Abstract
Major depressive disorder (MDD) is a mood disorder that has become a global health emergency according to the World Health Organization (WHO). It affects 280 million people worldwide and is a leading cause of disability and financial loss. Patients with MDD present immunoendocrine alterations like cortisol resistance and inflammation, which are associated with alterations in neurotransmitter metabolism. There are currently numerous therapeutic options for patients with MDD; however, some studies suggest a high rate of therapeutic failure. There are multiple hypotheses explaining the pathophysiological mechanisms of MDD, in which several systems are involved, including the neuroendocrine and immune systems. In recent years, inflammation has become an important target for the development of new therapeutic options. Extracellular monomeric ubiquitin (emUb) is a molecule that has been shown to have immunomodulatory properties through several mechanisms including cholinergic modulation and the generation of regulatory T cells. In this perspective article, we highlight the influence of the inflammatory response in MDD. In addition, we review and discuss the evidence for the use of emUb contained in Transferon as a concomitant treatment with selective serotonin reuptake inhibitors (SSRIs).
1. Introduction
Major depressive disorder (MDD) is a mood disorder that has become a global health emergency, according to the World Health Organization (WHO), as it affects 280 million people around the world. It is the leading cause of disability and causes an excessive overall economic burden on health and social care [1]. Symptoms of MDD include irritability, feelings of profound sadness, and emptiness, all persisting for at least two weeks. Patients may experience a loss of pleasure in previously enjoyed activities, poor concentration, feelings of excessive guilt, low self-esteem, hopelessness about the future, thoughts about dying or suicide, disrupted sleep, changes in appetite or weight, and a general feeling of exhaustion or low energy [1,2].
An intricate combination of social, psychological, genetic, biological, and environmental factors may cause MDD [3]. People who have experienced adversity in their lives are more susceptible to developing depression [1]. MDD is linked to and influenced by deteriorated health due to cardiovascular disease, cancer, diabetes, chronic infections, and respiratory diseases [4]. Likewise, MDD can cause cardiovascular, metabolic, or immunological complications [5,6,7].
MDD is the second leading cause of disability worldwide, as described in the Global Burden of Disease Study [8]. This is aggravated by the fact that between 76% and 85% of patients do not receive treatment due to stigma, ignorance, or inaccessibility to mental health care [9]. It was estimated that during the 2010–2018 period, the monetary burden on the US government for adult depression cases was increased by 37.9% [10]. According to an economic analysis, costs resulting from MDD are estimated at approximately $382.4 billion, of which nearly two-thirds of the economic burden of MDD is attributed to indirect costs [11], such as absenteeism in the workplace, unemployment, all-cause mortality, and disability [10,11].
The treatment for MDD is fundamentally based on psychotherapy and pharmacotherapy, and is usually complemented with diet, exercise, etc. [12]. Several treatment guidelines for MDD suggest that moderate to severe depressive episodes should be treated with pharmacotherapy or a combination of pharmacotherapy and psychotherapy [12,13]. Mild MDD may initially be treated solely with psychotherapy; nonetheless, it is important to always take into consideration patient preferences and their previous treatment history [2,13]. Additionally, an initial conservative strategy of vigilance and non-pharmacological treatment may also be chosen for mild MDD [13].
Several drugs from different groups have been used as pharmacotherapy in patients with MDD; some examples are shown in Table 1. In a general sense, the effects of these drugs are based on improving mood and increasing motivation [14]. Antidepressants are often used in combination with psychological treatment and lifestyle changes [1,2]; they must be taken daily, and usually start to take effect in 2–4 weeks [1,2,14] This treatment ideally should be continued for four to nine months, and the duration of medication will depend on many factors, such as the symptoms and the patient’s risk of developing another depressive episode [12]. In some cases, antidepressants must be prescribed for years to prevent future relapses [1,12]. In this context, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, a large-scale efficacy trial, reported that up to 50% of patients with MDD required additional treatment to first-line treatment and approximately 30% of patients failed to remit even after four sequential therapies, suggesting a high prevalence of treatment resistance [15,16]. In another study, 15% of patients with MDD failed to remit, and 35% had multiple episodes during 23 years of follow-up; recurrence rates ranged from 40% to 85% [17]. MDD frequently occurs comorbidly with diabetes and cardiovascular disease, which is a prototypical example of physical–mental comorbidity. This interaction can exacerbate both conditions, as well as increase the rate of therapeutic failure [18,19,20]. Other factors that contribute to therapeutic failure are poor quality of life, increased symptomatology, polypharmacy, lower adherence to self-care regimens, increased risk for developing functional impairment, higher morbidity and mortality, and higher medical costs [18].

Table 1.
Types of antidepressants.
The high relapse rate observed in patients with MDD generates the need to explore new therapeutic alternatives. In this manuscript, we will review some theories about the pathophysiology of MDD, focusing on inflammation. As will be addressed below, inflammation has become relevant in the pathophysiology of MDD, so it may be a therapeutic target. Finally, the possibility of ubiquitin as a potential therapeutic agent in MDD will be explored due to its immunomodulatory capacity.
2. Neuroimmunoendocrine and Inflammation Alterations in MDD
The pathophysiology of major depressive disorder (MDD) involves a complex interplay between various systems, including the immune, nervous, and endocrine systems. In recent years, several hypotheses have been proposed to explain the pathophysiology of MDD, as described below.
2.1. Hypothesis of MDD Pathophysiology
Stress is one of the most important factors in the development of MDD, particularly stress in early life and chronic stress in susceptible individuals [21]. The pathophysiology of MDD is characterized by monoamine depletion, glucocorticoid receptor (GR) resistance, elevated levels of corticotropin-releasing hormone (CRH) and cortisol, and an overstimulation of glutamate receptors [22,23].
Several hypotheses have been proposed over time to explain the pathophysiology of MDD [24] (Figure 1), such as the classic “monoamine hypothesis”, which proposes that MDD is the result of the selective depletion of monoamines (serotonin, dopamine, and noradrenaline) in several areas of the central nervous system (CNS) [25,26]. Another theory is the “glutamate hypothesis”, which postulates that this neurotransmitter is also relevant in the formation of depressive symptoms and cognitive impairment through overstimulation of NMDA (N-methyl-D-aspartate) glutamate receptors, alteration of the AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor, altered glutamate reuptake, and dysfunction of the endocannabinoid system [27]. Another theory that has been put forward is the “neuroendocrine hypothesis” involving hyperactivity of the hypothalamic–pituitary–adrenal (HPA) axis, with an increase in cortisol production and a consequent reduction in hippocampal neurogenesis and plasticity in the cortex [27,28]. Recently, the neuroinflammation hypothesis has emphasized the mechanisms of microglial activation and the consequent production of proinflammatory cytokines, which in turn causes the hyperactivation of areas of the limbic system such as the amygdala, hippocampus, and cingulate gyrus, as well as metabolic alterations in the production of neurotransmitters induced by the activation of the enzyme indolamine-2,3-dioxygenase [29]. All of these theories described above attempt to explain MDD pathophysiology and symptoms, however, the inflammatory theory has become increasingly important and has been proposed as a central element in the pathophysiology of MDD [30,31]. One of the first pieces of evidence linking inflammation and behavioral changes was reported when immunotherapy with interferon (IFN)-α was introduced as a treatment for hepatitis C, in which the presence of depressive symptoms and even suicidal ideation was observed in patients receiving IFN-α treatment, as well as some reports of cognitive alterations and alterations in EEG patterns [32,33].
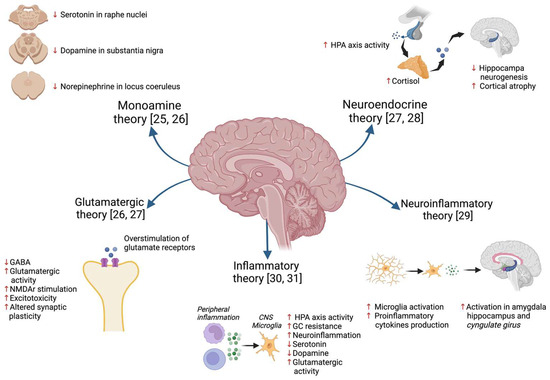
Figure 1.
A description of some of the hypotheses that have been proposed to explain the pathophysiology of depression [25,26,27,28,29,30,31].
According to the results of several studies in human and animal models, cytokines have a neuromodulator function and induce mood disorders like depression or anxiety and cognitive impairments, such as memory loss. Table 2 shows examples of psychiatric effects induced by cytokines [34]. Studies in murine models and in humans have demonstrated that proinflammatory cytokines modify the metabolism of neurotransmitters such as serotonin and dopamine in brain regions such as the hypothalamus, locus coeruleus, and central amygdala [35,36]. Additionally, it has been shown that an increase in serum proinflammatory cytokines induces sickness behavior, characterized by a febrile response, anorexia, lack of motivation, social deprivation, and reduced movement [37,38]. Additionally, it has been documented that during systemic or chronic inflammatory diseases (e.g., chronic infections, autoimmune diseases, or chronic degenerative diseases) there is an increase in circulating proinflammatory cytokines that stimulate the brain and cause anxiety, anhedonia, social withdrawal, fatigue, and sleep disturbances [38,39,40].

Table 2.
Psychiatric effects induced by cytokines.
Regarding the neuromodulator function of other cytokines, it has been described in murine models that IL-4 deficiency can lead to spatial learning and memory deficits [41]. Similarly, impaired short-term memory in a Y-maze test has been observed in IL-17A-deficient mice [42]. Both IL-4 and IL-17A mediate these effects by inducing the production of the brain-derived neurotrophic factor (BDNF), which promotes neurogenesis and is important in promoting learning behaviors [42,43]. Additionally, in a murine model, it was observed that the injection of IL-17A into the brain during the fetal stage of mice induces a significant impairment of social interaction in adulthood [44]. Finally, interferon-γ (IFN-γ) has been reported to regulate social behavioral brain connections. In several study models, it was observed that the inhibition of IFN-γ receptors or the STAT1 pathway in the prefrontal cortex caused a decrease in social interaction [45].
Cytokines are not the only component of the immune system involved in the development of psychiatric disorders. The complement system also regulates neurobiological processes such as neurogenesis, cell migration in the cerebral and cerebellar cortex, and synaptic pruning [46]. It was recently reported that the complement system protein C3 regulates conditioned fear behaviors in mice, while C3aR modulates unconditioned or innate anxiety behaviors [47]. Other complement molecules involved in the pathophysiology of psychiatric disorders have been described, such as the C1-inhibitor or C4A in schizophrenia [48,49].
2.2. HPA Axis and Glucocorticoid Resistance
As mentioned above, one of the hypotheses proposed to explain the pathophysiology of MDD involves alterations of the HPA axis. The hypothalamus–pituitary–adrenal (HPA) axis connects the nervous and endocrine systems and is formed by the hypothalamus, pituitary, and adrenal glands [50]. The HPA axis regulates the stress response by releasing CRH by the hypothalamus, which promotes corticotropin (ACTH) release by the pituitary gland. ACTH stimulates the adrenal glands to release cortisol, triggering the “fight or flight” response. This axis has a negative feedback regulation system that mediates the production of glucocorticoids (Figure 2) [51]. Activation of the HPA axis regulates different processes such as digestion, immune responses, mood, emotions, and energy metabolism [50].
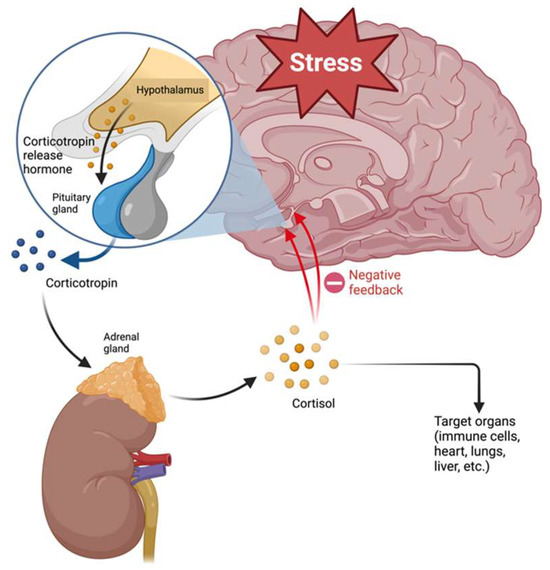
Figure 2.
Regulation of hypothalamus–pituitary–adrenal axis.
It has been reported that MDD patients have cortisol secretion disturbances, such as hyperactivity of the HPA axis or inversion of the circadian cycle in cortisol production, and it has been estimated that 90% of MDD patients have hypercortisolism [28,52]. Moreover, constant exposure to cortisol can lead to cognitive impairment, inducing irreversible changes in the neural system [53,54,55]. In this respect, hypercortisolism due to chronic stress affects the hippocampus, and it depends on stress duration. Consequently, if the stress lasts longer, there will be more damage to the hippocampus, ranging from modification of plasticity to neurotoxicity (Figure 3) [56,57].
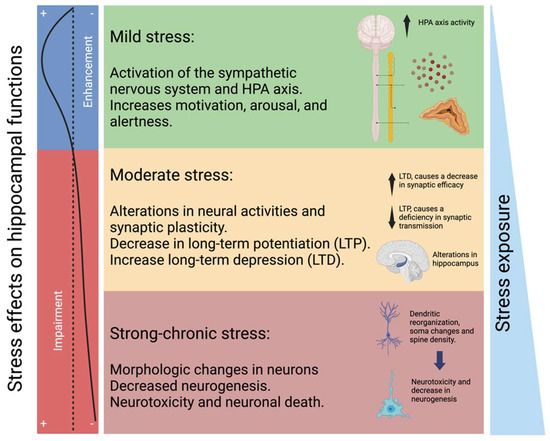
Figure 3.
Changes in the hippocampus according to the magnitude of stress. Mild stress conditions are associated with short-term neurochemical alterations through the sympathetic nervous system, affecting motivational, arousal, and alertness functions. Moderate stress induces relatively more prolonged alterations in neural activities and synaptic plasticity. Chronic and strong stress conditions can affect hippocampal morphology, neurogenesis, and neurotoxicity. Mild stress can enhance hippocampal functions. Prolonged stress can induce alterations in hippocampal functions (left panel).
Another feature observed in patients with MDD is glucocorticoid resistance, characterized by decreased sensitivity in glucocorticoid receptors such as cortisol receptors [58]. Several mechanisms have been proposed to explain glucocorticoid resistance, including alterations in glucocorticoid receptor (GR) function, changes in GR expression, alterations in glucocorticoid bioavailability through modification of serum protein binding, deficiencies in HPA axis feedback, and immune system inhibition [59,60]. It is worth mentioning that proinflammatory cytokines can also give feedback to the hypothalamus and anterior pituitary and thus increase HPA axis activity through modulation of GR function and expression [61]. It is well known that cortisol inhibits the immune response by inhibiting the expression of transcription factors such as NF-κB or decreasing the expression of adhesion molecules for diapedesis [62]. High levels of circulating glucocorticoids have been observed in patients with MDD and may coexist with elevated levels of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α [58,61,63]. The simultaneous presence of elevated levels of glucocorticoids and cytokines in patients with MDD creates a complex interaction between the immune system and the HPA axis, in which a paradoxical phenomenon is observed in leukocytes. For instance, monocytes can generate proinflammatory cytokines but are ineffective in eliminating pathogens [64,65]. To explain this phenomenon, several studies report that elevated glucocorticoid levels in MDD induce glucocorticoid resistance, resulting in the inadequate regulation of the HPA axis, and this glucocorticoid resistance favors proinflammatory signaling pathways that are not inhibited by cortisol as normally occurs in the negative feedback loop [63,66]. A study in mice reported that chronic stress induces an increase in sympathetic activity in the bone marrow, which promotes the generation of Ly6C monocytes characterized by their resistance to glucocorticoids and their increased inflammatory capacity, as well as the production of more reactive oxygen species [64].
Some authors have considered that the normalization of HPA axis activity in patients with MDD may be a target for developing new therapeutic strategies [50]. This is an advantage since antidepressants such as SSRIs only reduce HPA axis hyperactivity by 30% after 52 weeks of treatment [67].
2.3. Proinflammatory Cytokines and Neurotransmitter Metabolism
Cytokines are glycoproteins that act as messenger molecules to regulate inflammation and other cellular functions [68]. Cytokines are produced by different immune cells, parenchymal cells, endothelial and epithelial cells, fibroblasts, adipocytes, and stromal cells [68]. In addition, microglia, astrocytes, and neurons in the brain also produce cytokines. Several mechanisms by which peripheral cytokines stimulate the brain have been reported [69,70]: (i) by stimulating receptors in the blood–brain barrier (BBB) and producing metabolites in the brain; (ii) by accessing the brain through the circumventricular organs; (iii) by being carried through transporters in the BBB; and (iv) through stimulation of afferent fibers of the vagus nerve (Figure 4).
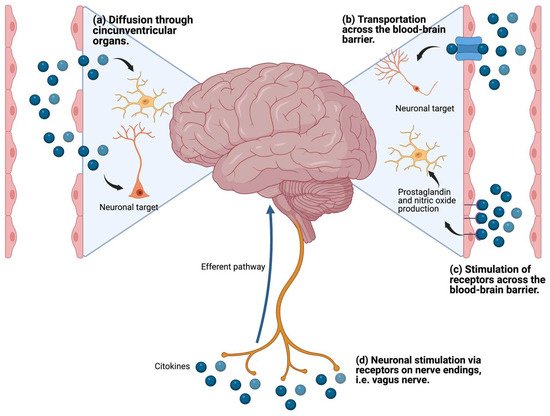
Figure 4.
Mechanisms of communication of peripheral proinflammatory cytokines with the brain.
Inflammation can be triggered in response to stressors such as infection, injury, surgery, and social stress, causing increased production of inflammatory mediators such as cytokines; the intensity and duration of the stimulus determines the level of inflammatory mediators release and the effects they cause [71]. As described above, proinflammatory cytokines produced in the periphery can stimulate the brain and generate an inflammatory response caused by the activation of neurons, microglia, and astrocytes [72]. During acute and chronic stress, proinflammatory cytokines induce changes in the metabolism of tryptophan, a precursor of serotonin, in the periphery and brain [73]. One of the mechanisms involved in the activation of indolamine 2,3-dioxygenase (IDO) is found in macrophages and microglia cells. IDO promotes tryptophan metabolism to the kynurenine pathway, resulting in decreased serotonin levels in the periphery and brain [74,75]. In addition, kynurenine produced in the periphery can cross the BBB and be metabolized by kynurenine produced in the brain by activated astrocytes and microglia [76]. Kynurenine is metabolized by astrocytes by kynurenine aminotransferase II (KAT II) and generates quinolinic acid which causes decreased production of dopamine and glutamate, as well as a blockade of α7nAChR cholinergic receptors, which is associated with cognitive dysfunction [76,77]. Similarly, activated microglia metabolizes kynurenine by kynurenine 3-monooxygenase (KMO) and 3-hydroxy anthranilic acid oxygenase (3-HAO) generates kynurenic acid, which stimulates NMDA receptors and causes lipid peroxidation, oxidative stress, excitotoxicity, and neurodegeneration [76,77] (Figure 5). In addition, it has been reported that chronic stress induces a decrease in serotonergic system function with an increase in the expression of SERT and p11 in peripheral blood mononuclear cells (PBMCs) [78]. On the other hand, the oxidative stress response caused during inflammation inhibits the production of tetrahydrobiopterin, a cofactor necessary for the activity of aromatic hydroxylase enzymes, which participate in the synthesis of neurotransmitters such as serotonin, dopamine, and norepinephrine, so that their inhibition causes a deficit in the production of monoamines [76,79]. These metabolic changes are related to the development of sickness behavior in patients with systemic inflammatory responses caused by injury or infection [38]. However, as soon as the inflammation is corrected the symptoms subside [38]. In cases of chronic stress, as in MDD, the above-mentioned alterations in the HPA axis such as glucocorticoid resistance, cause dysregulation of the inflammatory response and a low-grade chronic inflammation state, which generates a dopamine and serotonin deficit [80].
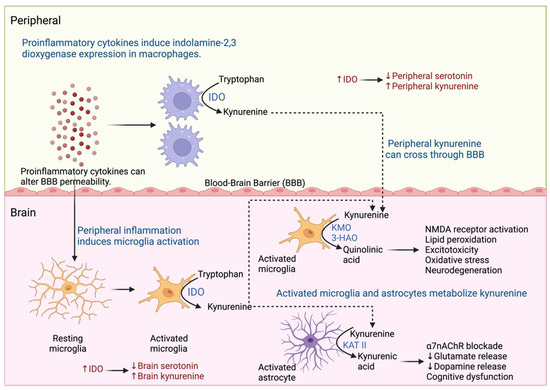
Figure 5.
Kynurenine pathway in MDD. Inflammation promotes the generation of metabolites of the kynurenine pathway in the periphery and brain. Macrophages and microglia express indolamine 2,3-dioxygenase (IDO) and metabolize tryptophan to kynurenine. Kynurenine is metabolized by activated astrocytes by kynurenine aminotransferase II (KAT II) and activates microglia by kynurenine 3-monooxygenase (KMO) and 3-hydroxy anthranilic acid oxygenase (3-HAO), which generate neurotoxic metabolites that cause neurodegeneration and cognitive dysfunction.
In the last two decades, there has been increasing evidence that MDD is associated with systemic immune system activation, reflected in inflammatory markers, immune cell numbers, and antibody titers [81]. Several meta-analyses have demonstrated that patients with MDD have higher levels of proinflammatory cytokines such as IL-1, IL-6, IL-12, IFN-γ, and TNF-α, and inflammatory mediators such as the circulating C-reactive protein [82,83,84,85,86,87]. Several studies that have evaluated cytokine concentration in MDD patients have shown that MDD patients frequently have higher levels of IL-6 and TNF-α compared to healthy volunteers [82,86]. However, in studies that quantify Th1 and Th2 cytokines in patients with MDD, there is a discrepancy regarding which cytokines are elevated. While some studies have shown an increase in Th1 cytokines, others have shown an increased concentration of Th2 cytokines [88,89,90,91]. These differences in results may be explained by the characteristics of the population studied, the heterogeneity of the pharmacological and nonpharmacological interventions, as well as the comorbidities of the individuals who participated in the study.
3. Inflammation as a Therapeutic Target for MDD
In recent years, an emphasis has been placed on the multidisciplinary treatment of patients with MDD [12]. The importance and benefits of physical activity and an adequate diet have been highlighted [92,93]. As discussed above, the pathophysiology of MDD is complex, and one of the points that should be underscored is inflammation. Several reports about the relationship of the brain–gut axis and microbiota with MDD have recently been published [93,94]. In this respect, it is important to note that gut microbiota is regulated by serotonin production, as well as T-regulatory cells and the cytokines they release [95,96]. It has been observed that patients with MDD have dysbiosis, which causes the production of proinflammatory cytokines and, consequently, the activation of Th1 and Th17 cells [97,98]. Furthermore, there is evidence of an elevation in HPA axis activation and cortisol release, which induces a decrease in serotonin released in the intestine and an increase in intestinal permeability [99]. Recent evidence proposes that increased intestinal permeability promotes the development of chronic low-grade inflammation through dysbiosis and the translocation of intestinal bacteria [100]. In addition to the production of proinflammatory cytokines, the normal levels of gut microbiota metabolites such as propionate, butyrate, and acetate are diminished, and there is also a decrease in serotonin production [101]. These changes have been related to the symptoms observed in MDD [99,101]. This is why dietary interventions have become crucial in managing MDD patients. Moreover, a key link has been established between physical activity and a decrease in proinflammatory cytokines in conjunction with a contribution to proper hippocampal function and, as a result, the down-regulation of the production of stress hormones such as cortisol [102,103].
As mentioned above, inflammation plays an important role in the pathophysiology of MDD, and cytokines such as IL-1, IL-6, or TNF-α are involved. Subsequently, several mechanisms for low chronic inflammation have been investigated as new alternatives for treating MDD [104,105]. Proinflammatory cytokines have dose-dependent effects; it has been reported that a concentration below 10 nM triggers a local inflammatory response. Reaching a concentration of 10 nM of proinflammatory cytokines generates systemic effects that affect the brain and trigger behavioral changes such as sickness behavior. Finally, a concentration of proinflammatory cytokines higher than 10 nM is associated with toxic effects on the heart and endothelium [106] (Figure 6).
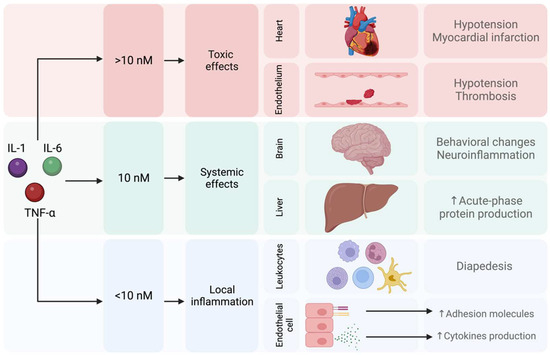
Figure 6.
Local, systemic, and toxic effects induced by proinflammatory cytokines.
In addition, it is well known that the inflammatory response has intrinsic regulatory mechanisms, such as the production of anti-inflammatory cytokines by regulatory cells [107]. However, other regulatory mechanisms, such as cortisol release or the cholinergic anti-inflammatory pathway (CAP) that regulate inflammation, have been targeted as new therapeutic options [104,105].
3.1. Cholinergic Anti-Inflammatory Pathway and Inflammatory Reflex
Acetylcholine (Ach) down-regulates the inflammatory response by suppressing the production of proinflammatory cytokines in peripheral immune cells through α7 nicotinic acetylcholine receptors (α7nAchRs) [108]. Cholinergic and catecholaminergic signals regulate lymphocyte activation in chronic inflammatory and autoimmune diseases [109]. CAP is a neural mechanism that inhibits the release of proinflammatory cytokines via afferent signaling by the vagus nerve and α7 receptors [110]. The CAP begins with afferent signaling from the vagus nerve, activated by cytokines, PAMPs, or DAMPs which, in turn, stimulate the nucleus of the solitary tract. Afterward, vagus efferent signals descend to the spleen to regulate the production of proinflammatory cytokines through Ach released by splenic T lymphocytes and dopamine released by the suprarenal gland (Figure 7) [110,111].
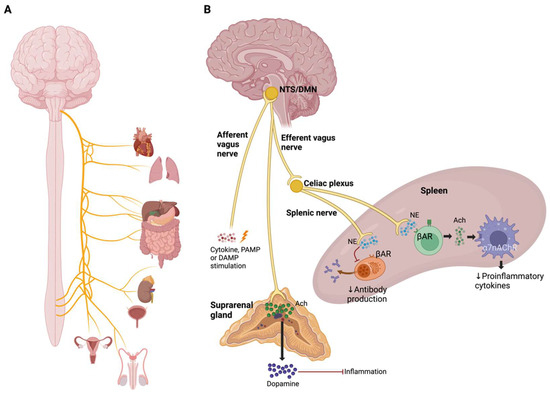
Figure 7.
Cholinergic anti-inflammatory pathway. (A) Organs that are innervated by the vagus nerve. (B) Mechanisms of the cholinergic anti-inflammatory pathway. Afferent stimulation of the vagus nerve by cytokines, PAMPs, or DAMPs integrates into the nucleus of the solitary tract (NTS), which triggers an efferent response from the dorsal motor nucleus of the vagus nerve (DMN) to the spleen and the adrenal gland.
Vagus nerve stimulation increases Ach release by choline acetyltransferase (ChAT) in T cells. This Ach released interacts with α7nAChR expressed by macrophages resulting in the inhibition of NF-κB translocation, JAK2-STAT3 pathway activation and inflammasome activation, and, in consequence, causes suppression of proinflammatory cytokine production [109].
Norepinephrine and epinephrine inhibit the production of proinflammatory cytokines by stimulating β2-adrenoceptors, and both also stimulate the synthesis of anti-inflammatory cytokines [112,113]. However, it is also described that epinephrine has pro-inflammatory effects by promoting cytokine production in macrophages in a dose-dependent manner [114,115].
3.2. Extracellular Monomeric Ubiquitin (emUb)
In the 1940’s, it was described a process to obtain dialyzable leucocyte extracts (DLEs) from leukocyte lysate. Initially, DLE was described as a compound of low molecular weight peptides [116,117]. Subsequently, a standardized method to produce DLE from dialysis of human dialyzable leucocyte extracts (hDLEs) was developed in the National Polytechnic Institute in Mexico City, denominated “Transferon Oral®” [118]. This hDLE Transferon is considered a complex drug (CD).
CDs are drugs in which the active pharmaceutical ingredient (API) is a heterogeneous substance composed of different but closely related molecules and, owing to its complexity, its mechanism of action (MOA), and its pharmacokinetic profile (PK) cannot be fully characterized [119]. The API of this immunomodulator is a mixture of human peptides with a molecular weight (MW) lower than 10 kDa, which is highly reproducible among batches, as described by extensive physicochemical and biological characterization [120,121,122,123].
Recently, a peptidome analysis of Transferon Oral® was performed, and ubiquitin (Ub) was identified as one of its main components. This immunomodulator has a peptide concentration of 2 mg/5 mL, of which 1 μg is monomeric ubiquitin. The efficacy of this immunomodulator has been demonstrated in an HSV-1 infection assay in mice, showing improved survival in hDLE-Transferon treated mice when orally administrated. A similar assay using deubiquitinized Transferon (Ub was removed with an anti-ubiquitin antibody) showed protection from HSV-1 death, although less than whole hDLE-Transferon and monomeric Ub. These results suggest that the other components of this immunomodulator found in lower concentrations also have activity on the immune system [118].
Ub is a small protein weighing 8.5 kDa that is highly conserved in eukaryotic cells. Ub performs its functions in conjugation with a myriad of proteins [124]. Ub has intracellular and extracellular functions; intracellular functions are carried out by the binding of Ub to different protein substrates, generating a monoubiquitinization to which more Ub can be attached depending on the process. Ubiquitination is involved in diverse cellular processes such as apoptosis, organelle biogenesis, cell cycle, transcription and DNA repair, ribosome regulation, modulation of cell surface receptors, ion channels, and secretory pathways [125]. The extracellular functions of Ub are carried out by a monomeric form of Ub (emUb), which is found in serum, cerebrospinal fluid, lung, alveolar lining fluid, and urine. It has been described that emUb regulates different processes such as cell differentiation and development, immune response and inflammation, muscle and neuronal degeneration, morphogenesis of neuronal networks, and response to stress and to extracellular modulators [126]. mUb is a highly stable small protein whose intracellular activities are related to protein recycling, the modulation of NF-κB, and DNA repair [127,128]. However, many questions remain about Ub when it is found extracellularly due to cellular physiological processes or some event involving cell rupture [126]. The relevance of emUb first attracted the attention of researchers when a correlation was observed between high levels of serum ubiquitin and an increase in the survival rate in sepsis and severe burn injury patients; subsequently, increased levels of emUb were also observed in inflammatory diseases [129,130,131]. In preclinical models, the intravenous administration of emUb reduces TNF-α plasma levels and the mortality induced by endotoxin in pigs, whereas, in a lung polytrauma pig model, it decreases IL-8, IL-10, TNF-α, and CXCL12 in the damaged organ [132,133]. In addition, in PBMC exposed to lipopolysaccharide (LPS), emUb prevents the increment of pro-inflammatory cytokine TNF-α [130].
The hDLE Transferon Oral has been demonstrated to increase IFN-γ levels and CD4+ cells in people with herpes zoster, reducing refractory pain compared to patients who only receive acyclovir [134], which suggests that this immunomodulator could be used in diseases with inflammatory imbalances. In a herpes simplex virus type I (HSV-1)-infected mouse model, it was observed that the hDLE increases IFN-γ and reduces TNF-α and IL-6, improving survival [135]. Furthermore, the effect of the subcutaneous administration of the immunomodulator on the conventional pharmacological treatment of puppies infected with the Canine Parvovirus (CPV) type II was evaluated. The use of this immunomodulator increased the CPV-infected puppies’ survival rate (∆ 40%), decreased the circulating neutrophils and lymphocyte levels, and reduced cortisol and epinephrine concentrations during the critical period of the infection compared to puppies treated with conventional drugs alone [136,137].
The mechanism of action (MOA) of emUb is not fully understood, but experimental data suggest that Ub blocks partially the CXCR4/CXCL12 axis [126,138,139,140]. CXCR4 is related to the production of TNF-α in macrophages by the MAPK and NF-κB signaling pathways [141]. It has been described that Ub is a CXCR4 partial agonist which is expressed on various tissues such as nerves and leucocytes [139]. In this sense, it was suggested that emUb may interacts with the CXCR4 expressed in the afferent endings of the vagus nerve in the stomach after oral administration and, subsequently, causes a slight activation of the CAP (as mentioned above). The CAP induces the inhibition of TNF-α release by spleen macrophages through ACh signaling pathways and decreases serum cortisol concentrations (Figure 8) [118,142,143]. In addition, emUb has been shown to induce immunomodulatory effects in macrophages and lymphocytes, such as decreased chemotaxis, proliferation, and cytokine secretion through CXCR4 receptor-mediated regulation of the inflammatory response triggered by damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs), in order to avoid a hyper-inflammatory response [131,144]. A third possible immunomodulatory mechanism may involves professional antigen-presenting cells like dendritic cells (DCs). The maturation of DCs is crucial for stimulating T-cell responses to foreign antigens and for maintaining immune tolerance to self antigens [145]. DC maturation requires the up-regulation of MHC class II (MHC-II) on the surface, as well as co-stimulatory molecules, such as CD80 and CD86 [145,146]. An important mechanism controlling surface expression of MHCII and CD86 is the ubiquitin-dependent degradation of these molecules [146,147]. Ubiquitination of MHC-II and CD86 is carried out by Membrane-Associated RING-CH-1 (MARCH1), which is an E3 ubiquitin ligase and promotes endocytosis and lysosomal degradation of MHC-II and CD86 [145,146,147]. Under basal conditions, MARCH1 is downregulated during DC maturation and IL-10 induces its expression. Thus, emUb administration reduces the surface expression of MHCII and CD86 on DCs and THP-1 macrophages [145,146,148]. Finally, it has been observed that ubiqutinization of MHC-II and CD86 promotes the differentiation of regulatory T cells [146].
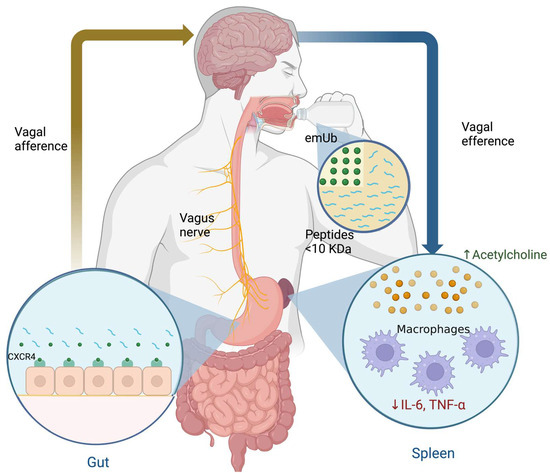
Figure 8.
Hypothesis on the immunomodulatory effects of extracellular monomeric ubiquitin (emUb) as one of the main low molecular weight peptide components of human dialyzable leucocyte extract in patients with major depressive disorder.
4. Use of emUb as a Part of the Peptide Composition of the hDLE in MDD Treatment
Since previous evidence in animal models and in humans showed that hDLE-Transferon has immunoregulatory properties that cause a decrease in circulating proinflammatory cytokines and cortisol, in addition to elevating IFN-γ levels. For this reason, the use of this immunomodulator was evaluated as a complementary treatment to selective serotonin receptor inhibitors (SSRIs) in patients with MDD.
First, the immunological phenotype of patients with MDD was identified in healthy volunteers and MDD patients, in which were determined the proinflammatory cytokines concentration in serum and 24 h urine cortisol concentration, as well as T subclasses, B and NK lymphocyte. Results showed that subjects with MDD had higher cortisol and TNF-α levels, increased CD3+CD8+ and NK percentages, decreased B lymphocyte counts, and no significant variations in CD3+CD4+ lymphocytes when compared to healthy individuals (Table 3). Additionally, in the same study, it was also observed that patients with MDD had higher levels of IL-4 and IL-13 and significantly lower concentrations of IL-2 and IFN-γ [149].

Table 3.
Variables evaluated in patients with MDD and healthy volunteers.
Subsequently, a 52-week follow-up of MDD patients and healthy volunteers was conducted, treating MDD patients with selective serotonin receptor inhibitors (SSRIs) in order to evaluate the long-term effect of SSRI administration on cortisol concentration and pro/anti-inflammatory cytokine profile (Table 4). The Hamilton Depressive Rating Scale (HDRS) and the Beck Depression Inventory (BDI) were applied monthly, and levels of IL-1β, IL-10, IL-2, IFN-γ, IL-4, and IL-13, as well as 24 h urine cortisol were determined at weeks 0, 5, 20, 20, 36, and 52 of treatment. At week 20 of treatment, HDRS and BDI indicated a remission of the depressive episode concomitant with increases in IL-2 and IL-1β but no change in cortisol. Moreover, a significant reduction in cortisol levels, with an increase in IL-1β and IFN-γ and a decrease in Th2 cytokines, were observed towards the end of clinical follow-up at week 52 [67].

Table 4.
Variables measured during 52-week follow-up in patients with MDD treated with SSIRs.
Finally, the administration of hDLE-Transferon (which contains emUB) as an adjuvant treatment to SSRIs in patients with MDD was evaluated. MDD patients received SSRIs or SSRIs plus hDLE. Serum concentrations of the proinflammatory cytokines IL-1β, IL-2, and IFN-γ; the anti-inflammatory cytokines IL-4, IL-13, and IL-10, as well as 24 h urine cortisol quantification was determined along 52 weeks of treatment (Table 5). After 52 weeks of treatment, it was detected a decrease of 30% of Urine cortisol in the SSRI-treated group, whereas in the SSRI plus hDLE-treated group urine cortisol was reduced by 54%. Cortisol reduction in patients treated with SSRI plus hDLE correlated with reduction of anti-inflammatory cytokines levels and increases levels of proinflammatory cytokines which suggests a decrease in HPA axis hyperactivity accompanied by a more physiological balance between pro and anti-inflammatory cytokines [150]. It is important to emphasize that patients treated with SSRI plus hDLE presented a higher IFN-γ concentration in comparison with patients that received SSRIs.

Table 5.
Variables were assessed at 52-week follow-up in patients with MDD treated with SSIR and SSIR + hDLE.
As mentioned above, the proposed mechanism of action of Transferon is an immunomodulatory effect acting on the CXCR4/CXCL12 axis and thus activating the CAP, which would generate the inhibition of proinflammatory cytokines released in the spleen through Ach release and decreased cortisol levels (Figure 8). Therefore, the effect of SSRI plus hDLE consumption in MDD patients may cause a significant decrease in the HPA axis activity and an increase in IFN-γ levels, which were better results than those seen in patients who only received SSRI treatment.
It has been described that serotonin promotes IFN-γ production, so the difference in IFN-γ levels observed in patients treated with the SSRI plus hDLE combination suggests that this treatment offers a better response in MDD patients. However, further studies are needed to understand the molecular mechanisms that cause clinical and biochemical improvements [151,152]. Despite the observed results of the use of SSRIs plus hDLE in patients with MDD, several questions remain to be resolved about the MOA by which the emUb regulates inflammatory processes, alone or as part of the peptide component of this immunomodulator. However, the fact that both modulate cytokines such as TNF-α, IL-6, IFN-γ, and cortisol strongly suggests that these biotherapeutics have the potential to complement the treatment of patients with MDD. More clinical trials evaluating treatment with SSRIs plus hDLE are required, since one of the limitations of the results is the size of the population evaluated.
5. Conclusions
Evidence shows that patients with MDD have altered proinflammatory molecules in circulation, glucocorticoid resistance, and significant variations in neurotransmitter levels in the brain and periphery. According to several reports, there is a high rate of therapeutic failure in MDD, so new therapeutic options need to be explored, and inflammation has become a critical target. The combination of SSRIs with hDLE has shown immunomodulatory effects, due to a probable CAP activation through the partial agonism of the CXCR4/CXCL12 axis. As a consequence, the inflammatory and cortisol imbalance is improved, inducing an improvement clinical and molecular status of patients, which makes this treatment a promising therapeutical option. However, further studies are needed to fully understand the effector mechanisms by which emUb contained in hDLE generates immunoregulatory effects in MDD patients. In addition, further clinical trials are needed to validate the potential use of emUB as an adjunctive treatment in MDD. Physicians should consider inflammation as a fundamental part of MDD pathophysiology, so it is important to treat possible causes of inflammation involving dietary habits, lifestyle, addictions, among other factors.
Author Contributions
Conceptualization, J.L.M.-G. and L.P.; investigation, J.L.M.-G., L.H.G.-M., T.H. and L.V.-C.; resources, L.P., S.M.P.-T., D.M.-C., D.O.-S. and L.V.-C.; data curation, J.L.M.-G., L.H.G.-M. and T.H.; writing—original draft preparation, J.L.M.-G., L.H.G.-M. and L.V.-C.; writing—review and editing, J.L.M.-G., L.H.G.-M., L.P., L.V.-C., G.P.-S., E.B.-V., M.C.M.-L., D.O.-S., S.A.-H. and T.H.; visualization, J.L.M.-G. and L.P.; supervision, L.P.; project administration, L.P. and M.C.M.-L.; funding acquisition, L.P. and M.C.M.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the CONAHCYT, with grant number: CF-2023-G-836 and SIP-IPN 20241740. J.L.M.-G. and S.A.-H. received support from the CONAHCYT with grant numbers 822954 and 594780, respectively. J.L.M.-G. is a doctoral student from the Programa de Doctorado en Ciencias en Inmunología, Instituto Politécnico Nacional (IPN). S.A.-H. is a doctoral student from the Programa de Doctorado en Ciencias Biomédicas, Universidad Nacional Autónoma de México (UNAM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
S.M.P.-T. is involved in the development and commercialization of Transferon. The other authors declare the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major Depressive Disorder. Nat. Rev. Dis. Prim. 2023, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major Depressive Disorder. Nat. Rev. Dis. Prim. 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Lopizzo, N.; Chiavetto, L.B.; Cattane, N.; Plazzotta, G.; Tarazi, F.I.; Pariante, C.M.; Riva, M.A.; Cattaneo, A. Gene–Environment Interaction in Major Depression: Focus on Experience-Dependent Biological Systems. Front. Psychiatry 2015, 6, 68. [Google Scholar] [CrossRef]
- Moussavi, S.; Chatterji, S.; Verdes, E.; Tandon, A.; Patel, V.; Ustun, B. Depression, Chronic Diseases, and Decrements in Health: Results from the World Health Surveys. Lancet 2007, 370, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Barton, D.A. Depression and the Link with Cardiovascular Disease. Front. Psychiatry 2016, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, Y.; Akhtar, M.A.; Kanawati, M.A.; Mucheke, R.; Mahfouz, M.; Al-Nufoury, M. Depression and Metabolic Syndrome: A Narrative Review. Cureus 2022, 14, e22153. [Google Scholar] [CrossRef] [PubMed]
- Sanches, A.; Costa, R.; Marcondes, F.K.; Cunha, T.S. Relationship among Stress, Depression, Cardiovascular and Metabolic Changes and Physical Exercise. Fisioter. Mov. 2016, 29, 23–36. [Google Scholar] [CrossRef]
- Ferrari, A. Global, Regional, and National Burden of 12 Mental Disorders in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- James, J.E. Mental Health. In The Health of Populations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 429–464. ISBN 978-0-12-802812-4. [Google Scholar]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef]
- Bermudes, R.A. Rapid Response Therapies for Major Depression Could Dramatically Reduce Societal Cost. Psychiatr. News 2023, 58. [Google Scholar] [CrossRef]
- Karrouri, R.; Hammani, Z.; Otheman, Y.; Benjelloun, R. Major Depressive Disorder: Validated Treatments and Future Challenges. World J. Clin. Cases 2021, 9, 9350. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, R.S. Major Depressive Disorder; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780323581318. [Google Scholar]
- Sheffler, Z.M.; Patel, P.; Abdijadid, S. Antidepressants; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sinyor, M.; Schaffer, A.; Levitt, A. The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Trial: A Review. Can. J. Psychiatry 2010, 55, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Gaynes, B.N.; Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Spencer, D.; Fava, M. The STAR*D Study: Treating Depression in the Real World. Cleve. Clin. J. Med. 2008, 75, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.H. A Review of Antidepressant Therapy in Primary Care: Current Practices and Future Directions. Prim. Care Companion J. Clin. Psychiatry 2013, 15, 23071. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.S.; Zizzi, F.B.; Cattaneo, A.; Comandini, A.; Di Dato, G.; Lubrano, E.; Pellicano, C.; Spallone, V.; Tongiani, S.; Torta, R. Management and Treatment of Patients With Major Depressive Disorder and Chronic Diseases: A Multidisciplinary Approach. Front. Psychol. 2020, 11, 542444. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Khullar, S.; Singh, M.; Kaur, G.; Mastana, S. Diabetes to Cardiovascular Disease: Is Depression the Potential Missing Link? Med. Hypotheses 2015, 84, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, N. Depression and Diabetes. Dialogues Clin. Neurosci. 2018, 20, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tafet, G.E.; Nemeroff, C.B. The Links Between Stress and Depression: Psychoneuroendocrinological, Genetic, and Environmental Interactions. J. Neuropsychiatry Clin. Neurosci. 2016, 28, 77–88. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural Basis of Major Depressive Disorder: Beyond Monoamine Hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Seki, K.; Yoshida, S.; Jaiswal, M. Molecular Mechanism of Noradrenaline during the Stress-Induced Major Depressive Disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [CrossRef]
- Bruno, A.; Dolcetti, E.; Rizzo, F.R.; Fresegna, D.; Musella, A.; Gentile, A.; De Vito, F.; Caioli, S.; Guadalupi, L.; Bullitta, S.; et al. Inflammation-Associated Synaptic Alterations as Shared Threads in Depression and Multiple Sclerosis. Front. Cell. Neurosci. 2020, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Cosci, F.; Chouinard, G. The Monoamine Hypothesis of Depression Revisited: Could It Mechanistically Novel Antidepressant Strategies? In Neurobiology of Depression; Elsevier: Amsterdam, The Netherlands, 2019; pp. 63–73. ISBN 9780128133330. [Google Scholar]
- Musazzi, L.; Treccani, G.; Popoli, M. Glutamate Hypothesis of Depression and Its Consequences for Antidepressant Treatments. Expert Rev. Neurother. 2012, 12, 1169–1172. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, A.Y.; Onaolapo, O.J. Glutamate and Depression: Reflecting a Deepening Knowledge of the Gut and Brain Effects of a Ubiquitous Molecule. World J. Psychiatry 2021, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. Stress, Hypercortisolism and Corticosteroid Receptors in Depression: Implicatons for Therapy. J. Affect. Disord. 2001, 62, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Afridi, R.; Suk, K. Neuroinflammatory Basis of Depression: Learning From Experimental Models. Front. Cell. Neurosci. 2021, 15, 691067. [Google Scholar] [CrossRef] [PubMed]
- Alesci, S.; Martinez, P.E.; Kelkar, S.; Ilias, I.; Ronsaville, D.S.; Listwak, S.J.; Ayala, A.R.; Licinio, J.; Gold, H.K.; Kling, M.A.; et al. Major Depression Is Associated with Significant Diurnal Elevations in Plasma Interleukin-6 Levels, a Shift of Its Circadian Rhythm, and Loss of Physiological Complexity in Its Secretion: Clinical Implications. J. Clin. Endocrinol. Metab. 2005, 90, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Chiu, W.C.; Su, Y.P.; Su, K.P.; Chen, P.C. Recurrence of Depressive Disorders after Interferon-Induced Depression. Transl. Psychiatry 2017, 7, e1026. [Google Scholar] [CrossRef]
- Amodio, P.; De Toni, E.N.; Cavalletto, L.; Mapelli, D.; Bernardinello, E.; Del Piccolo, F.; Bergamelli, C.; Costanzo, R.; Bergamaschi, F.; Poma, S.Z.; et al. Mood, Cognition and EEG Changes during Interferon Alpha (Alpha-IFN) Treatment for Chronic Hepatitis C. J. Affect. Disord. 2005, 84, 93–98. [Google Scholar] [CrossRef]
- Salvador, A.F.; de Lima, K.A.; Kipnis, J. Neuromodulation by the Immune System: A Focus on Cytokines. Nat. Rev. Immunol. 2021, 21, 526–541. [Google Scholar] [CrossRef]
- Brebner, K.; Hayley, S.; Zacharko, R.; Merali, Z.; Anisman, H. Synergistic Effects of Interleukin-1beta, Interleukin-6, and Tumor Necrosis Factor-Alpha: Central Monoamine, Corticosterone, and Behavioral Variations. Neuropsychopharmacology 2000, 22, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry 2009, 65, 732. [Google Scholar] [CrossRef] [PubMed]
- Derecki, N.C.; Cardani, A.N.; Yang, C.H.; Quinnies, K.M.; Crihfield, A.; Lynch, K.R.; Kipnis, J. Regulation of Learning and Memory by Meningeal Immunity: A Key Role for IL-4. J. Exp. Med. 2010, 207, 1067. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Brigas, H.C.; Temido-Ferreira, M.; Pousinha, P.A.; Regen, T.; Santa, C.; Coelho, J.E.; Marques-Morgado, I.; Valente, C.A.; Omenetti, S.; et al. Meningeal Γδ T Cell-Derived IL-17 Controls Synaptic Plasticity and Short-Term Memory. Sci. Immunol. 2019, 4, eaay5199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-Driven Microglia Modulate Stress Resilience through BDNF-Dependent Neurogenesis. Sci. Adv. 2021, 7, 9888–9905. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The Maternal Interleukin-17a Pathway in Mice Promotes Autism-like Phenotypes in Offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef]
- Filiano, A.J.; Xu, Y.; Tustison, N.J.; Marsh, R.L.; Baker, W.; Smirnov, I.; Overall, C.C.; Gadani, S.P.; Turner, S.D.; Weng, Z.; et al. Unexpected Role of Interferon-γ in Regulating Neuronal Connectivity and Social Behaviour. Nature 2016, 535, 425–429. [Google Scholar] [CrossRef]
- Coulthard, L.G.; Hawksworth, O.A.; Woodruff, T.M. Complement: The Emerging Architect of the Developing Brain. Trends Neurosci. 2018, 41, 373–384. [Google Scholar] [CrossRef]
- Westacott, L.J.; Humby, T.; Haan, N.; Brain, S.A.; Bush, E.L.; Toneva, M.; Baloc, A.I.; Moon, A.L.; Reddaway, J.; Owen, M.J.; et al. Complement C3 and C3aR Mediate Different Aspects of Emotional Behaviours; Relevance to Risk for Psychiatric Disorder. Brain Behav. Immun. 2022, 99, 70–82. [Google Scholar] [CrossRef]
- Allswede, D.M.; Zheutlin, A.B.; Chung, Y.; Anderson, K.; Hultman, C.M.; Ingvar, M.; Cannon, T.D. Complement Gene Expression Correlates with Superior Frontal Cortical Thickness in Humans. Neuropsychopharmacology 2018, 43, 525–533. [Google Scholar] [CrossRef]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; Van Doren, V.; et al. Schizophrenia Risk from Complex Variation of Complement Component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. Hpa Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M.; Schatzberg, A.F. HPA Axis in Major Depression: Cortisol, Clinical Symptomatology, and Genetic Variation Predict Cognition. Mol. Psychiatry 2017, 22, 527. [Google Scholar] [CrossRef]
- Gomez, R.G.; Fleming, S.H.; Keller, J.; Flores, B.; Kenna, H.; DeBattista, C.; Solvason, B.; Schatzberg, A.F. The Neuropsychological Profile of Psychotic Major Depression and Its Relation to Cortisol. Biol. Psychiatry 2006, 60, 472–478. [Google Scholar] [CrossRef]
- Lupien, S.J.; Gillin, C.J.; Hauger, R.L. Working Memory Is More Sensitive than Declarative Memory to the Acute Effects of Corticosteroids: A Dose-Response Study in Humans. Behav. Neurosci. 1999, 113, 420–430. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive Impairment in Depression: A Systematic Review and Meta-Analysis. Psychol. Med. 2014, 44, 2029–2040. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.J. Neurocognitive Effects of Stress: A Metaparadigm Perspective. Mol. Psychiatry 2023, 28, 2750–2763. [Google Scholar] [CrossRef]
- Kim, E.J.; Pellman, B.; Kim, J.J. Stress Effects on the Hippocampus: A Critical Review. Learn. Mem. 2015, 22, 411. [Google Scholar] [CrossRef]
- Pariante, C.M. Why Are Depressed Patients Inflamed? A Reflection on 20 Years of Research on Depression, Glucocorticoid Resistance and Inflammation. Eur. Neuropsychopharmacol. 2017, 27, 554–559. [Google Scholar] [CrossRef]
- Silverman, M.N.; Sternberg, E.M. Glucocorticoid Regulation of Inflammation and Its Functional Correlates: From HPA Axis to Glucocorticoid Receptor Dysfunction. Ann. N. Y. Acad. Sci. 2012, 1261, 55–63. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic Stress, Glucocorticoid Receptor Resistance, Inflammation, and Disease Risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, Cytokines and Brain Abnormalities in Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The Anti-Inflammatory and Immunosuppressive Effects of Glucocorticoids, Recent Developments and Mechanistic Insights. Mol. Cell. Endocrinol. 2011, 335, 2. [Google Scholar] [CrossRef]
- Perrin, A.J.; Horowitz, M.A.; Roelofs, J.; Zunszain, P.A.; Pariante, C.M. Glucocorticoid Resistance: Is It a Requisite for Increased Cytokine Production in Depression? A Systematic Review and Meta-Analysis. Front. Psychiatry 2019, 10, 423. [Google Scholar] [CrossRef]
- Weber, M.D.; Godbout, J.P.; Sheridan, J.F. Repeated Social Defeat, Neuroinflammation, and Behavior: Monocytes Carry the Signal. Neuropsychopharmacology 2017, 42, 46–61. [Google Scholar] [CrossRef]
- Irwin, M.R.; Miller, A.H. Depressive Disorders and Immunity: 20 Years of Progress and Discovery. Brain Behav. Immun. 2007, 21, 374–383. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Monsalves-Alvarez, M.; Henriquez, S.; Llanos, M.N.; Troncoso, R. Glucocorticoid Resistance in Chronic Diseases. Steroids 2016, 115, 182–192. [Google Scholar] [CrossRef]
- Hernández, M.E.; Mendieta, D.; Martínez-Fong, D.; Loría, F.; Moreno, J.; Estrada, I.; Bojalil, R.; Pavón, L. Variations in Circulating Cytokine Levels during 52 Week Course of Treatment with SSRI for Major Depressive Disorder. Eur. Neuropsychopharmacol. 2008, 18, 917–924. [Google Scholar] [CrossRef]
- Himmerich, H.; Patsalos, O.; Lichtblau, N.; Ibrahim, M.A.A.; Dalton, B. Cytokine Research in Depression: Principles, Challenges, and Open Questions. Front. Psychiatry 2019, 10, 30. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine Networks in Neuroinflammation. Nat. Rev. Immunol. 2016, 17, 49–59. [Google Scholar] [CrossRef]
- Bauer, M.E. Accelerated Immunosenescence in Rheumatoid Arthritis: Impact on Clinical Progression. Immun. Ageing 2020, 17, 6. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On Inflammatory Hypothesis of Depression: What Is the Role of IL-6 in the Middle of the Chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Corrigan, M.; O’Rourke, A.M.; Moran, B.; Fletcher, J.M.; Harkin, A. Inflammation in the Pathogenesis of Depression: A Disorder of Neuroimmune Origin. Neuronal Signal. 2023, 7, 20220054. [Google Scholar] [CrossRef]
- Herselman, M.F.; Bailey, S.; Bobrovskaya, L. The Effects of Stress and Diet on the “Brain–Gut” and “Gut–Brain” Pathways in Animal Models of Stress and Depression. Int. J. Mol. Sci. 2022, 23, 2013. [Google Scholar] [CrossRef]
- Miller, A.H. Mechanisms of Cytokine-Induced Behavioral Changes: Psychoneuroimmunology at the Translational Interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef]
- Huang, Y.S.; Ogbechi, J.; Clanchy, F.I.; Williams, R.O.; Stone, T.W. IDO and Kynurenine Metabolites in Peripheral and CNS Disorders. Front. Immunol. 2020, 11, 493984. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef] [PubMed]
- Becerril-Villanueva, E.; Olvera-Alvarez, M.I.; Alvarez-Herrera, S.; Maldonado-García, J.L.; López-Torres, A.; Ramírez-Marroquín, O.A.; González-Ruiz, O.; Nogueira-Fernández, J.M.; Mendoza-Contreras, J.M.; Sánchez-García, H.O.; et al. Screening of SERT and P11 MRNA Levels in Airline Pilots: A Translational Approach. Front. Psychiatry 2022, 13, 859768. [Google Scholar] [CrossRef] [PubMed]
- Vancassel, S.; Capuron, L.; Castanon, N. Brain Kynurenine and BH4 Pathways: Relevance to the Pathophysiology and Treatment of Inflammation-Driven Depressive Symptoms. Front. Neurosci. 2018, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Hassamal, S. Chronic Stress, Neuroinflammation, and Depression: An Overview of Pathophysiological Mechanisms and Emerging Anti-Inflammatories. Front. Psychiatry 2023, 14, 1130989. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A Meta-Analysis of Cytokines in Major Depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of Depression with C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom. Med. 2009, 71, 171–186. [Google Scholar] [CrossRef]
- Liu, Y.; Ho, R.C.M.; Mak, A. Interleukin (IL)-6, Tumour Necrosis Factor Alpha (TNF-α) and Soluble Interleukin-2 Receptors (SIL-2R) Are Elevated in Patients with Major Depressive Disorder: A Meta-Analysis and Meta-Regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef]
- Köhler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; et al. Peripheral Cytokine and Chemokine Alterations in Depression: A Meta-Analysis of 82 Studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory Markers in Depression: A Meta-Analysis of Mean Differences and Variability in 5166 Patients and 5083 Controls. Brain Behav. Immun. 2020, 87, 901. [Google Scholar] [CrossRef]
- Min, X.; Wang, G.; Cui, Y.; Meng, P.; Hu, X.; Liu, S.; Wang, Y. Association between Inflammatory Cytokines and Symptoms of Major Depressive Disorder in Adults. Front. Immunol. 2023, 14, 1110775. [Google Scholar] [CrossRef] [PubMed]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2023, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Halbreich, U.; Han, C.; Leonard, B.E.; Luo, H. Imbalance between Pro- and Anti-Inflammatory Cytokines, and between Th1 and Th2 Cytokines in Depressed Patients: The Effect of Electroacupuncture or Fluoxetine Treatment. Pharmacopsychiatry 2009, 42, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M.; Leonard, B.E.; Steinbusch, H.W.M.; Kim, Y.K. Th1, Th2, and Th3 Cytokine Alterations in Major Depression. J. Affect. Disord. 2005, 88, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yu, K. Th1, Th2, and Th17 Cells and Their Corresponding Cytokines Are Associated with Anxiety, Depression, and Cognitive Impairment in Elderly Gastric Cancer Patients. Front. Surg. 2022, 9, 996680. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.; Fahimi, A. Immune and Neuroprotective Effects of Physical Activity on the Brain in Depression. Front. Neurosci. 2018, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Tu, H.; Chen, T. The Microbiota–Gut–Brain Axis in Depression: The Potential Pathophysiological Mechanisms and Microbiota Combined Antidepression Effect. Nutrients 2022, 14, 2081. [Google Scholar] [CrossRef]
- Chang, L.; Wei, Y.; Hashimoto, K. Brain–Gut–Microbiota Axis in Depression: A Historical Overview and Future Directions. Brain Res. Bull. 2022, 182, 44–56. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Watson, J.; Reyes, J.; Trivedi, M.; Beurel, E. Th17 Cells Sense Microbiome to Promote Depressive-like Behaviors. Microbiome 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rodriguez, E.M.; Madorma, D.; O’Connor, G.; Mason, B.L.; Han, D.; Deo, S.K.; Oppenheimer, M.; Nemeroff, C.B.; Trivedi, M.H.; Daunert, S.; et al. Identification of a Signalling Mechanism by Which the Microbiome Regulates Th17 Cell-Mediated Depressive-like Behaviors in Mice. Am. J. Psychiatry 2020, 177, 974. [Google Scholar] [CrossRef] [PubMed]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling Cognition: The Gut Microbiota and Hypothalamic-Pituitary-Adrenal Axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut Microbiota and Its Metabolites in Depression: From Pathogenesis to Treatment. eBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Gujral, S.; Aizenstein, H.; Reynolds, C.F.; Butters, M.A.; Erickson, K.I. Exercise Effects on Depression: Possible Neural Mechanisms. Gen. Hosp. Psychiatry 2017, 49, 2. [Google Scholar] [CrossRef]
- Zhao, J.L.; Jiang, W.T.; Wang, X.; Cai, Z.D.; Liu, Z.H.; Liu, G.R. Exercise, Brain Plasticity, and Depression. CNS Neurosci. Ther. 2020, 26, 885. [Google Scholar] [CrossRef] [PubMed]
- Kohler, O.; Krogh, J.; Mors, O.; Eriksen Benros, M. Inflammation in Depression and the Potential for Anti-Inflammatory Treatment. Curr. Neuropharmacol. 2016, 14, 732–742. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The Role of Inflammation in Depression: From Evolutionary Imperative to Modern Treatment Target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Alvarez-Herrera, S.; Pavon, L. Interacciones Neuroendocrinoinmunológicas. In Inmunología Molecular, Celular y Traslacional; Pavón, L., Ed.; Wolters Kluwer: Mexico City, Mexico, 2021; pp. 227–242. ISBN 9788417949181. [Google Scholar]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of Inflammatory Reaction in Health and Disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef]
- Balkan, B.; Pogun, S. Nicotinic Cholinergic System in the Hypothalamus Modulates the Activity of the Hypothalamic Neuropeptides during the Stress Resp. Curr. Neuropharmacol. 2017, 15, 371–387. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. Neural Regulation of Immunity: Molecular Mechanisms and Clinical Translation. Nat. Neurosci. 2017, 20, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, P.S.; Rosas-Ballina, M.; Levine, Y.A.; Tracey, K.J. Rethinking Inflammation: Neural Circuits in the Regulation of Immunity. Immunol. Rev. 2012, 248, 188–204. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Tracey, K.J. The Vagus Nerve and the Inflammatory Reflex-Linking Immunity and Metabolism. Nat. Rev. Endocrinol. 2012, 8, 743–754. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Wang, H.; Czura, C.J.; Friedman, S.G.; Tracey, K.J. The Cholinergic Anti-Inflammatory Pathway: A Missing Link in Neuroimmunomodulation. Mol. Med. 2003, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Vida, G.; Peña, G.; Deitch, E.A.; Ulloa, L. A7-Cholinergic Receptor Mediates Vagal Induction of Splenic Norepinephrine. J. Immunol. 2011, 186, 4340–4346. [Google Scholar] [CrossRef]
- Zhou, J.; Yan, J.; Liang, H.; Jiang, J. Epinephrine Enhances the Response of Macrophages under LPS Stimulation. Biomed. Res. Int. 2014, 2014, 254686. [Google Scholar] [CrossRef]
- Staedtke, V.; Bai, R.Y.; Kim, K.; Darvas, M.; Davila, M.L.; Riggins, G.J.; Rothman, P.B.; Papadopoulos, N.; Kinzler, K.W.; Vogelstein, B.; et al. Disruption of a Self-Amplifying Catecholamine Loop Reduces Cytokine Release Syndrome. Nature 2018, 564, 273–277. [Google Scholar] [CrossRef]
- Macias, A.E.; Guaní-Guerra, E. Transfer Factor: Myths and Facts. Arch. Med. Res. 2020, 51, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Islas-Weinstein, L.; Maldonado-García, J.L. Immunomodulatory Supplements; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128187319. [Google Scholar]
- Vallejo-Castillo, L.; Favari, L.; Vázquez-Leyva, S.; Mellado-Sánchez, G.; Macías-Palacios, Z.; López-Juárez, L.E.; Valencia-Flores, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; et al. Sequencing Analysis and Identification of the Primary Peptide Component of the Dialyzable Leukocyte Extract “Transferon Oral”: The Starting Point to Understand Its Mechanism of Action. Front. Pharmacol. 2020, 11, 569039. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Stolk, P.; De Bruin, M.L.; Leufkens, H.G.M.; Crommelin, D.J.A.; De Vlieger, J.S.B. The EU Regulatory Landscape of Non-Biological Complex Drugs (NBCDs) Follow-on Products: Observations and Recommendations. Eur. J. Pharm. Sci. 2019, 133, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivero, E.; Merchand-Reyes, G.; Pavón, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Salinas-Jazmín, N.; Estrada-Parra, S.; Velasco-Velázquez, M.; Pérez-Tapia, S.M. Batch-to-Batch Reproducibility of TransferonTM. J. Pharm. Biomed. Anal. 2014, 88, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Medina-Rivero, E.; Vallejo-Castillo, L.; Vázquez-Leyva, S.; Pérez-Sánchez, G.; Favari, L.; Velasco-Velázquez, M.; Estrada-Parra, S.; Pavón, L.; Pérez-Tapia, S.M. Physicochemical Characteristics of TransferonTM Batches. Biomed. Res. Int. 2016, 2016, 7935181. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Leyva, S.; Vallejo-Castillo, L.; López-Morales, C.A.; Herbert-Pucheta, J.E.; Zepeda-Vallejo, L.G.; Velasco-Velázquez, M.; Pavón, L.; Pérez-Tapia, S.M.; Medina-Rivero, E. Identity Profiling of Complex Mixtures of Peptide Products by Structural and Mass Mobility Orthogonal Analysis. Anal. Chem. 2019, 91, 14392–14400. [Google Scholar] [CrossRef] [PubMed]
- Herbert-Pucheta, J.E.; López-Morales, C.A.; Medina-Rivero, E.; Estrada-Parra, S.; Pérez-Tapia, S.M.; Zepeda-Vallejo, L.G. Consistency of a Dialyzable Leucocyte Extract Manufactured at GMP Facilities by Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Biomed. Anal. 2021, 196, 113940. [Google Scholar] [CrossRef]
- Pickart, C.M.; Eddins, M.J. Ubiquitin: Structures, Functions, Mechanisms. Biochim. Biophys. Acta-Mol. Cell Res. 2004, 1695, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Z.J. The Novel Functions of Ubiquitination in Signaling. Curr. Opin. Cell Biol. 2004, 16, 119–126. [Google Scholar] [CrossRef]
- Mendoza-Salazar, I.; Fragozo, A.; González-Martínez, A.P.; Trejo-Martínez, I.; Arreola, R.; Pavón, L.; Almagro, J.C.; Vallejo-Castillo, L.; Aguilar-Alonso, F.A.; Pérez-Tapia, S.M. Almost 50 Years of Monomeric Extracellular Ubiquitin (EUb). Pharmaceuticals 2024, 17, 185. [Google Scholar] [CrossRef]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin Signalling in Neurodegeneration: Mechanisms and Therapeutic Opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Swatek, K.N.; Komander, D. Ubiquitin Modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Laporte, H.M.; Albee, L.J.; Baker, T.A.; Bach, H.H.; Vana, P.G.; Evans, A.E.; Gamelli, R.L.; Majetschak, M. Ubiquitin Urine Levels in Burn Patients. J. Burn Care Res. 2017, 38, e133–e143. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Krehmeier, U.; Bardenheuer, M.; Denz, C.; Quintel, M.; Voggenreiter, G.; Obertacke, U. Extracellular Ubiquitin Inhibits the TNF-Alpha Response to Endotoxin in Peripheral Blood Mononuclear Cells and Regulates Endotoxin Hyporesponsiveness in Critical Illness. Blood 2003, 101, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M. Extracellular Ubiquitin: Immune Modulator and Endogenous Opponent of Damage-Associated Molecular Pattern Molecules. J. Leukoc. Biol. 2011, 89, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Majetschak, M.; Cohn, S.M.; Nelson, J.A.; Burton, E.H.; Obertacke, U.; Proctor, K.G. Effects of Exogenous Ubiquitin in Lethal Endotoxemia. Surgery 2004, 135, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.A.; Romero, J.; Bach, H.H.; Strom, J.A.; Gamelli, R.L.; Majetschak, M. Effects of Exogenous Ubiquitin in a Polytrauma Model with Blunt Chest Trauma. Crit. Care Med. 2012, 40, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Parra, S.; Nagaya, A.; Serrano, E.; Rodriguez, O.; Santamaria, V.; Ondarza, R.; Chavez, R.; Correa, B.; Monges, A.; Cabezas, R.; et al. Comparative Study of Transfer Factor and Acyclovir in the Treatment of Herpes Zoster. Int. J. Immunopharmacol. 1998, 20, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Jazmin, N.; Estrada-Parra, S.; Becerril-Garcia, M.A.; Limon-Flores, A.Y.; Vazquez-Leyva, S.; Medina-Rivero, E.; Pavon, L.; Velasco-Velazquez, M.A.; Perez-Tapia, S.M. Herpes Murine Model as a Biological Assay to Test Dialyzable Leukocyte Extracts Activity. J. Immunol. Res. 2015, 2015, 146305. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Vallejo-Castillo, L.; Fragozo, A.; Vázquez-Leyva, S.; Pavón, L.; Pérez-Sánchez, G.; Soria-Castro, R.; Mellado-Sánchez, G.; Cobos-Marin, L.; Pérez-Tapia, S.M. Increased Survival in Puppies Affected by Canine Parvovirus Type II Using an Immunomodulator as a Therapeutic Aid. Sci. Rep. 2021, 11, 19864. [Google Scholar] [CrossRef]
- Muñoz, A.I.; Maldonado-García, J.L.; Fragozo, A.; Vallejo-Castillo, L.; Lucas-Gonzalez, A.; Trejo-Martínez, I.; Pavón, L.; Pérez-Sánchez, G.; Cobos-Marin, L.; Pérez-Tapia, S.M. Altered Neutrophil-to-Lymphocyte Ratio in Sepsis Secondary to Canine Parvoviral Enteritis Treated with and without an Immunomodulator in Puppies. Front. Vet. Sci. 2022, 9, 995443. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Marchese, A.; Majetschak, M. CXC Chemokine Receptor 4 Is a Cell Surface Receptor for Extracellular Ubiquitin. J. Biol. Chem. 2010, 285, 15566–15576. [Google Scholar] [CrossRef]
- Saini, V.; Staren, D.M.; Ziarek, J.J.; Nashaat, Z.N.; Campbell, E.M.; Volkman, B.F.; Marchese, A.; Majetschak, M. The CXC Chemokine Receptor 4 Ligands Ubiquitin and Stromal Cell-Derived Factor-1α Function through Distinct Receptor Interactions. J. Biol. Chem. 2011, 286, 33466–33477. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Davis, J.D.; Staren, D.M.; Volkman, B.F.; Majetschak, M. CXC Chemokine Receptor 4 Signaling upon Co-Activation with Stromal Cell-Derived Factor-1α and Ubiquitin. Cytokine 2014, 65, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xie, G.; Xiao, H.; Ding, F.; Bao, W.; Zhang, M. CXCR4 Knockdown Prevents Inflammatory Cytokine Expression in Macrophages by Suppressing Activation of MAPK and NF-ΚB Signaling Pathways. Cell Biosci. 2019, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Guyon, A. CXCL12 Chemokine and Its Receptors as Major Players in the Interactions between Immune and Nervous Systems. Front. Cell. Neurosci. 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, I.E.; Rodríguez, M.C.; Cerbón, M.; Ramos-Martínez, J.C.; Ramos-Martínez, E.G. Role of the Cholinergic Anti-Inflammatory Reflex in Central Nervous System Diseases. Int. J. Mol. Sci. 2021, 22, 13427. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Castro, E.A.; Talavera-Peña, A.K.; Reyes-Lagos, J.J.; Becerril-Villanueva, E.; Pérez-Sanchez, G.; de la Peña, F.R.; Maldonado-García, J.L.; Pavón, L. Modulation of Vagal Activity May Help Reduce Neurodevelopmental Damage in the Offspring of Mothers with Pre-Eclampsia. Front. Immunol. 2023, 14, 1280334. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, S.C. Ubiquitin Signaling in Immune Responses. Cell Res. 2016, 26, 457–483. [Google Scholar] [CrossRef]
- Liu, H.; Wilson, K.R.; Schriek, P.; Macri, C.; Blum, A.B.; Francis, L.; Heinlein, M.; Nataraja, C.; Harris, J.; Jones, S.A.; et al. Ubiquitination of MHC Class II Is Required for Development of Regulatory but Not Conventional CD4+ T Cells. J. Immunol. 2020, 205, 1207–1216. [Google Scholar] [CrossRef]
- Schriek, P.; Liu, H.; Ching, A.C.; Huang, P.; Gupta, N.; Wilson, K.R.; Tsai, M.H.; Yan, Y.; Macri, C.F.; Dagley, L.F.; et al. Physiological Substrates and Ontogeny-Specific Expression of the Ubiquitin Ligases MARCH1 and MARCH8. Curr. Res. Immunol. 2021, 2, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uribe, A.P.; Valencia-Martínez, H.; Carballo-Uicab, G.; Vallejo-Castillo, L.; Medina-Rivero, E.; Chacón-Salinas, R.; Pavón, L.; Velasco-Velázquez, M.A.; Mellado-Sánchez, G.; Estrada-Parra, S.; et al. CD80 Expression Correlates with IL-6 Production in THP-1-like Macrophages Costimulated with LPS and Dialyzable Leukocyte Extract (Transferon®). J. Immunol. Res. 2019, 2019, 2198508. [Google Scholar] [CrossRef] [PubMed]
- Pavón, L.; Sandoval-López, G.; Eugenia Hernández, M.; Loría, F.; Estrada, I.; Pérez, M.; Moreno, J.; Ávila, U.; Leff, P.; Antón, B.; et al. Th2 Cytokine Response in Major Depressive Disorder Patients before Treatment. J. Neuroimmunol. 2006, 172, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.E.; Mendieta, D.; Pérez-Tapia, M.; Bojalil, R.; Estrada-Garcia, I.; Estrada-Parra, S.; Pavón, L. Effect of Selective Serotonin Reuptake Inhibitors and Immunomodulator on Cytokines Levels: An Alternative Therapy for Patients with Major Depressive Disorder. Clin. Dev. Immunol. 2013, 2013, 267871. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Gutiérrez-Ospina, G.; López-Santiago, R.; Maldonado-Tapia, J.O.; Pérez-Tapia, S.M.; Moreno-Lafont, M.C. Behavioral and Neurochemical Shifts at the Hippocampus and Frontal Cortex Are Associated to Peripheral Inflammation in Balb/c Mice Infected with Brucella Abortus 2308. Microorganisms 2021, 9, 1937. [Google Scholar] [CrossRef]
- Maldonado-García, J.L.; Pérez-Sánchez, G.; Becerril-Villanueva, E.; Alvarez-Herrera, S.; Pavón, L.; Sánchez-Torres, L.; Gutiérrez-Ospina, G.; Girón-Pérez, M.I.; Damian-Morales, G.; Maldonado-Tapia, J.O.; et al. Imipramine Administration in Brucella Abortus 2308-Infected Mice Restores Hippocampal Serotonin Levels, Muscle Strength, and Mood, and Decreases Spleen CFU Count. Pharmaceuticals 2023, 16, 1525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).