Chemical Composition, Analgesic, and Anti-Inflammatory Properties of Pelargonium odoratissimum Essential Oils (L.) L’Hérit
Abstract
1. Introduction
2. Results
2.1. Phytochemical Composition of P. odoratissimum Essential Oils
2.2. Acute Toxicity Test
2.3. Analgesic Activity
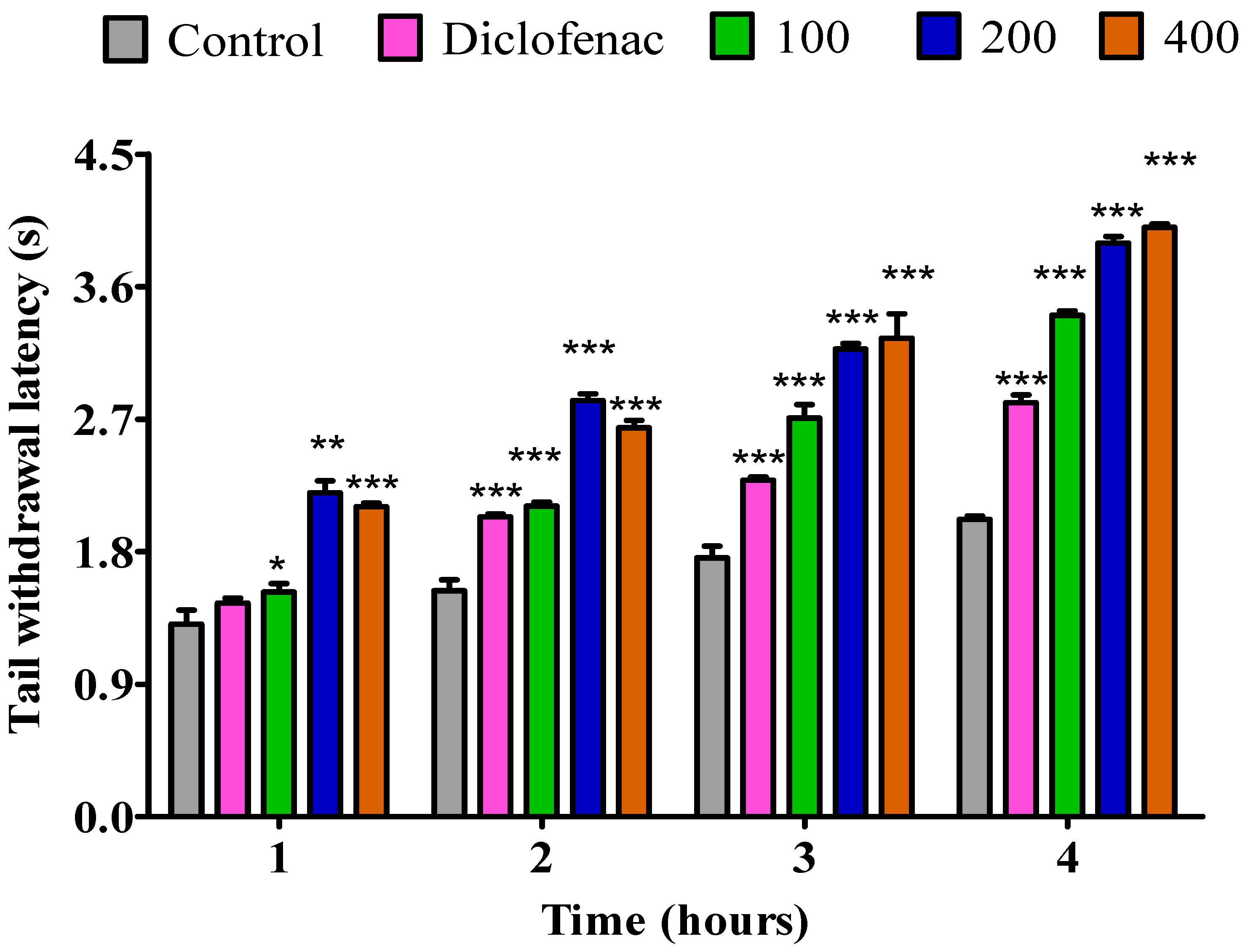
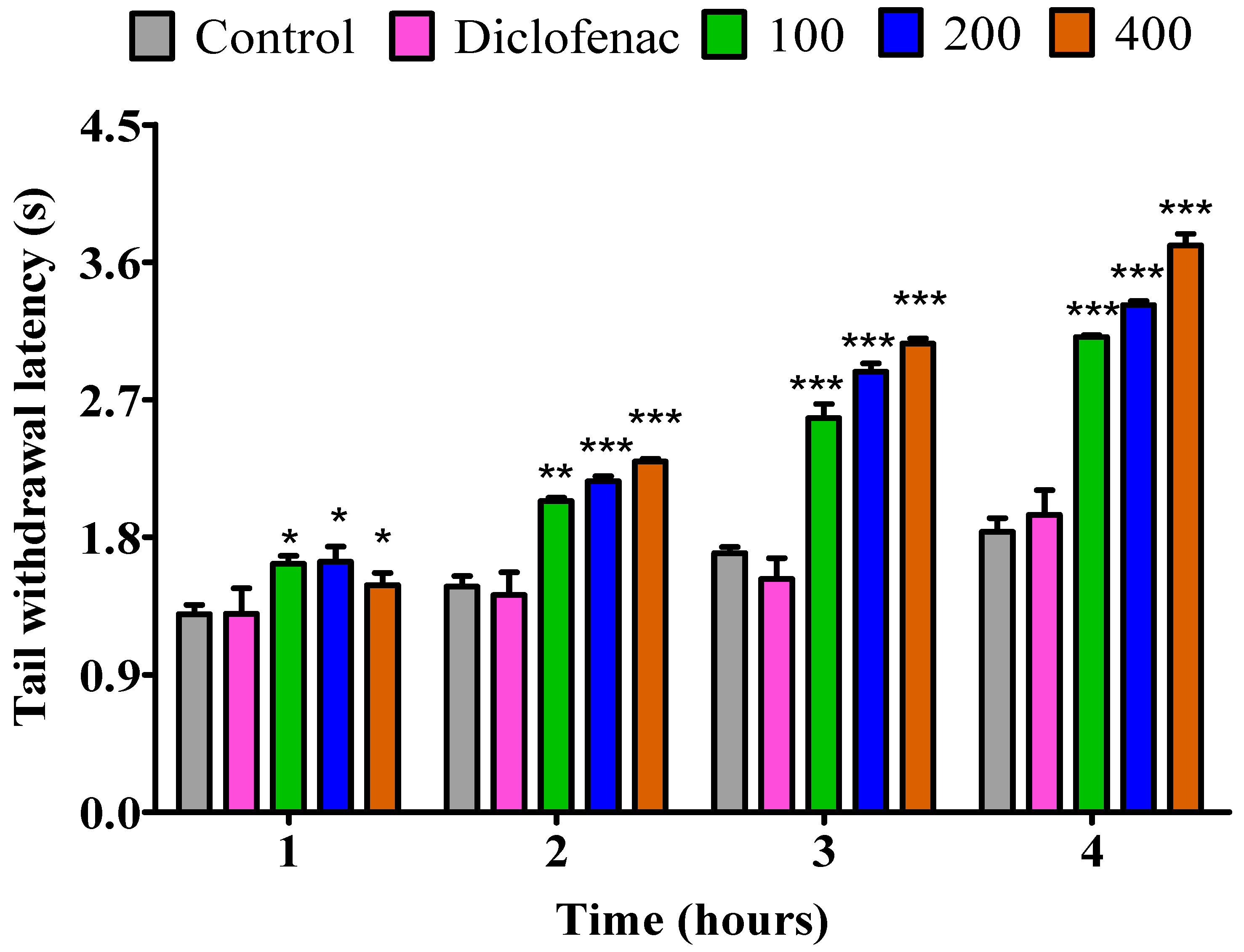
2.3.1. The Impact of Essential Oil from Fresh P. odoratissimum Leaves on Rat’s Tail Immersion in Hot Water
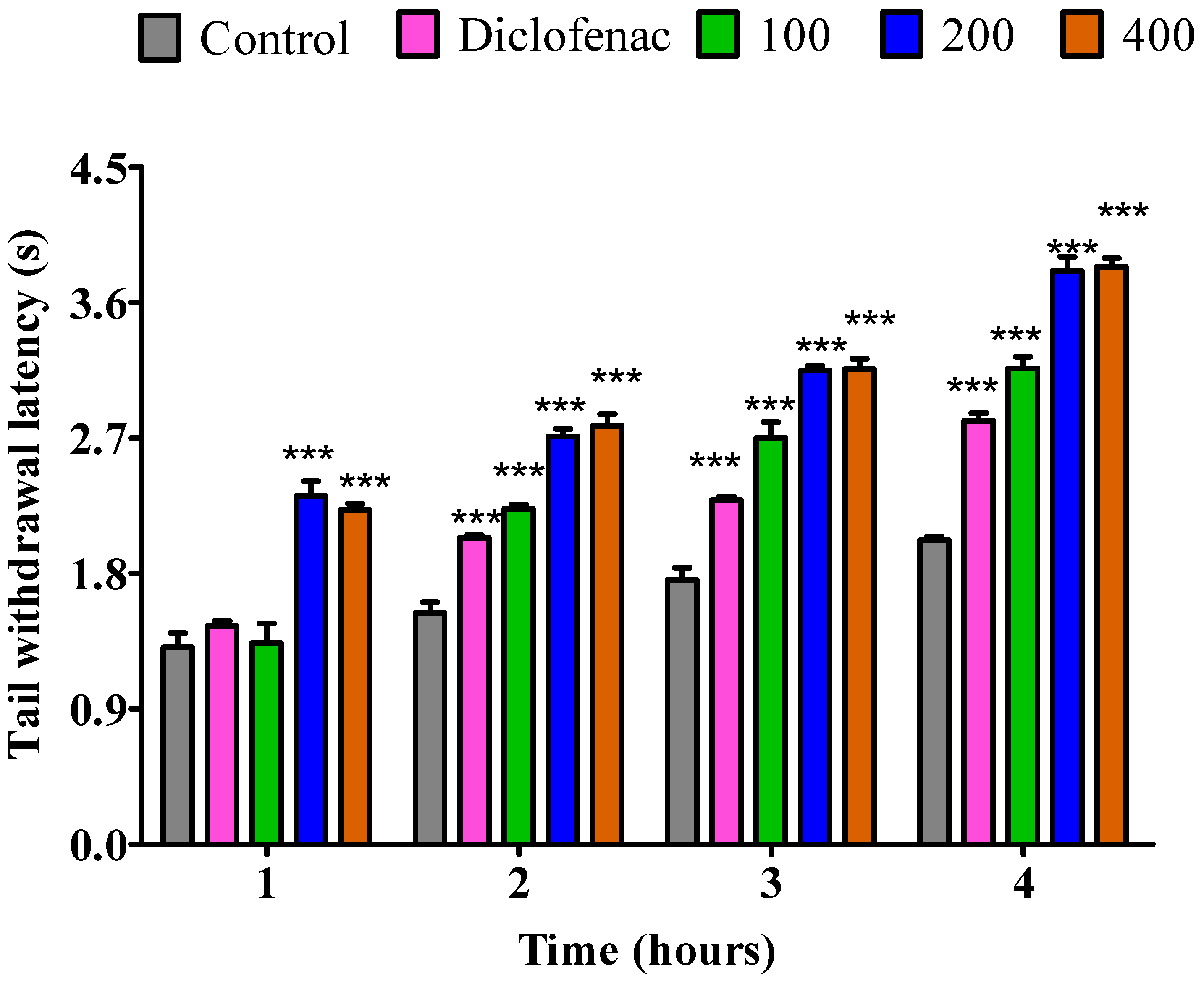
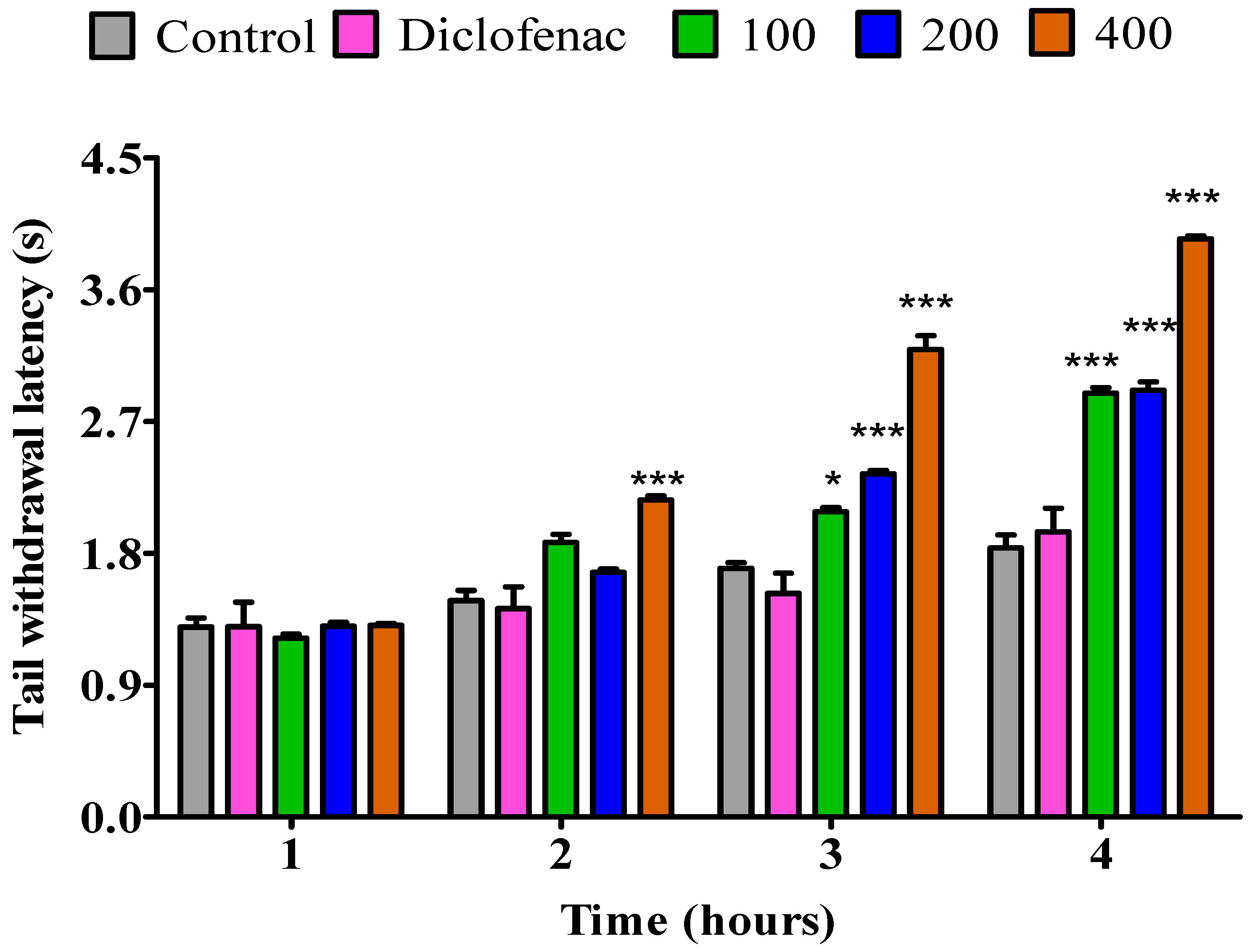
2.3.2. The Impact of Essential Oil from Dry P. odoratissimum Leaves on Rat’s Tail Immersion in Hot Water
2.3.3. The Impact of Essential Oil from Fresh P. odoratissimum Twigs on Rat’s Tail Immersion in Hot Water
2.3.4. The Impact of Essential Oil from Dry P. odoratissimum Twigs on Rat’s Tail Immersion in Hot Water
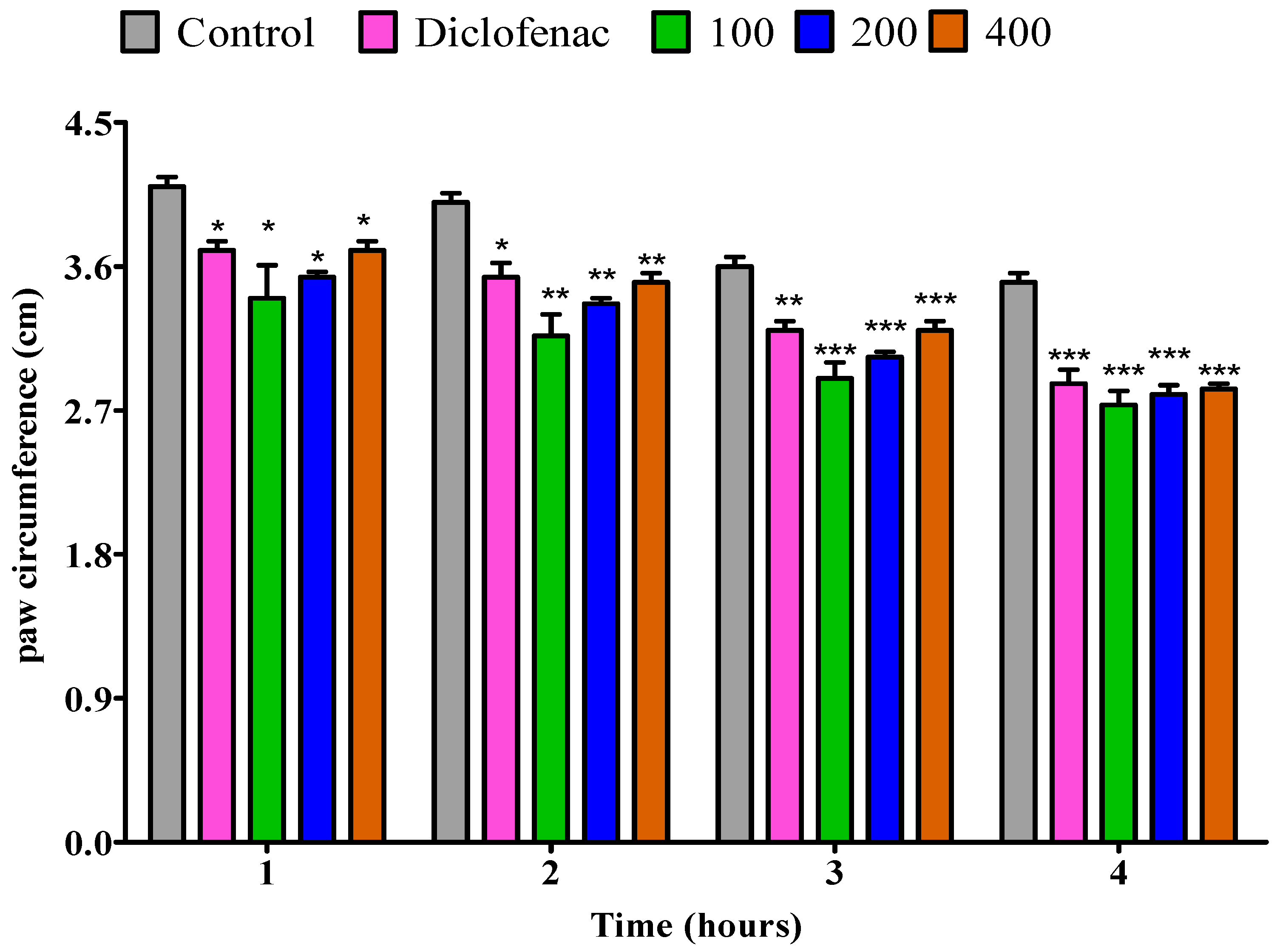
2.4. Anti-Inflammatory Effect
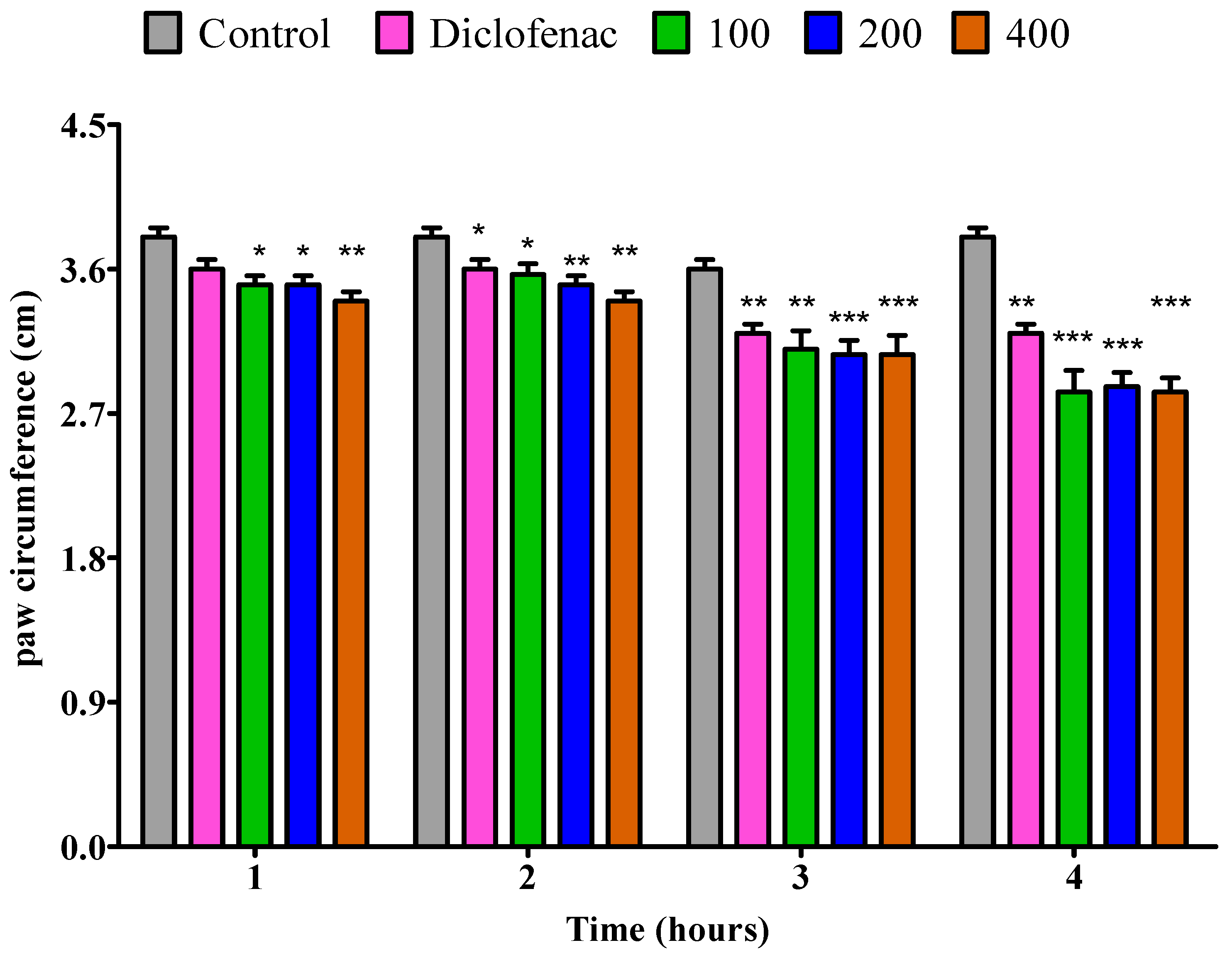
2.4.1. The Impact of the Essential Oil from Fresh P. odoratissimum Leaves on Rat’s Paw Edema Induced by Egg Albumin
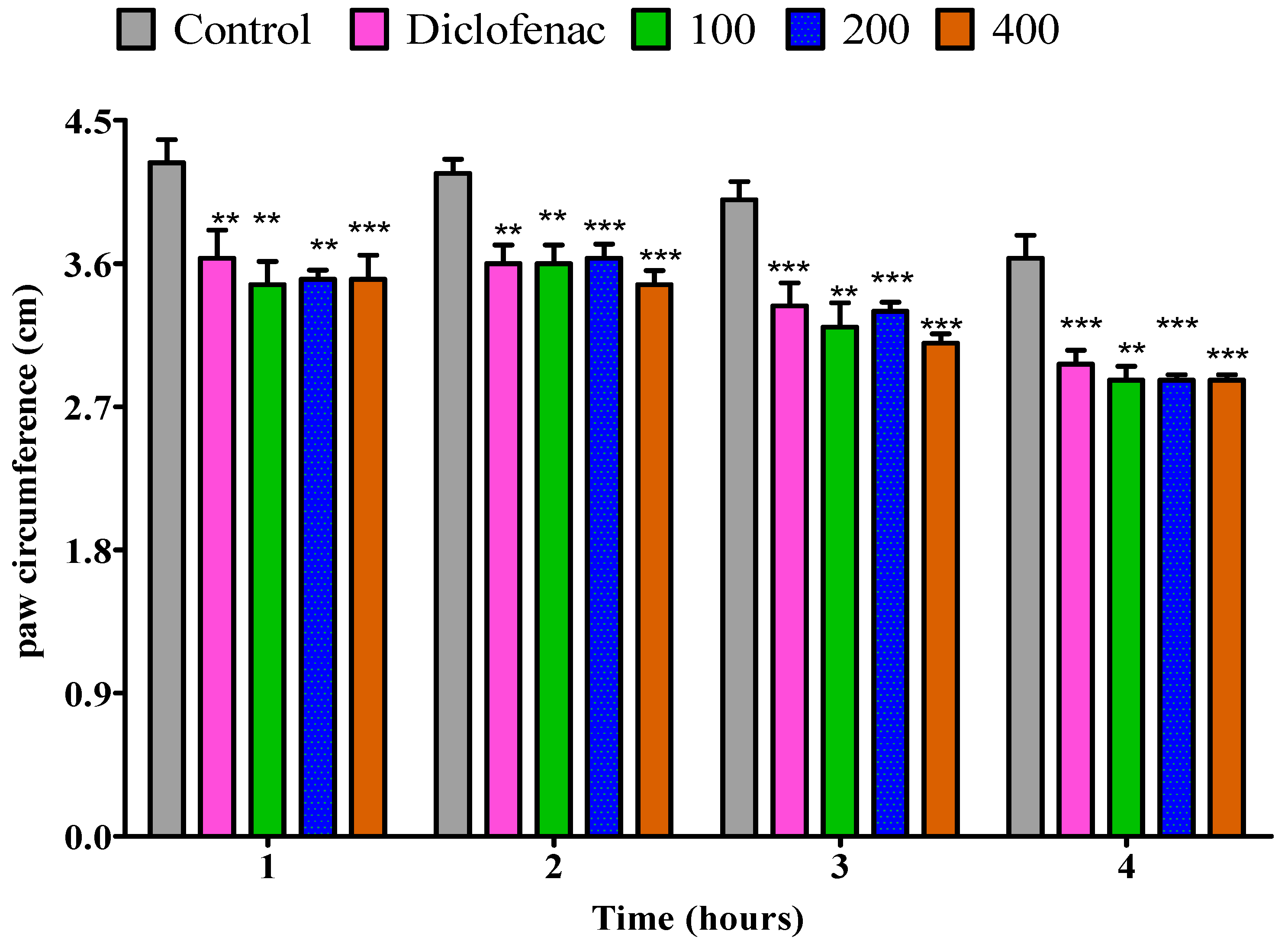
2.4.2. The Impact of Essential Oil from Dry P. odoratissimum Leaves on Rat’s Paw Edema Induced by Egg Albumin
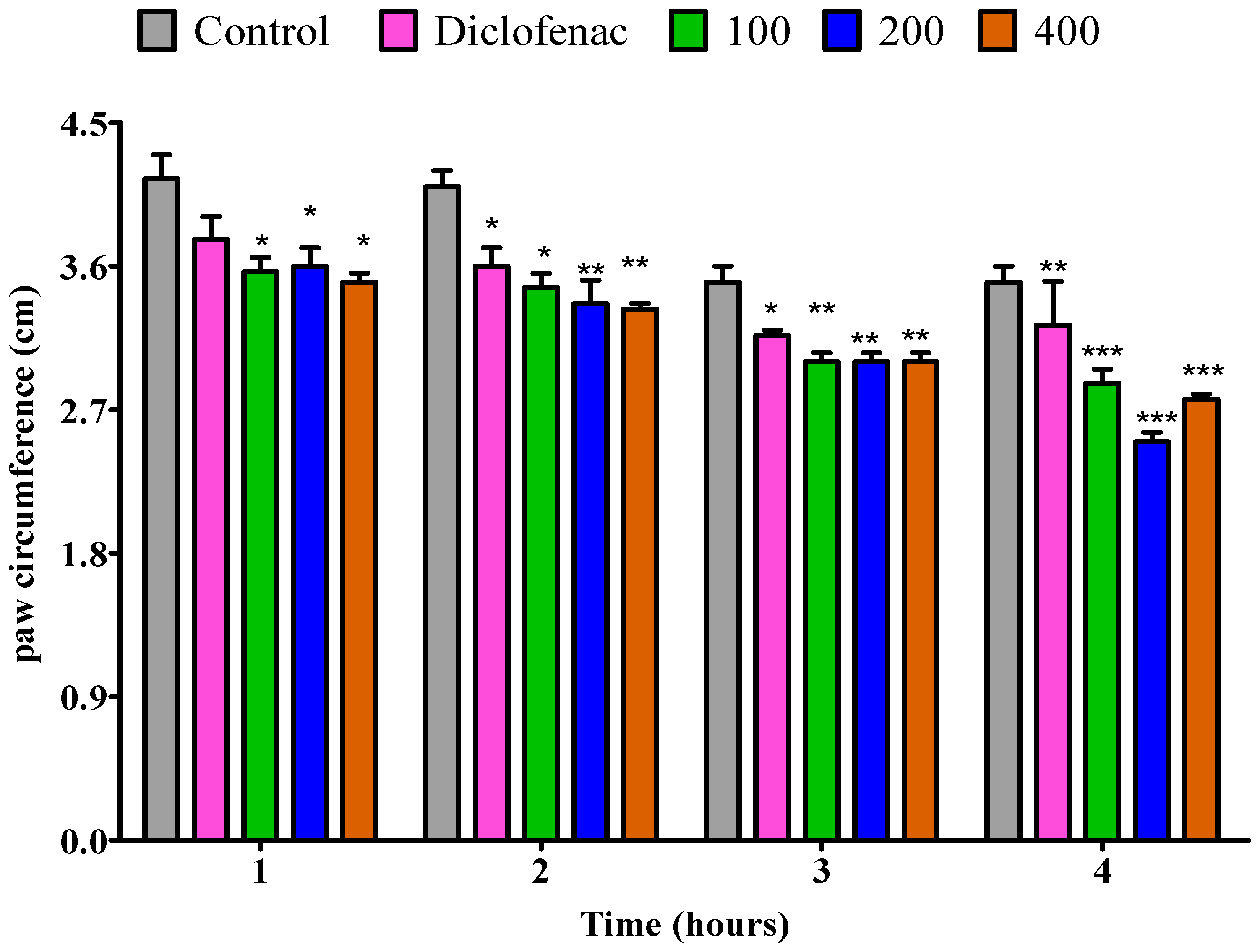
2.4.3. The Impact of Essential Oil from Fresh P. odoratissimum Twigs on Rat’s Paw Edema Induced by Egg Albumin
2.4.4. The Impact of Essential Oil from Dry P. odoratissimum Twigs on Rat’s Paw Edema Induced by Egg Albumin
3. Discussion
3.1. The Chemical Profile of Essential Oils from P. odoratissimum
3.2. Acute Toxicity and Analgesic Effect of P. odoratissimum Essential Oils
3.3. Anti-Inflammatory Activity of P. odoratissimum Essential Oils
4. Materials and Methods
4.1. Collection of P. odoratissimum and Authentication
4.2. Essential Oil Extraction
4.3. Essential Oil Analysis
4.4. Essential Oil Identification
4.5. Bioassays
4.5.1. Experimental Animals
4.5.2. Drug Used
4.5.3. Toxicity Evaluation
4.5.4. Analgesic Effect: Test of Tail Immersion
4.5.5. Impact of P. odoratissimum Essential Oils on Rat’s Paw Induced by Egg Albumin
4.5.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panda, S.; Sahoo, S.; Tripathy, K.; Singh, Y.D.; Sarma, M.K.; Babu, P.J.; Singh, M.C. Essential oils and their pharmacotherapeutics applications in human diseases. Adv. Tradit. Med. 2022, 22, 1–15. [Google Scholar] [CrossRef]
- Misra, P.; Agnihotry, S.; John, S.A.; Marker, S.; Srivastav, A.K.; Sagar, A.; Shukla, P.K. Therapeutic applications and pharmacological practices of essential oils. In Aromatherapy: The Science of Essential Oils, 1st ed.; Shukla, P.K., Srivastava, A.K., Chopra, D., Agnihotry, S., Misra, P., Singh, J., Eds.; Bentham Science Publishers: Singapore, 2024; pp. 207–275. Available online: https://www.benthamdirect.com/content/books/9789815136203.chapter-7 (accessed on 28 June 2025).
- Şenkal, B.C. The role of secondary metabolites obtained from medicinal and aromatic plants in our lives. ISPEC J. Agric. Sci. 2020, 4, 1071–1079. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plants extracts in different industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Hatvate, N.T.; Naren, P.; Khan, S.; Chavda, V.P.; Balar, P.C.; Gandhi, J.; Khatri, D.K. Essential oils for clinical aromatherapy: A comprehensive review. J. Ethnopharmacol. 2024, 330, 118180. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and essential oils: Holistic strategies in complementary and alternative medicine for integral wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential oils: Chemistry and pharmacological activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Pezantes-Orellana, C.; German Bermúdez, F.; Matías De la Cruz, C.; Montalvo, J.L.; Orellana-Manzano, A. Essential oils: A systematic review on revolutionizing health, nutrition, and omics for optimal well-being. Front. Med. 2024, 11, 1337785. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Staynova, R.; Koleva, N.; Ivanov, K.; Grekova-Kafalova, D. Public Perception and Usage Trends of Essential Oils: Findings from a Nationwide Survey. Cosmetics 2025, 12, 53. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Akacha, B.; Generalić Mekinić, I.; Ćmiková, N.; Ben Belgacem, A.; Bouteraa, M.T.; Ben Saad, R.; Mnif, W.; Kluz, M.I.; Kacaniova, M.; et al. Insight into Pelargonium odoratissimum essential oil preservative properties on ground beef. Foods 2024, 13, 3181. [Google Scholar] [CrossRef]
- Centre for Agriculture and Bioscience International Compendium. Pelargonium odoratissimum (Apple Geranium). Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.116183#core-collateral-ref-7 (accessed on 27 March 2016).
- South African National Biodiversity Institute Website. Available online: http://pza.sanbi.org/pelargonium-odoratissimum (accessed on 27 March 2016).
- Seeds and All: Planting Seeds for a Greener Tomorrow. Available online: https://seedsandall.co.za/product/apple-scented-geranium-pelargonium-odoratissimum-5-seed-pack (accessed on 27 March 2016).
- Abdelbaky, A.S.; Abd El-Mageed, T.A.; Babalghith, A.O.; Selim, S.; Mohamed, A.M. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Celi, D.; Quiroz, E.; Beltrán-Noboa, A.; Machado, A.; Tejera, E.; Fernandez-Soto, P. A chemical analysis of the Pelargonium species: P. odoratissimum, P. graveolens, and P. zonale identifies secondary metabolites with activity against gram-positive bacteria with multidrug-resistance. PLoS ONE 2024, 19, e0306637. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kedia, A.; Singh, A.; Yadav, S.; Singh, A.; Yadav, A.; Dubey, D. Antifungal, antiaflatoxin and antioxidant activity of plant essential oils and their In Vivo efficacy in protection of Chickpea Seeds. J. Food Qual. 2016, 39, 36–44. [Google Scholar] [CrossRef]
- Matusinsky, P.; Zouhar, M.; Pavela, R.; Novy, P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crops Prod. 2015, 67, 208–215. [Google Scholar] [CrossRef]
- Lo Pinto, M.; Agrò, A. Assessment of the insecticidal activity of five essential oils used against the subterranean termite Reticulitermes lucifugus (Rossi) (Blattodea, rhinotermitidae) in laboratory. J. Entomol. Zool. Stud. 2023, 11, 64–71. Available online: https://hdl.handle.net/10447/589011 (accessed on 31 August 2025).
- Abdulsamad, R.A.I. The effect of Pelargonium odoratissimum extract on some patients with diabetes. Bani Waleed Univ. J. Humanit. Appl. Sci. 2024, 9, 333–339. [Google Scholar] [CrossRef]
- Bumbulytė, G.; Būdienė, J.; Būda, V. Essential oils and their components control behaviour of yellow mealworm (Tenebrio molitor) larvae. Insects 2023, 14, 636. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Mbayachi, V.B.; Dai, W.K.; Khalil, M.; Ayejoto, D.A. Gas Chromatography/Mass Spectrometry. In Advanced Diagnostics in Combustion Science, 1st ed.; Tian, Z.Y., Ed.; Springer Nature: Singapore, 2023; pp. 33–69. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef]
- Gimenes, L.; Silva, J.C.R.L.; Facanali, R.; Hantao, L.W.; Siqueira, W.J.; Marques, M.O.M. Essential oils of new Lippia alba genotypes analyzed by flow-modulated comprehensive two-dimensional gas chromatography (GC × GC) and chemometric analysis. Molecules 2021, 26, 2332. [Google Scholar] [CrossRef]
- Szutt, A.; Dołhańczuk-Śródka, A.; Sporek, M. Evaluation of chemical composition of essential oils derived from different Pelargonium species leaves. Ecol. Chem. Eng. 2019, 26, 807–816. [Google Scholar] [CrossRef]
- Ospina, L.M.P.; Peláez, J.A.M.; Borrego-Muñoz, P.; Corona, W.F.C.; Villamizar, L.B. Chemical composition and antimicrobial activity of the essential oil of Pelargonium odoratissimum (L.) L’Herit. (Geraniaceae). Rev. Fac. Cienc. Básicas 2016, 12, 74–83. [Google Scholar] [CrossRef]
- Khalid, K.A.; Cai, W.; Ahmed, A.M.A. Effect of harvesting treatments and distillation methods on the essential oil of lemon Balm and Apple Geranium plants. J. Essent. Oil Bear. 2019, 12, 120–130. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Cutex quinque fasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Mugao, L. Factors influencing yield, chemical composition and efficacy of essential oils. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 169–178. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef] [PubMed]
- Pina, L.T.; Rabelo, T.K.; Borges, L.P.; Gonçalves, V.S.; Silva, A.S.; Oliveira, M.A.; Quintans, J.S.; Júnior, L.J.Q.; Scotti, L.; Scotti, M.T.; et al. Antihyperalgesic effect of γ-terpinene complexed in β-cyclodextrin on neuropathic pain model induced by tumour cells. Int. J. Pharm. 2024, 662, 124538. [Google Scholar] [CrossRef]
- Silva, G.H.O.; Amaral, C.F.; da Rocha, E.M.T.; Cuman, R.K.N.; de Souza Silva Comar, F.M. Effect of gamma-terpinene on the articular inflammatory response. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 1–8. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Jorjani, M. On the therapeutic targets and pharmacological treatments for pain relief following spinal cord injury: A mechanistic review. Biomed. Pharmacother. 2021, 139, 111563. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, Y.; Chi, P.; Liu, H.; Jing, Z.; Cao, H.; Du, Y.; Zhao, Y.; Qin, X.; Zhang, W.; et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy—A review. Pharmacol. Res.-Mod. Chin. Med. 2023, 6, 100210. [Google Scholar] [CrossRef]
- Golestaneh Talaei, M.; Jahandideh, M. Anti-inflammatory activity of Cymbopogon schoenanthus essential oil in animal models. Res. J. Pharmacogn. 2019, 6, 61–68. [Google Scholar] [CrossRef]
- Messaoudi, M.; Rebiai, A.; Sawicka, B.; Atanassova, M.; Ouakouak, H.; Larkem, I.; Egbuna, C.; Awuchi, C.G.; Boubekeur, S.; Ferhat, M.A.; et al. Effect of extraction methods on polyphenols, flavonoids, mineral elements, and biological activities of essential oil and extracts of Mentha pulegium L. Molecules 2021, 27, 11. [Google Scholar] [CrossRef]
- Bai, X.; Liu, L.; Zhang, J.; Chen, L.; Wu, T.; Aisa, H.A.; Maiwulanjiang, M. Spectrum–effect relationship between GC-QTOF-MS fingerprint and antioxidant, anti-inflammatory activities of Schizonepeta tenuifolia essential oil. Biomed. Chromatogr. 2021, 35, e5106. [Google Scholar] [CrossRef]
- Su, Y.H.; Lin, J.Y. Menthone supplementation protects from allergic inflammation in the lungs of asthmatic mice. Eur. J. Pharmacol. 2022, 931, 175222. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.B.; Alexander, H.D.; Linden, J.; Murray, E.K.; Gibson, D.S. Anti-inflammatory effects of phytocannabinoids and terpenes on inflamed Tregs and Th17 cells in vitro. Exp. Mol. Pathol. 2024, 139, 104924. [Google Scholar] [CrossRef]
- Matuka, T.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical composition and in vivo anti-inflammatory activity of essential oils from Citrus sinensis (L.) osbeck growing in South Africa. J. Essent. Oil-Bear. Plants 2020, 23, 638–647. [Google Scholar] [CrossRef]
- Khan, Z.; Nath, N.; Rauf, A.; Emran, T.B.; Mitra, S.; Islam, F.; Chandran, D.; Barua, J.; Khandaker, M.U.; Idris, A.M.; et al. Multifunctional roles and pharmacological potential of β-sitosterol: Emerging evidence toward clinical applications. Chem. Biol. Interact. 2022, 365, 110117. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.D.; Regan, M.C.; Myers, S.J.; Nocilla, K.A.; Akins, N.S.; Tahirovic, Y.A.; Wilson, L.J.; Dingledine, R.; Furukawa, H.; Traynelis, S.F.; et al. Novel GluN2B-selective NMDA receptor negative allosteric modulator possesses intrinsic analgesic properties and enhances analgesia of morphine in a rodent tail flick pain model. ACS Chem. Neurosci. 2023, 14, 917–935. [Google Scholar] [CrossRef]
- Ramos, A.G.B.; Menezes, I.R.A.D.; da Silva, M.S.A.; Torres Pessoa, R.; de Lacerda Neto, L.J.; Rocha Santos Passos, F.; Melo Coutinho, H.D.; Iriti, M.; Quintans-Júnior, L.J. Antiedematogenic and anti-inflammatory activity of the monoterpene isopulegol and its β-cyclodextrin (β-CD) inclusion complex in animal inflammation models. Foods 2020, 9, 630. [Google Scholar] [CrossRef]
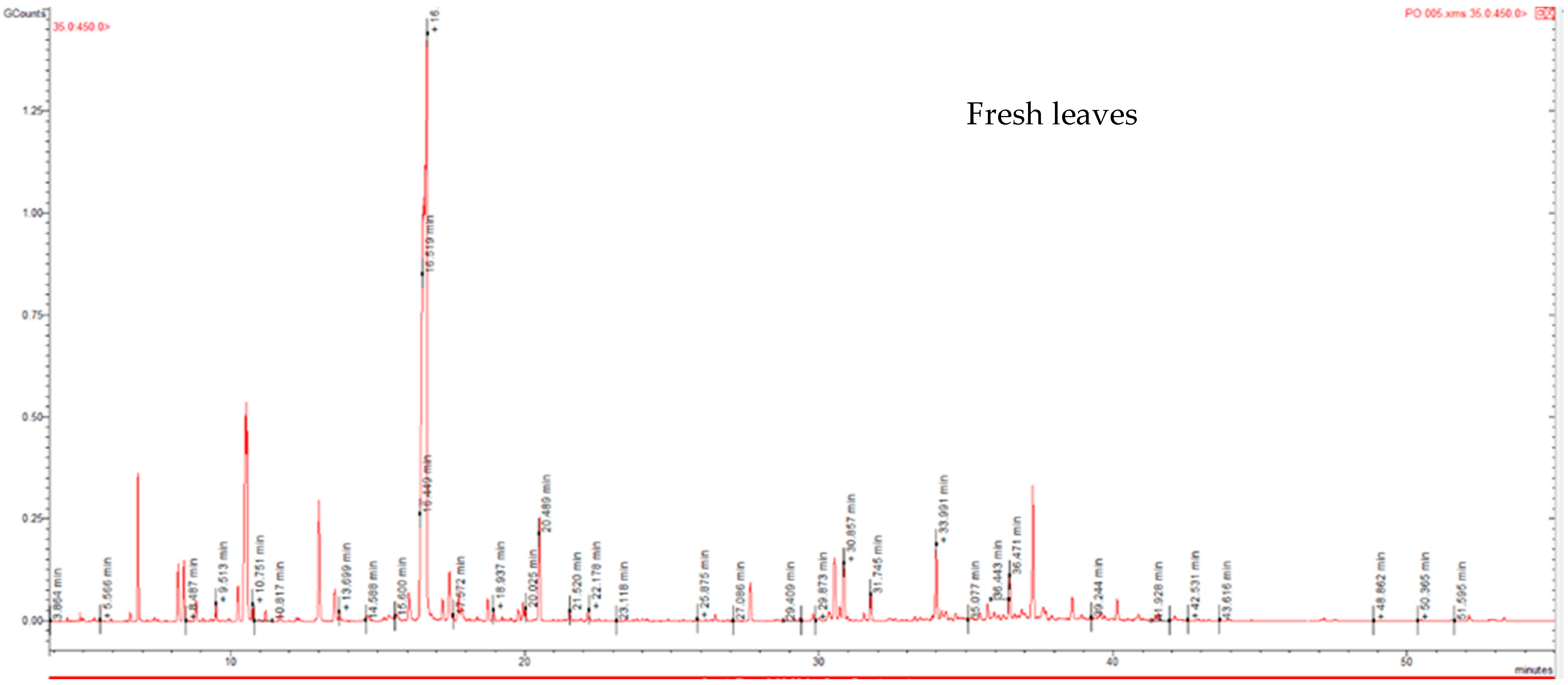
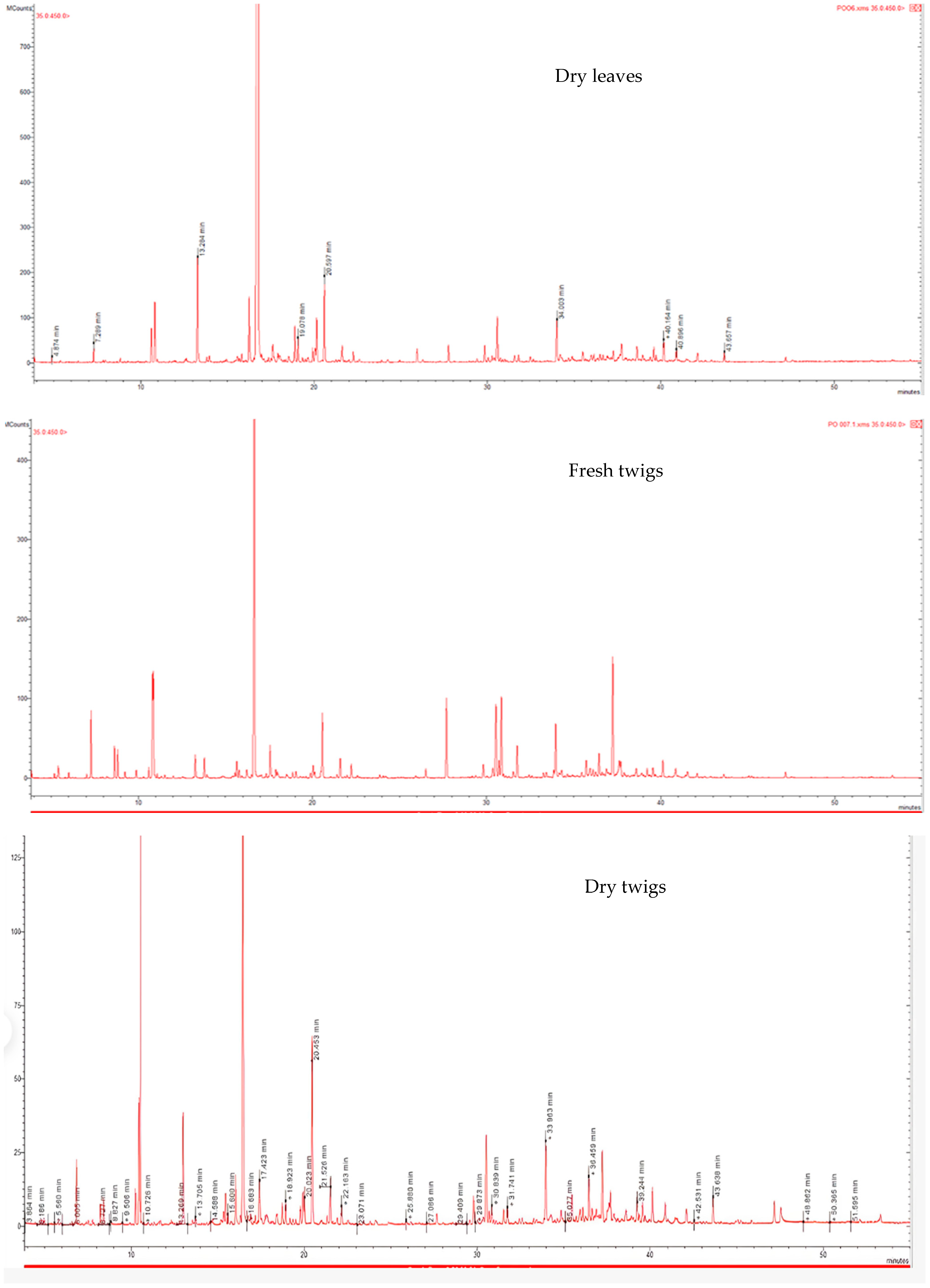

| No. | KIa | KIb | Compounds | Area (%) | |||
|---|---|---|---|---|---|---|---|
| Fresh Leaves | Dry Leaves | Fresh Twigs | Dry Twigs | ||||
| 1 | 762 | 763 | Toluene | - | - | 2.3 | - |
| 2 | 767 | 793 | 3-Methylheptane | - | - | 9.4 | - |
| 3 | 822 | 823 | 2,4-Dimethylheptane | - | - | 4.7 | 2.6 |
| 4 | 866 | 907 | m-Xylene | - | - | 2.0 | - |
| 5 | 883 | 888 | p-Xylene | - | - | 13.0 | 2.6 |
| 6 | 896 | 907 | o-Xylene | - | - | 4.3 | - |
| 7 | 939 | 939 | α-Pinene | 0.2 | - | - | - |
| 8 | 976 | 969 | Sabinene | - | 1.5 | - | 3.2 |
| 9 | 980 | 978 | β-Pinene | 0.2 | - | - | - |
| 10 | 1005 | 1007 | α-Phellandrene | 0.3 | - | - | - |
| 11 | 1026 | 1028 | p-Cymene | - | 0.5 | - | - |
| 12 | 1031 | 1030 | β-Phellandrene | 0.6 | - | 2.8 | - |
| 13 | 1040 | 1043 | cis-β-ocimene | 0.3 | - | - | - |
| 14 | 1053 | 1052 | trans-β-ocimene | 0.5 | - | - | - |
| 15 | 1062 | 1060 | γ-Terpinene | 6.8 | - | - | - |
| 16 | 1154 | 1153 | Menthone | 41.8 | 63.9 | - | - |
| 17 | 1164 | 1162 | isoMenthone | - | - | 46.3 | 45.7 |
| 18 | 1177 | 1174 | Terpinen-4-ol | 0.9 | - | - | - |
| 19 | 1189 | 1186 | Crypton | 2.2 | 2.3 | - | 4.5 |
| 20 | 1193 | 1195 | Myrtenal | 0.4 | 0.5 | - | - |
| 21 | 1210 | 1210 | Pseudocumohyroquinone | 0.2 | - | - | - |
| 22 | 1229 | 1225 | cis-Carveol | - | 0.5 | - | - |
| 23 | 1239 | 1237 | Cuminaldehyde | 1.4 | 1.9 | - | 2.0 |
| 24 | 1251 | 1249 | Piperitone | 3.6 | 4.6 | 5.3 | 10.0 |
| 25 | 1287 | 1288 | p-Cymen-7-ol | 0.6 | 0.7 | - | - |
| 26 | 1375 | 1379 | β-Elemene | 0.3 | - | - | - |
| 27 | 1380 | 1388 | β-Maaliene | - | 4.1 | - | - |
| 28 | 1380 | 1379 | β-Patchoulene | 1.3 | - | - | - |
| 29 | 1387 | 1405 | isoLongifolene | 0.7 | - | - | - |
| 30 | 1402 | 1405 | Longifolene | 0.7 | 0.8 | - | - |
| 31 | 1409 | 1410 | α-Gurjunene | 6.2 | - | - | - |
| 32 | 1461 | 1460 | Allo-aromadendrene | 1.7 | - | - | - |
| 33 | 1480 | 1483 | Germacrene D | - | 0.9 | 2.5 | - |
| 34 | 1484 | 1486 | γ-Selinene | 0.6 | - | - | 9.2 |
| 35 | 1485 | 1484 | α-Armophene | 0.4 | 0.5 | - | - |
| 36 | 1494 | 1498 | α-Selinene | 1.8 | 2.5 | 4.9 | 3.0 |
| 37 | 1499 | 1500 | α-Muurolene | - | 1.3 | - | - |
| 38 | 1505 | 1507 | α-Bulnesene | - | 0.7 | - | - |
| 39 | 1508 | 1509 | E,E-α-farnesene | 0.2 | - | - | - |
| 40 | 1513 | 1514 | γ-Cadinene | 0.6 | - | - | 5.2 |
| 41 | 1522 | 1523 | Lilial | 1.8 | 1.1 | - | - |
| 42 | 1524 | 1522 | δ-Cadinene | 1.2 | - | - | - |
| 43 | 1576 | 1578 | Spathulenol | 1.8 | 1.9 | - | 3.5 |
| 44 | 1576 | 1579 | Globulol | 0.4 | - | - | - |
| 45 | 1590 | 1592 | Viridiflorol | 0.7 | - | - | - |
| 46 | 1611 | 1607 | Tetradecanal | - | - | - | 2.0 |
| 47 | 1800 | 1806 | Nootkatone | 0.2 | - | - | - |
| Monoterpenes | 11.0 | 1.5 | 2.8 | 3.2 | |||
| Monoterpenoids | 47.3 | 70.2 | 51.6 | 55.7 | |||
| Sesquiterpenes | 17.2 | 10.8 | 7.4 | 17.4 | |||
| Sesquiterpenoids | 3.1 | 1.9 | - | 3.5 | |||
| Saturated hydrocarbons | - | - | 14.1 | 2.6 | |||
| Aromatics | 0.1 | 0.5 | 21.6 | 2.6 | |||
| Others | 5.2 | 5.3 | - | 8.5 | |||
| Total % of identified compounds | 83.9 | 90.2 | 97.5 | 93.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rungqu, P.; Oyedeji, O. Chemical Composition, Analgesic, and Anti-Inflammatory Properties of Pelargonium odoratissimum Essential Oils (L.) L’Hérit. Pharmaceuticals 2025, 18, 1428. https://doi.org/10.3390/ph18101428
Rungqu P, Oyedeji O. Chemical Composition, Analgesic, and Anti-Inflammatory Properties of Pelargonium odoratissimum Essential Oils (L.) L’Hérit. Pharmaceuticals. 2025; 18(10):1428. https://doi.org/10.3390/ph18101428
Chicago/Turabian StyleRungqu, Pamela, and Opeoluwa Oyedeji. 2025. "Chemical Composition, Analgesic, and Anti-Inflammatory Properties of Pelargonium odoratissimum Essential Oils (L.) L’Hérit" Pharmaceuticals 18, no. 10: 1428. https://doi.org/10.3390/ph18101428
APA StyleRungqu, P., & Oyedeji, O. (2025). Chemical Composition, Analgesic, and Anti-Inflammatory Properties of Pelargonium odoratissimum Essential Oils (L.) L’Hérit. Pharmaceuticals, 18(10), 1428. https://doi.org/10.3390/ph18101428







