Discovery of 1H-benzo[d]imidazole-(halogenated) Benzylidenebenzohydrazide Hybrids as Potential Multi-Kinase Inhibitors
Abstract
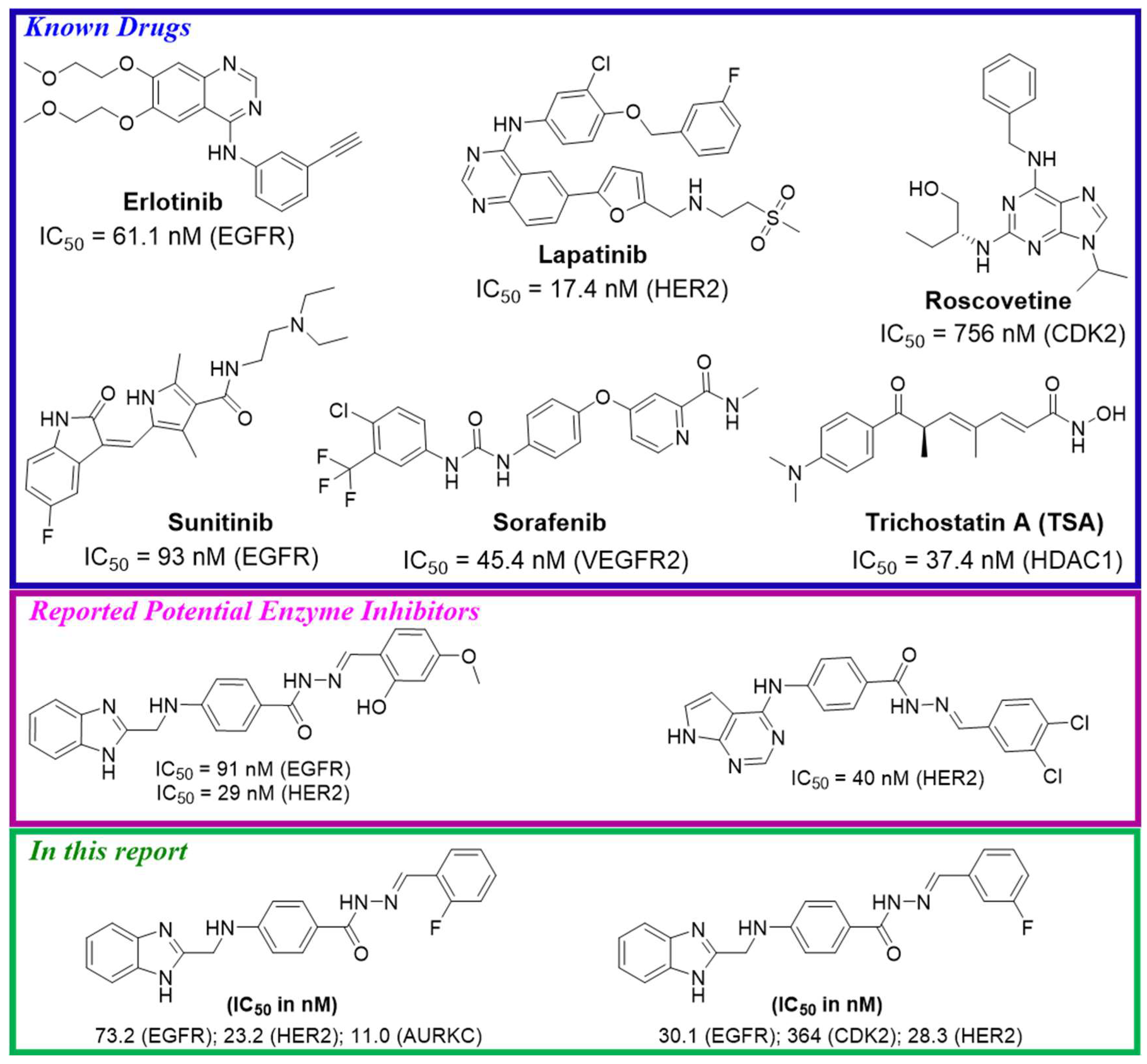
1. Introduction
2. Results and Discussion
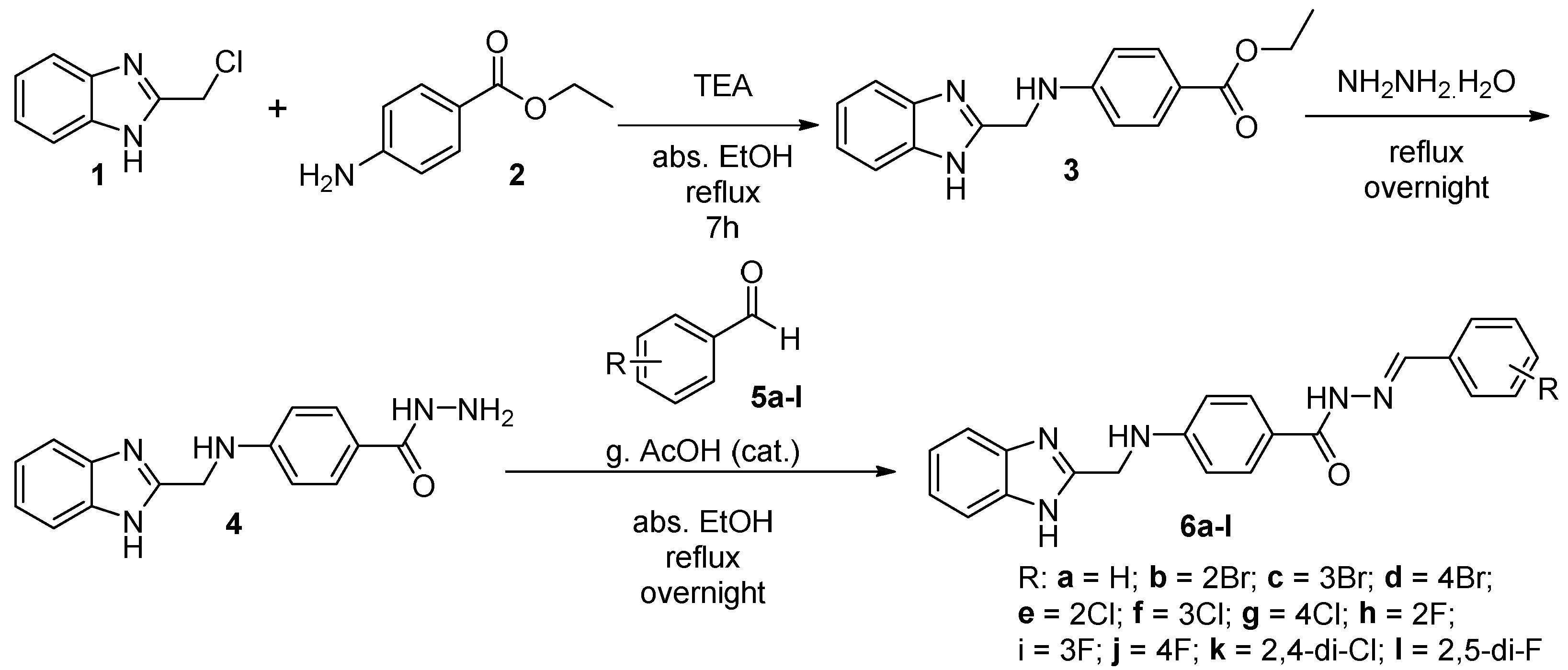
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Cytotoxicity
2.2.2. In Vitro Protein Kinase Inhibition Assays
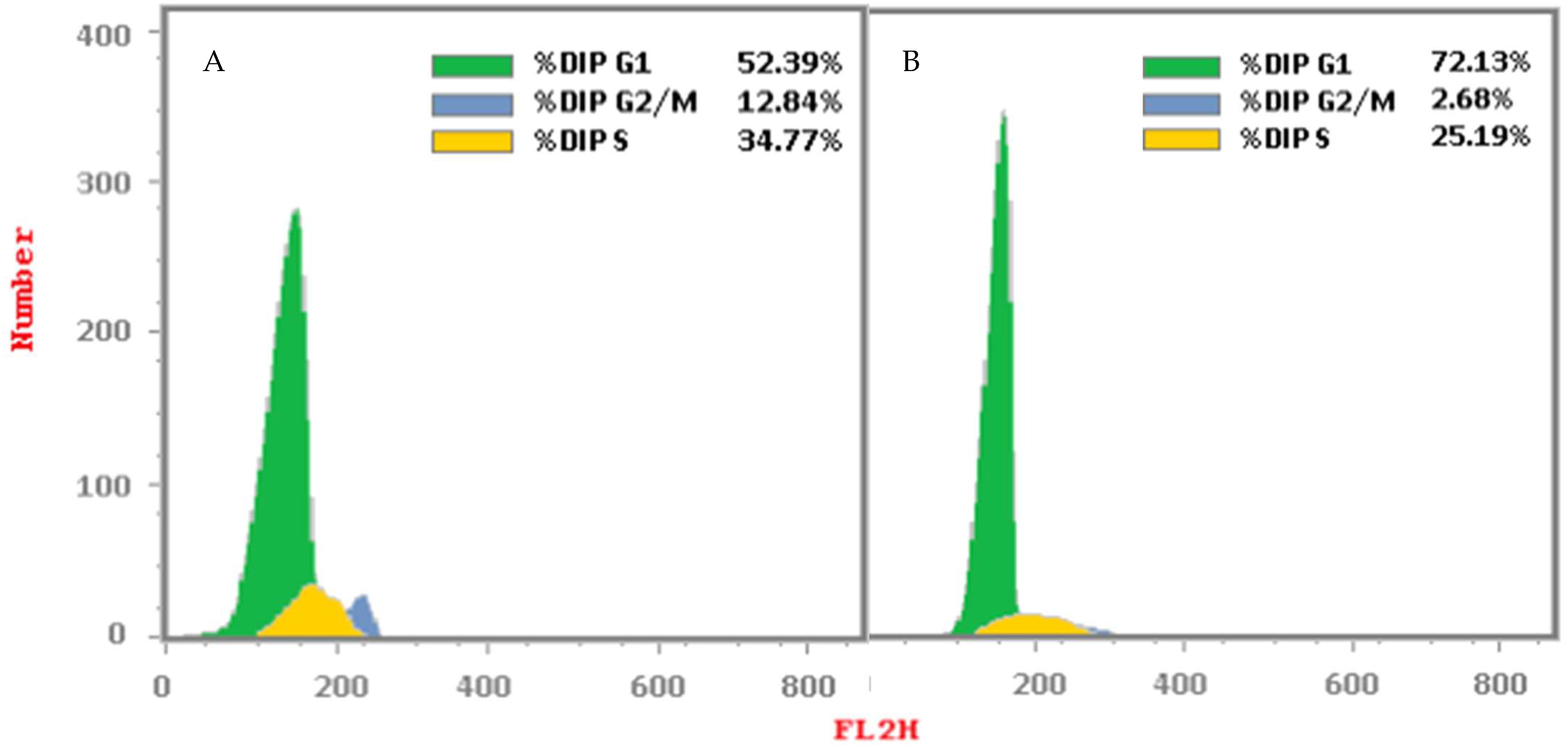
2.2.3. Cell Cycle Analysis
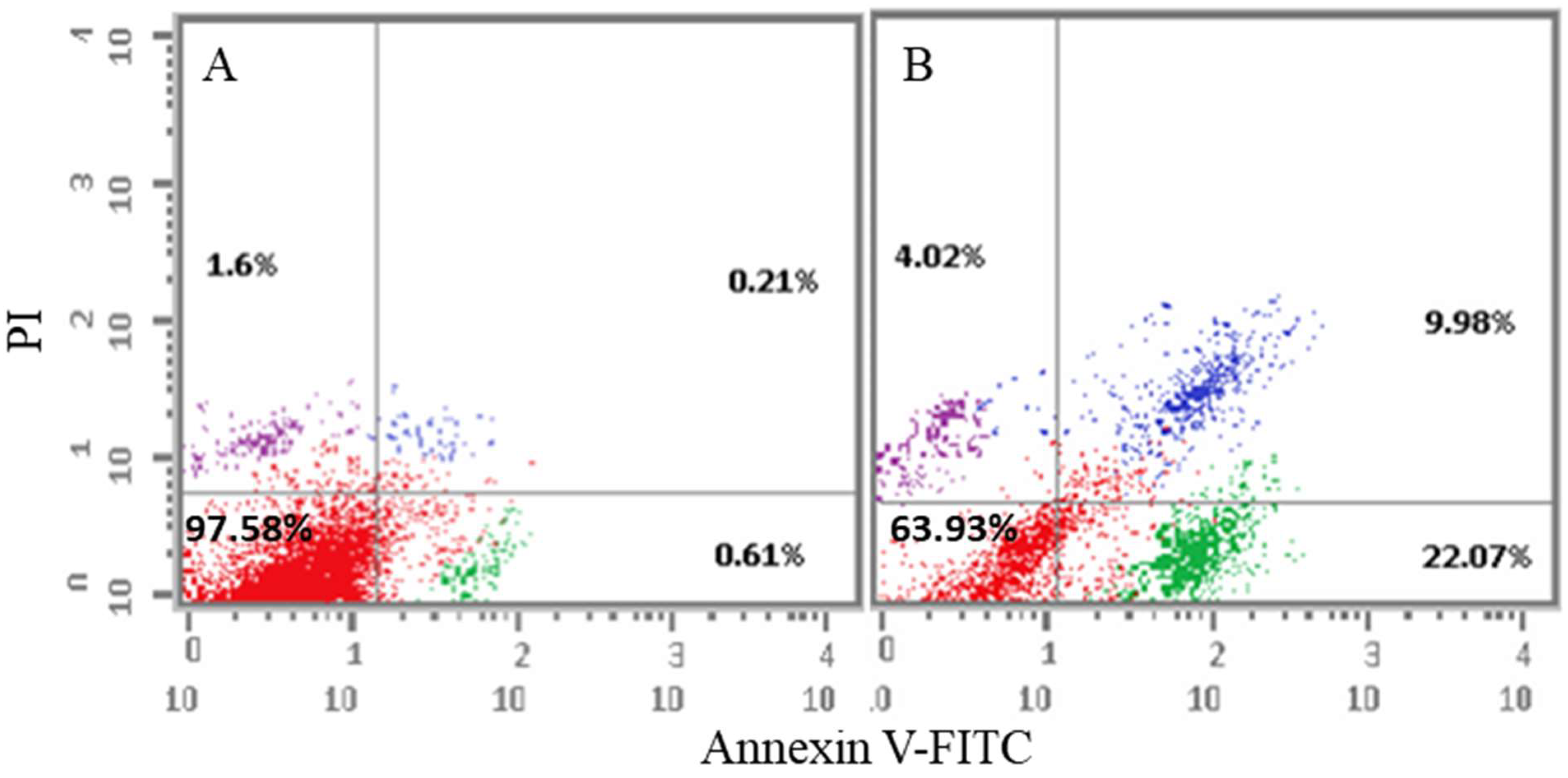
2.2.4. Apoptosis Analysis
Annexin-V/Propidium Iodide (PI) Staining Assay
Determination of Apoptotic Protein Levels
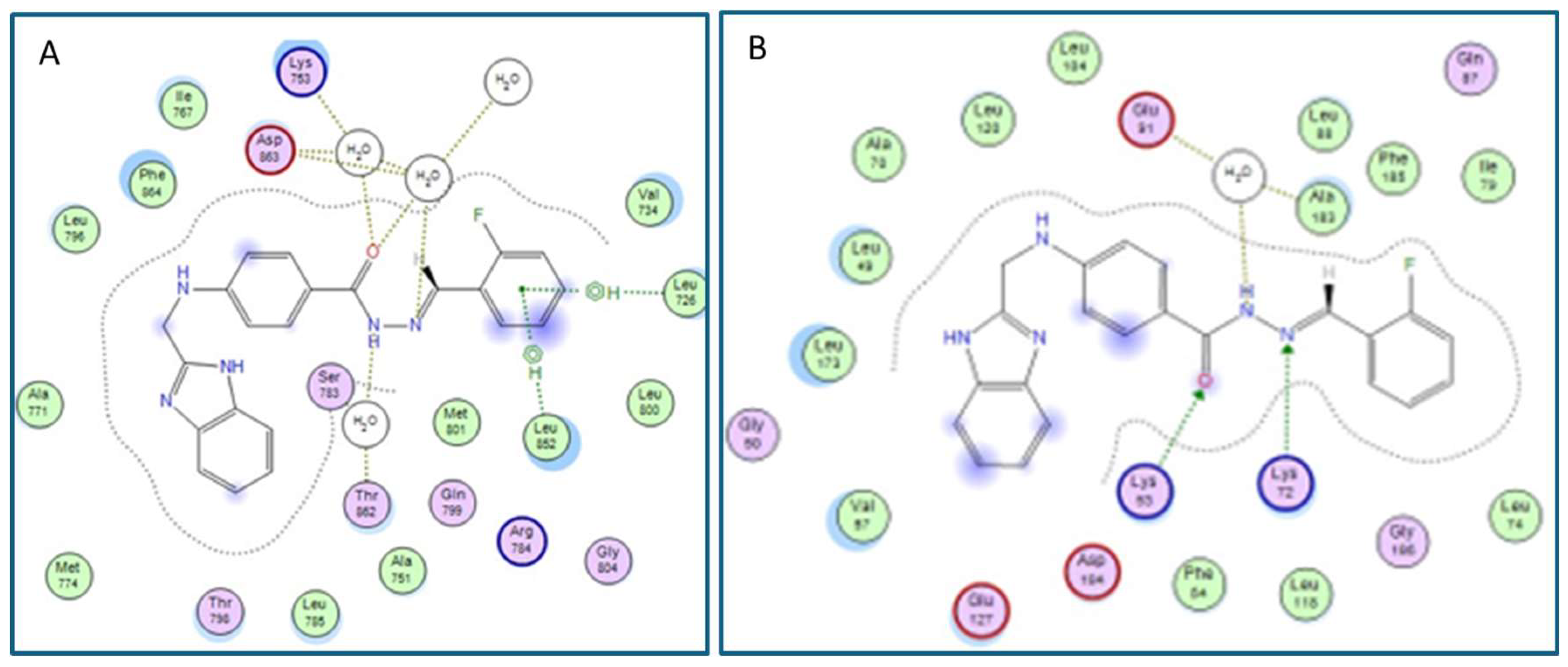
2.3. In Silico Studies
Molecular Docking
3. Materials and Methods
3.1. General
3.2. Chemistry
3.2.1. Synthesis of Ethyl 4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)benzoate (3)
3.2.2. Synthesis of 4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)benzohydrazide (4)
3.3. General Procedure for the Preparation of (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(substitutedbenzylidene)benzohydrazide (6a-l)
3.3.1. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-benzylidenebenzohydrazide (6a)
3.3.2. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(2-bromobenzylidene)benzohydrazide (6b)
3.3.3. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(3-bromobenzylidene)benzohydrazide (6c)
3.3.4. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(4-bromobenzylidene)benzohydrazide (6d)
3.3.5. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(2-chlorobenzylidene)benzohydrazide (6e)
3.3.6. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(3-chlorobenzylidene)benzohydrazide (6f)
3.3.7. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(4-chlorobenzylidene)benzohydrazide (6g)
3.3.8. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(2-fluorobenzylidene)benzohydrazide (6h)
3.3.9. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(3-fluorobenzylidene)benzohydrazide (6i)
3.3.10. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(4-fluorobenzylidene)benzohydrazide (6j)
3.3.11. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(2,4-dichlorobenzylidene)benzohydrazide (6k)
3.3.12. (E)-4-(((1H-benzo[d]imidazol-2-yl)methyl)amino)-N′-(2,5-difluorobenzylidene)benzohydrazide (6l)
3.4. Biological Evaluation
3.4.1. In Vitro Cytotoxicity Assay
3.4.2. In Vitro Enzyme Inhibitory Assays
3.4.3. Cell Cycle Analysis
3.4.4. Annexin-V/Propidium Iodide (PI) Double Staining Assay
3.4.5. Determination of Apoptotic Protein Levels
3.5. In Silico Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernandes, M.Z.; Cavalcanti, S.M.; Moreira, D.R.; de Azevedo Junior, W.F.; Leite, A.C. Halogen atoms in the modern medicinal chemistry: Hints for the drug design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.E.; Urner, L.M.; Kim, G.; Chow, K.; Baufeld, L.; Faull, K.; Cloughesy, T.F.; Clark, P.M.; Jung, M.E.; Nathanson, D.A. Development of a Potent Brain-Penetrant EGFR Tyrosine Kinase Inhibitor against Malignant Brain Tumors. ACS Med. Chem. Lett. 2020, 11, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Poznanski, J.; Winiewska-Szajewska, M.; Czapinska, H.; Poznańska, A.; Shugar, D. Halogen bonds involved in binding of halogenated ligands by protein kinases. Acta Biochim. Pol. 2016, 63, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R. Targeting the human epidermal growth factor receptor 2 (HER2) in the treatment of breast cancer: Recent advances and future directions. Rev. Recent. Clin. Trials 2007, 2, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kim, D.W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013, 369, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients With Metastatic Lung Adenocarcinoma With EGFR Mutations. J. Clin. Oncol. 2023, 41, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Fernández, D.; Soberanis Pina, P.; Turcott, J.G.; Chávez-Tapia, N.; Conde-Flores, E.; Cardona, A.F.; Arrieta, O. Management of diarrhea induced by EGFR-TKIs in advanced lung adenocarcinoma. Ther. Adv. Med. Oncol. 2023, 15, 17588359231192396. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef]
- Yang, J.C.; Shih, J.Y.; Su, W.C.; Hsia, T.C.; Tsai, C.M.; Ou, S.H.; Yu, C.J.; Chang, G.C.; Ho, C.L.; Sequist, L.V.; et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol. 2012, 13, 539–548. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, H.; Wang, S.; Dai, D.; Chen, F.; Meng, D.; Geng, P.; Tong, H.; Zhou, Y.; Pan, D.; et al. Effects of avitinib on the pharmacokinetics of osimertinib in vitro and in vivo in rats. Thorac. Cancer 2020, 11, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Foran, J.; Ravandi, F.; Wierda, W.; Garcia-Manero, G.; Verstovsek, S.; Kadia, T.; Burger, J.; Yule, M.; Langford, G.; Lyons, J.; et al. A phase I and pharmacodynamic study of AT9283, a small-molecule inhibitor of aurora kinases in patients with relapsed/refractory leukemia or myelofibrosis. Clin. Lymphoma Myeloma Leuk. 2014, 14, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.L.; Gianessi, C.A.; O’Malley, S.S.; Cavallo, D.A.; Shi, J.M.; Tetrault, J.M.; DeMartini, K.S.; Gueorguieva, R.; Pittman, B.; Krystal, J.H.; et al. Saracatinib Fails to Reduce Alcohol-Seeking and Consumption in Mice and Human Participants. Front. Psychiatry 2021, 12, 709559. [Google Scholar] [CrossRef] [PubMed]
- Massaro, F.; Molica, M.; Breccia, M. Ponatinib: A Review of Efficacy and Safety. Curr. Cancer Drug Targets 2018, 18, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Suda, K.; Koga, T.; Hamada, A.; Ohara, S.; Chiba, M.; Shimoji, M.; Takemoto, T.; Soh, J.; Mitsudomi, T. Foretinib can overcome common on-target resistance mutations after capmatinib/tepotinib treatment in NSCLCs with MET exon 14 skipping mutation. J. Hematol. Oncol. 2022, 15, 79. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.A., Jr.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J. Clin. Oncol. 2012, 30, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Meng, L.; Liu, B.; Zhang, Y.; Zhu, H.; Cui, J.; Sun, A.; Hu, Y.; Jin, J.; Jiang, H.; et al. Flumatinib versus Imatinib for Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia: A Phase III, Randomized, Open-label, Multi-center FESTnd Study. Clin. Cancer Res. 2021, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef]
- Kantarjian, H.; Giles, F.; Wunderle, L.; Bhalla, K.; O’Brien, S.; Wassmann, B.; Tanaka, C.; Manley, P.; Rae, P.; Mietlowski, W.; et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 2006, 354, 2542–2551. [Google Scholar] [CrossRef] [PubMed]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Sosman, J.A.; Quevedo, J.F.; Milhem, M.M.; Joshua, A.M.; Kudchadkar, R.R.; Linette, G.P.; Gajewski, T.F.; Lutzky, J.; Lawson, D.H.; et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: A randomized clinical trial. Jama 2014, 311, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Tunçbilek, M.; Kiper, T.; Altanlar, N. Synthesis and in vitro antimicrobial activity of some novel substituted benzimidazole derivatives having potent activity against MRSA. Eur. J. Med. Chem. 2009, 44, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Shingalapur, R.V.; Hosamani, K.M.; Keri, R.S. Synthesis and evaluation of in vitro anti-microbial and anti-tubercular activity of 2-styryl benzimidazoles. Eur. J. Med. Chem. 2009, 44, 4244–4248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Damu, G.L.; Cai, G.X.; Zhou, C.H. Design, synthesis and antimicrobial evaluation of novel benzimidazole type of Fluconazole analogues and their synergistic effects with Chloromycin, Norfloxacin and Fluconazole. Eur. J. Med. Chem. 2013, 64, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Luis, F.; Hernández-Campos, A.; Castillo, R.; Navarrete-Vázquez, G.; Soria-Arteche, O.; Hernández-Hernández, M.; Yépez-Mulia, L. Synthesis and biological activity of 2-(trifluoromethyl)-1H-benzimidazole derivatives against some protozoa and Trichinella spiralis. Eur. J. Med. Chem. 2010, 45, 3135–3141. [Google Scholar] [CrossRef]
- Luo, Y.; Yao, J.P.; Yang, L.; Feng, C.L.; Tang, W.; Wang, G.F.; Zuo, J.P.; Lu, W. Design and synthesis of novel benzimidazole derivatives as inhibitors of hepatitis B virus. Bioorg Med. Chem. 2010, 18, 5048–5055. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Bottegoni, G. Benzimidazole derivatives as kinase inhibitors. Curr. Med. Chem. 2014, 21, 2284–2298. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Benzimidazole-biologically attractive scaffold for protein kinase inhibitors. RSC Adv. 2014, 4, 12422–12440. [Google Scholar] [CrossRef]
- Woolley, D.W. Some biological effects produced by benzimidazole and their reversal by purines. J. Biol. Chem. 1944, 152, 225–232. [Google Scholar] [CrossRef]
- Demirel, S.; Ayhan Kilcigil, G.; Kara, Z.; Güven, B.; Onay Beşikci, A. Synthesis and Pharmacologic Evaluation of Some Benzimidazole Acetohydrazide Derivatives as EGFR Inhibitors. Turk. J. Pharm. Sci. 2017, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.J.; Siddiqui, A.A.; Khan, A.A.; Ali, Z.; Dewangan, R.P.; Pasha, S.; Yar, M.S. Design, synthesis, docking and QSAR study of substituted benzimidazole linked oxadiazole as cytotoxic agents, EGFR and erbB2 receptor inhibitors. Eur. J. Med. Chem. 2017, 126, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Juarez, J.; Li, J.; Manuia, M.; Niederst, M.J.; Tompkins, C.; Timple, N.; Vaillancourt, M.T.; Pferdekamper, A.C.; Lockerman, E.L.; et al. EGF816 Exerts Anticancer Effects in Non-Small Cell Lung Cancer by Irreversibly and Selectively Targeting Primary and Acquired Activating Mutations in the EGF Receptor. Cancer Res. 2016, 76, 1591–1602. [Google Scholar] [CrossRef]
- Brown, J.R. 3 Adriamycin and Related Anthracycline Antibiotics. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier: Amsterdam, The Netherlands, 1978; Volume 15, pp. 125–164. [Google Scholar]
- Daunorubicin. Available online: https://go.drugbank.com/drugs/DB00694 (accessed on 11 December 2023).
- Zorubicin. Available online: https://en.wikipedia.org/wiki/Zorubicin (accessed on 11 December 2023).
- Mirgany, T.O.; Rahman, A.F.M.M.; Alanazi, M.M. Design, synthesis, and mechanistic evaluation of novel benzimidazole-hydrazone compounds as dual inhibitors of EGFR and HER2: Promising candidates for anticancer therapy. J. Mol. Struct. 2024, 1309, 138177. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six—Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar]
- Alotaibi, A.A.; Asiri, H.H.; Rahman, A.F.M.M.; Alanazi, M.M. Novel pyrrolo[2,3-d]pyrimidine derivatives as multi-kinase inhibitors with VEGFR-2 selectivity. J. Saudi Chem. Soc. 2023, 27, 101712. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Alanazi, M.M.; Rahman, A.F.M.M. Discovery of New Pyrrolo[2,3-d]pyrimidine Derivatives as Potential Multi-Targeted Kinase Inhibitors and Apoptosis Inducers. Pharmaceuticals 2023, 16, 1324. [Google Scholar] [CrossRef]
- Bahadur, S.; Pandey, K.K. Synthesis of p-alkyl-(2-benzimidazolyl)-methyl-aminobenzoates and corresponding hydrazides as possible antimalarial agents. J. Indian. Chem. Soc. 1980, 57, 447–448. [Google Scholar]
- Alanazi, A.S.; Mirgany, T.O.; Alsfouk, A.A.; Alsaif, N.A.; Alanazi, M.M. Antiproliferative Activity, Multikinase Inhibition, Apoptosis- Inducing Effects and Molecular Docking of Novel Isatin–Purine Hybrids. Medicina 2023, 59, 610. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Hassan, G.S.; Al-Rashood, S.T.; Al-Warhi, T.; Altyar, A.E.; Alkahtani, H.M.; Almehizia, A.A.; Abdel-Aziz, H.A. Synthesis and in vitro anticancer activity of certain novel 1-(2-methyl-6-arylpyridin-3-yl)-3-phenylureas as apoptosis-inducing agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sabt, A.; Abdelhafez, O.M.; El-Haggar, R.S.; Madkour, H.M.F.; Eldehna, W.M.; El-Khrisy, E.; Abdel-Rahman, M.A.; Rashed, L.A. Novel coumarin-6-sulfonamides as apoptotic anti-proliferative agents: Synthesis, in vitro biological evaluation, and QSAR studies. J. Enzym. Inhib. Med. Chem. 2018, 33, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.M.; Aldawas, S.; Alsaif, N.A. Design, Synthesis, and Biological Evaluation of 2-Mercaptobenzoxazole Derivatives as Potential Multi-Kinase Inhibitors. Pharmaceuticals 2023, 16, 97. [Google Scholar] [CrossRef] [PubMed]

| Compound | In Vitro Cytotoxicity IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | R | HCT-116 | SI | HepG2 | SI | MCF-7 | SI | WI-38 |
| 6a | H | 24.62 ± 1.9 | 2.95 | 31.76 ± 2.2 | 2.29 | 26.31 ± 1.9 | 2.76 | 72.60 ± 4.0 |
| 6b | 2-Br | 42.90 ± 2.5 | 1.96 | 26.16 ± 1.9 | 3.22 | 31.82 ± 2.1 | 2.64 | 84.15 ± 4.3 |
| 6c | 3-Br | 10.21 ± 0.8 | 4.72 | 8.90 ± 0.6 | 5.41 | 7.82 ± 0.6 | 6.16 | 48.17 ± 2.7 |
| 6d | 4-Br | 30.26 ± 2.1 | 1.79 | 17.78 ± 1.3 | 3.05 | 25.18 ± 1.8 | 2.16 | 54.30 ± 3.1 |
| 6e | 2-Cl | 46.67 ± 2.7 | 1.33 | 28.80 ± 1.7 | 2.15 | 36.16 ± 2.3 | 1.71 | 61.86 ± 3.5 |
| 6f | 3-Cl | 63.72 ± 3.5 | 1.57 | 34.79 ± 2.1 | 2.87 | 45.61 ± 2.5 | 2.19 | >100 |
| 6g | 4-Cl | 35.29 ± 2.3 | 2.02 | 24.90 ± 1.7 | 2.86 | 22.51 ± 1.6 | 3.16 | 71.19 ± 3.5 |
| 6h | 2-F | 21.48 ± 1.6 | 4.33 | 12.94 ± 1.0 | 7.19 | 16.31 ± 1.2 | 5.70 | 92.98 ± 4.6 |
| 6i | 3-F | 13.44 ± 1.2 | 4.20 | 9.39 ± 0.8 | 6.01 | 11.64 ± 0.9 | 4.85 | 56.46 ± 3.0 |
| 6j | 4-F | 18.72 ± 1.4 | 2.08 | 14.02 ± 1.2 | 2.78 | 8.31 ± 0.7 | 4.70 | 39.03 ± 2.3 |
| 6k | 2,4-di-Cl | 67.83 ± 3.8 | 1.47 | 44.78 ± 2.4 | 2.23 | 48.64 ± 2.7 | 2.06 | >100 |
| 6l | 2,5-di-F | 39.84 ± 2.2 | 1.95 | 20.02 ± 1.5 | 3.88 | 29.81 ± 2.0 | 2.60 | 77.61 ± 4.1 |
| Sorafenib | 5.47 ± 0.3 | 1.95 | 9.18 ± 0.6 | 1.16 | 7.26 ± 0.3 | 1.47 | 10.65 ± 0.8 | |
| Doxorubicin | 5.23 ± 0.3 | 1.28 | 4.50 ± 0.2 | 1.49 | 4.17 ± 0.2 | 1.61 | 6.72 ± 0.5 | |
| Sunitinib | 17.91 ± 1.3 | 3.11 | 8.38 ± 0.5 | 6.64 | 24.06 ± 2.0 | 2.31 | 55.63 ± 3.3 | |
| Compound | In Vitro Protein Kinase Inhibition IC50 (nM) | ||||||
|---|---|---|---|---|---|---|---|
| EGFR | Her2 | VEGFR2 | CDK2 | AURKC | HDAC1 | mTOR | |
| 6c | 125.2 ± 0.041 | 55.6 ± 0.023 | 604.5 ± 0.022 | 938 ± 0.039 | 94.4 ± 0.036 | 2263 ± 0.077 | 1461 ± 0.05 |
| 6h | 73.2 ± 0.004 | 23.2 ± 0.001 | 194.5 ± 0.007 | 284 ± 0.012 | 11 ± 0.004 | 151.1 ± 0.005 | 413 ± 0.014 |
| 6i | 30.1 ± 0.03 | 28.3 ± 0.001 | 172.2 ± 0.006 | 364 ± 0.011 | 74.5 ± 0.003 | 96.6 ± 0.003 | 152 ± 0.005 |
| 6j | 166.4 ± 0.008 | 204.7 ± 0.009 | 307.2 ± 0.011 | 1448 ± 0.062 | 589.4 ± 0.022 | 473.3 ± 0.016 | 1305 ± 0.044 |
| Erlotinib | 61.1 ± 0.002 | - | - | - | - | - | - |
| Lapatenib | - | 17.4 ± 0.001 | - | - | - | - | - |
| Sorafenib | - | - | 45.4 ± 0.002 | - | - | - | - |
| Roscovetine | - | - | - | 756 ± 0.032 | - | - | - |
| TSA | - | - | - | - | 30.4 ± 0.001 | 37.4 ± 0.001 | - |
| Rapamycin | - | - | - | - | - | - | 208 ± 0.007 |
| Compound/Cell Line | DNA Content (%) | Cell Cycle Distribution Index (CDI) | ||
|---|---|---|---|---|
| %G0-G1 | %S | %G2/M | ||
| Cont. HepG2 | 52.39 | 34.77 | 12.84 | 0.91 |
| Compound 6i/HepG2 | 72.13 | 25.19 | 2.68 | 0.39 |
| Sample | Apoptosis | Necrosis | ||
|---|---|---|---|---|
| Alive Cell (%) | Early | Late | ||
| Cont. HepG2 | 97.58 | 0.61 | 0.21 | 1.6 |
| Compound 6i/HepG2 | 63.93 | 22.07 | 9.98 | 4.02 |
| Kinase Protein | Protein Expression (Pg/mL) (Folds) | ||
|---|---|---|---|
| Caspase-3 | Bax | Bcl-2 | |
| Control HepG2 | 99.904 ± 3.88 (1) | 71.075 ± 2.762 (1) | 15.668 ± 0.53 (1) |
| 6i/HepG2 | 388.497 ± 15.09 (3.9) | 513.731 ± 19.96 (7.22) | 2.073 ± 0.07 (0.132) |
| Saurosporine/HepG2 | 541.162 ± 21.02 (5.4) | 386.743 ± 15.03 (5.44) | 3.336 ± 0.11 (0.212) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirgany, T.O.; Asiri, H.H.; Rahman, A.F.M.M.; Alanazi, M.M. Discovery of 1H-benzo[d]imidazole-(halogenated) Benzylidenebenzohydrazide Hybrids as Potential Multi-Kinase Inhibitors. Pharmaceuticals 2024, 17, 839. https://doi.org/10.3390/ph17070839
Mirgany TO, Asiri HH, Rahman AFMM, Alanazi MM. Discovery of 1H-benzo[d]imidazole-(halogenated) Benzylidenebenzohydrazide Hybrids as Potential Multi-Kinase Inhibitors. Pharmaceuticals. 2024; 17(7):839. https://doi.org/10.3390/ph17070839
Chicago/Turabian StyleMirgany, Tebyan O., Hanadi H. Asiri, A. F. M. Motiur Rahman, and Mohammed M. Alanazi. 2024. "Discovery of 1H-benzo[d]imidazole-(halogenated) Benzylidenebenzohydrazide Hybrids as Potential Multi-Kinase Inhibitors" Pharmaceuticals 17, no. 7: 839. https://doi.org/10.3390/ph17070839
APA StyleMirgany, T. O., Asiri, H. H., Rahman, A. F. M. M., & Alanazi, M. M. (2024). Discovery of 1H-benzo[d]imidazole-(halogenated) Benzylidenebenzohydrazide Hybrids as Potential Multi-Kinase Inhibitors. Pharmaceuticals, 17(7), 839. https://doi.org/10.3390/ph17070839