Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions
Abstract
1. Introduction
2. Results
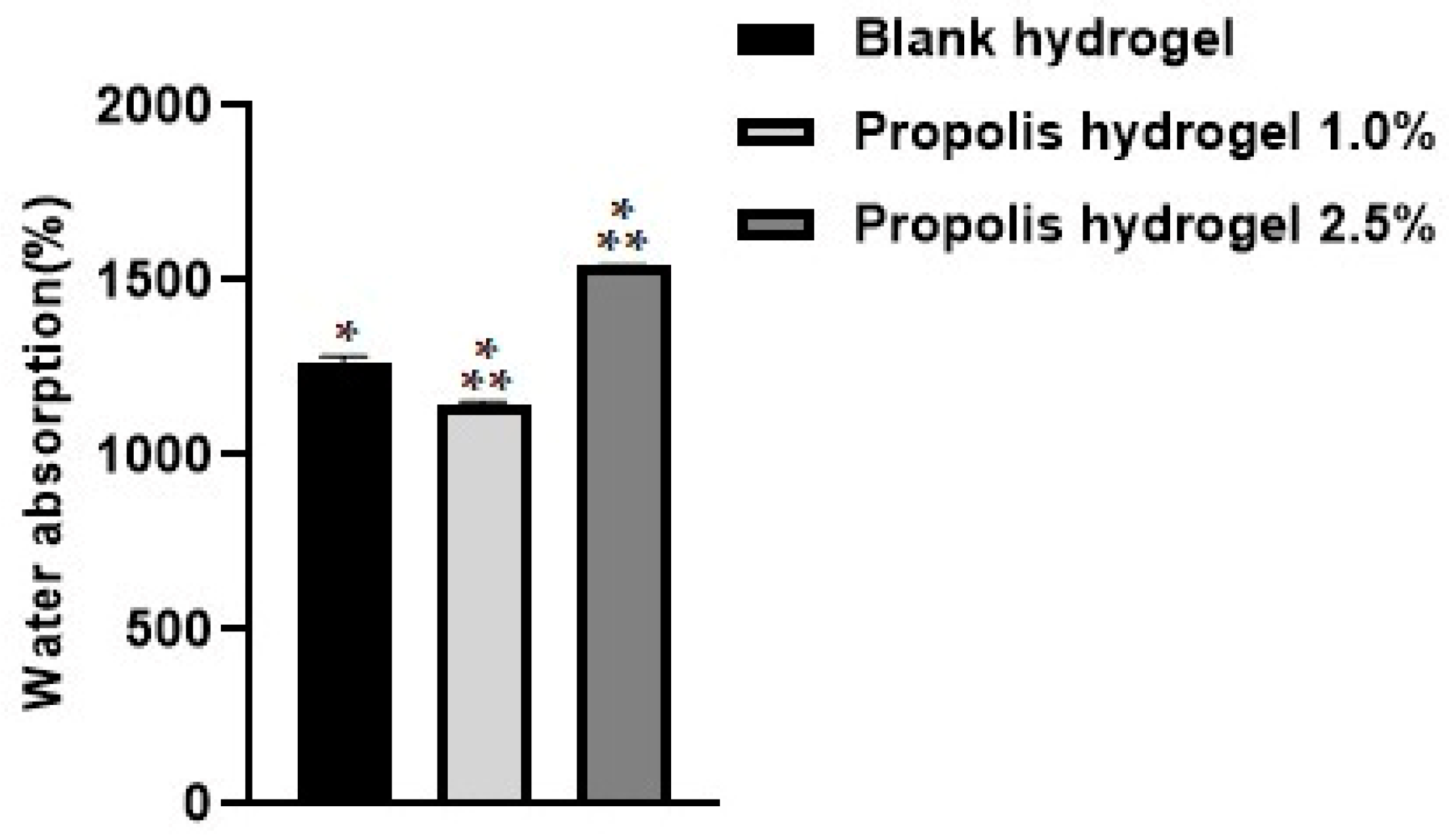
2.1. Water Absorption
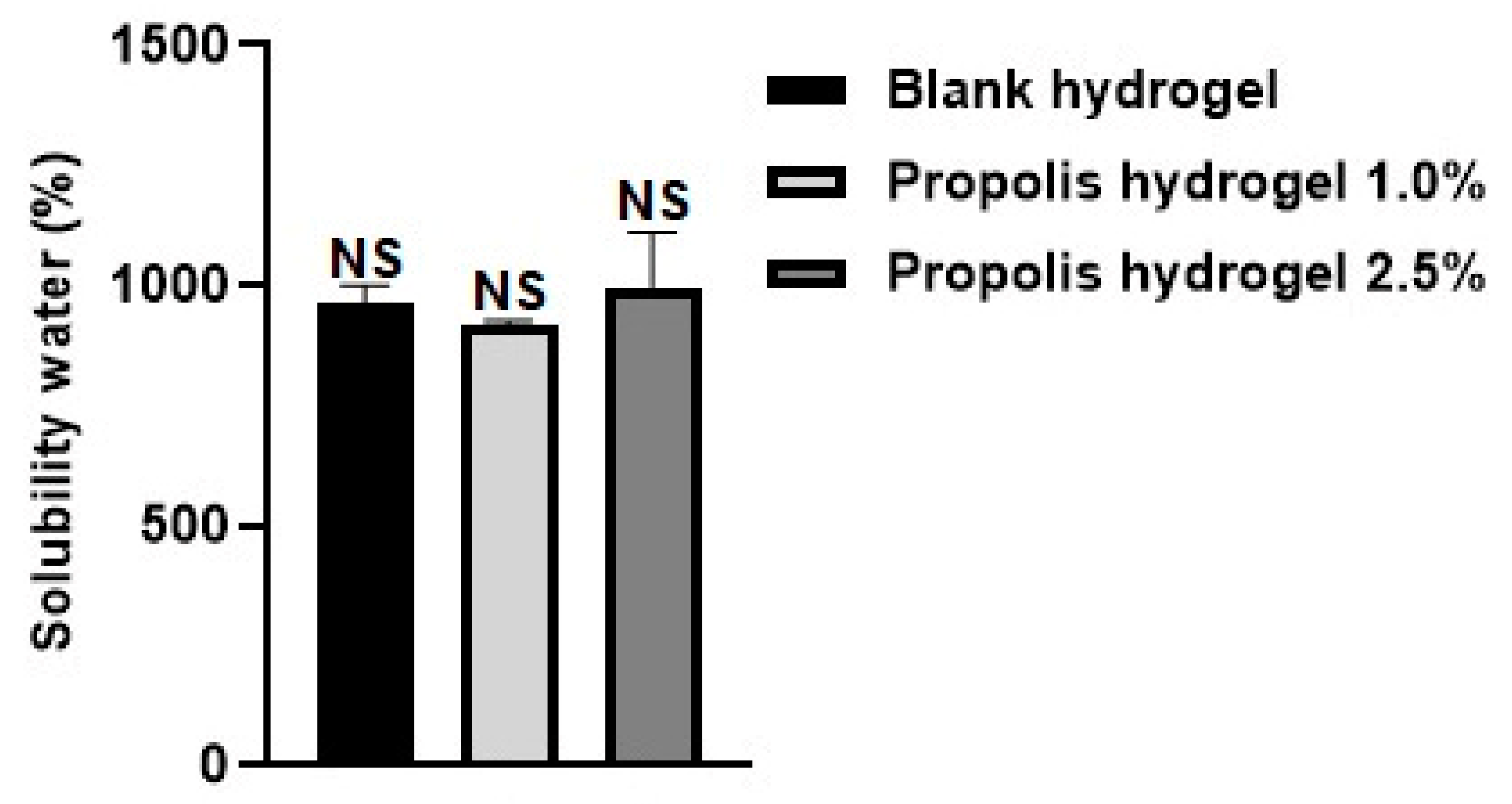
2.2. Solubility in Water
2.3. Porosity
2.4. Gel Fraction
2.5. Water Retention Capacity
2.6. Water Vapor Transmission
2.7. X-ray Diffractometry (XRD)
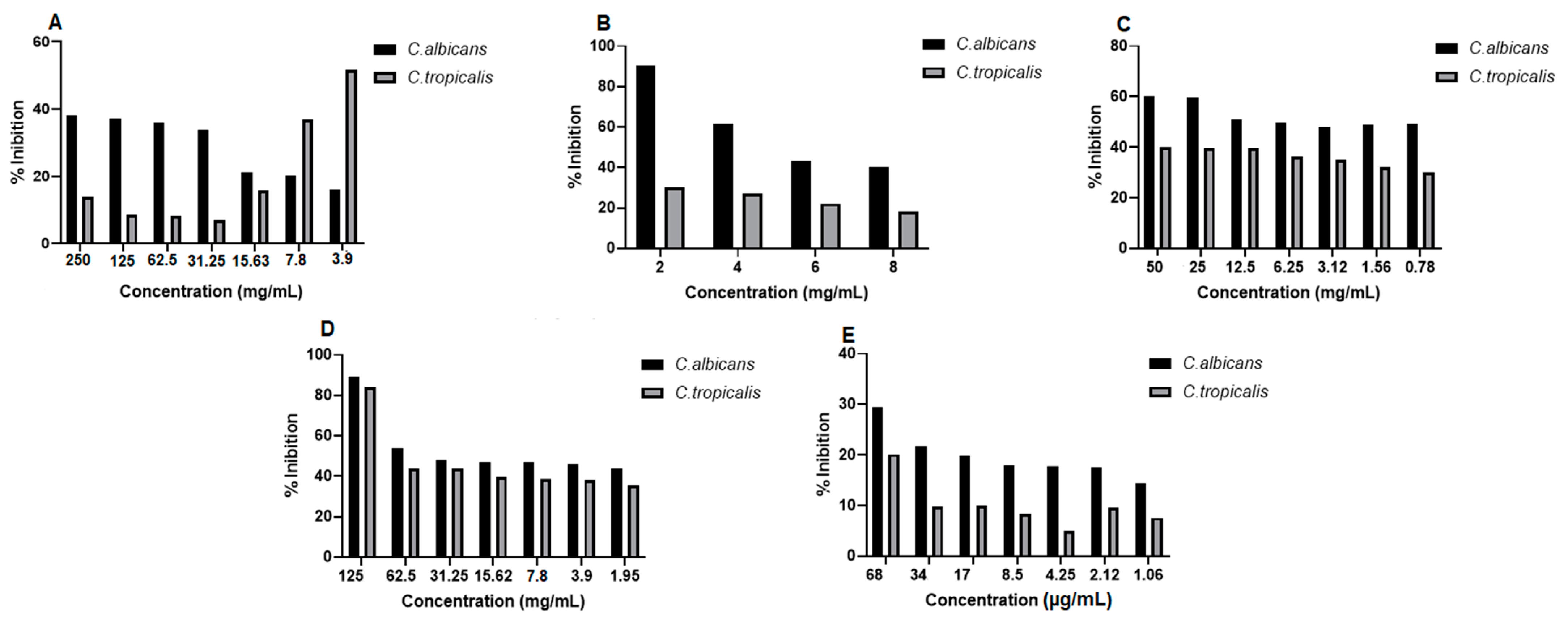
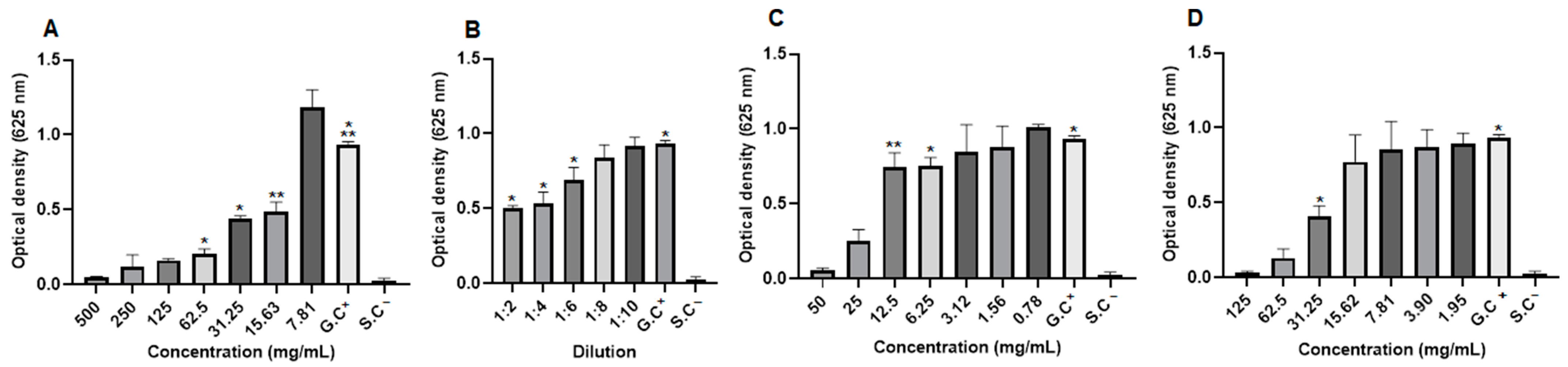
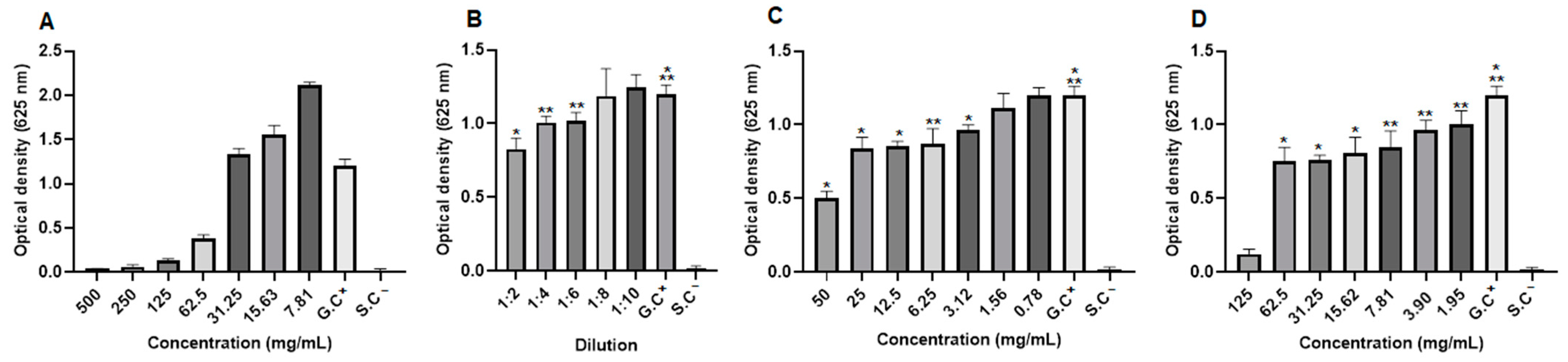
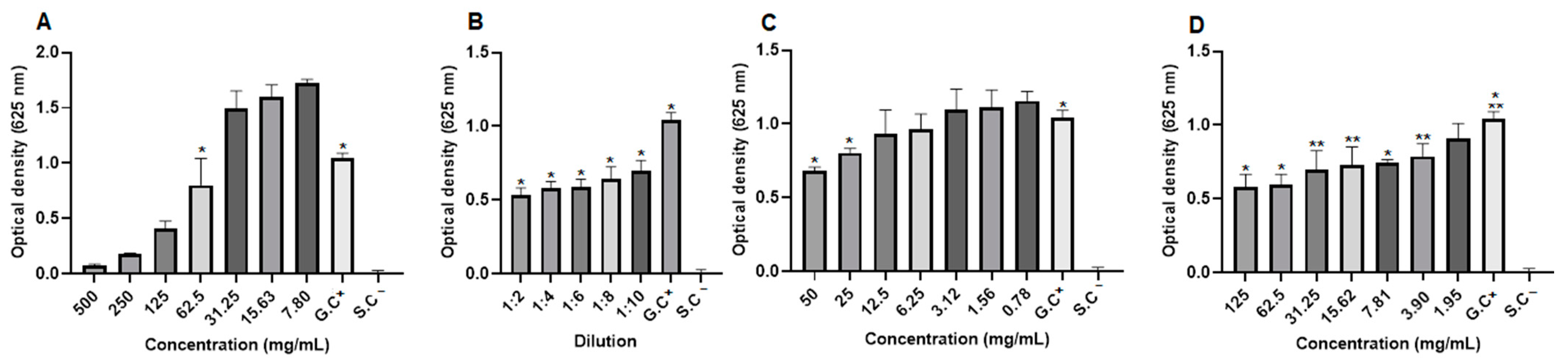
2.8. Antifungal Activity
2.9. Antibacterial Activity
2.10. Antioxidant Activity by Reduction of the Phosphomolybdenum Complex
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Natural Products
4.3. Hydrogel Synthesis
4.4. Water Absorption
4.5. Solubility in Water
4.6. Porosity
4.7. Gel Fraction
4.8. Water Retention Capacity
4.9. Water Vapor Transmission
4.10. X-ray Diffractometry (XDR)
4.11. Antifungal Activity
4.12. Antibacterial Activity
4.13. Antioxidant Activity by Reduction of the Phosphomolybdenum Complex
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Permyakova, E.S.; Solovieva, A.O.; Sitnikova, N.; Kiryukhantsev-Korneev, P.V.; Kutzhanov, M.K.; Sheveyko, A.N.; Ignatov, S.G.; Slukin, P.V.; Shtansky, D.V.; Manakhov, A.M. Polycaprolactone nanofibers functionalized by fibronectin/gentamicin and implanted silver for enhanced antibacterial properties, cell adhesion, and proliferation. Polymers 2024, 16, 261. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Wang, D.; Wang, H.; Chen, H.; Wu, X.; Li, T.; Qi, J.; Chen, T.; Wu, D.; Gao, Y. Revitalizing skin repair: Unveiling the healing power of livisin, a natural peptide calcium mimetic. Toxins 2024, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Zheremyan, E.A.; Ustiugova, A.S.; Karamushka, N.M.; Uvarova, A.N.; Stasevich, E.M.; Bogolyubova, A.V.; Kuprash, D.V.; Korneev, K.V. Breg-mediated immunoregulation in the skin. Int. J. Mol. Sci. 2024, 25, 583. [Google Scholar] [CrossRef] [PubMed]
- Julovi, S.M.; McKelvey, K.; Minhas, N.; Chan, Y.K.A.; Xue, M.; Jackson, C.J. Involvement of PAR-2 in the induction of cell-specific matrix metalloproteinase-2 by activated protein C in cutaneous wound healing. Int. J. Mol. Sci. 2024, 25, 370. [Google Scholar] [CrossRef]
- Quiñones-Vico, M.I.; Fernández-González, A.; Ubago-Rodríguez, A.; Moll, K.; Norrby-Teglund, A.; Svensson, M.; Gutiérrez-Fernández, J.; Torres, J.M.; Arias-Santiago, S. Antibiotics against Pseudomonas aeruginosa on human skin cell lines: Determination of the highest non-cytotoxic concentrations with antibiofilm capacity for wound healing strategies. Pharmaceutics 2024, 16, 117. [Google Scholar] [CrossRef]
- Witkowska, K.; Paczkowska-Walendowska, M.; Plech, T.; Szymanowska, D.; Michniak-Kohn, B.; Cielecka-Piontek, J. Chitosan-based hydrogels for controlled delivery of asiaticoside-rich centella asiatica extracts with wound healing potential. Int. J. Mol. Sci. 2023, 24, 17229. [Google Scholar] [CrossRef]
- Gyles, D.A.; Pereira, A.D.; Castro, L.D.; Brigida, A.S.; Nobre Lamarão, M.L.; Ramos Barbosa, W.L.; Carréra Silva, J.O.; Ribeiro-Costa, R.M. Polyacrylamide-metilcellulose hydrogels containing Aloe barbadensis extract as dressing for treatment of chronic cutaneous skin lesions. Polymers 2020, 12, 690. [Google Scholar] [CrossRef]
- Aguiar Junior, A.C.; Isaac, C.; Nicolosi, J.T.; Medeiros, M.M.M.d.; Paggiaro, A.O.; Gemperi, R. Analysis of the clinical care of patients with chronic ulcers of the lower limbs. Rev. Bras. Cir. Plást. 2015, 30, 2. [Google Scholar] [CrossRef]
- Verdú-Soriano, J.; Casado-Díaz, A.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Moreno-Moreno, P.; Dorado, G.; Quesada-Gómez, J.M.; Rodríguez-Mañas, L.; Lázaro-Martínez, J.L. Hard-to-Heal Wound healing: Superiority of hydrogel EHO-85 (containing Olea europaea Leaf extract) vs. a standard hydrogel. a randomized controlled trial. Gels 2023, 9, 962. [Google Scholar] [CrossRef]
- Chandika, P.; Kim, M.S.; Khan, F.; Kim, Y.M.; Heo, S.Y.; Oh, G.W.; Kim, N.G.; Jung, W.K. Wound healing properties of triple cross-linked poly (vinyl alcohol)/methacrylate kappa-carrageenan/chitooligosaccharide hydrogel. Carbohydr. Polym. 2021, 269, 118272. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, J.H.; Kang, K.K.; Sung, S.E.; Lee, S.; Sung, M.; Seo, M.S.; Park, J.H. Antioxidant and antimelanogenic activities of Lactobacillus kunkeei NCHBL-003 isolated from honeybees. Microorganisms 2024, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, W.; He, G.; Yang, J.; Li, J.; Ma, H.; Wang, S. Hydrogel-transformable antioxidant poly-γ-glutamic acid/polyethyleneimine hemostatic powder for efficient wound hemostasis. Gels 2024, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, R.A.; Alminderej, F.M.; Al-Harby, N.F.; Elmehbad, N.Y.; Mohamed, N.A. Design, synthesis, and characterization of novel bis-uracil chitosan hydrogels modified with zinc oxide nanoparticles for boosting their antimicrobial activity. Polymers 2023, 15, 980. [Google Scholar] [CrossRef] [PubMed]
- Díez-García, I.; Lemma, M.R.d.C.; Barud, H.S.; Eceiza, A.; Tercjak, A. Hydrogels based on waterborne poly(urethane-urea)s by physically cross-linking with sodium alginate and calcium chloride. Carbohydr. Polym. 2020, 250, 116940. [Google Scholar] [CrossRef] [PubMed]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo-sensitive poly (N-isopropylacrylamide-co-polyacrylamide) hydrogel for pH-responsive therapeutic delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef]
- Zhang, Y.; He, W.; Zhang, S.; Hu, X.; Sun, S.; Gao, H.; Kong, J. Poloxam thermosensitive hydrogels loaded with HFGF2-linked camelina lipid droplets accelerate skin regeneration in deep second-degree burns. Int. J. Mol. Sci. 2022, 23, 12716. [Google Scholar] [CrossRef]
- Lei, Q.; Zhang, Y.; Zhang, W.; Li, R.; Ao, N.; Zhang, H. A Synergy between dopamine and electrostatically bound bactericide in a poly (vinyl alcohol) hybrid hydrogel for treating infected wounds. Carbohydr. Polym. 2021, 272, 118513. [Google Scholar] [CrossRef]
- Amirian, J.; Zeng, Y.; Shekh, M.I.; Sharma, G.; Stadler, F.J.; Song, J.; Du, B.; Zhu, Y. In-situ crosslinked hydrogel based on amidated pectin/oxidized chitosan as potential wound dressing for skin repairing. Carbohydr. Polym. 2021, 251, 117005. [Google Scholar] [CrossRef]
- Garcia, L.V.; Silva, D.; Costa, M.M.; Armés, H.; Salema-Oom, M.; Saramago, B.; Serro, A.P. Antiseptic-loaded casein hydrogels for wound dressings. Pharmaceutics 2023, 15, 334. [Google Scholar] [CrossRef]
- Zandraa, O.; Ngwabebhoh, F.A.; Patwa, R.; Nguyen, H.T.; Motiei, M.; Saha, N.; Saha, T.; Saha, P. Development of dual crosslinked mumio-based hydrogel dressing for wound healing application: Physico-chemistry and antimicrobial activity. Int. J. Pharm. 2021, 607, 120952. [Google Scholar] [CrossRef]
- Elangwe, C.N.; Morozkina, S.N.; Olekhnovich, R.O.; Polyakova, V.O.; Krasichkov, A.; Yablonskiy, P.K.; Uspenskaya, M. V Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review. Int. J. Mol. Sci. 2023, 24, 4962. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in antibacterial hydrogel dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Kang, H.W. Fucoidan-loaded hydrogels facilitates wound healing using photodynamic therapy by in vitro and in vivo evaluation. Carbohydr. Polym. 2020, 247, 116624. [Google Scholar] [CrossRef] [PubMed]
- Chelu, M.; Musuc, A.M.; Aricov, L.; Ozon, E.A.; Iosageanu, A.; Stefan, L.M.; Prelipcean, A.-M.; Popa, M.; Moreno, J.C. Antibacterial Aloe vera based biocompatible hydrogel for use in dermatological applications. Int. J. Mol. Sci. 2023, 24, 3893. [Google Scholar] [CrossRef]
- Gounden, V.; Singh, M. Hydrogels and wound healing: Current and future prospects. Gels 2024, 10, 43. [Google Scholar] [CrossRef]
- Michalicha, A.; Belcarz, A.; Giannakoudakis, D.A.; Staniszewska, M.; Barczak, M. Designing composite stimuli-responsive hydrogels for wound healing applications: The state-of-the-art and recent discoveries. Materials 2024, 17, 278. [Google Scholar] [CrossRef]
- Grigore, A.; Vatasescu-Balcan, A.; Stoleru, S.; Zugravu, A.; Poenaru, E.; Engi, M.; Coman, O.A.; Fulga, I. Experimental research on the influence of ion channels on the healing of skin wounds in rats. Processes 2024, 12, 109. [Google Scholar] [CrossRef]
- Zakerikhoob, M.; Abbasi, S.; Yousefi, G.; Mokhtari, M.; Noorbakhsh, M.S. Curcumin-incorporated crosslinked sodium alginate-g-poly (N-isopropyl acrylamide) thermo-responsive hydrogel as an in-situ forming injectable dressing for wound healing: In vitro characterization and in vivo evaluation. Carbohydr. Polym. 2021, 271, 118434. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Pengsuk, C.; Lirdprapamongkol, K.; Thanyacharoen, T.; Techasakul, S.; Svasti, J.; Nooeaid, P. Turmeric herb extract-incorporated biopolymer dressings with beneficial antibacterial, antioxidant and anti-inflammatory properties for wound healing. Polymers 2023, 15, 1090. [Google Scholar] [CrossRef]
- Pawłowicz, K.; Paczkowska-walendowska, M.; Osmałek, T.; Cielecka-piontek, J. Towards the preparation of a hydrogel from lyophilisates of the Aloe arborescens aqueous extract. Pharmaceutics 2022, 14, 1489. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Bandeira, E.d.S.; Gomes, M.F.; Lynch, D.G.; Bastos, G.N.T.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Polyacrylamide hydrogel containing calendula extract as a wound healing bandage: In vivo test. Int. J. Mol. Sci. 2023, 24, 3806. [Google Scholar] [CrossRef] [PubMed]
- González-Montiel, L.; Figueira, A.C.; Medina-Pérez, G.; Fernández-Luqueño, F.; Aguirre-Álvarez, G.; Pérez-Soto, E.; Pérez-Ríos, S.; Campos-Montiel, R.G. Bioactive compounds, antioxidant and antimicrobial activity of propolis extracts during in vitro digestion. Appl. Sci. 2022, 12, 7892. [Google Scholar] [CrossRef]
- Alenezi, S.S.; Alenezi, N.D.; Ebiloma, G.U.; Natto, M.J.; Ungogo, M.A.; Igoli, J.O.; Ferro, V.A.; Gray, A.I.; Fearnley, J.; de Koning, H.P.; et al. The antiprotozoal activity of papua new Guinea propolis and its triterpenes. Molecules 2022, 27, 1622. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.M.; Camara, C.A.; Silva, E.M.S.D.; Santos, M.S.; Leite, A.B.; Queiroz, A.C.; Evelyn Da Silva, A.; Araújo, M.V.; Alexandre-Moreira, M.S.; Silva, T.M.S. Leismanicidal activity of propolis collected in the semiarid region of Brazil. Front. Pharmacol. 2021, 12, 702032. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Yañez, C.R.; Ruiz-Hurtado, P.A.; Reyes-Reali, J.; Mendoza-Ramos, M.I.; Vargas-Díaz, M.E.; Hernández-Sánchez, K.M.; Pozo-Molina, G.; Méndez-Catalá, C.F.; García-Romo, G.S.; Pedroza-González, A.; et al. Antifungal activity of Mexican propolis on clinical isolates of Candida species. Molecules 2022, 27, 5651. [Google Scholar] [CrossRef]
- Sousa, L.R.D.; Amparo, T.R.; Souza, G.H.B.d.; Ferraz, A.T.; Fonseca, K.d.S.; Azevedo, A.S.d.; Nascimento, A.M.d.; Andrade, Â.L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-trypanosoma cruzi potential of vestitol isolated from lyophilized red propolis. Molecules 2023, 28, 7812. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Souza, P.D.Q.d.; Pereira, R.R.; da Silva, E.O.; Barbosa, W.L.R.; Silva-Júnior, J.O.C.; Converti, A.; Ribeiro-Costa, R.M. Preliminary study on the chemical and biological properties of propolis extract from stingless bees from the northern region of Brazil. Processes 2024, 12, 700. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D.; Melliou, E.; Chinou, I. Antiproliferative activity of greek propolis. J. Med. Food 2010, 13, 286–290. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, E.H.; Jung, K.J.; Jin, C. Inhibition of angiogenesis by propolis. Arch. Pharm. Res. 2002, 25, 500–504. [Google Scholar] [CrossRef]
- Weng, M.S.; Ho, Y.S.; Lin, J.K. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing p21Waf1/Cip1 expression: Involvement of p38 mitogen-activated protein kinase. Biochem. Pharm. 2005, 69, 1815–1827. [Google Scholar] [CrossRef]
- Gogacz, M.; Peszke, J.; Natorska-Chomicka, D.; Makuch-Kocka, A.; Dos Santos Szewczyk, K. Anticancer effects of propolis extracts obtained with the cold separation method on PC-3 and DU-145 prostate cancer cell lines. Molecules 2022, 27, 8245. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.S.; Jin, C.; Jung, K.J.; Park, E.H. Estrogenic effects of ethanol and ether extracts of propolis. J. Ethnopharmacol. 2002, 82, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.K.; Kim, H.S.; Chung, S.T.; Eom, J.H.; Kim, K.A.; Oh, H.Y. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. Int. Immunopharmac. 2004, 4, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ebuchi, S.; Fujise, T.; Abesundara, K.J.; Doi, S.; Yamada, H.; Matsumoto, K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-o-caffeoylquinic acid. Biol. Pharm. Bull. 2004, 27, 1797–1803. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Adnyana, I.K.; Ishii, E.; Midorikawa, K.; Matsushige, K.; Kadota, S. Hepatoprotective and anti-Helicobacter pylori activities of constituents from Brazilian propolis. Phytomedicine 2001, 8, 16–23. [Google Scholar] [CrossRef]
- Cardile, V.; Panico, A.; Gentile, B.; Borrelli, F.; Russo, A. Effect of propolis on human cartilage and chondrocytes. Life Sci. 2003, 73, 1027–1035. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Elakkad, Y.E.; Elwakeel, A.A.; Allam, R.M.; Mousa, M.R. Formulation and characterization of propolis and tea tree oil nanoemulsion loaded with clindamycin hydrochloride for wound healing: In-vitro and in-vivo wound healing assessment. Saudi Pharm. J. 2021, 29, 1238–1249. [Google Scholar] [CrossRef]
- Said Dos Santos, R.; Bassi da Silva, J.; Rosseto, H.C.; Vecchi, C.F.; Campanholi, K.d.S.S.; Caetano, W.; Bruschi, M.L. Emulgels containing propolis and curcumin: The effect of type of vegetable oil, poly(acrylic acid) and bioactive agent on physicochemical stability, mechanical and rheological properties. Gels 2021, 7, 120. [Google Scholar] [CrossRef]
- Sato, T.; Mello, D.; Vasconcelos, L.; Valente, A.; Borges, A. Chitosan-based coacervate polymers for propolis encapsulation: Release and cytotoxicity studies. Int. J. Mol. Sci. 2020, 21, 4561. [Google Scholar] [CrossRef]
- Alarjani, K.M.; Yehia, H.M.; Badr, A.N.; Ali, H.S.; Al-Masoud, A.H.; Alhaqbani, S.M.; Alkhatib, S.A.; Rady, A.M.; Abdel-Maksoud, M. Antimicrobial impact of a propolis/PVA/chitosan composite and its prospective application against methicillin resistance bacterial infection. Front. Nanotechnol. 2024, 6, 1387933. [Google Scholar] [CrossRef]
- Phonrachom, O.; Charoensuk, P.; Kiti, K.; Saichana, N.; Kakumyan, P.; Suwantong, O. Potential use of propolis-loaded quaternized chitosan/pectin hydrogel films as wound dressings: Preparation, characterization, antibacterial evaluation, and in vitro healing assay. Inter. J. Biol. Macromolec. 2023, 241, 124633. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Yu, D.; Liu, W. Ultrastretchable, repairable and highly sensitive xanthan collagen nanosilver hydrogel for wide temperature flexible sensing. Chem. Eng. J. 2023, 470, 144385. [Google Scholar] [CrossRef]
- Ferreira, L.M.d.M.C.; Modesto, Y.Y.; Souza, P.D.Q.d.; Nascimento, F.C.d.A.; Pereira, R.R.; Converti, A.; Lynch, D.G.; Brasil, D.d.S.B.; da Silva, E.O.; Silva-Júnior, J.O.C.; et al. Characterization, biocompatibility and antioxidant activity of hydrogels containing propolis extract as an alternative treatment in wound healing. Pharmaceuticals 2024, 17, 575. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.R.; Kamoun, E.A.; Mohy Eldin, M.S.; El-Meligy, M.A. Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arabian J. Chem. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Design and optimization of process parameters of polyvinyl (alcohol)/chitosan/nano zinc oxide hydrogels as wound healing materials. Carbohydr. Polym. 2019, 207, 542–554. [Google Scholar] [CrossRef]
- Catanzano, O.; Gomez d’Ayala, G.; D’Agostino, A.; Di Lorenzo, F.; Schiraldi, C.; Malinconico, M.; Lanzetta, R.; Bonina, F.; Laurienzo, P. PEG-crosslinked-chitosan hydrogel films for in situ delivery of Opuntia ficus-indica extract. Carbohydr. Polym. 2021, 264, 117987. [Google Scholar] [CrossRef]
- Ajaz, N.; Abbas, A.; Afshan, R.; Irfan, M.; Khalid, S.H.; Asghar, S.; Munir, M.U.; Rizg, W.Y.; Majrashi, K.A.; Alshehri, S.; et al. In vitro and in vivo evaluation of hydroxypropyl-β-cyclodextrin-grafted-poly(acrylic acid)/poly(vinyl pyrrolidone) semi-interpenetrating matrices of dexamethasone sodium phosphate. Pharmaceuticals 2022, 15, 1399. [Google Scholar] [CrossRef]
- Zhang, R.; Lei, L.; Song, Q.; Li, X. Calcium ion cross-linking alginate/dexamethasone sodium phosphate hybrid hydrogel for extended drug release. Colloids Surf. B. 2019, 175, 569–575. [Google Scholar] [CrossRef]
- Augustine, R.; Dan, P.; Schlachet, I.; Rouxel, D.; Menu, P.; Sosnik, A. Chitosan Ascorbate Hydrogel Improves Water Uptake Capacity and Cell Adhesion of Electrospun Poly(Epsilon-Caprolactone) Membranes. Int. J. Pharm. 2019, 559, 420–426. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and impact of antioxidant hydrogel in chronic wound healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef]
- Kędzierska, M.; Jamroży, M.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Bańkosz, M.; Gruca, M.; Potemski, P.; Tyliszczak, B. Analysis of the influence of both the average molecular weight and the content of crosslinking agent on physicochemical properties of PVP-based hydrogels developed as innovative dressings. Int. J. Mol. Sci. 2022, 23, 11618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, Y.; Li, J.; Zhang, C.; Gao, F.; Ma, X.; Zhang, J.; Fu, C.; Geng, F. A Feasible Biocompatible Hydrogel Film Embedding Periplaneta Americana Extract for Acute Wound Healing. Int. J. Pharm. 2019, 571, 118707. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-peg hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, M.; Ding, J.; Lin, Y. Hydrogels: A promising therapeutic platform for inflammatory skin diseases treatment. J. Mat. Chem. B 2024, 12, 8007–8032. [Google Scholar] [CrossRef] [PubMed]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Preda, P.; Enciu, A.-M.; Adiaconita, B.; Mihalache, I.; Craciun, G.; Boldeiu, A.; Aricov, L.; Romanitan, C.; Stan, D.; Marculescu, C.; et al. New Amorphous Hydrogels with Proliferative Properties as Potential Tools in Wound Healing. Gels 2022, 8, 604. [Google Scholar] [CrossRef]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Li, Y.; Ding, X.; Li, D.; Shen, C.; Xu, F.J. Dual-crosslinked amorphous polysaccharide hydrogels based on chitosan/alginate for wound healing applications. Macromol. Rapid Commun. 2018, 39, 1800069. [Google Scholar] [CrossRef]
- Afrin, S.; Haque, P.; Islam, S.; Hossain, S. Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug. Gels 2022, 8, 340. [Google Scholar] [CrossRef]
- He, F.; Zhou, Q.; Wang, L.; Yu, G.; Li, J.; Feng, Y. Fabrication of a sustained release delivery system for pesticides using interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Appl. Clay. Sci. 2019, 183, 105347. [Google Scholar] [CrossRef]
- Barak, A.; Goel, Y.; Kumar, R.; Shukla, S.K. Removal of methyl orange over TiO2/polyacrylamide hydrogel. Mater. Today Proc. 2019, 12, 529–535. [Google Scholar] [CrossRef]
- Guebitz, G.M.; Nyanhongo, G.S. Enzymes as green catalysts and interactive biomolecules in wound dressing hydrogels. Trends Biotechnol. 2018, 36, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, P.; Tang, W.; Du, S.; Yu, M.; Lu, H.; Tan, H.; Xing, X. A Facile Injectable Carbon Dot/Oxidative Polysaccharide Hydrogel with Potent Self-Healing and High Antibacterial Activity. Carbohydr. Polym. 2021, 251, 117040. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, M.; Nanaki, S.; Zamboulis, A.; Papoulia, C.; Chrissafis, K.; Klonos, P.A.; Kyritsis, A.; Vergkizi-Nikolakaki, S.; Kostoglou, M.; Bikiaris, D.N. Super absorbent chitosan-based hydrogel sponges as carriers for caspofungin antifungal drug. Int. J. Pharm. 2021, 606, 120925. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Fang, Y.; Li, P.; Zhou, W.; Wang, Z.; Chen, M.; Liu, H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater. Sci. Eng. C 2020, 109, 110649. [Google Scholar] [CrossRef]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel wound dressings for bioactive treatment of acute and chronic wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Aldubaisi, Y.; Swami, V.; Swami, V.; Xu, G.; Vaughan, M.B.; Wolf, R.F.; Khandaker, M. Polycaprolactone electrospun nanofiber membrane with skin graft containing collagen and bandage containing MgO nanoparticles for wound healing applications. Polymers 2023, 15, 2014. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.H.; Lee, J.E.; Park, S.J.; Kim, K.; Kim, I.S.; Lee, Y.S.; Hwang, N.S.; Kim, B.G. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 2018, 178, 401–412. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.W.; Jang, Y.M.; Ko, S.C.; Park, W.S.; Choi, I.W.; Kim, Y.M.; Jung, W.K. Antimicrobial hydrogels based on PVA and diphlorethohydroxycarmalol (DPHC) derived from brown alga ishige okamurae: An in vitro and in vivo study for wound dressing application. Mater. Sci. Eng. C 2020, 107, 110352. [Google Scholar] [CrossRef]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-Haghi, A. Chemical composition and antimicrobial activities of Iranian propolis. Iran Biomed. J. 2018, 22, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Assis, D.S.; Ramos, L.D.P.; Hasna, A.A.; Queiroz, T.S.D.; Lima, N.D.; Berretta, A.A. Antimicrobial and antibiofilm effect of Brazilian green propolis aqueous extract against dental anaerobic bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef] [PubMed]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic constituents, antioxidant and antimicrobial activity and clustering analysis of propolis samples based on PCA from different regions of anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical variability and pharmacological potential of propolis as a source for the development of new pharmaceutical products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant sources responsible for the chemical composition and main bioactive properties of poplar-type propolis. Plants 2021, 10, 22. [Google Scholar] [CrossRef]
- Šturm, L.; Poklar Ulrih, N. Propolis flavonoids and terpenes, and their interactions with model lipid membranes: A review. Adv. Biom. Lipid Self-Assem. 2020, 32, 25–52. [Google Scholar] [CrossRef]
- Dwivedi, K.; Mandal, A.K.; Afzal, O.; Altamimi, A.S.A.; Sahoo, A.; Alossaimi, M.A.; Almalki, W.H.; Alzahrani, A.; Barkat, M.A.; Almeleebia, T.M.; et al. Emergence of nano-based formulations for effective delivery of flavonoids against topical infectious disorders. Gels 2023, 9, 671. [Google Scholar] [CrossRef]
- Marcucci, M.C.; Gutierrez- Gonçalves, M.E.J. Activities, a. atividades antimicrobiana e antioxidante da própolis do estado do Ceará antimicrobial and antioxidant activities of própolis from Ceará State. Rev. Fitos 2009, 4, 81–86. [Google Scholar] [CrossRef]
- Letullier, C.; Manduchet, A.; Dlalah, N.; Hugou, M.; Georgé, S.; Sforcin, J.M.; Cardinault, N. Comparison of the antibacterial efficiency of propolis samples from different botanical and geographic origins with and without standardization. J. Api. Res. 2020, 59, 19–24. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; Barreto, G.D.A.; Costa, S.S.; Silva, D.F.D.; Brandao, H.N.; Padilha, F.F. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Cabral, I.S.R.; Oldoni, T.L.C.; Prado, A.; Bezerra, R.M.N.; Alencar, S.M.D.; Ikegaki, M.; Rosalen, P.L. Phenolic composition, antibacterial and antioxidant activities of brazilian red propolis. Quím. Nova 2009, 32, 1523–1527. [Google Scholar] [CrossRef]
- Segueni, N.; Akkal, S.; Benlabed, K.; Nieto, G. Potential use of propolis in phytocosmetic as phytotherapeutic constituent. Molecules 2022, 27, 5833. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, M.C.; Laranjo, M.; Andrade, N.; Marques, M.; Costa, A.R.; Antunes, C.M. Antimicrobial, antibiofilm and toxicological assessment of propolis. Antibiotics 2023, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, I.S.; Lima, L.R.; Berretta, A.A.; Amorim, N.A.; Pratavieira, S.; Corrêa, T.Q.; Nogueira, F.A.R.; Barud, H.S. Antimicrobial formulation of a bacterial nanocellulose/propolis-containing photosensitizer for biomedical applications. Polymers 2023, 15, 987. [Google Scholar] [CrossRef] [PubMed]
- Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Recent advances in using natural antibacterial additives in bioactive wound dressings. Pharmaceutics 2023, 15, 644. [Google Scholar] [CrossRef]
- Pan, H.; Fan, D.; Duan, Z.; Zhu, C.; Fu, R.; Li, X. Non-Stick Hemostasis Hydrogels as Dressings with Bacterial Barrier Activity for Cutaneous Wound Healing. Mater. Sci. Eng. C 2019, 105, 110118. [Google Scholar] [CrossRef]
- Salgueiro, F.B.; Castro, R.N. Comparison Between the Chemical Composition and Antioxidant Capacity of Different Green Propolis Extracts. Quim. Nova 2016, 39, 1192–1199. [Google Scholar] [CrossRef]
- da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing potential of propolis in skin wounds evidenced by clinical studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid. Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bártolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef]
- Pandey, M.; Choudhury, H.; Segar Singh, S.K.; Annan, N.C.; Bhattamisra, S.K.; Gorain, B.; Amin, M.C.I.M. Budesonide-loaded pectin/polyacrylamide hydrogel for sustained delivery: Fabrication, characterization and in vitro release kinetics. Molecules 2021, 26, 2704. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M 100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- Leska, A.; Nowak, A.; Szulc, J.; Motyl, I.; Czarnecka-Chrebelska, K.H. Antagonistic activity of potentially probiotic lactic acid bacteria against honeybee (Apis mellifera L.) pathogens. Pathogens 2022, 11, 1367. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Metodologia dos Testes de Sensibilidade a Agentes Antimicrobianos por Diluição para Bactéria de Crescimento Aeróbico; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Duarte, M.C.T.; Figueira, G.M.; Sartoratto, A.; Rehder, V.L.G.; Delarmelina, C. Anti-candida activity of Brazilian medicinal plants. J. Ethnopharmacol. 2005, 97, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E 1. Analytical Biochemistry. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.M.d.M.C.; Cruz, N.F.d.; Lynch, D.G.; Costa, P.F.d.; Salgado, C.G.; Silva-Júnior, J.O.C.; Rossi, A.; Ribeiro-Costa, R.M. Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals 2024, 17, 1400. https://doi.org/10.3390/ph17101400
Ferreira LMdMC, Cruz NFd, Lynch DG, Costa PFd, Salgado CG, Silva-Júnior JOC, Rossi A, Ribeiro-Costa RM. Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals. 2024; 17(10):1400. https://doi.org/10.3390/ph17101400
Chicago/Turabian StyleFerreira, Lindalva Maria de Meneses Costa, Naila Ferreira da Cruz, Desireé Gyles Lynch, Patrícia Fagundes da Costa, Claudio Guedes Salgado, José Otávio Carréra Silva-Júnior, Alessandra Rossi, and Roseane Maria Ribeiro-Costa. 2024. "Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions" Pharmaceuticals 17, no. 10: 1400. https://doi.org/10.3390/ph17101400
APA StyleFerreira, L. M. d. M. C., Cruz, N. F. d., Lynch, D. G., Costa, P. F. d., Salgado, C. G., Silva-Júnior, J. O. C., Rossi, A., & Ribeiro-Costa, R. M. (2024). Hydrogel Containing Propolis: Physical Characterization and Evaluation of Biological Activities for Potential Use in the Treatment of Skin Lesions. Pharmaceuticals, 17(10), 1400. https://doi.org/10.3390/ph17101400







