Harmful Effects of Prescribed Opioids in Children and Adults: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Quality Assessment
3. Results
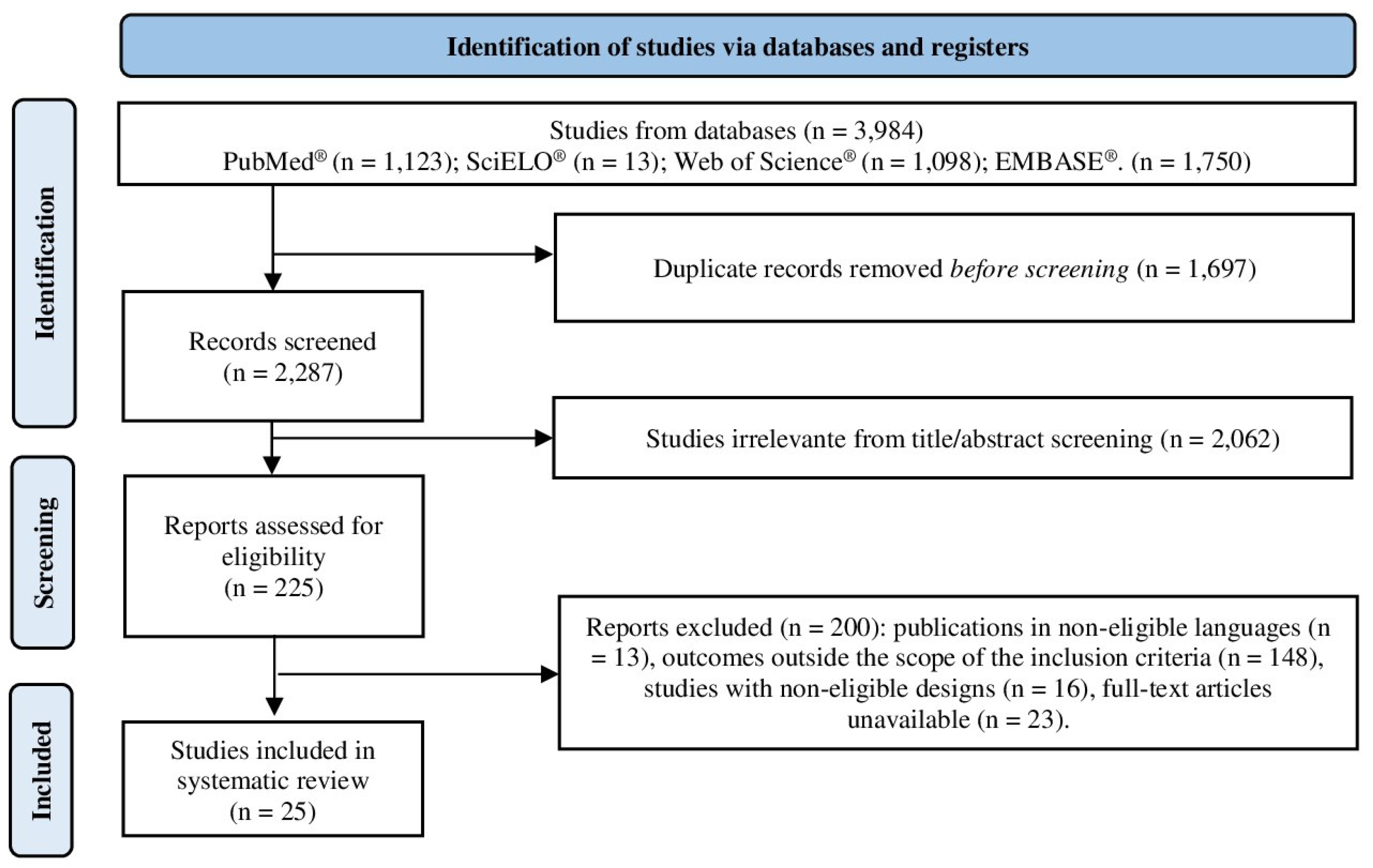
3.1. Study Selection
3.2. Data Synthesis
3.3. Study Characteristics
3.3.1. Harmful Effects Caused by Prescribed Opioids in Pediatric Patients
3.3.2. Harmful Effects Caused by Prescribed Opioids in the General Population
3.4. Risk of Bias
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carli, M.; Donnini, S.; Pellegrini, C.; Coppi, E.; Bocci, G. Opioid receptors beyond pain control: The role in cancer pathology and the debated importance of their pharmacological modulation. Pharmacol. Res. 2020, 159, 104938. [Google Scholar] [CrossRef]
- Machelska, H.; Celik, M.Ö. Opioid receptors in immune and glial cells—Implications for pain control. Front. Immunol. 2020, 11, 472575. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. Opioid receptors. Annu. Rev. Med. 2016, 67, 433–451. [Google Scholar] [CrossRef]
- Wang, Y.; Zhuang, Y.; DiBerto, J.F.; Zhou, X.E.; Schmitz, G.P.; Yuan, Q.; Jain, M.K.; Liu, W.; Melcher, K.; Jiang, Y.; et al. Structures of the entire human opioid receptor family. Cell 2023, 186, 413–427. [Google Scholar] [CrossRef] [PubMed]
- James, A.; Williams, J. Basic opioid pharmacology—An update. Br. J. Pain 2020, 14, 115–121. [Google Scholar] [CrossRef]
- Han, B.; Jones, C.M.; Einstein, E.B.; Dowell, D.; Compton, W.M. Prescription opioid use disorder among adults reporting prescription opioid use with or without misuse in the United States. J. Clin. Psychiatry 2024, 85, 56054. [Google Scholar] [CrossRef]
- Michienzi, A.E.; Borek, H.A. Emerging agents of substance use/misuse. Emerg. Med. Clin. N. Am. 2022, 40, 265–281. [Google Scholar] [CrossRef]
- Gustafsson, M.; Matos, C.; Joaquim, J.; Scholl, J.; van Hunsel, F. Adverse drug reactions to opioids: A study in a national pharmacovigilance database. Drug Saf. 2023, 46, 1133–1148. [Google Scholar] [CrossRef]
- Gustafsson, M.; Silva, V.; Valeiro, C.; Joaquim, J.; van Hunsel, F.; Matos, C. Misuse, abuse and medication errors’ adverse events associated with opioids—A systematic review. Pharmaceuticals 2024, 17, 1009. [Google Scholar] [CrossRef]
- Albores-Garcia, D.; Cruz, S.L. Fentanyl and other new psychoactive synthetic opioids. Challenges to prevention and treatment. Rev. Investig. Clin. Org. Hosp. Enfermedades Nutr. 2023, 75, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, A.; Chan, J.J.; Lian, J.; Wright, C.L.; Marin, A.M.; Rodriguez, E.K.; von Keudell, A.; Nazarian, A. Risk factors and pooled rate of prolonged opioid use following trauma or surgery: A systematic review and meta-(regression) analysis. J. Bone Jt. Surg. Am. 2018, 100, 1332–1340. [Google Scholar] [CrossRef]
- Hurtado, I.; Robles, C.; García-Sempere, A.; Llopis-Cardona, F.; Sánchez-Sáez, F.; Rodríguez-Bernal, C.; Peiró, S.; Sanfélix-Gimeno, G. Long-term use of prescription opioids for non-cancer pain and mortality: A population-based, propensity-weighted cohort study. Public Health 2024, 232, 4–13. [Google Scholar] [CrossRef]
- Basaran, M.B.; Koca, R.O.; Gormus, Z.I.S. Therapeutic innovations against opioid tolerance and addiction. Curr. Behav. Neurosci. Rep. 2024, 11, 201–210. [Google Scholar] [CrossRef]
- Volkow, N.D.; Blanco, C. The changing opioid crisis: Development, challenges and opportunities. Mol. Psychiatry 2021, 26, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Raffa, R.B.; Rosenblatt, M.H. Opioid withdrawal symptoms, a consequence of chronic opioid use and opioid use disorder: Current understanding and approaches to management. J. Clin. Pharm. Ther. 2020, 45, 892–903. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). About Prescription Opioids. 2024. Available online: https://www.cdc.gov/overdose-prevention/about/prescription-opioids.html (accessed on 15 March 2025).
- Substance Abuse and Mental Health Services Administration (SAMHSA). Key Substance Use and Mental Health Indicators in the United States: Results from the 2022 National Survey on Drug Use and Health. 2023. Available online: https://www.samhsa.gov/data/report/2022-nsduh-annual-national-report (accessed on 27 March 2025).
- Robert, M.; Jouanjus, E.; Khouri, C.; Fouilhé Sam-Laï, N.; Revol, B. The opioid epidemic: A worldwide exploratory study using the WHO pharmacovigilance database. Addiction 2023, 118, 771–775. [Google Scholar] [CrossRef]
- Ju, C.; Wei, L.; Man, K.K.C.; Wang, Z.; Ma, T.-T.; Chan, A.Y.L.; Brauer, R.; Chui, C.S.L.; Chan, E.W.; Jani, Y.H.; et al. Global, regional, and national trends in opioid analgesic consumption from 2015 to 2019: A longitudinal study. Lancet Public Health 2022, 7, e335–e346. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhang, Q.; Peng, J.; Yao, W.; Feng, W.; Wan, X. Global, regional, and national burden of opioid use disorder from 1990 to 2021: A statistical analysis of incidence, mortality, and disability-adjusted life years. BMC Public Health 2025, 25, 1988. [Google Scholar] [CrossRef]
- Han, F.; Liu, B.; Wang, L.; Zhu, S.; Li, X.; Kang, S.; Niu, X.; Song, J.; Wu, Y. Global, Regional, and National Epidemiology of Opioid Use Disorder Among Adolescents and Young Adults, 1990–2019. J. Adolesc. Health 2025, 76, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Strazdins, E.; Guessoum, A.; Westenberg, J.N.; Appenzeller-Herzog, C.; Cattaneo, M.E.G.V.; Krausz, R.M.; Dürsteler, K.M.; Lang, U.E.; Hemkens, L.G.; et al. Relative risks of adverse effects across different opioid agonist treatments—A systematic review and meta-analysis. Addiction 2025, 120, 1112–1126. [Google Scholar] [CrossRef]
- Zimmerman, A.; Laitman, A. Safe management of adverse effects associated with prescription opioids in the palliative care population: A narrative review. J. Clin. Med. 2024, 13, 2746. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- United States National Institutes of Health (NIH). Guidelines for the Review of Inclusion on the Basis of Sex/Gender, Race, Ethnicity, and Age in Clinical Research. 2019. Available online: https://grants.nih.gov/grants/peer/guidelines_general/Review_Human_subjects_Inclusion.pdf (accessed on 15 July 2025).
- United Nations International Children’s Emergency Fund (UNICEF). Convention on the Rights of the Child. 1989. Available online: https://www.unicef.org/child-rights-convention/convention-text (accessed on 15 July 2025).
- European Commission. EU Action on the Rights of the Child. 2022. Available online: https://ec.europa.eu/info/policies/justice-and-fundamental-rights/rights-child/eu-action-rights-child_en (accessed on 23 July 2025).
- Vadhel, A.; Bashir, S.; Mir, A.H.; Girdhar, M.; Kumar, D.; Kumar, A.; Mohan, A.; Malik, T.; Mohan, A. Opium alkaloids, biosynthesis, pharmacology and association with cancer occurrence. Open Biol. 2023, 13, 220355. [Google Scholar] [CrossRef]
- Mohebbi, E.; Haghdoost, A.A.; Noroozi, A.; Vardanjani, H.M.; Hajebi, A.; Nikbakht, R.; Mehrabi, M.; Kermani, A.J.; Salemianpour, M.; Baneshi, M.R. Awareness and attitude towards opioid and stimulant use and lifetime prevalence of the drugs: A study in 5 large cities of Iran. Int. J. Health Policy Manag. 2018, 8, 222–232. [Google Scholar] [CrossRef]
- Chen, Q.; Sterner, G.; Rhubart, D.; Newton, R.; Shaw, B.; Scanlon, D. Creating a robust coordinated data and policy framework for addressing substance use issues in the United States. Int. J. Drug Policy 2024, 134, 104629. [Google Scholar] [CrossRef] [PubMed]
- Marks, C.; Carrasco-Escobar, G.; Carrasco-Hernández, R.; Johnson, D.; Ciccarone, D.; Strathdee, S.A.; Smith, D.; Bórquez, A. Methodological approaches for the prediction of opioid use-related epidemics in the United States: A narrative review and cross-disciplinary call to action. Transl. Res. 2021, 234, 88–113. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.Z.Y.; Thornton, J.D.; Fenton, S.H.; Simmons, D.; Champagne-Langabeer, T. Helpful, unnecessary, or harmful: A systematic review of the effects of prescription drug monitoring program use on opioid prescriptions. Pharmacoepidemiol. Drug Saf. 2023, 2, 350–365. [Google Scholar] [CrossRef]
- Erkok, S.D.; Gallois, R.; Leegwater, L.; Gonzalez, P.C.; van Asten, A.; McCord, B. Combining surface-enhanced Raman spectroscopy (SERS) and paper spray mass spectrometry (PS-MS) for illicit drug detection. Talanta 2024, 278, 126414. [Google Scholar] [CrossRef]
- Muriel, J.; Barrachina, J.; Del Barco, G.; Carvajal, C.; Escorial, M.; Margarit, C.; Ballester, P.; Peiró, A.M. Impact of CYP2D6 genotype on opioid use disorder deprescription: An observational prospective study in chronic pain with sex-differences. Front. Pharmacol. 2023, 14, 1200430. [Google Scholar] [CrossRef] [PubMed]
- Templeton, K.J. Sex and gender issues in pain management. J. Bone Jt. Surg. Am. 2020, 102 (Suppl. S1), 32–35. [Google Scholar] [CrossRef]
- Khodneva, Y.; Muntner, P.; Kertesz, S.; Kissela, B.; Safford, M.M. Prescription opioid use and risk of coronary heart disease, stroke, and cardiovascular death among adults from a prospective cohort (REGARDS Study). Pain Med. 2016, 17, 444–455. [Google Scholar] [CrossRef][Green Version]
- Nicolakis, J.; Gmeiner, G.; Reiter, C.; Seltenhammer, M.H. Aspiration in lethal drug abuse—A consequence of opioid intoxication. Int. J. Leg. Med. 2020, 134, 2121–2132. [Google Scholar] [CrossRef]
- Takla, M.; Saadeh, K.; Tse, G.; Huang, C.L.H.; Jeevaratnam, K. Ageing and the autonomic nervous system. In Biochemistry and Cell Biology of Ageing: Part IV, Clinical Science; Springer: Berlin/Heidelberg, Germany, 2023; pp. 201–252. [Google Scholar]
- Chow, S.L.; Sasson, C.; Benjamin, I.J.; Califf, R.M.; Compton, W.M.; Oliva, E.M.; Robson, C.; Sanchez, E.J. Opioid use and its relationship to cardiovascular disease and brain health: A presidential advisory from the American Heart Association. Circulation 2021, 144, e218–e232. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.M.; Chowdhury, E.K.; Ernst, M.E.; Gilmartin-Thomas, J.; Reid, C.M.; Tonkin, A.; Neumann, J.; McNeil, J.J.; Kaye, D.M. Prescribed opioid use is associated with adverse cardiovascular outcomes in community-dwelling older persons. ESC Heart Fail. 2022, 9, 3973–3984. [Google Scholar] [CrossRef]
- Yaster, M.; McNaull, P.P.; Davis, P.J. The opioid epidemic in pediatrics: A 2020 update. Curr. Opin. Anaesthesiol. 2020, 33, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Pedapati, E.V.; Bateman, S.T. Toddlers requiring pediatric intensive care unit admission following at-home exposure to buprenorphine/naloxone. Pediatr. Crit. Care Med. 2011, 12, e102–e107. [Google Scholar] [CrossRef]
- Lavonas, E.J.; Banner, W.; Bradt, P.; Bucher-Bartelson, B.; Brown, K.R.; Rajan, P.; Green, J.L. Root causes, clinical effects, and outcomes of unintentional exposures to buprenorphine by young children. J. Pediatr. 2013, 163, 1377–1383. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Farnaghi, F.; Shariatmadari, S.F.; Jafari, N.; Mehregan, F.F.; Karimzadeh, P. Accidental children poisoning with methadone: An Iranian pediatric sectional study. Iran. J. Child Neurol. 2013, 7, 32–34. [Google Scholar]
- Bazmamoun, H.; Fayyaz, A.; Khajeh, A.; Sabzehei, M.K.; Khezrian, F. A study of methadone-poisoned children referred to Hamadan’s Besat Hospital/Iran. Iran. J. Child Neurol. 2014, 8, 34–37. [Google Scholar]
- Sharif, M.R.; Nouri, S. Clinical signs and symptoms and laboratory findings of methadone poisoning in children. Iran. J. Pediatr. 2015, 25, e206. [Google Scholar] [CrossRef]
- Borys, D.; Stanton, M.; Gummin, D.; Drott, T. Tapentadol toxicity in children. Pediatrics 2015, 135, e392–e396. [Google Scholar] [CrossRef]
- Hamedi, A.; Ataei, A.; Balali, M.R.; Ghahremani, S.; Ghahremani, S. A cross-sectional study on pediatric methadone poisoning in northeast of Iran. Asia Pac. J. Med. Toxicol. 2016, 5, 75–78. [Google Scholar]
- Stassinos, G.L.; Gonzales, L.; Klein-Schwartz, W. Characterizing the toxicity and dose-effect profile of tramadol ingestions in children. Pediatr. Emerg. Care 2019, 35, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Toce, M.S.; Burns, M.M.; O’Donnell, K.A. Clinical effects of unintentional pediatric buprenorphine exposures: Experience at a single tertiary care center. Clin. Toxicol. 2017, 55, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, S.; Miller, S.; Wang, B.; Wax, P.; Campleman, S.; Manini, A.F. Clinical predictors of adverse cardiovascular events for acute pediatric drug exposures. Clin. Toxicol. 2019, 58, 183–189. [Google Scholar] [CrossRef]
- Riasi, H.; Rabiee, N.; Chahkandi, T.; Arzanin, F.; Atary, S.K.; Salehi, F. Electrocardiographic changes in children with acute opioid poisoning: A cross-sectional study. Pediatr. Emerg. Care 2021, 37, e1082–e1086. [Google Scholar] [CrossRef]
- Farnaghi, F.; Gholami, N.; Hassanian-Moghaddam, H.; McDonald, R.; Zamanzadeh, R.; Zamani, N. Unintentional buprenorphine and methadone poisoning in children: A matched observational study. Clin. Toxicol. 2021, 59, 727–733. [Google Scholar] [CrossRef]
- Cohen, N.; Mathew, M.; Davis, A.; Brent, J.; Wax, P.; Schuh, S.; ToxIC Pediatric Opioid Exposure Study Group. Predictors of severe outcome following opioid intoxication in children. Clin. Toxicol. 2022, 60, 702–707. [Google Scholar] [CrossRef]
- Caré, W.; Tangre, A.; Dufayet, L.; Lekens, B.; Laborde-Casterot, H.; Langrand, J.; Vodovar, D. Exposure to immediate-release tramadol in children 6 years and under—A nationwide French poison control center study. Clin. Toxicol. 2022, 60, 750–758. [Google Scholar] [CrossRef]
- Emamhadi, M.; Sanaei-Zadeh, H.; Nikniya, M.; Zamani, N.; Dart, R.C. Electrocardiographic manifestations of tramadol toxicity with special reference to their ability for prediction of seizures. Am. J. Emerg. Med. 2012, 30, 1481–1485. [Google Scholar] [CrossRef]
- Eizadi-Mood, N.; Ozcan, D.; Sabzghabaee, A.M.; Mirmoghtadaee, P.; Hedaiaty, M. Does naloxone prevent seizure in tramadol intoxicated patients? Int. J. Prev. Med. 2014, 5, 302–305. [Google Scholar]
- Asadi, P.; Kasmaei, V.M.; Ziabari, S.Z.; Zohrevandi, B.; Manesh, A.M. Prevalence of tramadol consumption in first seizure patients; a one-year cross-sectional study. Emergency 2015, 3, 159. [Google Scholar] [PubMed]
- Farzaneh, E.; Amani, F.; Etemad, F. A clinico-epidemiologic study on patients with opium toxicity treated at Ardabil Hospitals, Iran, 2014–2015. Asia Pac. J. Med. Toxicol. 2016, 5, 111–114. [Google Scholar]
- Ghamsari, A.A.; Dadpour, B.; Najari, F. Frequency of electrocardiographic abnormalities in tramadol poisoned patients: A brief report. Emergency 2016, 4, 151–153. [Google Scholar]
- Moghadam, P.H.; Zarei, N.; Farsi, D.; Abbasi, S.; Mofidi, M.; Rezai, M.; Mahshidfar, B. Electrocardiographic changes in patients with tramadol-induced idiosyncratic seizures. Turk. J. Emerg. Med. 2016, 16, 151–154. [Google Scholar] [CrossRef]
- Ahmadimanesh, M.; Shadnia, S.; Rouini, M.R.; Sheikholeslami, B.; Ahsani Nasab, S.; Ghazi-Khansari, M. Correlation between plasma concentrations of tramadol and its metabolites and the incidence of seizure in tramadol-intoxicated patients. Drug Metab. Pers. Ther. 2018, 33, 75–83. [Google Scholar] [CrossRef]
- Bedson, J.; Chen, Y.; Ashworth, J.; Hayward, R.A.; Dunn, K.M.; Jordan, K.P. Risk of adverse events in patients prescribed long-term opioids: A cohort study in the UK clinical practice research Datalink. Eur. J. Pain 2019, 23, 908–922. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Ashkezari, M.D.; Farahmand, B.; Shokrzadeh, M. Demographic characteristics and functional performance of the kidneys and hearts of patients with acute tramadol toxicity. Drug Res. 2019, 69, 207–210. [Google Scholar] [CrossRef]
- Ahmadimanesh, M.; Naeini, M.B.; Rouini, M.R.; Shadnia, S.; Ghazi-Khansari, M. Assessment of tramadol pharmacokinetics in correlation with CYP2D6 and clinical symptoms. Drug Metab. Pers. Ther. 2020, 35, 20190021. [Google Scholar] [CrossRef]
- Caré, W.; Pinel, S.; Dufayet, L.; Langrand, J.; Micallef, J.; Vodovar, D. Trends in adverse drug reactions related to oral weak opioid analgesics in therapeutic use in adults: A 10-year French vigilances retrospective study. Fundam. Clin. Pharmacol. 2023, 37, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
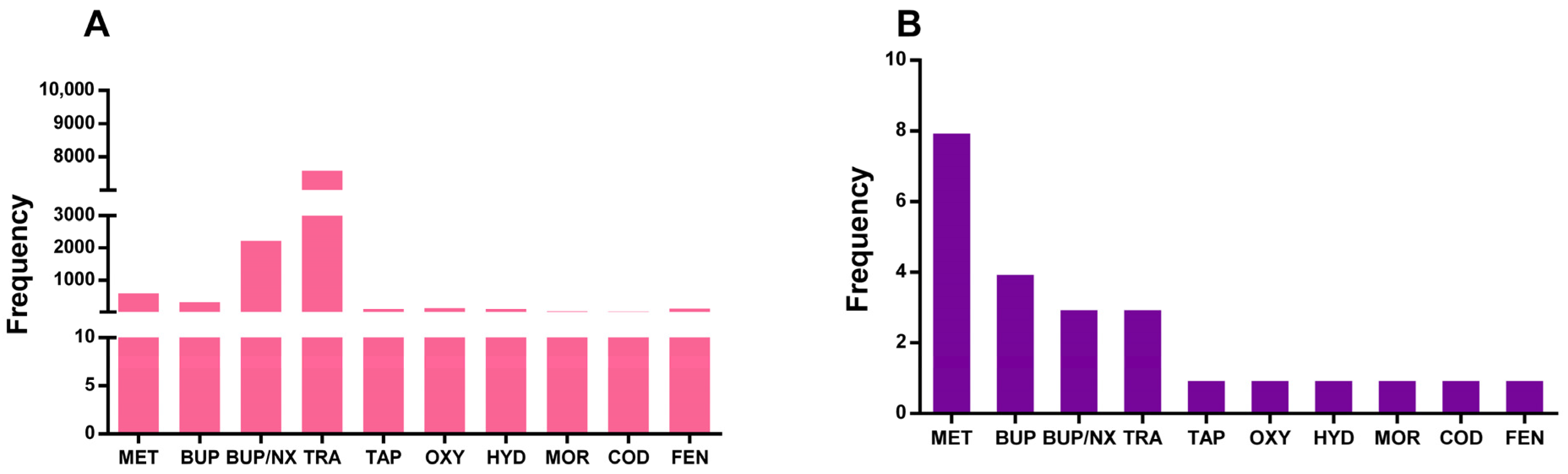
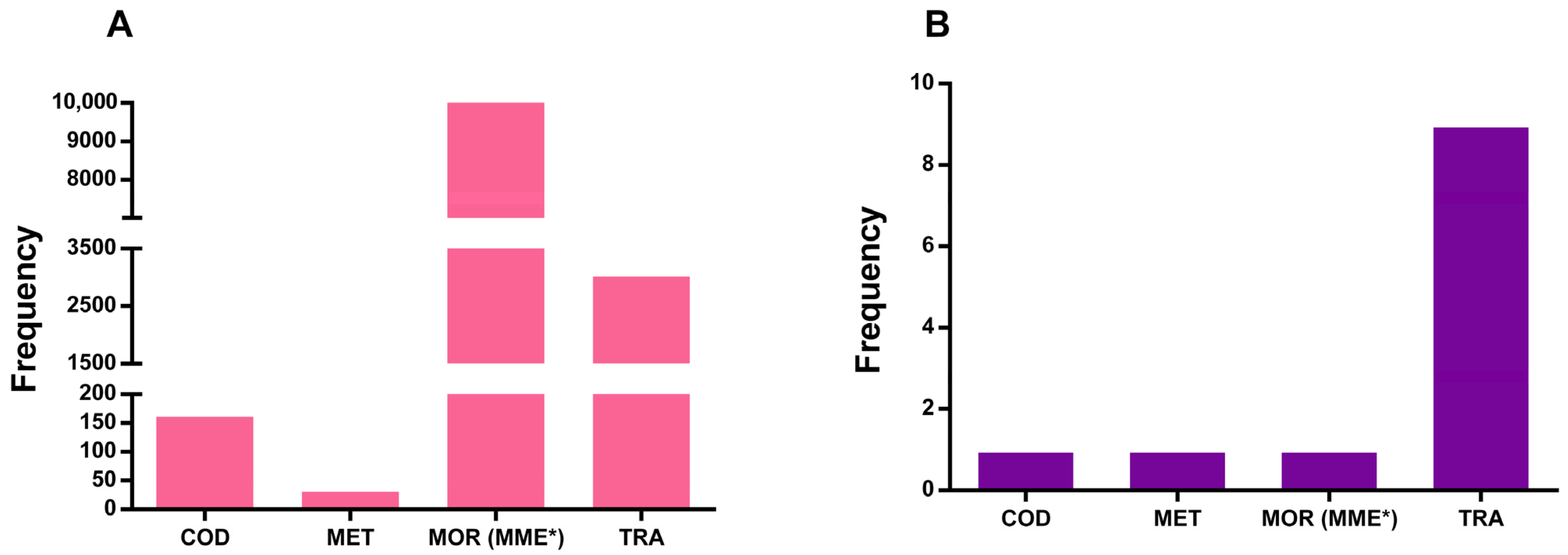
| Prescribed Opioid (%) | Pediatric Patients | General Population |
|---|---|---|
| BUP | 2.86 | - |
| BUP/NX | 0.83 | - |
| BUP/NX film | 1.06 | - |
| BUP/NX tablets | 18.43 | - |
| COD | 0.35 | 0.16 |
| FEN | 0.17 | - |
| HYD | 0.97 | - |
| MET | 3.92 | 0.03 |
| MOR | 0.42 | - |
| MOR (MME) * | - | 96.76 |
| Other opioids | 2.20 | - |
| OXY | 1.17 | - |
| TAP | 0.90 | - |
| TRA | 66.35 | 2.30 |
| Harmful Effect | Pediatric Patients (%) | General Population (%) |
|---|---|---|
| Gastrointestinal | 4.43 | 0.48 |
| Nausea and vomiting | 4.22 | 0.36 |
| Cardiac | 3.46 | 1.18 |
| Hypotension | 0.47 | 0.01 |
| Dominant S wave | - | 0.38 |
| Long QTc interval | - | 0.25 |
| Right axis deviation | 0.33 | |
| Sinus bradycardia | - | 0.52 |
| Tachycardia | 0.87 | 0.005 |
| Psychiatric | 1.80 | 0.04 |
| Agitation | 1.30 | - |
| Confusion | 0.53 | 0.03 |
| Nervous system | 18.87 | 1.22 |
| Cyanosis | 0.48 | - |
| Bradypnea | 1.03 | - |
| Dizziness | 0.22 | 0.34 |
| Drowsiness | 1.46 | 0.07 |
| Drowsiness/lethargy | 4.72 | - |
| Headache | - | 0.05 |
| Lethargy | 3.33 | - |
| Respiratory depression | 3.32 | - |
| Seizure | 0.33 | 0.62 |
| Respiratory, thoracic, and mediastinal | 2.43 | 0.01 |
| Aspiration pneumonia | 0.01 | 0.004 |
| Variation in oxygen saturation level | 0.60 | - |
| Ophtamological | 4.64 | 0.01 |
| Blurred vision | - | 0.01 |
| Miosis ** | 4.49 | - |
| Other signs and administration site conditions | 1.50 | 0.11 |
| Hyperhidrosis | - | 0.04 |
| Itching/pruritus | 0.70 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.S.; Costa, N.d.S.d.; Galiciolli, M.E.A.; Garlet, Q.I.; Joaquim, J.J.; Oliveira, C.S.; Matos, C. Harmful Effects of Prescribed Opioids in Children and Adults: A Systematic Review. Pharmaceuticals 2025, 18, 1429. https://doi.org/10.3390/ph18101429
Lima LS, Costa NdSd, Galiciolli MEA, Garlet QI, Joaquim JJ, Oliveira CS, Matos C. Harmful Effects of Prescribed Opioids in Children and Adults: A Systematic Review. Pharmaceuticals. 2025; 18(10):1429. https://doi.org/10.3390/ph18101429
Chicago/Turabian StyleLima, Luíza Siqueira, Nayara de S. da Costa, Maria Eduarda A. Galiciolli, Quelen I. Garlet, João José Joaquim, Cláudia S. Oliveira, and Cristiano Matos. 2025. "Harmful Effects of Prescribed Opioids in Children and Adults: A Systematic Review" Pharmaceuticals 18, no. 10: 1429. https://doi.org/10.3390/ph18101429
APA StyleLima, L. S., Costa, N. d. S. d., Galiciolli, M. E. A., Garlet, Q. I., Joaquim, J. J., Oliveira, C. S., & Matos, C. (2025). Harmful Effects of Prescribed Opioids in Children and Adults: A Systematic Review. Pharmaceuticals, 18(10), 1429. https://doi.org/10.3390/ph18101429














