Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity
Abstract
1. Introduction
2. Results
2.1. Characteristics of Honeys
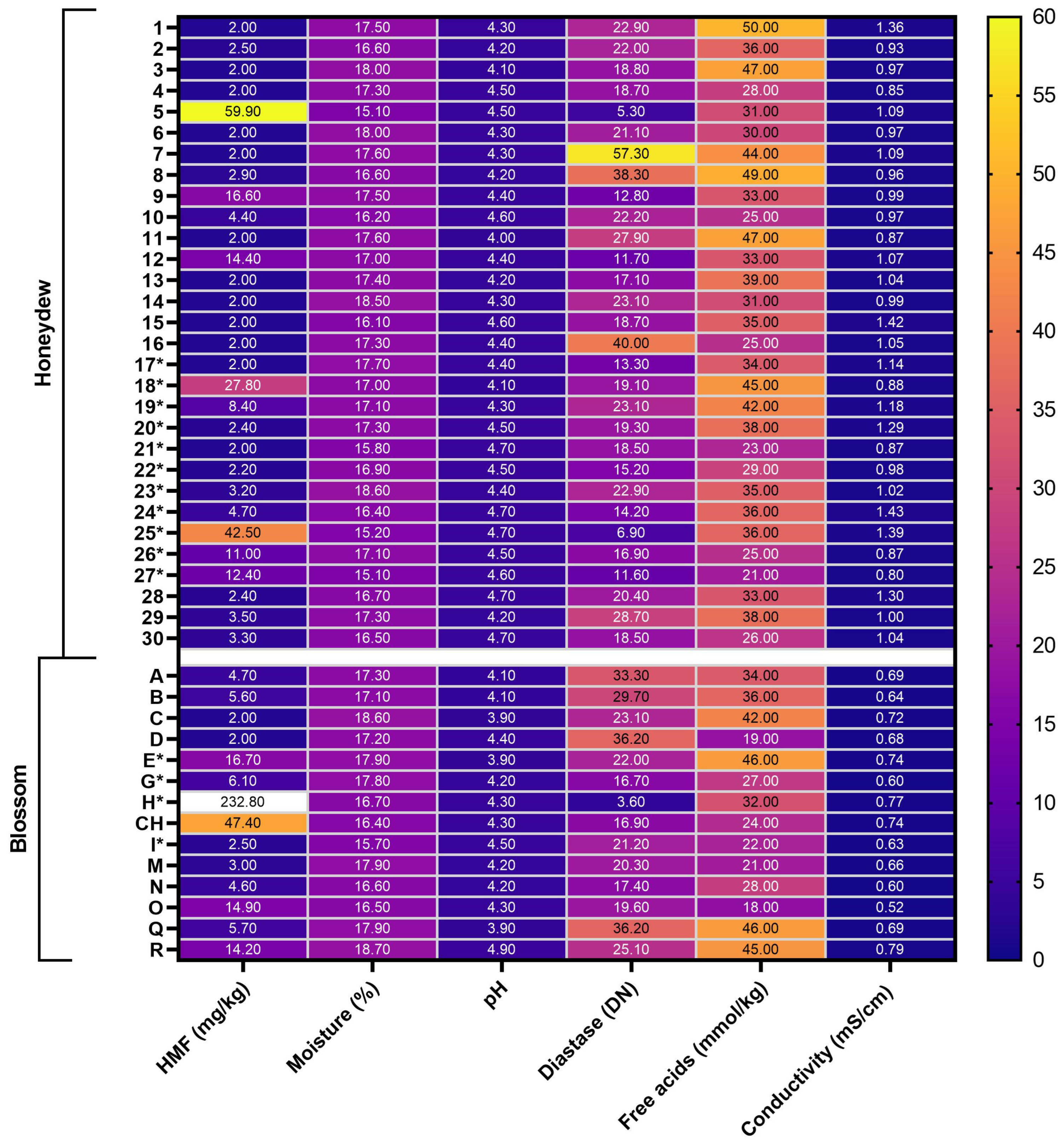
2.1.1. Physicochemical Analysis
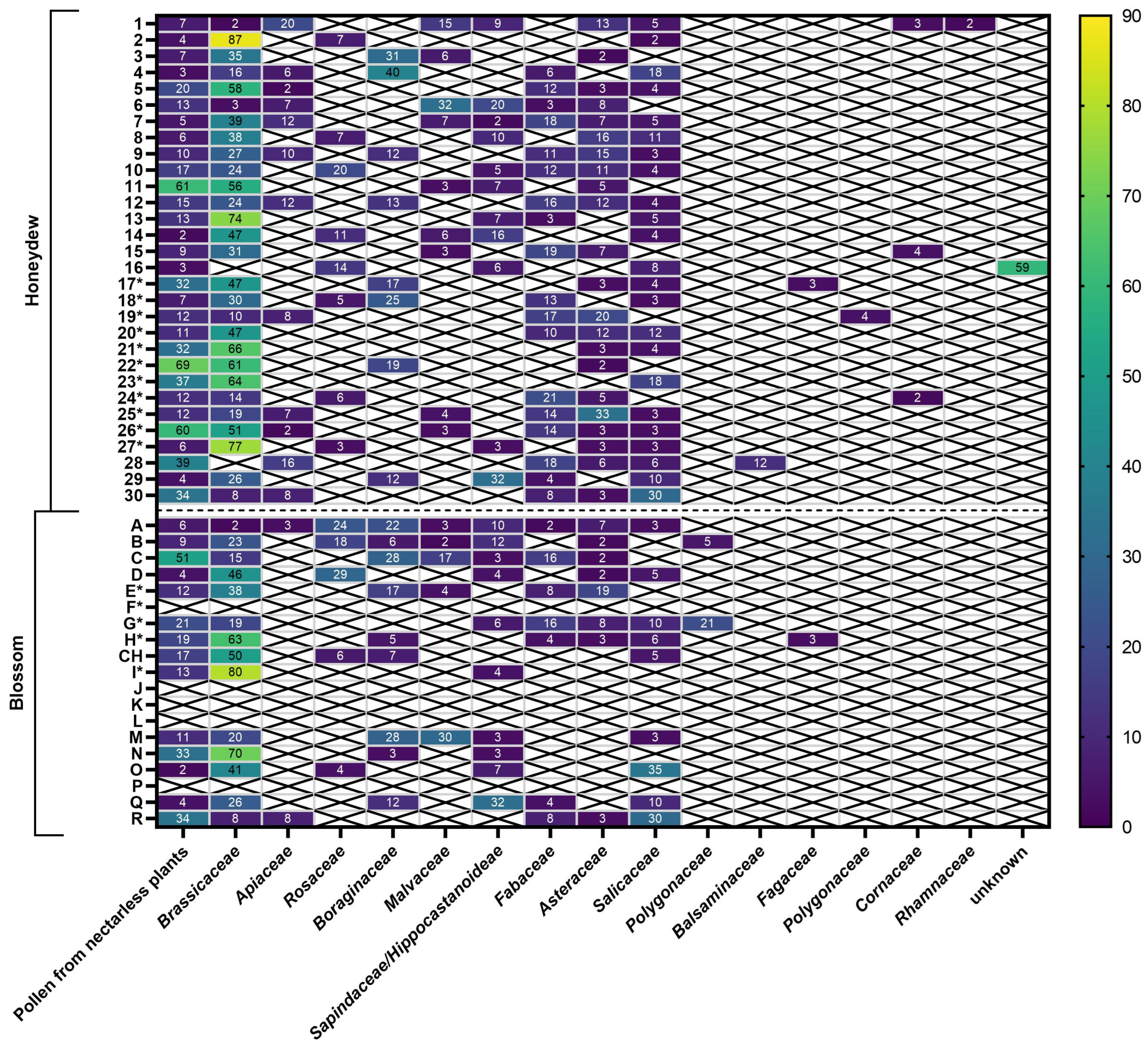
2.1.2. Melissopalynological Analysis
2.1.3. Presence of Chemical Substances
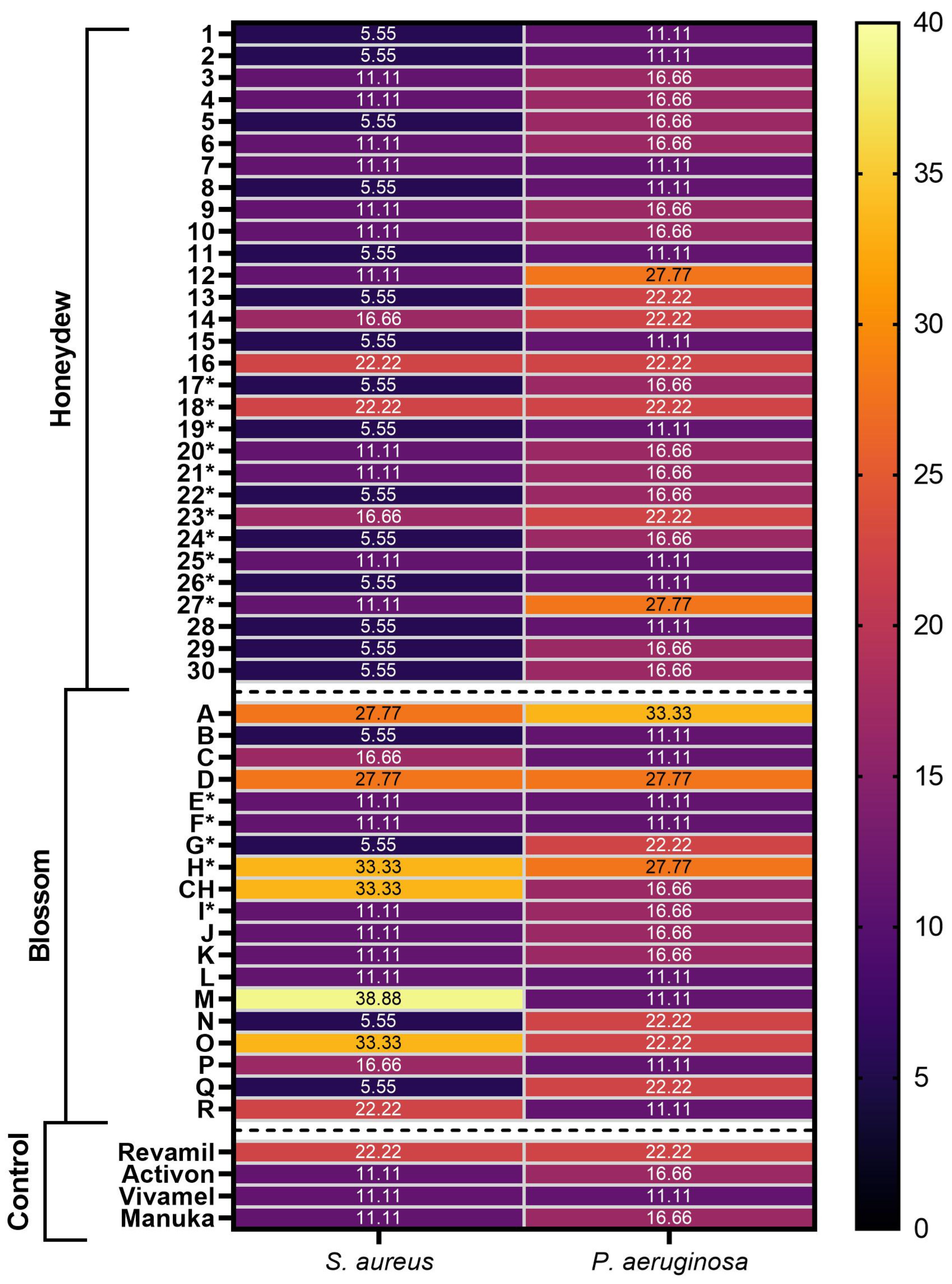
2.2. Antibacterial Activity of Honey Samples
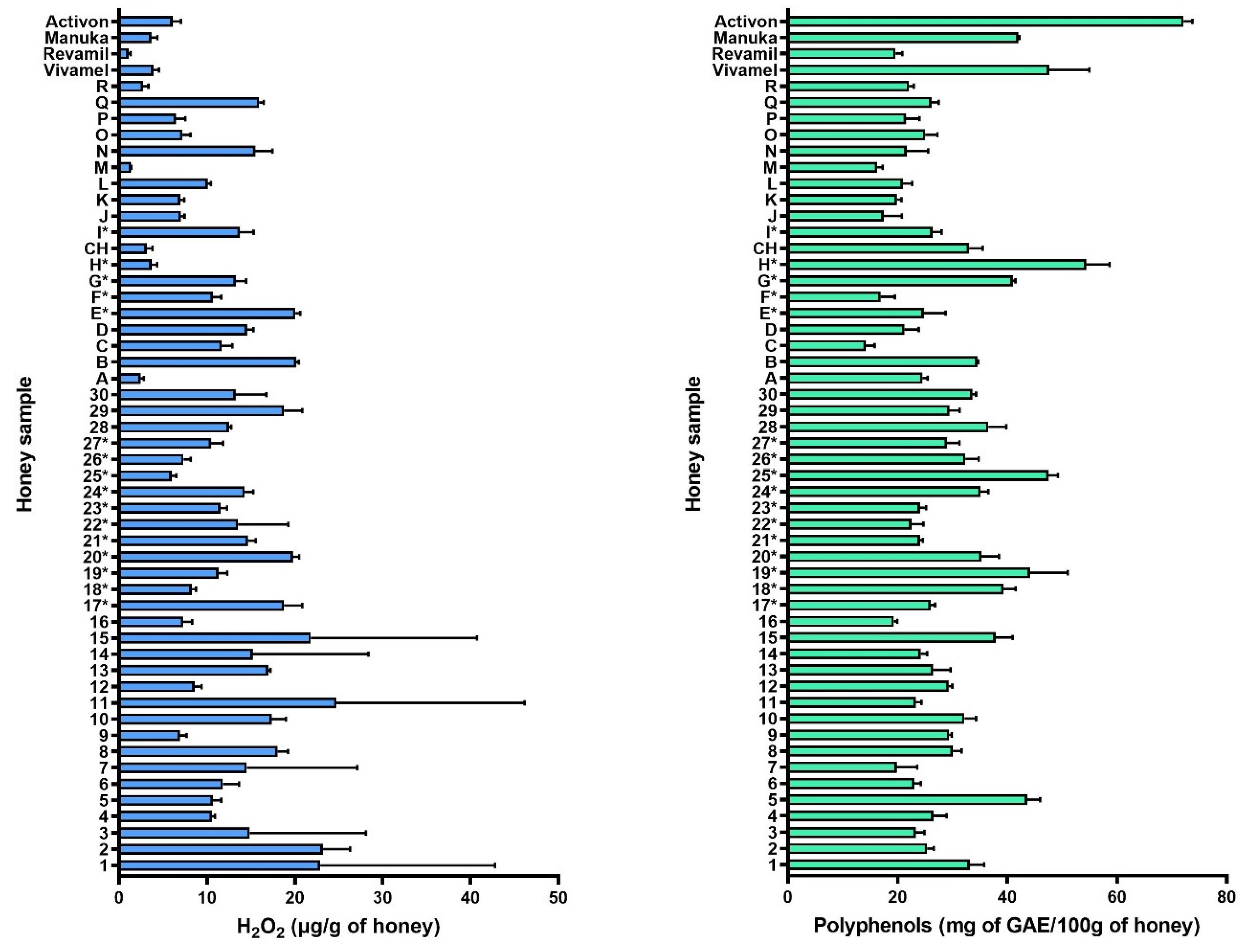
2.3. H2O2 and Total Polyphenolic Compound Contents in Honey Samples
2.4. Correlations of Parameters
3. Discussion
3.1. Quality Parameters
3.2. Undesirable Substances—Chemical Compounds
3.3. Antibacterial Activity of Samples
3.4. Relationships between Parameters
3.5. Mechanism of Antibacterial Activity of Honey
3.6. Holy Grail
4. Material and Methods
4.1. Honey Samples
4.2. Physical and Biochemical Analysis of Honeys
4.3. Bacterial Strains and Culture Conditions
4.4. Determination of H2O2 Content
4.5. Determination of Polyphenolic Content
4.6. In Vitro Antimicrobial Testing of Honey Samples
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Middendorp, J.J.; Sanchez, G.M.; Burridge, A.L. The Edwin Smith Papyrus: A Clinical Reappraisal of the Oldest Known Document on Spinal Injuries. Eur. Spine J. 2010, 19, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Dustmann, J.H. Antibacterial Effect of Honey. Apiacta 1979, 14, 7–11. [Google Scholar]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial Activity of Different Blossom Honeys: New Findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical Characteristics of Honey from Different Origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Bischofberger, A.M.; Pfrunder Cardozo, K.R.; Baumgartner, M.; Hall, A.R. Evolution of Honey Resistance in Experimental Populations of Bacteria Depends on the Type of Honey and Has No Major Side Effects for Antibiotic Susceptibility. Evol. Appl. 2021, 14, 1314–1327. [Google Scholar] [CrossRef] [PubMed]
- Troller, J.; Christian, J.H.B. Water Activity and Food; Elsevier Science: Amsterdam, The Netherlands, 1978; ISBN 978-0-12-700650-5. [Google Scholar]
- Chirife, J.; Zamora, M.; Motto, A. The Correlation between Water Activity and % Moisture in Honey: Fundamental Aspects and Application to Argentine Honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef]
- Beckh, G.; Wessel, P.; Lüllmann, C. Natürliche Bestandteile des Honigs: Hefen und deren Stoffwechselprodukte–Teil 2: Der Wassergehalt und die Wasseraktivität als Qualitätsparameter mit Bezug zum Hefewachstum. Dtsch. Lebensm. -Rundsch. 2004, 100, 14–17. [Google Scholar]
- The Water Activity of Honey and Related Sugar Solutions. Available online: https://ira.agroscope.ch/en-US/Page/Publikation?einzelpublikationId=12356&parentUrl=%2Fde-CH%2FPage%2FPublikationsliste%3Fguid%3D85894ecf-e9e1-4c3f-8499-314daea7244c%26page%3D1693 (accessed on 20 April 2024).
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, O.; Dolan, A.; Athman, R.; Power, A.; Gethin, G.; Cowman, S.; Humphreys, H. Comparison of the Antimicrobial Activity of Ulmo Honey from Chile and Manuka Honey against Methicillin-Resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2010, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Vasić, V.; Đurđić, S.; Tosti, T.; Radoičić, A.; Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž.; Trifković, J. Two Aspects of Honeydew Honey Authenticity: Application of Advance Analytical Methods and Chemometrics. Food Chem. 2020, 305, 125457. [Google Scholar] [CrossRef]
- Lachman, J.; Orsák, M.; Hejtmánková, A.; Kovářová, E. Evaluation of Antioxidant Activity and Total Phenolics of Selected Czech Honeys. LWT—Food Sci. Technol. 2010, 43, 52–58. [Google Scholar] [CrossRef]
- Alaerjani, W.M.A.; Abu-Melha, S.; Alshareef, R.M.H.; Al-Farhan, B.S.; Ghramh, H.A.; Al-Shehri, B.M.A.; Bajaber, M.A.; Khan, K.A.; Alrooqi, M.M.; Modawe, G.A.; et al. Biochemical Reactions and Their Biological Contributions in Honey. Molecules 2022, 27, 4719. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. A Current Perspective on Hydrogen Peroxide Production in Honey. A Review. Food Chem. 2020, 332, 127229. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Bartosz, G.; Stefaniuk, I.; Pichla, M.; Namieśnik, J.; Sadowska-Bartosz, I. Dietary Antioxidants as a Source of Hydrogen Peroxide. Food Chem. 2019, 278, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical Composition, Stability and Authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Salminen, S. Honeybees and Beehives Are Rich Sources for Fructophilic Lactic Acid Bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Thornburg, R.W. Tobacco Nectarin I. Purification and Characterization as a Germin-like, Manganese Superoxide Dismutase Implicated in the defensE of Floral Reproductive Tissues. J. Biol. Chem. 2000, 275, 36726–36733. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.J.; Thornburg, R.W. Tobacco Nectarin V Is a Flavin-Containing Berberine Bridge Enzyme-like Protein with Glucose Oxidase Activity. Plant Physiol. 2004, 134, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K. Unexpected Value of Honey Color for Prediction of a Non-Enzymatic H2O2 Production and Honey Antibacterial Activity: A Perspective. Metabolites 2023, 13, 526. [Google Scholar] [CrossRef]
- Almasaudi, S. The Antibacterial Activities of Honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- McLoone, P.; Warnock, M.; Fyfe, L. Honey: A Realistic Antimicrobial for Disorders of the Skin. J. Microbiol. Immunol. Infect. 2016, 49, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.-M.; Huang, J.-R.; Huang, H.-C.; Tzen, J.T.C.; Chou, W.-M.; Peng, C.-C. Facilitative Production of an Antimicrobial Peptide Royalisin and Its Antibody via an Artificial Oil-Body System. Biotechnol. Prog. 2011, 27, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Sojka, M.; Valachova, I.; Martinotti, S.; Ranzato, E.; Szep, Z.; Majtan, V.; Klaudiny, J.; Majtan, J. Bee-Derived Antibacterial Peptide, Defensin-1, Promotes Wound Re-Epithelialisation in Vitro and in Vivo. Sci. Rep. 2017, 7, 7340. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Grainger, M.N.C.; Manley-Harris, M.; Lane, J.R.; Field, R.J. Kinetics of the Conversion of Dihydroxyacetone to Methylglyoxal in New Zealand Mānuka Honey: Part II--Model Systems. Food Chem. 2016, 202, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J.; Klaudiny, J.; Bohova, J.; Kohutova, L.; Dzurova, M.; Sediva, M.; Bartosova, M.; Majtan, V. Methylglyoxal-Induced Modifications of Significant Honeybee Proteinous Components in Manuka Honey: Possible Therapeutic Implications. Fitoterapia 2012, 83, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The Origin of Methylglyoxal in New Zealand Manuka (Leptospermum scoparium) Honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.E.L.; Brown, H.L.; Jenkins, R.E. On the Antibacterial Effects of Manuka Honey: Mechanistic Insights. RRB 2015, 6, 215–224. [Google Scholar] [CrossRef][Green Version]
- Arena, E.; Ballistreri, G.; Tomaselli, F.; Fallico, B. Survey of 1,2-Dicarbonyl Compounds in Commercial Honey of Different Floral Origin. J. Food Sci. 2011, 76, C1203–C1210. [Google Scholar] [CrossRef]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl Compounds in Commonly Consumed Foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef]
- Laallam, H.; Boughediri, L.; Bissati, S.; Menasria, T.; Mouzaoui, M.S.; Hadjadj, S.; Hammoudi, R.; Chenchouni, H. Modeling the Synergistic Antibacterial Effects of Honey Characteristics of Different Botanical Origins from the Sahara Desert of Algeria. Front. Microbiol. 2015, 6, 01239. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the Standards for Medical Grade Honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Dzugan, M.; Ciszkowicz, E.; Tomczyk, M.; Miłek, M.; Lecka-Szlachta, K. Coniferous Honeydew Honey: Antibacterial Activity and Anti-Migration Properties against Breast Cancer Cell Line (MCF-7). Appl. Sci. 2024, 14, 710. [Google Scholar] [CrossRef]
- Kocyigit, A.; Aydogdu, G.; Balkan, E.; Yenigun, V.B.; Guler, E.M.; Bulut, H.; Koktasoglu, F.; Gören, A.C.; Atayoglu, A.T. Quercus Pyrenaica Honeydew Honey With High Phenolic Contents Cause DNA Damage, Apoptosis, and Cell Death Through Generation of Reactive Oxygen Species in Gastric Adenocarcinoma Cells. Integr. Cancer Ther. 2019, 18, 1534735419876334. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, N.A.; Hassan, Z.; Mustafa, M.Z.; Azman, W.N.W.; Hadie, S.N.H.; Ghani, N.; Mat Zin, A.A. The Potential Neuroprotective Effects of Stingless Bee Honey. Front. Aging Neurosci. 2023, 14, 1048028. [Google Scholar] [CrossRef] [PubMed]
- Fadzil, M.A.M.; Mustar, S.; Rashed, A.A. The Potential Use of Honey as a Neuroprotective Agent for the Management of Neurodegenerative Diseases. Nutrients 2023, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Che Mohd Nassir, C.M.N.; Abdul Hamid, H.; Hambali, A.; Abd Manan, N.; Mehat, M.Z.; Ismail, N.I.; Mustapha, M. Neuroprotective Potentials of Honey for Cerebral Small Vessel Disease. OBM Neurobiol. 2022, 6, 144. [Google Scholar] [CrossRef]
- Nweze, J.A.A.; Nweze, E.I.; Olovo, C.V.; Obi, O.J.; Chidebelu, P. Therapeutic Properties of Honey; IntechOpen: London, UK, 2019. [Google Scholar]
- Bucekova, M.; Buriova, M.; Pekarik, L.; Majtan, V.; Majtan, J. Phytochemicals-Mediated Production of Hydrogen Peroxide Is Crucial for High Antibacterial Activity of Honeydew Honey. Sci. Rep. 2018, 8, 9061. [Google Scholar] [CrossRef] [PubMed]
- Tsavea, E.; Vardaka, F.-P.; Savvidaki, E.; Kellil, A.; Kanelis, D.; Bucekova, M.; Grigorakis, S.; Godocikova, J.; Gotsiou, P.; Dimou, M.; et al. Physicochemical Characterization and Biological Properties of Pine Honey Produced across Greece. Foods 2022, 11, 943. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Search Results. CODEXALIMENTARIUS. Available online: https://www.fao.org/fao-who-codexalimentarius/search/en/?cx=018170620143701104933%3Aqq82jsfba7w&q=honey&cof=FORID%3A9 (accessed on 8 May 2024).
- European Parliament. Council Directive 2001/110/EC of 20 December 2001 Relating to Honey; European Parliament: Strasbourg, France, 2001; Volume 10. [Google Scholar]
- Vyhláška 76/2003 Sb., Kterou se Stanoví Požadavky pro Přírodní Sladidla, Med, Cukrovinky, Kakaový Prášek a Směsi. Available online: https://www.zakonyprolidi.cz/cs/2003-76 (accessed on 24 April 2024).
- Chakir, A.; Romane, A.; Marcazzan, G.L.; Ferrazzi, P. Physicochemical Properties of Some Honeys Produced from Different Plants in Morocco. Arab. J. Chem. 2016, 9, S946–S954. [Google Scholar] [CrossRef]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and Melissopalynological Characterization of Estonian Summer Honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef]
- EU Pesticides Database-MRLs. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 11 May 2024).
- Gunes, E.; Şahin, S.; Demir, C.; Borum, E.; Bilisik Tosunoglu, A. Determination of Phenolic Compounds Profile in Chestnut and Floral Honeys and Their Antioxidant and Antimicrobial Activities: Güneş et Al. J. Food Biochem. 2016, 41, e12345. [Google Scholar] [CrossRef]
- Jibril, F.; Abu Bakar, M.H.; Manivannan, L. Isolation and Characterization of Polyphenols in Natural Honey for the Treatment of Human Diseases. Bull. Natl. Res. Cent. 2019, 43, 4. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and Antimicrobial Effects of Phenolic Compounds Extracts of Northeast Portugal Honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef] [PubMed]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic Activity and Mode of Action of Flavonoids Isolated from Smaller Galangal and Amoxicillin Combinations against Amoxicillin-Resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, K. Chapter 12-Safety of Honey. In Regulating Safety of Traditional and Ethnic Foods; Prakash, V., Martín-Belloso, O., Keener, L., Astley, S., Braun, S., McMahon, H., Lelieveld, H., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 217–246. ISBN 978-0-12-800605-4. [Google Scholar]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and Biochemical Properties of Honeys from Arid Regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Bugarova, V.; Godocikova, J.; Majtan, J. Demanding New Honey Qualitative Standard Based on Antibacterial Activity. Foods 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.-J.; Sit, N.-W.; Ooi, P.A.-C.; Ee, K.-Y.; Lim, T.-M. The Antibacterial Potential of Honeydew Honey Produced by Stingless Bee (Heterotrigona itama) against Antibiotic Resistant Bacteria. Antibiotics 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Baloš, M.; Popov, N.; Vidakovic Knezevic, S.; Ljubojević Pelić, D.; Pelić, M.; Mihaljev, Ž.; Jakšić, S. Electrical Conductivity and Acidity of Honey. Arch. Vet. Med. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Masoura, M.; Passaretti, P.; Overton, T.W.; Lund, P.A.; Gkatzionis, K. Use of a Model to Understand the Synergies Underlying the Antibacterial Mechanism of H2O2-Producing Honeys. Sci. Rep. 2020, 10, 17692. [Google Scholar] [CrossRef] [PubMed]
- Bugarova, V.; Godocikova, J.; Bucekova, M.; Brodschneider, R.; Majtan, J. Effects of the Carbohydrate Sources Nectar, Sucrose and Invert Sugar on Antibacterial Activity of Honey and Bee-Processed Syrups. Antibiotics 2021, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Sasaki, M.; Nakamura, J.; Sasagawa, H.; Ohashi, K.; Takeuchi, H.; Natori, S. Change in the Expression of Hypopharyngeal-Gland Proteins of the Worker Honeybees (Apis mellifera L.) with Age and/or Role. J. Biochem. 1996, 119, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Valachova, I.; Kohutova, L.; Prochazka, E.; Klaudiny, J.; Majtan, J. Honeybee Glucose Oxidase--Its Expression in Honeybee Workers and Comparative Analyses of Its Content and H2O2-Mediated Antibacterial Activity in Natural Honeys. Naturwissenschaften 2014, 101, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Weston, R.J. The Contribution of Catalase and Other Natural Products to the Antibacterial Activity of Honey: A Review. Food Chem. 2000, 71, 235–239. [Google Scholar] [CrossRef]
- Dustmann: Über Die Katalaseaktivität in Bienenhonig-Google Scholar. Available online: https://scholar.google.com/scholar_lookup?title=%C3%9Cber%20die%20Katalaseaktivit%C3%A4t%20in%20Bienenhonig%20aus%20der%20Tracht%20der%20Heidekrautgew%C3%A4chse%20&publication_year=1971&author=J.H.%20Dustman (accessed on 11 May 2024).
- Tomás-Barberán, F.; Martos, I.; Ferreres, F.; Radovic, B.; Anklam, E. HPLC Flavonoid Profile as Markers for the Botanical Origin of European Honey. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Trifković, J.; Baošić, R.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Physicochemical Analysis and Phenolic Profile of Polyfloral and Honeydew Honey from Montenegro. RSC Adv. 2020, 10, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Olk, M.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Formation of Hydrogen Peroxide in Cell Culture Media by Apple Polyphenols and Its Effect on Antioxidant Biomarkers in the Colon Cell Line HT-29. Mol. Nutr. Food Res. 2009, 53, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska-Matysek, M.; Florek, M.; Wolanciuk, A.; Barłowska, J.; Litwińczuk, Z. Concentration of Minerals in Nectar Honeys from Direct Sale and Retail in Poland. Biol. Trace Elem. Res. 2018, 186, 579–588. [Google Scholar] [CrossRef]
- Rt, A.; Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in Honey: Environmental, Geographical and Botanical Aspects. J. Apic. Res. Bee World 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Świerczek-Zięba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.; Deshmukh, K.; Mulik, P.; Mulani, N.; Patwardhan, R. Resolving a Mechanism of Honey Antibacterial Action: Polyphenol/H2O2-Induced Oxidative Effect on Bacterial Cell Growth and on DNA Degradation. Indian J. Appl. Pure Bio. 2021, 36, 1–10. [Google Scholar]
- Brudzynski, K.; Abubaker, K.; Laurent, M.; Castle, A. Re-Examining the Role of Hydrogen Peroxide in Bacteriostatic and Bactericidal Activities of Honey. Front. Microbiol. 2011, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Juricova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Canini, A.; Majtan, J. Effect of Thermal Liquefying of Crystallised Honeys on Their Antibacterial Activities. Food Chem. 2018, 269, 335–341. [Google Scholar] [CrossRef] [PubMed]
- González-Miret, M.L.; Terrab, A.; Hernanz, D.; Fernández-Recamales, M.A.; Heredia, F.J. Multivariate Correlation between Color and Mineral Composition of Honeys and by Their Botanical Origin. J. Agric. Food Chem. 2005, 53, 2574–2580. [Google Scholar] [CrossRef]
- Škrovánková, S.; Snopek, L.; Mlček, J.; Volaří ková, E. Bioactive Compounds Evaluation in Different Types of Czech and Slovak Honeys. Potr. S. J. F. Sci. 2019, 13, 94–99. [Google Scholar] [CrossRef][Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudelka, L.; Sleha, R.; Janovska, S.; Radochova, V.; Bostik, P. Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity. Pharmaceuticals 2024, 17, 840. https://doi.org/10.3390/ph17070840
Pudelka L, Sleha R, Janovska S, Radochova V, Bostik P. Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity. Pharmaceuticals. 2024; 17(7):840. https://doi.org/10.3390/ph17070840
Chicago/Turabian StylePudelka, Ludovit, Radek Sleha, Sylva Janovska, Vera Radochova, and Pavel Bostik. 2024. "Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity" Pharmaceuticals 17, no. 7: 840. https://doi.org/10.3390/ph17070840
APA StylePudelka, L., Sleha, R., Janovska, S., Radochova, V., & Bostik, P. (2024). Czech Honeydew Honeys—A Potential Source of Local Medical Honey with Strong Antimicrobial Activity. Pharmaceuticals, 17(7), 840. https://doi.org/10.3390/ph17070840




