Therapeutic Evaluation of Alginate from Brown Seaweeds: A Comparative Study of Turbinaria ornata and Hormophysa cuneiformis
Abstract
1. Introduction
2. Results
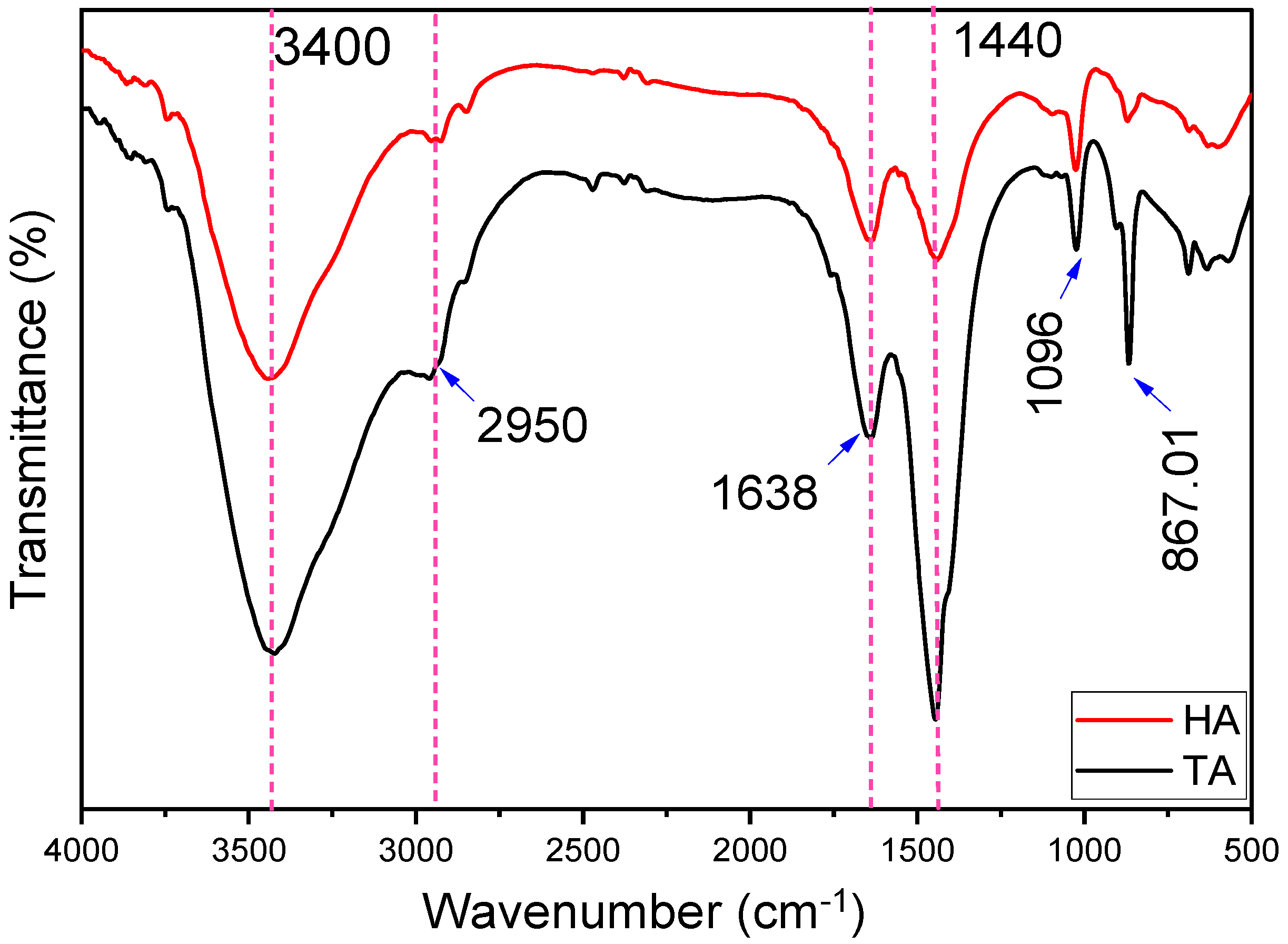
2.1. FTIR Characterization of Alginate Extracts
2.2. X-Ray Diffraction (XRD) Analysis of Alginate Extracted from Brown Algae
2.3. Thermal Stability and Decomposition Behavior of Alginates
2.4. Elemental Composition Analysis of the Extracted Alginate
2.5. Monosaccharide and Uronic Acid Composition of Alginate by HPLC
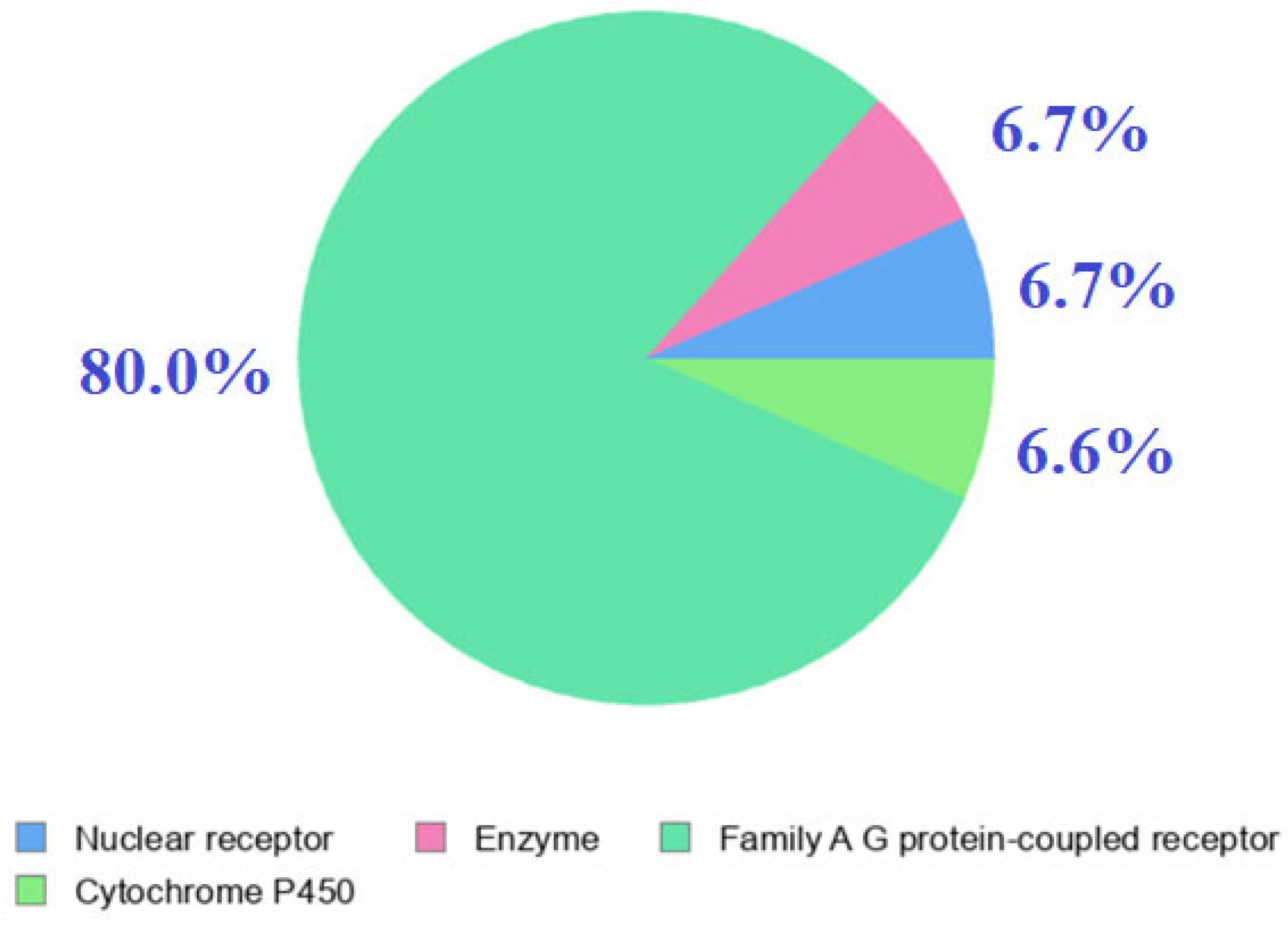
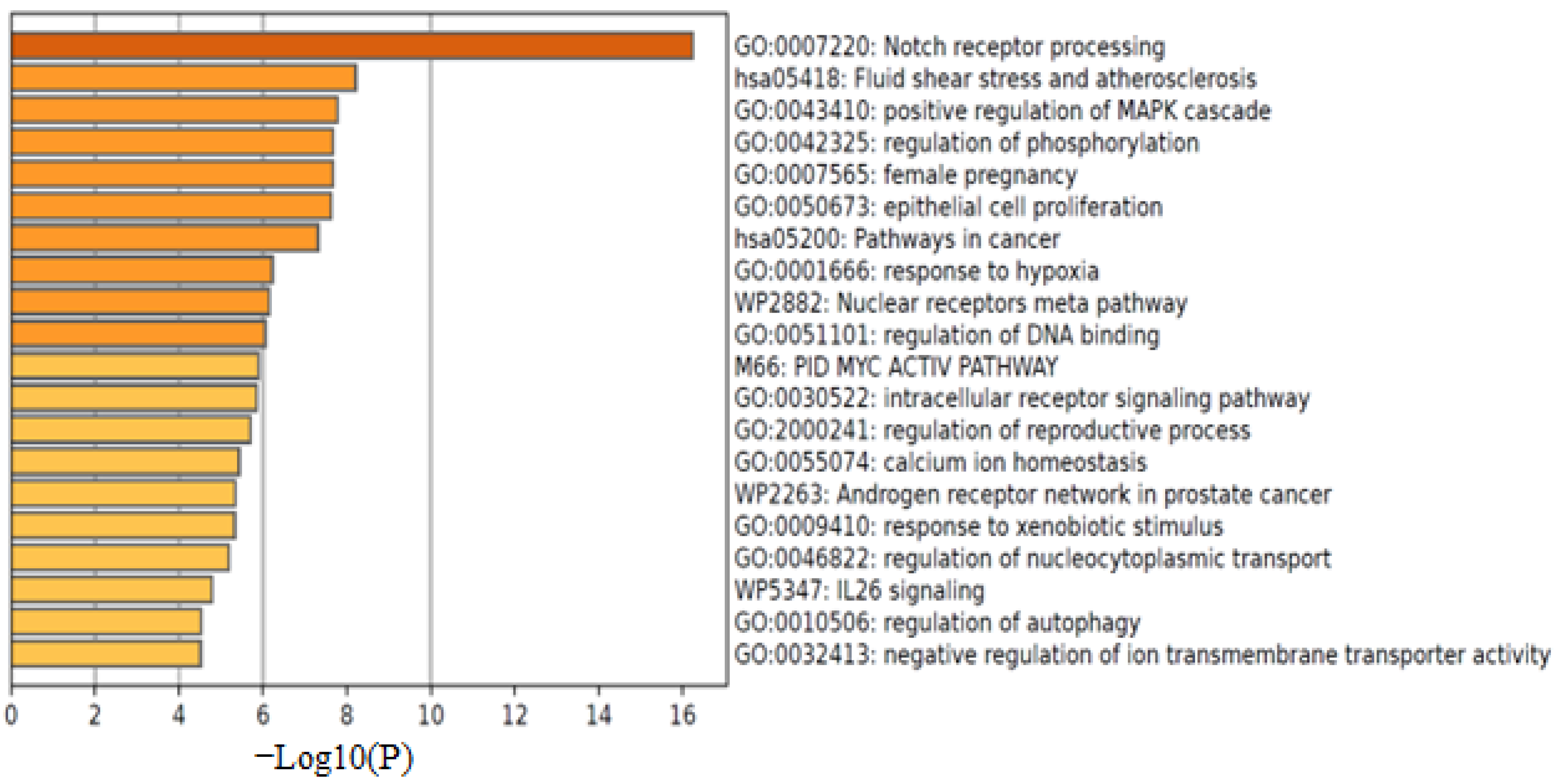
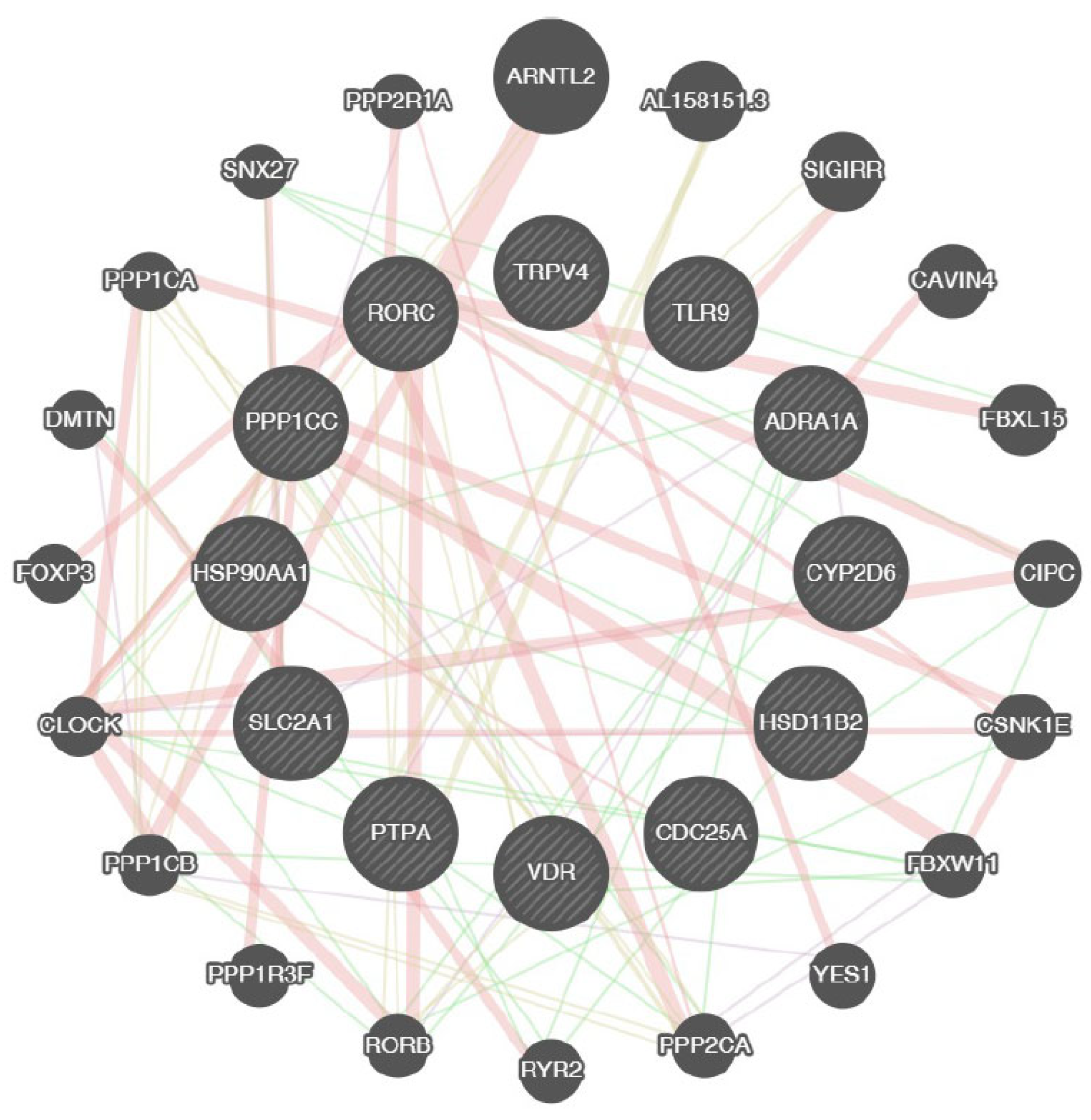
2.6. Computational Analysis
2.7. Butyl Choline Esterase Inhibitory Activity Assay
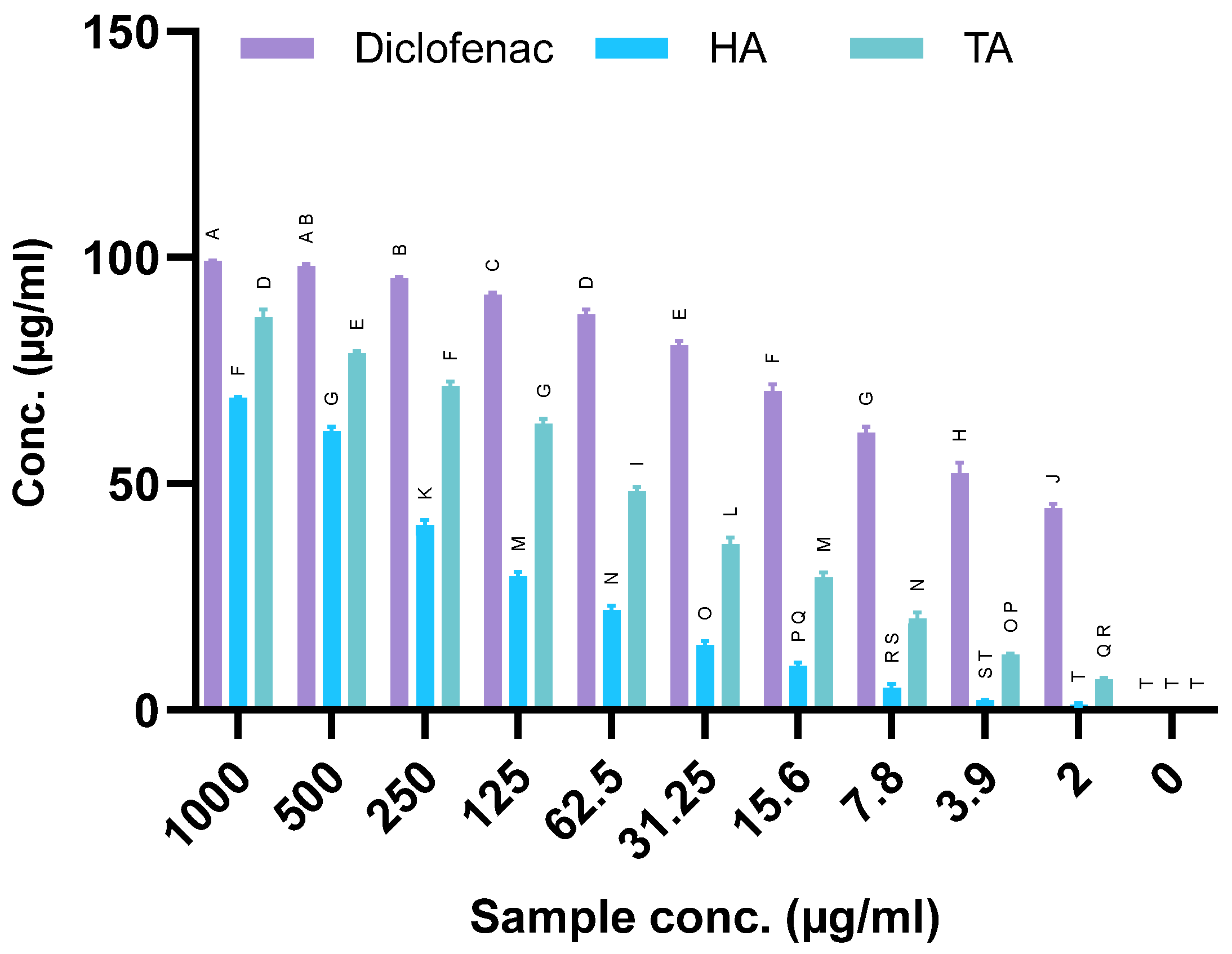
2.8. Anti-Inflammatory Activity
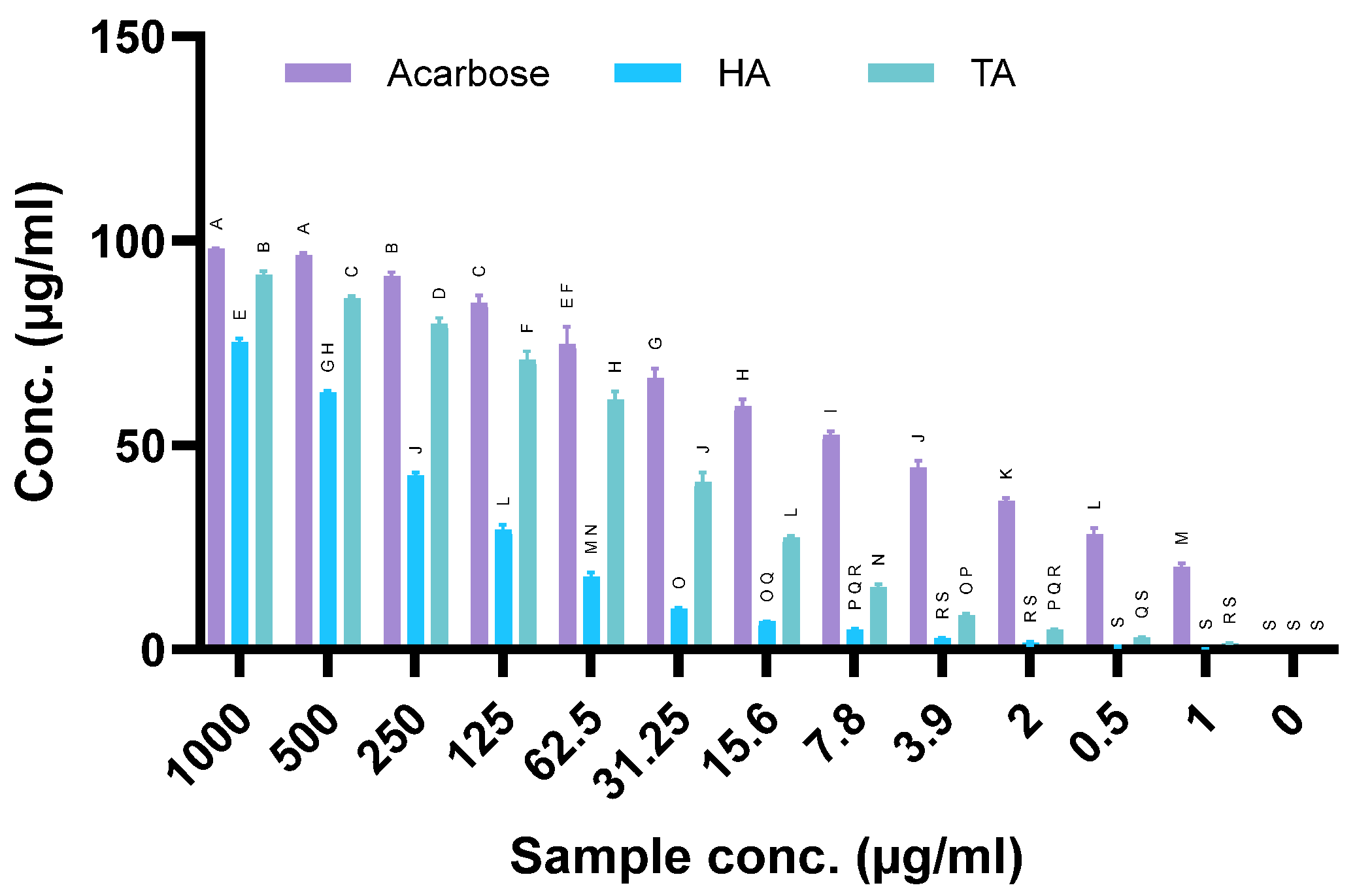
2.9. Antidiabetic Activity
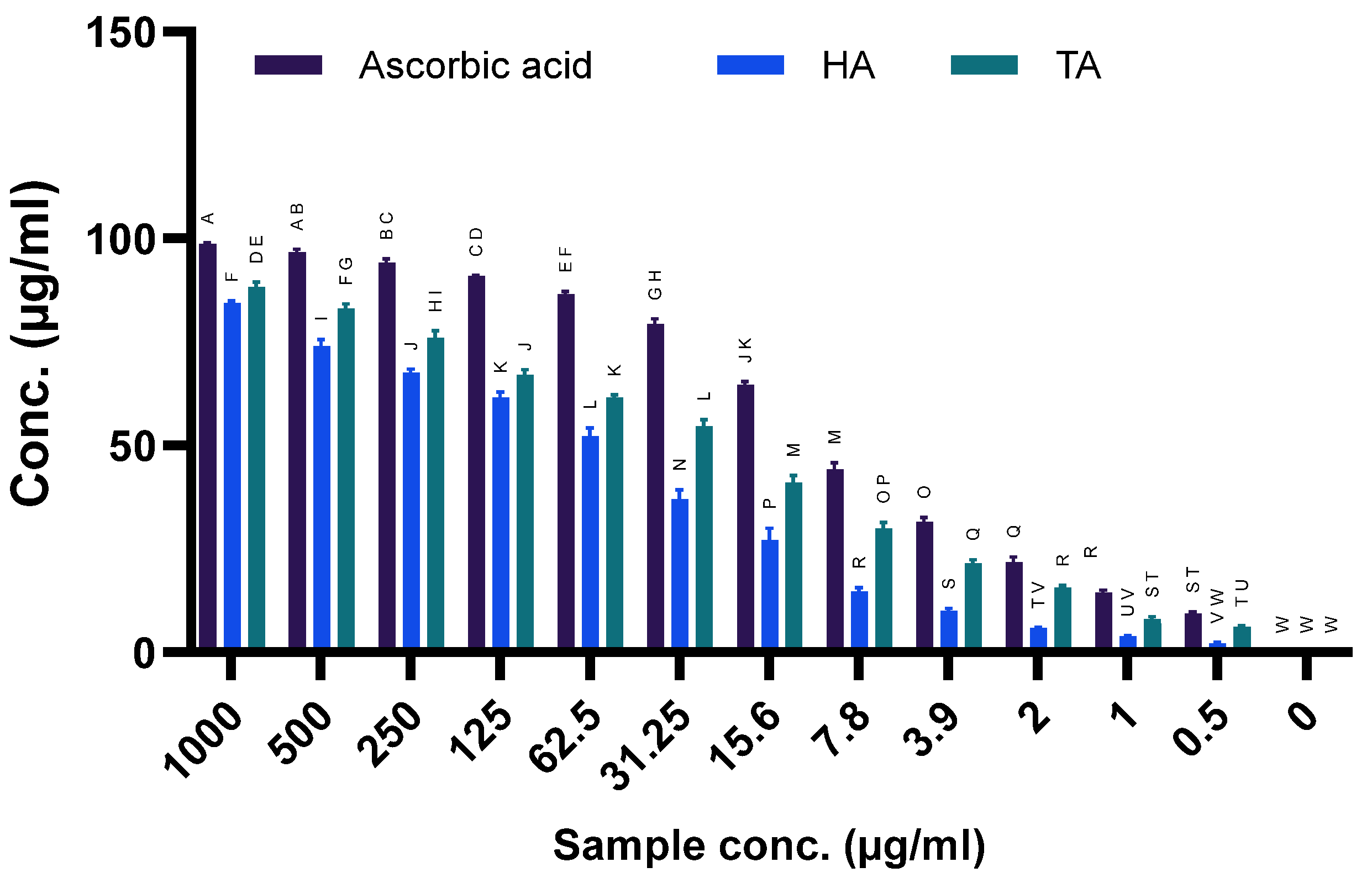
2.10. DDPH Scavenging
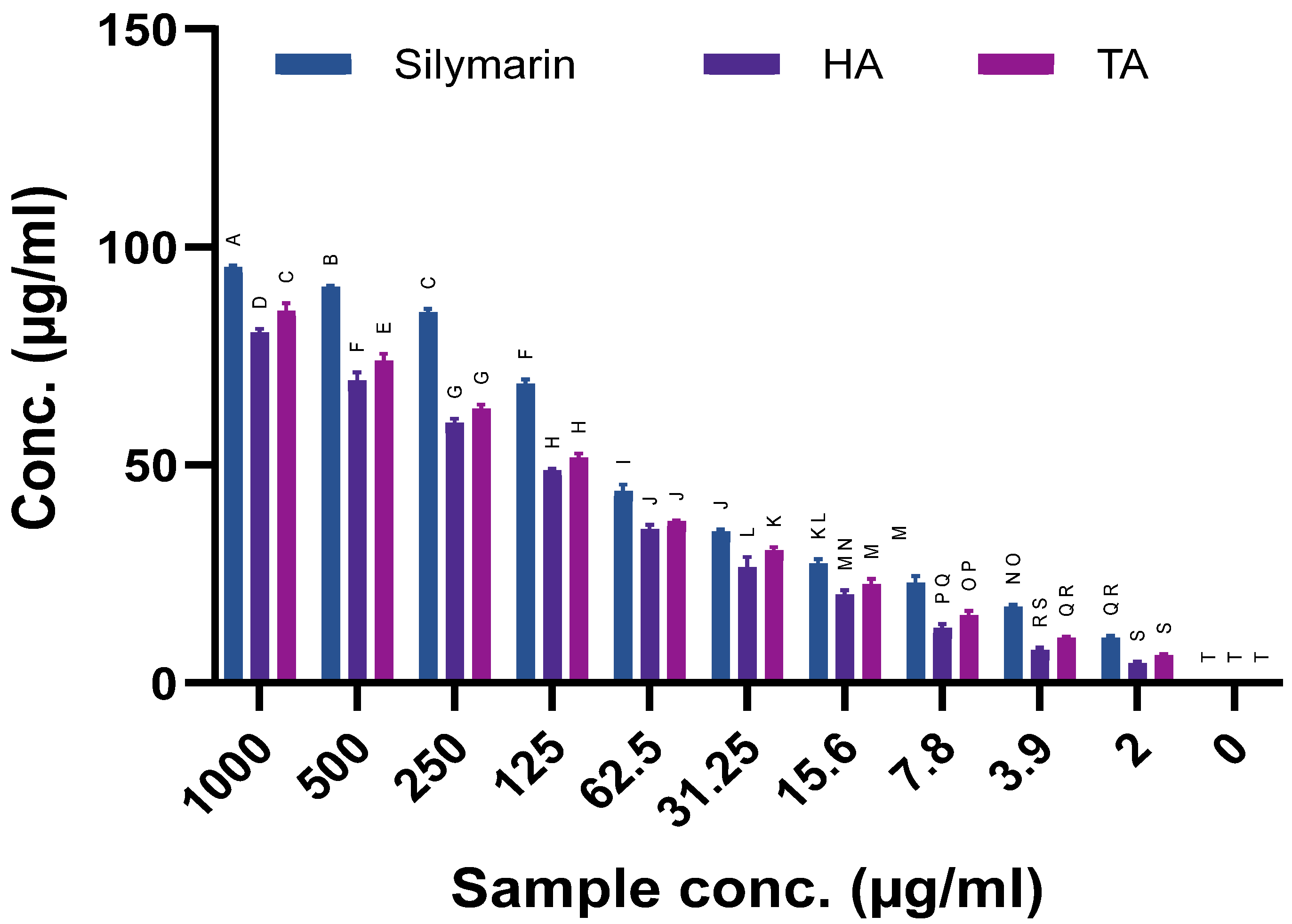
2.11. Hepatoprotective Activity by Alginate Extracted from Hormophysa cuneiformis and Turbinaria ornata
2.12. Comparative Potency Between Alginate Extracted from Hormophysa cuneiformis and Turbinaria ornata
3. Discussion
4. Materials and Methods
4.1. Collection of Marine Macroalgae
4.2. Extraction of Polysaccharides from Macroalgae
4.3. Physicochemical and Structural Characterization
4.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.3.2. X-Ray Diffraction (XRD) Analysis
4.3.3. Thermogravimetric Stability Assessment (TGA)
4.3.4. Elemental Composition Analysis
4.3.5. Monosaccharide Profiling via HPLC
4.3.6. Quantification of Uronic Acid Content
4.4. Computational Analysis
4.5. Butyl Choline Esterase Inhibitory Activity Assay
4.6. Anti-Inflammatory Activity
4.7. Antioxidant Assay
4.8. Antidiabetic Activity
4.9. Hepatoprotective Study in Hepatocytes Using MTT Assay
4.9.1. Isolation of Rat Hepatocytes
4.9.2. Experimental Design
4.9.3. MTT Viability Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| BChE | Butyrylcholinesterase |
| COX-1 | Cyclooxygenase-1 |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| DNSA | 3,5-Dinitrosalicylic acid |
| FTIR | Fourier Transform Infrared Spectroscopy |
| XRD | X-ray Diffraction |
| HPLC | High-Performance Liquid Chromatography |
| DTNB | 5,5-Dithiobis-(2-nitrobenzoic acid) |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| ROS | Reactive Oxygen Species |
| MOE | Molecular Operating Environment |
| GPX4 | Glutathione Peroxidase 4 |
| SOD1 | Superoxide Dismutase 1 |
| T. Alginate | Alginate from Turbinaria ornata |
| TGA | Thermogravimetric Analysis |
| Tris-HCl | Tris(hydroxymethyl)aminomethane Hydrochloride Buffer |
| UV | Ultraviolet |
| IC50 | Half Maximal Inhibitory Concentration |
| EC50 | Half Maximal Effective Concentration |
| PDB | Protein Data Bank |
| Akt | Protein Kinase B |
| Nrf2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| NF-κB | Nuclear Factor Kappa B |
| PI3K | Phosphoinositide 3-Kinase |
| HA | Hormophysa cuneiformis alginate |
| TA | Turbinaria ornata alginate |
References
- El-Sheekh, M.; Kassem, W.M.A.; Alwaleed, E.A.; Saber, H. Optimization and Characterization of Brown Seaweed Alginate for Antioxidant, Anticancer, Antimicrobial, and Antiviral Properties. Int. J. Biol. Macromol. 2024, 278, 134715. [Google Scholar] [CrossRef]
- Pandey, A.K.; Ezewudo, E.; Hoque, N.; Pandey, A.T.; Menon, S.; Simon, N.; Rasouli, B.; Habibi, E.; Sarker, S.D.; Nahar, L. A Review of Food Hydrocolloids on Cardiovascular Health: Alginate, Astragalus Polysaccharides, Carrageenan, Fucoidan, Lunasin, and Psyllium. Int. J. Biol. Macromol. 2025, 215, 144505. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Introductory Chapter: Alginates-A. In Alginates: Recent Uses of This Natural Polymer; IntechOpen: Rijeka, Croatia, 2020; p. 3. [Google Scholar]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Lorbeer, A.J.; Charoensiddhi, S.; Lahnstein, J.; Lars, C.; Franco, C.M.M.; Bulone, V.; Zhang, W. Sequential Extraction and Characterization of Fucoidans and Alginates from Ecklonia radiata, Macrocystis pyrifera, Durvillaea potatorum, and Seirococcus axillaris. J. Appl. Phycol. 2017, 29, 1515–1526. [Google Scholar] [CrossRef]
- Souto-Prieto, A.; Martinez-Sanz, M.; Ferreiro, T.; Parada-Pena, P.; Abuin-Arias, L.; Cobos, A.; Lopez-Sanchez, P. Insights into the Structuring Ability of Two Brown Seaweeds (Laminaria digitata and Saccharina latissima) for Applications as Natural Texturisers. Algal Res. 2024, 80, 103548. [Google Scholar] [CrossRef]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Fu, L.; Wang, Z.; Cao, Z.; Zhao, L.; Seswita-Zilda, D.; Zhang, A.; Zhang, Q.; Li, J. A Novel Bifunctional Alginate Lyase and Antioxidant Activity of the Enzymatic Hydrolysates. J. Agric. Food Chem. 2024, 72, 4116–4126. [Google Scholar] [CrossRef]
- Zhu, B.; Ni, F.; Xiong, Q.; Yao, Z. Marine Oligosaccharides Originated from Seaweeds: Source, Preparation, Structure, Physiological Activity and Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 60–74. [Google Scholar] [CrossRef]
- Colliec-Jouault, S.; Zykwinska, A. Marine Glycosaminoglycans (GAGs) and GAG-Mimetics: Applications in Medicine and Tissue Engineering. In Extracellular Sugar-Based Biopolymers Matrices; Springer: Berlin/Heidelberg, Germany, 2019; pp. 625–648. [Google Scholar]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of New Butyrylcholinesterase Inhibitors via Structure-Based Virtual Screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Sardana, S.; Gupta, R.; Madan, K.; Bisht, D.; Rana, V.S.; Bhargava, S.; Sethiya, N.K. Advance Drug Delivery and Combinational Drug Approaches for Hepatoprotective Action of Berberine: A Progressive Overview with Underlying Mechanism. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad002. [Google Scholar] [CrossRef]
- Ma, M.; Liu, Y.; Zhang, S.; Yuan, Y. A Comprehensive Review of Propylene Glycol Alginate in the Food Industry: Synthesis, Safety, Composite Hydrocolloids and Application. Trends Food Sci. Technol. 2025, 157, 104900. [Google Scholar] [CrossRef]
- Xiao, L.; Gong, H.; Tan, X.; Chen, P.; Yang, Y.; Zhu, H.; Zhong, S. Physicochemical Characterization and Antitumor Activity in Vitro of a Polysaccharide from Christia Vespertilionis. Int. J. Biol. Macromol. 2025, 290, 139095. [Google Scholar] [CrossRef] [PubMed]
- Savini, E. Design and Development of Biomineralized Nanostructured Devices from Natural Sources for Biomedical Applications. Ph.D. Thesis, Alma Mater Studiorum Università di Bologna, Bologna, Italy, 2016. [Google Scholar]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and Evaluation of Alginate, Gelatin, and Hyaluronic Acid Hybrid Hydrogels for Tissue Engineering Applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.-B.; Zuo, Y.; Lv, G.-Y.; Mu, Y.-H.; Li, H. Morphology, Hydrogen-Bonding and Crystallinity of Nano-Hydroxyapatite/Polyamide 66 Biocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 843–848. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, X.; Chen, J.; Kang, Z.; Ye, J.; Xie, D. Evolution of Phase, Morphology, Physicochemical Properties, and Biological Properties of HA Ceramic with the Increase of Crystallinity. Ceram. Int. 2024, 50, 33153–33163. [Google Scholar] [CrossRef]
- Tan, X.; Chen, P.; Xiao, L.; Gong, Z.; Qin, X.; Nie, J.; Zhu, H.; Zhong, S. Extraction, Purification, Structural Characterization, and Anti-Inflammatory Activity of a Polysaccharide from Lespedeza formosa. Int. J. Biol. Macromol. 2025, 300, 140154. [Google Scholar] [CrossRef]
- Osman, N.; Suliman, T.; Osman, K. Characterization of Native Alginates of Common Alginophytes from the Red Sea Coast of Sudan. Int. J. Second. Metab. 2020, 7, 266–274. [Google Scholar] [CrossRef]
- Rashedy, S.H.; Abd El Hafez, M.S.M.; Dar, M.A.; Cotas, J.; Pereira, L. Evaluation and Characterization of Alginate Extracted from Brown Seaweed Collected in the Red Sea. Appl. Sci. 2021, 11, 6290. [Google Scholar] [CrossRef]
- Ma, W.; Wei, S.; Peng, W.; Sun, T.; Huang, J.; Yu, R.; Zhang, B.; Li, W. Antioxidant Effect of Polygonatum sibiricum Polysaccharides in D-galactose-induced Heart Aging Mice. BioMed Res. Int. 2021, 2021, 6688855. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and Modification of Macroalgal Polysaccharides for Current and Next-Generation Applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef]
- Ross, A.B.; Hall, C.; Anastasakis, K.; Westwood, A.; Jones, J.M.; Crewe, R.J. Influence of Cation on the Pyrolysis and Oxidation of Alginates. J. Anal. Appl. Pyrolysis 2011, 91, 344–351. [Google Scholar] [CrossRef]
- Sun, J. Investigations on Biologically Active Carbohydrates from Natural Sources. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 2016. [Google Scholar]
- Donati, I.; Christensen, B.E. Alginate-Metal Cation Interactions: Macromolecular Approach. Carbohydr. Polym. 2023, 321, 121280. [Google Scholar] [CrossRef] [PubMed]
- ALNasser, M.N.; Alboraiy, G.M.; Alsowig, E.M.; Alqattan, F.M. Cholinesterase Inhibitors from Plants and Their Potential in Alzheimer’s Treatment: Systematic Review. Brain Sci. 2025, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liao, Y.; Tian, D.; Liao, J.; Chen, Q.; Yin, J. Small-Molecule Fluorescent Probes for Butyrylcholinesterase. ChemMedChem 2025, 20, e202400875. [Google Scholar] [CrossRef]
- Sang, Z.; Huang, S.; Tan, W.; Ban, Y.; Wang, K.; Fan, Y.; Chen, H.; Zhang, Q.; Liang, C.; Mi, J. Discovery of Novel Butyrylcholinesterase Inhibitors for Treating Alzheimer’s Disease. Acta Pharm. Sin. B 2025, 15, 2134–2155. [Google Scholar] [CrossRef]
- Jia, C.; Chai, J.; Zhang, S.; Sun, Y.; He, L.; Sang, Z.; Chen, D.; Zheng, X. The Advancements of Marine Natural Products in the Treatment of Alzheimer’s Disease: A Study Based on Cell and Animal Experiments. Mar. Drugs 2025, 23, 91. [Google Scholar] [CrossRef]
- Huang, J.; Ma, M.; Qin, M.; Li, X.; Ren, Y. Structural Characterization and Molecular Docking Studies of Fresh Coconut Meat Polysaccharides. Int. J. Mol. Sci. 2025, 26, 10222. [Google Scholar] [CrossRef]
- Pei, H.; He, Z.; Chen, W.; Zhao, Y.; Li, J.; Wang, R.; Zong, Y.; Du, R. Network Pharmacology and Molecular Docking Analysis on the Mechanism of Cordyceps Militaris Polysaccharide Regulating Immunity through TLR4/TNF-α Pathwayss. J. Biochem. Mol. Toxicol. 2023, 37, e23345. [Google Scholar] [CrossRef]
- Aksu, A.; Cetinkaya, S.; Yenidünya, A.F.; Çetinus, Ş.A.; Gezegen, H.; Tuezuen, B. Immobilization of Pectinase on Chitosan-Alginate-Clay Composite Beads: Experimental, DFT and Molecular Docking Studies. J. Mol. Liq. 2023, 390, 122947. [Google Scholar] [CrossRef]
- Sang, V.T.; Dai Hung, N.; Se-Kwon, K. Pharmaceutical Properties of Marine Polyphenols: An Overview. ACTA Pharm. Sci. 2019, 57, 217. [Google Scholar] [CrossRef]
- Vo, T.S.; Ngo, D.H.; Kim, S.-K. Brown Algal Polyphenol and Its Pharmaceutical Properties. In Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 223–243. [Google Scholar]
- Shafay, S.E.L.; El-Sheekh, M.; Bases, E.; El-Shenody, R. Antioxidant, Antidiabetic, Anti-Inflammatory and Anticancer Potential of Some Seaweed Extracts. Food Sci. Technol. 2021, 42, e20521. [Google Scholar] [CrossRef]
- Bases, E.; El-Sheekh, M.M.; El Shafay, S.M.; El-Shenody, R.; Nassef, M. Therapeutic Anti-Inflammatory Immune Potentials of Some Seaweeds Extracts on Chemically Induced Liver Injury in Mice. Sci. Rep. 2025, 15, 4370. [Google Scholar] [CrossRef] [PubMed]
- Newehy, A.S.E.; Gheda, S.F.; Ismail, M.M.; Aldisi, D.; Abulmeaty, M.M.A.; Elshobary, M.E. Fucoidan-Based Gold Nanoparticles: Antioxidant and Anticancer Potential from Turbinaria Decurrens and Sargassum Cinereum. Pharmaceutics 2025, 17, 826. [Google Scholar] [CrossRef]
- Barakat, K.M.; Ismail, M.M.; Abou El Hassayeb, H.E.; El Sersy, N.A.; Elshobary, M.E. Chemical Characterization and Biological Activities of Ulvan Extracted from Ulva Fasciata (Chlorophyta). Rend. Lincei. Sci. Fis. Nat. 2022, 33, 829–841. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Lotfali, E.; Bagheri, R.; Pircheraghi, G. Alginate-Based Multifunctional Films Incorporated with Sulfur Quantum Dots for Active Packaging Applications. Colloids Surf. B Biointerfaces 2022, 215, 112519. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Dima, Ş.; Apetrei, C. Determination of Antioxidant Capacity of Glutathione Encapsulated in Alginate Microcapsules Using Spectrophotometric and Electrochemical Methods. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135735. [Google Scholar] [CrossRef]
- Atya, M.E.; El-Hawiet, A.; Alyeldeen, M.A.; Ghareeb, D.A.; Abdel-Daim, M.M.; El-Sadek, M.M. In Vitro Biological Activities and in Vivo Hepatoprotective Role of Brown Algae-Isolated Fucoidans. Environ. Sci. Pollut. Res. 2021, 28, 19664–19676. [Google Scholar] [CrossRef]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and Antioxidant Activity of Quercetin Loaded Chitosan/Alginate Particles in Vitro and in Vivo in a Model of Paracetamol-Induced Toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Mohammed, C.; Lalgee, L.; Kistow, M.; Jalsa, N.; Ward, K. On the Binding Affinity and Thermodynamics of Sodium Alginate-Heavy Metal Ion Interactions for Efficient Adsorption. Carbohydr. Polym. Technol. Appl. 2022, 3, 100203. [Google Scholar] [CrossRef]
- Aleem, A.A. The Marine Algae of Alexandria, Egypt (139 P.); University of Alexandria: Alexandria, Egypt, 1993; pp. 1–55. [Google Scholar]
- Belous, O.S.; Kanaan, H. Marine Algae of the Lebanese Coast; Raidy Printing Group SAL: Fayadieh, Lebanon, 2015; ISBN 9953032491. [Google Scholar]
- Jha, B.; Reddy, C.R.K.; Thakur, M.C.; Rao, M.U. Seaweeds of India: The Diversity and Distribution of Seaweeds of Gujarat Coast; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 3, ISBN 9048124883. [Google Scholar]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2019. Available online: http://www.algaebase.org (accessed on 9 June 2019).
- Gomaa, M.; Fawzy, M.A.; Hifney, A.F.; Abdel-Gawad, K.M. Use of the Brown Seaweed Sargassum Latifolium in the Design of Alginate-Fucoidan Based Films with Natural Antioxidant Properties and Kinetic Modeling of Moisture Sorption and Polyphenolic Release. Food Hydrocoll. 2018, 82, 64–72. [Google Scholar] [CrossRef]
- Bojorges, H.; Martínez-Abad, A.; Martinez-Sanz, M.; Rodrigo, M.D.; Vilaplana, F.; López-Rubio, A.; Fabra, M.J. Structural and Functional Properties of Alginate Obtained by Means of High Hydrostatic Pressure-Assisted Extraction. Carbohydr. Polym. 2023, 299, 120175. [Google Scholar] [CrossRef] [PubMed]
- Yacou, C.; Altenor, S.; Carene, B.; Gaspard, S. Chemical Structure Investigation of Tropical Turbinaria Turbinata Seaweeds and Its Derived Carbon Sorbents Applied for the Removal of Hexavalent Chromium in Water. Algal Res. 2018, 34, 25–36. [Google Scholar] [CrossRef]
- Kendall, W.F., Jr.; Darrabie, M.D.; El-Shewy, H.M.; Opara, E.C. Effect of Alginate Composition and Purity on Alginate Microspheres. J. Microencapsul. 2004, 21, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Wekre, M.E.; Holmelid, B.; Underhaug, J.; Pedersen, B.; Kopplin, G.; Jordheim, M. Characterization of High Value Products in the Side-Stream of Laminaria Hyperborea Alginate Production-Targeting the Phenolic Content. Algal Res. 2023, 72, 103109. [Google Scholar] [CrossRef]
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: A promising therapeutic target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Alaa, A.-M.; El-Azab, A.S.; Abou-Zeid, L.A.; ElTahir, K.E.H.; Abdel-Aziz, N.I.; Ayyad, R.R.; Al-Obaid, A.M. Synthesis, Anti-Inflammatory, Analgesic and COX-1/2 Inhibition Activities of Anilides Based on 5, 5-Diphenylimidazolidine-2, 4-Dione Scaffold: Molecular Docking Studies. Eur. J. Med. Chem. 2016, 115, 121–131. [Google Scholar]
- Gomha, S.M.; Muhammad, Z.A.; Abdel-aziz, M.R.; Abdel-aziz, H.M.; Gaber, H.M.; Elaasser, M.M. One-pot Synthesis of New Thiadiazolyl-pyridines as Anticancer and Antioxidant Agents. J. Heterocycl. Chem. 2018, 55, 530–536. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; El-Shishtawy, R.M.; Elaasser, M.M.; Asiri, A.M. Synthesis of Novel Chalcone-Based Phenothiazine Derivatives as Antioxidant and Anticancer Agents. Molecules 2020, 25, 4566. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Elkomy, N.M.I.M.; El-Shaibany, A.; Elnagar, G.M.; Abdelkhalek, A.S.; Al-Mahbashi, H.; Elaasser, M.M.; Raweh, S.M.; Aldiyarbi, M.A.; Raslan, A.E. Evaluation of Acute Oral Toxicity, Anti-Diabetic and Antioxidant Effects of Aloe Vera Flowers Extract. J. Ethnopharmacol. 2023, 309, 116310. [Google Scholar] [CrossRef]
- Reese, J.A.; Byard, J.L. Isolation and Culture of Adult Hepatocytes from Liver Biopsies. In Vitro 1981, 17, 935–940. [Google Scholar] [CrossRef]
- Wilson, A.P. Cytotoxicity and Viability Assays. In Animal Cell Culture: A Practical Approach; Oxford University Press: Oxford, UK, 2000; pp. 175–220. [Google Scholar]

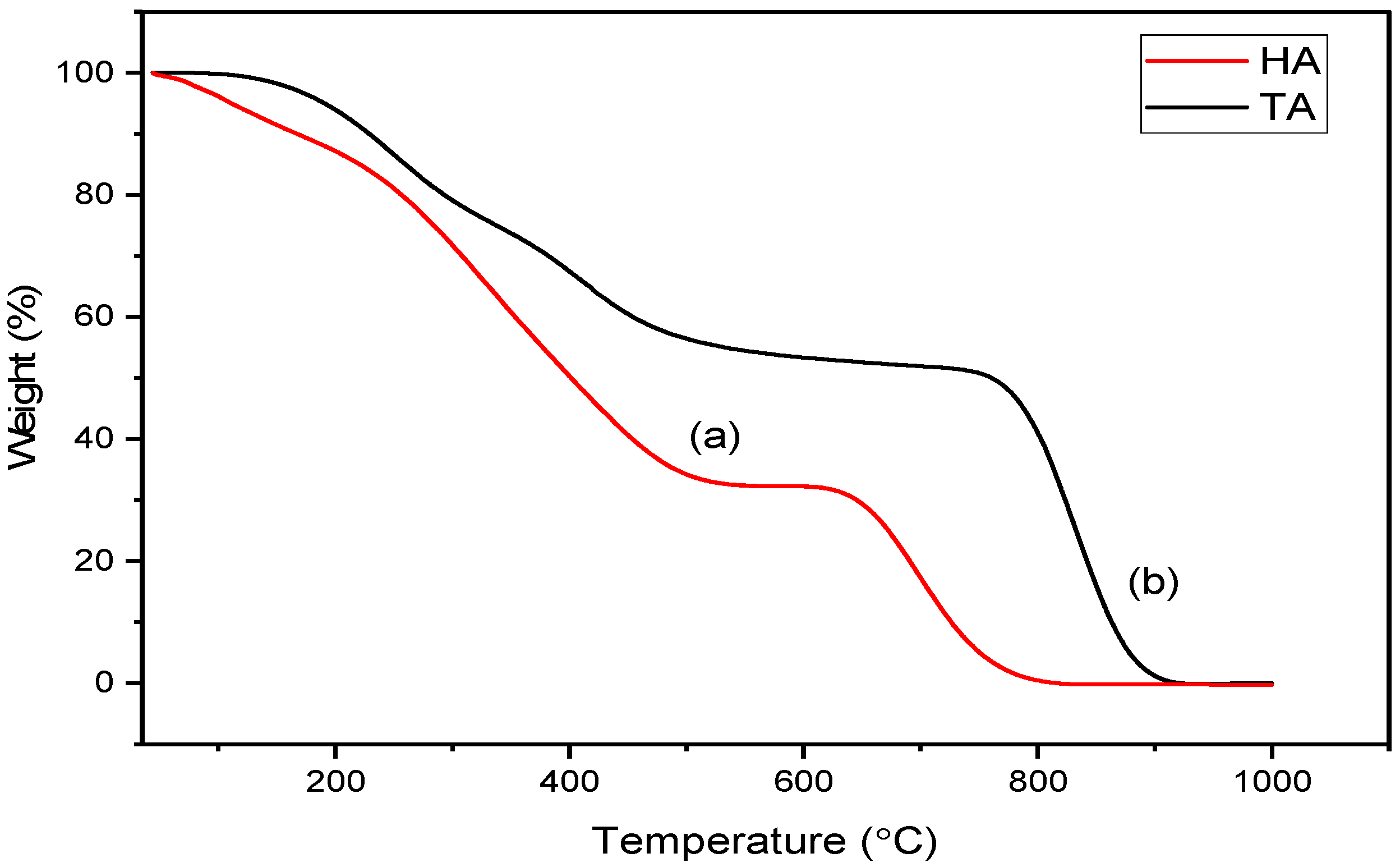

| Samples | 1st Stage (°C) | Weight Loss 1 (%) | 2nd Stage (°C) | Weight Loss 2 (%) | 3rd Stage (°C) | Weight Loss 3 (%) | Total Weight Loss (%) |
|---|---|---|---|---|---|---|---|
| HA | 45–167 | 8.95 | 168–581 | 52.41 | 582–996 | 29.57 | 90.93 |
| TA | 46–338 | 22.92 | 338–654 | 20.42 | 655–989 | 47.95 | 90.79 |
| Sample | C% | H% | N% | S% |
|---|---|---|---|---|
| HA | 11.98 | 2.16 | 2.69 | 0.97 |
| TA | 11.19 | 2.29 | 5.19 | 1.63 |
| Sugar | HA (µg/g) | TA (µg/g) |
|---|---|---|
| Rhamnose | 4.78 | 3.55 |
| Galactose | 7.74 | 6.37 |
| Fucose | 3.23 | 4.71 |
| Fructose | 4.02 | - |
| Glucose | 11.32 | 2.84 |
| Mannose | 4.39 | 3.01 |
| Uronic acid | 10.69 | 11.85 |
| Alginate Target Proteins | Common Name | Uniport ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| Alginate | |||||
| P-glycoprotein 1 | ABCB1 | P08183 | CHEMBL4302 | Primary active transporter | 0.11573667 |
| Gamma-secretase | PSEN2 PSENEN NCSTN APH1A PSEN1 APH1B | P49810 Q9NZ42 Q92542 Q96BI3 P49768 Q8WW43 | CHEMBL2094135 | Protease | 0.11573667 |
| Protein kinase C alpha | PRKCA | P17252 | CHEMBL299 | Kinase | 0.11573667 |
| Kappa Opioid receptor | OPRK1 | P41145 | CHEMBL237 | Family A G protein-coupled receptor | 0.11573667 |
| Protein kinase C delta (by homology) | PRKCD | Q05655 | CHEMBL2996 | Kinase | 0.11573667 |
| Cytochrome P450 19A1 | CYP19A1 | P11511 | CHEMBL1978 | Cytochrome P450 | 0.11573667 |
| Serotonin 2b (5-HT2b) receptor | HTR2B | P41595 | CHEMBL1833 | Family A G protein-coupled receptor | 0.11573667 |
| Alpha-2a adrenergic receptor | ADRA2A | P08913 | CHEMBL1867 | Family A G protein-coupled receptor | 0.11573667 |
| Adrenergic receptor alpha-2 | ADRA2C | P18825 | CHEMBL1916 | Family A G protein-coupled receptor | 0.11573667 |
| Alpha-2b adrenergic receptor | ADRA2B | P18089 | CHEMBL1942 | Family A G protein-coupled receptor | 0.11573667 |
| Dopamine D1 receptor | DRD1 | P21728 | CHEMBL2056 | Family A G protein-coupled receptor | 0.11573667 |
| Dopamine D2 receptor | DRD2 | P14416 | CHEMBL217 | Family A G protein-coupled receptor | 0.11573667 |
| Alpha-1d adrenergic receptor | ADRA1D | P25100 | CHEMBL223 | Family A G protein-coupled receptor | 0.11573667 |
| Serotonin 2a (5-HT2a) receptor | HTR2A | P28223 | CHEMBL224 | Family A G protein-coupled receptor | 0.11573667 |
| Serotonin 2c (5-HT2c) receptor | HTR2C | P28335 | CHEMBL225 | Family A G protein-coupled receptor | 0.11573667 |
| Dopamine D3 receptor | DRD3 | P35462 | CHEMBL234 | Family A G protein-coupled receptor | 0.11573667 |
| Cytochrome P450 2D6 | CYP2D6 | P10635 | CHEMBL289 | Cytochrome P450 | 0.11573667 |
| Serotonin 6 (5-HT6) receptor | HTR6 | P50406 | CHEMBL3371 | Family A G protein-coupled receptor | 0.11573667 |
| Alpha-1a adrenergic receptor (by homology) | ADRA1A | P35348 | CHEMBL229 | Family A G protein-coupled receptor | 0.11573667 |
| Serotonin 1b (5-HT1b) receptor (by homology) | HTR1B | P28222 | CHEMBL1898 | Family A G protein-coupled receptor | 0.11573667 |
| Transient receptor potential cation channel subfamily V member 4 (by homology) | TRPV4 | Q9HBA0 | CHEMBL3119 | Voltage-gated ion channel | 0.11573667 |
| Protein phosphatase 2C alpha | PPM1A | P35813 | CHEMBL2437 | Phosphatase | 0.11573667 |
| Glucose transporter | SLC2A1 | P11166 | CHEMBL2535 | Electrochemical transporter | 0.11573667 |
| Brain adenylate cyclase 1 | ADCY1 | Q08828 | CHEMBL2899 | Enzyme | 0.11573667 |
| Protein phosphatase 2C beta | PPM1B | O75688 | CHEMBL2845 | Phosphatase | 0.11573667 |
| Protein-tyrosine phosphatase 1B | PTPN1 | P18031 | CHEMBL335 | Phosphatase | 0.11573667 |
| Serine/threonine protein phosphatase PP1-gamma catalytic subunit | PPP1CC | P36873 | CHEMBL4438 | Phosphatase | 0.11573667 |
| Crystal Structure of Human Butyryl Cholinesterase in Complex with a Choline Molecule (PDB: 1P0M) | ||||
|---|---|---|---|---|
| Compound/ Drug | Docking Score (kcal/mol) | Amino Acids Involved in Binding | 2D | 3D |
| Rivastigmine (Control) | −6.6908 kcal/mol | GLY 117 (A) H-acceptor | 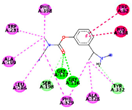 | 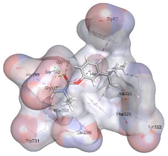 |
| Alginate | −7.5459 kcal/mol | HIS 438 (A) ionic | 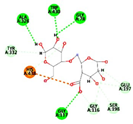 |  |
| Structure of human pancreatic alpha-amylase in complex with the carbohydrate inhibitor acarbose (PDB: 1b2y) | ||||
| Co-crystalized ligand | −8.5414 kcal/mol | GLU 233 (A) H-donor LYS 200 (A) H-acceptor GLN 63 (A) H-acceptor TRP 59 (A) H-pi TRP 59 (A) H-pi | 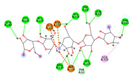 | 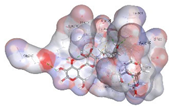 |
| Acarbose (Control) | −8.8135 kcal/mol | HIS 201 (A) H-donor GLU 233 (A) H-donor GLU 233 (A) H-donor ASP 197 (A) H-donor ASP 300 (A) H-donor ASP 197 (A) H-donor TRP 59 (A) H-donor THR 163 (A) H-donor LYS 200 (A) H-acceptor ARG 195 (A) H-acceptor HIS 299 (A) H-acceptor HIS 305 (A) H-acceptor GLN 63 (A) H-acceptor GLU 233 (A) ionic GLU 233 (A) ionic ASP 300 (A) ionic TYR 62 (A) H-pi | 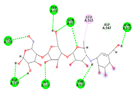 | 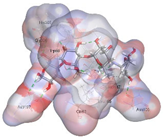 |
| Alginate | −6.5440 kcal/mol | ASP 197 (A) H-donor ASP 197 (A) H-donor ASP 300 (A) H-donor HIS 305 (A) H-acceptor HIS 305 (A) ionic HIS 305 (A) ionic | 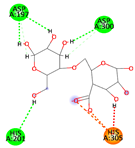 | 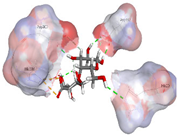 |
| Human COX-1 Crystal Structure (PDB: 6Y3C) | ||||
| Co-crystalized ligand | −4.1243 kcal/mol | ARG 83 (A) H-acceptor ARG 83 (A) H-acceptor ARG 83 (A) ionic ARG 83 (A) ionic ARG 120 (A) ionic ARG 120 (A) ionic | 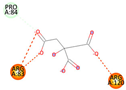 | 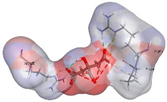 |
| Aspirin (Control) | −4.8185 kcal/mol | ARG 120 (A) ionic | 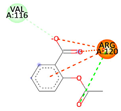 | 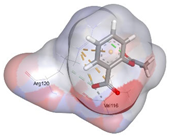 |
| Alginate | −6.1609 kcal/mol | GLU 524 (A) H-donor PRO 84 (A) H-donor ARG 120 (A) H-acceptor ARG 83 (A) H-acceptor ARG 83 (A) H-acceptor ARG 120 (A) ionic ARG 120 (A) ionic ARG 120 (A) ionic | 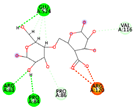 | 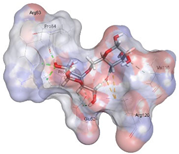 |
| Crystal Structure of human Superoxide Dismutase I (hSOD1) in complex with a napthalene-catechol linked compound (PDB: 5YTO) | ||||
| Co-crystalized ligand | −3.6198 kcal/mol | GLN 15 (D) H-donor GLY 10 (D) H-donor | 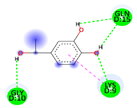 | 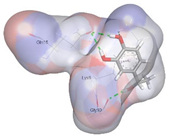 |
| Vitamin C (control) | −3.9119 kcal/mol | ASP 11 (D) H-donor GLY 12 (D) H-donor GLY 10 (D) H-acceptor | 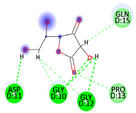 | 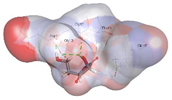 |
| Alginate | −5.0492 kcal/mol | GLY 10 (D) H-donor ASN 53 (D) H-acceptor LYS 9 (D) ionic | 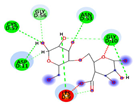 | 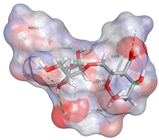 |
| Crystal structure of human GPX4 in complex with GXpep-1 (PDB: 5H5Q) | ||||
| Vitamin C (control) | −3.7975 kcal/mol | ASP 128 (A) H-donor ASP 128 (A) H-donor LYS 58 (A) H-acceptor |  | 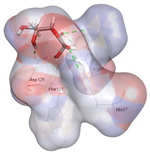 |
| Alginate | −4.7917 kcal/mol | HIS 42 (A) H-donor ARG 60 (A) H-acceptor ARG 60 (A) ionic | 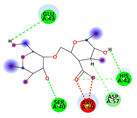 | 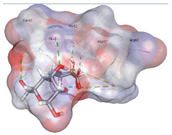 |
| Activity | H. cuneiformis Alginate (IC50 µg/mL) | T. ornata Alginate (IC50 µg/mL) | More Potent |
|---|---|---|---|
| BChE Inhibition | 39.01 | 107.38 | HA |
| COX-1 Inhibition | 360.22 | 69.61 | TA |
| α-Amylase Inhibition | 341.48 | 45.14 | TA |
| Antioxidant Activity | 58.22 | 25.89 | TA |
| Hepatoprotective Activity | 138.36 | 118.21 | TA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Sheekh, M.M.; Bases, E.; El Shafay, S.M.; El-Shenody, R.A.; Elshobary, M.E.; Abdel Wahab, A.H.A.; Yousuf, W.E.; Essa, D.I.; Alkafaas, S.S. Therapeutic Evaluation of Alginate from Brown Seaweeds: A Comparative Study of Turbinaria ornata and Hormophysa cuneiformis. Pharmaceuticals 2025, 18, 1720. https://doi.org/10.3390/ph18111720
El-Sheekh MM, Bases E, El Shafay SM, El-Shenody RA, Elshobary ME, Abdel Wahab AHA, Yousuf WE, Essa DI, Alkafaas SS. Therapeutic Evaluation of Alginate from Brown Seaweeds: A Comparative Study of Turbinaria ornata and Hormophysa cuneiformis. Pharmaceuticals. 2025; 18(11):1720. https://doi.org/10.3390/ph18111720
Chicago/Turabian StyleEl-Sheekh, Mostafa M., Eman Bases, Shimaa M. El Shafay, Rania A. El-Shenody, Mostafa E. Elshobary, Abdel Hady A. Abdel Wahab, Wesam E. Yousuf, Dorya I. Essa, and Samar Sami Alkafaas. 2025. "Therapeutic Evaluation of Alginate from Brown Seaweeds: A Comparative Study of Turbinaria ornata and Hormophysa cuneiformis" Pharmaceuticals 18, no. 11: 1720. https://doi.org/10.3390/ph18111720
APA StyleEl-Sheekh, M. M., Bases, E., El Shafay, S. M., El-Shenody, R. A., Elshobary, M. E., Abdel Wahab, A. H. A., Yousuf, W. E., Essa, D. I., & Alkafaas, S. S. (2025). Therapeutic Evaluation of Alginate from Brown Seaweeds: A Comparative Study of Turbinaria ornata and Hormophysa cuneiformis. Pharmaceuticals, 18(11), 1720. https://doi.org/10.3390/ph18111720









