Polyphenols as Modulators of Gastrointestinal Motility: Mechanistic Insights from Multi-Model Studies
Abstract
1. Introduction
2. Methods
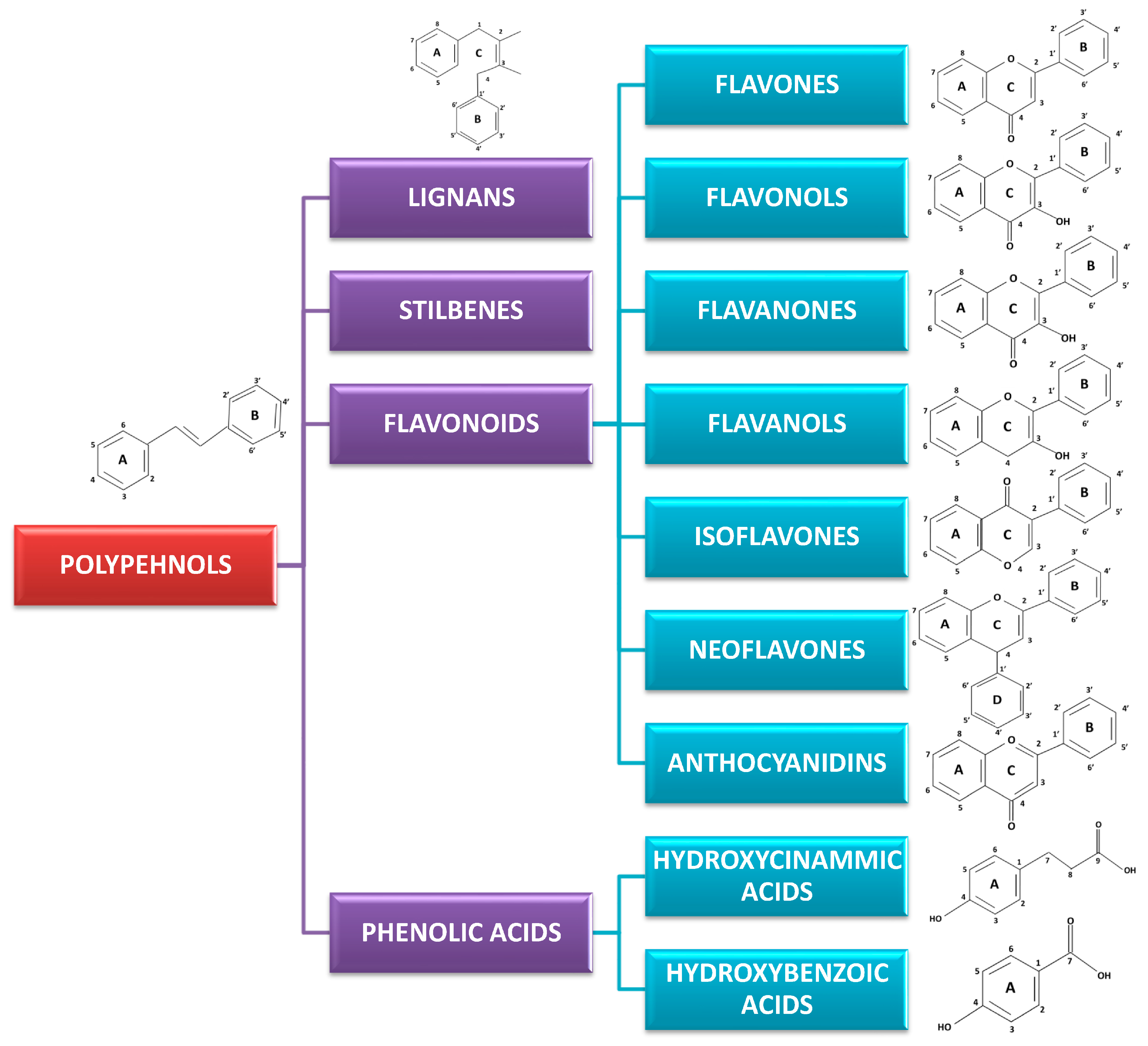
3. Classification and Chemistry of Polyphenols
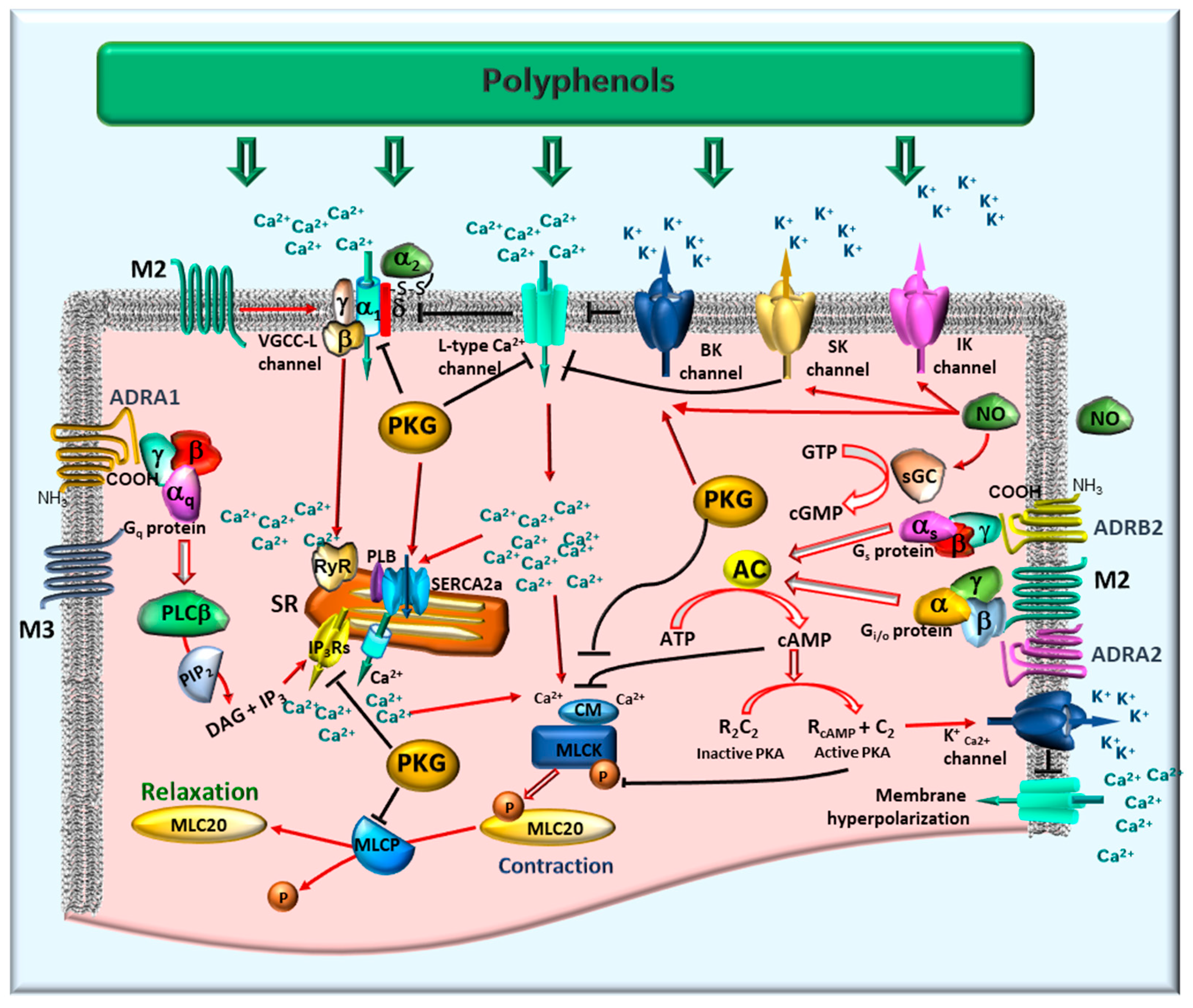
4. Mechanisms of Action of Polyphenols on Gastrointestinal Smooth Muscle
4.1. Excitation-Contraction Coupling and the Role of Calcium
4.2. Relaxation Via Potassium Channels and Membrane Hyperpolarization
- Large-conductance calcium-activated potassium (BK) channels: Sensitive to both membrane depolarization and elevations in intracellular calcium.
- ATP-sensitive potassium (KATP) channels: Functionally link cellular metabolic status to membrane potential.
- Voltage-gated and inward rectifier potassium channels: Contribute significantly to the maintenance of basal tone and electrical excitability.
4.3. Regulation by Nitric Oxide and the cGMP Pathway
4.4. Modulation of Contractile Machinery: MLCK and MLCP
4.5. Neurotransmitter Receptor-Mediated Modulation
- Adrenergic receptors (ADRA): These receptors can elicit either relaxation or contraction depending on their specific distribution and subtype. ADRA1 receptors typically promote contraction and sphincter tone. In contrast, ADRA2 receptors inhibit neurotransmitter release, thereby diminishing contraction, while ADRB2 receptors mediate smooth muscle relaxation. Collectively, these subtypes regulate gut motility and tone in response to sympathetic innervation [28].
- Muscarinic acetylcholine receptors (mAChRs): The M2 and M3 subtypes are predominantly involved in intestinal smooth muscle. M3 receptors serve as the principal mediators of smooth muscle contraction. Their activation, coupled to Gq/11 proteins, stimulates phosphoinositide hydrolysis, leading to the production of inositol trisphosphate (IP3). IP3 then triggers Ca2+ release from the sarcoplasmic reticulum (SR), elevating intracellular Ca2+ concentration. This Ca2+ increase activates calmodulin and, consequently, MLCK, thereby promoting MLC20 phosphorylation and initiating contraction [36]. M2 receptors constitute approximately 80% of muscarinic receptors in GI smooth muscle. Coupled to Gi/o proteins, they primarily inhibit adenylate cyclase (AC), resulting in reduced cyclic AMP levels. Although M2 receptors do not directly induce contraction, they enhance the contractile response by increasing the Ca2+ sensitivity of the contractile apparatus and modulating ion channel activity, specifically by inhibiting K+ currents and modulating VGCCs. The synergistic activation of M2 and M3 receptors leads to membrane depolarization via the generation of non-selective cationic and chloride currents, further facilitating Ca2+ influx and contraction [36]. Significantly, the contractile contribution of M2 receptors is conditional on M3 receptor activation; antagonism of M3 receptors typically abolishes the contractile response, indicating that M3 receptors are indispensable for initiating contraction, whereas M2 receptors serve to modulate and sustain it [37].
- Upper GI smooth muscle of porcine expresses both M2 and M3, with variable ratios in different GI regions [38]. The coexistence of M2 and M3 receptors in the intestine ensures a balanced cholinergic control [39]. Immunohistochemical studies and mRNA expression analysis in colonic samples confirmed the presence of M1, M2, and M3 receptors, with M2 and M3 immunoreactivity particularly abundant in circular and longitudinal muscle layers, as well as within elements of the enteric nervous system [40]. The functional significance of M2 receptors in the GI tract has been supported by pharmacological studies and animal models, which demonstrate that M2, together with M3, contributes to cholinergic control of intestinal smooth muscle contraction and serves as a presynaptic autoreceptor modulating acetylcholine release [36].
- Opioid receptors (μ, δ, κ) play an important role in modulating smooth muscle contraction and GI motility, primarily leading to the inhibition of both motility and secretion [44].
4.6. Inflammatory and Oxidative Stress Modulation
5. Effects by Flavonoid Subclass: Unraveling Structure-Activity Relationships in GI Motility Modulation
| Compound | Tissue/Model | Main Effect/Mechanism | EC50 and/or Reported Concentration | Reference |
|---|---|---|---|---|
| Luteolin | Mouse colon/Murine model (ex vivo) | Relaxation/L-type Ca2+ channel antagonism | 10–30 µM | [10] |
| Apigenin | Mouse stomach; guinea pig ileum (ex vivo) | Relaxation/Ca2+ channel antagonism | not specified | [49] |
| 12.5 µM | [50] | |||
| 1.02 µM/0.1–100 µM | [9] | |||
| Naringenin | Rat colon (ex vivo); Rat uterus (in vivo); Guinea pig intestinal (ex vivo) | Relaxation/BK activation, hyperpolarization | 0.1–20 µM | [19] |
| 46.3 µM/1–1000 μM | [14] | |||
| not specified | [51] | |||
| Hesperidin | Rat ileum/cecum (in vivo and ex vivo) | Contraction/MLCK activation, MLC20-P activation, COX-2/iNOS inhibition | 2.5–160 µM | [15] |
| not specified | [52] | |||
| Hesperetin | Rat jejunum (ex vivo) | Relaxation/KATP and NO pathway, prostaglandin modulation | 10–100 µM | [53] |
| Quercetin | Guinea pig intestine, Mouse stomach, Human gastric strips (ex vivo); Rat colon and ileum (ex vivo) | Relaxation/Ca2+ channel antagonism, NO/opioid signaling; directly through KATP channels | 10–300 µM | [19] |
| 4.3 µM/0.1–100 µM | [9] | |||
| 150–300 µM | [12] | |||
| 5.4 µM/0.1–100 µM | [13] | |||
| Kaempferol | Rat colon, jejunum, trachea, bladder (in silico, in vivo, ex vivo) | Relaxation/Ca2+ channel antagonism, KV channels | not specified | [11] |
| 150–300 µM | [12] | |||
| not specified | [54] | |||
| Myricetin | Rat colon and ileum (ex vivo) | Relaxation/Ca2+ channel blockade KATP channel activation | 150–300 µM | [12] |
| not specified | [11] | |||
| Isorhamnetin | Rat jejunum, bladder, rat ileum (in silico, ex vivo, and in vivo) | Relaxation/Ca2+ antagonism | not specified | [55] |
| Catechin | Rabbit jejunum, Mouse stomach; Rat fundus (ex vivo); Humans (in vivo) | Relaxation/Ca2+ channel blockade, NO generation | 7.39 mM/1–30 mM | [16] |
| 13 µM/0.1–100 µM | [9] | |||
| 20 µM | [56] | |||
| Epicatechin | Rat jejunum (ex vivo, in vivo) | Relaxation/Ca2+ channel antagonism, MLCK binding | not specified | [17] |
| Daidzein | Rat jejunum; Guinea pig stomach (ex vivo) | Relaxation/Ca2+ channel antagonism, adrenergic signaling | 1–300 µM | [57] |
| 5–160 μM | [18] | |||
| Genistein | Guinea pig intestine, Mouse stomach (ex vivo) | Relaxation/Excitation-contraction uncoupling | 10–300 µM | [19] |
| 1.5 µM/0.1–100 µM | [9] | |||
| Formononetin | Rat aorta (ex vivo) | Relaxation/Endothelium/NO-dependent mechanism and -independent via BK, KATP activation | 0.1–100 μM | [58] |
| Pelargonidin | Endothelial cells (ex vivo) | Relaxation/NO release from the endothelium | not specified | [59] |
| Cyanidin-3-O-rutinoside | Rat ileum (ex vivo) | Relaxation/VGCC blockade or interference with intracellular Ca2+ mobilization | 3.23 mg/mL 0.01–3 mg/mL | [60] |
| Resveratrol | Rat uterus; Rat interstinal artery; Human gastric strips (ex vivo) | Relaxation/BK activation; L-type Ca2+ inhibition | 0.01–50 μM | [26] |
| not specified | [61] | |||
| 178 μM/0.1–100 µM | [25] | |||
| Dibenzocyclooctadiene lignans | Guinea pig ileum (ex vivo) | Relaxation/muscarinic receptors, intracellular Ca2+ mobilization and L-type Ca2+ inhibition | EC20 = 2.2 μM EC50 = 6.6 μM | [62] |
| Arctigenin | Rat ileum (ex vivo) | Relaxation/L-type Ca2+ inhibition | 1–40 μM | [63] |
| Trachelogenin | Rat ileum (ex vivo) | Relaxation/L-type Ca2+ inhibition | 0.5–20 μM | [63] |
| Caffeic acid | Rat aorta, uterus, and ileum (ex vivo) | Relaxation/Serotonergic, muscarinic receptors; L-type Ca2+ channels inhibition | 2.1 mM/0.03–7 mM | [23] |
| Rosmarinic acid | Mouse colon (in vivo) | Relaxation/Downregulation of MLCK, ROCK | not specified | [24] |
| Extract/Plant | Tissue/Model | Main Effect/Mechanism | EC50 and/or Reported Concentration | Reference |
|---|---|---|---|---|
| Zingiber officinale (Ginger) | Mouse ileum, colon, LES; Rat colon (ex vivo); Humans, mouse (in vivo) | Relaxation/LES Contraction M3 and 5-HT3 receptor non-competitive antagonism L-type Ca2+ channel inhibition | 0.001–1000 µM | [64] |
| 2.7 µM/1–30 µM | [65] | |||
| not specified | [66] | |||
| 25–100 µM | [67] | |||
| not specified | [68] | |||
| 3–30 µM | [69] | |||
| not specified | [70] | |||
| Curcuma longa (Tumeric) | Mouse ileum and colon, pulmonary artery, and ileum; Rat uterus (ex vivo) | Relaxation/Ca2+ channel blockade; non-competitive antagonism of cholinergic, histaminergic, and serotonergic receptors | 0.021; 0.089 µM | [71] |
| 0.03; 0.04 mg/mL | [72] | |||
| 0.110 mg/mL/0.03–0.3 mg/mL | [73] | |||
| 12.9 µM | [74] | |||
| not specified | [75] | |||
| Bidens tripartita | Porcine jejunum (ex vivo) | Contraction/Enhanced ACh response; | 0.001–100 μM | [76] |
| Roman Chamomile | Guinea pig ileum; rat gut; human gut (ex vivo) | Relaxation/Direct smooth muscle relaxation | 2–200 µg/mL | [77] |
| Catha edulis | Rat colon and ileum (ex vivo) | Relaxation/Ca2+ channel antagonism | 0.05–0.5 mg/mL | [12] |
| Tamarix dioica | Rat and rabbit jejunum, trachea, aorta (ex vivo) | Relaxation/KATP channel activation; Ca2+ channel antagonism | 0.334 mg/mL/0.3–3.0 mg/mL | [11] |
| Citrullus lanatus | Rabbit jejunum (in silico, ex vivo, in vivo) | Relaxation/Ca2+ channel antagonism | 0.02482 mg/mL | [54] |
| Cucumis melo | Rabbit jejunum, trachea (in silico, ex vivo, in vivo) | Relaxation/Ca2+ antagonism, MAPK/PI3K targets | 0.3–3 mg/mL | [78] |
| Achillea millefolium | Guinea pig ileum (ex vivo) | Relaxation/Ca2+ channel antagonism (quercetin, apigenin) | 4.5 μM | [50] |
| Baccharis conferta | Guinea pig ileum (ex vivo) | Relaxation/histamine-dependent | 123 and 234 μM | [49] |
| Berberis lycium | Rabbit jejunum, bladder, rat ileum (in silico, ex vivo, in vivo) | Relaxation/Ca2+ antagonism | 0.3–3 mg/mL | [55] |
| Melissa officinalis | Rat ileum (ex vivo), chicken gut | Contraction/Relaxation/Potentiation of ACh-induced contraction | 19 ng/mL/2.5–75 ng/mL | [79] |
| 0.1–100 µg/mL | [80] | |||
| Salvia sclarea | Rat ileum, trachea (in silico, ex vivo) | Relaxation/Ca2+ channel antagonism | 2.4–5.8 mg/mL/0.005–1.5 mg/mL | [81] |
5.1. Flavones: Potent Modulators of GI Smooth Muscle Activity
5.1.1. Luteolin
5.1.2. Apigenin
5.2. Flavanones: Diverse Influences on GI Motility
5.2.1. Naringenin
5.2.2. Hesperidin
5.2.3. Hesperetin
5.3. Flavonols: Prevalent Modulators of GI Motility
5.3.1. Quercetin
5.3.2. Kaempferol
5.3.3. Myricetin
5.3.4. Isorhamnetin
5.4. Flavanols: Key Calcium Antagonists in GI Smooth Muscle
5.4.1. Catechin
5.4.2. Epicatechin
5.5. Isoflavones—Unique Structures with Diverse Pharmacological Profiles
5.5.1. Daidzein
5.5.2. Genistein
5.5.3. Formononetin
5.6. Anthocyanins—Limited Direct Contractility Data
5.6.1. Pelargonidin
5.6.2. Cyanidin-3-O-Rutinoside
6. Effects of Stilbenes: Resveratrol as a Multi-Targeted Spasmolytic Agent
7. Effects of Lignans: Multi-Pathway Modulation of Smooth Muscle Contractility
7.1. Dibenzocyclooctadiene Lignans
7.2. Dibenzylbutyrolactone Lignans
8. Effects of Phenolic Acids: Diverse Mechanisms of Motility Modulation
8.1. Hydroxycinnamic Acids: Modulators of Contractility and Inflammation
8.1.1. Caffeic Acid
8.1.2. Rosmarinic Acid
9. Effects of Polyphenol-Rich Extracts and Mixed Compositions: Diverse Actions on GI Motility
9.1. Zingiber officinale (Ginger) Extract
9.2. Curcuma longa (Turmeric) Extract
9.3. Bidens tripartita
9.4. Roman Chamomile (Chamaemelum nobile)
9.5. Catha edulis
9.6. Tamarix dioica
9.7. Citrullus lanatus (Watermelon Seeds)
9.8. Cucumis melo (Melon Seeds)
9.9. Achillea millefolium (Yarrow)
9.10. Baccharis conferta
9.11. Berberis lycium
9.12. Melissa officinalis (Lemon Balm)
9.13. Salvia sclarea
10. Compounds Lacking Direct Experimental Data
11. Classification of Experimental Models
12. Clinical Implications and Limitations
13. Conclusions
14. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-AP | 4-aminopyridine |
| 5-HT | Serotonin receptors |
| AC | Adenylate cyclase |
| ACh | Acetylcholine |
| ADRA | Adrenergic receptors |
| BK | Large-conductance calcium-activated potassium channels |
| cGMP | Cyclic guanosine monophosphate |
| COX-2 | Cyclooxygenase-2 |
| CREB | cAMP response element-binding protein |
| DAG | diacylglycerol |
| EC50 | half-maximal effective concentration |
| eNOS | Endothelial nitric oxide synthase |
| GI | Gastrointestinal |
| IBS | Irritable bowel syndrome |
| IK | Intermediate-conductance calcium-activated potassium channels |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IP3 | Inositol trisphosphate |
| IP3R | Inositol trisphosphate potassium receptor |
| KATP | ATP-sensitive potassium channels |
| LES | Lower esophageal sphincter |
| L-NAME | N(G)-nitro-L-arginine methyl ester |
| M2, M3 | Muscarinic acetylcholine receptors |
| MLC20 | 20 kDa regulatory light chains |
| MLCK | Myosin light chain kinase |
| MLCP | Myosin light chain phosphatase |
| MPO | Myeloperoxidase |
| NMR | Nuclear magnetic resonance spectroscopy |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| ODQ | NO-sensitive guanylyl cyclase |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PKG | Protein kinase G |
| PLB | phospholamban |
| PLCβ | phospholipase C beta |
| ROCK | Rho-associated protein kinase |
| RyR | Ryanodine receptor |
| SERCA2a | sarco/endoplasmic reticulum Ca2+-ATPase 2a |
| sGC | Soluble guanylate cyclase |
| SK | Small-conductance calcium-activated potassium channels |
| SR | Sarcoplasmic reticulum |
| TEA | Triethylamine |
| TNF | Tumor necrosis factor |
| TTX | Tetrodotoxin |
| VGCC | Voltage-gated calcium channel |
References
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable Bowel Syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Zogg, H.; Ghoshal, U.C.; Ro, S. Current Treatment Options and Therapeutic Insights for Gastrointestinal Dysmotility and Functional Gastrointestinal Disorders. Front. Pharmacol. 2022, 13, 808195. [Google Scholar] [CrossRef] [PubMed]
- Nita, A.F.; Chanpong, A.; Nikaki, K.; Rybak, A.; Thapar, N.; Borrelli, O. Recent Advances in the Treatment of Gastrointestinal Motility Disorders in Children. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 1285–1300. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Arias-Sánchez, R.A.; Torner, L.; Fenton Navarro, B. Polyphenols and Neurodegenerative Diseases: Potential Effects and Mechanisms of Neuroprotection. Molecules 2023, 28, 5415. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Amira, S.; Rotondo, A.; Mulè, F. Relaxant Effects of Flavonoids on the Mouse Isolated Stomach: Structure-Activity Relationships. Eur. J. Pharmacol. 2008, 599, 126–130. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, Y.; Wan, L.; Ye, J.; Lu, H.-L.; Huang, X.; Xu, W.-X. Luteolin Suppresses Colonic Smooth Muscle Motility via Inhibiting L-Type Calcium Channel Currents in Mice. Gen. Physiol. Biophys. 2020, 39, 49–58. [Google Scholar] [CrossRef]
- Imtiaz, S.; Aleem, A.; Saqib, F.; Ormenisan, A.; Elena Neculau, A.; Anastasiu, C. The Potential Involvement of an ATP-Dependent Potassium Channel-Opening Mechanism in the Smooth Muscle Relaxant Properties of Tamarix Dioica Roxb. Biomolecules 2019, 9, 722. [Google Scholar] [CrossRef]
- Nigusse, T.; Zhang, L.; Wang, R.; Wang, X.; Li, J.; Liu, C. Flavonoids in a Crude Extract of Catha edulis Inhibit Rat Intestinal Contraction via Blocking Ca 2+ Channels. Neurogastroenterol. Motil. 2019, 31, e13602. [Google Scholar] [CrossRef]
- Modzelewska, B.; Drygalski, K.; Kleszczewski, T.; Chomentowski, A.; Koryciński, K.; Kiełczewska, A.; Pawłuszewicz, P.; Razak Hady, H. Quercetin Relaxes Human Gastric Smooth Muscles Directly through ATP-sensitive Potassium Channels and Not Depending on the Nitric Oxide Pathway. Neurogastroenterol. Motil. 2021, 33, e14093. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pan, A.; Zuo, W.; Guo, J.; Zhou, W. Relaxant Effect of Flavonoid Naringenin on Contractile Activity of Rat Colonic Smooth Muscle. J. Ethnopharmacol. 2014, 155, 1177–1183. [Google Scholar] [CrossRef]
- Xiong, Y.; Chu, H.; Lin, Y.; Han, F.; Li, Y.; Wang, A.; Wang, F.; Chen, D.; Wang, J. Hesperidin Alleviates Rat Postoperative Ileus through Anti-Inflammation and Stimulation of Ca2+-Dependent Myosin Phosphorylation. Acta Pharmacol. Sin. 2016, 37, 1091–1100. [Google Scholar] [CrossRef]
- Ghayur, M.N.; Khan, H.; Gilani, A.H. Antispasmodic, Bronchodilator and Vasodilator Activities of (+)-Catechin, a Naturally Occurring Flavonoid. Arch. Pharm. Res. 2007, 30, 970–975. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Qamar, M.; Ziora, Z.M. Antispasmodic Activity of the Ethanol Extract of Citrullus lanatus Seeds: Justifying Ethnomedicinal Use in Pakistan to Treat Asthma and Diarrhea. J. Ethnopharmacol. 2022, 295, 115314. [Google Scholar] [CrossRef]
- Chen, D.; Xiong, Y.; Tang, Z.; Lv, B.; Lin, Y. Inhibitory Effects of Daidzein on Intestinal Motility in Normal and High Contractile States. Pharm. Biol. 2012, 50, 1561–1566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gharzouli, K.; Holzer, P. Inhibition of Guinea Pig Intestinal Peristalsis by the Flavonoids Quercetin, Naringenin, Apigenin and Genistein. Pharmacology 2004, 70, 5–14. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, N.J.; Yun, H.; Han, Y.T. Recent Advances in Synthesis of 4-Arylcoumarins. Molecules 2018, 23, 2417. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Meena, A.; Singh, P.; Kondratyuk, T.P.; Marler, L.E.; Pezzuto, J.M.; Negi, A.S. Neoflavonoids and Tetrahydroquinolones as Possible Cancer Chemopreventive Agents. Chem. Biol. Drug Des. 2012, 80, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Vos, R.; Tack, J. Effects of Capsaicin on the Sensorimotor Function of the Proximal Stomach in Humans. Aliment. Pharmacol. Ther. 2004, 19, 415–425. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Silva, A.; Pereira-de-Morais, L.; Rodrigues da Silva, R.E.; de Menezes Dantas, D.; Brito Milfont, C.G.; Gomes, M.F.; Araújo, I.M.; Kerntopf, M.R.; Alencar de Menezes, I.R.; Barbosa, R. Pharmacological Screening of the Phenolic Compound Caffeic Acid Using Rat Aorta, Uterus and Ileum Smooth Muscle. Chem. Biol. Interact. 2020, 332, 109269. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Xu, S.; Li, X.; Zhang, Y.; Gao, X. Rosmarinic Acid Alleviates Intestinal Inflammatory Damage and Inhibits Endoplasmic Reticulum Stress and Smooth Muscle Contraction Abnormalities in Intestinal Tissues by Regulating Gut Microbiota. Microbiol. Spectr. 2023, 11, e0191423. [Google Scholar] [CrossRef]
- Modzelewska, B.; Drygalski, K.; Hady, H.R.; Kiełczewska, A.; Chomentowski, A.; Koryciński, K.; Głuszyńska, P.; Kleszczewski, T. Resveratrol Relaxes Human Gastric Smooth Muscles Through High Conductance Calcium-Activated Potassium Channel in a Nitric Oxide-Independent Manner. Front. Pharmacol. 2022, 13, 823887. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, H.F.; Wang, L.D.; Jin, S.; Dou, X.C.; Tian, Z.F.; Ma, Q. Resveratrol and Genistein Inhibition of Rat Isolated Gastrointestinal Contractions and Related Mechanisms. World J. Gastroenterol. 2014, 20, 15335. [Google Scholar] [CrossRef]
- Dolejší, E.; Janoušková, A.; Jakubík, J. Muscarinic Receptors in Cardioprotection and Vascular Tone Regulation. Physiol. Res. 2024, 73, S389. [Google Scholar] [CrossRef]
- Seiler, R.; Rickenbacher, A.; Shaw, S.; Balsiger, B.M. α- and β-Adrenergic Receptor Mechanisms in Spontaneous Contractile Activity of Rat Ileal Longitudinal Smooth Muscle. J. Gastrointest. Surg. 2005, 9, 227–235. [Google Scholar] [CrossRef]
- Idrizaj, E.; Traini, C.; Vannucchi, M.G.; Baccari, M.C. Nitric Oxide: From Gastric Motility to Gastric Dysmotility. Int. J. Mol. Sci. 2021, 22, 9990. [Google Scholar] [CrossRef]
- Ren, J.; Xin, F.; Liu, P.; Zhao, H.-Y.; Zhang, S.-T.; Han, P.; Huang, H.-X.; Wang, W. Role of BK Ca in Stretch-Induced Relaxation of Colonic Smooth Muscle. Biomed. Res. Int. 2016, 2016, 9497041. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, D.H.; Son, S.M.; Choi, S.-Y.; You, R.Y.; Kim, C.H.; Choi, W.; Kim, H.S.; Lim, Y.J.; Han, J.Y.; et al. Physiological Function and Molecular Composition of ATP-Sensitive K+ Channels in Human Gastric Smooth Muscle. J. Smooth Muscle Res. 2020, 56, 29–45. [Google Scholar] [CrossRef]
- He, W.; Peng, Y.; Zhang, W.; Lv, N.; Tang, J.; Chen, C.; Zhang, C.; Gao, S.; Chen, H.; Zhi, G.; et al. Myosin Light Chain Kinase Is Central to Smooth Muscle Contraction and Required for Gastrointestinal Motility in Mice. Gastroenterology 2008, 135, 610–620.e2. [Google Scholar] [CrossRef]
- Kishi, H.; Ye, L.H.; Nakamura, A.; Okagaki, T.; Iwata, A.; Tanaka, T.; Kohama, K. Structure and Function of Smooth Muscle Myosin Light Chain Kinase. In Advances in Experimental Medicine and Biology; Kluwer Academic; Plenum Publishers: Dordrecht, The Netherlands, 1998; Volume 453, pp. 229–234. [Google Scholar]
- Lang, R.J.; Harvey, J.R.; McPhee, G.J.; Klemm, M.F. Nitric Oxide and Thiol Reagent Modulation of Ca2+-Activated K+ (BKCa) Channels in Myocytes of the Guinea-Pig Taenia Caeci. J. Physiol. 2000, 525 Pt 2, 363–376. [Google Scholar] [CrossRef]
- Modzelewska, B.; Sipowicz, M.A.; Saavedra, J.E.; Keefer, L.K.; Kostrzewska, A. Involvement of K+ATP Channels in Nitric Oxide-Induced Inhibition of Spontaneous Contractile Activity of the Nonpregnant Human Myometrium. Biochem. Biophys. Res. Commun. 1998, 253, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, Y.; Komori, S.; Matsuyama, H.; Kitazawa, T.; Unno, T. Functions of Muscarinic Receptor Subtypes in Gastrointestinal Smooth Muscle: A Review of Studies with Receptor-Knockout Mice. Int. J. Mol. Sci. 2021, 22, 926. [Google Scholar] [CrossRef]
- Ehlert, F.; Pak, K.; Griffin, M. Muscarinic Agonists and Antagonists: Effects on Gastrointestinal Function. In Muscarinic Recept; Springer: Berlin/Heidelberg, Germany, 2012; pp. 343–374. [Google Scholar] [CrossRef]
- Hanack, C.; Pfeiffer, A. Upper Gastrointestinal Porcine Smooth Muscle Expresses M2- and M3-Receptors. Digestion 1990, 45, 196–201. [Google Scholar] [CrossRef]
- Iino, S.; Nojyo, Y. Muscarinic M2 Acetylcholine Receptor Distribution in the Guinea-Pig Gastrointestinal Tract. Neuroscience 2006, 138, 549–559. [Google Scholar] [CrossRef]
- Harrington, A.M.; Peck, C.J.; Liu, L.; Burcher, E.; Hutson, J.M.; Southwell, B.R. Localization of Muscarinic Receptors M1R, M2R and M3R in the Human Colon. Neurogastroenterol. Motil. 2010, 22, 999-e263. [Google Scholar] [CrossRef] [PubMed]
- Culp, D.J.; Luo, W.; Richardson, L.A.; Watson, G.E.; Latchney, L.R. Both M1 and M3 Receptors Regulate Exocrine Secretion by Mucous Acini. Am. J. Physiol. Physiol. 1996, 271, C1963–C1972. [Google Scholar] [CrossRef]
- Barnes, P.J. Muscarinic Receptor Subtypes in Airways. Life Sci. 1993, 52, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Attinà, T.M.; Oliver, J.J.; Malatino, L.S.; Webb, D.J. Contribution of the M3 Muscarinic Receptors to the Vasodilator Response to Acetylcholine in the Human Forearm Vascular Bed. Br. J. Clin. Pharmacol. 2008, 66, 300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K. YFa and Analogs: Investigation of Opioid Receptors in Smooth Muscle Contraction. World J. Gastroenterol. 2011, 17, 4523. [Google Scholar] [CrossRef]
- Fleming, M.A.; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Muro, P.; Zhang, L.; Li, S.; Zhao, Z.; Jin, T.; Mao, F.; Mao, Z. The Emerging Role of Oxidative Stress in Inflammatory Bowel Disease. Front. Endocrinol 2024, 15, 1390351. [Google Scholar] [CrossRef] [PubMed]
- Sabotič, J.; Bayram, E.; Ezra, D.; Gaudêncio, S.P.; Haznedaroğlu, B.Z.; Janež, N.; Ktari, L.; Luganini, A.; Mandalakis, M.; Safarik, I.; et al. A Guide to the Use of Bioassays in Exploration of Natural Resources. Biotechnol. Adv. 2024, 71, 108307. [Google Scholar] [CrossRef]
- Stromsnes, K.; Lagzdina, R.; Olaso-gonzalez, G.; Gimeno-mallench, L.; Gambini, J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action and Biological Effects in in Vitro Studies, Animal Models and Humans. Biomedicines 2021, 9, 1074. [Google Scholar] [CrossRef]
- Weimann, C.; Göransson, U.; Pongprayoon-Claeson, U.; Claeson, P.; Bohlin, L.; Rimpler, H.; Heinrich, M. Spasmolytic Effects of Baccharis conferta and Some of Its Constituents. J. Pharm. Pharmacol. 2002, 54, 99–104. [Google Scholar] [CrossRef]
- Lemmens-Gruber, R.; Marchart, E.; Rawnduzi, P.; Engel, N.; Benedek, B.; Kopp, B. Investigation of the Spasmolytic Activity of the Flavonoid Fraction of Achillea millefolium s.l. on Isolated Guinea-Pig Ilea. Arzneimittelforschung 2011, 56, 582–588. [Google Scholar] [CrossRef]
- Veloso, C.A.G.; Figueiredo, I.A.D.; da Silva, G.R.; Miranda de Melo, J.I.; da Silva, M.S.; Tavares, J.F.; Cavalcante, F.d.A.; Costa, V.C.d.O. Flavonoids from Varronia dardani (Taroda) J.S. Mill (Cordiaceae) and the Evaluation of Spasmolytic Activity of Its Crude Ethanolic Extract. Nat. Prod. Res. 2021, 35, 4197–4201. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Gu, Y. Hesperidin Improves Colonic Motility in Loeramide-Induced Constipation Rat Model via 5-Hydroxytryptamine 4R/CAMP Signaling Pathway. Digestion 2020, 101, 692–705. [Google Scholar] [CrossRef]
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Antispasmodic Effect of Selected Citrus Flavonoids on Rat Isolated Jejunum Specimens. Eur. J. Pharmacol. 2016, 791, 640–646. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F. Scientific Basis for Medicinal Use of Citrullus lanatus (Thunb.) in Diarrhea and Asthma: In Vitro, in Vivo and in Silico Studies. Phytomedicine 2022, 98, 153978. [Google Scholar] [CrossRef] [PubMed]
- Hussain Shah, S.A.; Aleem, A. Investigations of Plausible Pharmacodynamics Supporting the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Berberis Lycium Royle. Via in Silico, in Vitro, and in Vivo Studies. J. Ethnopharmacol. 2023, 305, 116115. [Google Scholar] [CrossRef] [PubMed]
- Rocha, B.S.; Gago, B.; Barbosa, R.M.; Laranjinha, J. Dietary Polyphenols Generate Nitric Oxide from Nitrite in the Stomach and Induce Smooth Muscle Relaxation. Toxicology 2009, 265, 41–48. [Google Scholar] [CrossRef]
- Zhu, H.L.; Ito, Y.; Teramoto, N. Effects of Tyrosine Kinase Inhibitors on Voltage-Dependent Ba(2+) Currents in the Guinea-Pig Gastric Antrum. J. Physiol. Pharmacol. 2006, 57, 415–424. [Google Scholar] [PubMed]
- Wu, J.H.; Li, Q.; Wu, M.Y.; Guo, D.J.; Chen, H.L.; Chen, S.L.; Seto, S.W.; Au, A.L.S.; Poon, C.C.W.; Leung, G.P.H. Formononetin, an Isoflavone, Relaxes Rat Isolated Aorta through Endothelium-Dependent and Endothelium-Independent Pathways. J. Nutr. Biochem. 2010, 21, 613–620. [Google Scholar] [CrossRef]
- Stoclet, J.C.; Kleschyov, A.; Andriambeloson, E.; Diebolt, M.; Andriantsitohaina, R. Endothelial No Release Caused by Red Wine Polyphenols. J. Physiol. Pharmacol. 1999, 50, 535–540. [Google Scholar]
- Miladinovic, B.; Brankovic, S.; Kostic, M.; Milutinovic, M.; Kitic, N.; Šavikin, K.; Kitic, D. Antispasmodic Effect of Blackcurrant (Ribes nigrum L.) Juice and Its Potential Use as Functional Food in Gastrointestinal Disorders. Med. Princ. Pract. 2018, 27, 179. [Google Scholar] [CrossRef]
- Parlar, A.; Arslan, S.O. Resveratrol Normalizes the Deterioration of Smooth Muscle Contractility after Intestinal Ischemia and Reperfusion in Rats Associated With an Antioxidative Effect and Modulating Tumor Necrosis Factor Alpha Activity. Ann. Vasc. Surg. 2019, 61, 416–426. [Google Scholar] [CrossRef]
- Yang, J.M.; Ip, P.S.P.; Che, C.T.; Yeung, J.H.K. Relaxant Effects of Schisandra Chinensis and Its Major Lignans on Agonists-Induced Contraction in Guinea Pig Ileum. Phytomedicine 2011, 18, 1153–1160. [Google Scholar] [CrossRef]
- Koech, P.K.a.; Boldizsár, I.; Dobolyi, A.; Varró, P. Effects of Dibenzylbutyrolactone Lignans Arctigenin and Trachelogenin on the Motility of Isolated Rat Ileum. Toxicol. Rep. 2022, 9, 1222–1232. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of Action of Gingerols and Shogaols on 5-HT3 Receptors: Binding Studies, Cation Uptake by the Receptor Channel and Contraction of Isolated Guinea-Pig Ileum. Eur. J. Pharmacol. 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Pertz, H.; Lehmann, J.; Roth-Ehrang, R.; Elz, S. Effects of Ginger Constituents on the Gastrointestinal Tract: Role of Cholinergic M 3 and Serotonergic 5-HT 3 and 5-HT 4 Receptors. Planta Med. 2011, 77, 973–978. [Google Scholar] [CrossRef]
- Fahimi, F.; Khodadad, K.; Amini, S.; Naghibi, F.; Salamzadeh, J.; Baniasadi, S. Evaluating the Effect of Zingiber Officinalis on Nausea and Vomiting in Patients Receiving Cisplatin Based Regimens. Iran. J. Pharm. Res. 2011, 10, 379–384. [Google Scholar]
- Cai, Z.-X.; Tang, X.-D.; Wang, F.-Y.; Duan, Z.-J.; Li, Y.-C.; Qiu, J.-J.; Guo, H.-S. Effect of Gingerol on Colonic Motility via Inhibition of Calcium Channel Currents in Rats. World J. Gastroenterol. 2015, 21, 13466–13472. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in Gastrointestinal Disorders: A Systematic Review of Clinical Trials. Food Sci. Nutr. 2019, 7, 96–108. [Google Scholar] [CrossRef]
- Promdam, N.; Khuituan, P.; Panichayupakaranant, P. Effects of Standardized [6]-Gingerol Extracts and [6]-Gingerol on Isolated Ileum and Lower Esophageal Sphincter Contractions in Mice. Food Chem. 2022, 378, 132077. [Google Scholar] [CrossRef]
- Sun, X.; Nie, F.; Sun, J.; Zhang, J.; Wang, Y. Medicinal Plants for Chemotherapy-Induced Nausea and Vomiting: A Systematic Review of Antiemetic, Chemosensitizing, and Immunomodulatory Mechanisms. Ther. Clin. Risk Manag. 2025, 21, 1187–1218. [Google Scholar] [CrossRef]
- Micucci, M.; Aldini, R.; Cevenini, M.; Colliva, C.; Spinozzi, S.; Roda, G.; Montagnani, M.; Camborata, C.; Camarda, L.; Chiarini, A.; et al. Curcuma longa L. as a Therapeutic Agent in Intestinal Motility Disorders. 2: Safety Profile in Mouse. PLoS ONE 2013, 8, e80925. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aldini, R.; Budriesi, R.; Roda, G.; Micucci, M.; Ioan, P.; D’Errico-Grigioni, A.; Sartini, A.; Guidetti, E.; Marocchi, M.; Cevenini, M.; et al. Curcuma longa Extract Exerts a Myorelaxant Effect on the Ileum and Colon in a Mouse Experimental Colitis Model, Independent of the Anti-Inflammatory Effect. PLoS ONE 2012, 7, e44650. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Shah, A.J.; Ghayur, M.N.; Majeed, K. Pharmacological Basis for the Use of Turmeric in Gastrointestinal and Respiratory Disorders. Life Sci. 2005, 76, 3089–3105. [Google Scholar] [CrossRef]
- Jamil, Q.U.A.; Iqbal, S.M.; Jaeger, W.; Studenik, C. Vasodilating, Spasmolytic, Inotropic and Chronotropic Activities of Curcuminoids from Curcuma longa in Isolated Organ Preparations of Guinea Pigs. J. Physiol. Pharmacol. 2018, 69, 441–449. [Google Scholar] [CrossRef]
- Rahimi, R. Herbal Medicines for the Management of Irritable Bowel Syndrome: A Comprehensive Review. World J. Gastroenterol. 2012, 18, 589. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Chłopecka, M.; Latek, U.; Karlik, W.; Tomczykowa, M.; Strawa, J.; Tomczyk, M. Evaluation of the Effects of Bidens Tripartita Extracts and Their Main Constituents on Intestinal Motility—An Ex Vivo Study. J. Ethnopharmacol. 2020, 259, 112982. [Google Scholar] [CrossRef]
- Sándor, Z.; Mottaghipisheh, J.; Veres, K.; Hohmann, J.; Bencsik, T.; Horváth, A.; Kelemen, D.; Papp, R.; Barthó, L.; Csupor, D. Evidence Supports Tradition: The in Vitro Effects of Roman Chamomile on Smooth Muscles. Front. Pharmacol. 2018, 9, 323. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Tallei, T.E.; Simal–Gandara, J. Mechanistic Insights of Cucumis melo L. Seeds for Gastrointestinal Muscle Spasms through Calcium Signaling Pathway–Related Gene Regulation Networks in WGCNA and in Vitro, in Vivo Studies. Comput. Biol. Med. 2023, 155, 106596. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghannadi, A.; Malekshahi, K. Relaxant Effect of Essential Oil of Melissa Officinalis and Citral on Rat Ileum Contractions. Fitoterapia 2003, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Posłuszny, M.A.; Chłopecka, M.; Suor-Cherer, S.; Cisse, S.; el Amine Benarbia, M.; Mendel, M. Modulation of Chicken Gut Contractility by Melissa Officinalis—Ex Vivo Study. Poult. Sci. 2023, 102, 103045. [Google Scholar] [CrossRef]
- Randjelović, M.; Branković, S.; Jovanović, M.; Kitić, N.; Živanović, S.; Mihajilov-Krstev, T.; Miladinović, B.; Milutinović, M.; Kitić, D. An In Vitro and In Silico Characterization of Salvia sclarea L. Methanolic Extracts as Spasmolytic Agents. Pharmaceutics 2023, 15, 1376. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-Inflammatory and Anticancer Effects of Anthocyanins in In Vitro and In Vivo Studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Gao, F.; Yang, P.; Wang, W.; Wang, K.; Zhao, L.; Wang, Y.; Liao, X. Unveiling the Multifaceted Roles of Anthocyanins: A Review of Their Bioavailability, Impacts on Gut and System Health, and Industrial Implications. Curr. Res. Food Sci. 2025, 11, 101137. [Google Scholar] [CrossRef]
- Lete, I.; Allué, J. The Effectiveness of Ginger in the Prevention of Nausea and Vomiting during Pregnancy and Chemotherapy. Integr. Med. Insights 2016, 11, 11–17. [Google Scholar] [CrossRef]
- Lutomski, J.; McCarthy, F.P.; Greene, R.A. Hyperemesis Gravidarum: Current Perspectives. Int. J. Womens Health 2014, 6, 719. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the Prevention of Nausea and Vomiting: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Ersoy, S.; Orhan, I.; Turan, N.N.; Şahan, G.; Ark, M.; Tosun, F. Endothelium-Dependent Induction of Vasorelaxation by Melissa officinalis L. ssp. Officinalis in Rat Isolated Thoracic Aorta. Phytomedicine 2008, 15, 1087–1092. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Ghasemipour Afshar, E. Tangeretin: A Mechanistic Review of Its Pharmacological and Therapeutic Effects. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190191. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Shi, W.; Liu, H.; Yang, J.; Yuan, X.; Wu, L. The Multifunctional Effects of Nobiletin and Its Metabolites In Vivo and In Vitro. Evid.-Based Complement. Alternat Med. 2016, 2016, 2918796. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.J.; Lai, W.F. Chemical and Biological Properties of Biochanin A and Its Pharmaceutical Applications. Pharmaceutics 2023, 15, 1105. [Google Scholar] [CrossRef] [PubMed]
- Song, T.T.; Hendrich, S.; Murphy, P.A. Estrogenic Activity of Glycitein, a Soy Isoflavone. J. Agric. Food Chem. 1999, 47, 1607–1610. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food Anthocyanins: Malvidin and Its Glycosides as Promising Antioxidant and Anti-Inflammatory Agents with Potential Health Benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a B-Ring 5’-O-Methylated Derivative of Delphinidin, Stimulates Osteoblastogenesis and Reduces SRANKL-Induced Bone Loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E.; Probst, Y.C.; Kent, K.; Netzel, M.E. A Systematic Literature Review of the Effect of Anthocyanins on Gut Microbiota Populations. J. Hum. Nutr. Diet. 2019, 32, 32–53. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomentowski, A.; Drygalski, K.; Kleszczewski, T.; Berczyńska, M.; Tylicka, M.; Kapała, J.; Raciborska, A.; Zubrzycki, P.; Hady, H.R.; Modzelewska, B. Polyphenols as Modulators of Gastrointestinal Motility: Mechanistic Insights from Multi-Model Studies. Pharmaceuticals 2025, 18, 1564. https://doi.org/10.3390/ph18101564
Chomentowski A, Drygalski K, Kleszczewski T, Berczyńska M, Tylicka M, Kapała J, Raciborska A, Zubrzycki P, Hady HR, Modzelewska B. Polyphenols as Modulators of Gastrointestinal Motility: Mechanistic Insights from Multi-Model Studies. Pharmaceuticals. 2025; 18(10):1564. https://doi.org/10.3390/ph18101564
Chicago/Turabian StyleChomentowski, Andrzej, Krzysztof Drygalski, Tomasz Kleszczewski, Marta Berczyńska, Marzena Tylicka, Jacek Kapała, Agnieszka Raciborska, Przemysław Zubrzycki, Hady Razak Hady, and Beata Modzelewska. 2025. "Polyphenols as Modulators of Gastrointestinal Motility: Mechanistic Insights from Multi-Model Studies" Pharmaceuticals 18, no. 10: 1564. https://doi.org/10.3390/ph18101564
APA StyleChomentowski, A., Drygalski, K., Kleszczewski, T., Berczyńska, M., Tylicka, M., Kapała, J., Raciborska, A., Zubrzycki, P., Hady, H. R., & Modzelewska, B. (2025). Polyphenols as Modulators of Gastrointestinal Motility: Mechanistic Insights from Multi-Model Studies. Pharmaceuticals, 18(10), 1564. https://doi.org/10.3390/ph18101564






