1. Introduction
Recent epidemiology surveys revealed that nearly 100 million Americans suffer from some sort of chronic pain; among them, more than 30 million patients have migraines [
1,
2]. Migraine is a complex neurological disorder characterized by severe headaches and other accompanying symptoms which can seriously impair patients’ quality of life. Cortical spreading depression (CSD) is considered an electrophysiological correlate of migraine aura, but there is a known link between CSD and other neurological disorders, like stroke and traumatic brain injury (TBI) [
3]. CSD is characterized by a massive increase in extracellular K
+ and glutamate, as well as ionic and pH shifts [
4]. CSD is also able to activate central and peripheral nociceptive pathways, as we previously demonstrated in agreement with former literature data [
5,
6]. Despite the high prevalence and severity of migraine symptoms, including aura, available therapies (i.e., triptans) reduce pain intensity and duration in only about 30% of patients [
7]. This limitation for the treatment of migraine pain emphasizes the need to discover and validate novel, more effective targets.
Heat shock protein 90 (Hsp90) is a ubiquitous and highly expressed molecular chaperone, responsible for the regulation of protein folding of specific client proteins like transcription factors, nuclear hormone receptors, and signaling kinases [
8]. Hsp90 plays an essential role in many cellular processes, including cell cycle control, cell survival, hormones and other signaling pathways [
9]. The recognition of Hsp90 as a promoter for uncontrolled cell replication has led to the investigation of Hsp90 inhibitors as potential anti-cancer therapies; numerous second- and third-generation Hsp90 inhibitors are currently involved in clinical trials for the treatment of cancer [
10,
11]. Importantly, overexpression of Hsp90 has been linked to many other pathological conditions beyond cancer [
7]. Elevated levels of Hsp90 were observed during viral infection, inflammation, and certain neurodegenerative diseases [
12].
Novel results suggest that Hsp90 is a central regulator of acute signal transduction, which may link Hsp90 to the above disease states. It can directly regulate numerous G protein regulatory kinases (GRKs), as well as G protein coupled receptors (GPCRs). Moreover, Hsp90 can influence the localization of these proteins within different cell compartments. For instance, Hsp90 was shown to direct mature Gα subunits of GPCRs specifically to lipid rafts and mitochondria [
13]. He et al. confirmed that Hsp90 has an impact on cannabinoid signaling by regulating the translocation of Gα
i2 protein of cannabinoid type-2 receptor [
14]. Hsp90 has been shown to form a complex with small GTPases, like Raf kinase, but it is also associated with members of the Rab, Rho, and Rac kinase families [
15]. It is also known that Hsp90 influences the ERK-MAPK pathway signaling in the brain, but represses it in the spinal cord, influencing opioid signaling and opioid-induced analgesia [
16,
17]. These complex findings support that Hsp90 possesses a diverse range of cellular functions far beyond its originally identified role in protein folding and chaperoning. One of the recently recognized roles of Hsp90 is its involvement in pain signaling and analgesia [
18].
Hsp90 has been shown by several research groups to regulate the initiation and perpetuation of different types of pain [
18]. Hsp90 inhibitors significantly reduced hyperalgesia and allodynia caused by inflammatory-related pain models, such as peripheral neuropathy [
19,
20]. The administration of an Hsp90 inhibitor also mitigated the mechanical allodynia associated with monoarthritis in rats [
21]. The available results suggest that the pharmacological blockade of Hsp90 possesses analgesic effects either indirectly by suppression of inflammatory cascades or more directly by neuroprotection and the promotion of antinociceptive signaling [
18]. These findings support the therapeutic potential of Hsp90 inhibition in the treatment of pain. In our former work, we have demonstrated that the inhibition of Hsp90 can preserve blood–brain barrier integrity after CSD induction [
22]. To date, no study has investigated the possible analgesic effect of Hsp90 inhibitors in any headache model; therefore, this study addressed this gap in knowledge by investigating how Hsp90 inhibition might affect headache-like behaviors induced by cortical injection of KCl (1M) to model CSD and the potential interactions of Hsp90 with the endocannabinoid system.
3. Discussion
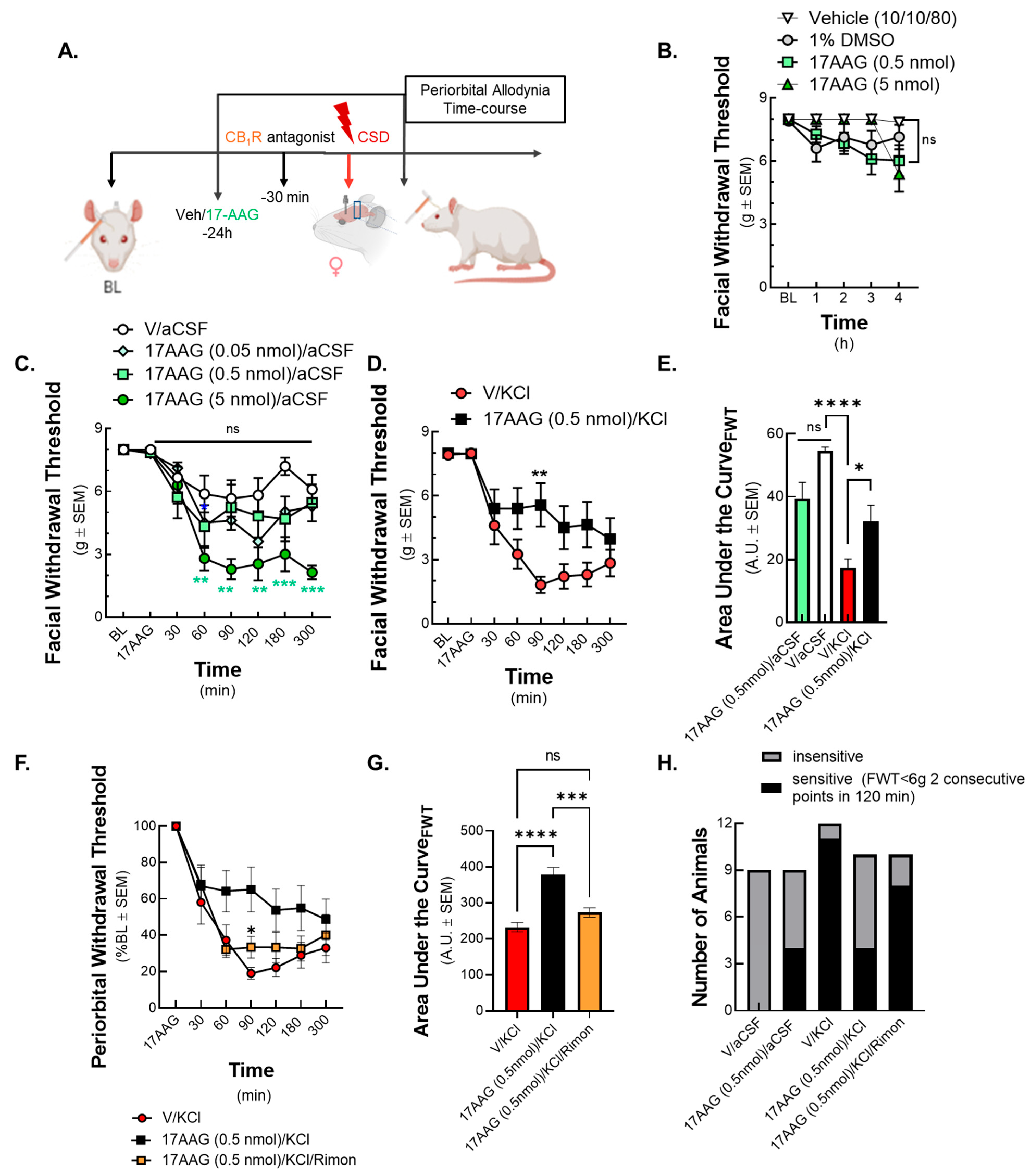
Emerging reports confirm the role of Hsp90 in pain signaling and analgesia, but our pioneering work describes the contribution of Hsp90 in migraine-like pain after CSD. Our former work indicated the effect of Hsp90 inhibition 24 h after its administration in mice models [
17]. Therefore, 17-AAG was administered in the same setting in the current CSD model and showed anti-allodynic effect in the KCl-induced CSD in female Sprague Dawley rats. This effect was mitigated by the CB
1R antagonist rimonabant and was accompanied by increased expression levels of NAPE-PLD, responsible for AEA synthesis, and reduced expression of the degradative enzyme FAAH in the periaqueductal grey (PAG), without influencing the intrinsic signaling capacity of the CB
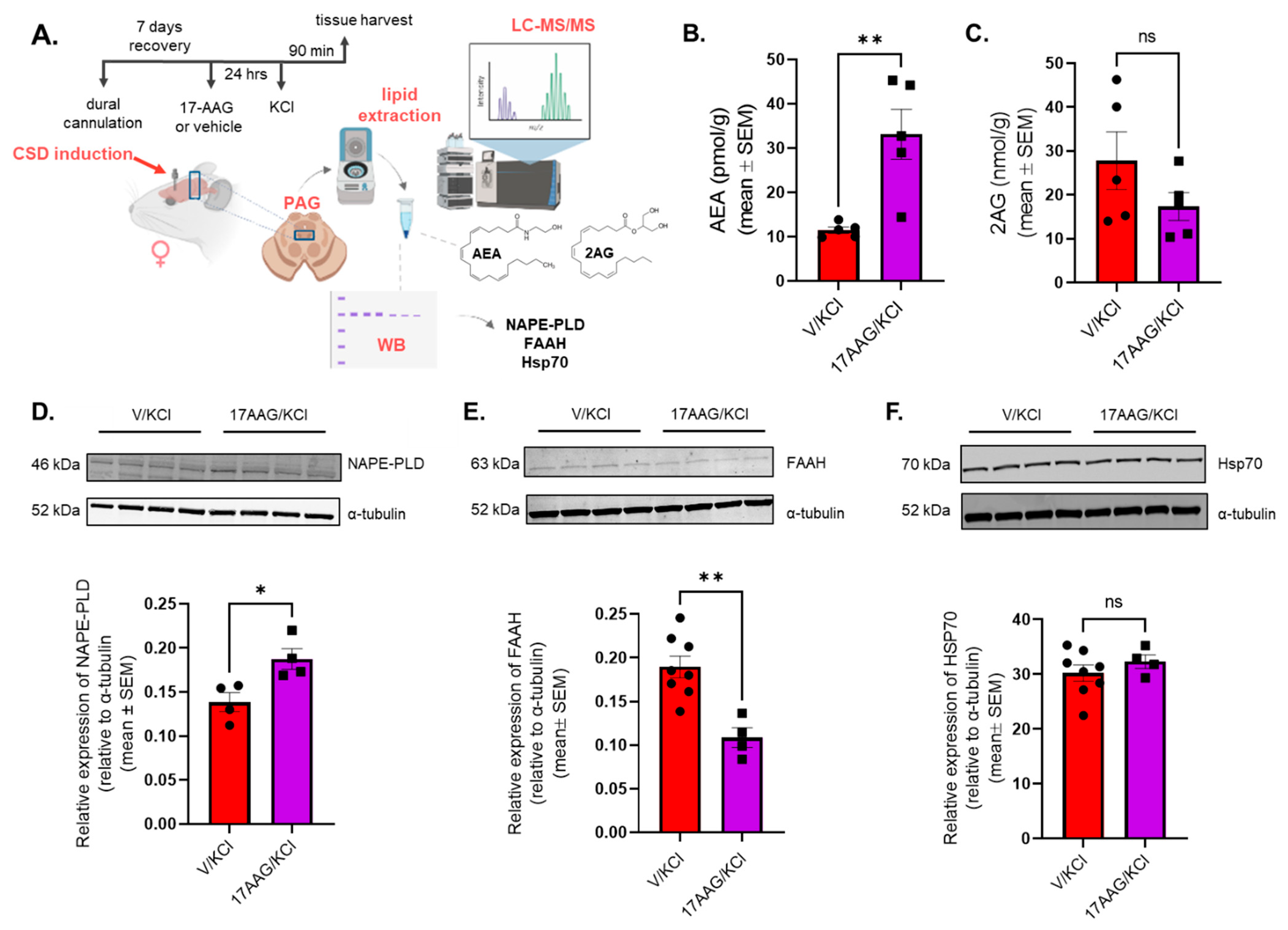
1R confirmed by GTPγS binding assay. These later observations also support the 24 h pretreatment schema of 17-AAG. This time frame could possibly allow 17-AAG to modulate the activity of Hsp90-related proteins, including transcription factors, leading to the modification in total protein expression, manifested as changes in the total level of AEA metabolic enzymes. Together, these results explain our observed increase in AEA levels in the PAG with Hsp90 inhibitor treatment. These results suggest that Hsp90 promotes pain transmission during the CSD event in the PAG, perhaps by repressing the levels of AEA, and therefore can be a new, potential target in the treatment of migraine-like headache pain.
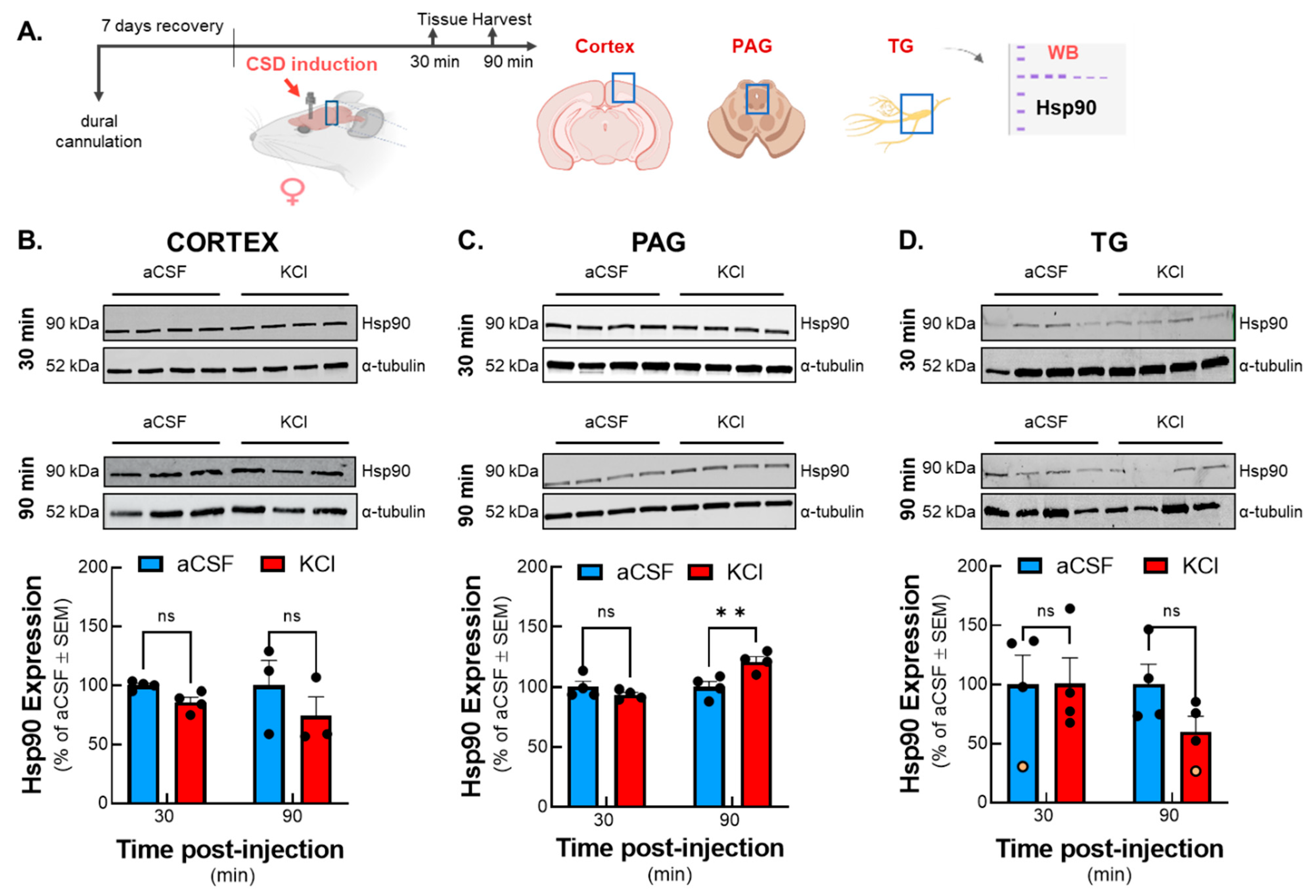
Our study mainly focused on one brain area, the periaqueductal grey (PAG). PAG is one of the most important brain centers, responsible for the ascending and descending modulation of pain perception, and it has a special role in the induction and maintenance of migraine-like pain [
31]. The activation of PAG during and after migraine is well documented [
32]. The PAG region can remain active post-cessation of pain by treatment with triptans [
33], implicating it as an important region modulating nociception from the trigeminovascular system. Our former results using the rodent model of CSD also confirm the importance of PAG area in the development of cortical spreading depression [
27]. It is well known that Hsp90 can be upregulated in several disease states in various central and peripheral tissues [
8]. However, we found elevated Hsp90 levels specifically in the PAG within the examined pain centers after induction of CSD, which could indicate increased cellular stress from the noxious stimulus. Considering the impact of CSD leading to the onset of neurogenic inflammation, the result of Hsp90 protein levels not being elevated in the other regions associated with ascending pain transmission was not anticipated and could suggest a specific role for Hsp90 in the PAG post-CSD. Knowing that PAG is a primary site of central sensitization in CSD model, our data raise the possibility that Hsp90 might be one of the key molecular mediators of this process. Since elevated expression of Hsp90 was observed exclusively in PAG samples, the rest of our study focused on that specific pain-related nucleus.
Hsp90 inhibition has proven useful in pain research, specifically in inflammatory pain [
19,
20]. More recently, the pharmacological blockade of Hsp90 has also been shown to enhance the antinociceptive properties of morphine in post-surgical and neuropathic pain models through opioid receptor signal modulation [
18]. The isoform-selective inhibitors of Hsp90 have been reported to improve the opioid therapeutic index through engagement of spinal circuits [
34]. These results indicate that Hsp90 may have a role in distinct nociceptive signaling processes. Our data support that administration of the Hsp90 inhibitor 17-AAG can lessen the facial sensitivity caused by CSD and it can also reduce the number of allodynic animals. Since the literature suggests a link between Hsp chaperones and the endocannabinoid system, we tested if the anti-allodynic effect of 17-AAG is CB receptor-dependent. The inhibition of CB
1R with rimonabant before CSD induction reduced the effect of 17-AAG, indicating the involvement of CB
1 receptor in the analgesic activity of Hsp90 blockade.
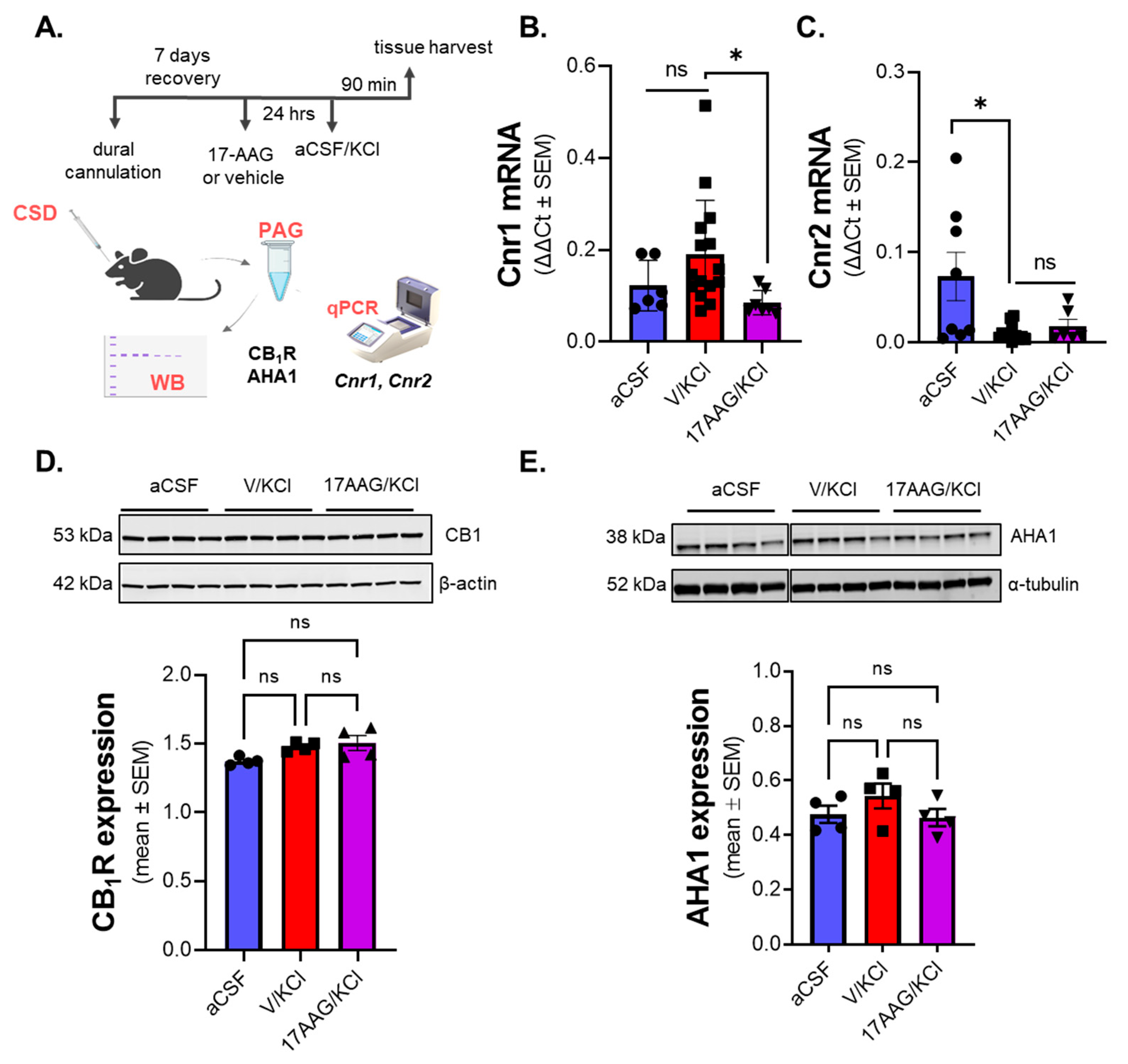
These behavioral observations encouraged further investigation of the interaction between Hsp90 and the endocannabinoid system. 17-AAG pretreatment did significantly change the
Cnr1 mRNA expression in PAG compared to the non-treated CSD group. However, total protein levels of CB
1R between those groups were unchanged. Moreover, there was no significant difference in the expression of AHA1, a co-chaperone of Hsp90 which has been reported to directly influence CB
1R expression in vitro [
25]. This result raised the possibility that Hsp90 inhibition might affect the functionality of CB
1R, rather than absolute amount. Using a
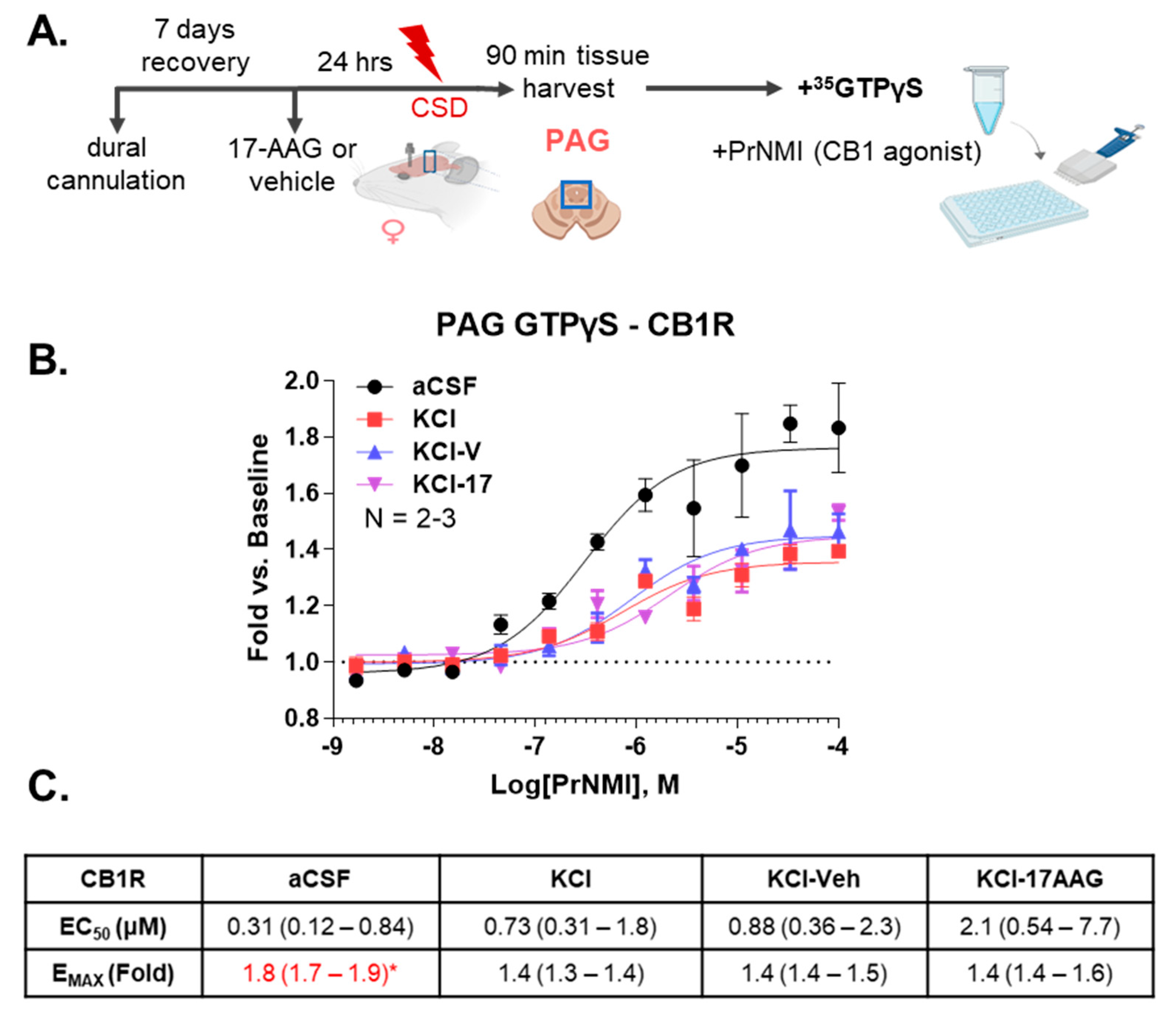
35S-GTPγS functional assay, we observed the efficacy of PrNMI was decreased after CSD induction. However, this effect was not altered by Hsp90 inhibitor treatment. Taken together, these results suggest that Hsp90 inhibition reduces allodynia in the CSD model in a CB
1R-dependent way, without alterations in CB
1R expression or activity.
To further explore the antinociceptive mechanism of Hsp90 inhibition, the level of major endocannabinoids in PAG was measured by LC-MS. The LC-MS data revealed an elevated level of AEA in PAG after 17-AAG treatment, suggesting that endocannabinoid lipids are directly impacted by Hsp90 inhibition. The level of AEA is tightly regulated by the enzymes NAPE-PLD and FAAH in the central nervous system [
29]. Our Western results show that Hsp90 inhibition increased NAPE-PLD expression while reducing FAAH levels in the PAG in post-CSD rats, thus providing a possible mechanism for elevated AEA. How 17-AAG inhibition elicits these changes in expression requires more investigation; however, the observed changes in the total level of AEA enzymes suggest that Hsp90 inhibition can possibly influence the transcription/translation machinery. During the 24 h pretreatment timeline, 17-AAG could modulate Hsp90-related transcription factors and consequently alter the protein expression. Some research points to Hsp90 as playing a role in regulating mRNA levels and protein half-life of the transcription factor HNF4A. This transcription factor promotes NAPE-PLD expression [
35]; Hsp90 chaperone machinery database:
https://www.picard.ch/Hsp90Int/index.php (accessed on 6 November 2023). Moreover, 17-AAG has been reported to downregulate STAT3, a promoter of FAAH expression [
36,
37]. Interestingly, cycloheximide, an inhibitor of protein translation abolished the additive effect of 17-AAG on the morphine-induced antinociception in mice, implying the role of protein translation in the effect of Hsp90 inhibition [
17]. Future studies may build on these reports to elucidate the exact Hsp90/eCB enzyme interaction.
Hsp70 is a known intracellular chaperone of AEA in the cytosol [
30]. Some papers have also reported increased Hsp70 levels after inhibition of Hsp90 by 17-AAG administration [
16]. Therefore, expression of Hsp70 was also measured by Western immunoblotting after 17-AAG treatment. We found no difference in levels of Hsp70 between the 17-AAG-treated and the vehicle group in PAG tissues 90 min post KCl induction, which may reflect different routes of administration, species differences, or sex differences. Overall, we can conclude that 17-AAG inhibition of Hsp90 increases AEA in the PAG tissue via increased synthesis and decreased hydrolysis of AEA and not by changing transport via Hsp70; data cannot rule out actions through Hsp90/70 interactions.
It is well known that AEA possesses analgesic effects through the activation of CB
1R [
38]. Interestingly, Lau and co-workers showed that exogenous application of AEA, but not 2-AG, produced a reduction in inhibitory GABAergic transmission in PAG neurons, and this disinhibition can lead to the analgesic effect of AEA [
28]. These former data align well with our results. The increased AEA level in PAG after 17-AAG administration can contribute to the anti-allodynic effect of Hsp90 blockade; however, its impact on GABA transmission needs to be investigated in future studies.
In addition to cannabinoid receptor activity, AEA has been reported to play a role in additional phenomena associated with migraine. A study published by Akerman and co-workers shows that AEA inhibited CGRP-induced and NO-induced dural vessel dilation, the effect of which was reversed by CB
1R antagonist AM251 [
39]. It was also reported that AEA induced a significant decrease in the nociceptive behavior in nitroglycerine-induced migraine model in male Sprague Dawley rats by reducing neuronal activation in nucleus trigeminal caudalis [
40]. Using tract tracing and gene-editing techniques may help answer the question of what circuits are most impacted by Hsp90 inhibition in the PAG. Stimulation of the TRPV1 receptor by endocannabinoids in the PAG can also lead to analgesic effects [
41]. Given the local increase in AEA within the PAG and our rimonabant results, this mechanism is unlikely but cannot be fully eliminated.
Review papers have emphasized the importance of peripheral FAAH inhibition as a possible therapeutic avenue for migraine pain [
42,
43]. Recent work has noted the importance of the central endocannabinoid system, especially 2-AG, in PAG during headache [
44]. It is also known that increased functional activity of PAG was observed during migraine pain, which can play a role in central sensitization [
32]. This work now demonstrates a significant contribution of the central AEA-endocannabinoid system in PAG during migraine-like pain.
Targeting FAAH-regulated AEA signaling has potential in the development of new agents against migraine pain. The results published by Cupini et al. showed increased FAAH activity and reduced AEA levels in platelets obtained from migraineur women [
45]. The extensive research for compounds that can modulate endocannabinoid tone resulted in several inhibitors targeting FAAH. The global FAAH inhibitor, URB597, as well as the peripherally restricted FAAH inhibitor, URB937, significantly inhibited nociceptive processing in nitroglycerine-induced migraine models [
43]. Recent safety concerns about individual FAAH inhibitors have delayed the progress of that research [
46], though clinical trials are on-going with different individual agents (clinicaltrials.gov)
Our study is the first to describe a direct region-specific modulation of the FAAH-NAPE-PLD-AEA system by an Hsp90 inhibitor, further contributing to a mechanistic understanding of CSD-associated headache disorders and the importance of CB
1R activity in the PAG for mitigating headache-associated allodynia. Hsp90’s ubiquitous expression and broad network of client protein interactions complicates its candidacy as a selective drug target. Although early generation Hsp90 inhibitors failed in clinical trial settings due to hepatotoxicity, this adverse effect has not been observed with more recent compounds that selectively target the C-terminal domain or disrupt co-chaperone interactions [
18]. Overall, Hsp90 inhibitor, 17-AAG has displayed great utility in the preclinical setting both as a pharmacological probe and as a mechanistic tool for elucidating pathways involved in neuroinflammation and GPCR signaling. Given the specific activity observed in the PAG, this study further supports investigation of next-generation Hsp90 inhibitors in headache-associated pain models. Their potential to modulate other receptor systems implicated in headache pathophysiology may be leveraged for therapeutic development.
Limitations: The authors acknowledge existing limitations of this study. First, only female Sprague Dawley rats were tested in this paper since migraine affects females to males at a ratio of approximately 3:1. Further experiments need to be performed in the future to test our findings in male animals. Second, the Hsp90 inhibitor, 17-AAG, was administered 24 h before cortical injections of KCl or aCSF through the guide cannula onto dura mater. Future studies are therefore warranted to examine the effect of other routes and different timelines of Hsp90 inhibitor administration. Furthermore, this paper mainly focuses on the PAG brain area. There is a possibility that other brain regions are also involved in the interaction of Hsp90 with the endocannabinoid system. Lastly, all antibody-based assays can be biased by lot variability, changes in access to antigen binding, or non-specific binding; thus, the differences in antibody detection may reflect those changes, rather than modification in total protein expression.
4. Material and Methods
4.1. Drugs and Reagents
7-(Allylamino)-17-demethoxygeldanamycin (17-AAG) (#AAJ66960MC) was purchased from Alfa Aesar (Haverhill, MA, USA). Rimonabant (SR141716A) was purchased from Cayman Chemicals (Ann Arbor, MI, USA).
4.2. Animals
Intact, female Sprague Dawley rats (200–250 g, n = 175) were purchased from Envigo (Indianapolis, IN, USA) and housed in a climate-controlled room on a regular 12/12 h light/dark cycle with lights on at 7:00 am with food and water available ad libitum. Animals were initially housed 3 per cage but individually housed after the dural cannulation. All procedures were performed during the 12 h light cycle and according to the policies and recommendations of the International Association for the Study of Pain and the NIH guidelines for laboratory animals, and with IACUC approval from the University of Arizona (Approval Code#: 17-223, approval date: 2 September 2023). Justification for animal numbers was consistent with NIH policy (NOT-OD-15-102), and experiments were randomized to blinded treatment groups to give 80% power to detect a treatment effect size of 20% compared to a baseline response of 5% at a significance level of 0.05 [
47]. Numbers required to achieve statistical power were determined by G.Power3.1 [
44]. To minimize bias, animals were randomly assigned to treatment groups. Female rats were used as headache disorders affect females to males at a ratio of nearly 3:1 [
48]. The stage of estrus cycle was not determined in this study.
4.3. Dural Cannulation
Dural cannulation was performed as previously described [
6,
23,
49]. Briefly, anesthesia was induced with intraperitoneal 45:5:2 mg/kg cocktail of ketamine/xylazine/acepromazine. Rats were placed in a stereotactic frame (Stoelting Co., Wood Dale, IL, USA), and a 1.5 to 2 cm incision was made to expose the skull. A 0.66 to 1 mm hole (Pinprick/KCl: −6 mm A/P, −3 mm M/L from bregma) was made with a hand drill (DH-0 Pin Vise; Plastics One, Roanoke, VA, USA) to carefully expose the dura. A guide cannula (0.5 mm from top of skull, 22 GA, #C313G; Protech International Inc., Boerne, TX, USA) was inserted into the hole and sealed into place with glue. Two additional 1 mm holes were made caudal to the cannula to receive stainless-steel screws (#MPX-080-3F-1M; Small Parts, Miami Lakes, FL, USA), and dental acrylic was used to fix the cannula to the screws. A dummy cannula (#C313DC; Plastics One) was inserted to ensure patency of the guide cannula. Rats were housed individually and allowed 6–8 d to recover. Cannula placement and dural integrity at screw placement were confirmed postmortem; animals with incorrect placement (beyond V1M) or dural damage were excluded based on a priori criteria (no animals were excluded on these bases).
4.4. Cortical Injection of KCl or aCSF
Cortical injections were performed using a Hamilton injector (30 GA, 80308 701 SN, Hamilton Company, Reno, NV, USA) customized to project 1.0 mm beyond the dura into the occipital cortex [
6]. The injector was inserted through the guide cannula to deliver a focal injection of 0.5 µL of 1 M KCl or artificial CSF (aCSF) into the cerebral cortex. aCSF comprised 145 mM NaCl, 2.7 mM KCl, 1 mM MgCl
2, 1.2 mM CaCl
2, and 2 mM Na
2HPO
4 (pH 7.4), and the solution was passed through a 0.2 µm syringe filter before injection. Cortical injections were always considered at t = 0 min.
4.5. Pre-Cortical Injection Treatments
A total of 5 uL of 17-AAG was applied onto the dura mater 24 h before cortical injections at concentrations of 0.05, 0.5, and 5 nmol utilizing the guide cannula in place for cortical injections. 17-AAG was dissolved in 100% DMSO to a concentration of 10 mM stock, and then diluted in saline for final concentration (0.05 and 0.5 nmol). The 5 nmol concentration dose was made from the same 10 mM stock into DMSO-Tween80-saline (1:1:80, v/v/v) due to solubility difficulties. Rimonabant (CB1R inverse agonist/antagonist, 1 mg/kg, 1 mL/kg) was injected intraperitoneally 30 min before cortical KCl injection.
4.6. Assessment of Periorbital Mechanical Allodynia
Periorbital allodynia was evaluated at baseline, after 17-AAG injection (acute response) and after cortical injection (t = 30, 60, 90, 120, 180, and 300 min) by an observer blinded to drug condition. Rats were grouped based on their post-surgical baseline to ensure equivalent pre-injection thresholds (6–8 g). Rats were acclimated to the testing box 1 h prior to evaluation of periorbital mechanical allodynia with calibrated von Frey filaments (1.0, 1.4, 2.0, 4.0, 6.0, 8.0 g) as previously described by Fioravanti and coworkers [
49]. Behavioral responses were determined by applying calibrated von Frey filaments perpendicularly to the midline of the forehead at the level of the eyes with enough force to cause the filament to slightly bend while held for 5 sec while the animals were freely moving. A response was indicated by a sharp withdrawal of the head, vocalization, or severe batting at the filament with attempts to bite it. The withdrawal threshold was determined using a modified version of the Dixon up–down method. Briefly, the stimulus was increased one increment after a negative response, and it was decreased one increment after a positive response. The stimulus was progressively increased until a positive response was detected, and then decreased until a negative result was obtained. This “up-down” method was repeated until three changes in behavior were observed. Animals with a post-surgical baseline threshold of <80% maximum (6g) were considered sensitive according to inclusion/exclusion criteria and removed from behavioral analyses (n = 7).
4.7. Tissue Harvest
Rats were anesthetized with a ketamine/xylazine mix (80:10 mg/kg, i.p.), and then transcardially perfused with ice cold 0.1 M phosphate buffer at flow rates so not to burst microvasculature (i.e., 3.1 mL/min). After decapitation, periaqueductal grey (PAG) was dissected (from bregma AP: −6.5 mm to −8.3 mm, ML: ±0.5 mm, DV: −5.0 mm to −6.0 mm), flash-frozen in liquid nitrogen and stored at −80 °C until further use.
4.8. Tissue Preparation for Western Immunoblotting
On the day of preparation, samples were placed in ice-cold lysis buffer (20 mm Tris-HCl, 50 mM NaCl, 2 mM MgCl2 × 6H2O, 1% v/v NP40, 0.5% v/v sodium deoxycholate, 0.1% v/v SDS; pH 7.4) supplemented with protease and phosphatase inhibitor cocktail (Halt™ Protease and Phosphatase Inhibitor Cocktail, ThermoScientific, Waltham, MA, USA). All subsequent steps were performed on ice or at 4 °C. The samples were sonicated, and then centrifuged at 12,000× g for 10 min. The supernatant was collected from the samples and BCA Assay was performed to determine the protein content (Pierce™ BCA Protein Assay Kit, Thermo Scientific).
4.9. Western Immunoblotting (WB)
Samples were thawed on ice. Total protein (20 μg) from tissue supernatant was loaded into TGX precast gels (10% CriterionTM, BioRad, Hercules, CA, USA) and transferred to nitrocellulose membrane (AmershamTM ProtranTM, GE Healthcare, Chicago, IL, USA). After transfer, the membrane was blocked at room temperature for 1 h in blocking buffer (5% dry milk in Tris-buffered saline with Tween 20 (TBST)). The following primary antibodies were diluted in blocking buffer (5% BSA in TBST): β-actin (Abcam, Waltham, MA, USA, ab6276, 1:2000), FAAH (Abcam, Waltham, MA, USA, ab54615, 1:1000), AHA1 (Abcam, Waltham, MA, USA, ab83036, 1:1000), heat shock protein 90 (Cell Signaling, Danvers, MA, USA, 4874S, 1:5000, pan-antibody), heat shock protein 70 (Cell Signaling, Danvers, MA, USA, 48725, 1:7000), cannabinoid receptor 1 (Abcam, Waltham, MA, USA, ab259323, 1:500), and NAPE-PLD (ThermoScientific, Waltham, MA, USA, PA5-115616, 1:500) α-tubulin (Cell Signaling, Danvers, MA, USA, 3873S, 1:10,000). The membrane was incubated in diluted primary antibody for 48 h at 4˚C. The membrane was washed three times in TBST for 5 min each, and then incubated with Goat anti-Mouse IRDye-680 (LiCor, Lincoln, NE, USA, 926–68020) or Goat anti-Rabbit IRDye-800 (LiCor, 926-32211) in 5% milk in TBST for 1 h rocking at room temperature (25–27 °C). The membrane was washed again three times for 5 min each and imaged with an Azure Sapphire laser imager (Azure Biosystems, Dublin, OH, USA). The Western image was produced using Azure Capture software and analyzed using Azure Spot Western Analysis software. Bands were quantified using ImageJ FIJI software 2.9.0 open access from NIH.
4.10. RT-qPCR
Total RNA was extracted from the tissue using RNeasy Mini Kit from Qiagen (Germantown, MD, USA) (#74104) per the manufacturer’s protocol, with final reconstitution of the RNA in nuclease-free water and storage at −80 °C until analysis. The RNA was quantified using a NanoDrop ND-1000 Spectrophotometer (ThermoFisher). Single-stranded complementary DNA (cDNA) was synthesized using a high-capacity cDNA reverse transcription kit (27-874-06) from Fisher Scientific, following the manufacturer’s protocol. The amplified cDNA was added to the RT2 SYBR Green Master Mix (330500; Qiagen) and gene-specific primers. RT2 qPCR primer assays (330001; Qiagen) detected the expression of Cnr1, a CB1 gene, (PPR52793A), and Cnr2, a CB2 gene (PPR50293A), as well as TUBA1a, a α-tubulin gene (PPR50840B). Gene expression was normalized to α-tubulin gene expression. The quantitative PCR (qPCR) was performed on the LightCycler 96 Real-Time PCR System (Roche, Indianapolis, IN, USA), using the following thermal program: 95 °C for 10 min, 45 cycles of 95 °C for 15 s, and 60 °C for 1 min and a melt curve analysis. The melt curves were analyzed to confirm selectivity and supported by agarose gel electrophoresis.
4.11. 35S-GTPγS Coupling
The
35S-GTPγS coupling assay was performed as described in our previous work [
50,
51]. Briefly, whole PAG was Dounce-homogenized in homogenization buffer (20 mM HEPES, pH 7; 100 mM NaCl; 2 mM MgCl
2; 1 mM EDTA; and 1 mM DTT). Tissue membrane was then centrifuged at 20,000×
g for 30 min at 4 °C. Pellet was resuspended in 1 mL of assay buffer (20 mM HEPES, pH 7; 150 mM NaCl; 2 mM MgCl
2; and 100 µM GDP) and Dounce-homogenized once more. Protein content was quantified by a BCA assay. 10 µg of PAG tissue membrane was combined with 25 pM of
35S-GTPγS (PerkinElmer, Shelton, CT, USA, #NEG030H250UC) and drug in a 200 µL reaction volume in the presence of 100 µM GDP in assay buffer. All drug dilutions and GTPγS were made in an assay buffer. The reactions were incubated at 30 °C for 2 h; then, they were collected onto GF/B filter plates (PerkinElmer) using a Brandel Cell Harvester. The plates were dried, Microscint PS (PerkinElmer) added, and the plates read in a 96-well format Microbeta2 scintillation counter (PerkinElmer). Each group was normalized to its own average baseline, and then fit to a 3-variable (Hill Slope = 1) agonist model using GraphPad Prism 9.0. The potency (EC
50) and efficacy (E
MAX) with the associated 95% confidence intervals were quantified and reported for each group.
4.12. Quantification of 2-AG and AEA by LC-MS
PAG samples for liquid chromatography/mass spectrometry (LC-MS) were purified by organic solvent extraction, as described in our previous work [
44]. Briefly, tissues were harvested, snap-frozen in pre-weighted tubes, and stored at −80 °C. On the day of the experiment, samples were weighed and homogenized in 1 mL of chloroform/methanol (2:1
v/
v) supplemented with phenylmethylsulfonyl fluoride (PMSF) at 1 mM final concentration to inhibit the degradation of endocannabinoids by endogenous enzymes. The homogenization was carried out by a Dounce homogenizer. Homogenates were then mixed with 0.3 mL of 0.7%
w/
v NaCl, vortexed, and then centrifuged for 10 min at 3200×
g at 4 °C. The aqueous phase and debris were collected and extracted two more times with 0.8 mL of chloroform. The organic phases from the three extractions were pooled together and AN internal standard was added to each sample. The mixture of internal standard solutions were prepared by serial dilution of d
4-AEA- and d
5-2-AG- in acetonitrile. Furthermore, 6 µL of 30% glycerol in methanol per sample was added to each sample, and then the organic solvents were evaporated under nitrogen gas. Dried samples were reconstituted with chloroform (0.2 mL) and mixed with 1 mL of ice-cold acetone to precipitate proteins. The mixtures were then centrifuged for 5 min at 1800×
g at 4 °C. The organic layer of each sample was collected and evaporated under nitrogen. Analysis of 2-AG and AEA was performed on an Ultivo triple quadrupole mass spectrometer combined with a 1290 Infinity II UPLC system (Agilent, Palo Alto, CA, USA), as described in our previous paper [
44]. The instrument was run in electrospray positive mode with a gas temperature of 150 °C at a flow of 5 L/min, nebulizer at 15 psi, capillary voltage of 4500 V, sheath gas at 400 °C with a flow of 12 L/min and a nozzle voltage of 300 V. The following transitions were monitored: 348.3→287.3 and 62, 352.3→287.4 and 65.9, 379.3→287.2 and 269.2, and 384.3→287.2 and 296.1 for AEA, d
4-AEA, 2-AG, and d
5-2-AG-. The first fragment listed was utilized for quantification and the second fragment was applied for confirmation. The first 3 min of analysis time was diverted to waste. Chromatographic separation used the following isocratic system: 21% 1 mM ammonium fluoride and 79% methanol on an Acquity UPLC BEH C-18 1.7u 2.1 × 100 mm column (Waters, Milford, MA, USA) maintained at 60 °C. After each injection, the column was washed with 90% methanol for one minute and then re-equilibrated for 5 min prior to the next injection. Samples were maintained at 4 °C. Mixed calibration solutions were made by serial dilution of AEA and 2-AG stock solutions in 80% C
2H
3N. Calibration curves for each analysis were prepared by mixing 10 µL of internal standard solution and 20 µL of standard solution. Prior to analysis, dried samples were dissolved in 200 µL of 80:20 C
2H
3N:H
2O then vortexed and sonicated, followed by centrifugation at 15,800×
g at 4 °C for 5 min. Supernatant was transferred to autosampler vials and 5 µL was injected for analysis.
4.13. Statistical Analysis
GraphPad Prism 9.5.0 software (GraphPad Software) was used for statistical analysis. To determine the numbers needed for each experiment, G.Power3.1 was used for 80% power to detect a 20% effect (ρ = 0.2) when alpha = 0.05. Unless otherwise stated, the data were expressed as mean ± S.E.M. Groups were assessed for normality and sphericity before statistical comparison. Periorbital allodynia measurements were assessed using a repeated-measure two-way ANOVA to analyze differences between treatment groups over time with a Bonferroni test applied post hoc [
27]. Molecular studies were compared by unpaired
t-test or one-way ANOVA with Tukey’s post-test, as indicated. Differences were considered significant if
p ≤ 0.05. To limit a biased interpretation of data, researchers conducting and analyzing the experiments were blinded to the protocol, with another researcher responsible for randomizing and treating rats to each experiment (e.g., double-blind).