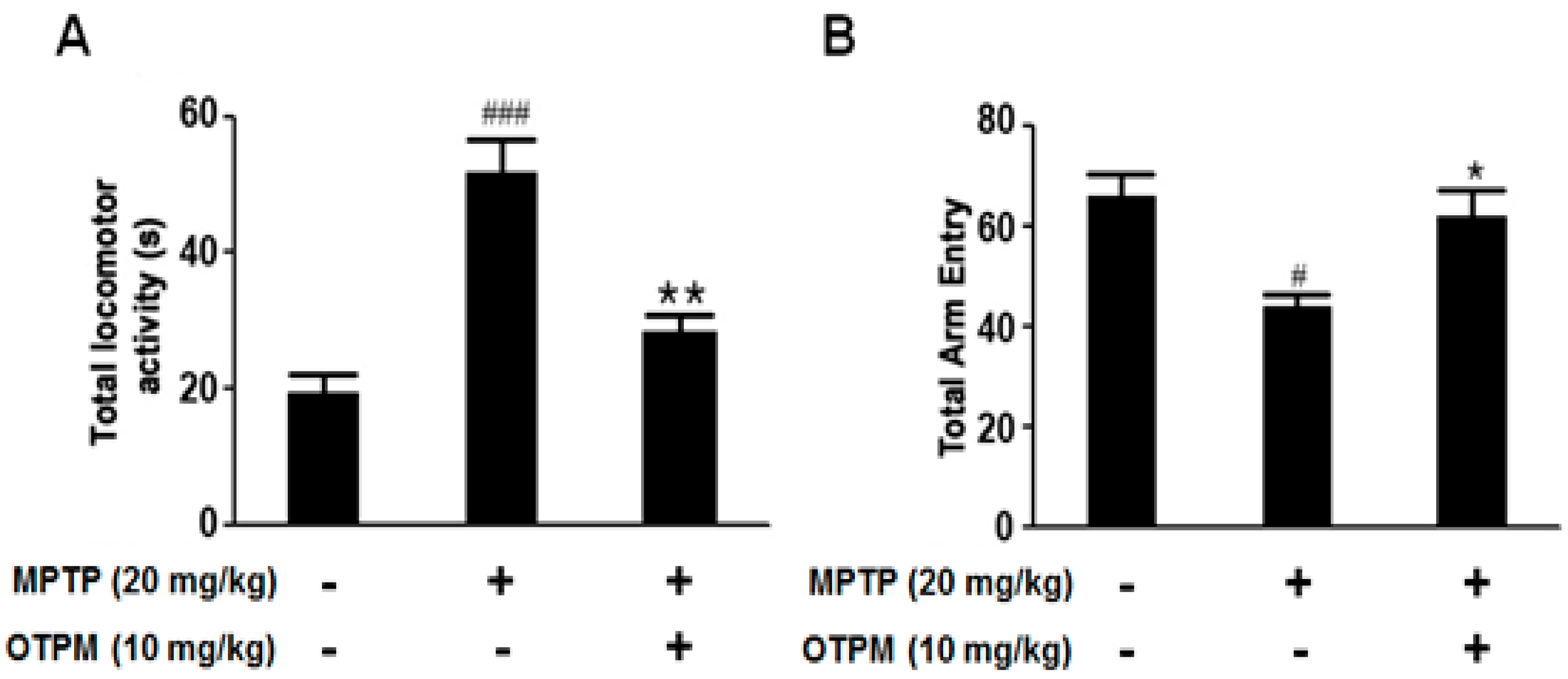
2.7. Improvement of Behavior by OTPM in the MPTP-Induced PD Mouse Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of OTPM
4.3. Cell Culture and Treatment
4.4. Animals and Treatment
4.5. Cell Viability and NO Assays
4.6. Total RNA Extraction and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.7. Western Blot Analysis
4.8. Immunocytochemistry
4.9. Immunohistochemistry
4.10. Behavioral Experiments: Pole Test and Y-Maze Test
4.11. Statistical Analysis
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balakrishnan, R.; Vijayraja, D.; Mohankumar, T.; Manimaran, D.; Ganesan, P.; Choi, D.K.; Elangovan, N. Isolongifolene Mitigates Rotenone-Induced Dopamine Depletion and Motor Deficits through Anti-Oxidative and Anti-Apoptotic Effects in a Rat Model of Parkinson‘s Disease. J. Chem. Neuroanat. 2021, 112, 101890. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Tamilselvam, K.; Sulthana, A.; Mohankumar, T.; Manimaran, D.; Elangovan, N. Isolongifolene attenuates oxidative stress and behavioral impairment in rotenone-induced rat model of Parkinson‘s disease. Int. J. Nutr. Pharmacol. Neurol. Dis. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Lotharius, J.; Brundin, P. Impaired Dopamine Storage Resulting from α-Synuclein Mutations May Contribute to the Pathogenesis of Parkinson‘s Disease. Hum. Mol. Genet. 2002, 11, 2395–2407. [Google Scholar] [CrossRef] [PubMed]
- Jurcău, M.C.; Andronie-Cioara, F.L.; Jurcău, A.; Marcu, F.; Ţiț, D.M.; Pașcalău, N.; Nistor-Cseppentö, D.C. The Link between Oxidative Stress, Mitochondrial Dysfunction and Neuroinflammation in the Pathophysiology of Alzheimer’s Disease: Therapeutic Implications and Future Perspectives. Antioxidants 2022, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Essa, M.M.; Guizani, N.; Balakrishnan, R.; Hemalatha, T.; Manivasagam, T.; Justin-Thenmozhi, A.; Elangovan, N.; Velusamy, T. Protective effect of Zizyphus spinachristi on MPP+-induced oxidative stress. Front. Biosci. 2018, 10, 285–299. [Google Scholar]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory Mechanisms in Parkinson‘s Disease: Potential Environmental Triggers, Pathways, and Targets for Early Therapeutic Intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson‘s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson‘s Disease. Antioxidans 2020, 9, 1007. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 267. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Machado, V.; Zöller, T.; Attaai, A.; Spittau, B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson‘s Disease-Lessons from Transgenic Mice. Int. J. Mol. Sci. 2016, 17, 151. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Helweg, L.P.; Greiner, J.F.W.; Kaltschmidt, C. NF-κB in Neurodegenerative Diseases: Recent Evidence from Human Genetics. Front. Mol. Neurosci. 2022, 15, 954541. [Google Scholar] [CrossRef] [PubMed]
- Dolatshahi, M.; Ranjbar Hameghavandi, M.H.; Sabahi, M.; Rostamkhani, S. Nuclear Factor-Kappa B (NF-κB) in Pathophysiology of Parkinson Disease: Diverse Patterns and Mechanisms Contributing to Neurodegeneration. Eur. J. Neurosci. 2021, 54, 4101–4123. [Google Scholar] [CrossRef] [PubMed]
- Jannat, K.; Balakrishnan, R.; Han, J.H.; Yu, Y.J.; Kim, G.W.; Choi, D.K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells 2023, 12, 2652. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Azam, S.; Cho, D.Y.; Su-Kim, I.; Choi, D.K. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson‘s Disease: Current Knowledge and Future Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef]
- Shadfar, S.; Kim, Y.G.; Katila, N.; Neupane, S.; Ojha, U.; Bhurtel, S.; Srivastav, S.; Jeong, G.S.; Park, P.H.; Hong, J.T.; et al. Neuroprotective Effects of Antidepressants via Upregulation of Neurotrophic Factors in the MPTP Model of Parkinson‘s Disease. Mol. Neurobiol. 2018, 55, 554–566. [Google Scholar] [CrossRef]
- Mendonça, I.P.; de Paiva, I.H.R.; Duarte-Silva, E.P.; de Melo, M.G.; da Silva, R.S.; de Oliveira, W.H.; da Costa, B.L.d.S.A.; Peixoto, C.A. Metformin and Fluoxetine Improve Depressive-like Behavior in a Murine Model of Parkinsońs Disease through the Modulation of Neuroinflammation, Neurogenesis and Neuroplasticity. Int. Immunopharmacol. 2022, 102, 108415. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, N.; Lei, J.; Jing, B.; Li, M.; Tian, H.; Xue, B.; Li, X. Fluoxetine Shows Neuroprotective Effects against LPS-Induced Neuroinflammation via the Notch Signaling Pathway. Int. Immunopharmacol. 2022, 113, 109417. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, E.; Cho, D.Y.; Ko, H.M.; Baek, H.Y.; Choi, D.K.; Kim, E.; Park, J.Y. Synthesis of 4-(3-Oxo-3-Phenylpropyl)Morpholin-4-Ium Chloride Analogues and Their Inhibitory Activities of Nitric Oxide Production in Lipopolysaccharide-Induced BV2 Cells. Bioorg. Med. Chem. Lett. 2021, 36, 127780. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.W.; Lee, J.K.; Im, W.B.; Jin, B.K.; Yoon, S.H. Simplified Heterocyclic Analogues of Fluoxetine Inhibit Inducible Nitric Oxide Production in Lipopolysaccharide-Induced BV2 Cells. Biol. Pharm. Bull. 2011, 34, 538–544. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Kim, Y.S.; Kim, G.W.; Kim, W.J.; Hong, S.M.; Kim, C.G.; Choi, D.K. Standardized Extract of Glehnia Littoralis Abrogates Memory Impairment and Neuroinflammation by Regulation of CREB/BDNF and NF-κB/MAPK Signaling in Scopolamine-Induced Amnesic Mice Model. Biomed. Pharmacother. 2023, 165, 115106. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Im, J.H.; Balakrishnan, R. Paeoniflorin Exercise-Mimetic Potential Regulates the Nrf2/HO-1/BDNF/CREB and APP/BACE-1/NF-κB/MAPK Signaling Pathways to Reduce Cognitive Impairments and Neuroinflammation in Amnesic Mouse Model. Biomed. Pharmacother. 2025, 189, 118299. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; de Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Kim, Y.S.; Kang, S.I.; Choi, D.K. Green Oat Cognitaven® Attenuates Mild Cognitive Impairment by Activating the CREB/BDNF/Nrf2/HO-1 Pathway and Modulating NF-κB/MAPK Signaling. Biomed. Pharmacother. 2025, 189, 118295. [Google Scholar] [CrossRef]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Simonyi, A.; Kotzbauer, P.T.; Gu, Z.; Sun, G.Y. Cytosolic Phospholipase A2 Plays a Crucial Role in ROS/NO Signaling during Microglial Activation through the Lipoxygenase Pathway. J. Neuroinflamm. 2015, 12, 199. [Google Scholar] [CrossRef]
- Mander, P.; Brown, G.C. Activation of Microglial NADPH Oxidase Is Synergistic with Glial INOS Expression in Inducing Neuronal Death: A Dual-Key Mechanism of Inflammatory Neurodegeneration. J. Neuroinflamm. 2005, 2, 20. [Google Scholar] [CrossRef]
- Lee, S.M.; Yune, T.Y.; Kim, S.J.; Kim, Y.C.; Oh, Y.J.; Markelonis, G.J.; Oh, T.H. Minocycline Inhibits Apoptotic Cell Death via Attenuation of TNF-α Expression Following INOS/NO Induction by Lipopolysaccharide in Neuron/Glia Co-Cultures. J. Neurochem. 2004, 91, 568–578. [Google Scholar] [CrossRef]
- Brown, G.C.; Vilalta, A. How Microglia Kill Neurons. Brain Res. 2015, 1628, 288–297. [Google Scholar] [CrossRef]
- Ghoshal, A.; Das, S.; Ghosh, S.; Mishra, M.K.; Sharma, V.; Koli, P.; Sen, E.; Basu, A. Proinflammatory Mediators Released by Activated Microglia Induces Neuronal Death in Japanese Encephalitis. Glia 2007, 55, 483–496. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, H.; Wilson, B.C.; Shi, J.S.; Hong, J.S.; Gao, H.M. Fluoxetine Protects Neurons against Microglial Activation-Mediated Neurotoxicity. Park. Relat. Disord. 2012, 18, S213–S217. [Google Scholar] [CrossRef] [PubMed]
- Mojiri-Forushani, H.; Khajehali, E.; Adelipour, M.; Mohammadi, A. Inhibitory Effects of Fluoxetine on the Secretion of Inflammatory Mediators and JAK/STAT3 and JNK/TLR4 Gene Expression. Mol. Biol. Rep. 2023, 50, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Joshi, L.; Plastira, I.; Bernhart, E.; Reicher, H.; Triebl, A.; Köfeler, H.C.; Sattler, W. Inhibition of Autotaxin and Lysophosphatidic Acid Receptor 5 Attenuates Neuroinflammation in LPS-Activated BV-2 Microglia and a Mouse Endotoxemia Model. Int. J. Mol. Sci. 2021, 22, 8519. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.K.; Chung, Y.C.; Hyun, C.G. Anti-Inflammatory Effects of 6-Methylcoumarin in LPS-Stimulated RAW 264.7 Macrophages via Regulation of MAPK and NF-κB Signaling Pathways. Molecules 2021, 26, 5351. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Wan, C.X.; Xia, Y.Z.; Zhang, C.; Chen, M.H.; Wang, Z.D.; Li, Z.R.; Li, X.M.; Geng, Y.-D.; et al. Anti-Neuroinflammatory Effect of Sophoraflavanone G from Sophora Alopecuroides in LPS-Activated BV2 Microglia by MAPK, JAK/STAT and Nrf2/HO-1 Signaling Pathways. Phytomedicine 2016, 23, 1629–1637. [Google Scholar] [CrossRef]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.M.; Huang, B.X.; Lv, Q.K.; Lei, L.C.; et al. BHBA Suppresses LPS-Induced Inflammation in BV-2 Cells by Inhibiting NF-κB Activation. Mediat. Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef]
- Choi, J.W.; Jo, S.W.; Kim, D.E.; Paik, I.Y.; Balakrishnan, R. Aerobic Exercise Attenuates LPS-Induced Cognitive Dysfunction by Reducing Oxidative Stress, Glial Activation, and Neuroinflammation. Redox Biol. 2024, 71, 103101. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.W.; Ren, W.Z.; Liu, J.X.; Li, S.N.; Fu, S.P.; Zeng, Y.L.; Xu, S.Y.; Yan, X.; Gao, Y.J.; et al. GLP-2 Attenuates LPS-Induced Inflammation in BV-2 Cells by Inhibiting ERK1/2, JNK1/2 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 190. [Google Scholar] [CrossRef]
- Ge, Y.T.; Zhong, A.Q.; Xu, G.F.; Lu, Y. Resveratrol Protects BV2 Mouse Microglial Cells against LPS-Induced Inflammatory Injury by Altering the MiR-146a-5p/TRAF6/NF-κB Axis. Immunopharmacol. Immunotoxicol. 2019, 41, 549–557. [Google Scholar] [CrossRef]
- Choi, W.Y.; Sim, J.H.; Lee, J.Y.; Kang, D.H.; Lee, H.Y. Increased Anti-Inflammatory Effects on LPS-Induced Microglia Cells by Spirulina Maxima Extract from Ultrasonic Process. Appl. Sci. 2019, 9, 2144. [Google Scholar] [CrossRef]
- Mitra, S.; Ghosh, N.; Sinha, P.; Chakrabarti, N.; Bhattacharyya, A. Alteration of Nuclear Factor-KappaB Pathway Promote Neuroinflammation Depending on the Functions of Estrogen Receptors in Substantia Nigra after 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine Treatment. Neurosci. Lett. 2016, 616, 86–92. [Google Scholar] [CrossRef]
- Perkins, N.D. Integrating Cell-Signalling Pathways with NF-κB and IKK Function. Nat. Rev. Mol. Cell Biol. 2007, 8, 49–62. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Z.; Cao, X.; Ding, L.; Wen, Z.S.; Bian, J.S. HNO Suppresses LPS-Induced Inflammation in BV-2 Microglial Cells via Inhibition of NF-κB and P38 MAPK Pathways. Pharmacol. Res. 2016, 111, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Korn, S.H.; Wouters, E.F.M.; Vos, N.; Janssen-Heininger, Y.M.W. Cytokine-Induced Activation of Nuclear Factor-ΓB Is Inhibited by Hydrogen Peroxide through Oxidative Inactivation of IκB Kinase. J. Biol. Chem. 2001, 276, 35693–35700. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Li, H.; Tian, Z.; Xu, C.; Liu, J.; Guo, Y. Disruption of NF-κB Signaling by Fluoxetine Attenuates MGMT Expression in Glioma Cells. Onco Targets. Ther. 2015, 8, 2199–2208. [Google Scholar] [CrossRef]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Kim, N.; Jung, H.C.; Song, I.S.; Kim, J.S. Fluoxetine Inhibits NF-κB Signaling in Intestinal Epithelial Cells and Ameliorates Experimental Colitis and Colitis-Associated Colon Cancer in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G9–G19. [Google Scholar] [CrossRef]
- Tian, M.; Yang, M.; Li, Z.; Wang, Y.; Chen, W.; Yang, L.; Li, Y.; Yuan, H. Fluoxetine Suppresses Inflammatory Reaction in Microglia under OGD/R Challenge via Modulation of NF-κB Signaling. Biosci. Rep. 2019, 39, BSR20181584. [Google Scholar] [CrossRef]
- Yang, J.M.; Rui, B.B.; Chen, C.; Chen, H.; Xu, T.J.; Xu, W.P.; Wei, W. Acetylsalicylic Acid Enhances the Anti-Inflammatory Effect of Fluoxetine through Inhibition of NF-κB, P38-MAPK and ERK1/2 Activation in Lipopolysaccharide-Induced BV-2 Microglia Cells. Neuroscience 2014, 275, 296–304. [Google Scholar] [CrossRef]
- Mustapha, M.; Taib, C.N.M. MPTP-Induced Mouse Model of Parkinson‘s Disease: A Promising Direction of Therapeutic Strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [CrossRef]
- Gudapati, K.; Singh, A.; Clarkson-Townsend, D.; Feola, A.J.; Allen, R.S. Behavioral Assessment of Visual Function via Optomotor Response and Cognitive Function via Y-Maze in Diabetic Rats. J. Vis. Exp. 2020, 2020, e61806. [Google Scholar] [CrossRef]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson‘s Disease: Its Role in Neuronal Death and Implications for Therapeutic Intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in Neuroinflammatory and Neurodegenerative Diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Ko, H.W.; Bok, E.; Park, E.S.; Huh, S.H.; Nam, J.H.; Jin, B.K. The Role of Neuroinflammation on the Pathogenesis of Parkinson‘s Disease. BMB Rep. 2010, 43, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and Oxidative Stress: Co-Conspirators in the Pathology of Parkinson‘s Disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Halliday, G.M.; Stevens, C.H. Glia: Initiators and Progressors of Pathology in Parkinson‘s Disease. Mov. Disord. 2011, 26, 6–17. [Google Scholar] [CrossRef]
- Gao, H.-M.; Liu, B.; Zhang, W.; Hong, J.-S. Critical Role of Microglial NADPH Oxidase-Derived Free Radicals in the in Vitro MPTP Model of Parkinson‘s Disease. FASEB J. 2003, 17, 1–22. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, I.S.; More, S.V.; Kim, B.W.; Bahk, Y.Y.; Choi, D.K. Gastrodin Protects Apoptotic Dopaminergic Neurons in a Toxin-Induced Parkinson‘s Disease Model. Evid.-Based. Complement. Alternat. Med. 2013, 2013, 514095. [Google Scholar] [CrossRef]
- Ogawa, N.; Hirose, Y.; Ohara, S.; Ono, T.; Watanabe, Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res. Commun. Chem. Pathol. Pharmacol. 1985, 50, 435–441. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-M.; Balakrishnan, R.; Ko, H.M.; Park, J.-Y.; Kumar, H.; Kim, B.; Yoon, S.-H.; Choi, D.-K. Neuroprotective Effects of Fluoxetine Derivative 4-[3-Oxo-3-(2-trifluoromethyl-phenyl)-propyl]-morpholinium Chloride (OTPM) as a Potent Modulator of Motor Deficits and Neuroinflammatory Pathways in LPS-Induced BV-2 Microglial Cells and MPTP-Induced Parkinsonian Models. Pharmaceuticals 2025, 18, 1799. https://doi.org/10.3390/ph18121799
Kang S-M, Balakrishnan R, Ko HM, Park J-Y, Kumar H, Kim B, Yoon S-H, Choi D-K. Neuroprotective Effects of Fluoxetine Derivative 4-[3-Oxo-3-(2-trifluoromethyl-phenyl)-propyl]-morpholinium Chloride (OTPM) as a Potent Modulator of Motor Deficits and Neuroinflammatory Pathways in LPS-Induced BV-2 Microglial Cells and MPTP-Induced Parkinsonian Models. Pharmaceuticals. 2025; 18(12):1799. https://doi.org/10.3390/ph18121799
Chicago/Turabian StyleKang, Seong-Mook, Rengasamy Balakrishnan, Hyun Myung Ko, Ju-Young Park, Hemant Kumar, Byungwook Kim, Sung-Hwa Yoon, and Dong-Kug Choi. 2025. "Neuroprotective Effects of Fluoxetine Derivative 4-[3-Oxo-3-(2-trifluoromethyl-phenyl)-propyl]-morpholinium Chloride (OTPM) as a Potent Modulator of Motor Deficits and Neuroinflammatory Pathways in LPS-Induced BV-2 Microglial Cells and MPTP-Induced Parkinsonian Models" Pharmaceuticals 18, no. 12: 1799. https://doi.org/10.3390/ph18121799
APA StyleKang, S.-M., Balakrishnan, R., Ko, H. M., Park, J.-Y., Kumar, H., Kim, B., Yoon, S.-H., & Choi, D.-K. (2025). Neuroprotective Effects of Fluoxetine Derivative 4-[3-Oxo-3-(2-trifluoromethyl-phenyl)-propyl]-morpholinium Chloride (OTPM) as a Potent Modulator of Motor Deficits and Neuroinflammatory Pathways in LPS-Induced BV-2 Microglial Cells and MPTP-Induced Parkinsonian Models. Pharmaceuticals, 18(12), 1799. https://doi.org/10.3390/ph18121799







