Is Camphor the Future in Supporting Therapy for Skin Infections?
Abstract
1. Introduction
2. Camphor’s Chemical Properties and Methods of Production
3. Camphor Occurrence
4. Camphor as an Additional Ingredient in Products
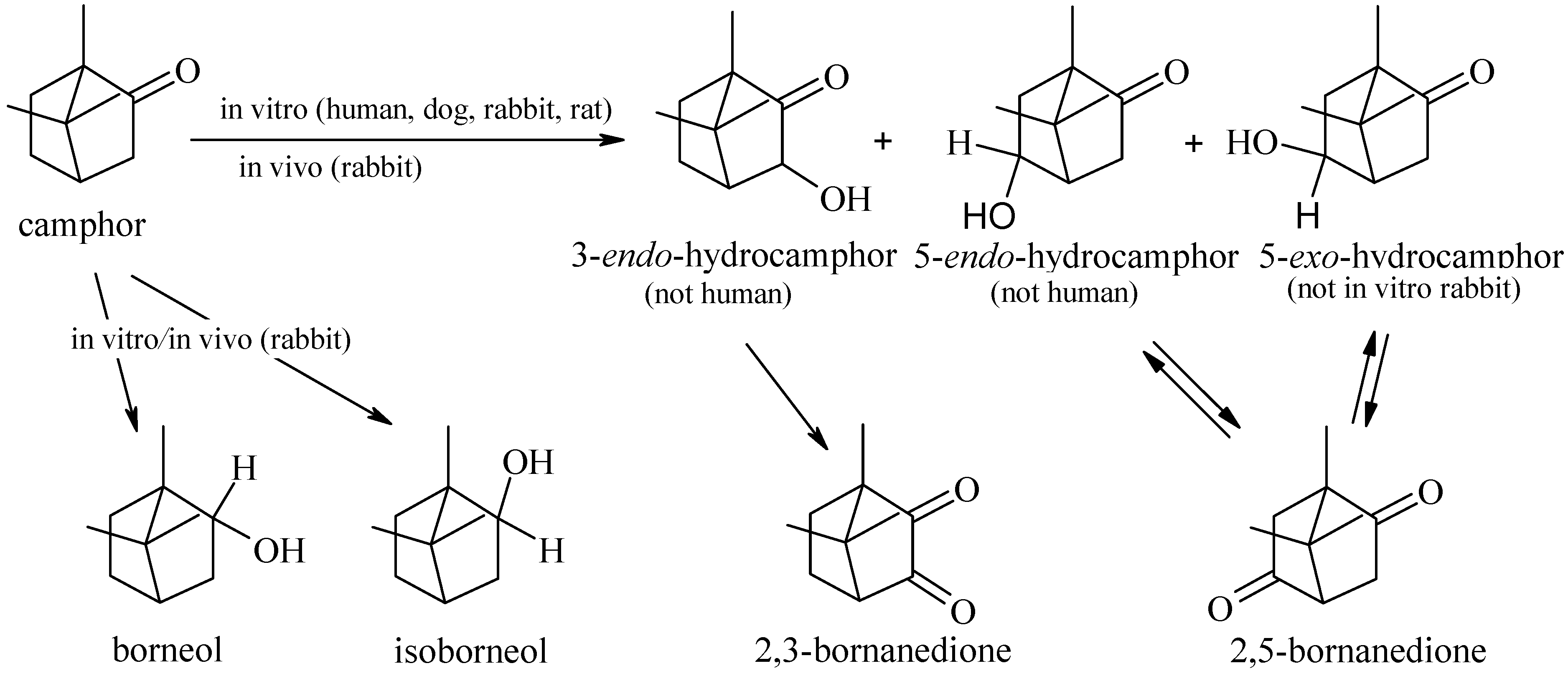
5. Camphor Metabolism
6. Molecular Activity of Camphor
Molecular Effects of Camphor-Containing Essential Oils on Pathogen Cells
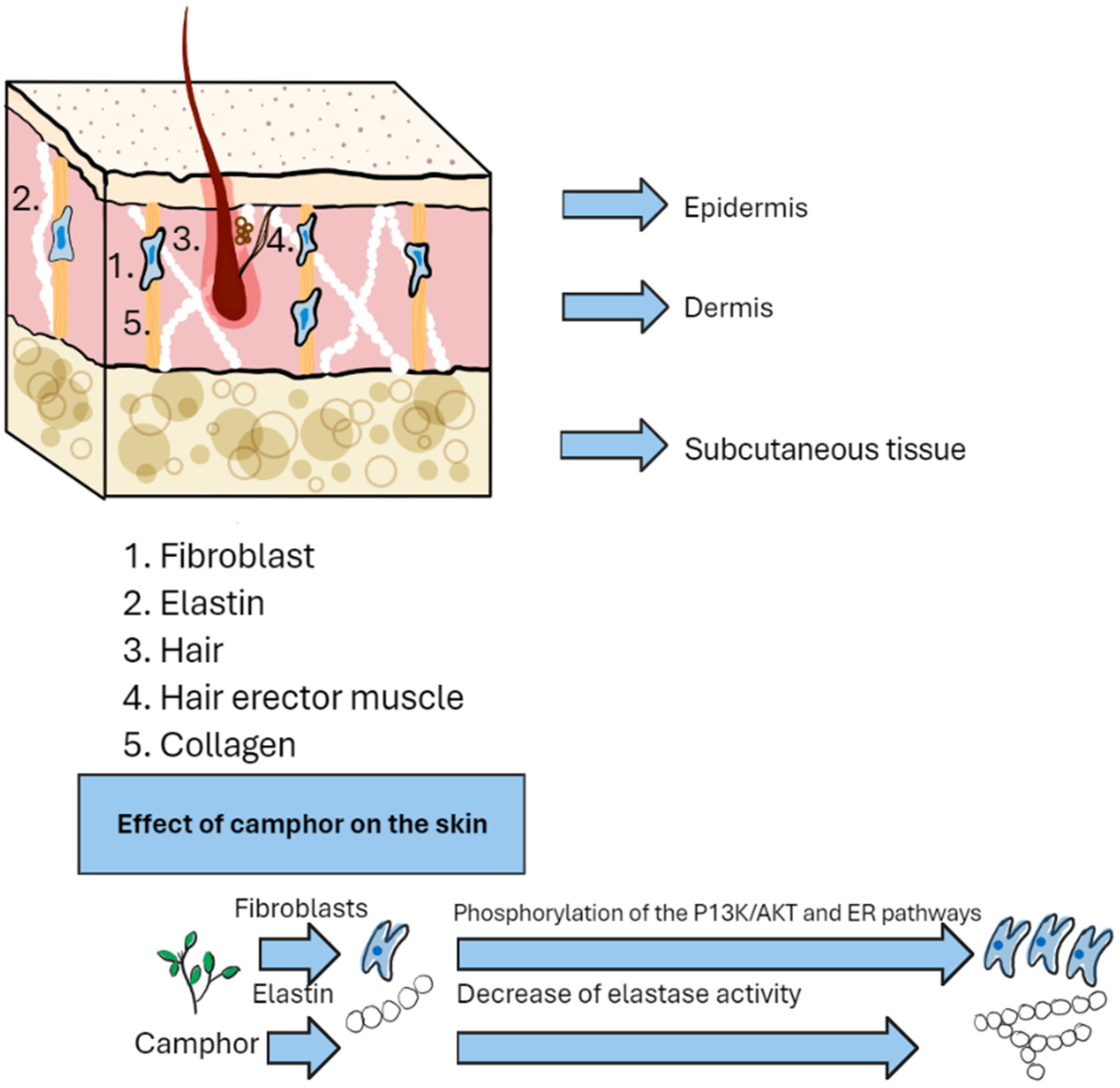
7. Mechanisms of Camphor Action on the Skin
Methods of Treating Skin Diseases
8. Antimicrobial Activity of Camphor
8.1. Interactions of Camphor-Containing Essential Oils with Antimicrobial Drugs
8.2. Antimicrobial Activity of Novel Camphor-Based Derivates
9. Future Perspectives of Antimicrobial Usage of Camphor Derivates
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nurzyńska-Wierdak, R.; Pietrasik, D.; Walasek-Janusz, M. Essential Oils in the Treatment of Various Types of Acne—A Review. Plants 2023, 12, 90. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Murthykumar, K.; Nirmalkumar, H.; Chandrasekar, H. Role of camphor in oral health care. Int. J. Pharm. Sci. Health Care 2014, 2, 1–5. [Google Scholar]
- Bubley, J.A.; Alharthi, M.; Arbiser, J.L. Successful Treatment of Palmoplantar Psoriasis with Chemical Peeling and Gentian Violet. JAAD Case Rep. 2021, 17, 28–30. [Google Scholar] [CrossRef]
- Kang, N.J.; Han, S.C.; Yoon, S.H.; Sim, J.Y.; Maeng, Y.H.; Kang, H.K.; Yoo, E.S. Cinnamomum camphora Leaves Alleviate Allergic Skin Inflammatory Responses In Vitro and In Vivo. Toxicol. Res. 2019, 35, 279–285. [Google Scholar] [CrossRef]
- Abdollahi, D.; Jafariazar, Z.; Afshar, M. Effect of Monoterpenes on Ex Vivo Transungual Delivery of Itraconazole for the Management of Onychomycosis. J. Cosmet. Dermatol. 2020, 19, 2745–2751. [Google Scholar] [CrossRef]
- Li, Z.; Gan, Y.; Kang, T.; Ke, B.; Zhao, Y.; Huang, T.; Chen, Y.; Liu, J. Camphor Attenuates Hyperalgesia in Neuropathic Pain Models in Mice. J. Pain Res. 2023, 16, 785–795. [Google Scholar] [CrossRef]
- Silva-Filho, S.E.; de Silva-Comar, F.M.S.; Wiirzler, L.A.M.; do Pinho, R.J.; Grespan, R.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Camphor on the Behavior of Leukocytes In Vitro and In Vivo in Acute Inflammatory Response. Trop. J. Pharm. Res. 2014, 13, 2031–2037. [Google Scholar] [CrossRef]
- Gabbanini, S.; Lucchi, E.; Carli, M.; Berlini, E.; Minghetti, A.; Valgimigli, L. In Vitro Evaluation of the Permeation through Reconstructed Human Epidermis of Essentials Oils from Cosmetic Formulations. J. Pharm. Biomed. Anal. 2009, 50, 370–376. [Google Scholar] [CrossRef]
- Narayan, S.; Singh, N. Camphor Poisoning—An Unusual Cause of Seizure. Med. J. Armed Forces India 2012, 68, 252–253. [Google Scholar] [CrossRef]
- Manoguerra, A.; Erdman, A.; Wax, P.; Nelson, L.; Martin Caravati, E.; Cobaugh, D.; Chyka, P.; Olson, K.; Booze, L.; Woolf, A.; et al. Camphor Poisoning: An Evidence-Based Practice Guideline for out-of-Hospital Management. Clin. Toxicol. 2006, 44, 357–370. [Google Scholar] [CrossRef]
- Babaei, M.; Hesari, A.K.; Soltani, S. Evaluation of the Camphor Effects on Histological Parameters of Skin in Adult Mice and the Protective Role of Vitamin E. J. Basic Res. Med. Sci. 2021, 8, 53–62. [Google Scholar]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Del Giudice, P. Skin Infections Caused by Staphylococcus Aureus. Acta Derm. Venereol. 2020, 100, 208–215. [Google Scholar] [CrossRef]
- Amy Stanway MB ChB Streptococcal Skin Infection. Available online: https://dermnetnz.org/Topics/Streptococcal-Skin-Infections (accessed on 23 January 2024).
- Klitgaard, K.; Nielsen, M.W.; Ingerslev, H.C.; Boye, M.; Jensen, T.K. Discovery of Bovine Digital Dermatitis-Associated Treponema Spp. in the Dairy Herd Environment by a Targeted Deep-Sequencing Approach. Appl. Environ. Microbiol. 2014, 80, 4427–4432. [Google Scholar] [CrossRef]
- Könönen, E.; Wade, W.G. Actinomyces and Related Organisms in Human Infections. Clin. Microbiol. Rev. 2015, 28, 419–442. [Google Scholar] [CrossRef]
- Gardini, G.; Gregori, N.; Matteelli, A.; Castelli, F. Mycobacterial Skin Infection. Curr. Opin. Infect. Dis. 2022, 35, 79–87. [Google Scholar] [CrossRef]
- Espinosa-Hernández, V.M.; Morales-Pineda, V.; Martínez-Herrera, E. Skin Infections Caused by Emerging Candida Species. Curr. Fungal Infect. Rep. 2020, 14, 99–105. [Google Scholar] [CrossRef]
- Blaise, G.; Nikkels, A.; Hermanns-Lê, T.; Nikkels-Tassoudji, N.; Piérard, G.E. Corynebacterium-associated Skin Infections. Int. J. Dermatol. 2008, 47, 884–890. [Google Scholar] [CrossRef]
- Ghahramani, G. Superficial Staphylococcal and Streptococcal Infections. In Inpatient Dermatology; Springer: Cham, Switzerland, 2018; pp. 99–103. [Google Scholar] [CrossRef]
- Allen, C.H.; Patel, B.; Endom, E.E. Primary Bacterial Infections of the Skin and Soft Tissues Changes in Epidemiology and Management. Clin. Pediatr. Emerg. Med. 2004, 5, 246–255. [Google Scholar] [CrossRef]
- Bikowski, J. Secondarily Infected Wounds and Dermatoses: A Diagnosis and Treatment Guide. J. Emerg. Med. 1999, 17, 197–206. [Google Scholar] [CrossRef]
- Siddiqui, A.H.; Koirala, J. Methicillin-Resistant Staphylococcus aureus. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Bekka-Hadji, F.; Bombarda, I.; Djoudi, F.; Bakour, S.; Touati, A. Chemical Composition and Synergistic Potential of Mentha pulegium L. and Artemisia Herba Alba Asso. Essential Oils and Antibiotic against Multi-Drug Resistant Bacteria. Molecules 2022, 27, 1095. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Vikas Kumar, G.; Yadav, H.; Anupurba, S. Catheter-Related Blood Stream Infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic Resistance in Hospital-Acquired ESKAPE-E Infections in Low- and Lower-Middle-Income Countries: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]
- Sancineto, L.; Piccioni, M.; De Marco, S.; Pagiotti, R.; Nascimento, V.; Braga, A.L.; Santi, C.; Pietrella, D. Diphenyl Diselenide Derivatives Inhibit Microbial Biofilm Formation Involved in Wound Infection. BMC Microbiol. 2016, 16, 220. [Google Scholar] [CrossRef]
- Apinundecha, C.; Teethaisong, Y.; Suknasang, S.; Ayamuang, I.O.; Eumkeb, G. Synergistic Interaction between Boesenbergia rotunda (L.) Mansf. Essential Oil and Cloxacillin on Methicillin-Resistant Staphylococcus Aureus (MRSA) Inhibition. Evid.-Based Complement. Altern. Med. 2023, 2023, 3453273. [Google Scholar] [CrossRef]
- Roman, H.; Niculescu, A.G.; Lazăr, V.; Mitache, M.M. Antibacterial Efficiency of Tanacetum Vulgare Essential Oil against ESKAPE Pathogens and Synergisms with Antibiotics. Antibiotics 2023, 12, 1635. [Google Scholar] [CrossRef]
- Langan, S.M.; Mulick, A.R.; Rutter, C.E.; Silverwood, R.J.; Asher, I.; García-Marcos, L.; Ellwood, E.; Bissell, K.; Chiang, C.Y.; El Sony, A.; et al. Trends in Eczema Prevalence in Children and Adolescents: A Global Asthma Network Phase I Study. Clin. Exp. Allergy 2023, 53, 337–352. [Google Scholar] [CrossRef]
- Flohr, C.; French’, L.; Bissonnette, R.; Taieb, A.; Deleuran, M.; Austin, J. Global Report on Atopic Dermatitis 2022; International League of Dermatological Societies (ILDS): London, UK, 2022. [Google Scholar]
- Grossi, A.P.; Ruggieri, A.; Del Vecchio, A.; Comandini, A.; Corio, L.; Calisti, F.; Di Loreto, G.; Almirante, B. Skin Infections in Europe: A Retrospective Study of Incidence, Patient Characteristics and Practice Patterns. Int. J. Antimicrob. Agents 2022, 60, 106637. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Kolkar, K.P.; Meti, N.T.; Chalannavar, R.K. Camphor Tree, Cinnamomum camphora (L.); Ethnobotany and Pharmacological Updates. Biomedicine 2021, 41, 181–184. [Google Scholar] [CrossRef]
- Zuccarini, P.; Soldani, G. Camphor: Benefits and Risks of a Widely Used Natural Product. Acta Biol. Szeged. 2009, 53, 77–82. [Google Scholar] [CrossRef]
- Ponomarev, D.A.; Mettee, H. Camphor and Its Industrial Synthesis. Chem. Educ. J. 2017, 18, 2–5. [Google Scholar]
- Mahdy, A.-H.S.; Zayed, S.E.; Abo-Bakr, A.M.; Hassan, E.A. Camphor: Synthesis, Reactions and Uses as a Potential Moiety in the Development of Complexes and Organocatalysts. Tetrahedron 2022, 121, 132913. [Google Scholar] [CrossRef]
- Calderini, E.; Drienovská, I.; Myrtollari, K.; Pressnig, M.; Sieber, V.; Schwab, H.; Hofer, M.; Kourist, R. Simple Plug-In Synthetic Step for the Synthesis of (−)-Camphor from Renewable Starting Materials. ChemBioChem 2021, 22, 2951–2956. [Google Scholar] [CrossRef]
- Lang, P.T.; Harned, A.M.; Wissinger, J.E. Oxidation of Borneol to Camphor Using Oxone and Catalytic Sodium Chloride: A Green Experiment for the Undergraduate Organic Chemistry Laboratory. J. Chem. Educ. 2011, 88, 652–656. [Google Scholar] [CrossRef]
- Aswandi, A.; Kholibrina, C.R. New Insights into Sumatran Camphor (Dryobalanops aromatica Gaertn) Management and Conservation in Western Coast Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 739, 012061. [Google Scholar] [CrossRef]
- Renvall, P.; Niemela, T. Ocotea Usambarensis and Its Fungal Decayers in Natural Stands. Bull. Jard. Bot. Natl. Belgique/Bull. Natl. Plantentuin België 1993, 62, 403–414. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.-S.; Park, S.-H.; Park, H. Phytochemistry and Applications of Cinnamomum camphora Essential Oils. Molecules 2022, 27, 2695. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, T.; Liao, X.; Zhou, Y.; Chen, S.; Chen, J.; Xiong, W. Extraction of Camphor Tree Essential Oil by Steam Distillation and Supercritical CO2 Extraction. Molecules 2022, 27, 5385. [Google Scholar] [CrossRef]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Chanotiya, C.S.; Alam, M.; Samad, A. Chemical Characterization and Antifungal Activity of Cinnamomum camphora Essential Oil. Ind. Crops Prod. 2013, 49, 628–633. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Zhang, R.; Ye, S.; Zhao, Z.; Huang, Y.; Xu, X.; Lan, W.; Yang, D. Metabolomics Analysis to Evaluate the Antibacterial Activity of the Essential Oil from the Leaves of Cinnamomum camphora (Linn.) Presl. J. Ethnopharmacol. 2020, 253, 112652. [Google Scholar] [CrossRef]
- Tsiftsoglou, O.S.; Atskakani, M.-E.; Krigas, N.; Stefanakis, M.K.; Gounaris, C.; Hadjipavlou-Litina, D.; Lazari, D. Exploring the Medicinal Potential of Achillea Grandifolia in Greek Wild-Growing Populations: Characterization of Volatile Compounds, Anti-Inflammatory and Antioxidant Activities of Leaves and Inflorescences. Plants 2023, 12, 613. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Klein, R.A.; Khlebnikov, A.I.; Quinn, M.T. Composition and Biological Activity of the Essential Oils from Wild Horsemint, Yarrow, and Yampah from Subalpine Meadows in Southwestern Montana: Immunomodulatory Activity of Dillapiole. Plants 2023, 12, 2643. [Google Scholar] [CrossRef]
- Oftadeh, M.; Sendi, J.J.; Ebadollahi, A. Toxicity and Deleterious Effects of Artemisia Annua Essential Oil Extracts on Mulberry Pyralid (Glyphodes pyloalis). Pestic. Biochem. Physiol. 2020, 170, 104702. [Google Scholar] [CrossRef]
- Shahrivari, S.; Alizadeh, S.; Ghassemi-Golezani, K.; Aryakia, E. A Comprehensive Study on Essential Oil Compositions, Antioxidant, Anticholinesterase and Antityrosinase Activities of Three Iranian Artemisia Species. Sci. Rep. 2022, 12, 7234. [Google Scholar] [CrossRef]
- Ghasemi, G.; Alirezalu, A.; Ishkeh, S.R.; Ghosta, Y. Phytochemical Properties of Essential Oil from Artemisia Sieberi Besser (Iranian Accession) and Its Antioxidant and Antifungal Activities. Nat. Prod. Res. 2021, 35, 4154–4158. [Google Scholar] [CrossRef]
- Ghorbani, S.; Kosari-Nasab, M.; Mahjouri, S.; Talebpour, A.H.; Movafeghi, A.; Maggi, F. Enhancement of In Vitro Production of Volatile Organic Compounds by Shoot Differentiation in Artemisia spicigera. Plants 2021, 10, 208. [Google Scholar] [CrossRef]
- Usami, A.; Ono, T.; Marumoto, S.; Miyazawa, M. Comparison of Volatile Compounds with Characteristic Odor in Flowers and Leaves of Nojigiku (Chrysanthemum japonense). J. Oleo Sci. 2013, 62, 631–636. [Google Scholar] [CrossRef]
- Guo, S.; Geng, Z.; Zhang, W.; Liang, J.; Wang, C.; Deng, Z.; Du, S. The Chemical Composition of Essential Oils from Cinnamomum camphora and Their Insecticidal Activity against the Stored Product Pests. Int. J. Mol. Sci. 2016, 17, 1836. [Google Scholar] [CrossRef]
- Najafian, S.; Afshar, M.; Radi, M. Annual Phytochemical Variations and Antioxidant Activity within the Aerial Parts of Lavandula Angustifolia, an Evergreen Medicinal Plant. Chem. Biodivers. 2022, 19, e202200536. [Google Scholar] [CrossRef]
- Karaca, N.; Şener, G.; Demirci, B.; Demirci, F. Synergistic Antibacterial Combination of Lavandula latifolia Medik. Essential Oil with Camphor. Z. Naturforsch. C 2021, 76, 169–173. [Google Scholar] [CrossRef]
- Purkayastha, J.; Nath, S.C. Composition of the Camphor-Rich Essential Oil of Ocimum Basilicum L. Native to Northeast India. J. Essent. Oil Res. 2006, 18, 332–334. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical Composition of the Essential Oil of Camphor Basil (Ocimum kilimandscharicum Guerke). Glob. J. Med. Plant Res. 2013, 1, 207–209. [Google Scholar]
- Sufer, Ö.; Ceylan, A.; Onbasili, D.; Çelik Yuvali, G.; Bozok, F. Chemical Compounds and Biological Activity of Turkish Santolina chamaecyparissus L. Essential Oil by Microwave Assisted Distillation. Kastamonu Üniv. Orman Fakültesi Derg. 2021, 21, 165–175. [Google Scholar] [CrossRef]
- Bozyel, M.E.; Canli, K.; Benek, A.; Simsek, O.; Akata, I.; Altuner, E.M. Biochemical Composition, a Nd in Vitro Antimicrobial and Antioxidant Activities of Salva Fruticosa, an Ethnomedicinal Plant. Appl. Ecol. Environ. Res. 2023, 21, 3243–3256. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ciccarelli, D.; Giovanelli, S.; Pistelli, L.; Flamini, G.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Bertelloni, F.; Ebani, V.V. Antimicrobial Activity and Composition of Five Rosmarinus (Now salvia spp. and Varieties) Essential Oils. Antibiotics 2021, 10, 1090. [Google Scholar] [CrossRef]
- Tosun, A.; Khan, S.; Kim, Y.; Calín-Sánchez, A.; Hysenaj, X.; Carbonell-Barrachina, A. Essential Oil Composition and Anti-Inflammatory Activity of Salvia officinalis L (Lamiaceae) in Murin Macrophages. Trop. J. Pharm. Res. 2014, 13, 937. [Google Scholar] [CrossRef]
- Ivanova, S.; Pashova, S.; Dyankov, S.; Georgieva, Y.; Ivanov, K.; Benbassat, N.; Koleva, N.; Bozhkova, M.; Karcheva-Bahchevanska, D. Chemical Composition and Future Perspectives of Essential Oil Obtained from a Wild Population of Stachys germanica L. Distributed in the Balkan Mountains in Bulgaria. Int. J. Anal. Chem. 2023, 2023, 4275213. [Google Scholar] [CrossRef]
- Izadi, Z.; Esna-Ashari, M.; Piri, K.; Davoodi, P. Chemical Composition and Antimicrobial Activity of Feverfew (Tanacetum parthenium) Essential Oil. Int. J. Agric. Biol. 2010, 12, 759–763. [Google Scholar]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of Chemical Composition of Essential Oils in Wild Populations of Thymus algeriensis Boiss. et Reut., a North African Endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Valussi, M. Efficacy, Safety and Tolerability of Tiger Balm® Ointments: A Systematic Review and a Meta-Analysis of Prevalence. J. Pharm. Pharmacogn. Res. 2020, 8, 1–17. [Google Scholar] [CrossRef]
- Goodnough, C.L.; Wu, Y.; Gross, E.R. Topical Analgesic BENGAY® Reduces Myocardial Infarct Size in Rodents. Br. J. Anaesth. 2021, 127, e163–e166. [Google Scholar] [CrossRef]
- Stinson, R.J.; Morice, A.H.; Ahmad, B.; Sadofsky, L.R. Ingredients of Vicks VapoRub Inhibit Rhinovirus-Induced ATP Release. Drugs Context 2023, 12, 1–18. [Google Scholar] [CrossRef]
- Vlahovic, T.C.; Dawes, C.L. Myths in Treating Onychomycosis. In Onychomycosis: An Illustrated Guide to Diagnosis and Treatment; Springer: Cham, Switzerland, 2017; pp. 215–222. [Google Scholar] [CrossRef]
- Derby, R.; Rohal, P.; Jackson, C.; Beutler, A.; Olsen, C. Novel Treatment of Onychomycosis Using Over-the-Counter Mentholated Ointment: A Clinical Case Series. J. Am. Board Fam. Med. 2011, 24, 69–74. [Google Scholar] [CrossRef]
- Snell, M.; Klebert, M.; Önen, N.F.; Hubert, S. A Novel Treatment for Onychomycosis in People Living With HIV Infection: Vicks VapoRub™ Is Effective and Safe. J. Assoc. Nurses AIDS Care 2016, 27, 109–113. [Google Scholar] [CrossRef]
- Coiffard, L.; Couteau, C. Soap and Syndets: Differences and Analogies, Sources of Great Confusion. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11432–11439. [Google Scholar] [CrossRef]
- Patil, J.N.; Patil, K.C.; Patil, P.K.; Chaudhari, H.S. Formulation and evaluation of camphor aloe soap. World J. Pharm. Res. 2023, 12, 1257–1273. [Google Scholar] [CrossRef]
- Kędzia, A.; Kędzia, A.W. Activity of camphor oil to anaerobic bacteria. Postępy Fitoter. 2009, 3, 147–151. [Google Scholar]
- Santos, C.D.; Cabot, J.C. Persistent Effects after Camphor Ingestion: A Case Report and Literature Review. J. Emerg. Med. 2015, 48, 298–304. [Google Scholar] [CrossRef]
- Wojtunik-kulesza, K.A. Toxicity of Selected Monoterpenes and Essential Oils Rich in These Compounds. Molecules 2022, 27, 1716. [Google Scholar] [CrossRef]
- Martin, D.; Valdez, J.; Boren, J.; Mayersohn, M. Dermal Absorption of Camphor, Menthol, and Methyl Salicylate in Humans. J. Clin. Pharmacol. 2004, 44, 1151–1157. [Google Scholar] [CrossRef]
- Robertson, J.S.; Hussain, M. Metabolism of Camphors and Related Compounds. Biochem. J. 1969, 113, 57–65. [Google Scholar] [CrossRef]
- Park, T.J.; Seo, H.K.; Kang, B.J.; Kim, K.T. Noncompetitive Inhibition by Camphor of Nicotinic Acetylcholine Receptors. Biochem. Pharmacol. 2001, 61, 787–793. [Google Scholar] [CrossRef]
- Zárybnický, T.; Boušová, I.; Ambrož, M.; Skálová, L. Hepatotoxicity of Monoterpenes and Sesquiterpenes. Arch. Toxicol. 2018, 92, 1–13. [Google Scholar] [CrossRef]
- Uc, A.; Bishop, W.P.; Sanders, K.D. Camphor Hepatotoxicity. South Med. J. 2000, 93, 596–598. [Google Scholar] [CrossRef]
- Köppel, C.; Tenczer, J.; Schirop, T.; Ibe, K. Camphor Poisoning—Abuse of Camphor as a Stimulant. Arch. Toxicol. 1982, 51, 101–106. [Google Scholar] [CrossRef]
- Zehetner, P.; Höferl, M.; Buchbauer, G. Essential Oil Components and Cytochrome P450 Enzymes: A Review. Flavour Fragr. J. 2019, 34, 223–240. [Google Scholar] [CrossRef]
- Santhanakrishnan, T.S. Tetrahedron Report Number 172. Tetrahedron 1984, 40, 3597–3609. [Google Scholar] [CrossRef]
- Leibman, K.C.; Ortiz, E. Mammalian Metabolism of Terpenoids. I. Reduction and Hydroxylation of Camphor and Related Compounds. Drug Metab. Dispos. 1973, 1, 543–551. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9780815370965. [Google Scholar]
- Gyoubu, K.; Miyazawa, M. In Vitro Metabolism of (−)-Camphor Using Human Liver Microsomes and CYP2A6. Biol. Pharm. Bull. 2007, 30, 230–233. [Google Scholar] [CrossRef][Green Version]
- He, R.; Li, H.; Chu, Z.; Wang, F.; Du, F.; Xu, F. Circulating Metabolites of Borneolum syntheticum (Bingpian) Inhibit Foam-Cell Formation in Macrophages Induced by Oxidized Low-Density Lipoprotein. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Xie, F.; Chai, J.K.; Hu, Q.; Yu, Y.H.; Ma, L.; Liu, L.Y.; Zhang, X.L.; Li, B.L.; Zhang, D.H. Transdermal Permeation of Drugs with Differing Lipophilicity: Effect of Penetration Enhancer Camphor. Int. J. Pharm. 2016, 507, 90–101. [Google Scholar] [CrossRef]
- Cui, Y.; Li, L.; Zhang, L.; Li, J.; Gu, J.; Gong, H.; Guo, P.; Tong, W. Enhancement and Mechanism of Transdermal Absorption of Terpene-Induced Propranolol Hydrochloride. Arch. Pharm. Res. 2011, 34, 1477–1485. [Google Scholar] [CrossRef]
- Steenen, S.A.; Van Wijk, A.J.; Van Der Heijden, G.J.M.G.; Van Westrhenen, R.; De Lange, J.; De Jongh, A. Propranolol for the Treatment of Anxiety Disorders: Systematic Review and Meta-Analysis. J. Psychopharmacol. 2016, 30, 128–139. [Google Scholar] [CrossRef]
- Mothana, R.A.; Al-Rehaily, A.J.; Schultze, W. Chemical Analysis and Biological Activity of the Essential Oils of Two Endemic Soqotri Commiphora Species. Molecules 2010, 15, 689–698. [Google Scholar] [CrossRef]
- Celen Yuceturk, S.; Aydogan Turkoglu, S.; Kockar, F.; Kucukbay, F.Z.; Azaz, A.D. Essential Oil Chemical Composition, Antimicrobial, Anticancer, and Antioxidant Effects of Thymus Convolutus Klokov in Turkey. Z. Naturforsch. Sect. C J. Biosci. 2021, 76, 193–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhao, Y.; Gu, W.; Zhu, Y.; Wang, S. Novel Camphor-Based Pyrimidine Derivatives Induced Cancer Cell Death through a ROS-Mediated Mitochondrial Apoptosis Pathway. RSC Adv. 2019, 9, 29711–29720. [Google Scholar] [CrossRef]
- Rawat, A.; Rawat, M.; Prakash, O.; Kumar, R.; Punetha, H.; Rawat, D.S. Comparative Study on Eucalyptol and Camphor Rich Essential Oils from Rhizomes of Hedychium spicatum Sm. and Their Pharmacological, Antioxidant and Antifungal Activities. An. Acad. Bras. Cienc. 2022, 94, e20210932. [Google Scholar] [CrossRef]
- Pishgahzadeh, E.; Shafaroodi, H.; Asgarpanah, J. Analgesic and Antiinflammatory Activities of the Essential Oil from Artemisia Sieberi Besser. Braz. J. Pharm. Sci. 2019, 55, e17011. [Google Scholar] [CrossRef]
- Liao, J.; Xie, X.; Wang, W.; Gao, Y.; Cai, Y.; Peng, J.; Li, T.; Yi, Q.; He, C.; Wang, L. Anti-Inflammatory Activity of Essential Oil from Leaves of Blumea balsamifera (L.) DC through Inhibiting TLR4/NF-KB Signaling Pathways and NLRP3 Inflammasome Activation in LPS-Induced RAW264.7 Macrophage Cells. J. Essent. Oil Bear. Plants 2021, 24, 160–176. [Google Scholar] [CrossRef]
- Tran, T.A.; Ho, M.T.; Song, Y.W.; Cho, M.; Cho, S.K. Camphor Induces Proliferative and Anti-Senescence Activities in Human Primary Dermal Fibroblasts and Inhibits UV-Induced Wrinkle Formation in Mouse Skin. Phyther. Res. 2015, 29, 1917–1925. [Google Scholar] [CrossRef]
- Pejčić, M.; Stojanović-Radić, Z.; Genčić, M.; Dimitrijević, M.; Radulović, N. Anti-Virulence Potential of Basil and Sage Essential Oils: Inhibition of Biofilm Formation, Motility and Pyocyanin Production of Pseudomonas Aeruginosa Isolates. Food Chem. Toxicol. 2020, 141, 111431. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and Antibacterial Activities of the Essential Oils Obtained from Seven Iranian Populations of Rosmarinus Officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Erdogan Eliuz, E.A.; Ayas, D.; Goksen, G. In Vitro Phototoxicity and Antimicrobial Activity of Volatile Oil Obtained from Some Aromatic Plants. J. Essent. Oil Bear. Plants 2017, 20, 758–768. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated Healing by Topical Administration of Salvia officinalis Essential Oil on Pseudomonas Aeruginosa and Staphylococcus Aureus Infected Wound Model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Yamani, H.A.; Pang, E.C.; Mantri, N.; Deighton, M.A. Antimicrobial Activity of Tulsi (Ocimum tenuiflorum) Essential Oil and Their Major Constituents against Three Species of Bacteria. Front. Microbiol. 2016, 7, 195885. [Google Scholar] [CrossRef]
- Fatma, G.; Mouna, B.F.; Mondher, M.; Ahmed, L. In-Vitro Assessment of Antioxidant and Antimicrobial Activities of Methanol Extracts and Essential Oil of Thymus Hirtus Sp. Algeriensis. Lipids Health Dis. 2014, 13, 114. [Google Scholar] [CrossRef]
- Shakeri, A.; Sharifi, M.J.; Fazly Bazzaz, B.S.; Emami, A.; Soheili, V.; Sahebkar, A.; Asili, J. Bioautography Detection of Antimicrobial Compounds from the Essential Oil of Salvia Pachystachys. Curr. Bioact. Compd. 2018, 14, 80–85. [Google Scholar] [CrossRef]
- Nguir, A.; Mabrouk, H.; Douki, W.; Ben Ismail, M.; Ben Jannet, H.; Flamini, G.; Hamza, M.A. Chemical Composition and Bioactivities of the Essential Oil from Different Organs of Ferula communis L. Growing in Tunisia. Med. Chem. Res. 2016, 25, 515–525. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical Composition of Mentha Pulegium and Rosmarinus Officinalis Essential Oils and Their Antileishmanial, Antibacterial and Antioxidant Activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Aly, M.S.A.; Hegazy, M.M. Essential Oil Constituents and Biological Activities of the Halophytic Plants, Suaeda Vermiculata Forssk and Salsola Cyclophylla Bakera Growing in Saudi Arabia. J. Essent. Oil Bear. Plants 2019, 22, 82–93. [Google Scholar] [CrossRef]
- Stappen, I.; Ali, A.; Tabanca, N.; Khan, I.; Wanner, J.; Gochev, V.; Singh, V.; Lal, B.; Jaitak, V.; Kaul, V.; et al. Antimicrobial and Repellent Activity of the Essential Oils of Two Lamiaceae Cultivated in Western Himalaya. Curr. Bioact. Compd. 2015, 11, 23–30. [Google Scholar] [CrossRef]
- Adewinogo, S.O.; Sharma, R.; Africa, C.W.J.; Marnewick, J.L.; Hussein, A.A. Chemical Composition and Cosmeceutical Potential of the Essential Oil of Oncosiphon suffruticosum (L.) Källersjö. Plants 2021, 10, 1315. [Google Scholar] [CrossRef]
- Bertella, A.; Benlahcen, K.; Abouamama, S.; Pinto, D.C.G.A.; Maamar, K.; Kihal, M.; Silva, A.M.S. Artemisia Herba-Alba Asso. Essential Oil Antibacterial Activity and Acute Toxicity. Ind. Crops Prod. 2018, 116, 137–143. [Google Scholar] [CrossRef]
- Tawfeeq, A.A.; Mahdi, M.F.; Abaas, I.S.; Alwan, A.H. Isolation, quantification, and identification of rosmarinic acid, gas chromatography-mass spectrometry analysis of essential oil, cytotoxic effect, and antimicrobial investigation of rosmarinus officinalis leaves. Asian J. Pharm. Clin. Res. 2018, 11, 126. [Google Scholar] [CrossRef]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential Oil Compositions and Antibacterial and Antioxidant Activities of Five Lavandula stoechas Cultivars Grown in Thailand. Chem. Biodivers. 2019, 16, e1900371. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Bourhia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Majid Mahmood, H.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical Profiling, Antioxidant, and Antimicrobial Activity against Drug-Resistant Microbes of Essential Oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- Asili, J.; Emami, S.A.; Eynolghozat, R.; Noghab, Z.S.; Bazzaz, B.S.F.; Sahebkar, A. Chemical Composition and In Vitro Efficacy of Essential Oil of Seven Artemisia Species Against ESBL Producing Multidrug-Resistant Escherichia coli. J. Essent. Oil Bear. Plants 2015, 18, 124–145. [Google Scholar] [CrossRef]
- Siqueira, I.B.; Teixeira Barbosa, A.A.; Jain, S.; Miranda Fernandes, R.P.; Tavares Silva, A.R.S.; Ferreira Barbosa, F.H.; Schimieguel, D.M.; Blank, A.F.; Sacramento, A.G.; de Castro Nizio, D.A.; et al. In Vitro Antibacterial Activity of Essential Oils of Croton tetradenius Baill. From the Brazilian Caatinga Biome and Its Synergistic Effect With Ciprofloxacin and Meropenem. J. Essent. Oil Bear. Plants 2021, 24, 12–21. [Google Scholar] [CrossRef]
- Pandey, M.; Pandey, A.; Shukla, S.K.; Kumar, R.; Pathak, A.; Mishra, R.K.; Dikshit, A. A Comparative Analysis of In Vitro Growth Inhibition of Waterborne Bacteria with Bioactive Plant Lippia nodiflora L. and Camphor. Desalin. Water Treat. 2016, 57, 26250–26256. [Google Scholar] [CrossRef]
- Mhiri, R.; Kchaou, M.; Belhadj, S.; El Feki, A.; Allouche, N. Characterization of Aromatic Compounds and Biological Activities of Essential Oils from Tunisian Aromatic Plants. J. Food Meas. Charact. 2018, 12, 839–847. [Google Scholar] [CrossRef]
- Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical Composition and Antimicrobial Activity of Essential Oil of Algerian Tetraclinis articulata (Vahl) Masters. J. Essent. Oil Res. 2016, 28, 42–48. [Google Scholar] [CrossRef]
- Mahboubi, M.; Valian, M.; Kazempour, N. Chemical Composition, Antioxidant and Antimicrobial Activity of Artemisia Sieberi Oils from Different Parts of Iran and France. J. Essent. Oil Res. 2015, 27, 140–147. [Google Scholar] [CrossRef]
- Ovidi, E.; Laghezza Masci, V.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus Nobilis, Salvia Sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Farhat, A.; Benmoussa, H.; Bachoual, R.; Nasfi, Z.; Elfalleh, W.; Romdhane, M.; Bouajila, J. Efficiency of the Optimized Microwave Assisted Extractions on the Yield, Chemical Composition and Biological Activities of Tunisian Rosmarinus officinalis L. Essential Oil. Food Bioprod. Process. 2017, 105, 224–233. [Google Scholar] [CrossRef]
- Carvalho, M.F.N.N.; Leite, S.; Costa, J.P.; Galvão, A.M.; Leitão, J.H. Ag(I) Camphor Complexes: Antimicrobial Activity by Design. J. Inorg. Biochem. 2019, 199, 110791. [Google Scholar] [CrossRef]
- Grimsey, L.; Van Vuuren, S.F.; Wright, M.H.; Cock, I.E. Selected South African Combretum Spp. Extracts Inhibit Methicillin-Resistant Staphylococcus Aureus and ESBL Strains of Escherichia Coli and Klebsiella Pneumoniae. S. Afr. J. Bot. 2024, 165, 49–58. [Google Scholar] [CrossRef]
- Ouakouak, H.; Benarfa, A.; Messaoudi, M.; Begaa, S.; Sawicka, B.; Benchikha, N.; Simal-Gandara, J. Biological Properties of Essential Oils from Thymus Algeriensis Boiss. Plants 2021, 10, 786. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Zulfiqar, A.; Khan, I.A.; Efferth, T.; Salgueiro, L. Chemical Composition and Biological Activities of Artemisia Judaica Essential Oil from Southern Desert of Jordan. J. Ethnopharmacol. 2016, 191, 161–168. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.-H.; Lee, J. Antibiofilm and Antihyphal Activities of Cedar Leaf Essential Oil, Camphor, and Fenchone Derivatives against Candida Albicans. Front. Microbiol. 2017, 8, 278535. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Camphor and Eucalyptol—Anticandidal Spectrum, Antivirulence Effect, Efflux Pumps Interference and Cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical Composition, Antimicrobial, and Cytotoxic Properties of Five Lamiaceae Essential Oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- Satyal, P.; Dosoky, N.S.; Poudel, A.; Setzer, W.N. Essential Oil Constituents and Their Biological Activities from the Leaves of Cassia Fistula Growing in Nepal. Open Access J. Med. Aromat. Plants 2012, 3, 1–4. [Google Scholar]
- Agour, A.; Mssillou, I.; Mechchate, H.; Es-safi, I.; Allali, A.; El Barnossi, A.; Al Kamaly, O.; Alshawwa, S.Z.; El Moussaoui, A.; Bari, A.; et al. Brocchia cinerea (Delile) Vis. Essential Oil Antimicrobial Activity and Crop Protection against Cowpea Weevil Callosobruchus maculatus (Fab.). Plants 2022, 11, 583. [Google Scholar] [CrossRef]
- Asres, K.; Tadesse, S.; Mazumder, A.; Bucar, F. Essential Oil of Plectranthus Cylindraceus Hochst. Ex. Benth from Ethiopia: Chemical Composition and Antimicrobial Activity. J. Essent. Oil Bear. Plants 2013, 16, 136–143. [Google Scholar] [CrossRef]
- Al-Maharik, N.; Jaradat, N.; Hawash, M.; Al-Lahham, S.; Qadi, M.; Shoman, I.; Jaber, S.; Rahem, R.A.; Hussein, F.; Issa, L. Chemical Composition, Antioxidant, Antimicrobial and Anti-Proliferative Activities of Essential Oils of Rosmarinus Officinalis from Five Different Sites in Palestine. Separations 2022, 9, 339. [Google Scholar] [CrossRef]
- Beniaich, G.; Hafsa, O.; Maliki, I.; Bin Jardan, Y.A.; El Moussaoui, A.; Chebaibi, M.; Agour, A.; Zouirech, O.; Nafidi, H.-A.; Khallouki, F.; et al. GC-MS Characterization, In Vitro Antioxidant, Antimicrobial, and In Silico NADPH Oxidase Inhibition Studies of Anvillea Radiata Essential Oils. Horticulturae 2022, 8, 886. [Google Scholar] [CrossRef]
- Mohamadi Sani, A.; Khiabani, A.; Yaghooti, F. Chemical Composition and Antimicrobial Activity of the Essential Oil of Artemisia aucheri Aerial Parts. J. Essent. Oil Bear. Plants 2016, 19, 875–884. [Google Scholar] [CrossRef]
- Bahadirli, N.P. Comparison of Chemical Composition and Antimicrobial Activity of Salvia Fruticosa Mill. and S. Aramiensis Rech. Fill. (Lamiaceae). J. Essent. Oil Bear. Plants 2022, 25, 716–727. [Google Scholar] [CrossRef]
- Drobac, M.; Kukic-Markovic, J.; Milenkovic, M.; Niketic, M.; Petrovic, S. The Chemical Composition, Antimicrobial and Antiradical Properties of the Essential Oil of Achillea Grandifolia Aerial Parts from Serbia. Bot. Serbica 2021, 45, 233–240. [Google Scholar] [CrossRef]
- El Hachlafi, N.; Fikri-Benbrahim, K.; Al-Mijalli, S.H.; Elbouzidi, A.; Jeddi, M.; Abdallah, E.M.; Assaggaf, H.; Bouyahya, A.; Alnasser, S.M.; Attar, A.; et al. Tetraclinis articulata (Vahl) Mast. Essential Oil as a Promising Source of Bioactive Compounds with Antimicrobial, Antioxidant, Anti-Inflammatory and Dermatoprotective Properties: In Vitro and in Silico Evidence. Heliyon 2024, 10, e23084. [Google Scholar] [CrossRef]
- Sanae, A.A.; Ferdinand, K.E.; Bruno, E.; Hamzaoui Najia, E.; Amal, B.; Malika, M.S.; Touriya, Z. The Antifungal Effect of the Main Monoterpenes of the Essential Oil of Artemisia herba alba Var. Huguetii (Caball.) Maire of the Region of Ouarzazate-Morocco When Tested against Strains of Candida. Res. J. Chem. Environ. 2020, 24, 111–117. [Google Scholar]
- Chaturvedi, T.; Kumar, A.; Kumar, A.; Verma, R.S.; Padalia, R.C.; Sundaresan, V.; Chauhan, A.; Saikia, D.; Singh, V.R.; Venkatesha, K. Chemical Composition, Genetic Diversity, Antibacterial, Antifungal and Antioxidant Activities of Camphor-Basil (Ocimum kilimandscharicum Guerke). Ind. Crops Prod. 2018, 118, 246–258. [Google Scholar] [CrossRef]
- Benali, T.; Habbadi, K.; Khabbach, A.; Marmouzi, I.; Zengin, G.; Bouyahya, A.; Chamkhi, I.; Chtibi, H.; Aanniz, T.; Achbani, E.H.; et al. GC–MS Analysis, Antioxidant and Antimicrobial Activities of Achillea Odorata Subsp. Pectinata and Ruta Montana Essential Oils and Their Potential Use as Food Preservatives. Foods 2020, 9, 668. [Google Scholar] [CrossRef]
- Hendel, N.; Napoli, E.; Sarri, M.; Saija, A.; Cristani, M.; Nostro, A.; Ginestra, G.; Ruberto, G. Essential Oil from Aerial Parts of Wild Algerian Rosemary: Screening of Chemical Composition, Antimicrobial and Antioxidant Activities. J. Essent. Oil Bear. Plants 2019, 22, 1–17. [Google Scholar] [CrossRef]
- Santomauro, F.; Donato, R.; Sacco, C.; Pini, G.; Flamini, G.; Bilia, A. Vapour and Liquid-Phase Artemisia Annua Essential Oil Activities against Several Clinical Strains of Candida. Planta Med. 2016, 82, 1016–1020. [Google Scholar] [CrossRef]
- Qasem, A.; Assaggaf, H.; Montesano, D.; Khalil, Z.; Al-Mijalli, S.H.; El Baaboua, A.; El Omari, N.; El Menyiy, N.; Bakrim, S.; Sheikh, R.A.; et al. Determination of Chemical Compounds and Investigation of Biological Properties of Matricaria Chamomilla Essential Oils, Honey, and Their Mixture. Molecules 2022, 27, 5850. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. Review of Environmental Effects of Oxybenzone and Other Sunscreen Active Ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef]
- Berganayeva, G.; Kudaibergenova, B.; Litvinenko, Y.; Nazarova, I.; Sydykbayeva, S.; Vassilina, G.; Izdik, N.; Dyusebaeva, M. Medicinal Plants of the Flora of Kazakhstan Used in the Treatment of Skin Diseases. Molecules 2023, 28, 4192. [Google Scholar] [CrossRef]
- Xu, H.; Blair, N.T.; Clapham, D.E. Camphor Activates and Strongly Desensitizes the Transient Receptor Potential Vanilloid Subtype 1 Channel in a Vanilloid-Independent Mechanism. J. Neurosci. 2005, 25, 8924–8937. [Google Scholar] [CrossRef]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid Agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Green, B.G. Sensory Characteristics of Camphor. J. Investig. Dermatol. 1990, 94, 662–666. [Google Scholar] [CrossRef]
- Kotaka, T.; Kimura, S.; Kashiwayanagi, M.; Iwamoto, J. Camphor Induces Cold and Warm Sensations with Increases in Skin and Muscle Blood Flow in Human. Biol. Pharm. Bull. 2014, 37, 1913–1918. [Google Scholar] [CrossRef]
- Alpizar, Y.A.; Gees, M.; Sanchez, A.; Apetrei, A.; Voets, T.; Nilius, B.; Talavera, K. Bimodal Effects of Cinnamaldehyde and Camphor on Mouse TRPA1. Pflug. Arch. Eur. J. Physiol. 2013, 465, 853–864. [Google Scholar] [CrossRef]
- Denda, M.; Sokabe, T.; Fukumi-Tominaga, T.; Tominaga, M. Effects of Skin Surface Temperature on Epidermal Permeability Barrier Homeostasis. J. Investig. Dermatol. 2007, 127, 654–659. [Google Scholar] [CrossRef]
- Janjua, N.R.; Mogensen, B.; Andersson, A.M.; Petersen, J.H.; Henriksen, M.; Skakkebæk, N.E.; Wulf, H.C. Systemic Absorption of the Sunscreens Benzophenone-3, Octyl-Methoxycinnamate, and 3-(4-Methyl-Benzylidene) Camphor after Whole-Body Topical Application and Reproductive Hormone Levels in Humans. J. Investig. Dermatol. 2004, 123, 57–61. [Google Scholar] [CrossRef]
- Selescu, T.; Ciobanu, A.C.; Dobre, C.; Reid, G.; Babes, A. Camphor Activates and Sensitizes Transient Receptor Potential Melastatin 8 (TRPM8) to Cooling and Icilin. Chem. Senses 2013, 38, 563–575. [Google Scholar] [CrossRef]
- Gannu, R.; Vishnu, Y.V.; Kishan, V.; Rao, Y.M. In Vitro Permeation of Carvedilol through Porcine Skin: Effect of Vehicles and Penetration Enhancers. PDA J. Pharm. Sci. Technol. 2008, 62, 256–263. [Google Scholar]
- Patel, D.R.; Joshi, A.; Patel, H.H.; Stagni, G. Development and In-Vivo Evaluation of Ondansetron Gels for Transdermal Delivery. Drug Dev. Ind. Pharm. 2015, 41, 1030–1036. [Google Scholar] [CrossRef]
- Vaghardoost, R.; Mousavi Majd, S.G.; Tebyanian, H.; Babavalian, H.; Malaei, L.; Niazi, M.; Javdani, A. The Healing Effect of Sesame Oil, Camphor and Honey on Second Degree Burn Wounds in Rat. World J. Plast. Surg. 2018, 7, 67–71. [Google Scholar]
- Haught, J.M.; Jukic, D.M.; English, J.C. Hydroxyethyl Starch-Induced Pruritus Relieved by a Combination of Menthol and Camphor. J. Am. Acad. Dermatol. 2008, 59, 151–153. [Google Scholar] [CrossRef]
- Gavale, A.G.; Wagh, P.R. Herbal Drugs Use in a Skin Disorders Review Article. World J. Pharm. Res. 2023, 12, 2655–2676. [Google Scholar] [CrossRef]
- Phillips, B. The phenol-camphor treatment of dermatophytosis. Br. J. Dermatol. 1944, 56, 219–227. [Google Scholar] [CrossRef]
- Scalia, S.; Tursilli, R.; Iannuccelli, V. Complexation of the Sunscreen Agent, 4-Methylbenzylidene Camphor with Cyclodextrins: Effect on Photostability and Human Stratum Corneum Penetration. J. Pharm. Biomed. Anal. 2007, 44, 29–34. [Google Scholar] [CrossRef]
- Schmidt, T.; Ring, J.; Abeck, D. Photoallergic Contact Dermatitis Due to Combined UVB (4-Methylbenzylidene camphor/octyl methoxycinnamate) and UVA (Benzophenone-3/butyl methoxydibenzoylmethane) Absorber Sensitization. Dermatology 1998, 196, 354–357. [Google Scholar] [CrossRef]
- Cohen, M.; Wolfe, R.; Mai, T.; Lewis, D. A Randomized, Double Blind, Placebo Controlled Trial of a Topical Cream Containing Glucosamine Sulfate, Chondroitin Sulfate, and Camphor for Osteoarthritis of the Knee. J. Rheumatol. 2003, 30, 523–528. [Google Scholar]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Centini, M.; Anselmi, C. 4-Methylbenzylidene Camphor Microspheres: Reconstituted Epidermis (Skinethic®) Permeation and Distribution. Int. J. Cosmet. Sci. 2015, 37, 298–305. [Google Scholar] [CrossRef]
- Somade, O.T. Camphor Toxicity: A Review of Recent Findings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 775–790. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef]
- Khine, H.; Weiss, D.; Graber, N.; Hoffman, R.S.; Esteban-Cruciani, N.; Avner, J.R. A Cluster of Children with Seizures Caused by Camphor Poisoning. Pediatrics 2009, 123, 1269–1272. [Google Scholar] [CrossRef]
- Ernst, E. Adverse Effects of Herbal Drugs in Dermatology. Br. J. Dermatol. 2000, 143, 923–929. [Google Scholar] [CrossRef]
- Cutillas, A.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. Essential Oils from Spain: Determination of Composition, Antioxidant Capacity, Antienzymatic, and Antimicrobial Bioactivities. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Rupasinghe, H.P.V. Essential Oils from Origanum Vulgare and Salvia officinalis Exhibit Antibacterial and Anti-Biofilm Activities against Streptococcus Pyogenes. Microb. Pathog. 2018, 117, 118–127. [Google Scholar] [CrossRef]
- Canlı, K.; Yetgin, A.; Benek, A.; Bozyel, M.E.; Murat Altuner, E. In Vitro Antimicrobial Activity Screening of Ethanol Extract of Lavandula stoechas and Investigation of Its Biochemical Composition. Adv. Pharmacol. Sci. 2019, 2019, 3201458. [Google Scholar] [CrossRef]
- Aouf, A.; Bouaouina, S.; Abdelgawad, M.A.; Abourehab, M.A.S.; Farouk, A. In Silico Study for Algerian Essential Oils as Antimicrobial Agents against Multidrug-Resistant Bacteria Isolated from Pus Samples. Antibiotics 2022, 11, 1317. [Google Scholar] [CrossRef]
- Ghavam, M. In Vitro Biological Potential of the Essential Oil of Some Aromatic Species Used in Iranian Traditional Medicine. Inflammopharmacology 2022, 30, 855–874. [Google Scholar] [CrossRef]
- Ivarsen, E.; Fretté, X.C.; Christensen, K.B.; Christensen, L.P.; Engberg, R.M.; Grevsen, K.; Kjaer, A. Bioassay-Guided Chromatographic Isolation and Identification of Antibacterial Compounds from Artemisia annua L. That Inhibit Clostridium Perfringens Growth. J. AOAC Int. 2014, 97, 1282–1290. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Rashad, Y.M.; Abdulkhair, W.M. Evaluation of the Antimicrobial Potential of Selected Medicinal Plant Extracts against Some Plant and Human Pathogens. J. Pure Appl. Microbiol. 2014, 8, 159–168. [Google Scholar]
- Oukil, N.; Hamri, S.; Bedjou, F. Antimicrobial Effects of Combinations between Essential Oils, Antibiotics, and Major Components of Essential Oils. Phytothérapie 2023, 21, 10–18. [Google Scholar] [CrossRef]
- Costa, J.; Sousa, S.; Galvão, A.; Mata, J.; Leitão, J.; Carvalho, M. Key Parameters on the Antibacterial Activity of Silver Camphor Complexes. Antibiotics 2021, 10, 135. [Google Scholar] [CrossRef]
- Peraman, R.; Tiwari, A.K.; Geetha Vani, M.; Hemanth, J.; Geetha Sree, Y.; Karthik, K.; Ashby, C.R.; Padmanabha Reddy, Y.; Pemmidi, R.V. New Camphor Hybrids: Lipophilic Enhancement Improves Antimicrobial Efficacy against Drug-Resistant Pathogenic Microbes and Intestinal Worms. Med. Chem. Res. 2018, 27, 1728–1739. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Galvão, A.M.; Guerreiro, S.I.; Leitão, J.H.; Suarez, A.C.; Carvalho, M.F.N.N. Antibacterial Activity of Silver Camphorimine Coordination Polymers. Dalton Trans. 2016, 45, 7114–7123. [Google Scholar] [CrossRef]
- Laczkowski, K.; Misiura, K.; Biernasiuk, A.; Malm, A.; Siwek, A.; Plech, T.; Ciok-Pater, E.; Skowron, K.; Gospodarek, E. Synthesis, In Vitro Biological Screening and Molecular Docking Studies of Novel Camphor-Based Thiazoles. Med. Chem. 2014, 10, 600–608. [Google Scholar] [CrossRef]
- Jitrangsri, K.; Lertsuphotvanit, N.; Kabthong, N.; Phaechamud, T. Metronidazole-Loaded Camphor-Based In Situ Forming Matrix for Periodontitis Treatment. AAPS PharmSciTech 2023, 24, 185. [Google Scholar] [CrossRef]
- Rani, P.; Kumari, C.; Kumar, K.D.; Kumar, R. Effect of Camphor Incorporation on the Material and Antibacterial Properties of Soy Protein Isolate Films. J. Res. Updates Polym. Sci. 2023, 12, 162–170. [Google Scholar] [CrossRef]
- Santos, T.B.; Vieira, A.A.; Paula, L.O.; Santos, E.D.; Radi, P.A.; Khouri, S.; Maciel, H.S.; Pessoa, R.S.; Vieira, L. Flexible Camphor Diamond-like Carbon Coating on Polyurethane to Prevent Candida Albicans Biofilm Growth. J. Mech. Behav. Biomed. Mater. 2017, 68, 239–246. [Google Scholar] [CrossRef]
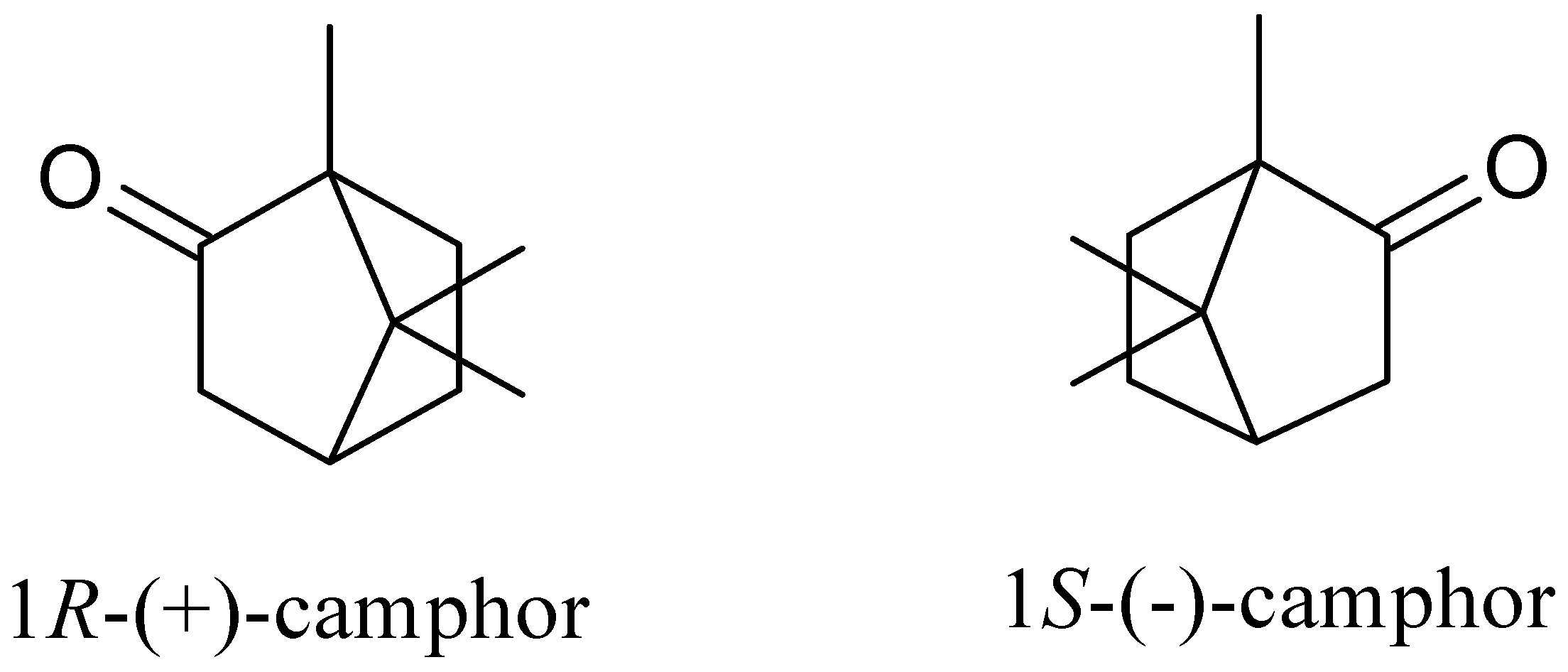

| Plant Cultivar | Source of EO | Percentage [%] | References |
|---|---|---|---|
| Achillea grandifolia | Inflorescences | 10.0–70.5 | [46] |
| Leaves | 5.5–83.2 | ||
| Achillea millefolium L. | Inflorescences | 13.0 | [47] |
| Artemisia annua | Leaves and stems | 11.4 | [48] |
| Artemisia haussknechtii | Aerial parts | 11.8 | [49] |
| Artemisia khorassanica | Aerial parts | 74.2 | [49] |
| Artemisia sieberi | Aerial parts | 33.6 | [50] |
| Artemisia spicigera | Whole plant | 29.6 | [51] |
| Chrysanthemum japonense | Flowers | 47.6 | [52] |
| Leaves | 39.1 | ||
| Cinnamomum camphora | Leaves | 93.1 | [33] |
| Branch | 53.6 | ||
| Wood | 53.2 | ||
| Cinnamomum camphora | Barks | 51.3 | [53] |
| Leaves | 40.5 | ||
| Fruits | 28.1 | ||
| Lavandula angustifolia | Leaves and inflorescences | 10.7–18.8 | [54] |
| Lavandula latifolia | Whole plant | 12.2 | [55] |
| Ocimum basilicum L. | Aerial Parts | 42.1 | [56] |
| Ocimum kilimandscharicum Guerke | Leaves | 45.9 | [57] |
| Santolina chamaecyparissus L. | Aerial part | 17.7 | [58] |
| Salvia fruticosa | Aerial part | 20.3 | [59] |
| Salvia jordanii | Aerial part | 33.4 | [60] |
| Salvia officinalis L. | Whole plant | 26.6 northern Albania | [61] |
| 43.8 southern Albania | |||
| Salvia rosmarinus | Aerial part | 3.3–42.2 | [60] |
| Stachys germanica L. | Aerial parts | 52.9 | [62] |
| Tanacetum parthenium L. | Aerial parts | 53.4–52.9 | [63] |
| Stems and leaves | 47.9–49.6 | ||
| Inflorescence | 11.6–11.5 | ||
| Unripe seeds | 12.3–12.4 | ||
| Ripe seeds | 12.3–10.3 | ||
| Thymus algeriensis | Whole plant | 6.8–19.9 (vegetative stage) | [64] |
| 8.1–15.7 (flowering stage) |
| Bacteria | Plant Cultivar | Camphor Concentration [%] | MIC Value | Ref. |
|---|---|---|---|---|
| P. aeruginosa | Salvia officinalis | 16.4% | 0.125 mg/mL | [102] |
| Salvia officinalis | 16.6% | 5 mg/mL | [99] | |
| Curcuma aeruginosa | 29.39% | 0.125 mg/mL | [103] | |
| 1 mg/mL | ||||
| Thymus hirtus ssp. algeriensis | 19.2% | 0.022 mg/mL | [104] | |
| Salvia pachystachys | 31.0% | 5 mg/mL | [105] | |
| Ferula communis | 18.3% | 0.156 mg/mL | [106] | |
| Rosmarinus officinalis | 18.74% | 2 mg/mL | [107] | |
| Pulicaria undulata | 44.48% | 6.25 mg/mL | [108] | |
| Rosmarinus officinalis | 26.5% | 2.56 mg/mL | [109] | |
| Oncosiphon suffruticosum | 31% | 6.4 mg/mL | [110] | |
| A. baumannii | Artemisia herba-alba Asso | 50.7% | 10 mg/mL | [111] |
| 20 mg/mL | ||||
| Rosmarinus officinalis | 23.04% | 12.5 mg/mL | [112] | |
| K. pneumoniae | Lavandula stoechas ‘avonview’ | 35.30% | 0.39 mg/mL | [113] |
| Lavandula stoechas | 36.69% | 0.5 mg/mL | [101] | |
| Withania frutescens | ~24–25% | 0.006125 mg/mL | [114] | |
| E. coli | Rosmarinus officinalis | 18.743% | 1 mg/mL | [107] |
| Thymus hirtus ssp. algeriensis | 19.2% | 1.8 mg/mL | [104] | |
| Rosmarinus officinalis | 26.5% | 1.28 mg/mL | [109] | |
| Artemisia annua | ~30% | 2.5 mg/mL | [115] | |
| Croton tetradenius | ~14% | 5.6 mg/mL | [116] | |
| Withania frutescens | ~24–25% | 0.006125 mg/mL | [114] | |
| Lavandula stoechas | 36.69% | 1 mg/mL | [101] | |
| - | pure camphor | 2.75 mg/mL | [117] |
| Bacteria | Plant Cultivar | Camphor Conc. [%] | ZOI Value for EO [mm] | EO Content per Disc | ZOI Value for Positive Control | Positive Control Content per Disc | Ref. |
|---|---|---|---|---|---|---|---|
| P. aeruginosa | Curcuma aeruginosa | 29.39% | 5.7 | 10 µL | 7.6 | 10 µg GM | [103] |
| Withania frutescens | ~24–25% | 16.11 | 10 µL | - | 20 µg STR, 1.67 mg AMP | [114] | |
| Rosmarinus officinalis | 19.75% | 7.0 | 50 µL | ND | 10 µg PCN | [118] | |
| Artemisia absinthium | 39.1% | 12.0 | |||||
| Tetraclinis articulata | 20.1% | 20.0 | 6 µL | 20.0 | 10 µg CPL | [119] | |
| 15.0 | 30 µg CHL | ||||||
| 32.0 | 30 µg GM | ||||||
| - | 20/10 µg AMC | ||||||
| Pulicaria undulata | 44.48% | 24.0 | 20 µL | 17.0 | ND GM | [108] | |
| 16.0 | ND TET | ||||||
| Artemisia sieberi Besser | 42.9% | 10.3 | 5 µL | ND | ND | [120] | |
| 41.6% | 8.6 | ||||||
| 37.1% | 10.3 | ||||||
| - | pure camphor | 10.4 | ND | ND | ND | ||
| A. baumannii | Artemisia herba-alba Asso | 50.47% | 30.6 | 6 µL | 13.6 | 10 µg STR | [111] |
| 27.3 | 10.0 | ||||||
| Rosmarinus officinalis | 23.04% | 34.0 | ND | - | - | [112] | |
| Artemisia herba-alba | 32.0% | 15.3 | 10 µL | 14.0 | ND CIP | [24] | |
| 12.0 | ND AKN | ||||||
| Salvia officinalis | 17.1% | 13.67 | 10 µL | ND | 20 µg GM | [121] | |
| K. pneumoniae | Tetraclinis articulata | 20.1% | 29.0 | 6 µL | 22.0 | 10 µg CPL | [119] |
| 30.0 | 30 µg CHL | ||||||
| 39.0 | 30 µg GM | ||||||
| 30.0 | 20/10 µg AMC | ||||||
| Rosmarinus officinalis | 24.82% | 13.12 | 20 µL | 6.0 | ND OTC | [100] | |
| 21.41% | 9.06 | ||||||
| Rosmarinus officinalis | 16.99% | 8.67 | 5 mg | 28.0 | 5 mg NAL | [122] | |
| 20.4% | 9.0 | ||||||
| Lavandula stoechas ‘avonview’ | 42.47% | 10.0 | 30 µL | 9.30 | ND CHL | [123] | |
| 35.30% | 8.03 | ||||||
| Combretum hereroense | ND | 18.2 | 10 µL | 18.8 | 30 µg CFX | [124] | |
| E. coli | Rosmarinus officinalis | 16.99% | 12.67 | 5 mg | 30.0 | 5 mg NAL | [122] |
| 20.4% | 13.67 | ||||||
| Thymus hirtus ssp. algeriensis | 19.2% | 30.0 | 10 µL | 15.0 | ND STR | [104] | |
| 27.0 | ND CHL | ||||||
| Salvia officinalis | 17.1% | 16.67 | 10 µL | ND | 20 µg GM | [121] | |
| Pulicaria undulata | 44.48% | 17.0 | 20 µL | 22.0 | ND GM | [108] | |
| 16.0 | ND TET | ||||||
| Artemisia sieberi Besser | 42.9% | 12.8 | 5 µL | ND | ND | [120] | |
| 41.6% | 12.4 | ||||||
| 37.1% | 12.3 | ||||||
| - | pure camphor | 12.91 | ND | ND | ND | ||
| Thymus algeriensis | 17.45–32.56% | 13.0 | 40 µL | 27.0 | 25 µg AMX | [125] | |
| Rosmarinus officinalis | 26.5% | 14.2 | ND | 21.5 | ND CIP | [109] | |
| Combretum hereroense | ND | 15.8 | 10 µL | 23.2 | 30 µg CFX | [124] |
| Plant Cultivar | Camphor Concentration [%] | MIC Value | Ref. |
|---|---|---|---|
| Salvia lavandulifolia | 29.1% | 0.156 mg/mL | [129] |
| 0.312 mg/mL | |||
| Curcuma aeruginosa | 29.39% | 0.25 mg/mL | [103] |
| - | pure camphor | 0.125 mg/mL | [128] |
| 0.35 mg/mL | |||
| Withania frutescens | 24.26% | 0.0004 mg/mL | [114] |
| Rosmarinus officinalis | 26.5% | 0.16 mg/mL | [109] |
| Cassia fistula | ~14% | 0.313 mg/mL | [130] |
| - | pure camphor | 0.156 mg/mL | |
| Tetraclinis articulata | 20.1% | 0.268 mg/mL | [119] |
| Brocchia cinerea | ~14% | 0.0168 mg/mL | [131] |
| Plectranthus cylindraceus Hochst. et Benth | 40.93% | 0.4 mg/mL | [132] |
| Rosmarinus officinalis | 24.3% | 0.025 mg/mL | [133] |
| 15.52% | 0.00156 mg/mL | ||
| 18.47% | 0.00078 mg/mL | ||
| Anvillea radiata | 21.41% | 0.01031 mg/mL | [134] |
| 0.02275 mg/mL | |||
| Artemisia aucheri | 18% | 0.4 mg/mL | [135] |
| Salvia fruticosa | 21.32% | 0.079 mg/mL | [136] |
| Achillea grandifolia | 23.4% | 0.16042 mg/mL | [137] |
| 0.525 mg/mL | |||
| Tetraclinis articulata | 15.97% | 0.125 mg/mL | [138] |
| Artemisia herba-alba var. huguetii | 18.38% | 0.00183 mg/mL | [139] |
| Plant Cultivar | Camphor Conc. [%] | ZOI Value for EO [mm] | EO Content per Disc | ZOI Value for Positive Control | Positive Control Content per Disc | Ref. |
|---|---|---|---|---|---|---|
| Curcuma aeruginosa | 29.39% | 11.6 | 10 µL | 10.54 | 10 µg COM | [103] |
| Ocimum kilimandscharicum Guerke | 52.0–57.2% | 4.33–5.33 | 8 µL | 24.33 | ND KTC | [140] |
| 4.66–6.33 | ||||||
| Achillea odorata subsp. pectinata | 45.01 | 25.33 | 12.5 µL | 18.66 | 10 µg AMB | [141] |
| Pulicaria undulata | 44.48% | 23.0 | 20 µL | 14.0 | ND NYT | [108] |
| Withania frutescens | 24.26% | 47.0 | 10 µL | 21.2 | ND FLC | [114] |
| Suaeda vermiculata Forssk | 28.74% | 19.0 | 50 µL | 16.0 | 10 µg CLT | [108] |
| Rosmarinus officinalis | 33.9–41.2% | 11.6–14.6 | 10 µL | 28.0 | 10 µg MIC | [142] |
| 18.0 | 20 µg AMB | |||||
| Artemisia annua | 17.0% | 8.5 | 15–30 µL | ND | ND | [143] |
| Artemisia siebieri Besser | 42.9% | 16.2 | 5 µL | - | - | [120] |
| 41.6% | 14.2 | |||||
| 37.1% | 16.0 | |||||
| - | pure camphor | 11.4 | ND | - | - | |
| Thymus algeriensis | 17.45–32.56% | 13.0 | 80 µL | 24.0 | 25 µg ITC | [125] |
| Rosmarinus officinalis | 26.5% | 18.2 | ND | 21.0 | ND FLC | [109] |
| 18.6 | 16.0 | |||||
| Tetraclinis articulata | 20.1% | 22.0 | 6 µL | 10.0 | 100 µg AMB | [125] |
| 18.0 | 50 µg ECC | |||||
| 19.0 | 50 µg MIC | |||||
| 10.0 | 50 µg CLT | |||||
| 0.0 | 1 µg 5FC | |||||
| Brocchia cinerea | ~14% | 42.33 | 20 µL | 21.0 | ND FLC | [131] |
| Matricaria chamomilla | 16.42% | 21.07 | 10 µL | 28.6 | 100 I.U. NYT | [144] |
| Tetraclinis articulata | 15.97% | 15.6 | 10 µL | ~10 | 20 µg FLC | [138] |
| ~20 | 20 µg CLT | |||||
| Artemisia herba-alba var. huguetii | 18.38% | 39.0 | 4.7 µg | 24.7 | ND FLC | [139] |
| Molecular Actions of Camphor | Effect | Ref. |
|---|---|---|
| Decrease in elastase activity | Increase in the amount of elastin | [98] |
| Induction of phosphorylation of PI3K/AKT and ER pathways | Inhibition of fibroblast apoptosis | |
| Action on β-galactosidase | Inhibition of skin aging | |
| Activation of TRPV1 | Desensitizing and analgesic effect | [147] |
| Activation of TRPM8 | Activation of the channel, enhancement of cold sensitivity, inhibition of the response to menthol | [154] |
| Activation of TRPV3 | Heat activation, suggestive sensitizing role | [148] |
| Activation and inhibition of TRPA1 | Agonist–antagonist action, probable role as a cold sensor in nociceptive neurons | [151] |
| Bacteria | Plant Cultivar | Camphor Concentration [%] | MIC Value | Ref. |
|---|---|---|---|---|
| S. aureus | Lavandula stoechas | 8.64% | 0.01795 mg/mL | [171] |
| Salvia officinalis | 16.4% | 0.125 mg/mL | [102] | |
| Ocimum tenuiflorum | 31.52% | 0.00225 mg/mL | [103] | |
| Lavandula stoechas | 36.69% | 0.5 mg/mL | [101] | |
| Rosmarinus officinalis | 26.5% | 0.32 mg/mL | [109] | |
| S. pyogenes | Satureja montana | 29.1% | 0.03 mg/mL | [129] |
| 0.63 mg/mL | ||||
| Makhlaseh | 49.0–56.9% | 8 mg/mL | [172] | |
| 4 mg/mL | ||||
| Salvia officinalis | 16.6% | 0.5 mg/mL | [170] | |
| Perovskia abrotanoides | 21.68% | 2.0 mg/mL | [173] | |
| Lavandula stoechas × viridis ‘St. Brelade’s’ | 7.13% | 6.25 mg/mL | [113] | |
| Clostridium spp. | Artemisia annua | >60% | ~1.6 mg/mL | [174] |
| Plant Cultivar | Camphor Conc. [%] | ZOI Value for EO [mm] | EO Content per Disc | ZOI Value for Positive Control [mm] | Positive Control Content per Disc | Ref. |
|---|---|---|---|---|---|---|
| Ocimum kilimanscharicum Guerke | 54.6% | 7.66 | 8 µL/disc | 18.0 | ND, TET | [140] |
| 52.0% | 6.66 | |||||
| 57.2% | 5.0 | |||||
| Lavandula stoechas | 8.64% | 12.0 | 5.83 mg/disc | 22.0 24.0 | ND, CIP, GM | [171] |
| 13.0 | 23.4 mg/disc | |||||
| 18.0 | 35.1 mg/disc | |||||
| Salvia officinalis | 36.92% | 8.0–13.0 | 20 µL/disc | ND | ND | [172] |
| Curcuma aeruginosa | 29.39% | 22.0 | 10 µL/disc | 7.28 | 10 µg GM | [103] |
| Artemisia sieberi Besser | 2.8–42.9% | 12.3–20.6 | 5 µL/disc | ND | ND | [120] |
| - | pure camphor | 12.0 | ND | ND | ND | |
| Thymus algeriensis | 17.45–32.56% | 18.0 | 40 µL/disc | 10.0 | 25 µg AMX | [125] |
| Rosmarinus officinalis | 26.5% | 25.2 | ND | 30.0 | 5 µg CIP | [109] |
| Cinnamomum camphora | 3% | 7.0 | ND | 15.0 | 10 µg STR | [175] |
| 6% | 11.0 | |||||
| 9% | 17.0 | |||||
| Combretum hereroense | ND | 21.4 | 10 µL/disc | 7.2 | 5 µg/MET | [124] |
| >15.0 | 10 µg CHL, GM 5 µg CIP |
| Camphor Source | MIC Value | ZOI Value | Pathogen | Resistance Mechanism | Ref. |
|---|---|---|---|---|---|
| Artemisia judaica EO (16.1% camphor) | 1.25 μL/mL | - | C. albicans | Resistance to azoles mediated by efflux pumps Cdr1 and Cdr2 | [101] |
| Artemisia herba-alba EO (32% camphor) | 1.2 μL/mL | - | S. aureus | MRSA—methicillin-resistant S. aureus, mediated by mecA gene resistance to various β-lactam antibiotics | [24] |
| Combretum hereroense EO | 0.17 mg/mL | [127] | |||
| Camphor quinoxalin-2,3(1H,4H)-dione | 24 μM | - | [134] | ||
| Artemisia herba-alba EO (32% camphor) | 1.2 µL/mL | - | A. baumannii | IRAB—imipenem-resistant A. baumannii | [24] |
| Rosmarinus officinalis EO (~23% camphor) | - | 9.33–12.67 mm vs. 30.0 mm nalidixic acid | E. coli | Resistance to fluoroquinolones mediated by gyrA and parC genes mutations | [131] |
| Artemisia annua EO (~30% camphor) | 2.5–10 mg/mL | - | E. coli | ESβL—extended spectrum β-lactamase | [132] |
| camphor-linked biphenyl quinoxalin-6-sulfonamide | 16 µg/mL | - | K. pneumoniae | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Madej, A.; Viscardi, S.; Grabarczyk, M.; Topola, E.; Kozłowska, J.; Mączka, W.; Wińska, K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals 2024, 17, 715. https://doi.org/10.3390/ph17060715
Duda-Madej A, Viscardi S, Grabarczyk M, Topola E, Kozłowska J, Mączka W, Wińska K. Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals. 2024; 17(6):715. https://doi.org/10.3390/ph17060715
Chicago/Turabian StyleDuda-Madej, Anna, Szymon Viscardi, Małgorzata Grabarczyk, Ewa Topola, Joanna Kozłowska, Wanda Mączka, and Katarzyna Wińska. 2024. "Is Camphor the Future in Supporting Therapy for Skin Infections?" Pharmaceuticals 17, no. 6: 715. https://doi.org/10.3390/ph17060715
APA StyleDuda-Madej, A., Viscardi, S., Grabarczyk, M., Topola, E., Kozłowska, J., Mączka, W., & Wińska, K. (2024). Is Camphor the Future in Supporting Therapy for Skin Infections? Pharmaceuticals, 17(6), 715. https://doi.org/10.3390/ph17060715








